Abstract
The retina responds to a wide range of light stimuli by adaptation of retinal signaling to background light intensity and the use of two different photoreceptors: rods that sense dim light and cones that sense bright light. Rods signal to rod bipolar cells that receive significant inhibition from amacrine cells in the dark, especially from a rod bipolar cell-activated GABAergic amacrine cell. This inhibition modulates the output of rod bipolar cells onto downstream neurons. However, it was not clear how the inhibition of rod bipolar cells changes when rod signaling is limited by an adapting background light and cone signaling becomes dominant. We found that both light-evoked and spontaneous rod bipolar cell inhibition significantly decrease with light adaptation. This suggests a global decrease in the activity of amacrine cells that provide input to rod bipolar cells with light adaptation. However, inhibition to rod bipolar cells is also limited by GABAergic connections between amacrine cells, which decrease GABAergic input to rod bipolar cells. When we removed this serial inhibition, the light-evoked inhibition to rod bipolar cells remained after light adaptation. These results suggest that decreased inhibition to rod bipolar cells after light adaptation is due to decreased rod pathway activity as well as an active increase in inhibition between amacrine cells. Together these serve to limit rod bipolar cell inhibition after light adaptation, when the rod pathway is inactive and modulation of the signal is not required. This suggests an efficiency mechanism in the retina to limit unnecessary signaling.
Keywords: retina, light, GABA, glycine, patch clamp
the retina responds to light stimuli that vary by 10 orders of magnitude, using several different mechanisms of light adaptation. One way that the retina achieves this is to have two different photosensors—rod photoreceptors that respond to dim light and cone photoreceptors that respond to brighter light. The information from photoreceptors is separated into parallel pathways: ON cone bipolar cells (BCs) that respond to the onset of bright light, OFF cone BCs that respond to the offset of bright light, and rod BCs that respond to the onset of dim light. These BC pathways receive inhibitory input from amacrine cells (ACs) to their axon terminals that shapes their response to light (Dong and Werblin 1998; Eggers and Lukasiewicz 2006b; Sagdullaev et al. 2006). However, previous recordings of light-evoked inhibition from BCs (Eggers and Lukasiewicz 2006a; Pang et al. 2004) were made in dark-adapted retinas where rod signals are most active. It is not clear what roles cone pathways versus rod pathways play in this inhibition.
Rod signals have a significantly slower time course than cone signals (Ashmore and Copenhagen 1980; Copenhagen et al. 1983; Schnapf and Copenhagen 1982), so we might expect that transitioning from rod-mediated to cone-mediated inhibition would speed up the time course of inhibition. Rod BCs receive large reciprocal inhibition onto GABA receptors from the GABAergic A17 AC, the only known AC to feed input solely to rod BCs (Fig. 1) (Chavez and Diamond 2008; Eggers and Lukasiewicz 2010; Hartveit 1999). However, over half of the synapses onto rod BC terminals come from nonreciprocal connections (Kim et al. 1998; Strettoi et al. 1990). These glycinergic and GABAergic AC inputs come at the onset of a light stimulus (Eggers and Lukasiewicz 2006a; Pang et al. 2004) and thus are presumably activated by ON cone BCs and would be active in light-adapted retinas. In dark-adapted retinas a large proportion of the inhibition to rod BCs is mediated by the GABAC receptor (GABACR), with smaller proportions mediated by the GABAA (GABAAR) and glycine (glycineR) receptors, but it is not clear how this changes with light adaptation (Eggers and Lukasiewicz 2006a, 2006b).
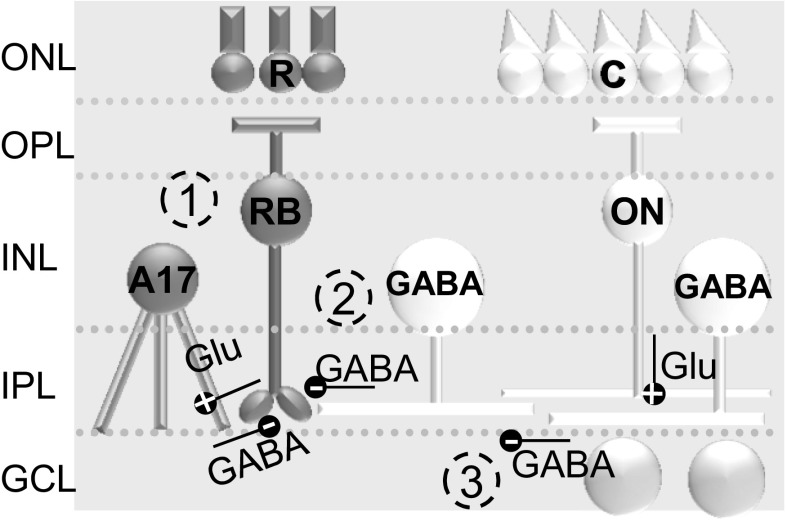
Fig. 1.
Potential pathways of rod bipolar cell (BC) inhibition. 1) In dark-adapted retinas where rod (R) pathways are active, rod BCs (RB) activate A17 amacrine cells (ACs) (+, glutamate) and receive significant GABAergic feedback inhibition (−, GABA) from those A17 ACs (dark gray pathway). 2) They also receive a small amount of glycinergic inhibition (not shown here) and GABAergic inhibition (−) that comes from cone (C)-activated pathways, activated (+) by ON cone BCs (ON). 3) Input from GABAergic ACs onto rod BCs is modulated by GABAA receptor-mediated serial connections between GABAergic ACs. ONL, outer nuclear layer; INL, inner nuclear layer; OPL, outer plexiform layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
Other rod-mediated inhibitory signals are transmitted through the OFF cone BC pathway. OFF cone BCs receive glycinergic inputs when rods are active through the rod BC-activated glycinergic AII ACs (Grunert and Wässle 1996; Haverkamp et al. 2003; Strettoi et al. 1994). Inputs from AII ACs are likely responsible for the large glycinergic currents seen in OFF cone BCs (Eggers et al. 2007; Ivanova et al. 2006), although they do not necessarily set the threshold for rod responses in the OFF pathway (Arman and Sampath 2012). However, dark-adapted OFF cone BCs also receive GABAergic inhibition and potentially glycinergic inhibition from other ACs (Eggers et al. 2007; Ivanova et al. 2006). It is not known how the inhibition in light-adapted conditions varies from this.
