Abstract
Obstructive sleep apnea (OSA) and dim light at night (dLAN) have both been independently associated with alterations in mood and cognition. We aimed to determine whether dLAN would interact with intermittent hypoxia (IH), a condition characteristic of OSA, to alter the behavioral, cognitive, and affective responses. Adult male mice were housed in either standard lighting conditions (14:10-h light-dark cycle; 150 lux:0 lux) or dLAN (150 lux:5 lux). Mice were then exposed to IH (15 cycles/h, 8 h/day, FiO2 nadir of 5%) for 3 wk, then tested in assays of affective and cognitive responses; brains were collected for dendritic morphology and PCR analysis. Exposure to dLAN and IH increased anxiety-like behaviors, as assessed in the open field, elevated plus maze, and the light/dark box. dLAN and IH increased depressive-like behaviors in the forced swim test. IH impaired learning and memory performance in the passive avoidance task; however, no differences were observed in spatial working memory, as assessed by y-maze or object recognition. IH combined with dLAN decreased cell body area in the CA1 and CA3 regions of the hippocampus. Overall, IH decreased apical spine density in the CA3, whereas dLAN decreased spine density in the CA1 of the hippocampus. TNF-α gene expression was not altered by IH or lighting condition, whereas VEGF expression was increased by dLAN. The combination of IH and dLAN provokes negative effects on hippocampal dendritic morphology, affect, and cognition, suggesting that limiting nighttime exposure to light in combination with other established treatments may be of benefit to patients with OSA.
Keywords: intermittent hypoxia, light at night, anxiety, depression, learning, memory
obstructive sleep apnea (osa) apnea is a major public health problem that has been associated with alterations in affect and cognitive function (5, 52). In South America, the prevalence of OSA is 32.8% of the population, and in the United States, physician-diagnosed OSA occurs in 5.7 percent of men and 2.8 percent of women (29, 45). OSA is characterized by brief episodes of repetitive upper airway obstruction leading to intermittent hypoxia (IH) during sleep. Approximately half of patients with OSA also suffer from depression and anxiety, and most experience memory deficits (1, 20, 23). Depression and anxiety changes are associated with structural changes in the brain; increased anxiety and depression correlate to reduced sleep quality (20). Individuals with OSA have neuropsychological deficits; levels of hypoxia during sleep can be used to predict performance of declarative and working memory (1). These changes in mood and learning and memory are consistent with responses previously reported in people exposed to high-altitude hypoxia (31, 36).
IH paradigms are used in mice to simulate the hypoxic stress seen in patients with OSA. The two main IH paradigms currently used include 1) a brief model that employs short-duration IH for a short total period and 2) chronic IH, in which animals are exposed for extended periods during each day for a prolonged total duration. These two models produce different outcomes in rodents (19). Brief IH stimulates neurogenesis and reduces depressive-like behaviors (34, 54). However, negative outcomes, such as impaired cognitive functions, increased inflammation, and oxidative stress are more common following chronic IH (19, 32, 44). Tumor necrosis factor-α (TNF-α) is one inflammatory gene that often increases in expression during IH; increased TNF-α gene expression occurs at the onset of IH treatment and begins to decrease with prolonged exposure (24). Additionally, vascular endothelial growth factor (VEGF) gene expression typically increases with exposure to IH, an observation that has been suggested to be the basis of both negative and positive outcomes observed in IH models (4, 49). Similarly, exposure to hypobaric hypoxia, mimicking high altitudes, ≤14 days induces dendritic atrophy in the hippocampus and cortex, whereas structural recovery occurs after 21 days of hypobaric hypoxia with a corresponding improvement in spatial memory (21, 44). Thus, hypoxia has markedly different effects depending on duration. Chronic IH models closely resemble deficits observed in most patients with OSA.
A relatively novel, but increasingly prevalent, daily stimulus experienced by virtually all people, is bright light at night. Electrical lighting has many benefits for modern society, including increased safety and productivity due to shift work. However, several studies have reported deleterious effects of light at night on individuals' health, including mood and obesity (10, 16, 46). In hamsters, dim light at night (dLAN) increases depressive-like behaviors and reduces CA1 dendritic spine density (2). Increased depressive-like behavior, as well as reduced anxiety, has also been observed in mice in response to light at night (2, 8). The mood alterations observed in response to light at night, particularly depression, are similar to the results of OSA (1, 20). In diurnal rodents dLAN also increases depressive-like behaviors and impairs cognition (9). Additionally, light at night is associated with increased body mass, suggesting changing environmental conditions could contribute to the occurrence of OSA by increasing obesity in the population (10).
Thus, we hypothesized that IH and dim light at night (dLAN) would interact to increase depression and anxiety-like behaviors and impair learning and memory. Given the overlap in affective and cognitive changes in rodents between light at night exposure and OSA, we expected exposure to dim light at night combined with IH treatment would negatively and additively affect outcome.
MATERIALS AND METHODS
Animals
Forty male Swiss-Webster mice (∼8 wk old) were obtained from Charles River Laboratories (Wilmington, MA). Mice were group-housed, five per cage in propylene cages (33 cm × 18 cm × 14 cm) at an ambient temperature of 22 ± 2°C, relative humidity of 50% ± 10%. Animals were given ad libitum Harlan Teklad 8640 food (Madison, WI) and filtered tap water. Upon arrival, mice were maintained under a 14:10-h light-dark cycle of illumination for 1 wk to allow acclimation to local conditions. Following this, mice were randomly assigned to either standard lighting conditions (n = 20) [14:10 h; 150 lux:0 lux (LD)] or exposed to dLAN (n = 20) (14:10; 150 lux:5 lux) for the remainder of the study. All experimental procedures were approved by The Ohio State University Institutional Animal Care and Use Committee.
Hypoxia Treatment
After 4 wk of acclimation to the experimental lighting conditions, mice were randomly assigned to receive intermittent hypoxia (IH) (n = 20) (15 cycles/h, 8 h/day, FiO2 nadir of 5%) or room air (RA) (n = 20), creating four experimental groups (n = 10/group). During this treatment, mice were moved to custom-designed Plexiglas chambers (31 cm × 19 cm × 18 cm) with a raised floor (6.5 cm); 10 mice were placed in one chamber at a time (30). Oxygen levels were controlled by connecting the cages via a regulator system to compressed air and nitrogen tanks. RA-exposed mice were housed in a similar cage, without connections to nitrogen or air tanks. Treatment occurred during the light phase (when these animals typically sleep) and lasted 3 wk; behavioral testing occurred during the final week of IH or RA treatment. Mice were removed from IH or RA treatment for 1 h during the first day of behavioral testing, which required illuminated conditions. Subsequent days of testing occurred at the end of treatment and during the dark phase. Normalization of behavior provoked by exposure to dLAN takes 2–4 wk of constant dark nights; thus, brief exposure to darkness during testing was expected to have a minimal effect on behavior (3).
