Abstract
Although interactions between the amygdala and prefrontal cortex (PFC) are critical for emotional guidance of behavior, the manner in which amygdala affects PFC function is not clear. Whereas basolateral amygdala (BLA) output neurons exhibit many characteristics associated with excitatory neurotransmission, BLA stimulation typically inhibits PFC cell firing. This apparent discrepancy could be explained if local PFC inhibitory interneurons were activated by BLA inputs. Here, we used in vivo juxtacellular and intracellular recordings in anesthetized rats to investigate whether BLA inputs evoke feedforward inhibition in the PFC. Juxtacellular recordings revealed that BLA stimulation evoked action potentials in PFC interneurons and silenced most pyramidal neurons. Intracellular recordings from PFC pyramidal neurons showed depolarizing postsynaptic potentials, with multiple components evoked by BLA stimulation. These responses exhibited a relatively negative reversal potential (Erev), suggesting the contribution of a chloride component. Intracellular administration or pressure ejection of the GABA-A antagonist picrotoxin resulted in action-potential firing during the BLA-evoked response, which had a more depolarized Erev. These results suggest that BLA stimulation engages a powerful inhibitory mechanism within the PFC mediated by local circuit interneurons.
Keywords: fast-spiking interneuron, parvalbumin, electrophysiology, in vivo intracellular recording, GABA
the basolateral amygdala (BLA) is critical for processing emotional and motivational information. Although the use of this information in decision making and goal-directed behaviors may involve BLA projections to the medial prefrontal cortex (mPFC) (Bechara et al. 1999; Ghods-Sharifi et al. 2009), the manner in which the BLA regulates PFC physiology is not clear. Indeed, data from anatomical and electrophysiological studies have been inconsistent. Elucidating synaptic mechanisms involved in the BLA control of PFC pyramidal cell firing would aid in understanding how emotion and motivation guide decision making.
BLA projections to the PFC consist primarily of excitatory fibers. BLA projection neurons are pyramidal-like cells with large apical dendrites and smaller basal dendrites (McDonald 1982). These neurons are likely excitatory, as they express glutamatergic cell markers, such as the vesicular glutamate transporter 1 and calcium-calmodulin kinase II (McDonald et al. 2002; Sosulina et al. 2006). Furthermore, BLA-originated axons form asymmetric synapses within the PFC (Gabbott et al. 2006; McDonald 1996). The vast majority of these synapses is on pyramidal cells, whereas inhibitory interneurons receive a relatively light innervation (Cunningham et al. 2008; Gabbott et al. 2006). Many of these synaptic contacts are found on dendritic spines (Bacon et al. 1996; Gabbott et al. 2006), which are typically observed in pyramidal neurons. Therefore, BLA activation might be expected to cause a direct excitation of PFC pyramidal neurons.
Despite anatomical data indicating an excitatory BLA–PFC projection, electrophysiological evidence is inconsistent with an excitatory pathway. BLA stimulation evokes a transient inhibition in 70–90% of PFC neurons, whereas only 5–8% of the recorded cells increased firing, and the rest did not respond (Floresco and Tse 2007; Ishikawa and Nakamura 2003; Pérez-Jaranay and Vives 1991). A possible explanation for this discrepancy is that BLA activation recruits feedforward mechanisms within the PFC to a greater extent than direct activation of pyramidal neurons. Indeed, a slice preparation, where BLA afferents coursing into the prelimbic cortex are electrically stimulated at the level of the infralimbic cortex, has revealed that stimulation of putative BLA afferents evokes disynaptic, inhibitory postsynaptic currents that may be driven by putative GABAergic interneurons (Ji et al. 2010; Orozco-Cabal et al. 2006; Sun and Neugebauer 2011). However, it is possible that electrical stimulation in slices activates non-BLA fibers that provide feedforward inhibition or directly innervate the recorded neurons. Although these data suggest the possibility of BLA-driven, feedforward inhibition via GABA interneuron recruitment, a confirmation of this arrangement, via direct demonstration that BLA stimulation in vivo activates GABAergic interneurons in the PFC, is necessary. Here, we tested this possibility with in vivo intracellular and juxtacellular recordings coupled to local drug infusion from PFC neurons, assessing their response to BLA stimulation in anesthetized rats.
MATERIALS AND METHODS
Animals and surgery.
Adult (300–450 g) male Sprague-Dawley and Long-Evans rats were obtained from Charles River Laboratories (Wilmington, MA) and housed with a 12-h light/dark cycle and food and water available ad libitum. All experiments were conducted in accordance with guidelines published in the U.S. Public Health Service Guide for the Use and Care of Animals, and all procedures were approved by the University of Maryland, Baltimore, Institutional Animal Care and Use Committee. Rats were anesthetized with chloral hydrate (400 mg/kg ip), followed by continuous, supplemental anesthesia (chloral hydrate, 24–30 mg·kg−1·h−1 ip) through an infusion pump (Bioanalytical Systems, West Lafayette, IN) during the recording session. Rats were placed on a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA), and core body temperature was maintained at 37–38°C with a heating pad and thermal probe (Fine Science Tools, Foster City, CA). A longitudinal incision was made over the skull, and the scalp was retracted. Burr holes were drilled in the skull over the mPFC and BLA. Stereotaxic coordinates for these sites were taken from a rat brain atlas (Paxinos and Watson 1998). PFC recording electrodes were advanced 3.2–2.7 mm rostral to bregma (AP), 0.8–1.0 mm lateral to midline (L), and 2.0–5.5 mm ventral to cortical surface (V). A concentric bipolar-stimulating electrode (0.5 mm diameter, 0.5 mm pole separation; NE-100X; Rhodes Medical Instruments, Summerland, CA) was lowered into the BLA (AP: −2.8–3.0 mm; L: 4.8–4.9 mm; V: 7.4–7.6 mm). In some experiments, an additional burr hole was drilled, and a stimulating electrode was placed in the ventral tegmental area (VTA; AP: −6.0 mm; L: 0.5 mm; V: 7.4 mm). Stimulating electrodes were connected to an ISO-Flex stimulus isolation unit (A.M.P.I., Jerusalem, Israel), driven by a Master-8 stimulator (A.M.P.I.).
Juxtacellular recordings.
Juxtacellular recording electrodes were pulled from 1.5 mm-diameter borosilicate glass with filament (World Precision Instruments, Sarasota, FL), using a vertical microelectrode puller (Stoelting, Wood Dale, IL). Electrode tips were broken to a tip diameter of ∼1 μm. Recording electrodes were filled with 0.5 M NaCl, containing 2% Neurobiotin (Vector Laboratories, Burlingame, CA), and lowered into the prelimbic and infralimbic divisions of the PFC with a hydraulic manipulator (Trent Wells, Coulterville, CA). Electrical signals were transmitted from a headstage to a NeuroData IR-283 intracellular amplifier (Cygnus Technologies, Delaware Water Gap, PA) and filtered through a HumBug 50/60-Hz noise eliminator (Quest Scientific, North Vancouver, Canada). Signals were monitored constantly on a digital oscilloscope (Fluke, Everett, WA), a multimeter (Tektronix, Beaverton, OR), and an audio monitor (Grass Technologies, Natus Neurology, Warwick, RI), digitized at 10 kHz with a Digidata 1322A (Axon Instruments, Union City, CA) and band-pass filtered (300 Hz–2 kHz) with AxoScope 9.0 software (Axon Instruments). Signals were recorded and saved on a personal computer (PC) for offline analyses. A 200-ms, 0.5-nA rectangular current pulse was delivered to the recording electrode to estimate its resistance (15–25 MΩ). The electrode was then advanced until a neuron's action potential was well isolated. Neurons with action-potential amplitudes of less than three times the noise were discarded. After recording spontaneous activity for 5 min, the BLA was stimulated with 1-mA, 0.5-ms pulses at 0.1 Hz. After stimulation, cells were filled using constant current (0.25–1 nA) and a current pulse (250 ms on, 250 ms off; 1.5–6 nA), injected through the bridge circuit of the recording amplifier (Pinault 1994). This typically resulted in the juxtacellular cell configuration within 1 min, at which point, action-potential firing was increased during the current pulse. Constant current was then decreased to 0–0.25 nA and current pulses reduced to 0.6–2 nA. Current pulses were continued for several minutes and typically resulted in the labeling of a single neuron. When more than one cell was labeled, recordings had more than one unit present. These cells were excluded from further analysis.