Additional components that can modulate BC inhibition are inhibitory connections between retinal ACs. BC inhibition is suppressed by GABAAR-mediated connections between ACs (Eggers and Lukasiewicz 2006a, 2010; Eggers et al. 2007; Roska et al. 1998; Zhang et al. 1997). Blocking GABAARs in the retina causes a large increase in inhibition to rod BCs mediated by GABACRs (Eggers and Lukasiewicz 2006a, 2010). These connections serve to limit the spatial extent of inhibition to BCs (Eggers and Lukasiewicz 2010), but it is not known how they change between rod- and cone-dominant conditions.
In this study we address these questions about how retinal inhibition changes with light adaptation by recording light-evoked and spontaneous inhibition from BCs in dark- and light-adapted conditions. Surprisingly, we found that rod BC inhibition is almost gone after the application of a rod-adapting background, while the OFF BC inhibition shows no significant change, suggesting a selective suppression of rod BC input. The retina potentially minimizes unnecessary signaling by suppressing rod BC inhibition in conditions where rod photoreceptors are not significantly active. This could be part of a smooth transition between light intensities.
METHODS
Preparation of retinal slices.
Animal protocols were approved by the University of Arizona Institutional Animal Care and Use Committee (IACUC). As described previously (Eggers and Lukasiewicz 2006a), C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME), aged 35–60 days, were euthanized with carbon dioxide and eyes were enucleated. The cornea and lens were removed, and the eyecup was incubated in cold extracellular solution (see Solutions and drugs) containing 800 U/ml of hyaluronidase for 20 min. The retina was removed from the eyecup, trimmed approximately square to remove the peripheral retina, and mounted onto 0.45-μm nitrocellulose filter paper (Millipore, Billerica, MA). The filter paper-mounted retina was sliced into 250-μm-thick slices and placed onto vacuum grease on glass coverslips after rotating 90°. Slices were taken from the central portion of the retina. All dissection and recording procedures were performed under infrared illumination to preserve the light sensitivity of the preparations.
Solutions and drugs.
Extracellular solution used as a control bath and for dissection contained (in mM) 125 NaCl, 2.5 KCl, 1 Mg2Cl, 1.25 NaH2PO4, 20 glucose, 26 NaHCO3, and 2 CaCl2 and was bubbled with a mixture of 95% O2-5% CO2. The pipette intracellular solution contained (in mM) 120 CsOH, 120 gluconic acid, 1 MgCl2, 10 HEPES, 10 EGTA, 10 TEA-Cl, 10 phosphocreatine-NA2, 4 Mg-ATP, and 0.5 Na-GTP with 50 μM Alexa Fluor 488 (Invitrogen, Carlsbad, CA) and was adjusted to pH 7.2 with CsOH. To block connections between ACs, 20 μM SR-95531 was used to block GABAARs. Antagonists were applied to the slice by a gravity-driven superfusion system (Cell Microcontrols, Norfolk, VA) at a rate of ∼1 ml/min. Unless otherwise indicated, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Whole cell recordings.
Glass coverslips containing retinal slices were placed in a custom chamber. The preparation was heated to 32°C by temperature-controlled thin-stage and inline heaters (Cell Microcontrols). Whole cell patch recordings were made from BCs and ACs from retinal slices as described previously (Eggers and Lukasiewicz 2006b). To isolate inhibitory currents, BCs were clamped at 0 mV, the reversal potential for currents mediated by nonselective cation channels. To isolate excitatory currents, ACs were clamped at −60 mV, the reversal potential for currents mediated by Cl− channels. Electrodes with resistances of 5–7 MΩ were pulled from borosilicate glass (World Precision Instruments, Sarasota, FL) with a P97 Flaming/Brown puller (Sutter Instruments, Novato, CA). Liquid junction potentials of 20 mV were corrected at the beginning of each recording.
Light-evoked inhibitory postsynaptic currents (L-IPSCs) and spontaneous IPSCs (sIPSCs) were recorded from BCs. Light-evoked excitatory postsynaptic currents (L-EPSCs) were recorded from A17 ACs. Responses were filtered with a 6-kHz four-pole low-pass Bessel filter on an MultiClamp 700B patch-clamp amplifier (Molecular Devices, Sunnyvale, CA) and digitized at 10 kHz with a Digidata 1140A data acquisition system (Molecular Devices) and Clampex software (Molecular Devices). Prospective rod and OFF BCs and A17 ACs were identified by their soma location in the upper inner nuclear layer (INL) for rod BCs, mid-INL for OFF BCs, and lower INL for A17 ACs and shape as oval for BCs or large and round for A17 ACs. BC (Ghosh et al. 2004) and AC (Menger and Wässle 2000; Nelson and Kolb 1985; Singer and Diamond 2003) morphology was confirmed by dendrites and axon location and shape at the end of the recording by imaging the Alexa fluorescence with an Intensilight fluorescence lamp and a Digitalsight camera operated by Elements software (Nikon Instruments, Tokyo, Japan).
Light stimulation.
To evoke L-IPSCs, full-field light stimuli were generated with a light-emitting diode (LED, λpeak = 525 nm) that was projected through the camera port of the microscope onto the retinal tissue. The maximum intensity of the light was 9.5 × 105 photons·μm−2·s−1, measured with an optometer (Gamma Scientific), and the duration of all light stimuli was 30 ms. Light intensity was controlled by varying the current through the LED. The relationship of current through the LED to light intensity was calibrated at many current levels, and the appropriate LED stimulus was chosen to give the desired light intensity. Background light adaptation was applied for 5 min before light-adapted responses were recorded.