Open Field
The open-field test in mice characterizes anxiety-like responses in a novel environment, as well as locomotor activity. Central tendency is the primary measure for anxiety-like responses, and it is defined as the proportion of time spent in the center of the open field. Locomotor activity is measured separately as the total number of beam breaks during testing. Mice were removed from the treatment chambers for ∼1 h during the light phase for behavioral testing and were allowed to acclimate to the room for 20 min before testing. Mice were tested for 20 min as previously described (8).
Light/Dark Box
The light/dark box also measures anxiety-like behavior and allows mice the option between an open area with a bright light and an enclosed, dark area. Following the open-field test, mice were placed in the dark side of a light/dark box (40 cm × 40 cm × 35 cm) and allowed to explore for 5 min. Latency to enter the light side of the box and total time spent in the light were recorded.
Elevated Plus Maze
The elevated plus maze measures anxiety-like behavior by assessing time spent in the open arms vs. time in the enclosed arms. Locomotor differences can also be assessed by using total entries into arms. The next day, mice were placed in the center of the elevated plus maze with two enclosed arms (50 cm × 10 cm × 40 cm) and two open arms (50 cm × 10 cm). The entire maze is elevated 40 cm off the ground. Animals were allowed to freely explore for 5 min and recorded on video. Video was scored on Observer software (Noldus, Leesburg, VA) by a condition-blind observer for latency to enter the open arms and the percentage of time spent in the open arms.
Forced-Swim Test
Following the elevated plus maze, mice were tested in the Porsolt forced-swim paradigm that assesses depressive-like behaviors. The main variable assessed was time spent immobile; this response is interpreted as behavioral despair and is reversible with antidepressant treatment (7). Mice were placed in an opaque, cylindrical tank (diameter = 24 cm, height = 53 cm) filled with room temperature (22 ± 2°C) water ∼17 cm deep. Mice were recorded for 10 min and then removed from the water, dried, and returned to a clean cage. The video was scored on Observer software (Noldus) by a condition-blind observer for latency to first float, the number of floating bouts, and total time floating.
Y-Maze
The Y-maze assesses spatial working memory, interpreted by the percent of spontaneous alternations made during the testing period. The following day, each mouse completed one 5-min trial in a black Plexiglas Y-maze (36 cm × 7 cm × 9 cm). Recording and analysis of behavior were conducted as described previously (18). The number of spontaneous alternations made by each animal was divided by the total possible alternation score for each animal, yielding a percentage score.
Object Recognition
The object recognition task assesses learning and memory in mice by familiarizing them with an object and then presenting them with both a novel and the familiar object. Learning and memory were assessed with object recognition, measured by the amount of time spent exploring the novel object compared with the familiar object. Mice were exposed to rat cages (45 cm × 24 cm × 22 cm) with blackened walls for 5 min. The following day mice were placed back in chambers for 10 min with two plastic funnels in both back corners, and activity was recorded. Cages and objects were cleaned with 70% ethanol between each trial. One hour after the first trial, animals were placed back in the recognition cages, this time with one funnel and an overturned miniature, metal bowl, and animal activity was recorded for 2 min. Both trials were scored on Observer software (Noldus) by a condition-blind observer for time spent exploring the cage, the left object, and the right object.
Passive Avoidance
The passive avoidance test also assesses learning and memory by allowing mice to form an association between escaping from an aversive stimulus (light) and a foot shock. Retention of this pairing was assessed 24 h after initial trial and latency to enter the dark chamber was used to assess retention. During the following 2 days, animals were placed in the passive avoidance chamber (Gemini Avoidance System, San Diego Instruments, San Diego, CA). Mice were placed in the right side of the chamber in a starting box; after 20 s, the chamber was illuminated as an electrically operated door opened to expose a dark chamber on the other side. Mice had a maximum trial length of 300 s to enter the dark chamber. After animals entered the dark side, the door closed, and mice received a 2-mA foot shock for 2 s. Then, mice were removed from the chamber and returned to a clean cage. Twenty-four hours later, mice were again placed in the chamber following the same procedure, but received no foot shock. Latency to enter the dark side of the chamber was observed for both trials.
Tissue Collection and Processing
Animals were euthanized during the light phase (1100 EST) by rapid decapitation under isoflurane sedation. At necropsy, adrenal glands were collected and weighed, and a trunk blood sample was obtained for corticosterone RIA. RIA was performed as previously described (8). Brain tissue was also collected, and half was placed in RNAlater (Applied Biosystems, Foster City, CA) for RNA extraction and gene analysis, while the other half was placed in Golgi-Cox staining using Rapid GolgiStain kit (FD NeuroTechnologies, Columbia, MD). The hemisphere of brain used for Golgi-Cox staining or RNAlater (Applied Biosystems, Foster City, CA) was randomly selected. Golgi-Cox-stained brains were stored, processed, and analyzed, as described previously (2).
Hippocampal VEGF gene expression was assayed using quantitative real-time PCR. Hemispheres were stored at 4°C for 2 wk, and then hippocampi were dissected out and stored at −80°C until extraction. Total RNA was extracted from ≤30 mg of individual hippocampi using a homogenizer (Ultra-Turrax TB; IKA Works, Wilmington, NC) with TRIzol reagent (Invitrogen, Carlsbad, CA), according to the manufacturer's guidelines. Then extracted RNA was suspended in 30 μl of RNase-free water, and the concentration of RNA was determined by spectrophotometer (NanoDrop-1000; Nanodrop Technologies, Wilmington, DE). RNA was stored at −80°C until use. Next cDNA was made by reverse transcription of 2 μg RNA with MMLV reverse-transcriptase enzyme (Invitrogen, Carlsbad, CA), according to the manufacturer's guidelines. PCR primers and TaqMan probes targeting VEGF-A and TNF-α was purchased at Assays-on-Demand Products for Gene Expression (Applied Biosystems). A TaqMan 18S ribosomal primer and probe set (labeled with VIC; Applied Biosystems) was used as the control gene for relative quantification. Amplifications were performed on an Applied Biosystems 7500 fast real-time PCR systems by using TaqMan universal PCR master mix. A 1:10 dilution of cDNA sample was used. The universal two-step RT-PCR cycling conditions used were 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, and 60°C for 1 min. Each individual sample was run in duplicate. Relative gene expression was calculated from a standard curve consisting of serial dilutions of mixed samples of cDNA (1:10, 1:100, 1:1,000, 1:10,000) and normalized to 18S rRNA gene expression.
Statistical Analysis
Main effects of light condition (LD, dLAN) and IH treatment (air, IH) and interactions between the two were assessed. Behavioral tests were analyzed using a two-way univariate ANOVA. A two-way, repeated-measures ANOVA was used to compare body mass across treatment. For variables with unequal variance, data were log transformed to run statistical analysis. Statistics were performed using SPSS 19 for Windows. Outliers determined by Z score (± 2 SE from mean) were removed from subsequent analysis. An a priori G*Power analysis indicated a sample size of 8 per group necessary to detect differences at the 0.8 level. Mean differences were considered statistically significant when P was ≤ 0.05.
RESULTS
Anxiety and Depression
Open field.
