Abstract
The median preoptic nucleus (MnPN) and the ventrolateral preoptic area (VLPO) are two hypothalamic regions that have been implicated in sleep regulation, and both nuclei contain sleep-active GABAergic neurons. Adenosine is an endogenous sleep regulatory substance, which promotes sleep via A1 and A2A receptors (A2AR). Infusion of A2AR agonist into the lateral ventricle or into the subarachnoid space underlying the rostral basal forebrain (SS-rBF), has been previously shown to increase sleep. We examined the effects of an A2AR agonist, CGS-21680, administered into the lateral ventricle and the SS-rBF on sleep and c-Fos protein immunoreactivity (Fos-IR) in GABAergic neurons in the MnPN and VLPO. Intracerebroventricular administration of CGS-21680 during the second half of lights-on phase increased sleep and increased the number of MnPN and VLPO GABAergic neurons expressing Fos-IR. Similar effects were found with CGS-21680 microinjection into the SS-rBF. The induction of Fos-IR in preoptic GABAergic neurons was not secondary to drug-induced sleep, since CGS-21680 delivered to the SS-rBF significantly increased Fos-IR in MnPN and VLPO neurons in animals that were not permitted to sleep. Intracerebroventricular infusion of ZM-241385, an A2AR antagonist, during the last 2 h of a 3-h period of sleep deprivation caused suppression of subsequent recovery sleep and reduced Fos-IR in MnPN and VLPO GABAergic neurons. Our findings support a hypothesis that A2AR-mediated activation of MnPN and VLPO GABAergic neurons contributes to adenosinergic regulation of sleep.
Keywords: median preoptic nucleus, ventrolateral preoptic area, subarachnoid space, c-Fos immunoreactivity, GABAergic neurons
adenosine is an endogenous sleep factor that has been shown to promote non-rapid eye movement (REM) sleep (for review, see Refs. 6, 7, 36, and 39). Extracellular concentrations of adenosine in the basal forebrain increase during wakefulness and decrease during sleep (37). Adenosine and adenosine agonists influence the activity of putative sleep- and wake-regulatory neurons (3, 40). Sleep is increased by administration of adenosine, adenosine receptor agonists, or drugs that increase extracellular adenosine levels in forebrain sites, including the substantia innominata (37, 38), magnocellular basal forebrain area (5), subarachnoid space ventral to the rostral basal forebrain (SS-rBF) (15, 43–45, 46), and median and lateral preoptic areas (28, 29, 30, 55).
Two brain regions implicated in the regulation of sleep are the median preoptic nucleus (MnPN) and the ventrolateral preoptic area (VLPO) of the hypothalamus. A subset of MnPN neurons exhibit sleep-related discharge with low discharge during waking, increased activity with the onset of sleep, and the highest discharge during non-REM and REM sleep (51). The number of MnPN neurons exhibiting c-Fos protein immunoreactivity (Fos-IR), a marker of neuronal activation, increases in proportion to the amount of preceding sleep and with increasing homeostatic sleep pressure (11–13). VLPO neurons also exhibit increased discharge during non-REM and REM sleep, as well as sleep-associated Fos-IR (48, 52). VLPO neurons appear critical for sleep induction and sleep maintenance, as excitotoxic VLPO lesions markedly decrease both non-REM and REM sleep (27). Excitotoxic lesion of preoptic area (POA) that primarily involves the medial region also causes chronic sleep loss (18).
There are four adenosine receptor subtypes, A1, A2A, A2B, and A3, all of which are coupled to G proteins (42). Inhibitory G protein-coupled adenosine A1 receptors and stimulatory G protein-coupled adenosine A2A receptors (A2AR) in the brain mediate the sleep-inducing effects of adenosine (reviewed in Refs. 6 and 36). Intracerebroventricular infusion of an A2AR agonist, 2-p-(2-carboxyethyl) phenylethylamino-5-N-ethylcarboxyamidoadenosine (CGS-21680), promotes sleep (10, 43–45, 46, 56). The largest increase in sleep occurs when CGS-21680 is administered to the SS-rBF (44). CGS-21680-induced sleep is associated with an increase in Fos-IR in VLPO neurons (46). A subset of VLPO neurons recorded in hypothalamic slice is excited by adenosine acting on the A2AR (9). Previous in vivo studies have not identified the neurotransmitter phenotype(s) of the neurons that are activated by A2AR agonist. The effects of A2AR agonists on MnPN neurons have not been previously reported.
We hypothesized that A2AR-dependent modulation of sleep is mediated through the activation of sleep-regulatory GABAergic neurons in the preoptic hypothalamus. We recorded sleep-wake behavior and quantified Fos-IR in GABAergic neurons in the MnPN and VLPO to 1) determine whether central administration of an A2AR receptor agonist, CGS-21680, promotes sleep and activates preoptic GABAergic neurons; 2) determine whether CGS-21680 administration activates MnPN and VLPO neurons independently of increased sleep, i.e., in animals that are not permitted sleep after drug treatment; and 3) determine whether intracerebroventricular infusion of an A2AR antagonist, ZM-241385, during sleep deprivation suppresses subsequent recovery sleep and attenuates the activation of preoptic GABAergic neurons during recovery sleep. Preliminary results have appeared previously in abstract form (21, 22).
METHODS
Experiments were performed on male, Sprague-Dawley rats, weighing between 300 and 350 g at the time of surgery. These rats were maintained on 12:12-h light-dark cycle (lights on at 0600) and with food and water available ad libitum. All experiments were conducted in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee, V.A. Greater Los Angeles Healthcare System.
Surgical Procedures
All surgical procedures were done under anesthesia (ketamine + xylazine: 80:10 mg/kg ip) and aseptic conditions. Details of the surgical procedure were described previously (23). In brief, rats were implanted with electroencephalogram (EEG) and dorsal neck electromyogram (EMG) electrodes for recording sleep-wake behavior. In addition, rats were implanted with a microinjection guide cannula (23G stainless-steel tube) in the lateral ventricle at coordinates, AP = −0.8 mm; L = 1.4 mm, and H = 3.6 mm (34), or the SS-rBF, at coordinates, AP = +1.5 mm; L = 1.8 mm and H = 8.3 mm (44).
Following implantation, a blocking stylet was inserted into the guide cannula to maintain patency until microinjection. Rats were permitted a 10–12-day postsurgical recovery period, after which they were connected to recording cables and placed in temperature-controlled (23 ± 2°C) and sound-attenuated recording chambers for adaptation.
Experimental Protocols
Experiment 1: effects of microinjection of CGS-21680 into the lateral ventricle on sleep and Fos-IR.
Three days before the experiments, the placement of intracerebroventricular cannula was tested by intracerebroventricular injection of angiotensin (200 ng in a volume of 5 μl). Only rats exhibiting a short latency (<2 min) drinking response to angiotensin were used.
Control rats (n = 7) received microinjection of vehicle containing 4% DMSO in artificial cerebrospinal fluid (aCSF) of the following composition in mM: 145 NaCl, 2.7 KCl, 1.3 MgSO4, 1.2 CaCl2, and 2 Na2HPO4; at pH, 7.2. Experimental rats received microinjection of either 8 nmol (n = 7) or 24 nmol (n = 7) CGS-21680 (Sigma, St. Louis, MO) in a volume of 5 μl over 10 min. All intracerebroventricular injections were initiated at 1400 (lights on at 0600). Drug or vehicle was injected during the second half of the light phase because baseline sleep amounts are reduced compared with the early light phase, increasing the likelihood of detecting drug-induced increases in sleep. The rats were left undisturbed for 2 h following intracerebroventricular injection, while EEG and EMG were continuously recorded.
Experiment-2: effects of microinjection of CGS-21680 into the SS-rBF on sleep and Fos-IR.
In this experiment, rats received microinjection of vehicle (n = 7, 4% DMSO in aCSF) or 8 nmol (n = 7) CGS-21680 in a volume of 5 μl over 10 min into the SS-rBF at 1400. Rats were left undisturbed for 2 h, while EEG and EMG were continuously recorded.
Experiment 3: effects of microinjection of CGS-21680 into the SS-rBF, followed by 2 h of sleep deprivation, on Fos-IR.
Rats were microinjected with 8 nmol of CGS-21680 (n = 7) or vehicle (n = 7) in a volume of 5 μl delivered over 10 min in the SS-rBF. To dissociate the direct effect of CGS-21680 on Fos-IR from the effect of increased sleep, groups of drug- and vehicle-treated rats were sleep deprived for 2 h postinjection. Rats were initially adapted to stimuli used to prevent sleep (tapping the cage and/or gentle movement of the cage) for about 20 min/day for 5 days before the experimental day. On the experimental day, both drug- and vehicle-treated rats were subjected to sleep deprivation by delivering arousing stimuli within 10 s of the appearance of non-REM sleep detected by visualizing the EEG. Animals were euthanized immediately after the 2-h sleep deprivation period.
Experiment 4: effects of infusion of A2AR antagonist ZM-241385 into the lateral ventricle on sleep and Fos-IR.