Data analysis and statistics.
L-IPSC and L-EPSC traces from a given response condition were averaged with Clampfit software (Molecular Devices), and the charge transfer (Q), peak amplitude, time to peak, and decay to 37% of the peak (D37) were measured in each condition. Q was measured over the length of the response, using the same time parameters in each condition for the same cell. This was typically 1 s. For the kinetic parameters (time to peak and D37) average traces were replaced with a 100-fold decimation substituted with an average of those points to smooth out small fluctuations due to spontaneous activity. For intensity-response curves, L-IPSC Qs were normalized to the response to the maximal light stimulus in dark-adapted conditions. The normalized data were plotted versus the log10 of the stimulus intensity.
sIPSC data were analyzed with Clampfit software. A sIPSC template was calculated for each data file with the average of >10 prototypical events from the recording. The software used this template to automatically detect spontaneous events. These events were manually accepted or rejected on the basis of rigid criteria. Events that were used to calculate the frequency were rejected if they appeared to be noise. Events that were used to calculate the average peak amplitude were rejected if they appeared to be noise or were overlapping. Frequency was calculated by dividing the number of events recorded by the recording time. The distributions of sIPSC amplitude values were compared with the Kolmogorov-Smirnov test.
For each cell, a normalized data value of the percentage of response to maximal light stimulation in the dark was calculated. Paired Student's t-tests were used to compare values between conditions for the same cell. Standard Student's t-tests were used to compare values between different cells. Differences were considered significant when P ≤ 0.05. All data are reported as means ± SE.
RESULTS
Switching from rod to cone pathways decreases rod BC inhibition.
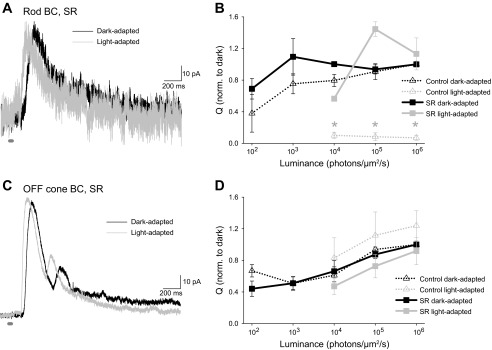
To determine how inhibition to rod BCs varies with light intensity, we recorded L-IPSCs in response to stimuli of increasing intensities with no background light (dark adapted) and compared these responses to L-IPSCs after a background light (950 photons·μm−2·s−1, light adapted) of an intensity that maximally activates the rods was applied (Wang and Kefalov 2009). This is an intensity that severely desensitizes and potentially saturates mouse rods, although they may be able to adapt to brighter backgrounds to retrieve a small amount of rod response (Naarendorp et al. 2010), possibly through rod-cone coupling. In rod BCs, L-IPSCs are a combination of currents mediated by glycineRs, GABAARs, and GABACRs (Eggers and Lukasiewicz 2006a, 2006b). Rod BC L-IPSCs at the highest light intensity tested were almost completely absent after light adaptation (Fig. 2A). At all intensities tested above the adapting background, the rod BC L-IPSC Q was significantly decreased by light adaptation (n = 6, P < 0.01; Fig. 2B). This suggests that inhibition to rod BCs potentially comes primarily from rod-dominant sources, like the A17 AC that makes a GABAergic feedback circuit with rod BCs. This is supported by our data (not shown) that only 29.2 ± 2.3% of the L-IPSC to rod BCs (n = 3, P < 0.01) remains after the application of DHT (50 μM), a drug that inactivates A17 ACs (Chavez et al. 2006). The decrease in inhibition was not due to a change in the tonic inhibition to rod BCs, as during light adaptation there is no significant change in the baseline current (dark baseline − light baseline = 2.3 ± 2.0 pA, P = 0.22).
Fig. 2.
Rod BC light-evoked inhibitory postsynaptic currents (L-IPSCs) are significantly decreased by adaptation with a rod-saturating light. A: example L-IPSCs in response to the maximum light intensity (30-ms light stimulus) used from rod BCs in a dark-adapted retina and with a rod-saturating background (dark gray bar shows timing of light stimulus). B: the charge transfer (Q) of L-IPSCs in response to many intensities of light was normalized to the response at the maximum light intensity in dark-adapted conditions. L-IPSCs in light-adapted conditions were significantly decreased at all intensities used (*P < 0.01, n = 6).
To assess the general activity of ACs giving input to rod BCs we also recorded sIPSCs in dark-adapted and light-adapted retinas. In the rod BC, sIPSCs in control conditions are mediated by glycineRs and GABAARs (Eggers and Lukasiewicz 2006a, 2006b). We found that sIPSCs in rod BCs were significantly decreased by light adaptation (Fig. 3A). This was caused by both a decrease in the frequency of sIPSCs (Fig. 3B; n = 7, P < 0.005) and a shift of the peak amplitude distribution to smaller peak amplitudes (Fig. 3C). We analyzed changes in the peak amplitude of sIPSCs for all rod BCs where more than five sIPSCs remained after light adaptation. The average peak amplitude of all rod BC sIPSCs was significantly decreased (Fig. 3D; n = 5, P < 0.05). This suggests that ACs that inhibit rod BCs are less spontaneously active when a rod-saturating background is applied, although their activity is not completely suppressed since spontaneous inputs remained in many rod BCs.
Fig. 3.
