Abstract
The insular cortex (IC) is widely believed to be an important forebrain structure involved in cognitive and sensory processes such as memory and pain. However, little work has been performed at the cellular level to investigate the synaptic basis of IC-related brain functions. To bridge the gap, the present study was designed to characterize the basic synaptic mechanisms for insular long-term potentiation (LTP). Using a 64-channel recording system, we found that an enduring form of late-phase LTP (L-LTP) could be reliably recorded for at least 3 h in different layers of IC slices after theta burst stimulation. The induction of insular LTP is protein synthesis dependent and requires activation of both GluN2A and GluN2B subunits of the NMDA receptor, L-type voltage-gated calcium channels, and metabotropic glutamate receptor 1. The paired-pulse facilitation ratio was unaffected by insular L-LTP induction, and expression of insular L-LTP required the recruitment of postsynaptic calcium-permeable AMPA receptors. Our results provide the first in vitro report of long-term multichannel recordings of L-LTP in the IC in adult mice and suggest its potential important roles in insula-related memory and chronic pain.
Keywords: long-term potentiation, insular cortex, multielectrode array, glutamate receptor, calcium-permeable AMPA receptor
insular cortex (IC) is an integrating forebrain structure involved in several sensory and cognitive processes, such as learning, memory, and sensory perception (Apkarian et al. 2011; Bermudez-Rattoni 2004; Craig 2011; Gal-Ben-Ari and Rosenblum 2012). Both electrophysiological recordings in experimental animals (Hanamori et al. 1998; Ogawa and Wang 2002) and brain imaging studies in human subjects (Brooks et al. 2005; Henderson et al. 2007; Schweinhardt et al. 2006) have consistently indicated important roles of the IC in pain processing. Additionally, the IC has also been implicated in the acquisition and storage of different learning and memory tasks such as conditioned taste aversion (CTA), novel taste learning, inhibitory avoidance, and object recognition memory (Berman and Dudai 2001; Bermudez-Rattoni et al. 1991, 2005; Yefet et al. 2006). Signaling mechanisms underlying aversive taste memory consolidation in the IC have been reported (for reviews, see Adaikkan and Rosenblum 2012; Guzman-Ramos and Bermudez-Rattoni 2011; Rosenblum 2008). However, less information is available regarding the synaptic basis of IC-mediated sensory and cognitive functions.
Long-term potentiation (LTP), a cellular model of memory and chronic pain (Bliss and Collingridge 1993; Citri and Malenka 2008; Sacktor 2012; Zhuo 2007, 2008), has been widely studied in a broad range of brain regions, such as hippocampus, amygdala, and neocortex (Rioult-Pedotti 2000; Rogan et al. 1997; Whitlock et al. 2006). Previous studies using the in vivo recording technique have reported the existence of LTP in the basolateral amygdala (BLA)-IC pathway, which is NMDA receptor dependent and related to behavioral performance in the CTA test (Escobar et al. 1998, 2002; Escobar and Bermudez-Rattoni 2000; Jones et al. 1999; Rodriguez-Duran et al. 2011). Our recent work in the anterior cingulate cortex (ACC), using both in vivo and in vitro electrophysiological recordings, demonstrates that excitatory synapses are highly plastic in adult animals and postsynaptic AMPA receptors contribute to the expression of cingulate LTP (for review, see Zhuo 2007, 2008). Unlike the ACC, however, little work has been performed on the basic mechanisms of in vitro insular LTP, including the possibility of late-phase LTP (L-LTP) induction.
In the present study, we wanted to investigate whether synaptic transmission in the IC can undergo L-LTP. Using a 64-channel multielectrode dish (MED64) recording system (Kang et al. 2012; Li et al. 2010), we were able to not only record both early LTP (E-LTP) and L-LTP (at least 3 h) but also monitor LTP spatially in both superficial and deep layers of the IC. Moreover, we demonstrate that theta burst stimulation (TBS) can induce protein synthesis-dependent insular LTP that requires multiple receptors/ion channels for its production. We found a requirement for NMDA receptors, L-type voltage-gated calcium channels, and metabotropic glutamate receptor subtype 1 (mGluR1). The expression of insular L-LTP was found to depend on postsynaptic incorporation of calcium-permeable AMPA receptors (CP-AMPARs). These observations are pertinent for the understanding of pain processing and other brain functions within the IC.
METHODS
Animals.
Experiments were carried out with male C57BL/6 mice (7–9 wk old, Orient Bio). All animals were fed in groups of four per cage under standard laboratory conditions (12:12-h light-dark cycle, temperature 22–26°C, air humidity 55–60%) with ad libitum water and mouse chow. The experimental procedures were approved by the Institutional Animal Care and Use Committee of Seoul National University. The number of animals used and their suffering were greatly minimized.
Drugs.
The chemicals and drugs used in this study were as follows: d-(−)-2-amino-5-phosphonopentanoic acid (AP5), anisomycin, [(R)-[(S)-1-(4-bromophenyl)-ethylamino]-(2,3-dioxo-1,2,3,4-tetrahydroquinoxalin-5-yl)-methyl]-phosphonic acid (NVP-AAM077), R-(R*,S*)-α-(4-hydroxyphenyl)-β-methyl-4-(phenylmethyl)-1-piperidine propanol (Ro 25-6981), nimodipine, 2-methyl-6-(phenylethynyl)-pyridine (MPEP), 7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester (CPCCOEt), (2S)-α-ethylglutamic acid (EGLU), (RS)-α-methylserine-O-phosphate (MSOP), and 1-naphthylacetyl spermine (NASPM). Among these AP5, NVP-AAM077, Ro 25-6981, MPEP, MSOP, and NASPM were dissolved in distilled water, while anisomycin, nimodipine, and CPCCOEt were prepared in dimethyl sulfoxide (DMSO) as stock solutions for frozen aliquots at −20°C. EGLU was dissolved in equimolar NaOH. All these drugs were diluted from the stock solutions to the final desired concentration in artificial cerebrospinal fluid (ACSF) before immediate use. AP5, anisomycin, Ro 25-6981, nimodipine, EGLU, MSOP, and NASPM were purchased from Tocris Cookson (Bristol, UK); MPEP and CPCCOEt were obtained from Abcam Biochemicals (Cambridge, UK). NVP-AAM077 was obtained from Sigma (St. Louis, MO). The doses for each compound were chosen based on our preliminary experiments as well as information in previous papers (Clem and Huganir 2010; Grover and Yan 1999; Liu et al. 2012; Toyoda et al. 2010; Wei et al. 1999; Wu et al. 2004; Zhao et al. 2005). For the pharmacological characterization of the induction of insular LTP, all agents were perfused from 30 min prior to TBS until 30 min after TBS application (lasting 1 h). With regard to the evaluation of the expression mechanism of insular LTP, NASPM was given at 5 min after TBS and continued for 30 min before washout. All slices in the pharmacological experiments were used in only one recording session.
Slice preparation.
The general procedures for making IC slices are similar to those described previously (Koga et al. 2012; Wei et al. 2002). Briefly, mice were anesthetized with gaseous isoflurane and decapitated. The whole brain was rapidly removed and immersed in a cold bath of oxygenated (95% O2-5% CO2) ACSF containing (in mM) 124 NaCl, 2.5 KCl, 1.0 NaH2PO4, 1 MgSO4, 2 CaCl2, 25 NaHCO3, and 10 glucose, pH 7.35–7.45. After cooling for 1–2 min, appropriate portions of the brain were then trimmed and the remaining brain block was glued onto the ice-cold stage of a vibrating tissue slicer (Leika VT1000S). Then three coronal IC slices (300 μm) were obtained at the level of the corpus callosum connection and transferred to an incubation chamber continuously perfused with oxygenated ACSF at 26°C. Slices were allowed to recover for at least 2 h before any electrophysiological recording was attempted.
Whole cell patch-clamp electrophysiology.
For whole cell patch-clamp electrophysiology, slices were individually transferred to a recording chamber on the stage of a BX51WI microscope (Olympus) equipped with infrared-differential interference contrast optics and superfused with the same ACSF at 2 ml/min for visualized whole cell patch-clamp recordings (Koga et al. 2012). Excitatory postsynaptic currents (EPSCs) were recorded from superficial-layer (layer II-III) pyramidal neurons with an Axon 200B amplifier (Axon Instruments), and local stimulations were delivered by a bipolar tungsten stimulating electrode placed in a deep layer (layer V-VI). The NMDA receptor-mediated components of EPSCs were pharmacologically isolated in Mg2+-free ACSF containing CNQX (20 μM) and picrotoxin (100 μM). The patch electrodes (2–5 MΩ) contained (in mM) 102 cesium gluconate, 5 TEA-chloride, 3.7 NaCl, 10 BAPTA, 0.2 EGTA, 20 HEPES, 2 MgATP, 0.3 NaGTP, and 5 QX-314 chloride (adjusted to pH 7.2 with CsOH). Neurons were voltage clamped at −30 mV, and NMDA receptor-mediated EPSCs were evoked at 0.05 Hz. Two doses of AP5 (50 and 100 μM) were sequentially bath applied to test the consequences on NMDA receptor-mediated EPSCs. Initial access resistance (typically 15–30 MΩ) was monitored throughout the experiment. Data were filtered at 1 kHz and digitized at 10 kHz and were discarded if the access resistance changed >15%.
Multichannel field potential recordings.
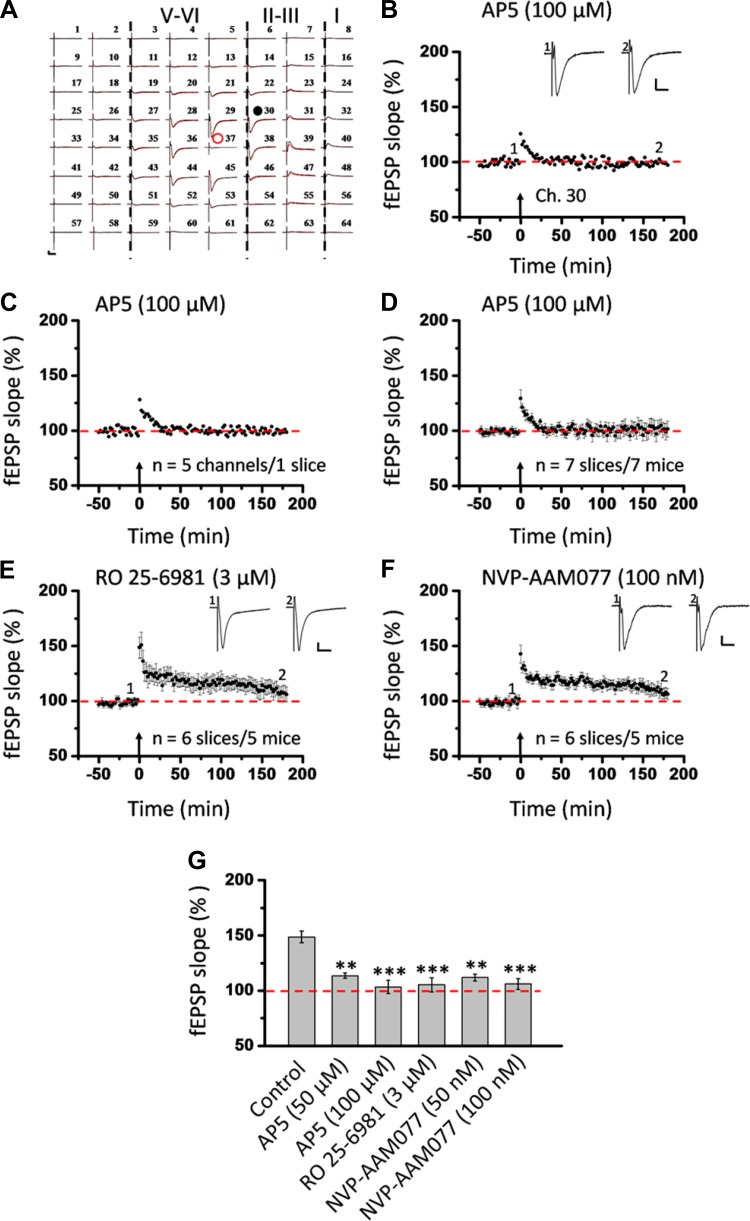
A commercial 64-channel recording system (MED64, Panasonic Alpha-Med Sciences) was used for extracellular field potential recordings in this study. The procedures for preparation of the MED64 probe and multichannel field potential recordings were similar to those described previously (Kang et al. 2012; Li et al. 2010). The MED64 probe had an array of 64 planar microelectrodes, each 50 × 50 μm in size, arranged in an 8 × 8 pattern (interelectrode distance 150 μm: Fig. 1A, right). Before use, the surface of the MED64 probe was treated with 0.1% polyethyleneimine (Sigma) in 25 mM borate buffer (pH 8.4) overnight at room temperature. After incubation, one slice was positioned on the MED64 probe in such a way that the IC area was entirely covered by the recording dish mounted on the stage of an inverted microscope (CKX41, Olympus). The relative location of the IC slice with the probe (see Fig. 1A, left, and Fig. 1B) followed the anatomical atlas (Paxinos and Franklin 2001). Once the slice was settled, a fine-mesh anchor (Warner Instruments, Harvard) was carefully positioned to ensure slice stabilization during recording. The slice was continuously perfused with oxygenated fresh ACSF at the rate of 2–3 ml/min with the aid of a peristaltic pump (Minipuls 3, Gilson) during the whole experimental period of electrophysiological recording.
