Abstract
Sensory systems need to tease out stimulation-evoked activity against a noisy background. In the olfactory system, the odor response profile of an olfactory sensory neuron (OSN) is dependent on the type of odorant receptor it expresses. OSNs also exhibit spontaneous activity, which plays a role in establishing proper synaptic connections and may also increase the sensitivity of the cells. However, where the spontaneous activity originates and whether it informs sensory-evoked activity remain unclear. We addressed these questions by examining patch-clamp recordings of genetically labeled mouse OSNs with defined odorant receptors in intact olfactory epithelia. We show that OSNs expressing different odorant receptors had significantly different rates of basal activity. Additionally, OSNs expressing an inactive mutant I7 receptor completely lacked spontaneous activity, despite being able to fire action potentials in response to current injection. This finding strongly suggests that the spontaneous firing of an OSN originates from the spontaneous activation of its G protein-coupled odorant receptor. Moreover, OSNs expressing the same receptor displayed considerable variation in their spontaneous activity, and the variation was broadened upon odor stimulation. Interestingly, there is no significant correlation between the spontaneous and sensory-evoked activity in these neurons. This study reveals that the odorant receptor type determines the spontaneous firing rate of OSNs, but the basal activity does not correlate with the activity induced by near-saturated odor stimulation. The implications of these findings on olfactory information processing are discussed.
Keywords: spontaneous activity, olfactory sensory neuron, odorant response, odorant receptor, patch clamp
all sensory systems face the challenge of deciphering stimulation-driven activity against a noisy background. In the olfactory system, a major source of basal activity comes from the primary olfactory sensory neurons (OSNs) (Joseph et al. 2012; Phillips et al. 2012). OSNs exhibit basal spontaneous activity in the absence of odorants that is required for correct wiring to the olfactory bulb (Yu et al. 2004), but whether spontaneous activity arises from the olfactory transduction cascade or from some other mechanisms remains unclear.
In rodents, each OSN expresses a single type of G protein-coupled odorant receptor from a repertoire of ∼1,200 (Buck and Axel 1991; Zhang and Firestein 2007), and the receptor type defines the response profile and the central target (glomerulus in the olfactory bulb) of OSNs (Mombaerts 2004; Sakano 2010). Upon odorant binding, odorant receptors activate a signaling cascade through a stimulatory G protein (Golf) and type III adenylyl cyclase (ACIII) to produce cAMP, the second messenger. The elevated cAMP level leads to opening of a cyclic nucleotide-gated (CNG) channel and subsequently a Ca2+-activated Cl− channel to shape the odorant response (Su et al. 2009; Touhara and Vosshall 2009).
Several lines of evidence suggest that the type of odorant receptor expressed heavily influences the spontaneous firing rate of OSNs. In Drosophila, for example, mutations that knock out specific odorant receptors cause significant reductions in spontaneous activity (Grosjean et al. 2011; Larsson et al. 2004). Furthermore, when single odorant receptors were introduced into a mutant neuron without the endogenous receptor, the spontaneous activity was determined by the donor receptor (Hallem et al. 2004). In mouse, inhibiting the odor transduction cascade also inhibits spontaneous activity of sensory neurons in both the main olfactory epithelium and the vomeronasal organ (Arnson and Holy 2011; Reisert 2010). Interestingly, mOR-EG neurons have been found to have significantly lower rates of spontaneous firing compared with M71 or I7 neurons (Reisert 2010). We wished to assess whether spontaneous firing of OSNs originates in the odorant receptors, and how the basal firing rate relates to sensory-evoked activity.
Here we address these questions by measuring the spontaneous and odorant-evoked activity in genetically labeled mouse OSNs with defined odorant receptors in intact olfactory epithelia (Grosmaitre et al. 2006; Ma et al. 1999). Neurons in this preparation closely resemble the natural configuration, with intact cilia, where signal transduction occurs, and with relatively long axons that reach the cribriform plate. We found that OSNs expressing different odorant receptors had significantly different rates of basal firing activity and OSNs with an inactive odorant receptor completely lacked spontaneous activity. Even among OSNs expressing the same receptor, the basal activity showed considerable variation, which became broadened upon odor stimulation, and the basal firing frequency did not correlate with the maximum, sensory-evoked activity. The results suggest that OSNs expressing the same receptor will carry temporally distinct spike trains into their common glomerulus.
MATERIALS AND METHODS
All animal handling procedures conformed to National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania.
Animals.
Several genetically modified mouse lines were used in this study. The transgenic I7(RDY)-IRES-YFP (I7-RDY; IRES = internal ribosome entry site; YFP = yellow fluorescent protein) line (in C57BL/6 genetic background) was generated by Dr. Hitoshi Sakano's lab (Imai et al. 2006) and acquired from the RIKEN Bioresource Center (no. RBRC02933). The transgenic mOR-EG-IRES-gapEGFP line (mOR-EG; in mixed C57BL/6 × DBA2J background) was provided by Dr. Touhara's lab (Oka et al. 2006). Gene-targeted MOR23-IRES-tauGFP (MOR23), SR1-IRES-tauGFP (SR1), M71-IRES-tauGFP (M71), and mI7→M71-IRES-tauGFP (I7; in mixed C57BL/6 × 129 background) mice were provided by Dr. Peter Mombaerts' lab (Bozza et al. 2002; Grosmaitre et al. 2009; Vassalli et al. 2002). Although the mouse lines were generated with embryos from a few different strains, they were all crossed to C57BL/6 mice for breeding. All experiments were performed on postnatal 3- to 6-wk-old mice.