Rod BC spontaneous IPSCs (sIPSCs) are significantly decreased by adaptation with a rod-saturating light. A: sIPSCs recorded from a rod BC in a dark-adapted retina and with a rod-saturating background (2 traces from each condition are shown, 1 in black and 1 in dark gray). B: for all rod BCs, the frequencies of sIPSCs decreased from 0.33 ± 0.13 Hz to 0.11 ± 0.06 Hz (n = 7, *P < 0.05). Triangles denote average frequencies. C: normalized histogram and cumulative probability histogram of peak amplitudes from the example in A. Light adaptation significantly decreased the peak amplitude [Kolmogorov-Smirnov test (K-S) P < 0.01]. Circles denote average ± SE peak amplitudes. D: for all rod BCs, the peak amplitudes of sIPSCs decreased from 17.1 ± 4.1 pA to 11.3 ± 2.5 pA (n = 5, *P < 0.05). Triangles denote average amplitudes.
Rod outputs are significantly decreased with light adaptation.
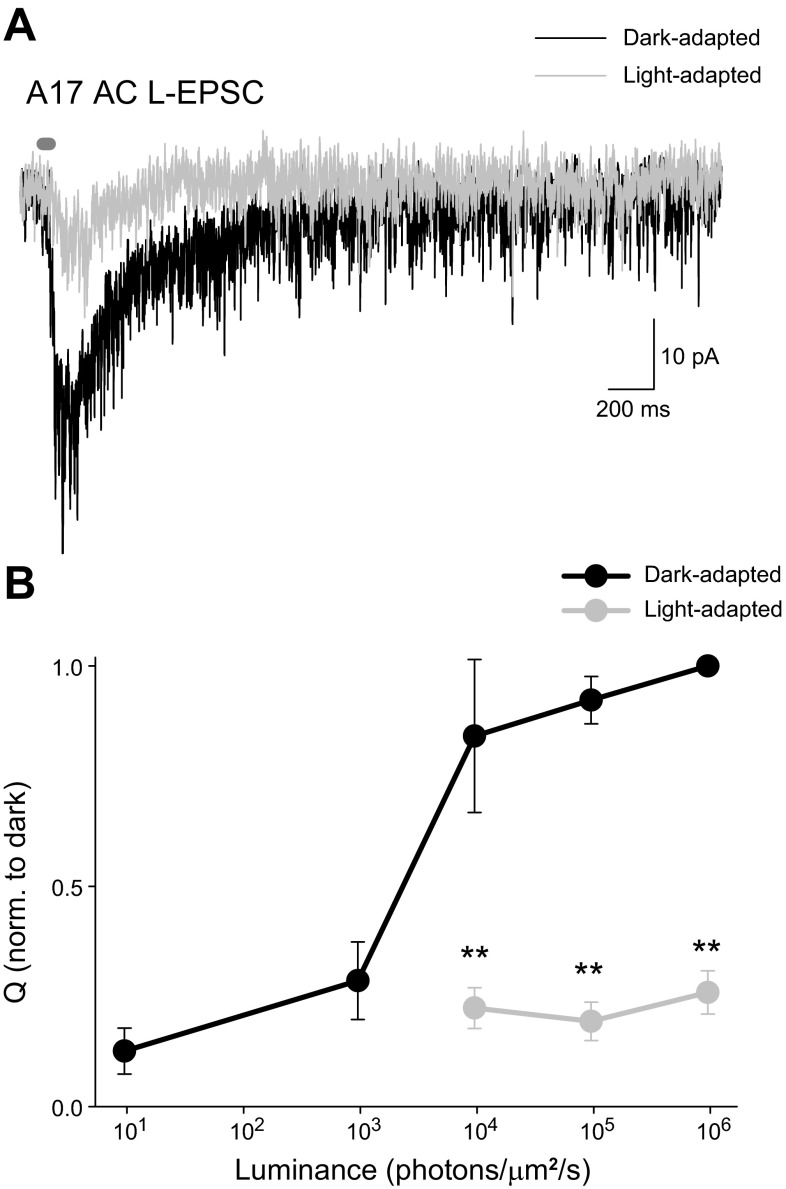
To ensure that the light intensities we used are significantly decreasing rod signals, we recorded L-EPSCs from A17 ACs in dark- and light-adapted conditions. A17 ACs receive all excitatory input from rod BCs and feedback GABAergic inhibition onto rod BC terminals. Thus L-EPSCs of A17 ACs are also a measure of the direct rod-activated feedback inhibition as well. We found that the Q (Fig. 4B; n = 4, P < 0.01) and peak amplitude (P < 0.05) of A17 AC L-EPSCs were significantly decreased with light adaptation at all intensities used. What little current remained was faster (D37 = 47 ± 3% of control, P < 0.01), suggesting it might be coming from rod-to-cone coupling instead of direct rod inputs, as cone signaling is faster than rod signaling (Ashmore and Copenhagen 1980; Copenhagen et al. 1983; Schnapf and Copenhagen 1982). On average, the Q of the A17 L-EPSCs in response to the maximum intensity was decreased to 26 ± 5% of control by light adaptation. In contrast, the rod BC L-IPSC was decreased to 10 ± 4% of control by light adaptation, a significantly larger decrease than that of the A17 L-EPSC (P < 0.05). This suggests that light adaptation is changing more facets of rod BC inhibition than just a decrease in rod signaling.
Fig. 4.
A17 AC light-evoked excitatory postsynaptic currents (L-EPSCs) are significantly decreased by adaptation with a rod-saturating light. A: example L-EPSCs from an A17 AC in a dark-adapted retina and with a rod-saturating background. B: the Q of L-EPSCs in response to many intensities of light was normalized to the response to the maximum light intensity in dark-adapted conditions. L-EPSCs in light-adapted conditions were significantly decreased at all intensities used (**P < 0.01, n = 4).
Switching from rod to cone pathways does not affect OFF cone BC L-IPSCs.