Three groups of rats were infused with either vehicle (n = 6) or one of two doses of ZM-241385, an adenosine A2AR antagonist (5 nmol, n = 6 or 25 nmol, n = 6), delivered into the lateral ventricle during the last 2 h of 3 h of sleep deprivation. Sleep deprivation was initiated 2 h after lights on at 0800. The rate of infusion was 0.4 μl/min. Following the end of sleep deprivation, intracerebroventricular infusion was discontinued, and rats were then left undisturbed and permitted recovery sleep, while EEG and EMG were continuously recorded for 2 h. Rats were euthanized immediately after the 2-h recovery sleep period.
Histology and Immunohistochemistry
At the end of all experiments, rats were given a lethal dose of pentobarbital sodium (100 mg/kg ip). Immediately after anesthetization, rats were injected with heparin (500 U ip) and perfused transcardially with 30–50 ml of 0.1 M PBS (pH 7.2) followed by 500 ml of 4% paraformaldehyde in PBS, containing 15% saturated picric acid solution (a final concentration of the picric acid was 0.2%). The brains were removed, postfixed for 20 min, and then equilibrated in 30% sucrose. Coronal sections encompassing the MnPN and VLPO were freeze-cut at 30-μm thickness and immunostained for c-Fos and glutamic acid decarboxylase (GAD).
c-Fos immunostaining.
Sections were first immunostained for c-Fos protein. Free-floating sections were incubated in 0.3% H2O2 in Tris-buffered saline (TBS) at room temperature (RT) for 30 min and then rinsed 3 times for 10 min each in TBS. The sections were placed in blocking solution (8% goat serum in TBS) for 1 h at RT. Sections were incubated in rabbit anti-c-Fos (PC-38; 1:20,000; Calbiochem, EMD Millipore, San Diego, CA) in 4% goat serum in TBS, for 40–48 h at 4°C. After rinsing in TBS 3 times for 10 min each, sections were incubated in biotinylated goat anti-rabbit secondary antibody (1:1,000; Vector Laboratories, Burlingame, CA, USA) in 4% goat serum in TBS, for 2 h at RT followed by rinsing with TBS. The sections were then incubated in avidin-biotin complex (1:500; Vector Laboratories) for 2 h at RT and then visualized with nickel-3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma, St. Louis, MO, USA). Black staining confined to the nucleus indicated presence of Fos-IR.
GAD immunostaining.
Sections were then washed in TBS followed by incubation in blocking solution (10% horse serum in TBS) containing avidin, (1:50; Vector Laboratories) for 1 h at RT. Sections were then incubated in the primary antibody, monoclonal mouse anti-GAD (MAB 5406; 1:400; Millipore, Temecula, CA) with biotin (1:50; Vector Laboratories) for 40–48 h at 4°C. The use of avidin and biotin (SP 2001; Vector Laboratories) in the GAD staining procedure reduced terminal staining substantially (20). The sections were then incubated in biotinylated horse anti-mouse secondary antibody (BA 2001; 1:400 Vector Laboratories) for 1 h at RT followed by rinsing with TBS. The sections were then incubated in avidin-biotin complex (1:150; Vector Laboratories) for 2 h at RT followed by DAB visualization to give a brown product.
All sections were rinsed with TBS, mounted on gelatin-coated microscope slides, dehydrated in graded alcohols, cleared in xylene, and coverslipped with DPX mounting medium. Tissues from control and experimental groups of each experiment were processed together using the same batch of reagents. Omission of the primary antibodies (anti-Fos and anti-GAD) in control sections did not yield staining in processes, proximal dendrites, or cell bodies.
Serial coronal sections were stained for Nissl (cresyl violet) for localization of microinjection sites. Sections stained for Fos-GAD were used for localization of microinjection sites for intracerebroventricular experiments.
Data Analysis
Sleep-wake scoring.
Bioelectric signals were amplified and band pass filtered at 0.3–100 Hz (EEG) or 10–300 Hz (EMG) (model 78 D, Grass Instruments, Quincy, MA). Bioelectrical signals were digitized at a sampling rate of 128 Hz for EEG and 256 Hz for EMG using a 1401 Plus data acquisition interface and Spike2 software (Cambridge Electronic Design, Cambridge, UK) and stored on a PC for off-line analysis.
The predominant sleep-wake states for each 10-s epoch were visually determined by an experienced scorer, blind to the experimental condition and group identity of the animals. Wake was defined as low-voltage, high-frequency activity combined with elevated neck muscle tone. non-REM sleep was defined as high-amplitude EEG with prominent activity in the 0.75- to 4.0-Hz range and relatively reduced muscle tone. REM sleep was defined as moderate-amplitude EEG with dominant theta frequency activity (6–8 Hz) combined with minimal neck EMG tonus, except for occasional brief twitches. Digitized EEG signals were subjected to a fast Fourier transform algorithm, after which a power spectrum was computed for the delta frequency range of 0.75–4.0 Hz. This was done for each 10-s epoch of scored non-REM sleep and waking in the 2-h recording period. Epochs containing artifacts were omitted from spectral analysis. For individual animals, non-REM sleep EEG delta power was expressed as a percentage of waking EEG delta power values computed for the 2 h of recording period. Non-REM sleep latency was defined by the time elapsed from the end of sleep deprivation period to the onset of six or more consecutive epochs of NREM sleep (≥60 s). REM sleep latency was defined by the time between the end of sleep deprivation and the onset of at least two consecutive epochs of REM sleep (≥20 s). Sleep data were averaged through the entire 120-min recording period for statistical analysis.
Cell counting and analyses.
A single person blind to the treatment conditions performed the counting and plotting of the immunoreactive neurons using the Neurolucida computer-aided plotting system (Micro Bright Field). Section outlines were drawn with ×20 magnification, whereas the identification and counting of different neuronal types, i.e., single- and double-labeled neurons, were done manually under ×400 magnification. Fos-IR was recognized by a black stain localized to the nucleus, whereas brown-stained soma and dendrites identified GAD-containing neurons. Neurons having a black nucleus and a brown cytoplasm were identified as double-labeled neurons.
Counts of Fos+, GAD+, and GAD+/Fos+ cells in MnPN and VLPO were performed using grids corresponding to the four areas of interest: rostral MnPN (rMnPN), caudal MnPN (cMnPN), core VLPO (cVLPO), and extended VLPO (eVLPO), as shown in Fig. 3 and described previously (20). For both the rMnPN and the cMnPN, cell counts were made in three sections and averaged to yield a single value for each rat. For the VLPO, cell counts were made bilaterally in three sections containing the largest part of the VLPO. Those six counts were then averaged to yield a single value for both the cVLPO and eVLPO (dorsal and medial combined boxes) for each rat.
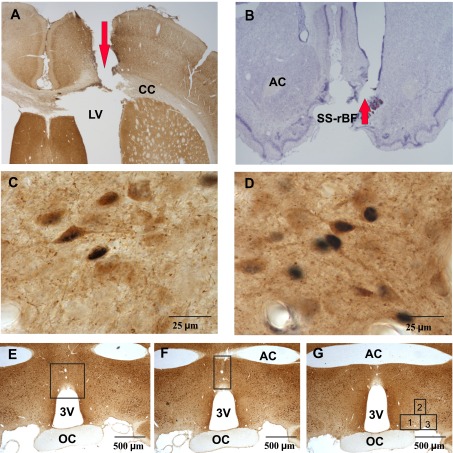
Fig. 3.
Locations of the microinjection sites and counting boxes. Photomicrographs of a coronal section showing cannula tract into lateral ventricle (A; ×20 magnification), tissue stained for c-Fos protein immunoreactivity (Fos-IR) and glutamic acid decarboxylase (GAD) and a coronal section showing cannula tract into the SS-rBF (×20 magnification), Nissl stained (B). C: photomicrograph (×1,000 magnification) showing neuronal staining for c-Fos and GAD in MnPN. D: photomicrograph (×1,000 magnification) showing neuronal staining for c-Fos and GAD in VLPO. Photomicrographs (×40 magnification), showing the locations of counting boxes for the rostral median preoptic nucleus (E), caudal median preoptic nucleus (F), and ventrolateral preoptic area (G). AC, anterior commissure; SS-rBF, subarachnoid space underlying the rostral basal forebrain; CC, corpus callosum; LV, lateral ventricle; 3V, third ventricle; OC, optic chiasm. The number 1 denotes core VLPO, while numbers 2 and 3 denote extended VLPO.
Statistical Analysis
The responses of various doses of CGS-21680 and ZM-24135 on single-labeled Fos+, GAD+, and double-labeled GAD+/Fos+ neurons in the MnPN and VLPO were compared with those obtained after vehicle treatments, using one-way ANOVA followed by Holm-Sidak test for pair-wise multiple comparisons. One-way ANOVA followed by Holm-Sidak tests were also used to determine the effects of CGS-21680 and ZM-241385 on sleep-wake parameters. The responses of an optimum dose of CGS-21680 into the SS-rBF on Fos-IR in GAD+ MnPN and VLPO neurons, as well as sleep-wake parameters, were compared with those observed after vehicle treatment using an independent t-test.