Electrophysiology.
We used the intact olfactory epithelium preparation described previously (Grosmaitre et al. 2006; Ma et al. 1999). Briefly, after deep anesthesia with ketamine-xylazine (200 and 20 mg/kg body wt), mice were quickly decapitated. The nose was dissected en bloc, and the olfactory mucosa was peeled from both sides of the septum and placed in oxygenated Ringer solution [containing (in mM) 124 NaCl, 3 KCl, 1.3 MgSO4, 2 CaCl2, 26 NaHCO3, 1.25 NaH2PO4, 5.5 glucose, and 4.47 sucrose; osmolarity ∼305 mosM and pH ∼7.4 bubbled with 95 O2-5% CO2]. The tissue was placed in a recording chamber with the mucus layer facing up and was continuously perfused with oxygenated Ringer solution at room temperature (23–25°C). Dendritic knobs of individual OSNs were visualized under an upright microscope (Olympus BX51WI) equipped with a CCD camera (Dage-MTI) and a ×40 water-immersive objective with an additional ×4 accessory lens. Recording pipettes for cell-attached patch were made from borosilicate glass (resistance 18–20 MΩ; Sutter Instruments) with a Flaming-Brown puller (Sutter Instruments). The pipette solution contained (in mM) 70 KCl, 53 KOH, 30 methanesulfonic acid, 5 EGTA, 10 HEPES, and 70 sucrose; pH ∼7.2, osmolarity ∼310 mosM. Recordings were started after the pipette formed a gigaseal with the cell membrane, and the signals were filtered at 5 kHz and sampled at 10 kHz. For perforated patch, 260 μM nystatin was included in the pipette solution. Under current-clamp mode, the signals were filtered at 2.9 kHz and sampled at 5 kHz. Further filtering off-line at 1.5 kHz did not change the response kinetics or amplitudes.
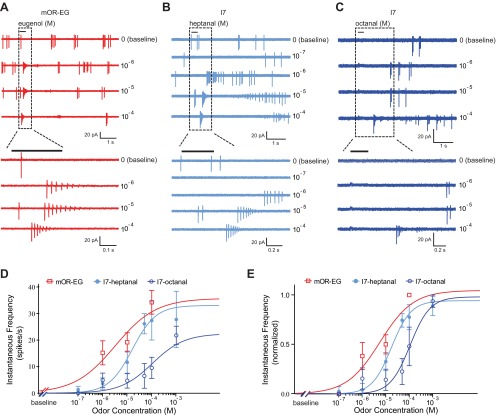
Odorants were delivered by Picospritzer (Toohey) at 20 psi through a multibarrel pipette, usually placed 25 μm away from the recording site. However, because I7 neurons responded to odor-free Ringer solution puffs under this condition, we had to move the puffing pipette to ∼70 μm away to eliminate the mechanical responses elicited by pressure ejection (Grosmaitre et al. 2007). This caused an additional delay (∼0.3 s) in the responses of I7 neurons, but it should not affect the firing rates induced by near-saturated odor stimulation for the following two reasons. First, odor puffs covered a relatively large area (∼200 μm in diameter), visualized by puffing food color solution. Second, increasing the heptanal concentration from 10−4 M to 10−3 M did not increase the responses of I7 neurons (see Fig. 3, D and E).
Fig. 3.
mOR-EG and I7 neurons respond with similar bursts of action potentials after stimulation with their respective ligands. A: an mOR-EG neuron fired bursts of action potentials with increasing instantaneous frequencies in response to increasing concentration of the ligand eugenol. B: an I7 neuron responded to increasing concentration of the primary ligand heptanal. C: an I7 neuron responded less sensitively to the secondary ligand octanal. Areas demarcated by rectangles are shown with expanded timescale at bottom. D: dose-response curves are fit with the Hill equation for mOR-EG (n = 14) and I7 (n = 9 for heptanal and n = 8 for octanal) neurons. The baseline instantaneous firing rate was subtracted from the sensory-evoked firing rates in each cell. E: the same data from D with responses from each cell normalized to the cell's maximum instantaneous firing rate. Note that the data point of mOR-EG neurons to 10−4 M eugenol loses the error bars because the largest response in each mOR-EG neuron occurred at this concentration. For I7 neurons, the maximum response was not always evoked by the highest concentration; therefore the error bars remain.
All odorants were dissolved in DMSO as a 0.5 M stock solution, which was then aliquoted and added to Ringer solution before the experiments to make up the final concentrations. Chemicals and odorants were purchased from Sigma-Aldrich.
Data analysis.
Data were acquired with an EPC-10 amplifier run by Pulse software (Heka Instruments, Lambrecht/Pfalz, Germany). Analysis of firing rates was performed with a custom program written in IGOR (WaveMetrics). Odorant responses were identified and quantified with a modified method described previously (Rospars et al. 2003; Tan et al. 2010). This method takes into account the period of inactivity in OSNs that typically follows the initial burst in response to odorants (Rospars et al. 2003). Briefly, basal firing frequency was defined as the mean frequency of one or more 30- to 60-s recording epochs for each OSN (up to 15 min). Instantaneous frequency was defined as the inverse of the interspike interval (ISI) between consecutive action potentials (2nd to 1st, 3rd to 2nd, and so forth). Basal instantaneous frequency of an OSN was defined as the average of the individual instantaneous frequencies over the entire recording epoch (1–15 min) and is reported as spikes per second. An odorant response was identified for a particular trial if the instantaneous frequency of three or more continuous spikes exceeded the threshold (median instantaneous frequency + 1.5 spikes/s determined from the preceding 30- to 60-s epoch) within 2 s after onset of odorant delivery. Odorant responses are reported as the mean of the individual instantaneous frequencies in the response (consecutive spikes with an instantaneous frequency above threshold). If no action potentials met response criteria during an odor trial, then the averaged instantaneous frequency over the 10-s window following odor delivery was assigned as the “response” for that trial. Dose-response curves were fit by the Hill equation, F = Fmax/[1 + (K1/2/C)nH], where F represents odor-induced response (measured by the instantaneous firing frequency after subtracting the baseline activity), Fmax the maximum response at near-saturating concentrations, K1/2 the concentration at which half of the maximum response was reached, C the concentration of odorant, and nH the Hill coefficient. Statistical analysis was performed in GraphPad Prism.
