Abstract
Dry eye syndrome is a painful condition caused by inadequate or altered tear film on the ocular surface. Primary afferent cool cells innervating the cornea regulate the ocular fluid status by increasing reflex tearing in response to evaporative cooling and hyperosmicity. It has been proposed that activation of corneal cool cells via a transient receptor potential melastatin 8 (TRPM8) channel agonist may represent a potential therapeutic intervention to treat dry eye. This study examined the effect of dry eye on the response properties of corneal cool cells and the ability of the TRPM8 agonist menthol to modify these properties. A unilateral dry eye condition was created in rats by removing the left lacrimal gland. Lacrimal gland removal reduced tears in the dry eye to 35% compared with the contralateral eye and increased the number of spontaneous blinks in the dry eye by over 300%. Extracellular single-unit recordings were performed 8–10 wk following surgery in the trigeminal ganglion of dry eye animals and age-matched controls. Responses of corneal cool cells to cooling were examined after the application of menthol (10 μM–1.0 mM) to the ocular surface. The peak frequency of discharge to cooling was higher and the cooling threshold was warmer in dry eye animals compared with controls. The dry condition also altered the neuronal sensitivity to menthol, causing desensitization to cold-evoked responses at concentrations that produced facilitation in control animals. The menthol-induced desensitization of corneal cool cells would likely result in reduced tearing, a deleterious effect in individuals with dry eye.
Keywords: cold cells, dry eye, rat, sensitization, trigeminal
tears provide nourishment to the anterior eye and are necessary for its protection from potentially damaging stimuli (Dartt 2009). Corneal primary afferent neurons involved in noxious stimulus-evoked tearing include both polymodal and mechanoreceptive neurons (Acosta et al. 2001a, 2004). Increased tearing caused by activation of these afferents is accompanied by irritation or pain (Acosta et al. 2001a, 2001b; Chen et al. 1995). In addition to polymodal and mechanoreceptive afferents, the cornea is innervated by neurons that are sensitive to innocuous cooling (Belmonte and Giraldez 1981; Brock et al. 2001; Hirata and Meng 2010). These corneal cool cells are also activated by menthol, an agonist to the transient receptor potential melastatin 8 (TRPM8) channel, and by hyperosmotic stimuli (Acosta et al. 2001a; Gallar et al. 1993; Hirata and Meng 2010; Madrid et al. 2006). Corneal cool cells are involved in a reflex that promotes tear production in response to drying of the ocular surface (Parra et al. 2010; Robbins et al. 2012). Activation of this tearing reflex does not appear to be accompanied by irritation or pain, which has led to the suggestion that TRPM8 agonists may be useful for the treatment of dry eye (Hirata and Meng 2010; Parra et al. 2010; Robbins et al. 2012).
Dry eye syndrome, a painful condition caused by an inadequate or altered tear film, may be the result of an inability of the lacrimal glands to produce an adequate quantity of tears with the proper composition (Abelson et al. 2009; Barabino and Dana 2007). Alternatively, dry eyes may result from an inability of sensory afferent neurons to monitor the corneal surface, resulting in insufficient neuronal drive to produce a sufficient quantity of tears (Dartt 2004, 2009; Mathers 2000; van Bijsterveld et al. 2003). Furthermore, even if the initial cause of dry eye is dysfunction of the lacrimal gland, it has been suggested that the dry eye condition itself may affect corneal afferents involved in tear regulation, initiating a vicious cycle that may lead to a further deterioration in lacrimal gland function and a worsening of the condition (Mathers 2000).