RESULTS
Effects of Intracerebroventricular Injection of CGS-21680
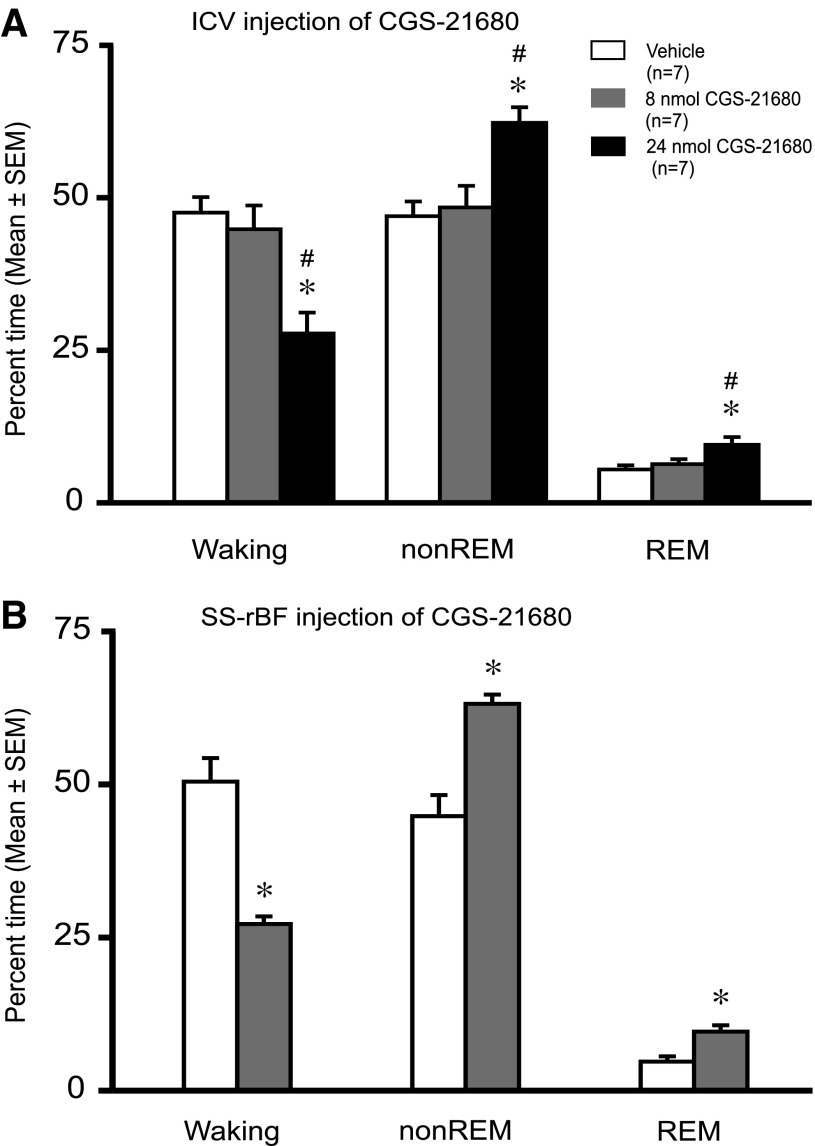
Intracerebroventricular administration of 24 nmol of CGS-21680 caused a significant decrease in % time awake and an increase in % time in non-REM sleep, compared with both the 8-nmol dose and vehicle (Fig. 1A). The 24-nmol dose also increased % time in REM sleep compared with the vehicle. There were no significant differences in sleep-wake amounts between 8 nmol CGS-21680 and vehicle. Delta power in non-REM sleep significantly increased in response to the higher dose of CGS-21680 (Fig. 2A). Latencies to non-REM and REM sleep onset decreased significantly after 24-nmol CGS-21680 administration, compared with both the 8-nmol dose and vehicle (Fig. 2, B and C).
Fig. 1.
Effects of CGS-21680 on sleep-wakefulness. Effects of microinjections of vehicle and CGS-21680 (8 nmol or 24 nmol) on % time spent in waking, non-rapid eye movement (REM) sleep, and REM sleep, when administered intracerebroventricularly (A) or into the subarachnoid space underlying the rostral basal forebrain (SS-rBF; B). A: means ± SE data (n = 7/group) for the 2-h postinjection recording period. CGS-21680 induced a dose-dependent increase in % time spent in non-REM sleep (F2,18 = 4.94; P = 0.019) and REM (F2,18 = 8.61; P = 0.002) sleep, whereas it resulted in a significant reduction in % time spent in waking (F2,18 = 10.34; P = 0.001). B: mean ± SE data (n = 7/group) for the 2 h postinjection recording period. In SS-rBF, a lower dose of CGS-21680 (8 nmol) significantly increased % time spent in non-REM sleep (t = −4.88; P = <0.001), as well as REM sleep (t = −3.675; P = 0.003) and decreased % time spent in waking (t = 5.734; P = <0.001). *Significantly different from vehicle, P < 0.05; #Significantly different from 8 nmol, P < 0.05.
Fig. 2.
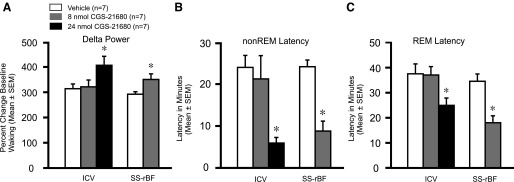
Effects of CGS-21680 on EEG delta power and non-REM and REM latencies. Effects of vehicle and CGS-21680 after intracerebroventricular (8 nmol or 24 nmol) and SS-rBF (8 nmol) microinjection on non-REM delta power (A), non-REM sleep latency (B), and REM sleep latency (C). Both intracerebroventriclar (ICV) and SS-rBF injections of CGS-21680 increased non-REM delta power (ICV: F2,18 = 3.555; P = 0.05; SS-rBF: t = −2.39; P = 0.034) and decreased latencies for both non-REM sleep (ICV: F2,18 = 7.14; P = 0.005; SS-rBF: t = 3.90; P = 0.002) and REM sleep (ICV: F2,18 = 3.24; P = 0.063; SS-rBF: t = 2.82; P = 0.015). The non-REM sleep delta power is expressed as the % change from baseline waking values. *Significantly different from vehicle, P < 0.05.
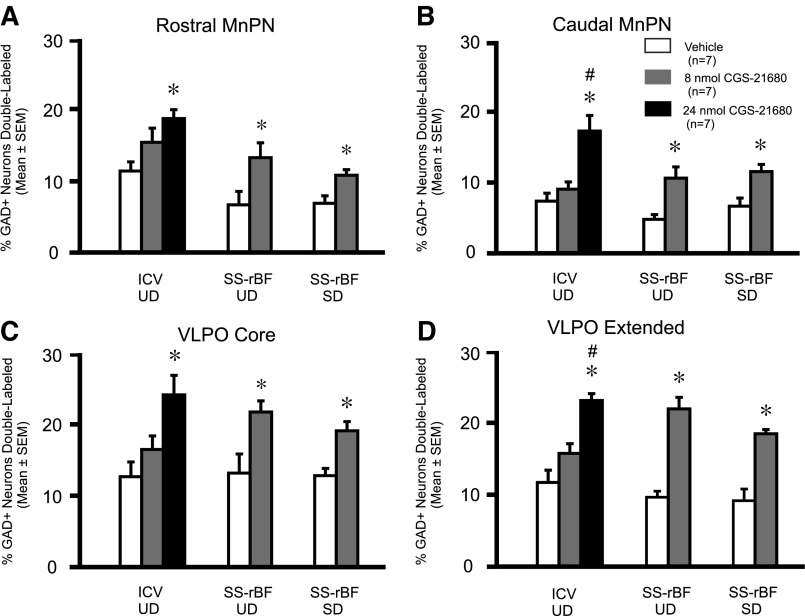
Examples of GAD and c-Fos immunostaining and locations of counting boxes in the preoptic area are shown in Fig. 3. Table 1 shows the single GAD-immunoreactive (IR), single Fos-IR, and double GAD+Fos-IR cell counts in the MnPN and VLPO in animals receiving intracerebroventricular injections of CGS-21680 or vehicle. Plotted in Fig. 4 is the percentage of GAD-IR neurons that were double-labeled for Fos-IR for the three groups. The numbers of single GAD-IR and single Fos-IR neurons in the MnPN and in the VLPO in vehicle and drug-treated animals were comparable (Table 1). However, the % GAD+ neurons double-labeled and the number of c-Fos/GAD dual immunoreactive neurons in both the MnPN and the VLPO were elevated in rats treated with CGS-21680 (24 nmol), compared with those treated with vehicle (Fig. 4 and Table 1). The number of c-Fos/GAD dual-immunoreactive neurons and the %GAD+ neurons double-labeled did not differ significantly between vehicle and 8 nmol CGS-21680-treated animals in all of the four areas examined (Table 1 and Fig. 4).
Table 1.