RESULTS
Spontaneous activity differs among OSNs expressing different odorant receptors.
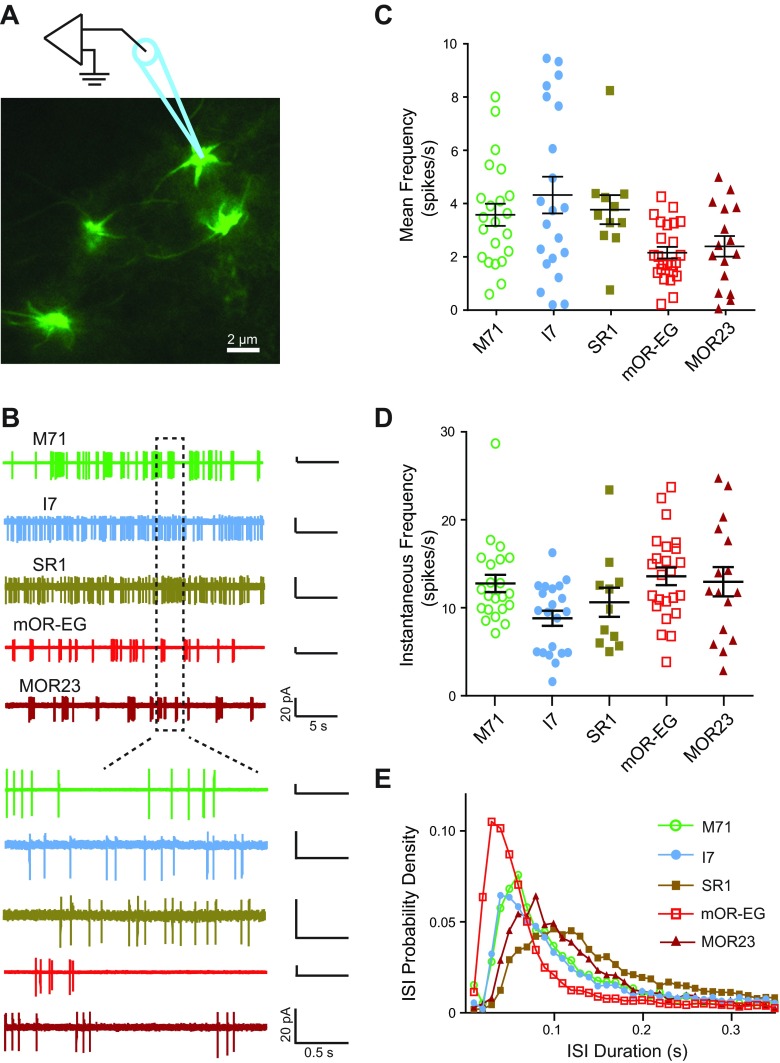
If odorant receptor type determines spontaneous activity, then neurons expressing different receptors should be distinguishable by the basal firing frequency. A previous report from dissociated OSNs suggests that this is the case, as I7 and M71 neurons exhibited higher rates of spontaneous activity than mOR-EG neurons (Reisert 2010). In addition to potential damage to the cilia and axons, dissociation of OSNs also removes olfactory cells from the surrounding sustentacular and microvillar cells, which potentially influence OSN firing rates (Hegg et al. 2009, 2010; Ogura et al. 2011). We therefore examined OSNs situated in their natural configuration in the intact olfactory epithelia (Fig. 1A). We performed cell-attached patch recordings from the dendritic knobs of OSNs that expressed odorant receptors M71 (n = 22), I7 (n = 21), SR1 (n = 11), mOR-EG (n = 24), or MOR23 (n = 16) (Fig. 1B). For each cell, the firing frequency and instantaneous frequency were averaged from 30- to 60-s epochs during the entire recording period (up to 15 min). We found a significant difference among the mean firing frequencies for OSNs expressing different odorant receptors (Fig. 1C; ANOVA: F = 4.135, P = 0.004; SR1 = 3.79 ± 0.57 Hz, I7 = 4.34 ± 0.69 Hz, M71 = 3.60 ± 0.57 Hz, mOR-EG = 2.18 ± 0.22 Hz, MOR23 = 2.42 ± 0.39 Hz). Between groups, I7 neurons had significantly higher frequencies than both mOR-EG and MOR23 neurons as determined by post hoc Tukey test (P < 0.005 and P < 0.05, respectively). We also found a significant difference in the instantaneous frequencies among OSNs (Fig. 1, D and E; ANOVA: F = 3.118, P = 0.02; SR1 = 10.73 ± 1.7 Hz, I7 = 8.89 ± 0.86 Hz, M71 = 12.87 ± 1.01 Hz, mOR-EG = 13.68 ± 1.01 Hz, MOR23 = 13.06 ± 1.7 Hz). The instantaneous frequency in I7 neurons again differed significantly from that recorded in mOR-EG neurons (P < 0.05, Tukey test). The higher instantaneous frequency in mOR-EG neurons reflects the fact that bursting was more prevalent in these than in I7 neurons (Fig. 1B), even though the overall firing rates were lower for mOR-EG cells (Fig. 1C). These data confirm that OSNs expressing different odorant receptors exhibit different spontaneous firing frequencies.