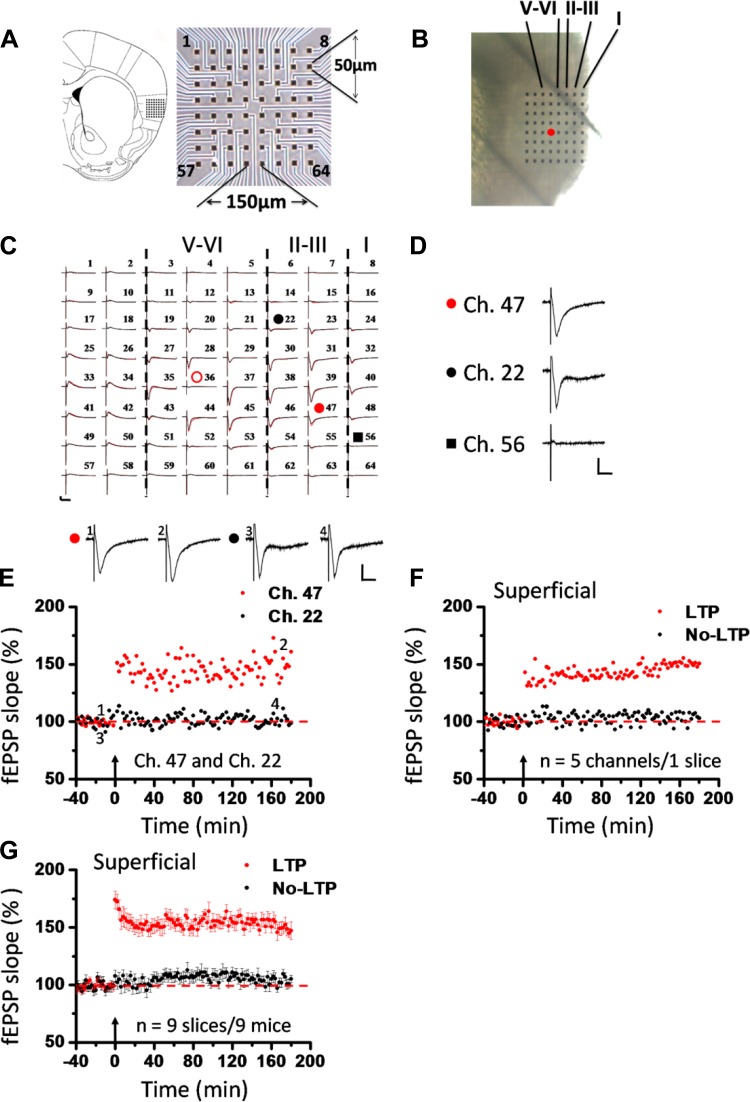
Fig. 1.
Induction of late-phase long-term potentiation (L-LTP) in the superficial layer of the insular cortex (IC). A: schematic diagram showing location of 1 MED64 probe on the coronal IC slice (left; adapted from Paxinos and Franklin 2001 with permission) as well as arrangement of the 8 × 8 recording array (interelectrode distance: 150 μm, electrode size: 50 × 50 μm; right). B: light microscopy photograph showing relative location of IC with the MED64 probe and the layer designation. Red dot indicates stimulation site in deep layer (layer V-VI). C: overview of multisite synaptic responses recorded at baseline (black) and 3 h after theta burst stimulation (TBS) (red). Red open circle denotes the stimulated channel (Ch. 36), while red and black filled circles mark the superficial channels undergoing (Ch. 47) and not undergoing (Ch. 22) L-LTP, respectively. Black rectangle represents the channel not exhibiting any response in the baseline state (Ch. 56). Vertical lines demarcate different layers. D: example traces of Ch. 47, Ch. 22, and Ch. 56 from C shown in an enlarged scale for the baseline state. E: results of 1 channel showing L-LTP (Ch. 47) and the other channel not showing L-LTP (Ch. 22) in 1 slice. Inset: representative field excitatory postsynaptic potentials (fEPSPs) at time points indicated by numbers in graph. F: summary of averaged data from 5 superficial channels in each category within the same slice. G: pooled data from 9 slices from 9 mice, separately illustrating the results of LTP-occurring channels and LTP-not occurring channels in the superficial layer. TBS application in the deep layer resulted in an enduring synaptic potentiation that could last for at least 3 h. However, some channels did not undergo LTP in response to the same protocol. Arrows in E–G indicate starting point of TBS application. Calibration in C–E: 100 μV, 10 ms. Error bars in G represent SE.
After a 20-min recovery, 1 of the 64 available planar microelectrodes was selected from the 64-switch box for stimulation by visual observation through a charge-coupled device camera (DP70, Olympus) connected to the inverted microscope (Fig. 1B, red dot). When not specified, monopolar, biphasic constant current pulses (0.1 ms in duration) generated by the data acquisition software (Mobius, Panasonic Alpha-Med Sciences) were applied to the deep layer (Fig. 1B, layer V-VI) of the IC slice at 0.008 Hz. The field excitatory postsynaptic potentials (fEPSPs) evoked at both the superficial layer (Fig. 1B, layer II-III) and deep layer of the IC were amplified by a 64-channel amplifier, displayed on the monitor screen, and stored on the hard disk of a microcomputer for off-line analysis. After the baseline synaptic responses were stabilized for at least 1 h, a TBS protocol (10 bursts at 5 Hz, 4 pulses at 100 Hz for each burst) was given at a stimulation intensity that was adjusted to elicit 40–60% of the maximal response. After TBS, the test stimulus was repeatedly delivered once every 2 min for at least 3 h to allow long-term monitoring of insular L-LTP induction and maintenance. In another set of experiments, paired-pulse facilitation (PPF) was recorded prior to TBS and 1 h and 3 h after TBS to determine the locus of insular LTP expression. The ratio of the slope of the second response to the slope of the first response was calculated and averaged. The interpulse interval varied between 25, 50, 75, 100, and 200 ms.
Data analysis.
Whole cell patch-clamp data were collected and analyzed with Clampex 10.2 and Clampfit 10.2 software (Axon Instruments). All multichannel electrophysiological data were analyzed off-line by the MED64 Mobius software. For quantification of the LTP data, the initial slope of fEPSPs was measured by taking the rising phase between 10% and 90% of the peak response, normalized and expressed as percent change from the baseline level. For comparison of the LTP magnitude between different treatments, the averaged value of the last 10 min of recordings was compared statistically. Here it is important to note that we used a statistical criterion for data inclusion in the present study. Specifically, for each channel, we performed a t-test by comparing the pre-LTP induction evoked responses (averaged value of the last 10-min baseline recording) to the postinduction responses (averaged value of the last 10 min of 3-h L-LTP recording). Only those channels reaching statistical significance at P < 0.05 were considered L-LTP-showing channels and were included in the final summarizing analysis for the control group and for those drugs having no effect on insular L-LTP. For those drugs blocking the induction of insular L-LTP, the channels showing reliable baseline responses before the conditioning were all included to avoid potential bias in the selection of channels. All data are presented as means ± SE. When necessary, statistical significance was assessed by unpaired Student's t-test, Mann-Whitney rank sum test, or one-way ANOVA [followed by post hoc Fisher's least significant difference (LSD) test] with SigmaPlot software. P < 0.05 was assumed statistically significant.
RESULTS
Long-term in vitro multichannel recordings of insular L-LTP.
To assess the possibility of L-LTP induction in acute IC slices, we employed a 64-channel recording system as described recently (Kang et al. 2012). The relative location of the MED64 probe within the IC slice is shown in Fig. 1, A and B. Generally, we concentrated our recording sites on the rostral IC region at the level of the corpus callosum connection, including three or four brain sections (300 μm in thickness) ranging from bregma +0.98 mm to bregma +1.18 mm according to the mouse atlas in stereotaxic coordinates (Paxinos and Franklin 2001) (Fig. 1A, left). The stimulation site is usually located in the deep layer (layer V-VI) of the IC slice (Fig. 1B, red dot). One representative example recording is illustrated in Fig. 1C. It is evident that widespread fEPSPs could be evoked in both superficial (layer II-III) and deep layers of the IC slice when an electrical stimulation (14 μA) was applied at channel (Ch.) 36, although some channels such as Ch. 56 showed no detectable response (Fig. 1D). After the baseline recording was stabilized for at least 1 h, a TBS protocol (see methods) was delivered at the same stimulation intensity. We found that TBS did not induce LTP in all recording sites. Some sites showed L-LTP (at least 3 h), while others were less potentiated or remained unchanged at the pre-TBS level. For example, Ch. 47 showed a long-lasting LTP (160.7% of baseline at 3 h after TBS) in response to TBS, but Ch. 22 did not undergo any potentiation (98.9% of baseline at 3 h after TBS), although both channels are located in the superficial layer (Fig. 1, C and E). The averaged data from five superficial channels for each condition of the same slice are plotted in Fig. 1F (LTP: 150.7% of baseline; no LTP: 103.1% of baseline at 3 h after TBS), while pooled data of nine slices from nine mice are presented in Fig. 1G. As demonstrated in the summarizing figure (Fig. 1G), those channels showing L-LTP after TBS first exhibited a strong acute potentiation (LTP vs. no LTP: 174.1 ± 7.4% vs. 107.5 ± 5.3% of baseline immediately after TBS; n = 9 slices/9 mice, P = 0.004, Mann-Whitney rank sum test) and then gradually declined to a relatively stable maintaining level of potentiation until the end of the 3-h recording (LTP vs. no LTP: 147.2 ± 7.6% vs. 105.1 ± 3.2% of baseline at 3 h after TBS; n = 9 slices/9 mice, P < 0.001, unpaired t-test).
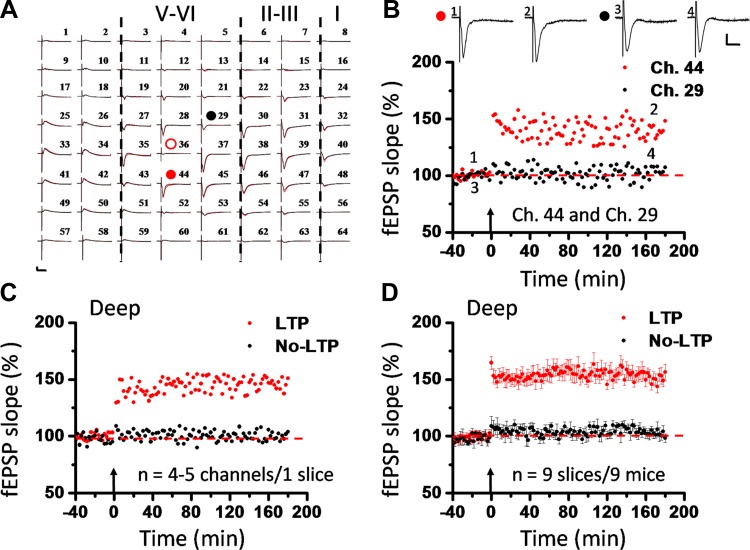
A notable finding is that TBS also induced L-LTP in the deep layer of the IC. Figure 2, A–C, show the corresponding data from the deep layer of the example slice of Fig. 1. In the same slice, Ch. 44 and Ch. 29 are both located in the deep layer (Fig. 2A) but exhibited different responses to the TBS as Ch. 47 and Ch. 22 did in the superficial layer (compare Fig. 1E and Fig. 2B). While Ch. 44 exhibited L-LTP lasting for 3 h, Ch. 29 failed to undergo any potentiation (Ch. 44 vs. Ch. 29: 148.4% vs. 107.3% of baseline at 3 h after TBS). Averaged data from four or five deep channels in this slice are shown in Fig. 2C (LTP: 151.0% of baseline; no LTP: 103.7% of baseline at 3 h after TBS). The pooled data from the deep layer of all slices demonstrated the same trend of L-LTP progression as the superficial layer (Fig. 2D). Interestingly, no obvious layer-related difference was observed in the magnitude of either acute potentiation (LTP vs. no LTP: 164.7 ± 5.6% vs. 109.0 ± 8.1% of baseline immediately after TBS; n = 9 slices/9 mice, P < 0.001, unpaired t-test) or L-LTP (LTP vs. no LTP: 156.3 ± 7.1% vs. 98.4 ± 3.6% of baseline at 3 h after TBS; n = 9 slices/9 mice, P < 0.001, Mann-Whitney rank sum test) (compare Fig. 1G and Fig. 2D). These results demonstrate, for the first time, simultaneous recording of L-LTP in different layers of the IC in adult mice.
Fig. 2.
Induction of L-LTP in deep layer of the IC. A: overview of spatial distribution of insular L-LTP induction in the deep layer (black lines, baseline; red lines, 3 h after TBS). Red open circle denotes the stimulated channel (Ch. 36), while red and black filled circles mark the deep channels showing (Ch. 44) and not showing (Ch. 29) L-LTP, respectively. Vertical lines demarcate different layers. B: results of 1 channel showing L-LTP (Ch. 44) and the other channel not showing L-LTP (Ch. 29) in 1 slice. Inset: representative fEPSPs at time points indicated by numbers in graph. C: summary of averaged data from 4 or 5 deep channels in each category within the same slice. D: pooled data from 9 slices from 9 mice, separately illustrating the results of LTP-occurring channels and LTP-not occurring channels in the deep layer. Similar results were obtained in the deep layer and in the superficial layer. No layer-related difference was detected in the induction of insular L-LTP. Arrows in B–D indicate starting point of TBS application. Calibration in A and B: 100 μV, 10 ms. Error bars in D represent SE.