Effects of intracerebroventricular administration of CGS-21680 on c-Fos-expression in GABAergic neurons of the MnPN and VLPO
| Area | Treatment (n = 7) | # GAD-IR Neurons | # Fos-IR Neurons | # Dual GAD/Fos-IR Neurons |
|---|---|---|---|---|
| MnPN Rostral | Vehicle | 89.0 ± 4.7 | 28.3 ± 4.4 | 11.4 ± 1.2 |
| 8 nmol CGS | 86.6 ± 6.4 | 29.9 ± 5.7 | 16.3 ± 2.9 | |
| 24 nmol CGS | 85.1 ± 3.2 | 30.4 ± 2.7 | 19.7 ± 1.5** | |
| ANOVA | F(2,18) =0.15 P = 0.85 | F(2,18) =0.057 P = 0.94 | F(2,18) =4.27 P < 0.05 | |
| MnPN Caudal | Vehicle | 74.6 ± 1.7 | 23.3 ± 4.78 | 6.0 ± 0.99 |
| 8 nmol CGS | 70.4 ± 2.5 | 23.8 ± 2.5 | 6.9 ± 0.7 | |
| 24 nmol CGS | 67.3 ± 4.5 | 24.9 ± 3.7 | 14.1 ± 1.9**# | |
| ANOVA | F(2,18) =1.32 P = 0.29 | F(2,18) =0.04 P = 0.96 | F(2,18) =11.16 P < 0.01 | |
| VLPO Core | Vehicle | 44.1 ± 2.3 | 7.4 ± 0.96 | 6.7 ± 1.25 |
| 8 nmol CGS | 38.5 ± 0.96 | 8.6 ± 1.37 | 7.8 ± 1.1 | |
| 24 nmol CGS | 35.7 ± 2.9* | 9.3 ± 1.21 | 11.0 ± 1.11* | |
| ANOVA | F(2,18) =3.70 P = 0.04 | F(2,18) =0.71 P = 0.50 | F(2,18) =3.85 P < 0.05 | |
| VLPO Extended | Vehicle | 97.6 ± 4.3 | 11.2 ± 1.7 | 12.9 ± 1.96 |
| 8 nmol CGS | 90.2 ± 5.4 | 14.1 ± 0.66 | 17.3 ± 2.3 | |
| 24 nmol CGS | 82.8 ± 4.8 | 14.1 ± 2.3 | 24.6 ± 0.8**# | |
| ANOVA | F(2,18) =2.27 P = 0.13 | F(2,18) =1.18 P = 0.32 | F(2,18) =0.51 P < 0.01 |
All values are expressed as means ± SE.
Significantly different from vehicle; #, significantly different from 8 nmol CGS-21680; *, #, P < 0.05,
, ##, P < 0.01 level of significance (Holm-Sidak test).
Fig. 4.
Effects of CGS-21680 on Fos-IR in MnPN and VLPO GABAergic neurons. Effects of CGS-21680 on the percentage of GAD+ neurons expressing Fos-IR (%GAD+ neurons double-labeled) in the MnPN (A and B) and VLPO (C and D) after intracerebroventricular or SS-rBF injections in undisturbed (UD) animals and after SS-rBF injections in animals that were sleep-deprived (SD) for 2 h after drug or vehicle administration. In UD animals, both ICV and SS-rBF injections of CGS-21680 increased the percentage of GAD+ neurons expressing Fos-IR in all of the preoptic area (POA) subregions examined; rostral MnPN (ICV: F2,18 = 5.66, P = 0.012; SS-rBF: t = −2.35, P = 0.037), caudal MnPN (ICV: F2,18 = 11.75, P = <0.001; SS-rBF: t = −3.37, P = 0.006), core VLPO (ICV: F2,18 = 6.69, P = 0.007; SS-rBF: t = −2.76, P = 0.017), and extended VLPO (ICV: F2,18 = 17.63, P = <0.001; SS-rBF: t = −6.78, P = <0.001). Like UD animals, CGS-21680 injection into the SS-rBF in SD animals also increased Fos-IR in GAD+ neurons in all of the POA-subregions examined (rostral MnPN: t = −2.98; P = 0.011; caudal MnPN: t = −3.19; P = 0.008; core VLPO: t = −3.83; P = 0.002; extended VLPO: t = −5.40; P = <0.001). *Significantly different from vehicle, P < 0.05. #Significantly different from 8 nmol, P < 0.05.
Effects of SS-rBF Injection of CGS-21680
Microinjection of an A2AR agonist into the SS-rBF has been shown to have more potent sleep-promoting effects than microinjection in the lateral ventricle (44), so we applied only the lower dose of CGS-21680 (8 nmol) in this site.
Administration of 8 nmol CGS-21680 to the SS-rBF significantly decreased waking and increased non-REM and REM sleep compared with vehicle (Fig. 1B). Latencies to both non-REM and REM sleep onset decreased significantly in response to administration of CGS-21680 to the SS-rBF (Fig. 2, B and C). The non-REM delta power was also significantly increased in response to drug (Fig. 2A).
The %GAD+ neurons double-labeled and number of dual c-Fos/GAD-IR neurons in the MnPN (rostral and caudal) and in the VLPO (core and extended) were elevated in CGS-21680 injected rats compared with those injected with vehicle (Fig. 4 and Table 2). No significant changes were detected in the number of single GAD-IR or single Fos-IR neurons in the VLPO and MnPN in the CGS-21680-treated group compared with the vehicle group (Table 2).
Table 2.
Effects of SS-rBF administration of CGS-21680 on c-Fos expression in GABAergic neurons of the MnPN and VLPO
| Area | Sleep-Wake Condition | Treatment (n = 7) | # GAD-IR Neurons | # Fos-IR Neurons | # Dual GAD/Fos-IR Neurons |
|---|---|---|---|---|---|
| Rostral MnPN | UD | Vehicle | 92.6 ± 3.5 | 17.4 ± 3.2 | 6.7 ± 1.97 |
| 8 nmol CGS | 84.3 ± 3.1 | 18.6 ± 2.6 | 12.9 ± 2.0 | ||
| t-test | t(12) =1.75 P = 0.10 | t(12) = −0.30 P = 0.76 | t(12) = −2.20 P < 0.05 | ||
| SD | Vehicle | 94.8 ± 2.3 | 20.2 ± 1.98 | 7.1 ± 1.12 | |
| 8 nmol CGS | 90.0 ± 4.4 | 21.9 ± 3.2 | 11.0 ± 1.1 | ||
| t-test | t(12) = 0.96 P = 0.35 | t(12) = −0.42 P = 0.67 | t(12) = −2.49 P < 0.05 | ||
| Caudal MnPN | UD | Vehicle | 73.5 ± 0.8 | 12.4 ± 2.7 | 3.7 ± 0.5 |
| 8 nmol CGS | 69.1 ± 2.9 | 11.8 ± 1.9 | 8.5 ± 1.5 | ||
| t-test | t(12) =1.40 P = 0.18 | t(12) =0.20 P = 0.84 | t(12) = −2.91 P < 0.05 | ||
| SD | Vehicle | 69.1 ± 2.9 | 11.8 ± 1.9 | 5.4 ± 0.96 | |
| 8 nmol CGS | 76.2 ± 1.5 | 18.0 ± 1.8 | 9.5 ± 0.76 | ||
| t-test | t(12) =1.11 P = 0.28 | t(12) =0.95 P = 0.35 | t(12) = −3.29 P < 0.01 | ||
| Core VLPO | UD | Vehicle | 44.4 ± 3.0 | 7.6 ± 1.0 | 7.2 ± 1.6 |
| 8 nmol CGS | 41.9 ± 3.2 | 9.1 ± 1.6 | 11.5 ± 1.0 | ||
| t-test | t(12) =0.56 P = 0.58 | t(12) = −0.80 P = 0.43 | t(12) = −2.25 P < 0.05 | ||
| SD | Vehicle | 46.5 ± 2.4 | 8.8 ± 1.4 | 6.8 ± 0.5 | |
| 8 nmol CGS | 43.0 ± 2.8 | 10.9 ± 2.3 | 10.1 ± 0.87 | ||
| t-test | t(12) =0.94 P = 0.36 | t(12) = −0.79 P = 0.44 | t(12) = −3.28 P < 0.01 | ||
| Extended VLPO | UD | Vehicle | 101.1 ± 9.3 | 12.1 ± 2.0 | 10.7 ± 1.2 |
| 8 nmol CGS | 86.3 ± 4.2 | 15.9 ± 1.6 | 24.0 ± 1.4 | ||
| t-test | t(12) =1.43 P = 0.17 | t(12) = −1.49 P = 0.16 | t(12) = −7.13 P < 0.01 | ||
| SD | Vehicle | 101.2 ± 4.7 | 12.9 ± 1.9 | 10.3 ± 1.8 | |
| 8 nmol CGS | 86.0 ± 5.56 | 16.6 ± 2.36 | 19.5 ± 1.5 | ||
| t-test | t(12) =2.08 P = 0.059 | t(12) = −1.20 P = 0.25 | t(12) = −3.94 P < 0.01 |
All values are expressed as means ± SE (t-test). UD indicates rats that were permitted undisturbed sleep during 2 h postinjection. SD indicates rats subjected to 2 h of sleep deprivation postinjection.