Fig. 1.
Olfactory sensory neurons (OSNs) genetically labeled for specific odorant receptor types display different spontaneous activity patterns. A: green fluorescent protein (GFP)-labeled MOR23 neurons (knobs and cilia) in an intact olfactory epithelium were visualized under fluorescent illumination. Schematic drawing shows patch-clamp electrode placement. B, top: spontaneous activities were recorded from M71, I7, SR1, mOR-EG, and MOR23 neurons in cell-attached configuration. Bottom: areas demarcated by rectangles are shown with expanded timescale. C: mean firing frequency of spontaneous activity is summarized for OSNs of each receptor type. D: instantaneous firing frequency is summarized for OSNs of each receptor type. In C and D, each symbol represents data from a single cell; black lines with error bars represent the mean ± SE for each cell type. E: probability density function of interspike intervals (ISIs) is plotted for each receptor type (bin = 0.01 s).
Disruption of odorant receptor-G protein coupling eliminates spontaneous activity in mutant I7 OSNs.
The differences in spontaneous activity recorded from OSNs expressing specific receptors suggest that receptor activity determines spontaneous firing. However, one alternative explanation is that downstream mechanisms such as spontaneous activation of Golf or ACIII contribute to the activity. To more definitively assess whether the receptor itself determines spontaneous activity, we performed cell-attached and perforated patch clamp on OSNs expressing a mutant receptor that is unable to activate G proteins.
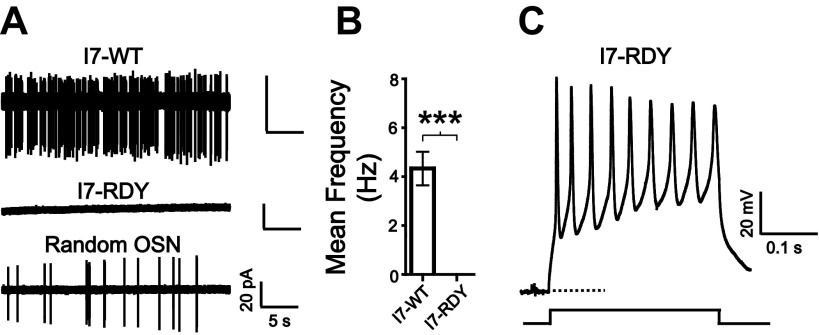
Odorant receptors are type A G protein-coupled receptors, which contain a conserved DRY (Asp-Arg-Tyr) motif near the cytoplasmic end of transmembrane domain III that is required for coupling of the receptors to their G proteins (Kobilka and Deupi 2007; Scheer et al. 1996). In a previous study, OSNs expressing a mutant I7 receptor in which the DRY sequence was changed to RDY (I7-RDY) resulted in loss of odorant-induced responses and disrupted axon targeting and synapse formation in the olfactory bulb (Imai et al. 2006).
We performed cell-attached recording on I7-RDY OSNs to determine whether these neurons had spontaneous activity. In sharp contrast to wild-type I7 neurons (I7-WT), which fired at ∼4 Hz (Fig. 2A, top, and Fig. 2B), we never detected a single spontaneous spike from the I7-RDY neurons (n = 34; Fig. 2A, middle, and Fig. 2B). Spontaneous spikes were readily detectable in randomly selected OSNs from the same olfactory epithelium of an I7-RDY mouse under identical conditions (Fig. 2A, bottom). Additionally, separate perforated patch-clamp recordings demonstrated that I7-RDY neurons were healthy and capable of activity, as they exhibited typical voltage-gated ionic currents and consistently fired action potentials in response to depolarizing currents (n = 6; Fig. 2C). These experiments provide strong evidence that the coupling between odorant receptor and G protein is required for spontaneous firing in OSNs.
Fig. 2.
Disruption of receptor-G protein interaction in I7-RDY neurons eliminates spontaneous activity. A, top: a wild-type I7 (I7-WT) neuron exhibited spontaneous firing in a cell-attached patch recording. Middle: an I7-RDY neuron exhibited no spontaneous firing under the same recording configuration. Bottom: a random non-I7-RDY neuron from the same epithelium showed spontaneous firing. B: summary of the mean (±SE) firing rates observed in I7-WT and I7-RDY neurons. ***P < 0.0001, nonparametric Mann-Whitney test. C: I7-RDY neurons are capable of firing action potentials in response to current injection in perforated patch-clamp recording. Dashed line marks −60 mV.
Basal activity of OSNs does not correlate with maximum firing rate upon odor stimulation.
Spontaneous activity in I7 and mOR-EG neurons was significantly different by measures of firing frequency and instantaneous frequency. These differences in background noise could have implications for the sensitivity of the receptors to odorants. We investigated the dose-response relationship of I7 and mOR-EG neurons in response to their respective preferred ligands, heptanal and eugenol, as well as I7 responses to the secondary ligand octanal, to see whether and how spontaneous activity correlates with sensory-evoked activity.