We previously recorded L-IPSCs from all BC types in dark-adapted retinas (Eggers et al. 2007) but did not investigate L-IPSCs in light-adapted retinas. To determine whether the decrease in L-IPSCs that we observe in rod BCs is specific to the rod pathway, we measured L-IPSCs from OFF cone BCs in dark- and light-adapted retinas. OFF cone BCs receive significant inhibition from rod BC-activated AII ACs (Grunert and Wässle 1996; Haverkamp et al. 2003; Strettoi et al. 1994) but also from other ACs (Eggers et al. 2007; Ivanova et al. 2006). Although the AII connections may not be the most important determinant of the very dim rod threshold observed in the OFF pathway (Arman and Sampath 2012), the connection between AII ACs and OFF cone BCs is very strong (Grunert and Wässle 1996; Haverkamp et al. 2003; Strettoi et al. 1994) and likely activated at brighter light intensities (Pang et al. 2012) through the gap junctional connection between ON cone BCs and AII ACs that remains active in bright light. They are therefore a good control for switching between rod and cone inhibition. We found that L-IPSCs of OFF cone BCs were not changed by our rod-adapting background at any of the intensities we tested (Fig. 5; P = 0.9, n = 8). There were also no significant changes in the timing of L-IPSCs (D37 P = 0.2; time to peak P = 0.3). This suggests that, unlike rod BCs, OFF cone BCs are receiving additional inhibition from other sources when their rod-mediated inhibition from AII ACs is decreased.
Fig. 5.
OFF cone BC L-IPSCs are not changed by adaptation with a rod-saturating light. A: example L-IPSCs from an OFF cone BC in a dark-adapted retina and with a rod-saturating background. B: the Q of L-IPSCs in response to many intensities of light was normalized to the response at the maximum light intensity in dark-adapted conditions. There was no significant difference between the L-IPSCs in dark- and light-adapted OFF cone BCs (P = 0.9, n = 8).
Serial connections between ACs limit cone-mediated inhibition to rod BCs.
We have previously shown that GABAAR-mediated connections between ACs can modulate inhibition to all BC types (Eggers and Lukasiewicz 2006a, 2010) in dark-adapted retinas. Blocking these connections with an antagonist to GABAARs causes an increase in GABAergic inhibition to the rod BC. To determine whether these serial connections are affected by light adaptation, we blocked GABAARs with SR-95531 and recorded L-IPSCs in response to multiple intensities in dark-adapted and light-adapted retinas (Fig. 6A). We expected that if the amount of inhibition between ACs is unaffected by a background light, rod BC L-IPSCs would still be suppressed in the light-adapted conditions when these serial connections were blocked. However, when these connections were blocked we did not see a decrease in rod BC L-IPSCs with light adaptation. Instead, there was no significant difference between the Q of dark-adapted and light-adapted L-IPSCs when GABAARs were blocked (Fig. 6B). This led to a light-adapted L-IPSC Q when GABAARs were blocked that was significantly greater than that in light-adapted L-IPSCs when GABAARs are active (Fig. 6B). Although the suppression of the A17 input onto GABACRs is presumably still present after GABAARs are blocked, blocking serial connections causes a significant increase in rod BC inhibition in the dark (Eggers and Lukasiewicz 2006a, 2010), so that this decrease is overcome by the increased GABA release (SR-95531 was 178 ± 23% of control at the highest light intensity in the dark-adapted retina). The L-IPSC at the maximum light intensity also became faster in light-adapted conditions. When GABAARs were blocked, the D37 of rod BC L-IPSCs was decreased from 200.2 ± 45.9 ms in the dark to 114.4 ± 14.0 ms in light-adapted conditions, an average of 67.2 ± 11.6% of dark-adapted D37. This suggests that cone pathways were being activated that were normally suppressed by serial connections between ACs in the light-adapted conditions.
Fig. 6.
When serial connections between ACs are blocked with a GABAA receptor (GABAAR) antagonist, rod BC L-IPSCs are not decreased by light adaptation. A: example L-IPSCs recorded from a rod BC in a dark-adapted retina in the presence of GABAAR antagonist SR-95531 (SR, 20 μM) and with a rod-saturating background. B: the Q of rod BC L-IPSCs in response to many intensities of light was normalized to the response to the maximum light intensity in dark-adapted conditions in the presence of SR-95531. Control data from Fig. 2 are added for comparison (dotted lines). Rod BC L-IPSCs in light-adapted, SR-95531 conditions were not significantly decreased and were significantly greater than L-IPSCs in control conditions (*P < 0.05, n = 5) at all intensities used. C: for comparison purposes, we also show L-IPSCs from an OFF cone BC, in SR-95531, in dark- and light-adapted conditions (n = 5). D: in contrast to rod BCs, OFF cone BCs did not change when light adapted in either control condition or presence of SR-95531.
We also recorded the response of OFF cone BC L-IPSCs to light adaptation after blocking GABAARs and also saw no differences between the Q of dark-adapted and light-adapted conditions (Fig. 6, C and D). Both in control conditions and when GABAA receptors were blocked, OFF cone BCs showed no significant decrease in L-IPSCs with light adaptation. When GABAARs were blocked, the D37 of OFF BC L-IPSCs was also decreased from 319.0 ± 42.4 ms to 178.3 ± 48.5 ms, an average of 52.7 ± 10.1% of the dark-adapted D37. This suggests that for rod BCs, but not for OFF BCs, activation of inhibitory connections between ACs after light adaptation is serving to limit inhibition.
Rod BC sIPSCs when GABAARs were blocked were reduced by light adaptation.