c-Fos Expression in Preoptic Neurons in CGS-21680-Treated Sleep-Deprived Rats
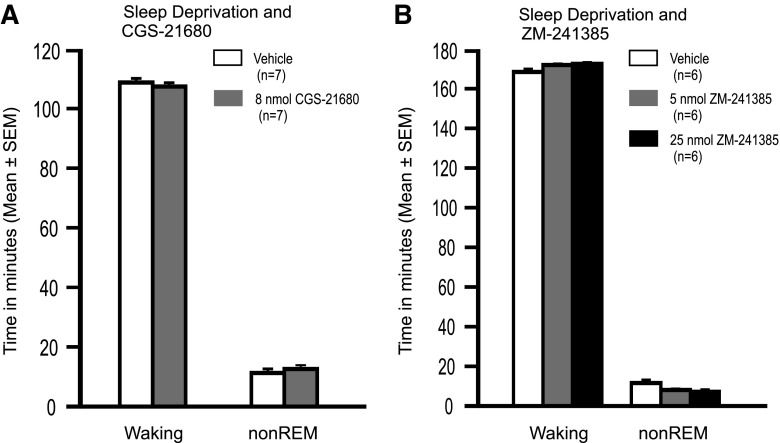
To determine whether treatment with CGS-21680 could activate MnPN and VLPO GABAergic neurons, independent of increasing sleep, groups of drug- and vehicle-treated rats (n = 7) were subjected to sleep deprivation for 2 h postinjection, and then killed without opportunity for recovery sleep. Drug and vehicle injections were made into the SS-rBF. Time (in minutes) spent awake and in non-REM sleep during the sleep deprivation period are shown in Fig. 5A. There were no significant differences between CGS-21680- and vehicle-treated rats. REM sleep was completely suppressed in both groups during sleep deprivation (data not shown).
Fig. 5.
Sleep-wake profiles of animals during sleep deprivation. A: average time (in minutes) spent awake and in non-REM sleep during 2-h sleep deprivation following SS-rBF administration of the A1R agonist, CGS21680, or vehicle. There were no significant between-group differences (time awake, t = 0.78, NS; time in non-REM sleep t = −0.72 NS). REM sleep was completely suppressed in all rats during sleep deprivation. B: average time spent awake and in non-REM sleep during 3 h of sleep deprivation in groups of rats that received intracerebroventricular infusion of vehicle or A2AR antagonist, ZM241385 (5 or 25 nmol) or vehicle during the final 2 h of the sleep deprivation period. Although there was a significant overall effect of drug and vehicle treatment on time spent in non-REM sleep during sleep deprivation (F2,15 = 4.73; P < 0.01), the maximum difference in mean time spent asleep across groups of 4.0 min would not be expected to have a significant functional impact on subsequent recovery sleep.
In spite of the comparable reductions in sleep achieved in the two groups, the percentage of GAD-IR neurons double immunolabeled for Fos-IR was significantly elevated in all MnPN and VLPO subregions examined in CGS-21680-treated vs. vehicle-treated rats (Fig. 4). The number of GAD/c-Fos dual IR cells was also elevated in the MnPN and VLPO of animals treated with drug (Table 2).
Effects of Intracerebroventricular Infusion of A2AR Antagonist ZM-241385
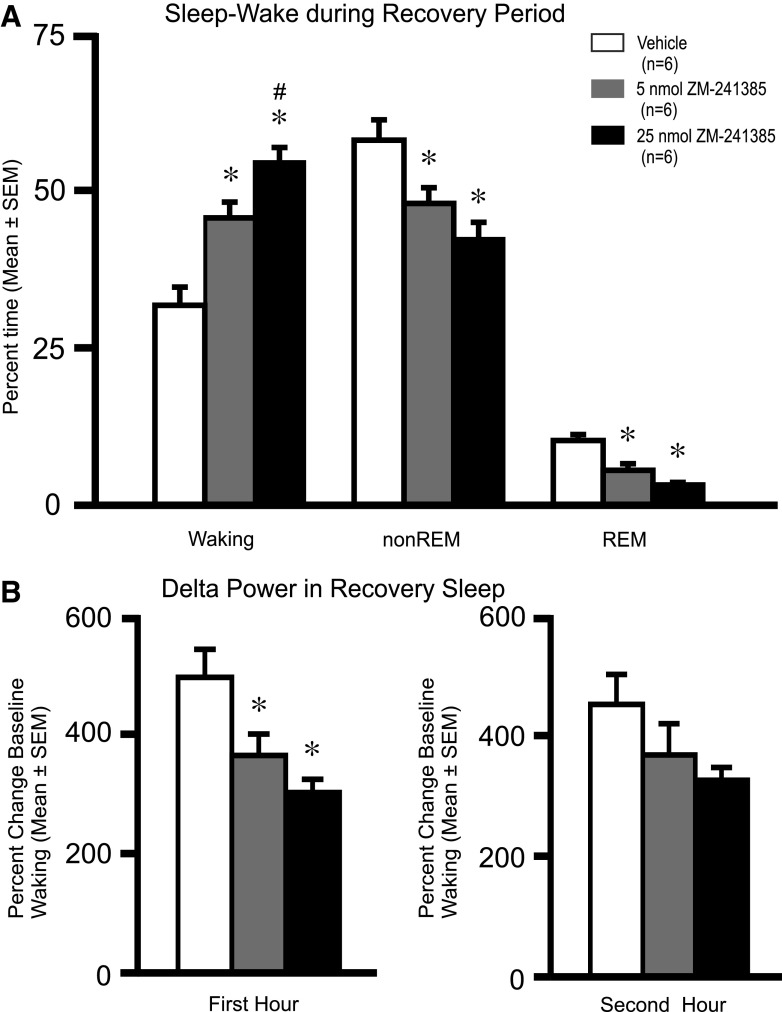
Intracerebroventricular infusion of ZM-241385 or vehicle (n = 6/group) was performed during the final 2 h of a 3-h period of sleep deprivation. Drug and vehicle were infused at a rate of 0.4 μl/min. Total amount of drug delivered was 5 nmol in one experimental group and 25 nmol in the other. Time spent awake and in non-REM sleep during the 3-h sleep deprivation period is shown in Fig. 5B.
Following the end of sleep deprivation and discontinuation of intracerebroventricular infusion, all animals were permitted the opportunity to have 2 h of undisturbed recovery sleep. Intracerebroventricular infusion of ZM-241385 at 5 and 25 nmol caused dose-dependent increases in % time awake during the 2-h recovery sleep opportunity (Fig. 6). The % time spent in non-REM and REM sleep during the recovery period were significantly reduced in response to both doses of ZM-241385 compared with vehicle (Fig. 6). The latency to sleep onset following the end of sleep deprivation was significantly increased in ZM-241385-treated rats (5 nmol; 23.1 ± 3.4 min and 25 nmol; 26.6 ± 1.7 min) compared with vehicle-treated rats (12.4 ± 2.7 min; F2,15 = 7.54; P < 0.02). In addition, EEG delta power during non-REM sleep was significantly lower during the first hour of recovery sleep in both groups receiving drug, compared with vehicle (Fig. 6B).
Fig. 6.
Effects of ZM-241385 on sleep-wakefulness during the recovery period. A: effects of intracerebroventricular infusions of vehicle or ZM-241385 (5 nmol or 25 nmol) on % time spent in waking (F2,15 = 19.48; P = <0.001), non-REM sleep (F2,15 = 7.99; P < 0.01), and REM sleep (F2,15 = 18.56; P = <0.001). The data are shown as means ± SE (n = 6/group) for the 2-h postinfusion recording period when rats were permitted undisturbed recovery sleep. ZM-241385 induced a dose-dependent reduction in % time spent in non-REM sleep and REM sleep, whereas it significantly increased % time spent in waking. B: EEG delta power in non-REM sleep was significantly decreased during the first hour of recovery sleep in ZM-241385-treated rats compared with vehicle controls (F2,15 = 7.96, P < 0.01). *Significantly different from vehicle, P < 0.05. #Significantly different from 5 nmol, P < 0.05.