We assessed the dose-response relationship by measuring the instantaneous frequency of OSNs in a manner reported previously (Tan et al. 2010). Instantaneous frequency is a more accurate measure of odorant responses than mean firing frequency because, at higher concentrations, the initial short burst of action potentials is followed by a period of silence (Fig. 3, A–C). Presumably the reduction in action potentials is due to a current shunt (a decreased membrane resistance) caused by the transduction current and/or progressive inactivation of voltage-gated Na+ channels caused by depolarization (Lynch and Barry 1991; Narusuye et al. 2003; Trotier and MacLeod 1983).
We delivered the ligand eugenol to a subset of mOR-EG neurons and the primary ligand heptanal to I7 neurons, each at concentrations ranging from 10−7 to 10−3 M (Fig. 3, A and B). Note that I7 neurons had longer response latencies because the puffing pipettes had to be placed farther away from the recording site to eliminate pressure-induced mechanical responses in these neurons (see materials and methods). Such mechanical responses were negligible in mOR-EG neurons. Because mOR-EG and I7 neurons exhibited different basal firing rates, we plotted the dose-response curves by subtracting the baseline firing rate from odor-induced responses in each cell (Fig. 3, D and E). At a low concentration (10−6 M), mOR-EG neurons showed higher instantaneous frequencies to eugenol than I7 responses to heptanal (P = 0.03, unpaired t-test), presumably reflecting the differences in their sensitivities to the ligands. Interestingly, with increasing concentrations the differences in mOR-EG and I7 responses became nonsignificant (P = 0.20 for 10−5 M and P = 0.60 for 10−4 M, unpaired t-test). As expected, the I7 responses to octanal showed less sensitivity than the responses to the primary ligand heptanal (Fig. 3, C–E).
While the average dose-response curves between mOR-EG and I7 neurons were similar, it is possible that individual neurons might differ in their odorant responses, since there are considerable variations in basal activity within OSNs expressing the same odorant receptor (Fig. 1, C and D). Would the OSNs with the highest basal firing rates also respond with the highest firing rates to its respective ligand? Is there an upper limit of sensory-evoked firing rates correlated with the basal activity?
To address these questions, we compared the spontaneous firing rate and the maximum sensory-evoked firing rate for individual I7 and mOR-EG neurons. Although mOR-EG neurons were predominantly rhythmically active while I7 neurons were tonically active at rest (Fig. 1A), they showed similar firing patterns upon odor stimulation (Fig. 4A). Within each subgroup, we found that neurons responded heterogeneously to odorants (Fig. 4, A and B). That is, responses did not converge toward a maximum for a receptor type, nor did low- or high-firing neurons respond in any discernible pattern. Indeed, in more than one case, neurons that had nearly identical instantaneous frequencies at baseline responded very differently when stimulated by odorants. We plotted the instantaneous firing frequency induced by near-saturating concentrations of ≥10−4 M (after subtracting the basal activity) versus the baseline spontaneous firing rate for each neuron (Fig. 4C). There was no significant correlation within each of the three subgroups, including mOR-EG neurons to eugenol (r = −0.04, n = 14, P = 0.904), I7 to heptanal (r = 0.43, n = 9, P = 0.242), and I7 to octanal (r = −0.35, n = 8, P = 0.400, Pearson correlation test, 2-tailed). When all cells in these three groups were pooled together, there was still no significant correlation (r = 0.11, n = 31, P = 0.57; nonparametric Spearman correlation test was used here because of the 2 distinct subgroups of I7 and mOR-EG neurons). These data suggest that the spontaneous firing rate does not correlate with the maximum firing rate of OSNs in response to near-saturated odor stimulation.
Fig. 4.
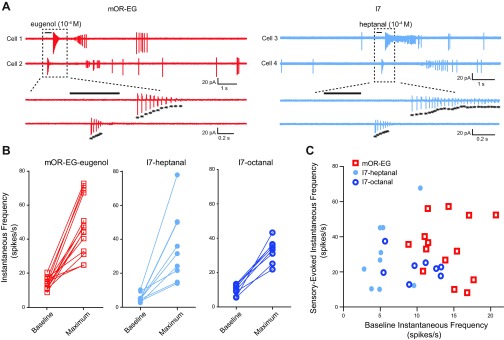
Baseline firing rates do not correlate with the maximum, sensory-evoked firing rates. A: 2 mOR-EG (left; cell 1 and cell 2) and 2 I7 (right; cell 3 and cell 4) neurons exhibited heterogeneous responses to a near-saturating concentration (10−4 M) of eugenol and heptanal, respectively. Areas demarcated by rectangles are shown on expanded timescale at bottom; asterisks mark individual action potentials. B: spontaneous and maximum sensory-evoked instantaneous firing rates are summarized for mOR-EG neurons to eugenol (left), I7 neurons to heptanal (center), and I7 neurons to octanal (right). Solid lines connect data from single cells. C: the sensory-induced instantaneous frequency with the baseline frequency subtracted is plotted against the basal activity for each I7 and mOR-EG neuron in B.
DISCUSSION
Using patch-clamp recordings from in situ OSNs in the intact olfactory epithelia, we show that odorant receptors are the source for spontaneous activity in OSNs. This conclusion is based on the following evidence: 1) the basal firing frequency of OSNs varies by the receptor type expressed (Fig. 1) and 2) disruption of odorant receptor-G protein coupling eliminates spontaneous activity in OSNs (Fig. 2). We further demonstrate that the baseline firing rate is not correlated with the maximum firing rate induced by near-saturated odor stimulation (Fig. 3 and Fig. 4).