Spatial analysis of insular LTP distribution.
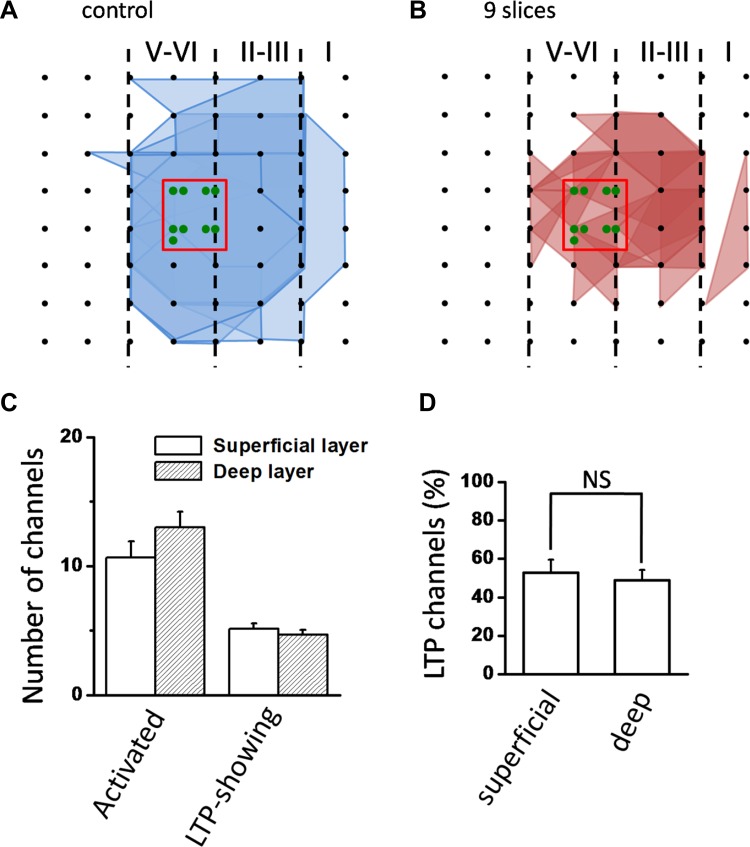
In addition to long-term recording of insular LTP across an extended timescale, we also performed spatial analysis of LTP distribution on the same slices mentioned above with a previously established method (Kang et al. 2012). We defined the generation of LTP in a channel according to the statistical criterion mentioned in methods. The spatial distribution of LTP-occurring channels versus activated channels (including both LTP-occurring and LTP-not occurring channels) was then displayed by plotting a polygonal graph on a matrix representing the 8 × 8 electrodes of the MED64 probe. The blue lines represent the activated channels and the red lines denote the LTP-occurring channels in Fig. 3, A and B (n = 9 slices/9 mice). In this way, the spatial map of LTP distribution within the whole recording screen can be easily discerned. We found that the LTP-occurring channels are mostly located in layer II-III and layer V-VI within 450 μm around the stimulation site (Fig. 3B). However, the spatial characteristics of insular L-LTP are indistinguishable between superficial and deep layers. In total, 96 channels (mean ± SE: 10.7 ± 1.2, 9 slices from 9 mice) exhibited clear synaptic responses in the superficial layer, with 46 channels (mean ± SE: 5.1 ± 0.5) exhibiting persistent LTP. In the deep layer, there were 42 LTP-occurring channels (mean ± SE: 4.7 ± 0.4, 9 slices from 9 mice) out of 117 fEPSP-showing channels (mean ± SE: 13.0 ± 1.2) (Fig. 3C). There was no significant layer difference in the induction ratio (% of LTP-occurring channels vs. all activated channels) of insular L-LTP (superficial layer vs. deep layer: 52.7 ± 6.8% vs. 48.9 ± 5.2%; n = 9 slices/9 mice, P = 0.127, unpaired t-test; Fig. 3D).
Fig. 3.
Spatial analysis of insular L-LTP distribution. A and B: polygonal diagrams of the channels that were activated (blue, A) and that showed L-LTP after TBS (red, B) in 9 slices from 9 mice. Black dots represent the 64 channels in the MED64 probe. Vertical lines indicate the layers in the IC slice. Overlapped blue regions denote frequently activated channels, while overlapped red regions indicate channels that show L-LTP. Red boxes in the center of the graphs mark stimulated channels, and number of green circles in the box indicates number of slices that were stimulated in each channel. Since the stimulation sites are usually located in these channels, they are not shown for other polygonal graphs. C: counts of the averaged number of channels that are activated and that undergo L-LTP in both superficial and deep layers. D: bar histogram of grouped data (n = 9 slices/9 mice) showing % of LTP-occurring channels in superficial and deep layers of the IC. There was no layer-related difference in the induction ratio of insular L-LTP. Error bars in C and D represent SE. NS, no significance.
Induction of insular L-LTP is protein synthesis dependent.
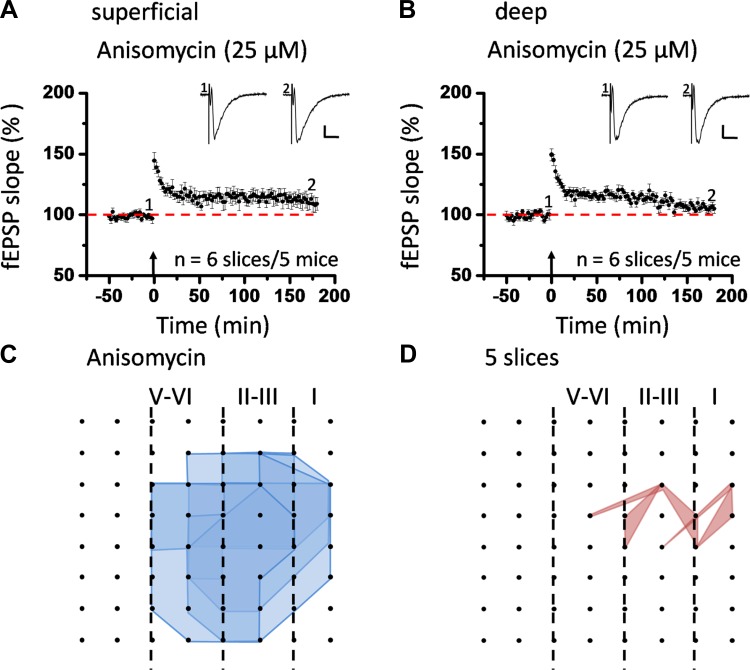
Previous research efforts on the mechanisms of L-LTP in the brain have reached the consensus that most forms of L-LTP are dependent on the synthesis of new proteins (Huang et al. 1994, 2000; Huang and Kandel 2007; Nguyen and Kandel 1996). To examine whether induction of insular L-LTP is also protein synthesis dependent and to confirm the long-term nature of the LTP recorded in this study, we applied anisomycin (25 μM) onto IC slices 30 min before TBS and indeed found a complete blockade of L-LTP (Fig. 4, A and B). In the superficial layer the magnitude of L-LTP was reduced to 109.0 ± 5.2% of baseline at 3 h after TBS (n = 6 slices/5 mice; Fig. 4A), while in the deep layer a similar abolition of L-LTP was observed (104.7 ± 3.6% of baseline, n = 6 slices/5 mice; Fig. 4B). Moreover, we checked the effect of anisomycin perfusion on spatial properties of L-LTP distribution across the insular network. We found that anisomycin resulted in a marked shrinkage of the LTP map compared with the activation map before TBS. Specifically, 101 channels (mean ± SE: 20.2 ± 1.7) were activated from the 5 slices analyzed, but only 10 channels (mean ± SE: 2.0 ± 1.0, n = 5 slices/4 mice) showed L-LTP and most of them were located in the superficial layer (Fig. 4, C and D). These results indicate that induction of L-LTP in the IC requires new protein synthesis in both temporal and spatial domains, which is consistent with previous findings in other synapses. On the basis of these data, we can term what we recorded as L-LTP.
Fig. 4.
Induction of insular L-LTP is dependent on new protein synthesis. A: grouped data from 6 slices from 5 mice for anisomycin (25 μM), showing a complete blockade of insular L-LTP in the superficial layer. B: summarized data in the deep layer (n = 6 slices/5 mice). Insets in A and B: representative fEPSPs at time points indicated by numbers in graph. Arrows indicate starting point of TBS application. Calibration: 100 μV, 10 ms. Error bars represent SE. C and D: spatial analysis of effect of anisomycin on LTP distribution in the IC. Shown are polygonal diagrams of the channels that were activated (blue, C) and that showed L-LTP after TBS (red, D) in the presence of anisomycin (n = 5 slices/4 mice). Anisomycin resulted in a great decrease in the number of LTP-occurring channels. LTP maps in this and subsequent figures are labeled as in Fig. 3.
Induction of insular L-LTP involves activation of both GluN2A and GluN2B receptor subtypes.
Next, we investigated whether the induction of insular LTP requires the activation of NMDA receptors. As expected, bath application of AP5 (100 μM) completely prevented the induction of LTP in the IC slices (Fig. 5). Figure 5, A–D, present the whole view plot, single-channel response in the superficial layer (Ch. 30 as indicated in Fig. 5A), average of five superficial channels in one slice, and grouped data from six slices, respectively, of L-LTP recordings in the presence of AP5. The potentiation was dramatically blocked (from 148.7 ± 5.2% to 103.4 ± 6.0% of baseline during the last 10 min of the 3-h LTP recording; n = 7 slices/7 mice, P < 0.001, 1-way ANOVA followed by Fisher's LSD test; Fig. 5G). A lower dose of AP5 (50 μM) resulted in less but still significant blockade of insular L-LTP (113.6 ± 2.3% of baseline during the last 10 min; n = 5 slices/4 mice, P < 0.01; Fig. 5G). Both GluN2A and GluN2B receptors have been reported to contribute to LTP at other synapses (Bartlett et al. 2007; Berberich et al. 2005; Fox et al. 2006; Liu et al. 2004; Volianskis et al. 2013; Zhang et al. 2008; Zhao et al. 2005; Zhuo 2009). Thus we determined the effects of NVP-AAM077 (100 nM) and Ro 25-6981 (3 μM) on the induction of LTP in acute IC slices. Previous studies have shown the relative selectivity of the two drugs at the above doses against GluN2A and GluN2B, respectively (Bartlett et al. 2007; Berberich et al. 2005; Volianskis et al. 2013; Wu et al. 2007; Zhao et al. 2005). Infusion of Ro 25-6981 at 3 μM produced a substantial inhibition of insular L-LTP generation in the superficial layer (105.3 ± 6.4% of baseline within the last 10 min; n = 6 slices/5 mice, P < 0.001, 1-way ANOVA followed by Fisher's LSD test; Fig. 5, E and G). Preexposure of the slice to NVP-AAM077 (100 nM) resulted in a similar blockade of insular L-LTP induction (106.1 ± 5.0% of baseline within the last 10 min; n = 6 slices/5 mice, P < 0.001, 1-way ANOVA followed by Fisher's LSD test; Fig. 5, F and G). A lower dose of NVP-AAM077 (50 nM) also significantly prevented the L-LTP initiation in the IC slice (111.9 ± 3.1% of baseline during the last 10 min; n = 5 slices/4 mice, P < 0.01; Fig. 5G).
Fig. 5.
Activation of both GluN2A and GluN2B subunits is required for L-LTP induction in the superficial layer of the IC. A: 1 sample of 64-channel recordings of insular L-LTP in the presence of d-(−)-2-amino-5-phosphonopentanoic acid (AP5, 100 μM). Black lines, baseline; red lines, 3 h after TBS. Red open circle denotes stimulated channel (Ch. 37), while black filled circle marks superficial channel not undergoing L-LTP (Ch. 30). Vertical lines demarcate different layers. B: results of 1 channel (Ch. 30) showing the failure of L-LTP induction in the presence of AP5. C: summary of averaged data from 5 channels in the superficial layer of the same slice. D: pooled data from 7 slices from 7 mice. E: pooled data (n = 6 slices/5 mice) of the effect of R-(R*,S*)-α-(4-hydroxyphenyl)-β-methyl-4-(phenylmethyl)-1-piperidine propanol (Ro 25-6981, 3 μM) on insular L-LTP induction in the superficial layer. F: pooled data (n = 6 slices/5 mice) for [(R)-[(S)-1-(4-bromophenyl)-ethylamino]-(2,3-dioxo-1,2,3,4-tetrahydroquinoxalin-5-yl)-methyl]-phosphonic acid (NVP-AAM077, 100 nM). G: bar histogram summarizing quantified data within last 10 min of the 3-h recording (170 min to 180 min after TBS). Bath infusion of all drugs could substantially block the induction of insular L-LTP. Administration of a lower dose of AP5 (50 μM, n = 5 slices/4 mice) or NVP-AAM077 (50 nM, n = 5 slices/4 mice) produced less but significant inhibition. Insets in B, E, and F: representative fEPSPs at time points indicated by numbers in graph. Arrows in B–F indicate starting point of TBS application. Calibration in A, B, E, and F: 100 μV, 10 ms. Error bars in D–G represent SE. **P < 0.01, ***P < 0.001.