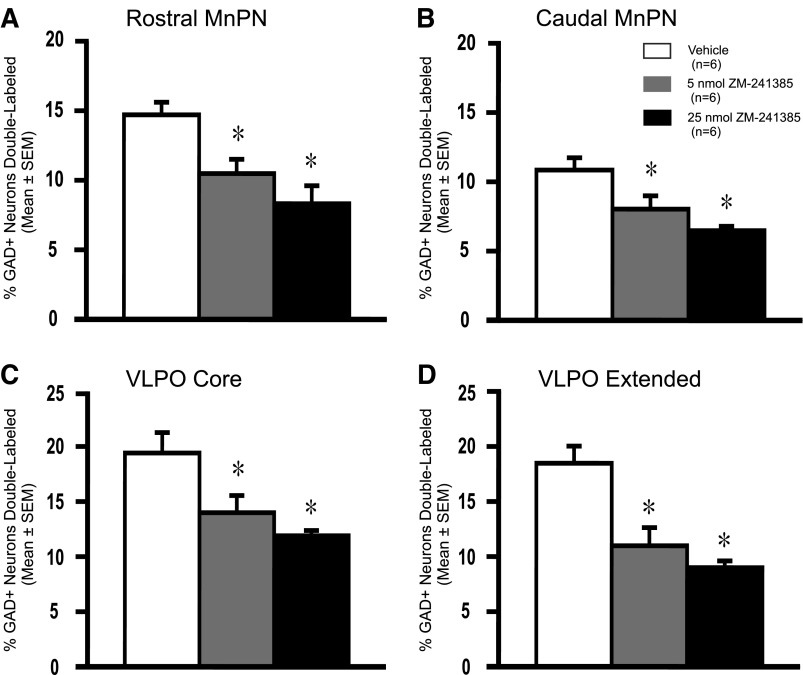
Intracerebroventricular infusion of ZM-241385 resulted in significant decreases in the %Fos+/GAD+ neurons in both the rostral and caudal portions of the MnPN (Fig. 7). Significant decreases in %GAD+ neurons double-labeled were also observed in the core and extended VLPO in response to drug (Fig. 7). Dual c-Fos/GAD-IR cell counts were also reduced in these preoptic nuclei in response to drug (Table 3). There were no significant differences in single-labeled GAD-IR or single-labeled Fos-IR cell counts between drug- and vehicle-treated animals in any of the preoptic nuclei examined (Table 3).
Fig. 7.
Effects of ZM-241385 on Fos-IR in the POA GABAergic neurons. Effects of intracerebroventricular infusion of ZM-241385 (5 nmol or 25 nmol) vs. vehicle on the percentage of GAD+ neurons expressing Fos-IR in the POA subregions studied; rostral MnPN (F2,15 = 8.95; P = 0.003), caudal MnPN (F2,15 = 8.14; P = 0.004), core VLPO (F2,15 = 7.14; P = 0.006), and extended VLPO (F2,15 = 13.50; P = <0.001). ZM-241385 significantly decreased the percentage of GAD+ neurons expressing Fos-IR in all of the POA subregions examined. Significantly different from vehicle, *P <0.05.
Table 3.
Effects of intracerebroventricular infusion of ZM-241385 during sleep deprivation on c-Fos expression in GABAergic neurons of the MnPN and VLPO during 2 h of recovery sleep
| Area | Treatment (n = 6) | # GAD-IR Neurons | # Fos-IR Neurons | # Dual GAD/Fos-IR Neurons |
|---|---|---|---|---|
| Rostral MnPN | Vehicle | 85.7 ± 1.8 | 27.5 ± 2.3 | 14.9 ± 1.2 |
| 5 nmol ZM | 88.9 ± 3.1 | 24.4 ± 2.4 | 10.4 ± 2.1* | |
| 25 nmol ZM | 90.7 ± 2.2 | 22.7 ± 2.8 | 8.3 ± 1.3** | |
| ANOVA (Holm-Sidak test) | F(2,15) =1.05 P = 0.37 | F(2,15) =0.97 P = 0.40 | F(2,15) =7.79 P < 0.01 | |
| Caudal MnPN | Vehicle | 70.1 ± 3.3 | 17.5 ± 2.3 | 8.5 ± 0.8 |
| 5 nmol ZM | 71.2 ± 3.1 | 16.9 ± 2.0 | 6.1 ± 0.7* | |
| 25 nmol ZM | 75.2 ± 2.8 | 14.1 ± 1.9 | 5.2 ± 0.2** | |
| ANOVA (Holm-Sidak test) | F(2,15) =0.90 P = 0.42 | F(2,15) =0.71 P = 0.50 | F(2,15) =7.00 P < 0.01 | |
| Core VLPO | Vehicle | 38.9 ± 2.7 | 7.1 ± 1.2 | 9.5 ± 1.2 |
| 5 nmol ZM | 40.5 ± 1.9 | 7.1 ± 1.2 | 6.6 ± 0.7* | |
| 25 nmol ZM | 42.8 ± 2.9 | 4.1 ± 0.9 | 5.8 ± 0.6** | |
| ANOVA (Holm-Sidak test) | F(2,15) =0.70 P = 0.51 | F(2,15) =2.35 P = 0.12 | F(2,15) =4.86 P < 0.05 | |
| Extended VLPO | Vehicle | 80.3 ± 6.2 | 13.5 ± 2.4 | 18.6 ± 2.7** |
| 5 nmol ZM | 87.1 ± 2.7 | 13.4 ± 2.4 | 10.9 ± 1.9** | |
| 25 nmol ZM | 91.5 ± 2.5 | 9.3 ± 1.5 | 9.0 ± 0.5** | |
| ANOVA (Holm-Sidak test) | F(2,15) =1.90 P = 0.18 | F(2,15) =1.26 P = 0.31 | F(2,15) =7.19 P < 0.01 |
All values are expressed as means ± SE.
P < 0.05,
P < 0.01 level of significance (Holm-Sidak test).
DISCUSSION
The intracerebroventricular administration of an adenosine A2AR agonist, CGS-21680, increased both non-REM and REM sleep amounts, increased delta power within non-REM sleep, and increased Fos-IR in GABAergic neurons in the MnPN and VLPO. Similar effects were observed after administration of this A2AR agonist to the SS-rBF, albeit at a lower dose. CGS-21680-induced increases in Fos-IR in MnPN and VLPO GABAergic neurons were observed, even in animals that were not allowed to sleep after drug administration. We also found that intracerebroventricular infusion of the adenosine A2AR antagonist ZM-241385 during sleep deprivation caused decreased recovery sleep and suppression of Fos-IR in MnPN and VLPO GABAergic neurons. Given that GABAergic neurons in the MnPN and VLPO play a crucial role in the generation of sleep, these findings support the hypothesis that activation of MnPN and VLPO GABAergic neurons is involved in the adenosinergic regulation of sleep.
This is the first study to demonstrate that in vivo central administration of A2AR agonists and antagonists alters c-Fos expression in GABAergic neurons in the VLPO and MnPN. This is also the first demonstration that activation of preoptic sleep-regulatory neurons in response to central A2AR agonists can occur independently of drug-induced sleep.
Adenosine is one of the several endogenous neuromodulators, including PGD2 (14, 43), IL-1 (4, 19), and growth hormone-releasing hormone (31, 35) that have been implicated in sleep regulation. The sleep-promoting effects of adenosine have been shown to involve A1-mediated inhibition of cholinergic and noncholinergic arousal-related neurons in the basal forebrain (3, 5, 15, 29, 37, 43–45, 46, 53). Additional sites of A1-mediated inhibition of arousal systems include hypocretin neurons in the perifornical lateral hypothalamus (2, 26, 41, 54), histaminergic neurons in the tuberomamillary nucleus (TMN) (32), and noradrenergic neurons in the locus coeruleus (LC) ( 33).
Work reported here and previously published studies indicate that A2AR-mediated activation of preoptic sleep regulatory neurons is an additional mechanism through which adenosine can promote sleep. Intracerebroventricular and SS-rBF infusion of A2AR agonists promote sleep (10, 43–45), increase c-Fos expression in VLPO neurons, and decrease Fos-IR in the TMN (46). Microinfusion of A2AR agonist into the lateral preoptic area, including the VLPO, promotes sleep (29). Bath application of adenosine in vitro reduces firing of some VLPO neurons via direct A1R effects but excites other VLPO neurons via effects on A2AR (9).
The MnPN and VLPO are sources of descending GABAergic projections to several wake-promoting regions in the posterior hypothalamus and brain stem. These include the TMN (47), dorsal raphe nucleus (DRN), and the LC (47, 49, 57). A subset of MnPN and VLPO neurons that project to the DRN and the adjacent ventrolateral periaqueductal gray, express Fos-IR during sleep (16, 59). Projections from the VLPO and MnPN to the hypocretin neuronal field in the perifornical lateral hypothalamus have also been described (60), and a subset of these projection neurons exhibit sleep-related Fos-IR (58). The activation and inactivation of MnPN neurons have been shown to suppress and activate, respectively, wake-active neurons in the lateral hypothalamus (50). Inactivation of MnPN increases c-Fos expression in hypocretin neurons of the hypothalamus and in serotonergic neurons of dorsal raphe (24). Activation of MnPN and VLPO GABAergic neurons by A2AR agonists, therefore, would be expected to result in the suppression of multiple wake-promoting neuronal systems, leading to increased sleep. Antagonism of A2AR in the MnPN and VLPO would be expected to have the opposite effects on wake-promoting neuronal activity leading to diminished propensity for sleep following sleep deprivation, as we have described here.