These experiments extend previous findings by establishing the odorant receptors as the source of spontaneous activity in rodent OSNs, as in insect OSNs. It has been shown that spontaneous activity of OSNs is receptor dependent in Drosophila by knocking out receptors in sensilla (Grosjean et al. 2011; Larsson et al. 2004). Likewise, recent work in locust shows that knocking out receptor activity by cooling the antenna eliminates spontaneous activity (Joseph et al. 2012). In rodents, however, experiments have not been so conclusive. Knocking out the downstream CNG channel resulted in no significant change in basal firing (Brunet et al. 1996). Recordings in ACIII knockouts also showed no reduction in baseline activity (Wong et al. 2000). Even in Golf knockouts, spontaneous activity persisted (Belluscio et al. 1998), as Gs likely compensates for Golf in these neurons because of its remarkable similarity. In contrast, a recent study on dissociated mouse OSNs reported a significantly lower firing rate in mOR-EG neurons compared with firing rates in I7 and M71 neurons and reported that the spontaneous activity could be reduced by application of inhibitory odorants (Reisert 2010). However, it could not be ruled out that spontaneous activation of the signaling proteins and/or channels such as G protein and ACIII contributed to the basal activity. Here, we add to those findings to provide convincing evidence that the receptor-G protein interaction is the source of spontaneous activity in OSNs.
Our results suggest that, while the odorant receptor is the source of spontaneous activity, basal firing can be independent of odor-evoked signaling pathways, as in the Golf, ACIII, or CNG knockouts. Interestingly, it has been shown that spontaneous activity is as important as odor-evoked activity when it comes to developing and maintaining synapses between OSNs and the olfactory bulb neurons (Yu et al. 2004), highlighting that the two signals might yield independent information. These results are consistent with recent studies implicating the involvement of the hyperpolarization-activated cyclic nucleotide-gated (HCN) ion channel, rather than the CNG channel, in mediating spontaneous activity and glomerular formation (Mobley et al. 2010; Nakashima et al. 2013). Our study suggests that the odorant receptor itself is required for maintaining the basal cAMP concentration that determines spontaneous activity. Spontaneous activation of odorant receptors could preferentially trigger an alternate G protein cascade other than the canonical Golf-cAMP odor cascade, perhaps through Gs-cAMP activation of the HCN channel (Nakashima et al. 2013). Curiously, the spontaneous activity of OSNs is reduced by niflumic acid, which blocks the Ca2+-activated Cl− channel, a target downstream of the CNG channel in the canonical transduction pathway (Reisert 2010). It is plausible that Ca2+ influx through HCN channels could also activate the same Cl− channel, which would contribute to the spontaneous firing of OSNs. This could explain why the spontaneous firing of OSNs is reduced by blocking the Cl− channel (Reisert 2010) but is not affected by knocking out the CNG channel (Brunet et al. 1996).
An alternative mechanism underlying the difference in spontaneous firing between I7-RDY neurons and OSNs lacking Golf, ACIII, or CNG channel is homeostatic processes, perhaps originating from the olfactory bulb, that could alter voltage-gated channel distributions, such as increasing sodium channel density, in response to global deficits in signaling (as in Golf, ACIII, or CNG knockouts) but not in response to deficits in only a few OSN types (as in I7-RDY neurons). Additional noncanonical targets of spontaneous activity and/or homeostatic mechanisms that could influence development and maintenance of OSN synapses include retinoic acid receptors (Oztokatli et al. 2012) and the β-site amyloid precursor protein cleaving enzyme 1 (Cao et al. 2012; Rajapaksha et al. 2011).
The firing rates we recorded are similar to those reported in freely breathing and tracheotomized rats (Duchamp-Viret et al. 2005) but are higher than those reported in dissociated OSNs (Reisert 2010). The fact that mOR-EG neurons have a relatively low firing rate compared with most other OSNs, particularly I7 neurons, is largely in agreement with a previous study (Reisert 2010). Interestingly, the firing rates observed here differ from those recorded in dissociated OSNs by merely adding a constant number (between 2.2 and 2.4 Hz for I7, M71, and mOR-EG neurons). The discrepancy in overall firing rates is likely attributed to the different preparations and recording configurations. In the intact olfactory epithelial preparations OSN cilia and axons remain relatively undisturbed in their natural configuration, whereas in dissociated preparations OSNs are isolated from surrounding sustentacular and microvillar cells, which may affect firing (Hegg et al. 2009, 2010; Ogura et al. 2011). It has been shown in perforated-patch recordings that OSNs in intact epithelium respond to their respective ligands 100% of the time (Grosmaitre et al. 2006), while the yield in dissociated OSNs is appreciably lower, suggesting that the intact epithelial preparation keeps OSNs healthier and is more physiologically relevant. It is also worth noting that all ex vivo preparations are probably kept in solutions different from that found in the natural environment, which may have some effects on spontaneous activity.