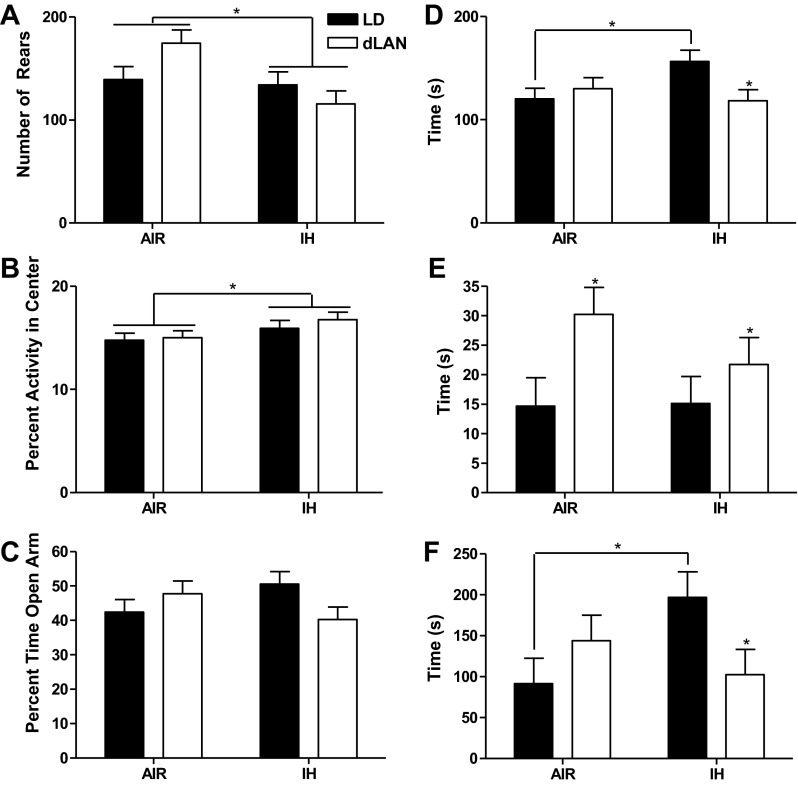
IH treatment increased central tendency in the first 5 min of open field compared with room air (F1,33 = 4.166; P < 0.05) (Fig. 1B). Central tendency was similar for mice housed in either dim light at night and dark nights (P > 0.05). In either lighting condition, IH treatment decreased rearing in the open field (F1,36 = 6.430, P < 0.05, n = 10/group), Fig. 1A. Mice housed in room air increased rearing compared with mice exposed to IH (P < 0.01). Lighting condition did not affect rearing (P > 0.05). Mice exposed to dLAN and room air increased rearing, whereas dLAN mice experiencing IH decreased rearing (F1,36 = 4.519; P < 0.05; Air/LD: n = 10; Air/dLAN: n = 10; IH/LD: n = 8; IH/dLAN: n = 9). Mice exposed to dLAN and room air displayed more rearing than mice housed in standard lighting conditions in room air (P < 0.05). Neither IH treatment nor lighting condition affected total locomotor activity during the 20-min test (P > 0.05; n = 10/group). IH treatment decreased anxiety-like behavior in terms of central tendency in the open field, whereas room air decreased anxiety-like behavior by increasing rearing.
Fig. 1.
The effects of intermittent hypoxia and dim light at night on anxiety and depressive-like behaviors in male mice. Means ± SE number of rears for the first 5 min of an open field test (A) and percent of activity in the center (B) in the open field test of anxiety-like behaviors. Total duration of test was 20 min. Means ± SE percent of time spent in the open arm of the elevated plus maze test for anxiety-like behaviors (C). Total duration of the test was 300 s. Means ± SE total time spent in light side of light-dark box (D) and latency to enter the light side of box (E). Total duration of the test was 300 s. Means ± SE latency to first floating bout in the forced swim test, a measure for depressive-like behavior (F). Total duration of test was 600 s, *P < 0.05 between room air (AIR) and intermittent hypoxia (IH) or light-dark (LD) and dim light at night (dLAN) for all behaviors.
Light/dark box.
IH treatment and room air exposure resulted in comparable latencies of mice to enter the light side of the light/dark box (P > 0.05). Overall, dLAN increased latency to enter the light side of the light/dark box compared with standard lighting conditions (F1,35 = 5.677; P < 0.05; Air/LD: n = 9; Air/dLAN: n = 10; IH/LD: n = 10; IH/dLAN: n = 10) (Fig. 1E). Mice in standard lighting conditions and room air decreased the time spent in the light compartment compared with IH (F1,33 = 5.072, P < 0.05; Air/LD: n = 10; Air/dLAN: n = 9; IH/LD: n = 9; IH/dLAN: n = 9). In IH-treated mice, dLAN decreased total time in the light compared with dark nights (P < 0.05). In standard lighting conditions, IH treatment increased total time in the light compared with room air animals housed in standard lighting conditions (P < 0.05). Mice in dark nights and exposed to IH increased time in the light side of the light/dark box compared with animals exposed to dLAN and room air (P < 0.05) (Fig. 1D). Mice exposed to both dLAN and room air increased total time in light compartment compared with mice exposed to dLAN and IH treatment (P < 0.05). IH alone did not alter anxiety-like behavior; however, dLAN increased anxiety-like behavior. Dark nights decreased anxiety-like behavior in conjunction with IH treatment, whereas mice exposed to dLAN and IH increased anxiety-like behavior.
Elevated plus maze.
Neither lighting condition nor IH or air treatment alone altered anxiety-like behavior on the open arm of the elevated plus maze (P > 0.05). Mice housed in standard lighting and exposed to room air spent less time on the open arm than animals in standard lighting and exposed to IH, whereas mice housed in dim light at night and room air spent more time than mice housed in dLAN and IH on the open arms of the elevated plus maze (F1,36 = 4.567; P < 0.05; n = 10/experimental group) (Fig. 1C). Mice housed in dLAN and treated with IH decreased time spent on the open arm compared with mice housed in dLAN and room air (P < 0.05). IH in dark nights decreased anxiety-like behavior, whereas IH in dLAN increased anxiety-like behavior.
Forced-swim test.
Individually, neither air treatment nor lighting condition affected the latency of mice to the first floating bout (P > 0.05). Mice exposed to dark nights decreased latency to float when exposed to room air compared with mice housed in dark nights and IH treatment; latency to first floating bout in dLAN was increased in mice exposed to room air, but decreased in mice exposed to dLAN and undergoing IH (F1,36 = 5.587; P < 0.05; n = 10/experimental group) (Fig. 1F). There were no differences in total time floating, swimming, climbing, or number of floating bouts (P > 0.05; n = 10/experimental group in each case). IH increased latency to first float; however, IH paired with dLAN decreased latency to float. This response suggests an elevated depressive-like response.
Learning and Memory
Object recognition.
There were no differences in the ratio of time spent exploring the new vs. old objects by treatment or lighting condition (P > 0.05; n = 10/group).
Y-maze.
There were no differences in the percentage of spontaneous alternation between treatments or between lighting conditions (P > 0.05; n = 10/group).
Passive avoidance.