The effect of dry eye on the corneal epithelium has been examined using several different animal models, including lacrimal gland removal (Barabino and Dana 2004; Fabiani et al. 2009; Kaminer et al. 2011). Although lacrimal gland removal has been used to assess the effectiveness of tear replacement therapies in treating the corneal barrier disruption, little is known regarding the effect of the dry eye condition on the properties of neurons innervating the cornea (Fujihara et al. 2001; Higuchi et al. 2010, 2012). Understanding the effect of dry eye on corneal cool cells is particularly important, since activation of these neurons has been suggested as a potential treatment for dry eye syndrome. In this study, we examined the effect of lacrimal gland removal-induced dry eye on the response properties of corneal cool cells and determined the ability of the TRPM8 agonist menthol to modify these properties.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley rats (Charles River) weighed 200–250 g at the time of surgery and 350–450 g at time of recording. Animals were housed in an environment with a controlled 12:12-h light-dark cycle and allowed free access to food and water. Animals were treated according to the policies and recommendations of the National Institutes of Health guidelines for the handling and use of laboratory animals. All procedures were approved by the Committee on Animal Research at the University of New England.
Surgery.
Under isoflurane anesthesia, a unilateral dry eye was created by removing the left exorbital and infraorbital lacrimal glands (Venable and Grafflin 1940). Carprofen (5 mg/kg sc) was administered once per day for 3 days to provide postsurgical analgesia.
Tear measurements.
In dry eye animals, tears were measured 4 and 8 wk postsurgery. Using fine forceps, cotton phenol red threads (Zone-Quick; Menicon, Waltham, MA) were placed in the lateral canthus of the eye for 30 s in unanesthetized animals. One measurement was taken from each eye. After removal, the length of color change on the phenol red threads was measured under a microscope to the nearest 0.1 mm.
Blink measurements.
Blinks were monitored every other week beginning the second week postsurgery in dry eye animals. Rats were placed in a 24 × 45 × 20-cm (length × width × height) chamber. After a 15-min acclimation period, spontaneous blinks in each eye were counted for 5 min.
Fluorescein staining.
Corneal fluorescein staining was performed to assess the degree of corneal damage. A 1% fluorescein solution (10 μl) was applied to the cornea in isoflurane-anesthetized dry eye animals. After 3 min, the eye was flushed with artificial tears to remove excess fluorescein and examined using cobalt blue light from a slitlamp (PocketScope; Welch Allyn, Skaneateles Falls, NY). The degree of staining was scored based on the 0–4 grading system (Lemp 1995; Nakamura et al. 2005; Suwan-apichon et al. 2006). A score of 0 indicated an absence of punctate fluorescein staining, a score of 1 indicated one-eighth or less of the corneal surface showed staining, a score of 2 indicated one-eighth to one-quarter of the corneal surface showed staining, a score of 3 indicated one-quarter to one-half of the corneal surface showed staining, and a score of 4 was given when greater than one-half of the corneal surface was stained. Corneas were examined every other week beginning the first week after surgery.
Electrophysiological recordings.
Corneal responsive neurons were recorded from the trigeminal ganglion 8–12 wk after removal of the lacrimal gland and in age-matched controls. Under isoflurane anesthesia, the femoral artery and vein were catheterized to monitor blood pressure and deliver drugs, and a tracheotomy was performed for ventilation. Urethane/chloralose anesthetics (500 mg/kg iv urethane and 50 mg/kg iv chloralose) were delivered to replace the isoflurane before recording. This was done to provide consistency with recordings performed in an earlier experiment (Kurose and Meng 2013). End-tidal CO2 was continuously monitored and maintained between 3.5 and 4.5%. Rats were placed in a stereotaxic apparatus, and a partial craniotomy was performed to allow for electrode penetration of the trigeminal ganglion (∼1.8–2.2 posterior and 1.6 lateral to bregma). Extracellular single-unit recordings were carried out using platinum-coated tungsten microelectrodes (500 kΩ; FHC, Bowdoinham, ME). Corneal cool cells were identified by responses that could be evoked by the placement of a cold metal probe (tip diameter ∼1 mm) placed near the receptive field. While the cool-responsive receptive fields of corneal cool cells were checked, the tip of the metal probe was kept 1–3 mm from the corneal surface.