At the completion of the recording session, the rat was given an overdose of chloral hydrate and transcardially perfused with cold saline, followed immediately by 4% paraformaldehyde in PBS (pH 7.4). Brains were postfixed for 24 h, rinsed in PBS, and placed in 30% sucrose with PBS for cryoprotection. Brains were then cut using a coronal matrix (ASI Instruments, Warren, MI) into separate blocks containing stimulating and recording electrode tracks. Thin sections (30–50 μm) were cut from tissue blocks on a freezing microtome. BLA sections were then mounted on gelatin-coated slides, Nissl stained, coverslipped, and examined under a microscope for verification of stimulating electrode placement. Neurobiotin-labeled neurons with a clearly visible apical dendrite were identified as pyramidal neurons. PFC sections were immunohistochemically labeled for parvalbumin (PV; 1:10,000 dilution, mouse anti-PV; Swant, Fribourg, Switzerland). PV and Neurobiotin were fluorescently tagged with cyanine 2 (Cy2; 1:600, anti-mouse secondary) and Cy3 (1:600, streptavidin conjugated to Cy3), respectively (Jackson ImmunoResearch Laboratories, West Grove, PA). PFC sections were then mounted in antiphotobleaching media (a gift from Dr. Adam Puche, Department of Anatomy and Neurobiology, University of Maryland School of Medicine, Baltimore, MD), coverslipped, and examined using confocal imaging.
Peristimulus time histograms were constructed for each neuron using consecutively acquired sweeps, centered around BLA stimulation. An inhibitory response was defined as complete suppression of action-potential firing within 200 ms of the stimulation and lasting at least 50 ms, as described by Floresco and Tse (2007). The “onset” of this period of suppression was quantified as the first 1-ms bin in which no action potentials were found. Excitatory responses were defined as two consecutive bins with action-potential firing >2 SD above the prestimulation mean. Data are presented as mean ± SD, unless noted otherwise.
To assess the role of GABA-A receptors on BLA-evoked inhibitory responses in mPFC pyramidal neurons, we used custom-built, double-barrel glass pipettes for local drug application. Pulled drug pipettes (1 mm outside diameter; 0.25 mm inside diameter), with 40–50 μm tip diameters, were fixed to juxtacellular recording electrodes (1–2 μm tip diameters). The distance between the recording electrode tip and drug pipette tip was 120–160 μm. The drug pipette was filled with artificial cerebral spinal fluid (aCSF) containing the GABA-A receptor antagonist picrotoxin (PTX; 1 mM) and <0.01% Chicago Sky Blue. Recording microelectrodes contained 2% Neurobiotin in 0.5 M NaCl and were of similar resistance as described for juxtacellular recordings. After isolating a mPFC neuron, spontaneous activity was monitored for 5 min to determine baseline activity. The BLA was then electrically stimulated (0.2 Hz, 0.5–1.0 mA, 0.5 ms duration) to evoke responses in mPFC neurons and establish a baseline response. To determine the effects of PTX on BLA-evoked excitation and inhibition, PTX was pressure ejected using a Toohey Spritzer Pressure System IIe (Toohey, Fairfield, NJ; 40 lb./in.2; 5–10 ms duration), 500 ms before BLA electrical stimulation every sweep, until ∼30–60 nl of PTX-containing aCSF was ejected.
Intracellular recordings.
Intracellular recording electrodes were pulled from 1 mm-diameter borosilicate glass tubes with filament (World Precision Instruments) on a Flaming/Brown horizontal puller (model P-97; Sutter Instrument, Novato, CA) to a resistance of 59–170 MΩ and filled with 2 M potassium acetate containing 2% Neurobiotin (Vector Laboratories). Recording electrodes were lowered into the prelimbic and infralimbic divisions of the mPFC with a hydraulic manipulator (Trent Wells). All recordings were made in current clamp. Electrical signals were transmitted from a headstage to an intracellular amplifier (NeuroData IR-283; Cygnus Technologies). Signals were monitored constantly on a digital oscilloscope (Fluke), a multimeter (Tektronix), and an audio monitor (Grass Technologies, Natus Neurology). Intracellular signals were acquired and digitized at 10 KHz, with a Digidata 1322A (Axon Instruments) and AxoScope 9.0 software (Axon Instruments), and recorded and saved on a PC for offline analyses.
Microelectrodes were advanced in the mPFC until a neuron was impaled. Electrical activity was recorded for 5 min before BLA stimulation. Neurons included in this study had a resting membrane potential more negative than −64 mV and action potentials that were either overshooting 0 mV (45 of 51) or with amplitudes ≥40 mV from threshold (48 of 51). Membrane-potential deflections to injected current pulses were used to determine the input resistance and time constant (estimated as the time to reach 63% of maximum voltage deflection in response to a −0.1 or −0.2 nA current pulse). After passive membrane property assessment, the BLA was stimulated at least 15 times, once every 10 s with single pulses (1.0 mA). Some neurons were stimulated with a range of current intensities (0.2–1.5 mA). The holding current was adjusted in several neurons to estimate reversal potential (Erev) of synaptic responses. In some cases (n = 3), the GABA-A antagonist PTX (200 μM; Sigma, St. Louis, MO) was included in the recording pipette to attenuate GABAergic responses.
A subset of experiments assessed the role of the mediodorsal (MD) thalamus in PFC responses evoked by BLA stimulation. A separate group of rats had a cannula implanted in the MD filled with 2% lidocaine (Abbott Laboratories, Abbott Park, IL) in saline, with Chicago Sky Blue (Sigma) added to verify the infusion site. After stable cell penetration and baseline recordings, the BLA was stimulated with a single pulse every 30 s for up to 30 min. After 15 repetitions, an infusion pump delivered 100 nl of 2% lidocaine over 10 s to the MD thalamus, while BLA stimulation continued.
Following BLA stimulation, Neurobiotin was injected into the cell by positive current pulses (100 ms, 0.5–1.2 nA, 2 Hz) for 10–20 min. The rat was given an overdose of sodium pentobarbital or chloral hydrate and transcardially perfused with cold saline, followed immediately by 4% paraformaldehyde in PBS (pH 7.4). Brains were postfixed for 24 h in the same fixative, rinsed in PBS, placed in 30% sucrose with PBS, and processed for Neurobiotin staining, as described in the juxtacellular recordings section below.
RESULTS
Juxtacellular recordings in the rat mPFC.
Juxtacellular recordings were gathered from 23 pyramidal neurons and seven putative interneurons (four PV-positive and three PV-negative) from 15 Sprague-Dawley and 10 Long-Evans rats. All pyramidal neurons included in this study were histologically identified on the basis of whether a clearly visible apical dendrite could be followed to superficial layers (Fig. 1A). The average firing rate of pyramidal neurons was 1.3 ± 1.6 Hz, whereas interneurons had a firing rate of 4.4 ± 4.9 Hz. PV-positive interneurons (Fig. 2A) had a firing rate of 6.1 ± 5.3 Hz, whereas PV-negative cells had a firing rate of 1.0 ± 0.6 Hz.