It is evident that OSNs (even those with the same receptor) show considerable variation in their basal activity (Fig. 1), which will introduce noise to the olfactory system. Surprisingly, the variation in firing rates among OSNs is further broadened upon odor stimulation (Fig. 4). One way to counter this noise would be through temporal and/or population averaging, and the structure of the olfactory system exhibits a remarkable convergence of thousands of OSNs expressing the same odorant receptor onto a single glomerulus innervated by ∼25 mitral/tufted cells. A previous study using two-photon microscopy to measure Ca2+ signals from axonal terminals of OSNs reveals that the activity pattern within a single glomerulus is spatially heterogeneous with small “hot spots” (Wachowiak et al. 2004). However, the time course of odor-induced Ca2+ signals is quite similar from different hot spots throughout a glomerulus, suggesting that temporal and/or population averaging occurs in these Ca2+ signals. Odor stimulation also induces uncorrelated fluctuations in adjacent hot spots, which may reflect the calcium influx induced by different firing patterns in individual OSNs. Recent studies suggest that, rather than interfering with information encoding, such noise can increase sensitivity to subthreshold weak stimuli (Ermentrout et al. 2008). For example, if a single OSN synapses onto a subset of mitral/tufted cells innervating the same glomerulus, the divergent firing rates of the input OSNs may bring temporally distinct spike trains to the associated mitral/tufted cells. The observed divergence in the firing rates of OSNs with the same receptor type would then broaden the dynamic range of individual glomeruli and the associated mitral/tufted cells in response to odorants. Indeed, there is evidence of such an increased dynamic range in Drosophila, where glomerular projection neurons (similar to mitral cells) fire with more sensitivity and reliability in response to a wider range of odorants than the olfactory receptor neurons that feed into them (Bhandawat et al. 2007). Additionally, a recent study in mice demonstrates that the spike timing of the mitral/tufted cells innervating the same glomerulus is differentially altered by odor stimulation (Dhawale et al. 2010). Further studies are required to understand how the olfactory system processes the temporally distinct firing patterns carried by OSNs expressing the same receptor.
GRANTS
This work was supported by National Institute on Deafness and Other Communication Disorders R01 Grants DC-006213 and DC-011554.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.C., A.S., and M.M. conception and design of research; T.C. and A.S. performed experiments; T.C. and A.S. analyzed data; T.C., A.S., and M.M. interpreted results of experiments; T.C. and M.M. prepared figures; T.C. and M.M. drafted manuscript; T.C. and M.M. edited and revised manuscript; T.C., A.S., and M.M. approved final version of manuscript.
REFERENCES
- Arnson HA, Holy TE. Chemosensory burst coding by mouse vomeronasal sensory neurons. J Neurophysiol 106: 409–420, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluscio L, Gold GH, Nemes A, Axel R. Mice deficient in Golf are anosmic. Neuron 20: 69–81, 1998 [DOI] [PubMed] [Google Scholar]
- Bhandawat V, Olsen SR, Gouwens NW, Schlief ML, Wilson RI. Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nat Neurosci 10: 1474–1482, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza T, Feinstein P, Zheng C, Mombaerts P. Odorant receptor expression defines functional units in the mouse olfactory system. J Neurosci 22: 3033–3043, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet LJ, Gold GH, Ngai J. General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron 17: 681–693, 1996 [DOI] [PubMed] [Google Scholar]
- Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell 65: 175–187, 1991 [DOI] [PubMed] [Google Scholar]
- Cao L, Rickenbacher GT, Rodriguez S, Moulia TW, Albers MW. The precision of axon targeting of mouse olfactory sensory neurons requires the BACE1 protease. Sci Rep 2: 231, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawale AK, Hagiwara A, Bhalla US, Murthy VN, Albeanu DF. Non-redundant odor coding by sister mitral cells revealed by light addressable glomeruli in the mouse. Nat Neurosci 13: 1404–1412, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchamp-Viret P, Kostal L, Chaput M, Lansky P, Rospars JP. Patterns of spontaneous activity in single rat olfactory receptor neurons are different in normally breathing and tracheotomized animals. J Neurophysiol 65: 97–114, 2005 [DOI] [PubMed] [Google Scholar]
- Ermentrout GB, Galan RF, Urban NN. Reliability, synchrony and noise. Trends Neurosci 31: 428–434, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean Y, Rytz R, Farine JP, Abuin L, Cortot J, Jefferis GS, Benton R. An olfactory receptor for food-derived odours promotes male courtship in Drosophila. Nature 478: 236–240, 2011 [DOI] [PubMed] [Google Scholar]
- Grosmaitre X, Fuss SH, Lee AC, Adipietro KA, Matsunami H, Mombaerts P, Ma M. SR1, a mouse odorant receptor with an unusually broad response profile. J Neurosci 29: 14545–14552, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosmaitre X, Santarelli LC, Tan J, Luo M, Ma M. Dual functions of mammalian olfactory sensory neurons as odor detectors and mechanical sensors. Nat Neurosci 10: 348–354, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosmaitre X, Vassalli A, Mombaerts P, Shepherd GM, Ma M. Odorant responses of olfactory sensory neurons expressing the odorant receptor MOR23: a patch clamp analysis in gene-targeted mice. Proc Natl Acad Sci USA 103: 1970–1975, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem EA, Ho MG, Carlson JR. The molecular basis of odor coding in the Drosophila antenna. Cell 117: 965–979, 2004 [DOI] [PubMed] [Google Scholar]