A custom-made chamber was placed around the eye with inflow and outflow portals to allow for the maintenance of a constant environment of artificial tears on the ocular surface. Controlled thermal stimulation with a 5-mm2 contact thermode was used to examine responses to cooling stimuli (TSAII; Medoc, Ramat Yishai, Israel). The thermode was positioned directly on the surface of the chamber, ∼0.4 mm from the surface of the cornea. From a holding temperature of 35°C, the cooling stimulus consisted of a ramp down to 20°C at a rate of 1.5°C/s. The total duration of the cooling stimulus was 150 s. From a holding temperature of 35°C, the heating stimulus consisted of a ramp up to 52°C at a rate of 3.4°C/s. The total duration of the heating stimulus was 20 s. Data were acquired by a CED Micro 1401 data acquisition unit, and isolated neurons were analyzed with Spike2 (Cambridge Electronic Design, Cambridge, UK).
Menthol application.
After control thermal stimulation of the cornea, the fluid in the chamber was removed and replaced with either vehicle or menthol (0.01–1.0 mM). A maximal concentration of 0.5 mM menthol was applied in dry eye animals, since preliminary experiments showed that 1 mM menthol in dry eye animals produced complete and long-lasting (>20 min) inhibition of activity. Cooling was applied 5 min after menthol application. After the cooling stimulus was applied, the chamber was drained and flushed several times with artificial tears. A minimum of 30 min was allowed between successive applications of menthol to allow for cold-evoked activity to return to baseline levels. Menthol (Sigma-Aldrich, St. Louis, MO) was made from a 10 mM stock solution in 40% ethanol and then diluted with artificial tears to the desired concentration. Vehicle consisted of 0.4% ethanol in artificial tears (in mM: 106.5 NaCl, 26.1 NaHCO3, 18.7 KCl, 1.0 MgCl2, 0.5 NaH2PO4, 1.1 CaCl2, and 10 HEPES; pH 7.45). In preliminary pilot studies, we found that high concentrations of ethanol (>10%) often suppressed cool cell activity; however, lower concentrations (4% ethanol) had no effect.
Statistical analysis.
Baseline activity was defined as the ongoing unit activity at the 35°C holding temperature for 30 s before the onset of each stimulus. To assess the responses evoked by cooling, the average frequency of cold-evoked activity during the beginning (0–30 s), middle (60–90 s), and end of the thermal stimulus (120–150 s) was calculated after the baseline activity was subtracted. To determine the effect of menthol on the average cold-evoked discharge during these three time periods, the frequency of activity during these time points in a predrug artificial tear control trial was subtracted from the frequency of activity evoked after vehicle or menthol application. The peak frequency was recorded as the highest frequency (spikes/s) value recorded during the cooling ramp. The cooling threshold was defined as the temperature (°C) at which the average number of spikes/s increased to a value greater than the mean frequency of the ongoing baseline activity plus three times its standard deviation (3SD) (Parra et al. 2010). Heat-evoked discharge was calculated by subtracting the average baseline activity from the average activity evoked during the stimulus. Menthol-evoked discharge was determined using the response magnitude (Rmag), which was calculated by subtracting the mean baseline activity plus 2SD from the activity evoked 0–30 and 30–120 s after drug application (Robbins et al. 2012).
Comparisons between multiple groups were made using either a one-way or two-way analysis of variance (ANOVA) with repeated measures after it was determined that the data set conformed to a normal distribution with equal variances, followed by Tukey's multiple comparison post hoc tests. In cases where the normality or equal variance tests failed, two groups were compared using the Mann-Whitney rank sum test, and multiple group comparisons were made using Kruskal-Wallis one-way ANOVA on ranks test, followed by Dunn's multiple comparison post hoc tests for nonparametric data. The association between the treatment group and the type of heat response (facilitation vs. inhibition) was evaluated using Pearson's χ2 test. Facilitation was defined as Rmag > 0 during the heat stimulus. Analysis was performed using SigmaStat version 3.5 (Systat Software, Chicago, IL). In all cases, data are represented as the treatment group mean ± SE, and P < 0.05 was considered to be statistically significant.
RESULTS
Dry eye condition.