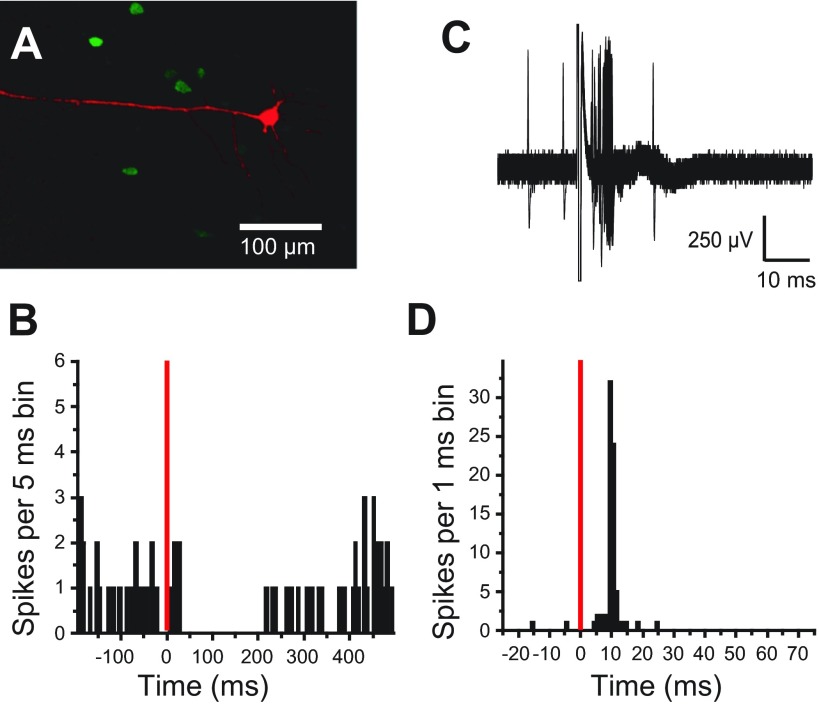
Fig. 1.
Pyramidal neuron responses to basolateral amygdala (BLA) stimulation. A: juxtacellularly labeled pyramidal neuron [Neurobiotin in red; parvalbumin (PV) in green]. The apical dendrite is oriented toward the apical surface. B: peristimulus time histogram (PSTH) constructed from consecutive sweeps, illustrating a typical pyramidal neuron response, a pause in spike firing (bin width = 5 ms). C: overlay of 100 traces showing excitatory response of a pyramidal neuron to BLA stimulation. D: PSTH plotting action-potential occurrences from the 100 consecutive sweeps shown in C with 1-ms bins. B and D: the stimulation artifact is represented by red, vertical lines.
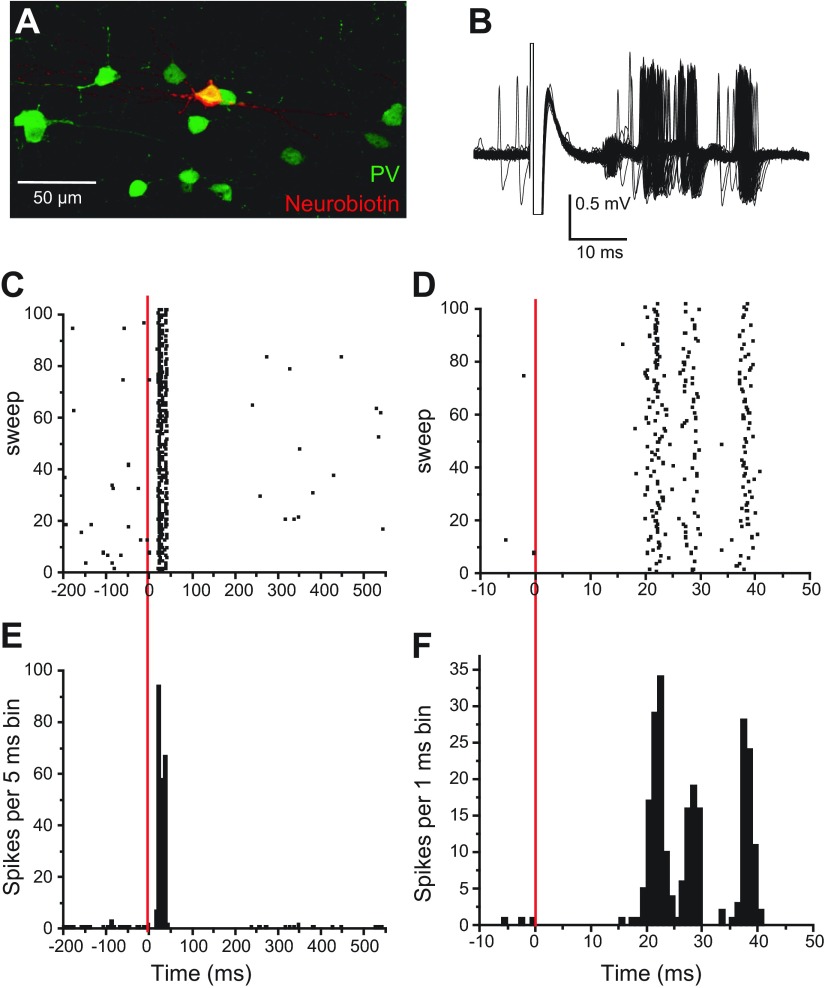
Fig. 2.
Interneuron responses to BLA stimulation. A: Neurobiotin-filled interneuron colabeled with PV. Neurobiotin is shown in red and PV in green so that colocalization results in a yellow-filled cell body. B: overlay of traces showing the excitatory response of a PV-positive interneuron to BLA stimulation. C–F: raster plots (C, D) and PSTH (E, F) of the excitatory response to BLA stimulation at 2 different time scales. This neuron fired at least 1 action potential during every sweep. The red, vertical lines represent the time of stimulation.
BLA stimulation inhibits most mPFC pyramidal neurons.
BLA stimulation suppressed spontaneous firing in most pyramidal neurons (n = 18 of 23; 78%). This pause had an onset of 17.6 ± 7.4 ms and lasted 276 ± 163 ms (Fig. 1B). The remaining pyramidal neurons (n = 5; 22%) displayed short-latency excitation (15.5 ± 5.9 ms; Fig. 1, C and D), typically consisting of a single action potential, followed by a pause in spike firing. Firing reliability (percent of stimulations that resulted in an action potential) was 63.4 ± 29%. As a control for specificity of the BLA stimulation site, four neurons were recorded from two rats, in which the stimulating electrode was placed in the caudal striatum overlying the BLA. PFC pyramidal neurons were unresponsive to caudal striatal stimulation, with intensities as high as 1.5 mA. The data indicate that although BLA stimulation can evoke firing in some PFC pyramidal neurons, the majority becomes inhibited, as reported previously.
BLA stimulation excites interneurons.
BLA stimulation evoked a short-latency excitation in all interneurons recorded. The response consisted of several action potentials per stimulation, followed by a pause in firing (n = 7; Fig. 2, B–F). Action potentials were elicited in almost every BLA stimulation trial (firing reliability: 94 ± 4.2%) in PV-positive interneurons. The latency to the first action potential was 20.7 ± 3.2 ms in all interneurons. There was not a significant difference between the latency of the first action potential in interneurons and inhibition onset in pyramidal neurons (t24 = 0.1647; P = 0.87). Thus BLA stimulation can excite local inhibitory interneurons in the mPFC.
In vivo intracellular recordings from PFC pyramidal neurons.
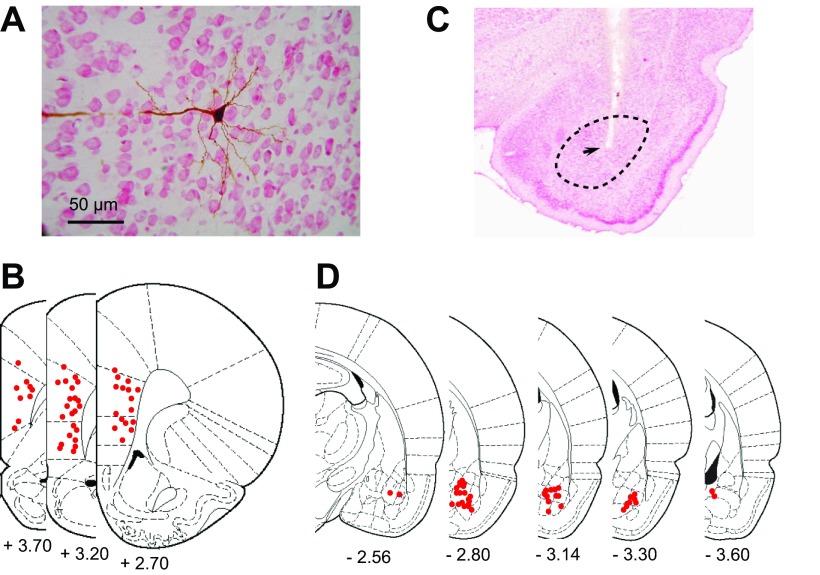
Juxtacellular data indicate that PV interneurons respond with an early excitation, and most pyramidal neurons respond with a longer latency inhibition. These observations lead to the prediction that pyramidal neurons exhibit a combination of glutamate and GABA components in response to BLA stimulation. To test for this possibility, we recorded synaptic responses to BLA stimulation with intracellular recordings from 49 pyramidal neurons located in deep layers of the mPFC (deep layer III–layer V; Fig. 3A). Most were found in the prelimbic cortex (n = 28), whereas others were located in the infralimbic cortex (n = 18) or at the ventral border of the anterior cingulate cortex (n = 3; Fig. 3, A and B). Stimulating electrodes were placed in the anterior subdivision of the BLA (BLAa; n = 18), posterior BLA (BLAp; n = 18), or border between these divisions (n = 10; Fig. 3, C and D). Several stimulation sites were found at the border of the BLAa and the central nucleus of the amygdala (CeA; n = 3). These data were included, since there is no evidence that the CeA projects to the PFC, and BLA neurons were almost certainly excited by adjacent stimulation. Several other stimulation sites, clearly within the CeA (n = 2) or the ventral endopiriform cortex (n = 2), were excluded from the analysis.