Similar inhibitory effects of NMDA receptor antagonists on insular L-LTP induction were found in the deep layer (Fig. 6). As shown in Fig. 6, A–C, bath perfusion of AP5 (100 μM), Ro 25-6981 (3 μM), or NVP-AAM077 (100 nM) led to the inability of deep-layer recording sites to exhibit clear L-LTP, with the averaged fEPSP slope measured during the last 10 min being 95.8 ± 2.2% (n = 6 slices/6 mice, P < 0.001), 98.5 ± 3.3% (n = 6 slices/5 mice, P < 0.001), and 101.6 ± 4.4% (n = 6 slices/5 mice, P < 0.001, 1-way ANOVA followed by Fisher's LSD test) for AP5, Ro 25-6981, and NVP-AAM077, respectively (Fig. 6I). Taken together, these results suggest that induction of L-LTP in the IC requires activation of both GluN2A and GluN2B receptor subunits.
Fig. 6.
Summary of effects of all drugs on insular L-LTP induction in the deep layer of the IC. A: pooled data from 6 slices from 6 mice for AP5 (100 μM). B: pooled data from 6 slices from 5 mice for Ro 25-6981 (3 μM). C: summarized data from 6 slices from 5 mice for NVP-AAM077 (100 nM). D: summarized data from 6 slices from 5 mice for nimodipine (10 μM). E: summarized data from 6 slices from 6 mice for 2-methyl-6-(phenylethynyl)-pyridine (MPEP, 10 μM). F: grouped data from 8 slices from 7 mice for 7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester (CPCCOEt, 100 μM). G: grouped data from 5 slices from 5 mice for (2S)-α-ethylglutamic acid (EGLU, 100 μM). H: pooled data from 6 slices from 6 mice for (RS)-α-methylserine-O-phosphate (MSOP, 100 μM). I: bar histogram summarizing quantified data within last 10 min of the 3-h recording (170 min to 180 min after TBS). Similar results were obtained for each drug in the deep layer as in the superficial layer. Insets in A–H: representative fEPSPs at time points indicated by numbers in graph. Arrows in A–H indicate starting point of TBS application. Calibration in A–E, G–H: 100 μV, 10 ms; calibration in F: 50 μV, 10 ms. Error bars in A–I represent SE. **P < 0.01, ***P < 0.001; NS, no significance.
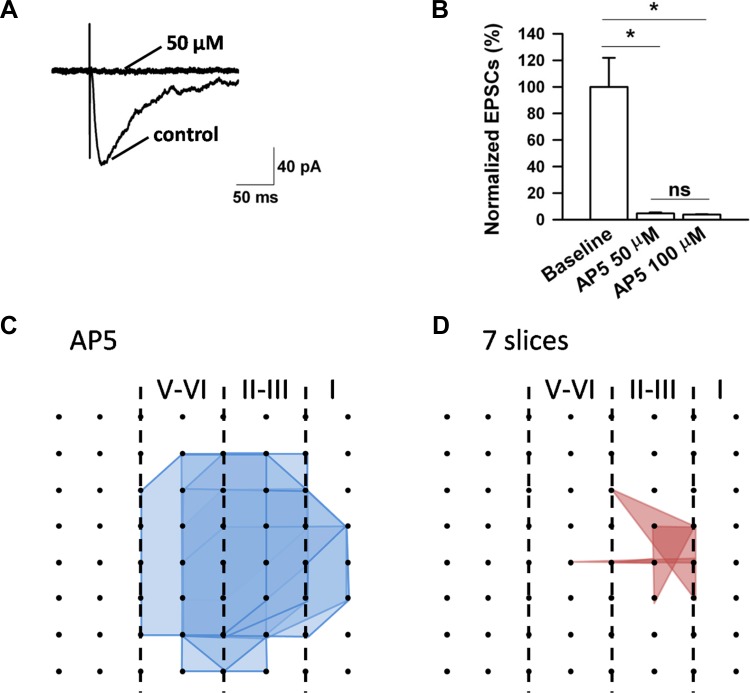
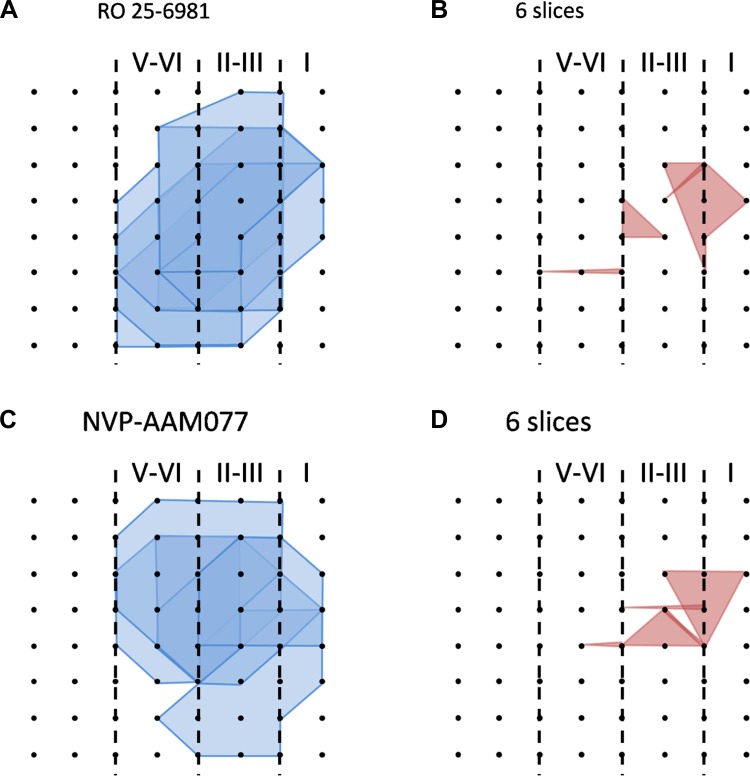
Because 50 μM AP5 did not abolish the induction of L-LTP, we decided to test the effects of AP5 on NMDA receptor-mediated EPSCs with whole cell patch-clamp recordings. We found that 50 μM AP5 completely blocked the NMDA responses. Similar results were obtained with 100 μM AP5 (Fig. 7, A and B). These results suggest that there are possible NMDA receptor-independent forms of L-LTP in the IC. Consistently, in the 100 μM AP5-treated group, we also found some individual channels undergoing L-LTP (see below for the number of LTP-occurring channels; averaged fEPSP slope 145.4 ± 8.1% of baseline at 3 h after TBS, n = 4 slices/4 mice). Furthermore, we evaluated the effect of AP5 on LTP distribution maps. The results showed that AP5 (100 μM) produced a dramatic reduction of the LTP-occurring map while the activation map remained unaffected. In total, 128 channels (mean ± SE: 18.3 ± 0.8, n = 7 slices/7 mice) exhibited clear fEPSPs but only 16 channels (mean ± SE: 2.3 ± 1.2) underwent L-LTP (Fig. 7, C and D). Therefore, similar to anisomycin, the blocking effects of AP5 on insular L-LTP have both temporal and spatial aspects. Spatial analysis of the LTP maps in the presence of Ro 25-6981 (n = 6 slices/5 mice) and NVP-AAM077 (n = 6 slices/5 mice) obtained results consistent with AP5, where much fewer LTP-occurring channels were detected and scattered mainly in the superficial layer (Fig. 8).
Fig. 7.
Effects of AP5 on NMDA receptor-mediated excitatory postsynaptic currents (EPSCs) and spatial analysis of insular L-LTP blockade by AP5. A: representative traces showing that AP5 at 50 μM completely blocked the NMDA receptor-mediated currents. Calibration: 40 pA, 50 ms. B: summarized data of normalized NMDA current amplitude in 3 different states. Asterisks indicate significant difference from baseline, while ns denotes no significant difference between the 2 doses (50 μM: n = 7 neurons/3 mice; 100 μM, n = 3 neurons/2 mice). Error bars represent SE. C and D: polygonal diagrams of channels that were activated (blue, C) and that showed L-LTP after TBS (red, D) in the presence of AP5 (100 μM, n = 7 slices/7 mice). It is evident that the spatial distribution of LTP-occurring channels was greatly diminished when IC slices were pretreated with AP5.
Fig. 8.
Spatial analysis of insular L-LTP blockade by Ro 25-6981 and NVP-AAM077. A and B: polygonal graphs of activated (blue, A) and LTP-occurring (red, B) channels among the insular network when TBS is applied in the presence of Ro 25-6981 (3 μM, n = 6 slices/5 mice). C and D: corresponding results for NVP-AAM077 (100 nM, n = 6 slices/5 mice). Both drugs reduced the number of L-LTP-occurring channels compared with the activation map.
Roles of L-type voltage-gated calcium channels.
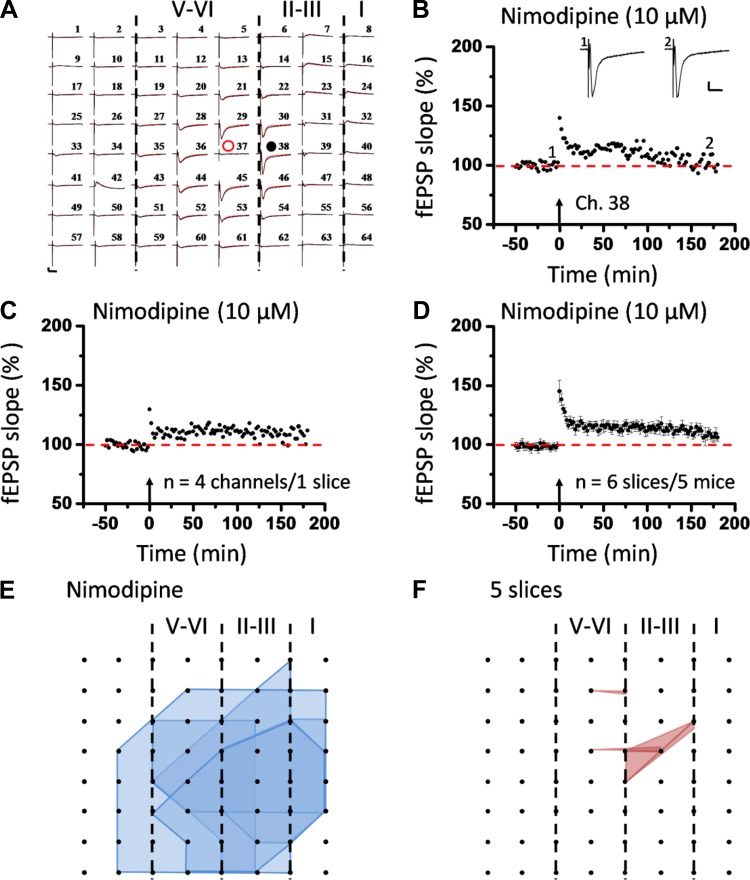
To determine any involvement of voltage-gated calcium channels in insular L-LTP induction, we perfused IC slices with nimodipine (a selective L-type voltage-gated calcium channel blocker, 10 μM) and found an effective, although not complete, attenuation of synaptic potentiation (Fig. 9). The whole 64-channel view of synaptic responses of one representative slice is illustrated in Fig. 9A for both before TBS and 3 h after TBS in the presence of nimodipine. Figure 9, B and C, show the single-channel data (Ch. 38 as indicated in Fig. 9A) and averaged data from four channels in the superficial layer of the same slice, respectively. Pooled results from a series of similar experiments are summarized in Fig. 9D. On average, the magnitude of insular L-LTP was significantly reduced to 106.0 ± 3.5% of baseline at 3 h after TBS (n = 6 slices/5 mice, P < 0.001, unpaired t-test). Nimodipine could equally prevent L-LTP induction in the deep layer of the IC slice (105.2 ± 3.3% of baseline within the last 10 min of recording; n = 6 slices/5 mice, P < 0.01, 1-way ANOVA followed by Fisher's LSD test; Fig. 6, D and I). Pretreatment with nimodipine also resulted in a much smaller LTP distribution map among the IC network, with only 11 LTP-occurring channels (mean ± SE: 2.2 ± 0.4, n = 5 slices/4 mice) out of 123 fEPSP-showing channels (mean ± SE: 24.6 ± 2.8; Fig. 9, E and F).
Fig. 9.
Induction of insular L-LTP in the superficial layer partially depends on L-type voltage-gated calcium channels. A: 1 sample of 64-channel recordings of insular L-LTP in the presence of nimodipine (10 μM). Black lines, baseline; red lines, 3 h after TBS. Red open circle denotes the stimulated channel (Ch. 37), while black filled circle marks the superficial channel not undergoing L-LTP (Ch. 38). Vertical lines demarcate different layers. B: results of 1 channel (Ch. 38) showing suppression of L-LTP induction by nimodipine. Sample fEPSP recordings taken at times indicated by corresponding numbers are shown above plot. C: summary of averaged data from 4 superficial channels of 1 slice. D: pooled data from 6 slices from 5 mice. Bath infusion of nimodipine resulted in a partial but significant inhibition of insular L-LTP induction. Arrows in B–D indicate starting point of TBS application. Calibration in A and B: 100 μV, 10 ms. Error bars in D represent SE. E and F: spatial analysis of nimodipine-induced L-LTP blockade. Shown are the polygonal graphs of activated (blue, E) and LTP-occurring (red, F) channels among the insular network when TBS is applied in the presence of nimodipine (n = 5 slices/4 mice). Nimodipine treatment resulted in a spatially compressed LTP distribution map in the IC.