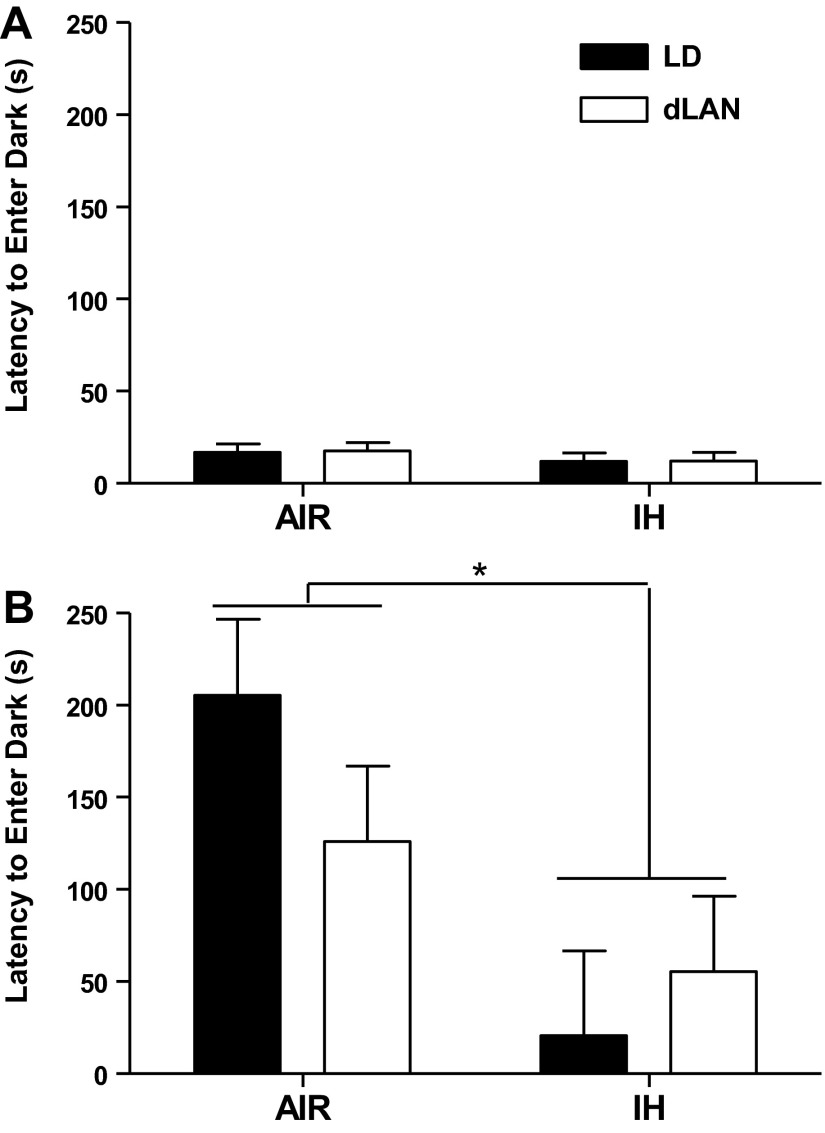
Only data for the second cohort of animals were analyzed because of an error in recording the first cohort. No differences in latency to enter the dark side of the chamber on the training day were observed (P > 0.05; n = 5/group) (Fig. 2). Overall, IH treatment decreased the latency for mice to enter the dark side of the chamber of the second day of testing compared with mice exposed to room air (F1,15 = 9.084; P < 0.05; Air/LD: n = 5; Air/dLAN: n = 5; IH/LD: n = 4; IH/dLAN: n = 5) (Fig. 2). Lighting conditions had no effect on latency to enter the dark side of the chamber on the second day of testing (P > 0.05). In both dLAN and standard lighting conditions, the latency to enter the dark side of the chamber increased in mice exposed to room air compared with mice exposed to IH (P < 0.05). Thus, IH treatment decreased learning and memory.
Fig. 2.
The effect of intermittent hypoxia and dim light at night on learning and memory in male mice. Means ± SE latency to enter the dark chamber on day 1 of testing (A) and day 2 of testing (B) of the passive avoidance test. Total duration of test was 300 s. *P < 0.05 between AIR and IH.
Body and Tissue Masses
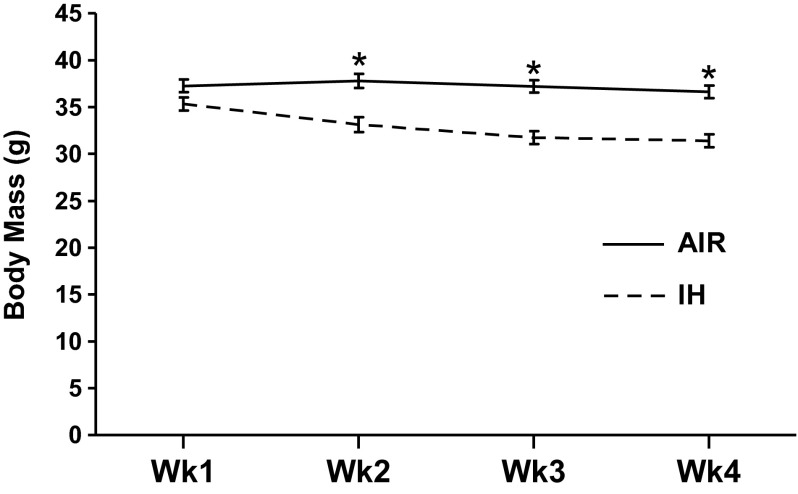
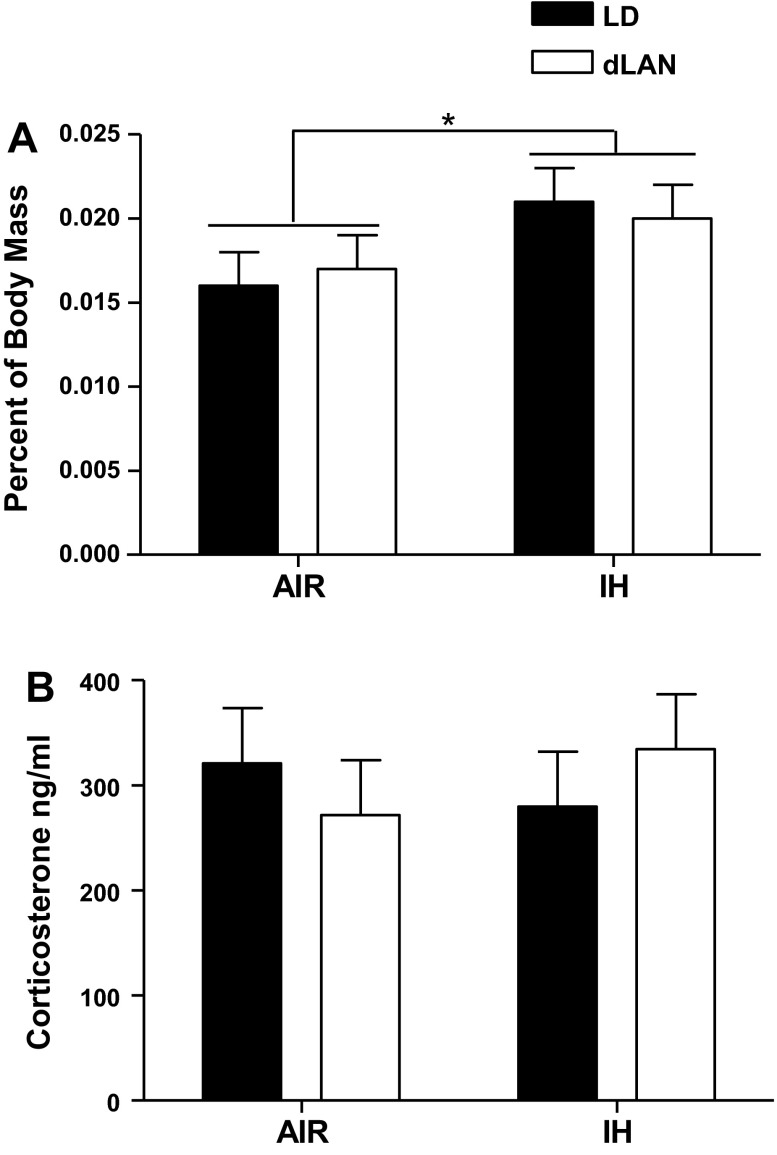
Across 3 wk of treatment, IH-treated animals had lower average body mass than air-treated mice (F1,35 = 19.404; P < 0.001, Air/LD: n = 10; Air/dLAN: n = 10; IH/LD: n = 9; IH/dLAN: n = 10) (Fig. 3). As reported previously, housing in dLAN increased body mass compared with housing in standard lighting conditions before the beginning of IH or air treatment (F1,36 = 5.676; P < 0.05; n = 10/group); this effect was removed by 3 wk of treatment (P > 0.05; Air/LD: n = 10; Air/dLAN: n = 10; IH/LD: n = 9; IH/dLAN: n = 10; data not shown). Body mass was not recorded for one animal at necropsy; the corrected organ masses were omitted from analyses (Air/LD: n = 10; Air/dLAN: n = 10; IH/LD: n = 9; IH/dLAN: n = 10). IH treatment increased body mass corrected adrenal glands masses (F1,35 = 8.833; P < 0.01; Fig. 4A). Lighting condition did not alter body mass-corrected adrenal gland masses (P > 0.05). IH treatment decreased body mass and increased adrenal gland masses.
Fig. 3.