Fig. 3.
Example of a prefrontal cortex (PFC) neuron that was recorded from and filled with Neurobiotin. A: neutral, red-stained coronal section with a Neurobiotin-filled pyramidal neuron. B: overlay of 3 sections from The Rat Brain in Stereotaxic Coordinates, illustrating sites of recorded neurons (red dots). C: Nissl-stained section, illustrating a representative stimulating-electrode placement in the BLA (arrow). The black, dashed circle represents the boundaries of the BLA. D: overlay of sections of the The Rat Brain in Stereotaxic Coordinates, illustrating placements of BLA-stimulating electrodes (red dots). [From Paxinos and Watson (1998).]
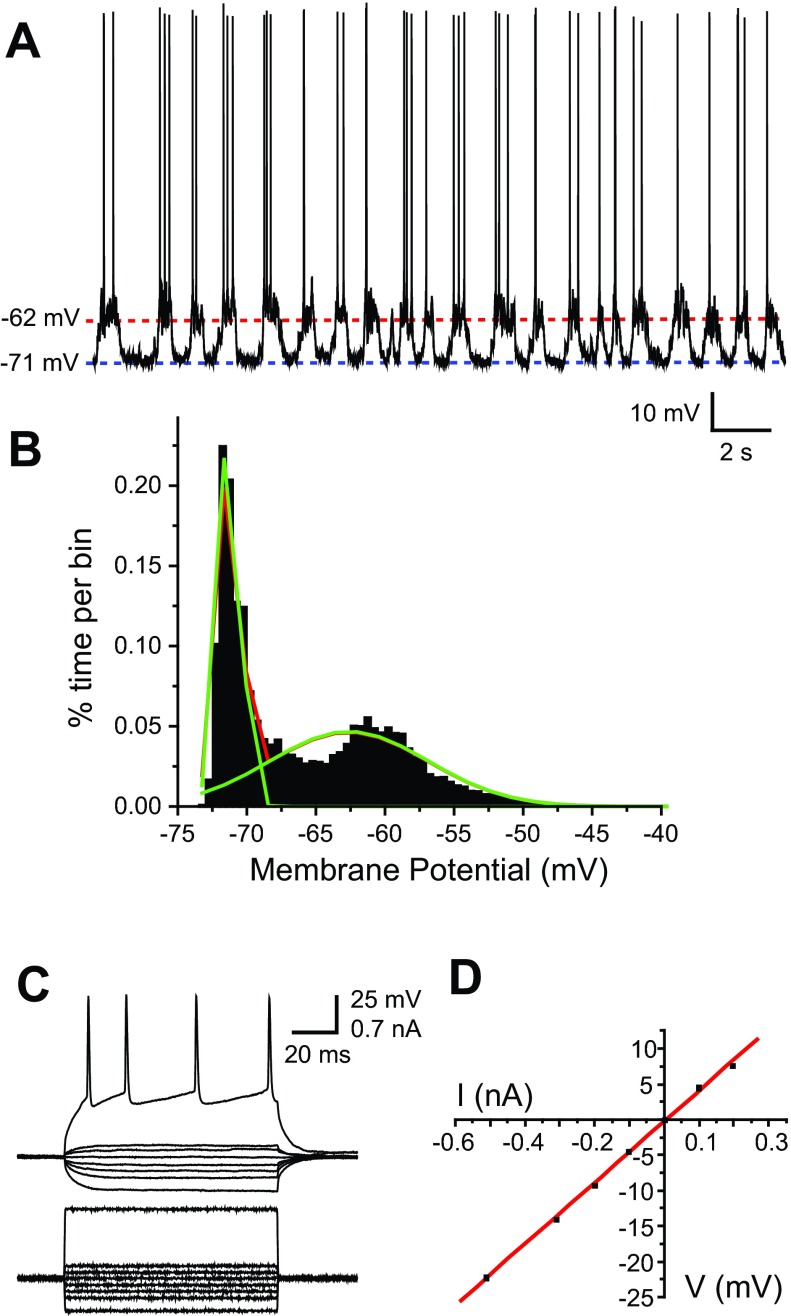
Basic electrophysiological measurements of PFC pyramidal neurons were within the range of what was reported previously reported (Lewis and O'Donnell 2000). Approximately one-half of the neurons (30 of 54) exhibited a bimodal membrane potential, consisting of a hyperpolarized down state and a depolarized up state (Fig. 4, A and B). Action potentials occurred only during the up state. Neurons were considered bimodal if the membrane potential could be fit to a dual Gaussian distribution (Fig. 4B), and neurons that did not meet bimodal criteria had a resting membrane potential similar to the down state of bimodal neurons (−77.1 ± 4.1 mV, n = 24). Current/voltage curves were built with depolarizing and hyperpolarizing current pulses to assess input resistance (Fig. 4, C and D). Neurons of the prelimbic and infralimbic regions in Sprague-Dawley rats were similar in all of these measures except for the down-state membrane potential, which was more negative in prelimbic neurons (Table 1; P = 0.01, Student's t-test). This difference in down-state membrane potential was not present in Long-Evans rats.
Fig. 4.
Passive membrane properties and spontaneous activity of PFC pyramidal neurons. A: trace showing spontaneous activity of a representative pyramidal neuron that displays up- and down-state transitions. B: histogram of membrane-potential values recorded in the trace shown in A, showing a bimodal distribution that can be fit to a dual Gaussian function (green lines). C: membrane-potential traces of an example neuron responding to injection of positive and negative, 100-ms current pulses. The spiking pattern seen in the most depolarized trace is typical of a regular spiking pyramidal neuron of the cerebral cortex, including spike-frequency adaptation. D: current (I)–voltage (V) plot of the traces in C. A linear function was fit to the plot, and its slope was used to estimate input resistance.
Table 1.