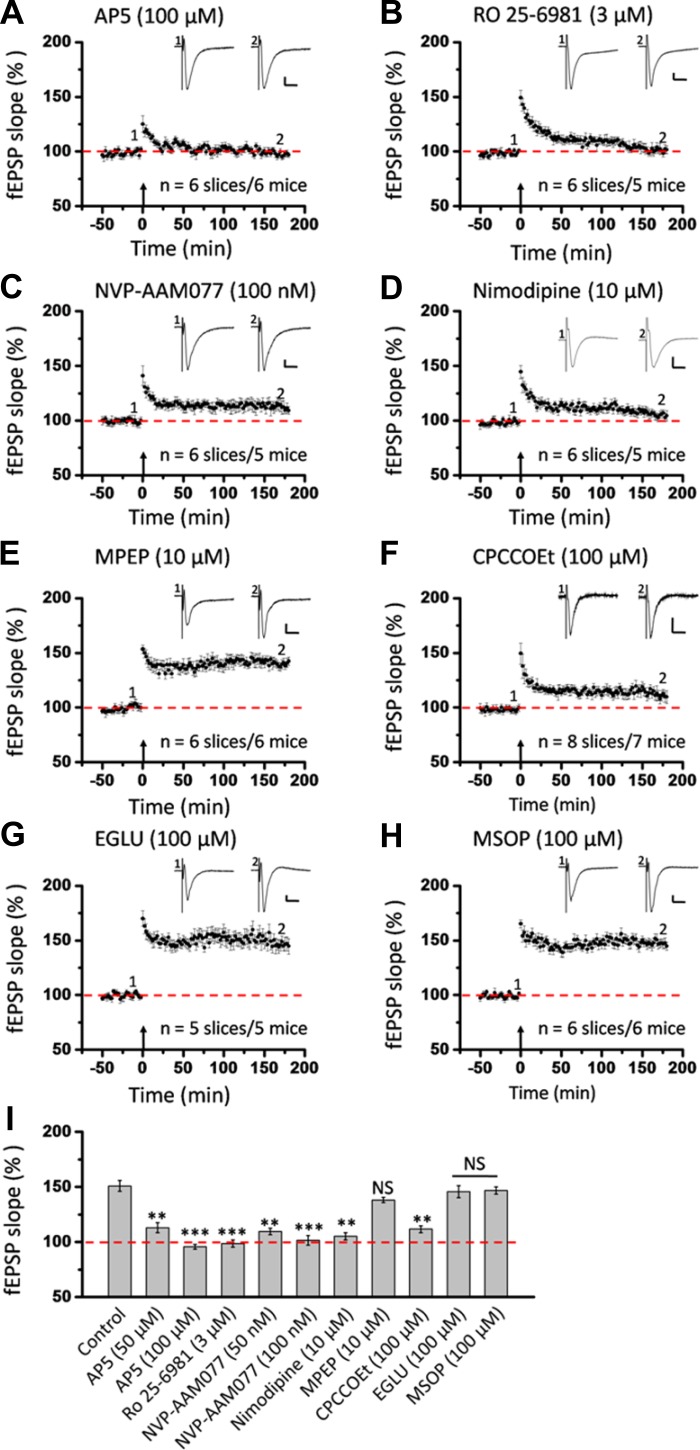
Selective involvement of mGluR1 in insular L-LTP induction.
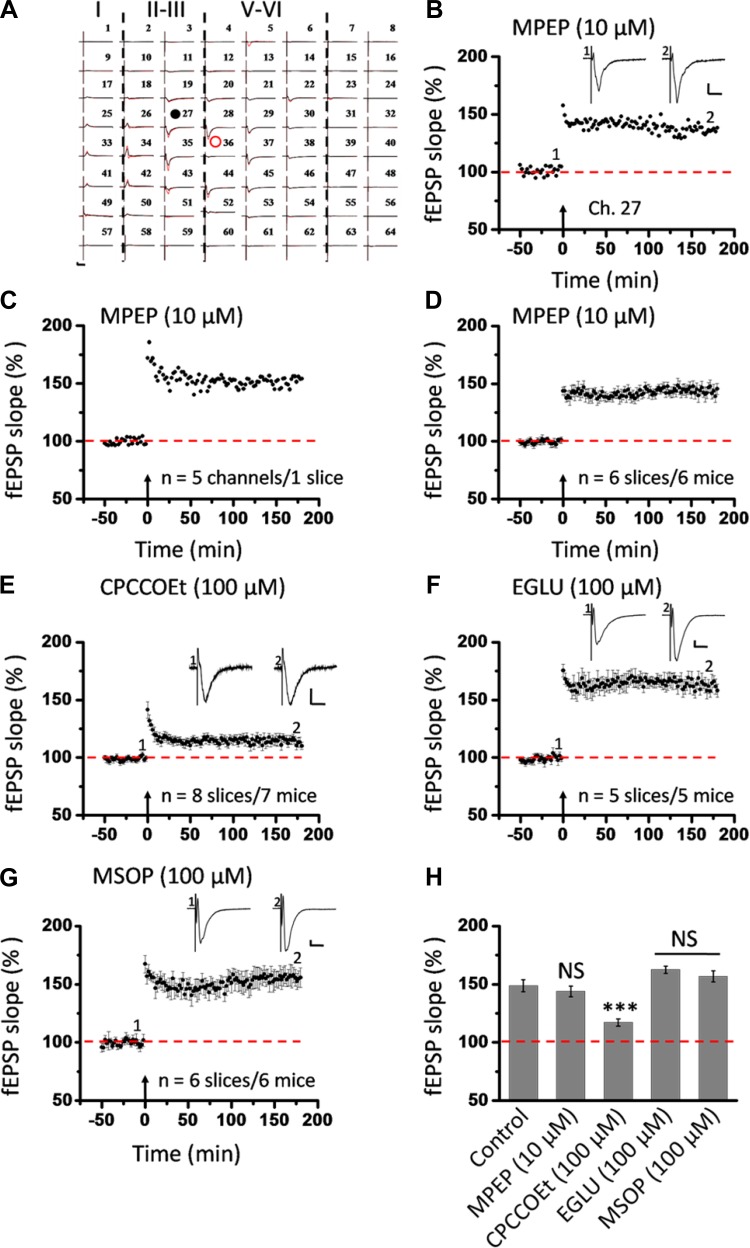
In addition to NMDA receptors, mGluRs have been reported to contribute to LTP induction and modulation in the brain (for reviews, see Anwyl 1999; Bordi and Ugolini 1999; Bortolotto et al. 1999). To test the mGluR dependence of insular L-LTP, we examined the consequences of administration of MPEP and CPCCOEt, two drugs selectively antagonizing activation of mGluR5 and mGluR1, respectively (Liu et al. 2012). Figure 10, A–D, illustrate the whole view response, single-channel (Ch. 27 as indicated in Fig. 10A) data within a slice, averaged recordings of five superficial channels from one slice, and grouped data from six slices from six mice, respectively, regarding the effect of MPEP (10 μM) on insular LTP. MPEP infusion failed to produce any effect on the development of insular L-LTP in the superficial layer (143.9 ± 4.5% of baseline during the last 10 min recording; n = 6 slices/6 mice, P = 0.684, 1-way ANOVA followed by Fisher's LSD test; Fig. 10H). By contrast, pretreatment with CPCCOEt (100 μM) gave a partial but significant inhibition of insular L-LTP (117.1 ± 3.2% of baseline within the last 10 min; n = 8 slices/7 mice, P < 0.001, 1-way ANOVA followed by Fisher's LSD test; Fig. 10, E and H).
Fig. 10.
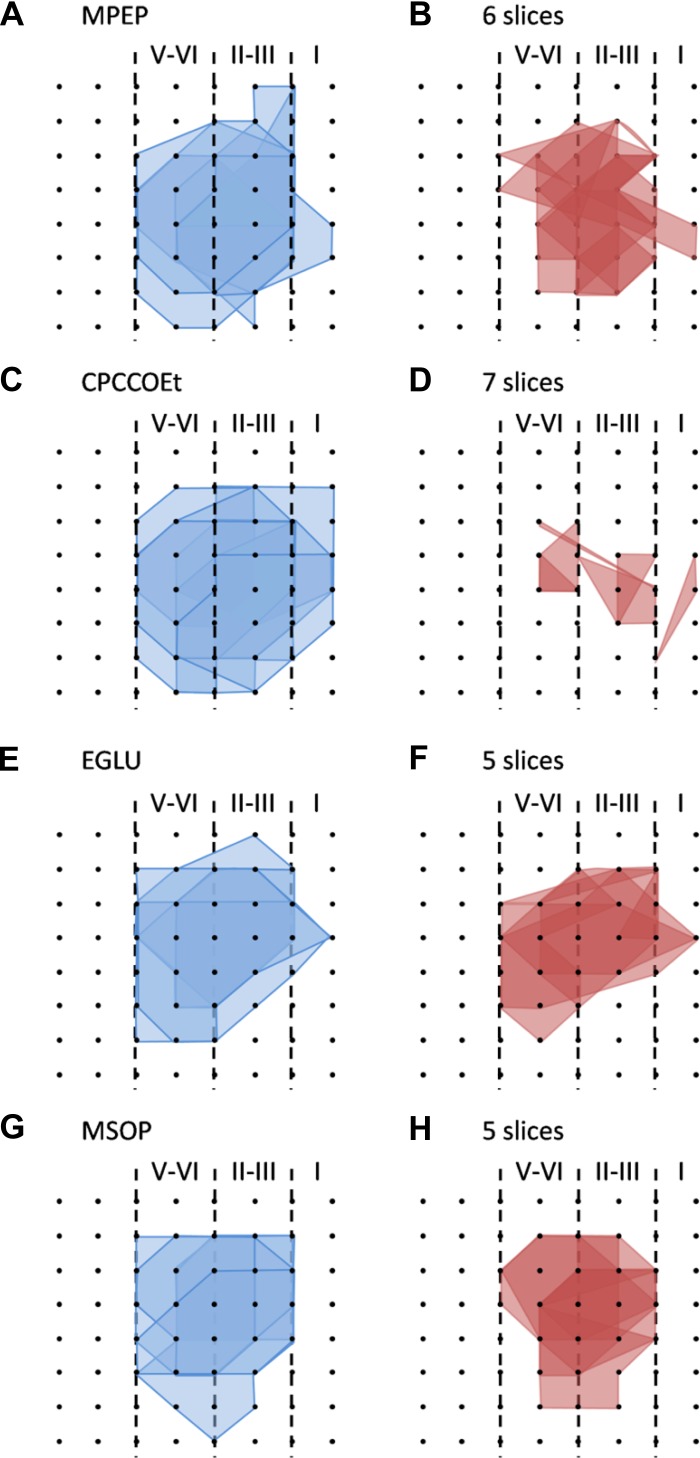
Selective involvement of metabotropic glutamate receptor (mGluR)1 in L-LTP induction in the superficial layer of the IC. A: 1 sample of 64-channel recordings of insular L-LTP in the presence of MPEP (10 μM). Black lines, baseline; red lines, 3 h after TBS. Red open circle denotes the stimulated channel (Ch. 36), while black filled circle marks the superficial channel showing L-LTP (Ch. 27). Vertical lines demarcate different layers. B: results of 1 channel (Ch. 27) showing normal induction of insular L-LTP in the presence of MPEP. C: summary of averaged data from 5 superficial channels of 1 slice. D: pooled data from 6 slices from 6 mice. E: pooled data (n = 8 slices/7 mice) for the effect of CPCCOEt (100 μM) on insular L-LTP induction in the superficial layer. F: summarized data (n = 5 slices/5 mice) for EGLU (100 μM). G: grouped data (n = 6 slices/6 mice) for MSOP (100 μM). H: bar histogram summarizing quantified data within last 10 min of the 3-h recording (170 min to 180 min after TBS). Application of antagonists for mGluR5 and group II and group III mGluRs had no effect on the induction of insular L-LTP. However, antagonism of mGluR1 activation resulted in a significant blockade of insular L-LTP. Insets in B and E–G: representative fEPSPs at time points indicated by numbers in graph. Arrows in B–G indicate starting point of TBS application. Calibration in A, B, F, G: 100 μV, 10 ms; calibration in E: 50 μV, 10 ms. Error bars in D–H represent SE. ***P < 0.001; NS, no significance.
Besides group I mGluRs, previous literatures suggest the modulatory role of group II and group III mGluRs in LTP (Grover and Yan 1999; Huang et al. 1997; O'Leary and O'Connor 1998; Wu et al. 2004). Therefore, we also tested the actions of EGLU (a selective group II mGluR antagonist; Wu et al. 2004) and MSOP (a group III mGluR antagonist; Grover and Yan 1999) on insular L-LTP. Neither EGLU (100 μM) nor MSOP (100 μM) exerted any inhibition of the initiation of insular L-LTP (EGLU: 162.5 ± 3.1% of baseline within the last 10 min, n = 5 slices/5 mice, P = 0.625; MSOP: 156.8 ± 4.8% of baseline within the last 10 min, n = 6 slices/6 mice, P = 0.552, 1-way ANOVA followed by Fisher's LSD test; Fig. 10, F–H).