Body mass was decreased during IH exposure. Means ± SE body mass for duration of IH treatment. *P < 0.05 between AIR and IH.
Fig. 4.
The effect of intermittent hypoxia and dim light at night on adrenal gland masses and corticosterone concentrations in mice. Means ± SE body corrected mass for adrenal glands (A) and means ± SE corticosterone concentrations (B) at end of experiment. *P < 0.05.
Corticosterone Concentrations
At the end of treatment, there were no differences in corticosterone levels between lighting conditions or air treatment (P > 0.05; n = 10/group) (Fig. 4B).
TNF-α and VEGF Expression
TNF-α expression in the hippocampus was not altered by exposure to IH or dLAN (P > 0.05; Air/LD: n = 10; Air/dLAN: n = 8; IH/LD: n = 9; IH/dLAN: n = 9). Hippocampal VEGF expression was not affected by IH or air treatment (P > 0.05). VEGF expression was lower in mice exposed to dark nights compared with dLAN (F1,33 = 8.833; P < 0.05; Air/LD: n = 10; Air/dLAN: n = 8; IH/LD: n = 10; IH/dLAN: n = 9).
Hippocampal Cell Morphology
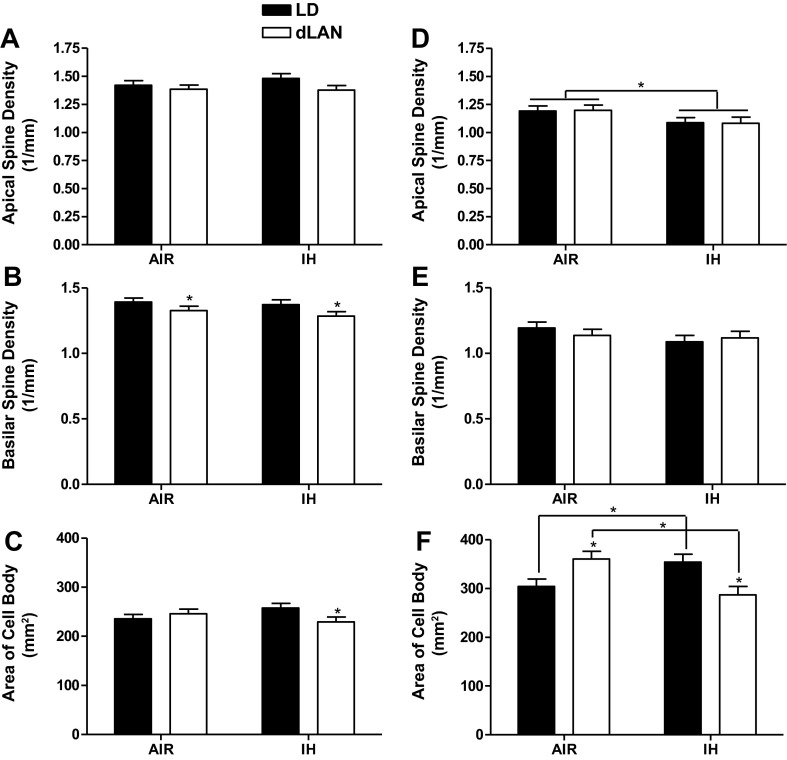
CA1 apical spine density was not altered by IH treatment or exposure to dLAN (P > 0.05; Air/LD: n = 8; Air/dLAN: n = 9; IH/LD: n = 8; IH/dLAN: n = 8) (Fig. 5A). There was no effect of IH treatment on basilar spine density (P > 0.05). Exposure to dLAN decreased basilar spine density compared with exposure to standard lighting (F1,29 = 5.404, P < 0.05; Air/LD: n = 10; Air/dLAN: n = 8; IH/LD: n = 8; IH/dLAN: n = 7) (Fig. 5B). Treatment and lighting condition did not individually alter cell body area (P > 0.05). In mice experiencing room air and dark nights, cell body area decreased compared with IH and dark nights, whereas with dLAN and room air, cell body area increased compared with dLAN and IH (F1,30 = 4.165; P = 0.05; Air/LD: n = 9; Air/dLAN: n = 9; IH/LD: n = 8; IH/dLAN: n = 8) (Fig. 5C). Mice treated with IH and standard lighting increased cell body area compared with IH and dLAN (P < 0.05). During the Golgi staining process, the sections from two animals were inadvertently destroyed and were, thus, omitted from analyses. For all analyses conducted in the CA1 of the hippocampus, three mice were omitted for lack of traceable neurons. In the CA1 of the hippocampus, dLAN decreased basilar spine density, and dLAN combined with IH treatment decreased cell body area.
Fig. 5.