Increased adenosinergic signaling occurring as a consequence of sustained wakefulness and involving A1R-mediated inhibition of arousal systems, is hypothesized to be a critical component of sleep homeostasis (6, 7, 36). The contribution of A2AR-mediated excitation of preoptic sleep regulatory neurons to changes in homeostatic sleep drive is not known. Intracebroventricular infusion of A2AR antagonist during sleep deprivation did result in reduced amounts of recovery sleep and reduced Fos-IR in MnPN and VLPO GABAergic neurons. Recovery sleep in ZM-241385-treated rats was characterized by prolonged latency to sleep onset and reduced EEG delta activity in non-REM sleep, suggestive of diminished homeostatic sleep pressure compared with equally sleep-deprived vehicle-treated rats. However, it remains unclear whether ZM-241385 effects on recovery sleep are the result of disfacilitation of MnPN and VLPO sleep regulatory neurons or reflect activation of arousal systems via A2AR-responsive circuits involving the ventral striatum (25).
A comprehensive in vitro study of the responses of GABAergic VLPO neurons to adenosine identified two functional cell types (9). Type 1 cells are inhibited by serotonin and adenosine A1AR agonists but are unresponsive to A2AR agonists. Type 2 cells are excited by serotonin and excited by A2AR agonists. The latter were hypothesized to be functionally important for sleep induction during the period of high homeostatic sleep pressure by virtue of their excitatory response to adenosine (9). We infer that the subset of GAD-IR neurons expressing Fos-IR in response to an A2AR agonist in the present study corresponds to type 2 neurons characterized in vitro. The existence of two functional GABAergic cell types is supported by in vivo electrophysiological findings that some sleep-active neurons in the lateral preoptic area exhibit increased discharge in response to sleep deprivation and others do not (1).
The alerting drug, caffeine, is a mixed A1 and A2AR antagonist. Although both A1 and A2A receptors appear to play a role in sleep regulation (see above), the alerting effects of caffeine are more dependent on A2AR mechanisms. It was demonstrated that caffeine increased wakefulness in both wild-type mice and A1 receptor knockout mice, but it had no wake-promoting effects in A2AR knockout mice (17). A recent study demonstrated that selective lesions of A2AR-expressing neurons in the nucleus accumbens (NAC) of mice, and knockdown of A2AR in the NAC of rats with focal RNA interference significantly attenuates the wake-promoting effects of caffeine (25). This suggests that A2AR-expressing neurons in the NAC have sleep-promoting properties and that caffeine blocks A2ARs in this site. It is possible that intracerebroventricular and SS-rBF infusion of A2AR agonists and antagonists in the current study could have targeted these NAC neurons, with observed changes in c-Fos expression in the MnPN and VLPO being a downstream consequence. However, SS-rBF drug administration might be expected to more directly affect MnPN and VLPO sleep-active GABAergic neurons, which lie in immediate proximity to the subarachnoid space where drugs were injected.
Although, the density of the A2AR is reported to be low in the POA compared with the striatum, nucleus accumbens, and olfactory tubercle (8), evidence at the system and cellular levels (see above) is consistent with the existence of functional A2AR on sleep regulatory neurons in the preoptic area. However, the experimental approach used in the current study cannot determine whether centrally administered A2AR agonist and antagonist directly targeted MnPN and VLPO neurons. Although the distribution of A2AR in MnPN and VLPO is unknown, on the basis of our current findings, one would expect that A2A receptors are differentially expressed in sleep-active, GABAergic vs. nonGABAergic neurons in these nuclei.
Perspectives and Significance
Our study demonstrates that the intracerebroventricular and SS-rBF administration of an A2AR agonist at doses capable of inducing sleep, increase c-Fos-IR in GABAergic neurons in subregions of the preoptic hypothalamus implicated in sleep regulation, namely, the MnPN and the VLPO. In contrast, intracerebroventricular infusion of adenosine A2AR antagonist suppresses Fos-IR in MnPN and VLPO GABAergic neurons and suppresses recovery sleep following sleep deprivation. These findings are consistent with a hypothesis that A2AR-mediated activation of preoptic area sleep-regulatory neurons contributes to the adenosinergic regulation of sleep. Further studies are needed to quantify the colocalization of A2AR on the sleep-active GABAergic population of the MnPN and VLPO neurons. The focal deletion of A2AR in MnPN and VLPO using Cre/Lox-P approaches may further help determine the relative contributions of A2AR signaling in the preoptic area to sleep regulation.
GRANTS
This work was supported by the U.S. Department of Veteran Affairs Medical Research Service and U.S. National Institutes of Health Grants MH-63323.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.K., D.M., M.N.A., and R.S. conception and design of research; S.K., S.R., and K.C.H. performed experiments; S.K. analyzed data; S.K. and R.S. interpreted results of experiments; S.K. prepared figures; S.K. drafted manuscript; S.K., K.-C.H., D.M., M.N.A., and R.S. edited and revised manuscript; R.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Keng-Tee Chew and Brian Angara for their excellent technical support.
REFERENCES
- 1. Alam MA, Alam MN, Kumar S, McGinty D, Szymusiak R. Effects of sleep deprivation and A2A adenosine receptor antagonist on single unit activity in the rat ventrolateral preoptic area (VLPO). Sleep 35: A 41, 2012 [Google Scholar]
- 2. Alam MN, Kumar S, Rai S, Methippara M, Szymusiak R, McGinty D. Role of adenosine A1 receptor in the perifornical-lateral hypothalamic area in sleep-wake regulation in rats. Brain Res 1304: 96– 104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alam MN, Szymusiak R, Gong H, King J, McGinty D. Adenosinergic modulation of basal forebrain neurons during sleep and waking: neuronal recording with microdialysis. J Physiol 521: 679– 690, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baker FC, Shah S, Stewart D, Angara C, Gong H, Szymusiak R, Opp MR, McGinty D. Interleukin-1β enhances non-rapid eye movement sleep and increases c-Fos protein expression in the median preoptic nucleus of the hypothalamus. Am J Physiol Regul Integr Comp Physiol 288: R998– R1005, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Basheer R, Porkka-Heiskanen T, Stenberg D, McCarley RW. Adenosine and behavioral state control: adenosine increases c-Fos protein and AP1 binding in basal forebrain of rats. Brain Res Mol Brain Res 73: 1– 10, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol 73: 379– 396, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol 45: 347– 360, 1995 [DOI] [PubMed] [Google Scholar]
- 8. DeMet E, DeMet A. Localization of adenosine A2A receptors in the rat brain (3H) ZM-241385. Naunyn Schmiedebergs Arch Pharmacol 366: 478– 481, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Gallopin T, Luppi PH, Cauli B, Urade Y, Rossier J, Hayaishi O, Lambolez B, Fort P. The endogenous somnogen adenosine excites a subset of sleep-promoting neurons via A2A receptors in the ventrolateral preoptic nucleus. Neuroscience 134: 1377– 1390, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Gerashchenko D, Okano Y, Urade Y, Inoué S, Hayaishi O. Strong rebound of wakefulness follows prostaglandin D2- or adenosine A2A receptor agonist-induced sleep. J Sleep Res 9: 81– 87, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Gong H, Szymusiak R, King J, Steininger T, McGinty D. Sleep-related c-Fos protein expression in the preoptic hypothalamus: effects of ambient warming. Am J Physiol Regul Integr Comp Physiol 279: R2079– R2088, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Gvilia I, Turner A, McGinty D, Szymusiak R. Preoptic area neurons and the homeostatic regulation of rapid eye movement sleep. J Neurosci 26: 3037– 3044, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gvilia I, Xu F, McGinty D, Szymusiak R. Homeostatic regulation of sleep: a role for preoptic area neurons. J Neurosci 26: 9426– 9433, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hayaishi O. Sleep-wake regulation by prostaglandins D2 and E2. J Biol Chem 263: 14593– 14596, 1988 [PubMed] [Google Scholar]
- 15. Hong ZY, Huang ZL, Qu WM, Eguchi N, Urade Y, Hayaishi O. An adenosine A2A receptor agonist induces sleep by increasing GABA release in the tuberomammillary nucleus to inhibit histaminergic systems in rats. J Neurochem 92: 1542– 1549, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Hsieh KC, Gvilia I, Kumar S, Uschakov A, McGinty D, Alam MN, Szymusiak R. c-Fos expression in neurons projecting from the preoptic and lateral hypothalamic area to the ventral periaqueducal gray in relation to sleep states. Neuroscience 188: 55– 67, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, Fredholm BB, Urade Y, Hayaishi O. Adenosine A2A, but not A1, receptors mediate the arousal effects of caffeine. Nat Neurosci 8: 858– 859 [DOI] [PubMed] [Google Scholar]