Similar results were obtained from the deep layer (Fig. 6, E–I). Specifically, the averaged fEPSP slope during the last 10 min of recording was as follows: MPEP 138.2 ± 2.4% of baseline (n = 6 slices/6 mice), CPCCOEt 111.6 ± 3.2% of baseline (n = 8 slices/7 mice), EGLU 145.9 ± 5.4% of baseline (n = 5 slices/5 mice), and MSOP 146.8 ± 3.2% of baseline (n = 6 slices/6 mice) (all P > 0.05 except CPCCOEt at P < 0.01, ANOVA followed by Fisher's LSD test; Fig. 6I). We also performed network analysis of the LTP distribution maps by counting the number of activated and LTP-occurring channels in the four drug-treated groups of IC slices. As expected, all drugs had no effect on the LTP maps except CPCCOEt, which caused a dramatic decrease in the number of LTP-occurring channels (mean ± SE: 3.3 ± 0.5, n = 7 slices/6 mice) compared with the fEPSP-showing channels before TBS (mean ± SE: 18.1 ± 1.3; Fig. 11). Taken together, these data indicate that induction of insular L-LTP is dependent on the activation of mGluR1 but with no involvement of other mGluR subtypes.
Fig. 11.
Spatial analysis of the effects of mGluR antagonists on LTP distribution maps in the IC. A and B: polygonal graphs of activated (blue, A) and LTP-occurring channels (red, B) among the insular network when TBS is applied in the presence of MPEP (10 μM, n = 6 slices/6 mice). C and D: corresponding results for CPCCOEt (100 μM, n = 7 slices/6 mice). E and F: data for EGLU (100 μM, n = 5 slices/5 mice). G and H: findings with MSOP (100 μM, n = 5 slices/5 mice). Among the 4 drugs tested, only CPCCOEt produced a significant shrinkage in the insular L-LTP map compared with the activation map.
Postsynaptic recruitment of CP-AMPARs during insular L-LTP.
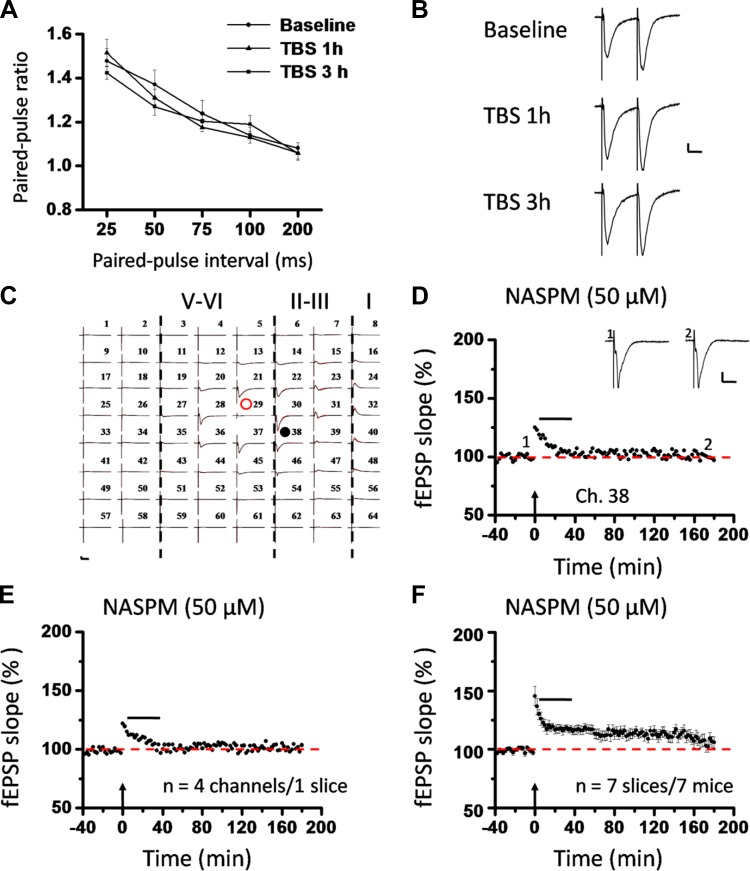
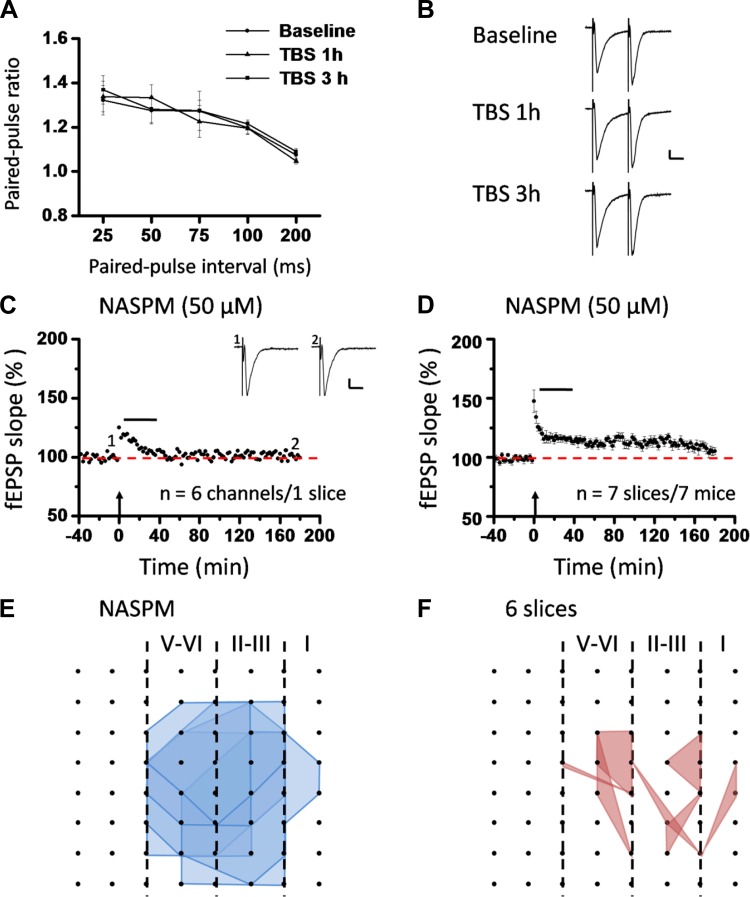
To investigate the locus of insular L-LTP expression, we first recorded the PPF during the baseline (prior to TBS) and at 1 h and 3 h after TBS. The paired-pulse ratio at various intervals is shown in Fig. 12, A and B, and Fig. 13, A and B, for superficial layer and deep layer, respectively. No significant difference was detected in either layer (n = 5–6 slices/4–6 mice, P = 0.258 and P = 0.772 at 50-ms interval for superficial layer and deep layer, 1-way ANOVA), indicating that the locus of L-LTP expression is most likely postsynaptic.
Fig. 12.
Roles of calcium-permeable AMPA receptors (CP-AMPARs) in postsynaptic expression of L-LTP in the superficial layer of the IC. A: paired-pulse ratios (slope of fEPSP2/slope of fEPSP1) recorded with intervals of 25, 50, 75, 100, and 200 ms in superficial layer of the IC. Comparison of the paired-pulse ratio revealed no significant difference under 3 conditions (baseline, TBS 1 h, and TBS 3 h), suggesting a postsynaptic locus of insular L-LTP expression. B: representative traces of paired-pulse facilitation (PPF) with an interval of 25 ms recorded in superficial layer of the IC. C: 1 example of 64-channel recordings of insular L-LTP with the CP-AMPAR blocker 1-naphthylacetyl spermine (NASPM, 50 μM) applied from 5 min after TBS until 35 min after TBS. Black lines, baseline; red lines, 3 h after TBS. Red open circle denotes the stimulated channel (Ch. 29), while black filled circle marks the superficial channel showing the L-LTP blockade by NASPM (Ch. 38). Vertical lines demarcate different layers. D: results of 1 channel (Ch. 38) showing suppression of insular L-LTP expression by NASPM. Sample fEPSP recordings taken at times indicated by corresponding numbers are shown above plot. E: summary of averaged data from 4 superficial channels of the same slice as C and D. F: pooled data from 7 slices from 7 mice. Horizontal bars in D–F denote period of NASPM application. Arrows in D–F indicate starting point of TBS application. Calibration in B–D: 100 μV, 10 ms. Error bars in A and F represent SE.
Fig. 13.
Roles of CP-AMPARs in postsynaptic expression of L-LTP in the deep layer of the IC and spatial analysis of the L-LTP distribution map changes. A: paired-pulse ratios (slope of fEPSP2/slope of fEPSP1) recorded with intervals of 25, 50, 75, 100, and 200 ms in the deep layer of the IC. Similar to the superficial layer, the paired-pulse ratio did not differ among the 3 conditions (baseline, TBS 1 h, and TBS 3 h), arguing against a presynaptic locus of insular L-LTP expression. B: raw traces of PPF with an interval of 25 ms recorded in the deep layer of the IC. C: summary of averaged data from 6 deep channels of 1 slice. D: pooled data from 7 slices from 7 mice, demonstrating the inability to express L-LTP in the deep layer of the IC when the synaptic trafficking of CP-AMPARs is blocked by NASPM infusion (50 μM). Horizontal bars in C and D denote period of NASPM application. Arrows in C and D indicate starting point of TBS application. Calibration in B and C: 100 μV, 10 ms. Error bars in A and D represent SE. E and F: spatial analysis of NASPM-induced L-LTP blockade. Shown are polygonal graphs of activated (blue, E) and LTP-occurring (red, F) channels among the insular network when NASPM is applied at 5 min after TBS (n = 6 slices/6 mice). Compared with the baseline state, very few channels showed L-LTP after NASPM application.
GluA2-lacking CP-AMPARs have been increasingly recognized as an important player in several types of synaptic plasticity and plasticity-related behaviors such as memory and pain processing (Clem and Huganir 2010; Descalzi et al. 2012; Gangadharan et al. 2011; for reviews, see Isaac et al. 2007; Liu and Zukin 2007; Man 2011). We next examined the possible involvement of CP-AMPARs in the expression of insular L-LTP. Bath application of NASPM (50 μM), a potent CP-AMPAR blocker (Clem and Huganir 2010; Lu et al. 2007), starting from 5 min after TBS to 35 min after TBS, reversed the potentiation in the superficial layer as qualified at 3 h after TBS (single channel: Ch. 38, 97.1% of baseline, Fig. 12, C and D; averaged 4 channels from 1 slice: 103.5% of baseline, Fig. 12E; pooled data: 106.1 ± 4.8% of baseline, n = 7 slices/7 mice, P < 0.001, unpaired t-test, Fig. 12F). Similarly, deep-layer L-LTP was also robustly blocked by a 30-min treatment with NASPM (averaged 6 channels from one slice: 99.5% of baseline, Fig. 13C; pooled data: 105.0 ± 3.0% of baseline, n = 7 slices/7 mice, P = 0.001, Mann-Whitney rank sum test, Fig. 13D). In addition, NASPM application also blocked insular L-LTP spatially (Fig. 13, E and F). Specifically, there are a total of 108 channels (mean ± SE: 18.0 ± 1.1) being activated from the 6 slices included in the spatial analysis, but only 22 channels (mean ± SE: 3.7 ± 1.1) showed L-LTP (Fig. 13F). Interestingly, the LTP-occurring channels were sparsely distributed across all layers. These findings indicate that postsynaptic recruitment of CP-AMPARs is a necessary step for insular L-LTP expression, which is in large consistence with previous results in other central synapses (Guire et al. 2008; Lu et al. 2007; Plant et al. 2006; Toyoda et al. 2007; but see Adesnik and Nicoll 2007).
DISCUSSION
It is believed that the IC is an important forebrain structure involved in key functions of the mammalian brain, including incentive evaluation, interoceptive awareness, pain perception, and learning and memory formation (Apkarian et al. 2011; Bermudez-Rattoni 2004; Craig 2011; Gal-Ben-Ari and Rosenblum 2012; Zhuo 2008). In addition, synaptic plasticity has become a widely accepted cellular model underlying a variety of higher brain functions and behaviors (Bliss and Collingridge 1993; Citri and Malenka 2008; Zhuo 2005, 2008). Surprisingly, to date little effort has been attempted to link these two aspects together by elucidating insular synaptic transmission and plasticity at the physiological level. The present study, to our knowledge, is the first report to systematically characterize the electrophysiological and pharmacological properties of insular LTP in acute IC slices from adult mice. We demonstrate that excitatory synapses in the IC are highly plastic and within-insula tetanic stimulation evokes protein synthesis-dependent L-LTP in the neighboring regions encompassing both superficial and deep layers of the IC. The induction of insular L-LTP requires activation of multiple mechanisms, including NMDA receptors, L-type voltage-gated calcium channels, and mGluR1 receptors. The expression of insular L-LTP is mainly mediated by postsynaptic CP-AMPAR-related mechanisms. Although much of the signaling pathways for the insular L-LTP remains to be determined, our study provides an important model for future investigations of molecular mechanisms for insular LTP as well as the possible functional significance.
Mapping L-LTP in the insular circuit.
A recent study using the whole cell patch-clamp recording technique shows that glutamate is the major excitatory transmitter in the IC and basal synaptic transmission is mainly mediated by postsynaptic AMPA and kainate receptor (KA) receptors (Koga et al. 2012). The present study extends the previous work by investigating synaptic L-LTP in adult IC slices. To map the L-LTP taking place within the IC circuit, we used a 64-channel multielectrode array recording system as recently described in a separate study (Kang et al. 2012). We found that TBS produced L-LTP at both superficial (II-III) and deep (V-VI) layers of the IC. Spatial analysis of LTP distribution showed that not every activated channel would undergo LTP, with the spreading scope of LTP-occurring channels being smaller than that of the fEPSP-showing (activated) channels. Notably, among these LTP-occurring channels, there was no apparent layer-related difference in either induction rate or maintenance magnitude of insular L-LTP.