Basic electrophysiological measures of mPFC pyramidal neurons during in vivo intracellular recordings
| Sprague-Dawley Rats |
Action Potential |
|||||||
|---|---|---|---|---|---|---|---|---|
| Down, mV | Up, mV | Firing rate, Hz | Rm, MΩ | [tau], ms | Threshold, mV | Amplitude, mV | Width, ms | |
| All | −75.6 ± 6.3 | −66.4 ± 7.6 | 1.6 ± 2.5 | 45.8 ± 14.0 | 8.5 ± 2.8 | −48.7 ± 6.2 | 60.6 ± 6.2 | 2.0 ± 0.36 |
| PL | −78.4 ± 4.1 | −68.7 ± 7.3 | 1.5 ± 2.2 | 44.1 ± 15.0 | 8.4 ± 3.2 | −49.1 ± 3.6 | 61.2 ± 7.0 | 2.2 ± 0.4 |
| IL | −68.8 ± 0.2 | −61.4 ± 0.7 | 0.3 ± 0.3 | 50.4 ± 12.1 | 8.3 ± 2 | −47.7 ± 9.9 | 60.4 ± 5.2 | 2.1 ± 0.2 |
| Long-Evans Rats | Action Potential | |||||||
|---|---|---|---|---|---|---|---|---|
| Down, mV | Up, mV | Firing rate, Hz | Rm, MΩ | [tau], ms | Threshold, mV | Amplitude, mV | Width, ms | |
| All | −78.7 ± 7.3 | −70.7 ± 6.6 | 0.5 ± 1.0 | 48.9 ± 25.5 | 7.3 ± 2.9 | −54.4 ± 7.2 | 51.5 ± 7.2 | 2.0 ± 0.4 |
| PL | −78.2 ± 6.7 | −70.6 ± 5.5 | 0.5 ± 0.9 | 50.8 ± 25.6 | 7.5 ± 2.8 | −54.1 ± 5.4 | 52.4 ± 7.6 | 2.0 ± 0.3 |
| IL | −81.0 ± 10.3 | −71.0 ± 11.1 | 0.6 ± 1.2 | 43.7 ± 25.1 | 6.9 ± 3.5 | −56.0 ± 6.7 | 48.4 ± 4.8 | 1.9 ± 0.6 |
Data from all neurons are presented separately for prelimbic (PL; n = 15 in Sprague-Dawley rats; n = 23 in Long-Evans rats) and infralimbic (IL; n = 8 in Sprague-Dawley rats; n = 7 in Long-Evans rats) cortices in Sprague-Dawley (total n = 23) and Long-Evans (total n = 30) rats. mPFC, medial prefrontal cortex; Rm, input resistance; τ, time constant. The down-state membrane potential in PL neurons was more hyperpolarized than IL neurons in Sprague-Dawley rats. No other differences were found. Values are represented as mean ± SD.
BLA stimulation evokes complex synaptic responses in PFC pyramidal neurons.
Electrical BLA stimulation (1.0 mA) evoked a complex depolarizing postsynaptic potential (dPSP) in PFC pyramidal neurons that included two to four clearly defined components and no action-potential firing (Fig. 5A). BLA-evoked responses in PFC pyramidal neurons were almost identical between Sprague-Dawley (n = 24) and Long-Evans (n = 25) rats in all measures of the BLA-evoked response; therefore, results on BLA-evoked responses from the two strains were pooled. The average onset latency was 11.2 ± 2.8 ms, peak amplitude was 7.9 ± 4.1 mV, and time to peak was 29.8 ± 10.4 ms. The dPSP duration (time-to-half-maximum amplitude) was 40.7 ± 16.4 ms. Most neurons (35 of 46) also exhibited a long-lasting return to the down state following the dPSP, during which action potentials were absent (Fig. 5B). The average duration of the long-lasting hyperpolarization (LLH) was 289.4 ± 136.1 ms (time from stimulation to one-half of the amplitude of the first up state immediately following the decay of the dPSP) and was highly variable within and between cells. BLA stimulation did not appear to otherwise affect up and down transitions. BLA response measures were not different between cells recorded from the prelimbic and infralimbic cortex or between BLAa and BLAp stimulation sites.
Fig. 5.
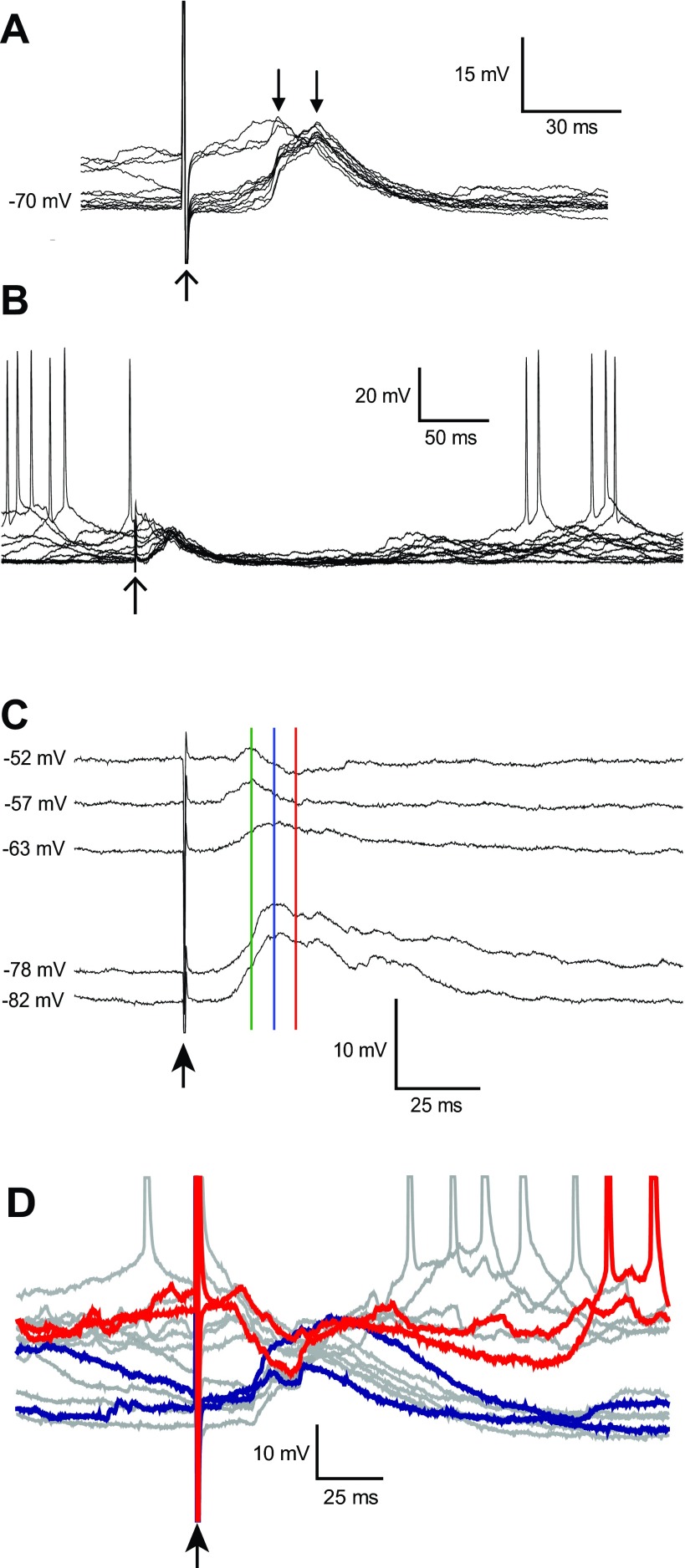
Synaptic responses evoked in PFC pyramidal neurons by BLA stimulation. A: overlay of traces showing depolarizing synaptic potentials in response to BLA stimulation. The time of stimulation and stimulus artifact are indicated with the upward-pointing arrow. When stimulation was delivered during the up state, the depolarizing postsynaptic potential (dPSP) was small compared with dPSPs evoked during the down state (downward-pointing arrows). B: overlay of several traces at a slower time scale, revealing the long-lasting hyperpolarization that follows the dPSP. C: several traces from a neuron, in which depolarization revealed a negative-reversing component in the BLA-evoked dPSP. The arrow indicates the time of stimulation, and membrane-potential values, at which responses were recorded, are indicated to the left of each trace. Depolarized traces revealed a segregation of components with different reversal potentials. The peak of the response observed at resting membrane potential (blue line) was depolarizing (down state: −78 mV). At depolarized membrane potentials, an early depolarizing component (green line) became segregated from a later hyperpolarizing component (red line). D: overlay of BLA-evoked responses in a pyramidal neuron with depolarized up states. BLA stimulation evoked a depolarizing response at negative membrane potentials (2 traces are highlighted in blue) and a hyperpolarizing response from the up state (2 representative responses are highlighted in red).