The effect of intermittent hypoxia and dim light at night on dendritic morphology. Apical spine density of neurons in CA1 (A) and CA3 (D) of the hippocampus (± SE). Basilar spine density of neurons in CA1 (B) and CA3 (E) of the hippocampus ( ± SE). Average cell body area of neurons in the CA1 (C) and CA3 (F) of the hippocampus ( ± SE). *P < 0.05.
Overall, IH treatment decreased apical spine density compared with room air (F1,31 = 5.005; P < 0.05, Air/LD: n = 10; Air/dLAN: n = 9; IH/LD: n = 9; IH/dLAN: n = 7) (Fig. 5D) in the CA3 region of the hippocampus, with one outlier removed based on z score. There was no effect of lighting condition on apical spine density (P > 0.05). Basilar spine density was not altered by treatment or lighting condition (P > 0.05; Air/LD: n = 10; Air/dLAN: n = 9; IH/LD: n = 9; IH/dLAN: n = 8) (Fig. 5E). Treatment and lighting condition did not individually alter cell body area (P > 0.05). In standard lighting conditions, IH increased area of the cell body compared with mice housed in standard lighting and room air (P < 0.05), mice housed in dLAN and exposed to air increased average area of the cell body in the CA3 of the hippocampus compared with mice exposed to dLAN and IH (F1,32 = 14.790; P < 0.01, Air/LD: n = 10; Air/dLAN: n = 9; IH/LD: n = 9; IH/dLAN: n = 8) (Fig. 5F). Mice exposed to room air and dLAN increased cell body area compared with mice exposed to room air and dark nights (P < 0.05). In mice exposed to IH treatment, exposure to dLAN decreased the area of cell bodies compared with mice in the standard lighting condition (P < 0.05). In the CA3, two animals did not have adequate staining for analysis and were omitted. In the CA3 of the hippocampus, IH decreased apical spine density, and dLAN and IH combined to decrease cell body area.
DISCUSSION
We examined the combinatorial effects of exposure to dLAN and IH on affective responses, learning and memory, and hippocampal morphology in this study. Overall, our results indicated that IH, combined with dLAN treatment, increases anxiety and depressive-like behaviors. IH reduced adiposity and increased adrenal masses. IH combined with dLAN decreased cell body area in the CA1 and CA3 regions of the hippocampus. Overall, IH exposure decreased apical spine density in the CA3 compared with room air, and dLAN decreased basilar spine density in the CA1 of the hippocampus compared with standard lighting conditions.
Relatively little affective assessment has been conducted in animal models of IH. Animals exposed to IH decreased anxiety-like behavior, contrary to reports of increased anxiety in patients with OSA (31, 36). Consistent with previous findings, dLAN housing alone decreased anxiety-like behavior (8). The combination of housing in dLAN and IH, however, increased anxiety-like behaviors consistent with anxiety observed in OSA patients, suggesting a role for dLAN in the patient population (31, 36). Previous studies examining IH treatment and sleep deprivation in rats reported that sleep deprivation alone or combined with IH increased corticosterone concentrations in rats (25). Sleep deprivation alone increased anxiety-like behavior in both mice and rats (25, 38). It is important in future studies to consider the possible role of stress in behavioral outcomes of sleep deprivation.
Elevated floating and decreased latencies to float in the forced swim test are interpreted as increased depressive-like behavior (28). Animals housed in standard lighting conditions and IH increased latency to float, indicating decreased depressive-like responses inconsistent with reports that OSA patients are more depressed than people without OSA (36). Animals experiencing both dLAN and IH increased depressive-like responses similar to patients with OSA (36). Because dLAN increases depressive-like responses in rodents (2, 8), presumably dLAN interacted with IH to alter behavior. Circadian disruption has been linked to depression in both clinical and animal studies (22, 40). Our results suggest that exposure to light at night might be exacerbating the negative effects of OSA on mood.
Mice lost mass following IH treatment, consistent with previous studies indicating the validity of our IH model (15, 39). IH increased adrenal gland masses consistent with a putative role played by the adrenal glands in IH-induced hypertension (13). IH also elevated catecholamine release from the adrenal medulla and increased oxidative stress (15, 17). Additionally, the adrenal gland is an important part of the stress response with activation of the autonomic nervous system and the hypothalamic-pituitary-adrenal (HPA) axis. Changes in adrenal masses again indicate a possible role for stress in the mice, although we did not observe differences in corticosterone at the end of treatment. However, it is possible that the HPA axis was still altered by changes in corticosterone earlier during treatment, as reported previously, leading to prolonged changes in adrenal gland masses (47). Increased adrenal masses have been reported in studies of paradoxical sleep deprivation (6, 42). IH, in a preconditioning context, has previously been reported to prevent the development of the poststress depression in rats by preventing the disturbance of the HPA axis (35), suggesting an ability of IH treatment to alter HPA axis functioning.
Consistent with previous studies of IH (44, 53) and patients with OSA (20, 37), we observed impaired learning and memory. The effects of IH and sleep deprivation were previously determined to have distinct effects on the central nervous system, although both have separately been associated with impaired memory function, specifically in spatial learning tasks (25–27). It was also reported that 3 days of IH exposure prior to acquisition decreased consolidation, consistent with other findings of IH impairing learning and memory after sustained exposure (48, 53). Differences in learning and memory were only observed in the passive avoidance task. Passive avoidance uses a shock to cause a negative association with a dark chamber. As mentioned, an increase in adrenal gland mass was provoked by IH, suggesting HPA axis dysregulation that could partially account for the observed deficits in retention. There were no differences in the results of spatial working memory or object recognition tests. Both tests require short durations of memory recall between 1 min and 1 h, whereas the passive avoidance task examined retention 24 h later. These results suggest that IH and dLAN may not affect shorter-term memory but might influence memory consolidation (48, 53).
Sleep architecture during IH treatment is disrupted in rats during the initial days of testing, but normalized by the end of the 14-day treatment (11). Despite normal sleep patterns at 14 days, rats displayed impaired performance in the Morris water maze, suggesting a role for IH, independent of chronic sleep disruption, in disrupting learning and memory (11).
The hippocampus has been widely recognized for its importance in affect, as well as learning and memory; thus, we assessed changes in dendritic morphology to address changes in behavior. IH exposure for 1–14 days decreased apical and basilar spine density; however, 21 days of IH exposure increased spine density to levels of normoxic rats (21). Brief durations of IH are also associated with decreased dendritic length and branching; dendritic branching is similar to normoxic animals at 21 days of IH (21, 44). dLAN is also associated with decreased dendritic length and apical and basilar spine density in the CA1 region of the hippocampus (2, 9). The current results support previous findings with decreased basilar spine density in the CA1 with dLAN and indicate that apical spine density in the CA3 does not recover by 21 days of IH exposure. Differences observed among the results of previous studies likely reflect variation in the total duration of IH during the day. Decreased spine density from both IH and dLAN suggests a combined role in the impaired learning and memory and increased depressive-like behavior observed in the present study.