Previously, the induction of LTP at the BLA-IC pathway was described by the use of electrophysiological recordings in anesthetized rats (Escobar et al. 1998, 2003). This form of in vivo insular LTP was found to be correlated with the animal's performance in the CTA task (Escobar and Bermudez-Rattoni 2000; Rodriguez-Duran et al. 2011). In addition, our earlier work on the roles of calcium/calmodulin-dependent protein kinase IV (CaMKIV) in fear memory showed a clear defect in in vitro LTP induction in IC slices prepared from CaMKIV−/− mice, but with significant potentiation being detectable in wild-type insular synapses (Wei et al. 2002). Nevertheless, only a short-term LTP was recorded in that study (40 min), and no spatial or pharmacological characterization of insular LTP was performed. Therefore, the present study confirmed and further extended these previous observations by presenting a much longer-lasting and widely distributed form of in vitro insular L-LTP, which lasts for at least 3 h and spreads to both superficial and deep layers of the IC slice. The insular L-LTP reported here is protein synthesis dependent, as evidenced by a significant blockade of the LTP induction temporally and a dramatic shrinkage of the LTP distribution map spatially in the presence of anisomycin.
Induction mechanisms of insular L-LTP.
Multiple mechanisms have been reported to contribute to synaptic potentiation (Bliss and Collingridge 1993; Citri and Malenka 2008), depending on the induction protocol, regions of the brain, recording methods, and age of animals (Rosenzweig and Barnes 2003; Yoshimura et al. 2003; Zhang et al. 2008). Compared with other central synapses such as the hippocampus and ACC, however, little information is available on the induction mechanisms of LTP in the IC. The present study reveals, for the first time, that in vitro induction of insular L-LTP depends on the activation of NMDA receptors and L-type voltage-gated calcium channels. Moreover, we demonstrate that both GluN2A and GluN2B subunits are involved in insular L-LTP. There is considerable controversy regarding the role of GluN2A and GluN2B subtypes in synaptic plasticity. On one hand, GluN2B receptor activation has been demonstrated to participate in LTD induction in either hippocampus (Duffy et al. 2008; Liu et al. 2004) or cortex (Massey et al. 2004; Toyoda et al. 2005), while on the other hand, there are several reports indicating its role in LTP induction in hippocampus (Bartlett et al. 2007; Fox et al. 2006; Tang et al. 1999; Zhang et al. 2008) and ACC (Zhao et al. 2005). Variable results have also been reported in terms of GluN2A and LTP (Bartlett et al. 2007; Liu et al. 2004; Massey et al. 2004; Yoshimura et al. 2003; Zhao et al. 2005) or LTD (Bartlett et al. 2007; Fox et al. 2006; Toyoda et al. 2005). Here we reveal the important role of both GluN2A and GluN2B in insular L-LTP induction, reminiscent of their joint involvement in cingulate LTP (Zhao et al. 2005) and LTP in adult hippocampus (Volianskis et al. 2013). Moreover, we report dramatic alterations in the spatial distribution of LTP-occurring channels across the IC network in the presence of all three NMDA receptor antagonists. These data are partially consistent with previous behavioral results concerning NMDA receptors and IC-related taste memory. For example, activation of the NMDA receptors in the IC has been reported to contribute to the acquisition, consolidation, and taste-illness association in the CTA task (Berman et al. 2000; Ferreira et al. 2002). Furthermore, novel taste learning elicited an apparent increase in tyrosine phosphorylation of the GluN2B subunit in the IC (Barki-Harrington et al. 2009; Rosenblum et al. 1997). Pharmacological blockade of GluN2B tyrosine phosphorylation attenuated the taste memory formation by altering the distribution pattern of NMDA receptors (Barki-Harrington et al. 2009). Thus it would be of great interest for future studies to examine any change of the GluN2B or GluN2A phosphorylation state associated with L-LTP induction in the IC.
In addition to ionotropic glutamate receptors, a large body of literature has shown an intimate relationship between mGluRs and LTP (Bordi and Ugolini 1999; Bortolotto et al. 1999; Huang et al. 1997; Liu et al. 2012). In particular, studies from knockout mice have revealed differential roles of mGluR5 and mGluR1 in hippocampal LTP, with mGluR5 predominantly contributing to NMDA receptor-dependent LTP in CA1 and dentate gyrus (Lu et al. 1997; but see Bortolotto et al. 2005) and mGluR1 mainly involved in NMDA receptor-independent LTP at mossy fiber synapses (Conquet et al. 1994). Our results showed that pharmacological inhibition of mGluR1 activation substantially reduced insular L-LTP induction and spatial distribution, whereas antagonism of mGluR5 had no effect. This selective involvement of mGluR1 stands in contrast to previous findings in in vivo electrophysiological recordings, where intracortical administration of the mGluR antagonist (RS)-α-methyl-4-carboxyphenylglycine (MCPG) could not disrupt the LTP induction in the BLA-IC pathway (Escobar et al. 2002). The reasons for the discrepancy might be attributable to differences in the recording method (in vitro vs. in vivo) and the specificity of the drug used (selective mGluR5 and mGluR1 antagonist vs. nonselective mGluR antagonist). Supporting the present electrophysiological data, however, behavioral studies revealed an impairment of CTA encoding by intra-IC injection of MCPG (Berman et al. 2000). Finally, we found no effect of selective group II and group III mGluR antagonists on the induction of insular L-LTP either temporally or spatially. It is unlikely that these negative results of mGluR antagonists were due to insufficient doses used in the present study, because the same doses of the drugs have been shown previously to be effective in vitro (Grover and Yan 1999; Wu et al. 2004). Therefore, it could be logically deduced that induction of insular L-LTP in the present conditions is indeed NMDA receptor- and mGluR1 dependent, but without any mediating or modulating role of group II and group III mGluRs.
Expression of insular LTP involves postsynaptic recruitment of CP-AMPARs.
The present findings provide new insights into the basic mechanisms for the expression of insular LTP in two aspects. First, our paired-pulse recordings showed no clear alterations in PPF in either superficial or deep layer after TBS, presenting direct electrophysiological evidence for a postsynaptic locus of insular L-LTP expression. Second, the present data suggest the involvement of CP-AMPARs trafficking in the expression of insular L-LTP. Transient synaptic incorporation of CP-AMPARs has been previously reported during the early phase of LTP, and rapid pharmacological inhibition of CP-AMPARs consistently yields a reversal of LTP expression in both hippocampus (Guire et al. 2008; Lu et al. 2007; Plant et al. 2006; but see Adesnik and Nicoll 2007) and ACC (Toyoda et al. 2007). Furthermore, genetically engineered mice lacking GluA2 subunit were capable of exhibiting numerous forms of LTP through a variety of different induction protocols (Asrar et al. 2009; Wiltgen et al. 2010). Here we show that expression of insular L-LTP might equally relate to the synaptic trafficking of CP-AMPARs. Moreover, CP-AMPAR-mediated calcium influx may provide another source of intracellular calcium required for the initiation of insular LTP in addition to the role of NMDA receptors and L-type voltage-gated calcium channels. However, NASPM application in naive slices did not affect the baseline synaptic transmission, indicating the lack of CP-AMPARs at insular synapses under basal conditions (Liu et al., unpublished observations). In the present study, we did not detect any depression of synaptic transmission below the baseline level by post-TBS NASPM treatment, which is in line with previous reports in the hippocampus (Lu et al. 2007; Plant et al. 2006) and ACC (Toyoda et al. 2007). The exact reasons for this phenomenon are not clear but might be due to the fact that in insular L-LTP CP-AMPARs are added to the existing component of calcium-impermeable AMPARs. Future studies are clearly warranted to reveal the exact postsynaptic mechanisms. Collectively, these results support the notion that activity-dependent insertion of CP-AMPARs into potentiated synapses may constitute an important mechanism employed by many central synapses for expression of LTP.
In conclusion, the present study is the first to demonstrate long-term in vitro multichannel recordings of L-LTP in adult mice IC and provide an initial assessment of the mechanisms underlying induction and expression of insular LTP. The main significance of this study is that it partially bridges the gap among previous behavioral, pharmacological, and biochemical studies performed in the IC by establishing a cellular/synaptic model of L-LTP of synaptic transmission in this area. Future intensive research efforts into more detailed mechanisms of insular LTP and their behavioral relevance might be helpful for facilitating our understanding of the synaptic basis for the involvement of the IC in several higher brain functions including pain perception and memory encoding.
GRANTS
This work was supported by the World-Class University (WCU) program of the Ministry of Education, Science and Technology in Korea through the National Research Foundation (R32-10142). M. Zhuo was supported by a Canadian Institutes of Health Research (CIHR) operating grant, Canada Research Chair (CRC), Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant 402555, and WCU. B.-K. Kaang is a Yonam Foundation Scholar and supported by WCU and the National Honor Scientist Program, Korea. G. L. Collingridge was supported by the Medical Research Council (MRC).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.-G.L., S.-J.K., T.-Y.S., K.K., and M.-M.Z. performed experiments; M.-G.L. and S.-J.K. analyzed data; M.-G.L., G.L.C., and M.Z. interpreted results of experiments; M.-G.L. and S.-J.K. prepared figures; M.-G.L. drafted manuscript; S.-J.K., G.L.C., B.-K.K., and M.Z. edited and revised manuscript; M.Z. conception and design of research; M.Z. approved final version of manuscript.