- Hegg CC, Irwin M, Lucero MT. Calcium store-mediated signaling in sustentacular cells of the mouse olfactory epithelium. Glia 57: 634–644, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegg CC, Jia C, Chick WS, Restrepo D, Hansen A. Microvillous cells expressing IP3 receptor type 3 in the olfactory epithelium of mice. Eur J Neurosci 32: 1632–1645, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T, Suzuki M, Sakano H. Odorant receptor-derived cAMP signals direct axonal targeting. Science 314: 657–661, 2006 [DOI] [PubMed] [Google Scholar]
- Joseph J, Dunn FA, Stopfer M. Spontaneous olfactory receptor neuron activity determines follower cell response properties. J Neurosci 32: 2900–2910, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilka BK, Deupi X. Conformational complexity of G-protein-coupled receptors. Trends Pharmacol Sci 28: 397–406, 2007 [DOI] [PubMed] [Google Scholar]
- Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43: 703–714, 2004 [DOI] [PubMed] [Google Scholar]
- Lynch JW, Barry PH. Properties of transient K+ currents and underlying single K+ channels in rat olfactory receptor neurons. J Gen Physiol 97: 1043–1072, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Chen WR, Shepherd GM. Electrophysiological characterization of rat and mouse olfactory receptor neurons from an intact epithelial preparation. J Neurosci Methods 92: 31–40, 1999 [DOI] [PubMed] [Google Scholar]
- Mobley AS, Miller AM, Araneda RC, Maurer LR, Muller F, Greer CA. Hyperpolarization-activated cyclic nucleotide-gated channels in olfactory sensory neurons regulate axon extension and glomerular formation. J Neurosci 30: 16498–16508, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P. Genes and ligands for odorant, vomeronasal and taste receptors. Nat Rev Neurosci 5: 263–278, 2004 [DOI] [PubMed] [Google Scholar]
- Nakashima N, Ishii TM, Bessho Y, Kageyama R, Ohmori H. Hyperpolarisation-activated cyclic nucleotide-gated channels regulate the spontaneous firing rate of olfactory receptor neurons and affect glomerular formation in mice. J Physiol 591: 1749–1769, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narusuye K, Kawai F, Miyachi E. Spike encoding of olfactory receptor cells. Neurosci Res 46: 407–413, 2003 [DOI] [PubMed] [Google Scholar]
- Ogura T, Szebenyi SA, Krosnowski K, Sathyanesan A, Jackson J, Lin W. Cholinergic microvillous cells in the mouse main olfactory epithelium and effect of acetylcholine on olfactory sensory neurons and supporting cells. J Neurophysiol 106: 1274–1287, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka Y, Katada S, Omura M, Suwa M, Yoshihara Y, Touhara K. Odorant receptor map in the mouse olfactory bulb: in vivo sensitivity and specificity of receptor-defined glomeruli. Neuron 52: 857–869, 2006 [DOI] [PubMed] [Google Scholar]
- Oztokatli H, Hornberg M, Berghard A, Bohm S. Retinoic acid receptor and CNGA2 channel signaling are part of a regulatory feedback loop controlling axonal convergence and survival of olfactory sensory neurons. FASEB J 26: 617–627, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ME, Sachdev RN, Willhite DC, Shepherd GM. Respiration drives network activity and modulates synaptic and circuit processing of lateral inhibition in the olfactory bulb. J Neurosci 32: 85–98, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapaksha TW, Eimer WA, Bozza TC, Vassar R. The Alzheimer's beta-secretase enzyme BACE1 is required for accurate axon guidance of olfactory sensory neurons and normal glomerulus formation in the olfactory bulb. Mol Neurodegener 6: 88, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J. Origin of basal activity in mammalian olfactory receptor neurons. J Gen Physiol 136: 529–540, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rospars JP, Lansky P, Duchamp A, Duchamp-Viret P. Relation between stimulus and response in frog olfactory receptor neurons in vivo. Eur J Neurosci 18: 1135–1154, 2003 [DOI] [PubMed] [Google Scholar]
- Sakano H. Neural map formation in the mouse olfactory system. Neuron 67: 530–542, 2010 [DOI] [PubMed] [Google Scholar]
- Scheer A, Fanelli F, Costa T, De Benedetti PG, Cotecchia S. Constitutively active mutants of the alpha 1B-adrenergic receptor: role of highly conserved polar amino acids in receptor activation. EMBO J 15: 3566–3578, 1996 [PMC free article] [PubMed] [Google Scholar]
- Su CY, Menuz K, Carlson JR. Olfactory perception: receptors, cells, and circuits. Cell 139: 45–59, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Savigner A, Ma M, Luo M. Odor information processing by the olfactory bulb analyzed in gene-targeted mice. Neuron 65: 912–926, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touhara K, Vosshall LB. Sensing odorants and pheromones with chemosensory receptors. Annu Rev Physiol 71: 307–332, 2009 [DOI] [PubMed] [Google Scholar]
- Trotier D, MacLeod P. Intracellular recordings from salamander olfactory receptor cells. Brain Res 268: 225–237, 1983 [DOI] [PubMed] [Google Scholar]
- Vassalli A, Rothman A, Feinstein P, Zapotocky M, Mombaerts P. Minigenes impart odorant receptor-specific axon guidance in the olfactory bulb. Neuron 35: 681–696, 2002 [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Denk W, Friedrich RW. Functional organization of sensory input to the olfactory bulb glomerulus analyzed by two-photon calcium imaging. Proc Natl Acad Sci USA 101: 9097–9102, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ST, Trinh K, Hacker B, Chan GC, Lowe G, Gaggar A, Xia Z, Gold GH, Storm DR. Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron 27: 487–497, 2000 [DOI] [PubMed] [Google Scholar]
- Yu CR, Power J, Barnea G, O'Donnell S, Brown HE, Osborne J, Axel R, Gogos JA. Spontaneous neural activity is required for the establishment and maintenance of the olfactory sensory map. Neuron 42: 553–566, 2004 [DOI] [PubMed] [Google Scholar]
- Zhang X, Firestein S. Comparative genomics of odorant and pheromone receptor genes in rodents. Genomics 89: 441–450, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]