Reduced pyramidal neuron soma size in both the CA1 and CA3 regions of the hippocampus of humans has been associated with major depressive disorder (41). In the present study, groups with larger CA1 and CA3 soma tended to reduce depressive-like behavior. Similarly, soma size was decreased in both CA1 and CA3 with housing in dLAN combined with IH. Again, these results indicate a negative combined role of IH and dLAN. These data also suggest that exposure to night-time lighting and the occurrence of sleep apnea, along with other stimuli, can alter brain architecture in adulthood. Thus, it seems reasonable to suggest that it is important to limit exposure to these stimuli to decrease negative outcomes associated with night time lighting and OSA.
Inflammation has been widely studied in conjunction with OSA and animal models of IH, but these studies focused on peripheral changes in inflammation. Elevated proinflammatory gene expression has been widely reported in peripheral tissues in response to IH in both animal (4, 19) and clinical studies (33, 43) in connection with hypertension and cardiac morbidity. Changes in inflammation could help explain changes in affect and cognition in patients with OSA; one clinical study reported a positive relationship between neuropsychological impairment and TNF-α. Sleep deprivation did not affect neuroinflammatory cytokine gene expression (51); indeed, in a study of ischemic recovery, acute sleep deprivation reduced inflammatory cytokine expression following the ischemic insult (50). Seven and 14 days of IH exposure increased TNF-α gene expression in the hippocampus compared with normoxic control rats, but expression declined after 14 days of IH (14). In common with changes in hippocampal dendritic morphology after 14 days, it is possible that the inflammatory response declines over time. In the current study, however, there were no changes in hippocampal proinflammatory gene expression assessed at 21 days.
Conclusions
Taken together, our data indicate that dLAN and IH sometimes have different effects when assessed separately; however, when the two factors are combined, they produce a negative outcome in affect, behavior, and dendritic morphology. Although the mechanism producing this negative outcome remains unspecified, our results suggest that chronic neuroinflammation is not the primary cause.
Perspectives and Significance
OSA is a prevalent condition affecting millions of individuals and is associated with adverse health consequences. Dim light at night is a relatively new environmental factor in terms of evolutionary history. It appears that light at night has negative outcomes on affect and can influence hippocampal morphology. Virtually all people in developed countries are now exposed to night-time lighting. It is likely that individuals with OSA are also experiencing this environmental factor. Our study suggests that the combination of dim light at night and intermittent hypoxia has negative outcomes on affect and behavior. Our data also suggest that simple interventions, such as limiting nighttime exposure to light in combination with treatments, such as continuous positive airway pressure (or CPAP) may potentially benefit patients with OSA.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: T.G.A., Z.M.W., U.J.M., and R.J.N. conception and design of research; T.G.A. performed experiments; T.G.A., Z.M.W., U.J.M., and R.J.N. analyzed data; T.G.A., Z.M.W., U.J.M., and R.J.N. interpreted results of experiments; T.G.A. prepared figures; T.G.A. drafted manuscript; T.G.A., Z.M.W., U.J.M., and R.J.N. edited and revised manuscript; T.G.A., Z.M.W., U.J.M., and R.J.N. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Hall Wang, Nick Queen, Letty Cooper, Ellen Hill, and Anthony Tomaro for their help with Golgi analysis, as well as Sally Wolfe and Stacey Beck for their excellent animal care. T. G. Aubrecht was supported by a NIDCR grant T32 DE014320 and HL093463 (to U. J. Magalang).
REFERENCES
- 1.Adams N, Strauss M, Schluchter M, Redline S. Relation of measures of sleep-disordered breathing to neuropsychological functioning. Am J Respir Crit Care Med 163: 1626–1631, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Bedrosian TA, Fonken LK, Walton JC, Haim A, Nelson RJ. Dim light at night provokes depression-like behaviors and reduces CA1 dendritic spine density in female hamsters. Psychoneuroendocrinology 36: 1062–1069, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Bedrosian TA, Weil ZM, Nelson RJ. Chronic dim light at night provokes reversible depression-like phenotype: possible role for TNF. Mol Psychiatry In press. [DOI] [PubMed] [Google Scholar]
- 4.Brogi E, Wu T, Namiki A, Isner JM. Indirect angiogenic cytokines upregulate VEGF and bFGF gene expression in vascular smooth muscle cells, whereas hypoxia upregulates VEGF expression only. Circulation 90: 649–652, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Cistulli PA. Craniofacial abnormalities in obstructive sleep apnoea: implications for treatment. Respirology 1: 167–174, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Coenen AML, Van Luijtelaar E. Stress induced by three procedures of deprivation of paradoxical sleep. Physiol Behav 35: 501–504, 1985 [DOI] [PubMed] [Google Scholar]
- 7.Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res 835: 18–26, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Fonken LK, Finy MS, Walton JC, Weil ZM, Workman JL, Ross J, Nelson RJ. Influence of light at night on murine anxiety- and depressive-like responses. Behav Brain Res 205: 349–354, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Fonken LK, Kitsmiller E, Smale L, Nelson RJ. Dim nighttime light impairs cognition and provokes depressive-like responses in a diurnal rodent. J Biol Rhythms 27: 319–327, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci USA 107: 18664–18669, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci 21: 2442–2450, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haensel A, Bardwell WA, Mills PJ, Loredo JS, Ancoli-Israel S, Morgan EE, Heaton RK, Dimsdale JE. Relationship between inflammation and cognitive function in obstructive sleep apnea. Sleep Breath 13: 35–41, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hui AS, Striet JB, Gudelsky G, Soukhova GK, Gozal E, Beitner-Johnson D, Guo SZ, Sachleben LR, Haycock JW, Gozal D. Regulation of catecholamines by sustained and intermittent hypoxia in neuroendocrine cells and sympathetic neurons. Hypertension 42: 1130–1136, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Hung MW, Tipoe GL, Poon AM, Reiter RJ, Fung ML. Protective effect of melatonin against hippocampal injury of rats with intermittent hypoxia. J Pineal Res 44: 214–221, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Jun J, Savransky V, Nanayakkara A, Bevans S, Li J, Smith PL, Polotsky VY. Intermittent hypoxia has organ-specific effects on oxidative stress. Am J Physiol Regul Integr Comp Physiol 295: R1274–R1281, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kloog I, Stevens RG, Haim A, Portnov BA. Nighttime light level co-distributes with breast cancer incidence worldwide. Cancer Causes Control 21: 2059–2068, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Kumar GK, Rai V, Sharma SD, Ramakrishnan DP, Peng YJ, Souvannakitti D, Prabhakar NR. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol 575: 229–239, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev 26: 91–104, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Levy P, Pepin JL, Arnaud C, Tamisier R, Borel JC, Dematteis M, Godin-Ribuot D, Ribuot C. Intermittent hypoxia and sleep-disordered breathing: current concepts and perspectives. Eur Respir J 32: 1082–1095, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Macey PM, Woo MA, Kumar R, Cross RL, Harper RM. Relationship between obstructive sleep apnea severity and sleep, depression and anxiety symptoms in newly-diagnosed patients. PLoS One 5: e10211, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maiti P, Muthuraju S, Ilavazhagan G, Singh SB. Hypobaric hypoxia induces dendritic plasticity in cortical and hippocampal pyramidal neurons in rat brain. Behav Brain Res 189: 233–243, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Mendlewicz J. Disruption of the circadian timing systems: molecular mechanisms in mood disorders. CNS Drugs 23: 15–26, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Millman RP, Fogel BS, McNamara ME, Carlisle CC. Depression as a manifestation of obstructive sleep apnea: reversal with nasal continuous positive airway pressure. J Clin Psychiatry 50: 348–351, 1989 [PubMed] [Google Scholar]