When GABAARs are blocked throughout the retina, GABAergic ACs are disinhibited, which increases GABAergic input mediated by GABACRs to rod BCs (Eggers and Lukasiewicz 2006a). However, in the absence of compounds that increase AC depolarization, such as kainate (Eggers and Lukasiewicz 2006b), rod BC sIPSCs are composed only of inputs onto GABAARs and glycineRs. Since GABAARs are blocked in the presence of SR-95531, we can monitor how glycinergic inhibition to rod BC sIPSCs changes with light adaptation. We found that glycineR sIPSCs were significantly reduced by light adaptation (Fig. 7A). This resulted in a significant decrease in the frequency of sIPSCs (Fig. 7B; n = 6, P < 0.01). Additionally, we observed a small shift of the peak amplitude distribution to smaller peak amplitudes (Fig. 7C). We analyzed changes in the peak amplitude of sIPSCs for all rod BCs where more than five sIPSCs remained after light adaptation. The average peak amplitude was significantly decreased (Fig. 7D; n = 4, P < 0.05). This suggests that glycinergic ACs that send inhibition to rod BCs are still less active when a rod-saturating background is applied, even when GABAARs are blocked so that GABAergic ACs are disinhibited. This makes sense, as we have previously shown that disinhibiting the GABAergic ACs affects only GABAergic, and not glycinergic, inputs to rod BCs (Eggers and Lukasiewicz 2006a). This decrease in glycinergic input is likely present in the results in Fig. 6, but it is not observed because the GABACR-mediated input dominated the rod BC L-IPSCs. In a separate experiment, we also compared GABAAR sIPSCs (in the presence of strychnine) and found that the sIPSC frequency was significantly decreased to 18 ± 5% (P < 0.05, n = 3) of the GABAAR frequency in the dark. Therefore the decrease of sIPSCs with light adaptation seen in Fig. 3 results from a decrease in both GABAAR and glycineR sIPSCs.
Fig. 7.
Rod BC sIPSCs in the presence of SR-95531 are significantly decreased by adaptation with a rod-saturating light. A: sIPSCs were recorded from a rod BC in a dark-adapted retina in the presence of SR-95531, and a rod-saturating background was applied (2 traces from each condition are shown, 1 in black and 1 in dark gray). B: the frequency of rod BC sIPSCs in the presence of SR-95531 decreased from 0.79 ± 0.41 Hz to 0.14 ± 0.08 Hz (n = 6, *P < 0.01). Triangles denote average frequencies. C: normalized histogram and cumulative probability histogram of peak amplitudes from the example in A. Light adaptation significantly decreased the peak amplitude (K-S P < 0.01). Circles denote average ± SE peak amplitudes. D: the peak amplitude of rod BC sIPSCs in the presence of SR-95531 decreased from 24.0 ± 3.2 pA to 17.2 ± 2.9 pA (n = 4, *P < 0.05). Triangles denote average amplitudes.
DISCUSSION
We have shown that, surprisingly, inhibition to rod BCs is significantly regulated by a change in background light level. Light-evoked and spontaneous inhibition to rod BCs are significantly reduced with a rod-adapting background, while light-evoked OFF BC inhibition is unchanged. This occurs by two mechanisms: a reduction in the A17 AC rod pathway-mediated inhibition as well as increased activity between GABAergic ACs with light adaptation that limits rod BC inhibition (Fig. 8). This suggests that rod BC inhibition is limited in circumstances where it is not needed, when the rods are significantly less active after light adaptation. This might allow the small signals from cone-rod coupling to pass through the rod signaling system in light-adapted conditions. In contrast, OFF cone BCs still receive strong inhibition after light adaptation, as they are active in both the rod and cone circuits.
Fig. 8.
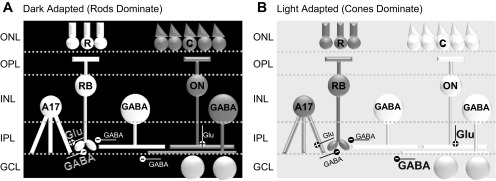
Distinct rod and cone pathways of rod BC inhibition. A: in dark-adapted retinas where rod pathways are active, rod BCs receive significant inhibition, likely from A17 ACs (white), and little inhibition from cone-activated pathways (dark gray). B: when rod pathways (dark gray) were saturated with an adapting background, inhibition from the cone pathway (white) was suppressed by inhibitory connections between GABAergic ACs, resulting in little inhibition to rod BCs.
In this study we found that light-evoked inhibition to rod BCs is almost nonexistent after light adaptation. We have previously shown that rod BCs receive significant inhibition in dark-adapted conditions from GABAergic and glycinergic ACs (Eggers and Lukasiewicz 2006a, 2006b). A significant portion of this inhibition comes from reciprocal connections with A17 ACs, with contributions from other ACs not activated by the rod pathways (Chavez and Diamond 2008; Chavez et al. 2010). Although rod signals cross over into the cone pathways, through activation of AII ACs and ON cone BCs by rod BCs, this is a secondary connection. Thus it makes sense that the contribution of direct rod pathway-activated inhibition from A17 ACs would be greater than that coming from the cone pathways, and rod BC inhibition from this source would show a significant decrease.
However, the observation that rod BC inhibition is almost gone in light-adapted conditions is somewhat puzzling because rod BCs receive inputs from non-rod pathway-activated ACs, most notably onto glycineRs that cannot be activated by the GABAergic A17 AC (Eggers and Lukasiewicz 2006a, 2006b). This implies that there must be some active suppression of rod BC inhibition with light adaptation in addition to a decrease due to rod saturation. Additionally, in dark-adapted retinas at brighter light intensities, where rods are presumably already saturated, rod BCs can also receive additional “inhibitory” input from the activation of the Cl− channel that is coupled to the EAAT glutamate transporter (Ichinose and Lukasiewicz 2012; Veruki et al. 2006). However, in light-adapted conditions rods are mostly saturated and not significantly responsive to light, so rod BCs should not be depolarized to a significant extent and this EAAT current is not likely to be active. Any inhibition received by a rod BC might be considered as excess, since the rod BC activation is already significantly reduced by rod adaptation (Fig. 4) and only small cone-rod coupling signals remain.