REFERENCES
- Adaikkan C, Rosenblum K. The role of protein phosphorylation in the gustatory cortex and amygdala during taste learning. Exp Neurobiol 21: 37–51, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adesnik H, Nicoll RA. Conservation of glutamate receptor 2-containing AMPA receptors during long-term potentiation. J Neurosci 27: 4598–4602, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Rev 29: 83–120, 1999 [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain 152: S49–S64, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asrar S, Zhou Z, Ren W, Jia Z. Ca2+ permeable AMPA receptor induced long-term potentiation requires PI3/MAP kinases but not Ca/CaM-dependent kinase II. PloS One 4: e4339, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barki-Harrington L, Elkobi A, Tzabary T, Rosenblum K. Tyrosine phosphorylation of the 2B subunit of the NMDA receptor is necessary for taste memory formation. J Neurosci 29: 9219–9226, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett TE, Bannister NJ, Collett VJ, Dargan SL, Massey PV, Bortolotto ZA, Fitzjohn SM, Bashir ZI, Collingridge GL, Lodge D. Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacology 52: 60–70, 2007 [DOI] [PubMed] [Google Scholar]
- Berberich S, Punnakkal P, Jensen V, Pawlak V, Seeburg PH, Hvalby Ø, Köhr G. Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. J Neurosci 25: 6907–6910, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman DE, Dudai Y. Memory extinction, learning anew, and learning the new: dissociations in the molecular machinery of learning in cortex. Science 291: 2417–2419, 2001 [DOI] [PubMed] [Google Scholar]
- Berman DE, Hazvi S, Neduva V, Dudai Y. The role of identified neurotransmitter systems in the response of insular cortex to unfamiliar taste: activation of ERK1–2 and formation of a memory trace. J Neurosci 20: 7017–7023, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez-Rattoni F. Molecular mechanisms of taste-recognition memory. Nat Rev Neurosci 5: 209–217, 2004 [DOI] [PubMed] [Google Scholar]
- Bermudez-Rattoni F, Introini-Collison IB, McGaugh JL. Reversible inactivation of the insular cortex by tetrodotoxin produces retrograde and anterograde amnesia for inhibitory avoidance and spatial learning. Proc Natl Acad Sci USA 88: 5379–5382, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez-Rattoni F, Okuda S, Roozendaal B, McGaugh JL. Insular cortex is involved in consolidation of object recognition memory. Learn Mem 12: 447–449, 2005 [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31–39, 1993 [DOI] [PubMed] [Google Scholar]
- Bordi F, Ugolini A. Group I metabotropic glutamate receptors: implications for brain diseases. Prog Neurobiol 59: 55–79, 1999 [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Collett VJ, Conquet F, Jia Z, van der Putten H, Collingridge GL. The regulation of hippocampal LTP by the molecular switch, a form of metaplasticity, requires mGlu5 receptors. Neuropharmacology 49: 13–25, 2005 [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Fitzjohn SM, Collingridge GL. Roles of metabotropic glutamate receptors in LTP and LTD in the hippocampus. Curr Opin Neurobiol 9: 299–304, 1999 [DOI] [PubMed] [Google Scholar]
- Brooks JC, Zambreanu L, Godinez A, Craig AD, Tracey I. Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage 27: 201–209, 2005 [DOI] [PubMed] [Google Scholar]
- Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, mechanisms. Neuropsychopharmacology 33: 18–41, 2008 [DOI] [PubMed] [Google Scholar]
- Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science 330: 1108–1112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conquet F, Bashir ZI, Davies CH, Daniel H, Ferraguti F, Bordi F, Franz-Bacon K, Reggiani A, Matarese V, Conde F, Collingridge GL, Crepel F. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature 372: 237–243, 1994 [DOI] [PubMed] [Google Scholar]
- Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Ann NY Acad Sci 1225: 72–82, 2011 [DOI] [PubMed] [Google Scholar]
- Descalzi G, Li XY, Chen T, Mercaldo V, Koga K, Zhuo M. Rapid synaptic potentiation within the anterior cingulate cortex mediates trace fear learning. Mol Brain 5: 6, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy S, Labrie V, Roder JC. d-Serine augments NMDA-NR2B receptor-dependent hippocampal long-term depression and spatial reversal learning. Neuropsychopharmacology 33: 1004–1018, 2008 [DOI] [PubMed] [Google Scholar]
- Escobar ML, Figueroa-Guzmán Y, Gómez-Palacio-Schjetnan A. In vivo insular cortex LTP induced by brain-derived neurotrophic factor. Brain Res 991: 274–279, 2003 [DOI] [PubMed] [Google Scholar]
- Escobar ML, Alcocer I, Bermudez-Rattoni F. In vivo effects of intracortical administration of NMDA and metabotropic glutamate receptors antagonists on neocortical long-term potentiation and conditioned taste aversion. Behav Brain Res 129: 101–106, 2002 [DOI] [PubMed] [Google Scholar]
- Escobar ML, Bermudez-Rattoni F. Long-term potentiation in the insular cortex enhances conditioned taste aversion retention. Brain Res 852: 208–212, 2000 [DOI] [PubMed] [Google Scholar]
- Escobar ML, Chao V, Bermudez-Rattoni F. In vivo long-term potentiation in the insular cortex: NMDA receptor dependence. Brain Res 779: 314–319, 1998 [DOI] [PubMed] [Google Scholar]
- Ferreira G, Gutierrez R, De La Cruz V, Bermudez-Rattoni F. Differential involvement of cortical muscarinic and NMDA receptors in short- and long-term taste aversion memory. Eur J Neurosci 16: 1139–1145, 2002 [DOI] [PubMed] [Google Scholar]
- Fox CJ, Russell KI, Wang YT, Christie BR. Contribution of NR2A and NR2B NMDA subunits to bidirectional synaptic plasticity in the hippocampus in vivo. Hippocampus 16: 907–915, 2006 [DOI] [PubMed] [Google Scholar]
- Gal-Ben-Ari S, Rosenblum K. Molecular mechanisms underlying memory consolidation of taste information in the cortex. Front Behav Neurosci 5: 87, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadharan V, Wang R, Ulzhofer B, Luo C, Bardoni R, Bali KK, Agarwal N, Tegeder I, Hildebrandt U, Nagy GG, Todd AJ, Ghirri A, Haussler A, Sprengel R, Seeburg PH, MacDermott AB, Lewin GR, Kuner R. Peripheral calcium-permeable AMPA receptors regulate chronic inflammatory pain in mice. J Clin Invest 121: 1608–1623, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover LM, Yan C. Evidence for involvement of group II/III metabotropic glutamate receptors in NMDA receptor-independent long-term potentiation in area CA1 of rat hippocampus. J Neurophysiol 82: 2956–2969, 1999 [DOI] [PubMed] [Google Scholar]
- Guire ES, Oh MC, Soderling TR, Derkach VA. Recruitment of calcium-permeable AMPA receptors during synaptic potentiation is regulated by CaM-kinase I. J Neurosci 28: 6000–6009, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Ramos K, Bermudez-Rattoni F. Post-learning molecular reactivation underlies taste memory consolidation. Front Syst Neurosci 5: 79, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamori T, Kunitake T, Kato K, Kannan H. Responses of neurons in the insular cortex to gustatory, visceral, and nociceptive stimuli in rats. J Neurophysiol 79: 2535–2545, 1998 [DOI] [PubMed] [Google Scholar]
- Henderson LA, Gandevia SC, Macefield VG. Somatotopic organization of the processing of muscle and cutaneous pain in the left and right insula cortex: a single-trial fMRI study. Pain 128: 20–30, 2007 [DOI] [PubMed] [Google Scholar]
- Huang LQ, Rowan MJ, Anwyl R. mGluR II agonist inhibition of LTP induction, and mGluR II antagonist inhibition of LTD induction, in the dentate gyrus in vitro. Neuroreport 8: 687–693, 1997 [DOI] [PubMed] [Google Scholar]
- Huang YY, Li XC, Kandel ER. cAMP contributes to mossy fiber LTP by initiating both a covalently mediated early phase and macromolecular synthesis-dependent late phase. Cell 79: 69–79, 1994 [DOI] [PubMed] [Google Scholar]
- Huang YY, Martin KC, Kandel ER. Both protein kinase A and mitogen-activated protein kinase are required in the amygdala for the macromolecular synthesis-dependent late phase of long-term potentiation. J Neurosci 20: 6317–6325, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. 5-Hydroxytryptamine induces a protein kinase A/mitogen-activated protein kinase-mediated and macromolecular synthesis-dependent late phase of long-term potentiation in the amygdala. J Neurosci 27: 3111–3119, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac JT, Ashby MC, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron 54: 859–871, 2007 [DOI] [PubMed] [Google Scholar]
- Jones MW, French PJ, Bliss TV, Rosenblum K. Molecular mechanisms of long-term potentiation in the insular cortex in vivo. J Neurosci 19: RC36, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SJ, Liu MG, Chen T, Ko HG, Baek GC, Lee HR, Lee K, Collingridge GL, Kaang BK, Zhuo M. Plasticity of metabotropic glutamate receptor-dependent long-term depression in the anterior cingulate cortex after amputation. J Neurosci 32: 11318–11329, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga K, Sim SE, Chen T, Wu LJ, Kaang BK, Zhuo M. Kainate receptor-mediated synaptic transmissions in the adult rodent insular cortex. J Neurophysiol 108: 1988–1998, 2012 [DOI] [PubMed] [Google Scholar]
- Li XY, Ko HG, Chen T, Descalzi G, Koga K, Wang H, Kim SS, Shang Y, Kwak C, Park SW, Shim J, Lee K, Collingridge GL, Kaang BK, Zhuo M. Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science 330: 1400–1404, 2010 [DOI] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 304: 1021–1024, 2004 [DOI] [PubMed] [Google Scholar]
- Liu MG, Lu D, Wang Y, Chen XF, Li Z, Xu Y, Jin JH, Wang RR, Chen J. Counteracting roles of metabotropic glutamate receptor subtypes 1 and 5 in regulation of pain-related spatial and temporal synaptic plasticity in rat entorhinal-hippocampal pathways. Neurosci Lett 507: 38–42, 2012 [DOI] [PubMed] [Google Scholar]
- Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci 30: 126–134, 2007 [DOI] [PubMed] [Google Scholar]
- Lu Y, Allen M, Halt AR, Weisenhaus M, Dallapiazza RF, Hall DD, Usachev YM, McKnight GS, Hell JW. Age-dependent requirement of AKAP150-anchored PKA and GluR2-lacking AMPA receptors in LTP. EMBO J 26: 4879–4890, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YM, Jia Z, Janus C, Henderson JT, Gerlai R, Wojtowicz JM, Roder JC. Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J Neurosci 17: 5196–5205, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man HY. GluA2-lacking, calcium-permeable AMPA receptor-inducers of plasticity? Curr Opin Neurobiol 21: 291–298, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci 24: 7821–7828, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PV, Kandel ER. A macromolecular synthesis-dependent late phase of long-term potentiation requiring cAMP in the medial perforant pathway of rat hippocampal slices. J Neurosci 16: 3189–3198, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H, Wang XD. Neurons in the cortical taste area receive nociceptive inputs from the whole body as well as the oral cavity in the rat. Neurosci Lett 322: 87–90, 2002 [DOI] [PubMed] [Google Scholar]
- O'Leary DM, O'Connor JJ. Priming of long-term potentiation by prior activation of group I and II metabotropic glutamate receptors in the rat dentate gyrus in vitro. Brain Res 809: 91–96, 1998 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KB. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 2001, p. 350 [Google Scholar]
- Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, Collingridge GL, Isaac JT. Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci 9: 602–604, 2006 [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science 290: 533–536, 2000 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Duran LF, Castillo DV, Moguel-Gonzalez M, Escobar ML. Conditioned taste aversion modifies persistently the subsequent induction of neocortical long-term potentiation in vivo. Neurobiol Learn Mem 95: 519–526, 2011 [DOI] [PubMed] [Google Scholar]
- Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature 390: 604–607, 1997 [DOI] [PubMed] [Google Scholar]
- Rosenblum K. Conditioned taste aversion and taste learning: molecular mechanisms. In: Learning and Memory: a Comprehensive Reference, edited by Byrne JH. Oxford, UK: Elsevier, 2008, p. 217–234 [Google Scholar]
- Rosenblum K, Berman DE, Hazvi S, Lamprecht R, Dudai Y. NMDA receptor and the tyrosine phosphorylation of its 2B subunit in taste learning in the rat insular cortex. J Neurosci 17: 5129–5135, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol 69: 143–179, 2003 [DOI] [PubMed] [Google Scholar]
- Sacktor TC. Memory maintenance by PKMzeta—an evolutionary perspective. Mol Brain 5: 31, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinhardt P, Glynn C, Brooks J, McQuay H, Jack T, Chessell I, Bountra C, Tracey I. An fMRI study of cerebral processing of brush-evoked allodynia in neuropathic pain patients. Neuroimage 32: 256–265, 2006 [DOI] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature 401: 63–69, 1999 [DOI] [PubMed] [Google Scholar]
- Toyoda H, Wu LJ, Zhao MG, Xu H, Zhuo M. Time-dependent postsynaptic AMPA GluR1 receptor recruitment in the cingulate synaptic potentiation. Dev Neurobiol 67: 498–509, 2007 [DOI] [PubMed] [Google Scholar]
- Toyoda H, Zhao MG, Zhuo M. Roles of NMDA receptor NR2A and NR2B subtypes for long-term depression in the anterior cingulate cortex. Eur J Neurosci 22: 485–494, 2005 [DOI] [PubMed] [Google Scholar]
- Toyoda H, Zhao MG, Mercaldo V, Chen T, Descalzi G, Kida S, Zhuo M. Calcium/calmodulin-dependent kinase IV contributes to translation-dependent early synaptic potentiation in the anterior cingulate cortex of adult mice. Mol Brain 3: 27, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volianskis A, Bannister N, Collett VJ, Irvine MW, Monaghan DT, Fitzjohn SM, Jensen MS, Jane DE, Collingridge GL. Different NMDA receptor subtypes mediate induction of long-term potentiation and two forms of short-term potentiation at CA1 synapses in rat hippocampus in vitro. J Physiol 591: 955–972, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Li P, Zhuo M. Loss of synaptic depression in mammalian anterior cingulate cortex after amputation. J Neurosci 19: 9346–9354, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Qiu CS, Liauw J, Robinson DA, Ho N, Chatila T, Zhuo M. Calcium-calmodulin-dependent protein kinase IV is required for fear memory. Nat Neurosci 5: 573–579, 2002 [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science 313: 1093–1097, 2006 [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Royle GA, Gray EE, Abdipranoto A, Thangthaeng N, Jacobs N, Saab F, Tonegawa S, Heinemann SF, O'Dell TJ, Fanselow MS, Vissel B. A role for calcium-permeable AMPA receptors in synaptic plasticity and learning. PloS One 5: e12818, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Rowan MJ, Anwyl R. An NMDAR-independent LTP mediated by group II metabotropic glutamate receptors and p42/44 MAP kinase in the dentate gyrus in vitro. Neuropharmacology 46: 311–317, 2004 [DOI] [PubMed] [Google Scholar]
- Wu LJ, Xu H, Ren M, Cao X, Zhuo M. Pharmacological isolation of postsynaptic currents mediated by NR2A- and NR2B-containing NMDA receptors in the anterior cingulate cortex. Mol Pain 3: 11, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yefet K, Merhav M, Kuulmann-Vander S, Elkobi A, Belelovsky K, Jacobson-Pick S, Meiri N, Rosenblum K. Different signal transduction cascades are activated simultaneously in the rat insular cortex and hippocampus following novel taste learning. Eur J Neurosci 24: 1434–1442, 2006 [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Ohmura T, Komatsu Y. Two forms of synaptic plasticity with distinct dependence on age, experience, and NMDA receptor subtype in rat visual cortex. J Neurosci 23: 6557–6566, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XH, Wu LJ, Gong B, Ren M, Li BM, Zhuo M. Induction- and conditioning-protocol dependent involvement of NR2B-containing NMDA receptors in synaptic potentiation and contextual fear memory in the hippocampal CA1 region of rats. Mol Brain 1: 9, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, Jia Y, Shum F, Xu H, Li BM, Kaang BK, Zhuo M. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron 47: 859–872, 2005 [DOI] [PubMed] [Google Scholar]
- Zhuo M. Targeting central plasticity: a new direction of finding painkillers. Curr Pharm Des 11: 2797–2807, 2005 [DOI] [PubMed] [Google Scholar]
- Zhuo M. A synaptic model for pain: long-term potentiation in the anterior cingulate cortex. Mol Cells 23: 259–271, 2007 [PubMed] [Google Scholar]
- Zhuo M. Cortical excitation and chronic pain. Trends Neurosci 31: 199–207, 2008 [DOI] [PubMed] [Google Scholar]
- Zhuo M. Plasticity of NMDA receptor NR2B subunit in memory and chronic pain. Mol Brain 2: 4, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]