The amplitude of the response was affected by the membrane potential at the time of BLA stimulation. Responses recorded during the up state were significantly smaller in amplitude than those recorded during the down state (Fig. 5A; up = 5.3 ± 2.4 mV; down = 10.4 ± 4.4 mV; Student's t-test, P < 0.0002; n = 35). This difference could be due to the up state being close to the Erev of the response. In several neurons (n = 11), we assessed the Erev by repeating stimulation, while depolarizing the neuron with current injection. Some neurons (n = 6) fired spikes during the dPSP, and their data were excluded from the Erev analysis. The remaining neurons (n = 6) had an average Erev of −55.3 ± 6.8 mV. In several neurons (n = 4), a component of the response was hyperpolarizing from depolarized membrane potentials (Fig. 5C). In two of those neurons, the response consisted of an early depolarizing component (time to peak: 20 ± 0.4 ms; Erev = −51 ± 5.6 mV) and a later hyperpolarizing component (time to negative peak: 34 ± 1.4 ms; Erev = −60.9 ± 1.6 mV). In neurons in which action potentials occurred during the BLA-evoked response when depolarizing current was injected (n = 6), these action potentials had an average latency (time-to-spike threshold) of 21.6 ± 5.8 ms, which is earlier than the time to the original peak in these neurons (27.8 ± 6.2 ms; measured without current injected). In a subset of cells (n = 3), BLA-evoked responses during the up state were hyperpolarizing, whereas in the down state, the responses were depolarizing, and action potentials were not observed during the response (Fig. 5D). The Erev of this response was −70.0 ± 0.7 mV. These results suggest that the PFC pyramidal neuron response to BLA stimulation contains a combination of synaptic events that includes an early excitatory component, followed by one or more inhibitory events, with Erev near the chloride equilibrium potential.
The GABA-A antagonist PTX unmasks a short-latency inhibitory component of the BLA response.
To determine whether GABA contributes to the negative-reversing component of BLA responses, the GABA-A receptor antagonist PTX (200 μM) was added to the internal recording solution. PTX is a noncompetitive GABA-A receptor antagonist that can block the flow of the chlorine ion through the receptor channel from inside of the cell (Akaike et al. 1985; Cupello et al. 1991; Inomata et al. 1988; Metherate and Ashe 1993). Therefore, PTX was expected to attenuate presumed GABAergic components of the BLA response and unmask any excitatory component shunted by feedforward inhibition. The presence of PTX in the recording electrode did not affect onset latency, amplitude, or time to peak of BLA-evoked responses. However, the decay to one-half of the amplitude was longer in cells recorded with PTX (62 ± 15 ms; n = 3) than in cells without PTX (41 ± 16 ms; Student's independent t-test, P < 0.05). Two of three PTX-treated pyramidal neurons remained spontaneously active throughout the recording. In these cells, BLA stimulation evoked action-potential firing during the dPSP (Fig. 6A), suggesting that GABA-A blockade unmasked excitatory components in the response. Following the initial postsynaptic response, there was a brief period of hyperpolarization and pause in action-potential firing (270 ± 125.9 ms), similar to the LLH recorded without PTX in the electrode. The third neuron treated with PTX did not remain spontaneously active and did not fire action potentials during the response. However, the Erev of the dPSP was −39.4 mV, more depolarized than that of untreated cells by >2 SD. PTX in the recording electrode may have blocked GABAergic components, revealing an underlying excitatory drive onto pyramidal neurons.
Fig. 6.
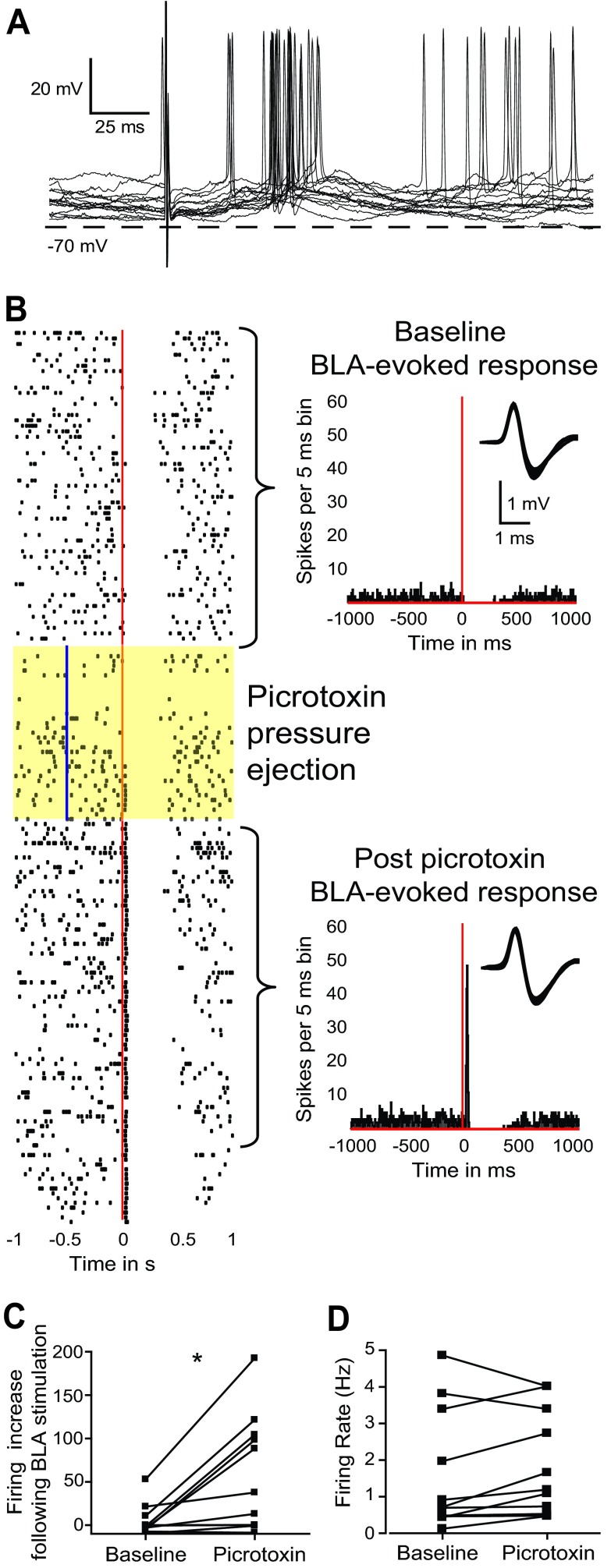
Picrotoxin (PTX) reveals a GABA component in the BLA-evoked dPSP and short-latency, BLA-evoked excitation. A: overlay of traces showing action potentials evoked by BLA stimulation in representative pyramidal neurons recorded with an electrode containing PTX (200 μM). B: raster plot (left) and PSTH (right), illustrating a response of a juxtacellularly recorded pyramidal neuron to BLA stimulation, before, during, and after local pressure ejection of PTX (vertical blue line in highlighted sweeps). The raster plot depicts consecutive sweeps (from top to bottom), with the red line indicating the time of BLA stimulation. The top PSTH shows baseline responses. Bin width is 5 ms, and the BLA stimulation time is shown with a vertical red line. The bottom PSTH shows responses after PTX pressure ejection, which unmasked a short-latency excitation in response to BLA stimulation. Insets in both PSTH illustrate the waveform of the neuron recorded. C: PTX significantly increased the magnitude of the early BLA-evoked excitatory response. The graph illustrates the firing increase by BLA stimulation (measured as the subtraction of firing/bin in the bins showing increased firing minus the prestimulation firing/bin) at baseline (left) and following PTX (right; *P < 0.05). D: PTX did not modify basal firing rates in all tested neurons.
To increase the number of neurons tested with PTX, we conducted juxtacellular recordings of BLA-evoked responses in pyramidal neurons, before, during, and after local administration of PTX via pressure ejection (1 mM; 30–60 nl). Although local PTX did not modify BLA-evoked responses in four out of 11 neurons, it revealed or enhanced an excitatory response in the rest. In four out of 11 cells, PTX unmasked short-latency excitation in response to BLA stimulation (Fig. 6B). In the remaining three neurons, BLA stimulation evoked a short-latency excitatory response that was enhanced by PTX pressure ejection (t10 = 3.216; P < 0.01). The enhancement was quantified by first determining the poststimulus bins, showing firing >2 SD above the prestimulation mean, and then subtracting the prestimulation firing/bin from the firing/bin in the excitatory response. The resultant firing increase is shown in Fig. 6C for pre- and post-PTX sweeps. PTX produced a slight increase in firing rate in nine of 11 neurons, but this effect was not significant (t10 = 1.387; P = 0.19; Fig. 6D), suggesting that PTX unmasking and potentiation of short-latency excitation were not due to general increases in neuronal discharges. PTX did not affect inhibitory responses in PFC neurons. These results are consistent with a BLA–PFC pathway that contains excitatory and inhibitory components, with GABA-A receptors selectively inhibiting BLA-evoked, short-latency excitatory responses in PFC neurons but not longer-lasting inhibitory components.