- 18. John J, Kumar V. Effect of NMDA lesions of the medial preoptic neurons on sleep and other functions. Sleep 21: 587– 598, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Krueger JM, Walter J, Dinarello CA, Wolff SM, Chedid L. Sleep-promoting effects of endogenous pyrogen (interleukin-1). Am J Physiol Regul Integr Comp Physiol 246: R994– R999, 1984 [DOI] [PubMed] [Google Scholar]
- 20. Kumar S, Alam MN, Rai S, Bashir T, McGinty D, Szymusiak R. Central nervous system sites of the sleep promoting effects of eszopiclone in rats. Neuroscience 181: 67– 78, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Kumar S, Rai S, Alam MN, McGinty D, Szymusiak R. Central administration of adenosine A2A receptor agonist activates GABAergic neurons in the rat preoptic hypothalamus. Sleep 33: A 54, 2010 [Google Scholar]
- 22. Kumar S, Rai S, Alam MN, McGinty D, Szymusiak R. Effects of intracerebroventricular (ICV) infusion of an adenosine A2A receptor antagonist on sleep and preoptic neuronal activity in rats. Sleep 35: A 41, 2012 [Google Scholar]
- 23. Kumar S, Szymusiak R, Bashir T, Rai S, McGinty D, Alam MN. Effects of serotonin on perifornical-lateral hypothalamic area neurons in rat. Eur J Neurosci 25: 201– 212, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Kumar S, Szymusiak R, Bashir T, Suntsova N, Rai S, McGinty D, Alam MN. Inactivation of median preoptic nucleus causes c-Fos expression in hypocretin- and serotonin-containing neurons in anesthetized rat. Brain Res 25: 201– 212, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lazarus M, Shen HY, Cherasse Y, Qu WM, huang ZL, Bass CE, Winsky-Sommerer R, Semba K, Fredholm BB, Boison D, Hayaishi O, Urade Y, Chen JF. Arousal effects of caffeine depends on adenosine A2A receptors in the shell of the nucleus accumbens. J Neurosci 31: 10067– 10075, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu ZW, Gao XB. Adenosine inhibits activity of hypocretin/orexin neurons by the A1 receptor in the lateral hypothalamus: a possible sleep-promoting effect. J Neurophysiol 97: 837– 848, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lu J, Greco MA, Shiromani P, Saper CB. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci 20: 3830– 3842, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mendelson WB. Sleep-inducing effects of adenosine microinjections into the medial preoptic area are blocked by flumazenil. Brain Res 852: 479– 481, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Methippara MM, Kumar S, Alam MN, Szymusiak R, McGinty D. Effects on sleep of microdialysis of adenosine A1 and A2A receptor analogs into the lateral preoptic area of rats. Am J Physiol Regul Integr Comp Physiol 289: R1715– R1723, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Morairty S, Rainnie DG, McCarley RW, Greene RW. Disinhibition of ventrolateral preoptic area sleep-active neurons by adenosine: a new mechanism for sleep promotion. Neuroscience 123: 451– 457, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Obal F, Jr, Alfoldi P, Cady AB, Johannsen L, Sary G, Krueger JM. Growth hormone-releasing factor enhances sleep in rats and rabbits. Am J Physiol Regul Integr Comp Physiol 255: R310– R316, 1988 [DOI] [PubMed] [Google Scholar]
- 32. Oishi Y, Huang ZL, Fredholm BB, Urade Y, Hayaishi O. Adenosine in the tuberomammillary nucleus inhibits the histaminergic system via A1 receptors and promotes non-rapid eye movement sleep. Proc Natl Acad Sci USA 105: 19992– 19997, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pan WJ, Osmanovic SS, Shefner SA. Characterization of the adenosine A1 receptor-activated potassium current in rat locus ceruleus neurons. J Pharmacol Exp Ther 273: 537– 544, 1995 [PubMed] [Google Scholar]
- 34. Paxinos G, Watson C. The Rat Brain: in Stereotaxic Coordinates. San Diego, CA: Academic, 1998 [Google Scholar]
- 35. Peterfi Z, McGinty D, Sarai E, Szymusiak R. Growth hormone-releasing hormone activates sleep regulatory neurons of the rat preoptic hypothalamus. Am J Physiol Regul Integr Comp Physiol 298: R147– R156, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Porkka-Heiskanen T, Alanko L, Kalinchuk Stenberg D. A. Adenosine and sleep. Sleep Med Rev 6: 321– 332, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science 276: 1265– 1268, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Portas CM, Thakkar M, Rainnie DG, Greene RW, McCarley RW. Role of adenosine in behavioral state modulation: a microdialysis study in the freely moving cat. Neuroscience 79: 225– 235, 1997 [DOI] [PubMed] [Google Scholar]
- 39. Radulovacki M. Adenosine sleep theory: how I postulated it. Neurol Res 27: 137– 138, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Rainnie DG, Grunze HC, McCarley RW, Green RW. Adenosine inhibition of mesopontine cholinergic neurons: implications for EEG arousal. Science 263: 689– 692, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rai S, Kumar S, Alam MA, Szymusiak R, McGinty D, Alam MN. A1 receptor mediated adenosinergic regulation of perifornical-lateral hypothalamic area in freely behaving rats. Neuroscience 167: 40– 48, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ribeiro JA, Sebastião AM, Mendonça A. Adenosine receptors in the nervous system: pathophysiological implications. Prog Neurobiol 68: 377– 392, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Satoh S, Matsumura H, Hayaishi O. Involvement of adenosine A2A receptor in sleep-promotion. Eur J Pharmacol 351: 155– 162, 1998 [DOI] [PubMed] [Google Scholar]
- 44. Satoh S, Matsumura H, Koike N, Tokunaga Y, Maeda O, Hayaishi T. Region-dependent difference in the sleep-promoting potency of an adenosine A2a receptor agonist. Eur J Neurosci 11: 1587– 1597, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Satoh S, Matsumura H, Suzuki O, Hayaishi T. Promotion of sleep mediated by the A2A-adenosine receptor and possible involvement of this receptor in the sleep induced by prostaglandin D2 in rats. Proc Natl Acad Sci USA 93: 5980– 5984, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Scammell TE, Gerashchenko DY, Mochizuki T, McCarthy MT, Estabrooke IV, Sears CA, Saper CB, Urade Y, Hayaishi O. An adenosine A2a agonist increases sleep and induces Fos in ventrolateral preoptic neurons. Neuroscience 107: 653– 663, 2001 [DOI] [PubMed] [Google Scholar]
- 47. Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci 18: 4705– 4721, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science 271: 216– 219, 1996 [DOI] [PubMed] [Google Scholar]
- 49. Steininger T, Gong H, McGinty D, Szymusiak R. Subregional organization of preoptic area/anterior hypothalamic projections to arousal-related monoaminergic cell groups. J Comp Neurol 429: 638– 653, 2001 [PubMed] [Google Scholar]
- 50. Suntsova N, Guzman-Marin R, Kumar S, Alam MN, Szymusiak R, McGinty D. The median preoptic nucleus reciprocally modulates activity of arousal-related and sleep-related neurons in the perifornical lateral hypothalamus. J Neurosci 27: 1616– 1630, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Suntsova N, Szymusiak R, Alam MN, Guzman-Marin R, McGinty D. Sleep-waking discharge patterns of median preoptic nucleus neurons in rats. J Physiol 43: 665– 667, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Szymusiak R, Alam MN, Steininger TL, McGinty D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res 803: 178– 188, 1998 [DOI] [PubMed] [Google Scholar]
- 53. Thakkar MM, Delgiacco RA, Strecker RE, McCarley RW. Adenosinergic inhibition of basal forebrain wakefulness-active neurons: a simultaneous unit recording and microdialysis study in freely behaving cats. Neuroscience 122: 1107– 1113, 2003 [DOI] [PubMed] [Google Scholar]
- 54. Thakkar MM, Engemann SC, Walsh KM, Sahota PK. Adenosine and the homeostatic control of sleep: effects of A1 receptor blockade in the perifornical lateral hypothalamus on sleep-wakefulness. Neuroscience 153: 875– 880, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Ticho SR, Radulovacki M. Role of adenosine in sleep and temperature regulation in the pre-optic area of rats. Pharmacol Biochem Behav 40: 33– 40, 1991 [DOI] [PubMed] [Google Scholar]
- 56. Urade Y, Eguchi N, Qu WM, Sakata M, Huang ZL, Chen JF, Schwarzschild MA, Fink Hayaishi O. JS. Sleep regulation in adenosine A2A receptor-deficient mice. Neurology 61: S94– S96, 2003 [DOI] [PubMed] [Google Scholar]
- 57. Uschakov A, Gong H, McGinty D, Szymusiak R. Efferent projections from the median preoptic nucleus to sleep- and arousal regulatory nuclei in the rat brain. Neuroscience 150: 104– 120, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Uschakov A, Gong H, McGinty D, Szymusiak R. Sleep-active neurons in the preoptic area project to the hypothalamic paraventricular nucleus and the perifornical lateral hypothalamus. Eur J Neurosci 23: 3284– 3296, 2006 [DOI] [PubMed] [Google Scholar]
- 59. Uschakov A, McGinty D, Szymusiak R, McKinley MJ. Functional correlates of activity in neurons projecting from the lamina terminalis to the ventrolateral periaqueductal gray. Eur J Neurosci 30: 2347– 2355, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yoshida K, McCormack S, Espana R, Crocker A, Scammell T. Afferents to the orexin neurons of the rat brain. J Comp Neurol 494: 845– 861, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]