Our results suggest that light-adapted rod BC inhibition is decreased, at least in part, by an increase in the activation of serial GABAAR-mediated synapses between ACs. We have previously shown that serial synapses between ACs are preferentially activated by large light stimuli (Eggers and Lukasiewicz 2010). In this study we only used full-field light stimuli, so we did not test for any differences in the spatial extent of the stimulus. However, the dependence on stimulus size of serial synapse activation suggests that rod BC inhibition could be preferentially activated in light-adapted retinas by a small light stimulus. If this were the case, it could allow a small inhibitory signal to dampen any responses in the rod BCs coming from rod-cone coupling (DeVries and Baylor 1995; Wu and Yang 1988), without requiring a large inhibitory input from wide-field ACs. This is an interesting topic for exploration in a future study.
Another mechanism that could potentially be reflected in our results showing decreases in light-evoked inhibition to rod BCs is dopamine modulation of inhibitory receptors. Light adaptation of the retina leads to increases in levels of dopamine release (Bloomfield and Dacheux 2001; Doyle et al. 2002). Previous studies have shown that GABACR-mediated currents are reduced by dopamine (Dong and Werblin 1994; Wellis and Werblin 1995). Since GABACRs are the primary carriers of current in the rod BCs (Eggers and Lukasiewicz 2006a; Euler and Wässle 1998; Shields et al. 2000), a reduction in GABACR currents could also cause a significant decrease in rod BC inhibition with light adaptation. Additionally, another study has shown that GABAARs can be potentiated by dopamine (Feigenspan and Bormann 1994), which would also serve to decrease inhibition to rod BCs through the enhancement of communication through serial synapses that ordinarily suppress rod BC inhibition (Eggers and Lukasiewicz 2006a, 2010).
We also found that both GABAAR- and glycineR-mediated spontaneous inhibitory inputs to rod BCs were significantly decreased, but not eliminated, by light adaptation. Spontaneous inputs to rod BCs come from both the spontaneous fusion of vesicles of neurotransmitter in the presynaptic ACs that is independent of Ca2+ and the Ca2+-triggered fusion of vesicles that reflect the general state of activity in the presynaptic AC in the absence of a light stimulus. As the rate of non-Ca2+-triggered fusion of vesicles likely does not change with light adaptation state, the decrease in spontaneous release with light adaptation shows that the general depolarization level of ACs releasing GABA and glycine onto rod BCs is decreased with light adaptation. Although the small changes in glycineR sIPSC amplitude could also reflect a postsynaptic change in receptor properties, these changes could also reflect reduced simultaneous fusion of two vesicles of neurotransmitter in the ACs, an additional presynaptic mechanism. A decrease in spontaneous inhibition, in addition to revealing a decrease in the general activity of presynaptic ACs, can also decrease the synaptic “noise” of a cell, which would increase the signal-to-noise ratio. While the light-adapting intensity used here eliminated rod BC L-IPSCs, at more moderately adapting intensities this could be an important mechanism.
Together all of the mechanisms we have discussed here—a decrease in rod pathway-mediated inhibition, an increase in the activation of serial connections between ACs, and a potential decrease in GABACR response due to dopamine—could contribute to a selective decrease in rod BC inhibition in the light-adapted retina, when rods and rod BCs are not active. This could be an additional adaptive mechanism that the retina uses to prioritize cone pathway signaling.
GRANTS
This work was supported by National Institutes of Health Grants EY-018131 (E. D. Eggers) and T32-GM-8400 (R. E. Mazade).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.D.E. conception and design of research; E.D.E. and R.E.M. performed experiments; E.D.E., R.E.M., and J.S.K. analyzed data; E.D.E. interpreted results of experiments; E.D.E. and R.E.M. prepared figures; E.D.E. drafted manuscript; E.D.E., R.E.M., and J.S.K. edited and revised manuscript; E.D.E., R.E.M., and J.S.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank members of the Eggers laboratory for helpful discussion and comments on this manuscript and Adam Bernstein for technical assistance.
REFERENCES
- Arman AC, Sampath AP. Dark-adapted response threshold of OFF ganglion cells is not set by OFF bipolar cells in the mouse retina. J Neurophysiol 107: 2649–2659, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore JF, Copenhagen DR. Different postsynaptic events in two types of retinal bipolar cell. Nature 288: 84–86, 1980 [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Dacheux RF. Rod vision: pathways and processing in the mammalian retina. Prog Retin Eye Res 20: 351–384, 2001 [DOI] [PubMed] [Google Scholar]
- Chavez AE, Diamond JS. Diverse mechanisms underlie glycinergic feedback transmission onto rod bipolar cells in rat retina. J Neurosci 28: 7919–7928, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez AE, Grimes WN, Diamond JS. Mechanisms underlying lateral GABAergic feedback onto rod bipolar cells in rat retina. J Neurosci 30: 2330–2339, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez AE, Singer JH, Diamond JS. Fast neurotransmitter release triggered by Ca influx through AMPA-type glutamate receptors. Nature 443: 705–708, 2006 [DOI] [PubMed] [Google Scholar]
- Copenhagen DR, Ashmore JF, Schnapf JK. Kinetics of synaptic transmission from photoreceptors to horizontal and bipolar cells in turtle retina. Vision Res 23: 363–369, 1983 [DOI] [PubMed] [Google Scholar]
- DeVries SH, Baylor DA. An alternative pathway for signal flow from rod photoreceptors to ganglion cells in mammalian retina. Proc Natl Acad Sci USA 92: 10658–10662, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong CJ, Werblin FS. Dopamine modulation of GABAC receptor function in an isolated retinal neuron. J Neurophysiol 71: 1258–1260, 1994 [DOI] [PubMed] [Google Scholar]