VTA antidromic activation.
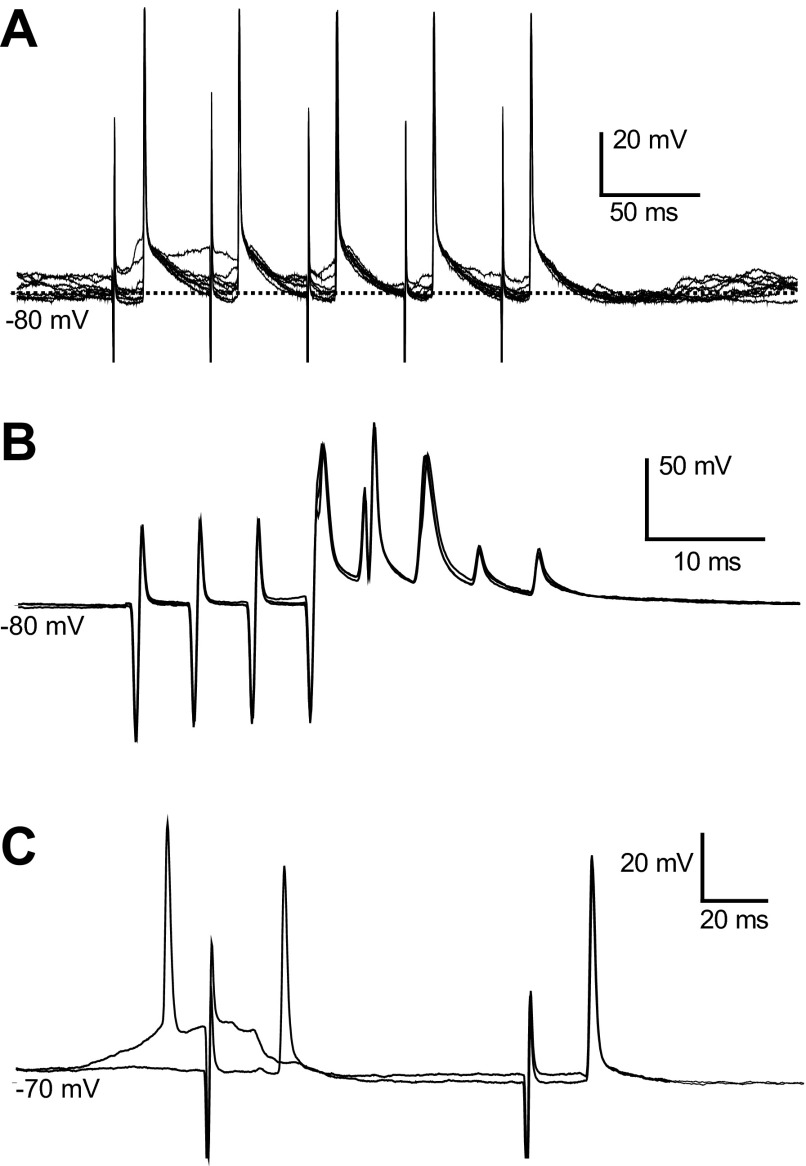
PFC pyramidal neurons send descending projections to the VTA, making synaptic contacts onto ascending projection neurons (Cowan et al. 1994). To test whether BLA inputs contact PFC neurons projecting to the VTA, a second stimulating electrode was placed in the VTA in several rats. Five PFC pyramidal neurons that were inhibited by BLA stimulation showed an antidromic response to VTA stimulation, indicating that these neurons project to the VTA. A response was deemed antidromic if the following criteria were met: constant spike latency, ability to follow high-frequency (>200 Hz) stimulation, and collision with a spontaneous action potential (Fig. 7). Unlike spontaneous and orthodromic action potentials, antidromic action potentials could be initiated directly out of the down state (Fig. 7, A and C). The average latency of antidromic action potentials was 11.5 ± 2.5 ms. Thus PFC neurons that are inhibited by BLA inputs include a population that projects to the VTA.
Fig. 7.
Antidromic activation from the ventral tegmental area (VTA). A: overlay of traces showing action potentials evoked by each pulse in a 20-Hz train delivered to the VTA. Action potentials were evoked with a constant latency of 9.8 ms and can be observed to rise from the down state. B: VTA-evoked action potentials follow 200 Hz stimulation. The 1st 3 action potential are full somatodendritic spikes, and the last 2 show only the initial segment component. C: overlay of 2 traces showing a spontaneous action potential (1st from left) colliding with an antidromically evoked action potential. BLA stimulation, immediately after the spontaneously occurring action potential, failed to evoke a spike.
DISCUSSION
In prefrontal cortical neurons recorded in vivo, BLA stimulation excited interneurons while inhibiting most pyramidal neurons. Juxtacellular recordings revealed that neurons excited by BLA stimulation included PV-positive interneurons. Furthermore, intracellular recordings from pyramidal neurons revealed a complex, multiple-component dPSP in response to BLA stimulation. Although the evoked dPSP was depolarizing from both the up and down states, it did not easily evoke action potentials. Intrasomatic depolarizing current was required to produce action potentials during the BLA-evoked response. Furthermore, current injection revealed at least one component with a negative Erev in every neuron tested. The GABA-A receptor antagonist PTX unmasked an excitatory drive onto pyramidal neurons, suggesting that GABA may be responsible for part of the evoked dPSP. Several BLA-responsive PFC pyramidal neurons were activated antidromically by VTA stimulation, suggesting that PFC neurons modulated by BLA inputs may affect mesocortical projections. These results provide direct evidence that PV PFC interneurons are activated by BLA stimulation.
Juxtacellular recordings revealed that BLA stimulation inhibits cell firing in most PFC pyramidal neurons and can evoke action potentials in few pyramidal neurons. These results are consistent with previous studies (Floresco and Tse 2007; Ishikawa and Nakamura 2003; Pérez-Jaranay and Vives 1991) and extend those findings by showing that BLA stimulation evoked action potentials in PFC PV interneurons. The short latency of BLA-evoked action potentials in interneurons indicates that they could be responsible for the inhibitory components observed with intracellular recordings in pyramidal neurons. Juxtacellularly recorded, evoked action potentials in pyramidal neurons occurred at short latencies, suggesting that excitatory drive can reach pyramidal neurons quickly, but it can also be suppressed swiftly by inhibition via local interneurons. It is possible that BLA activation of interneurons is so fast that it allows the suppression of monosynaptic, excitatory postsynaptic potentials (EPSPs). A fast feedforward mechanism with these characteristics has indeed been reported for corticostriatal projections (Mallet et al. 2005). As PTX did not modify longer-lasting, inhibitory components, it is conceivable that fast GABA-A inhibition may be responsible for transiently shunting BLA-driven pyramidal cell output, whereas slower BLA-evoked inhibition may be temporally sculpted by different inhibitory systems, such as GABA-B.
A potential confound is the possibility that electrical BLA stimulation activated terminals from PFC pyramidal neurons projecting to the amygdala that could produce EPSPs via local collaterals in the PFC. This is, however, unlikely, because PFC fibers innervating the amygdala target the intercalated cell mass at the border between CeA and lateral nuclei (Quirk et al. 2003). Furthermore, we did not observe any antidromically activated PFC neurons during the course of this study, as one would expect if antidromic activation were a factor. Together, the data indicate that feedforward inhibition plays a key role in regulating the flow of information from the BLA to the PFC.
Several BLA-inhibited PFC pyramidal neurons were antidromically activated by VTA stimulation. This observation suggests that BLA fibers can exert feedforward inhibitory control over PFC pyramidal neurons that provide direct excitatory input to the VTA. As PFC fibers target VTA dopamine (DA) neurons that project back to the PFC and VTA GABA neurons that project to the nucleus accumbens (Carr and Sesack 2000), the BLA can exert some control over mesocorticolimbic transmission. Although there are several means by which the amygdala may transfer information to the VTA, our data indicate that the BLA–PFC axis has the potential to regulate mesocortical and mesoaccumbal reward systems. This also raises the possibility that BLA may contribute to encoding of reward-prediction errors by VTA DA neurons by inhibiting PFC neurons that normally drive VTA DA neuron activity.