- 24.Niranjan R, Nath C, Shukla R. Melatonin attenuated mediators of neuroinflammation and alpha-7 nicotinic acetylcholine receptor mRNA expression in lipopolysaccharide (LPS) stimulated rat astrocytoma cells, C6. Free Radic Res 46: 1167–1177, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Perry JC, D'Almeida V, Antunes IB, Tufik S. Distinct behavioral and neurochemical alterations induced by intermittent hypoxia or paradoxical sleep deprivation in rats. Prog Neuropsychopharmacol Biol Psychiatry 32: 87–94, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Perry JC, D'Almeida V, Lima M, Godoi FRL, Vital MABF, Oliveira MGM, Tufik S. Intermittent hypoxia and sleep restriction: motor, cognitive and neurochemical alterations in rats. Behav Brain Res 189: 373–380, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Perry JC, D'Almeida V, Souza FG, Schoorlemmer GHM, Colombari E, Tufik S. Consequences of subchronic and chronic exposure to intermittent hypoxia and sleep deprivation on cardiovascular risk factors in rats. Respir Physiol Neurobiol 156: 250–258, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol 47: 379–391, 1978 [DOI] [PubMed] [Google Scholar]
- 29.Ram S, Seirawan H, Kumar SK, Clark GT. Prevalence and impact of sleep disorders and sleep habits in the United States. Sleep Breath 14: 63–70, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Ray AD, Magalang UJ, Michlin CP, Ogasa T, Krasney JA, Gosselin LE, Farkas GA. Intermittent hypoxia reduces upper airway stability in lean but not obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 293: R372–R378, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Rodway GW, Hoffman LA, Sanders MH. High-altitude-related disorders—Part I: Pathophysiology, differential diagnosis, and treatment. Heart Lung 32: 353–359, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Row BW. Intermittent hypoxia and cognitive function: implications from chronic animal models. Adv Exp Med Biol 618: 51–67, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 112: 2660–2667, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Rybnikova E, Mironova V, Pivina S, Tulkova E, Ordyan N, Vataeva L, Vershinina E, Abritalin E, Kolchev A, Nalivaeva N, Turner AJ, Samoilov M. Antidepressant-like effects of mild hypoxia preconditioning in the learned helplessness model in rats. Neurosci Lett 417: 234–239, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Rybnikova EA, Mironova VI, Pivina SG, Ordyan NE, Tyulkova EI, Samoilov MO. Hypoxic preconditioning prevents development of post-stress depressions in rats. Dokl Biol Sci 411: 431–433, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Shukitt-Hale B, Banderet LE, Lieberman HR. Relationships between symptoms, moods, performance, and acute mountain sickness at 4,700 meters. Aviat Space Environ Med 62: 865–869, 1991 [PubMed] [Google Scholar]
- 37.Shukitt-Hale B, Kadar T, Marlowe BE, Stillman MJ, Galli RL, Levy A, Devine JA, Lieberman HR. Morphological alterations in the hippocampus following hypobaric hypoxia. Hum Exp Toxicol 15: 312–319, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Silva RH, Kameda SR, Carvalho RC, Takatsu-Coleman AL, Niigaki ST, Abílio VC, Tufik S, Frussa-Filho R. Anxiogenic effect of sleep deprivation in the elevated plus-maze test in mice. Psychopharmacology 176: 115–122, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Singh SB, Selvamurthy W. Effect of intermittent chronic exposure to hypoxia on feeding behaviour of rats. Int J Biometeorol 37: 200–202, 1993 [DOI] [PubMed] [Google Scholar]
- 40.Stevens RG, Rea MS. Light in the built environment: potential role of circadian disruption in endocrine disruption and breast cancer. Cancer Causes Control 12: 279–287, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Uylings HB, Friedman L, Rajkowska G. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry 56: 640–650, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suchecki D, Tiba PA, Tufik S. Hormonal and behavioural responses of paradoxical sleep-deprived rats to the elevated plus maze. J Neuroendocrinol 14: 549–554, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Tamaki S, Yamauchi M, Fukuoka A, Makinodan K, Koyama N, Tomoda K, Yoshikawa M, Kimura H. Production of inflammatory mediators by monocytes in patients with obstructive sleep apnea syndrome. Intern Med 48: 1255–1262, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Titus AD, Shankaranarayana Rao BS, Harsha HN, Ramkumar K, Srikumar BN, Singh SB, Chattarji S, Raju TR. Hypobaric hypoxia-induced dendritic atrophy of hippocampal neurons is associated with cognitive impairment in adult rats. Neuroscience 145: 265–278, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med 11: 441–446, 2010 [DOI] [PubMed] [Google Scholar]
- 46.van Amelsvoort LG, Schouten EG, Kok FJ. Duration of shiftwork related to body mass index and waist to hip ratio. Int J Obes Relat Metab Disord 23: 973–978, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Wang TY, Chen XQ, Du JZ, Xu NY, Wei CB, Vale WW. Corticotropin-releasing factor receptor type 1 and 2 mRNA expression in the rat anterior pituitary is modulated by intermittent hypoxia, cold and restraint. Neuroscience 128: 111–119, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Ward CP, McCoy JG, McKenna JT, Connolly NP, McCarley RW, Strecker RE. Spatial learning and memory deficits following exposure to 24 h of sleep fragmentation or intermittent hypoxia in a rat model of obstructive sleep apnea. Brain Res 1294: 128–137, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warrington JP, Csiszar A, Mitschelen M, Lee YW, Sonntag WE. Whole brain radiation-induced impairments in learning and memory are time-sensitive and reversible by systemic hypoxia. PLos One 7: e30444, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weil ZM, Norman GJ, Karelina K, Morris JS, Barker JM, Su AJ, Walton JC, Bohinc S, Nelson RJ, DeVries AC. Sleep deprivation attenuates inflammatory responses and ischemic cell death. Exp Neurol 218: 129–136, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wisor JP, Schmidt MA, Clegern WC. Evidence for neuroinflammatory and microglial changes in the cerebral response to sleep loss. Sleep 34: 261–272, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea a population health perspective. Am J Respir Crit Care Med 165: 1217–1239, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Zhang JX, Lu XJ, Wang XC, Li W, Du JZ. Intermittent hypoxia impairs performance of adult mice in the two-way shuttle box but not in the Morris water maze. J Neurosci Res 84: 228–235, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Zhu XH, Yan HC, Zhang J, Qu HD, Qiu XS, Chen L, Li SJ, Cao X, Bean JC, Chen LH, Qin XH, Liu JH, Bai XC, Mei L, Gao TM. Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant-like effects in adult rats. J Neurosci 30: 12653–12663, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]