- Dong CJ, Werblin FS. Temporal contrast enhancement via GABAC feedback at bipolar terminals in the tiger salamander retina. J Neurophysiol 79: 2171–2180, 1998 [DOI] [PubMed] [Google Scholar]
- Doyle SE, Grace MS, McIvor W, Menaker M. Circadian rhythms of dopamine in mouse retina: the role of melatonin. Vis Neurosci 19: 593–601, 2002 [DOI] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. GABAA, GABAC and glycine receptor-mediated inhibition differentially affects light-evoked signalling from mouse retinal rod bipolar cells. J Physiol 572: 215–225, 2006a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Receptor and transmitter release properties set the time course of retinal inhibition. J Neurosci 26: 9413–9425, 2006b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Interneuron circuits tune inhibition in retinal bipolar cells. J Neurophysiol 103: 25–37, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, McCall MA, Lukasiewicz PD. Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina. J Physiol 582: 569–582, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Wässle H. Different contributions of GABAA and GABAC receptors to rod and cone bipolar cells in a rat retinal slice preparation. J Neurophysiol 79: 1384–1395, 1998 [DOI] [PubMed] [Google Scholar]
- Feigenspan A, Bormann J. Facilitation of GABAergic signaling in the retina by receptors stimulating adenylate cyclase. Proc Natl Acad Sci USA 91: 10893–10897, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wässle H. Types of bipolar cells in the mouse retina. J Comp Neurol 469: 70–82, 2004 [DOI] [PubMed] [Google Scholar]
- Grunert U, Wässle H. Glycine receptors in the rod pathway of the macaque monkey retina. Vis Neurosci 13: 101–115, 1996 [DOI] [PubMed] [Google Scholar]
- Hartveit E. Reciprocal synaptic interactions between rod bipolar cells and amacrine cells in the rat retina. J Neurophysiol 81: 2923–2936, 1999 [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Muller U, Harvey K, Harvey RJ, Betz H, Wässle H. Diversity of glycine receptors in the mouse retina: localization of the alpha3 subunit. J Comp Neurol 465: 524–539, 2003 [DOI] [PubMed] [Google Scholar]
- Ichinose T, Lukasiewicz PD. The mode of retinal presynaptic inhibition switches with light intensity. J Neurosci 32: 4360–4371, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova E, Muller U, Wässle H. Characterization of the glycinergic input to bipolar cells of the mouse retina. Eur J Neurosci 23: 350–364, 2006 [DOI] [PubMed] [Google Scholar]
- Kim IB, Lee MY, Oh S, Kim KY, Chun M. Double-labeling techniques demonstrate that rod bipolar cells are under GABAergic control in the inner plexiform layer of the rat retina. Cell Tissue Res 292: 17–25, 1998 [DOI] [PubMed] [Google Scholar]
- Menger N, Wässle H. Morphological and physiological properties of the A17 amacrine cell of the rat retina. Vis Neurosci 17: 769–780, 2000 [DOI] [PubMed] [Google Scholar]
- Naarendorp F, Esdaille TM, Banden SM, Andrews-Labenski J, Gross OP, Pugh EN., Jr Dark light, rod saturation, and the absolute and incremental sensitivity of mouse cone vision. J Neurosci 30: 12495–12507, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R, Kolb H. A17: a broad-field amacrine cell in the rod system of the cat retina. J Neurophysiol 54: 592–614, 1985 [DOI] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Paul DL, Wu SM. Rod, M-cone and M/S-cone inputs to hyperpolarizing bipolar cells in the mouse retina. J Physiol 590: 845–854, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Wu SM. Light-evoked current responses in rod bipolar cells, cone depolarizing bipolar cells and AII amacrine cells in dark-adapted mouse retina. J Physiol 558: 897–912, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roska B, Nemeth E, Werblin FS. Response to change is facilitated by a three-neuron disinhibitory pathway in the tiger salamander retina. J Neurosci 18: 3451–3459, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagdullaev BT, McCall MA, Lukasiewicz PD. Presynaptic inhibition modulates spillover, creating distinct dynamic response ranges of sensory output. Neuron 50: 923–935, 2006 [DOI] [PubMed] [Google Scholar]
- Schnapf JL, Copenhagen DR. Differences in the kinetics of rod and cone synaptic transmission. Nature 296: 862–864, 1982 [DOI] [PubMed] [Google Scholar]
- Shields CR, Tran MN, Wong RO, Lukasiewicz PD. Distinct ionotropic GABA receptors mediate presynaptic and postsynaptic inhibition in retinal bipolar cells. J Neurosci 20: 2673–2682, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JH, Diamond JS. Sustained Ca2+ entry elicits transient postsynaptic currents at a retinal ribbon synapse. J Neurosci 23: 10923–10933, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E, Dacheux RF, Raviola E. Cone bipolar cells as interneurons in the rod pathway of the rabbit retina. J Comp Neurol 347: 139–149, 1994 [DOI] [PubMed] [Google Scholar]
- Strettoi E, Dacheux RF, Raviola E. Synaptic connections of rod bipolar cells in the inner plexiform layer of the rabbit retina. J Comp Neurol 295: 449–466, 1990 [DOI] [PubMed] [Google Scholar]
- Veruki ML, Morkve SH, Hartveit E. Activation of a presynaptic glutamate transporter regulates synaptic transmission through electrical signaling. Nat Neurosci 9: 1388–1396, 2006 [DOI] [PubMed] [Google Scholar]
- Wang JS, Kefalov VJ. An alternative pathway mediates the mouse and human cone visual cycle. Curr Biol 19: 1665–1669, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellis DP, Werblin FS. Dopamine modulates GABAc receptors mediating inhibition of calcium entry into and transmitter release from bipolar cell terminals in tiger salamander retina. J Neurosci 15: 4748–4761, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SM, Yang XL. Electrical coupling between rods and cones in the tiger salamander retina. Proc Natl Acad Sci USA 85: 275–278, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Jung CS, Slaughter MM. Serial inhibitory synapses in retina. Vis Neurosci 14: 553–563, 1997 [DOI] [PubMed] [Google Scholar]