BLA-driven, feedforward inhibition in the mPFC may have a strong impact on behavior. The PFC is critical for goal-directed behaviors and response selection (Gruber et al. 2009; Matsumoto et al. 2003; Ostlund and Balleine 2005). Information that conveys emotional cues could be integrated in those processes by virtue of the BLA projection to the PFC. Indeed, BLA lesions impair decision making when response costs (e.g., effort, delays, risks) are important considerations (Ghods-Sharifi et al. 2009). It is probable that the dominant feedforward inhibition that we report here serves as a means to reduce background activity, allowing activated pyramidal neurons to contribute to the appropriate neural ensemble of PFC neurons for a particular behavior. Neuromodulators, such as DA, may play an important modulatory role in this pathway. DA has been shown to decrease BLA-evoked inhibition of PFC neurons (Floresco and Tse 2007). BLA-evoked inhibition may be dependent on the state of the DA mesocortical DA system. For example, BLA neurons increase their firing rate in response to omission of an expected reward or unexpected reward presentation (Roesch et al. 2010; Tye et al. 2010). Reward omission and unexpected reward result in inhibition and excitation of VTA DA neuron activity, respectively (Roesch et al. 2010; Schultz et al. 1997). Thus it is possible that BLA activity during reward omission and the associated DA neuron inhibition silence PFC neurons. On the other hand, BLA activity during unexpected reward and the associated phasic mesocortical DA responses would not be as effective in inhibiting PFC neurons, resulting in enhanced activation of PFC ensembles. Feedforward inhibition evoked in the PFC by strong BLA activation of local interneurons is likely a critical element in the selection of PFC neurons that can determine behaviorally appropriate outcomes.
GRANTS
Support for this work was provided by the National Institute of Mental Health Grant R01-MH57683 (to P. O'Donnell).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: P.O. conception and design of research; J.D. and H.A.T. performed experiments; J.D., H.A.T., and P.O. analyzed data; J.D. and P.O. interpreted results of experiments; J.D., H.A.T., and P.O. prepared figures; J.D. and H.A.T. drafted manuscript; P.O. edited and revised manuscript; P.O. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of J. Dilgen: Medical University of South Carolina, 171 Ashley Ave., Charleston, SC 29425.
REFERENCES
- Akaike N, Hattori K, Inomata N, Oomura Y. gamma-Aminobutyric-acid- and pentobarbitone-gated chloride currents in internally perfused frog sensory neurones. J Physiol 360: 367–386, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon SJ, Headlam AJ, Gabbott PL, Smith AD. Amygdala input to medial prefrontal cortex (mPFC) in the rat: a light and electron microscopy study. Brain Res 720: 211–219, 1996 [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci 19: 5473–5481, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci 20: 3864–3873, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan RL, Sesack SR, Van Bockstaele EJ, Branchereau P, Chan J, Pickel VM. Analysis of synaptic inputs and targets of physiologically characterized neurons in rat frontal cortex: combined in vivo intracellular recording and immunolabeling. Synapse 17: 101–114, 1994 [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Increasing interaction of amygdalar afferents with GABAergic interneurons between birth and adulthood. Cereb Cortex 18: 1529–1535, 2008 [DOI] [PubMed] [Google Scholar]
- Cupello A, Palm A, Rapallino MV, Hyden H. Can Cl− ions be extruded from a gamma-aminobutyric (GABA)-acceptive nerve cell via GABAA receptors on the plasma membrane cytoplasmic side? Cell Mol Neurobiol 11: 333–346, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Tse MT. Dopaminergic regulation of inhibitory and excitatory transmission in the basolateral amygdala-prefrontal cortical pathway. J Neurosci 27: 2045–2057, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Busby SJ. Amygdala input monosynaptically innervates parvalbumin immunoreactive local circuit neurons in rat medial prefrontal cortex. Neuroscience 139: 1039–1048, 2006 [DOI] [PubMed] [Google Scholar]
- Ghods-Sharifi S, St Onge JR, Floresco SB. Fundamental contribution by the basolateral amygdala to different forms of decision making. J Neurosci 29: 5251–5259, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber AJ, Hussain RJ, O'Donnell P. The nucleus accumbens: a switchboard for goal-directed behaviors. PLoS One 4: e5062, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata N, Tokutomi N, Oyama Y, Akaike N. Intracellular picrotoxin blocks pentobarbital-gated Cl− conductance. Neurosci Res 6: 72–75, 1988 [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Nakamura S. Convergence and interaction of hippocampal and amygdalar projections within the prefrontal cortex in the rat. J Neurosci 23: 9987–9995, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Sun H, Fu Y, Li Z, Pais-Vieira M, Galhardo V, Neugebauer V. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J Neurosci 30: 5451–5464, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BL, O'Donnell P. Ventral tegmental area afferents to the prefrontal cortex maintain membrane potential ‘up’ states in pyramidal neurons via D(1) dopamine receptors. Cereb Cortex 10: 1168–1175, 2000 [DOI] [PubMed] [Google Scholar]
- Mallet N, Le Moine C, Charpier S, Gonon F. Feedforward inhibition of projection neurons by fast-spiking GABA interneurons in the rat striatum in vivo. J Neurosci 25: 3857–3869, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Suzuki W, Tanaka K. Neuronal correlates of goal-based motor selection in the prefrontal cortex. Science 301: 229–232, 2003 [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cytoarchitecture of the central amygdaloid nucleus of the rat. J Comp Neurol 208: 401–418, 1982 [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Glutamate and aspartate immunoreactive neurons of the rat basolateral amygdala: colocalization of excitatory amino acids and projections to the limbic circuit. J Comp Neurol 365: 367–379, 1996 [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Muller JF, Mascagni F. GABAergic innervation of alpha type II calcium/calmodulin-dependent protein kinase immunoreactive pyramidal neurons in the rat basolateral amygdala. J Comp Neurol 446: 199–218, 2002 [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Ionic flux contributions to neocortical slow waves and nucleus basalis-mediated activation: whole-cell recordings in vivo. J Neurosci 13: 5312–5323, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cabal L, Pollandt S, Liu J, Vergara L, Shinnick-Gallagher P, Gallagher JP. A novel rat medial prefrontal cortical slice preparation to investigate synaptic transmission from amygdala to layer V prelimbic pyramidal neurons. J Neurosci Methods 151: 148–58, 2006 [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Lesions of medial prefrontal cortex disrupt the acquisition but not the expression of goal-directed learning. J Neurosci 25: 7763–7770, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (4th ed.). Oxford: Elsevier, 1998 [Google Scholar]
- Pérez-Jaranay JM, Vives F. Electrophysiological study of the response of medial prefrontal cortex neurons to stimulation of the basolateral nucleus of the amygdala in the rat. Brain Res 564: 97–101, 1991 [DOI] [PubMed] [Google Scholar]
- Pinault D. Golgi-like labeling of a single neuron recorded extracellularly. Neurosci Lett 170: 255–260, 1994 [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci 23: 8800–8807, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Calu DJ, Esber GR, Schoenbaum G. Neural correlates of variations in event processing during learning in basolateral amygdala. J Neurosci 30: 2464–2471, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science 275: 1593–1599, 1997 [DOI] [PubMed] [Google Scholar]
- Sosulina L, Meis S, Seifert G, Steinhauser C, Pape HC. Classification of projection neurons and interneurons in the rat lateral amygdala based upon cluster analysis. Mol Cell Neurosci 33: 57–67, 2006 [DOI] [PubMed] [Google Scholar]
- Sun H, Neugebauer V. mGluR1, but not mGluR5, activates feed-forward inhibition in the medial prefrontal cortex to impair decision making. J Neurophysiol 106: 960–973, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Cone JJ, Schairer WW, Janak PH. Amygdala neural encoding of the absence of reward during extinction. J Neurosci 30: 116–125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]