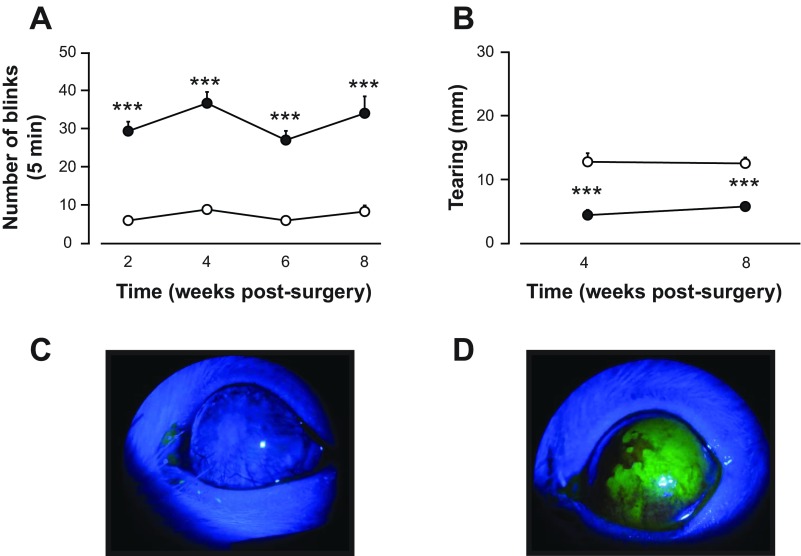
After lacrimal gland removal, spontaneous blinking, aqueous tear production, and fluorescein staining of the cornea were examined to assess the dry eye condition. Spontaneous blinking was monitored in alternate weeks beginning the second week after surgery and continuing over the course of 8 wk. A significant difference in the total number of blinks recorded during the 5-min observation period was found between the left and right eyes (Fig. 1A, P < 0.001, 2-way ANOVA with repeated measures). At the 2-wk time point, the number of blinks on the dry eye side was more than 4 times higher than the number of blinks recorded from the contralateral eye, an increase that persisted over the 8-wk observation period.
Fig. 1.
Effect of lacrimal gland removal on blinking, tearing, and corneal fluorescein staining. A: spontaneous blinking counted over a 5-min observation period was elevated in the eye ipsilateral to the side of gland removal (filled circles) compared with the contralateral eye (open circles) over an 8-wk period. B: tear measurements taken with a cotton thread in unanesthetized animals (see materials and methods) were lower on the side of lacrimal gland removal (filled circles) compared with the contralateral side (open circles). C: photomicrograph taken from a control animal demonstrating the absence of corneal fluorescein staining. D: corneal fluorescein staining in an animal 8 wk after lacrimal gland removal. In this case, the area of positive staining is >50% of the corneal surface, representing a score of 4 on the fluorescein rating scale. ***P < 0.001 vs. measurements taken from the contralateral side.
Using the cotton thread test, quantification of tears after lacrimal gland removal revealed a significant difference between the two eyes. Tears in the left eye were almost 3 times lower than those measured from the right eye at 4 wk postsurgery and remained suppressed at the 8-wk time point (Fig. 1B, P < 0.001, 2-way ANOVA with repeated measures). Fluorescein staining was examined before recording to determine the degree of corneal damage. All animals had greater than one-eighth of the cornea stained positively with fluorescein, with corresponding scores between 1 and 4 on the rating scale (Fig. 1, C and D).
Thermal responses in corneal cool cells.
A total of 22 neurons in 22 dry eye animals and 27 neurons in 25 lacrimal gland intact control animals were recorded from the trigeminal ganglion with corneal receptive fields. Receptive fields consisted of a small region of the cornea (<0.5 mm2) and did not cross over to the conjunctiva. As is typical of cool cells, neurons had ongoing activity at room temperature and at the chamber holding temperature of 35°C with a Peltier thermode. At this holding temperature, neurons recorded in dry eye animals did not display any difference in ongoing activity compared with controls (4.2 ± 1.0 vs. 5.3 ± 0.7 spikes/s for dry eye and control animals, respectively; P > 0.05, Mann-Whitney rank sum test).
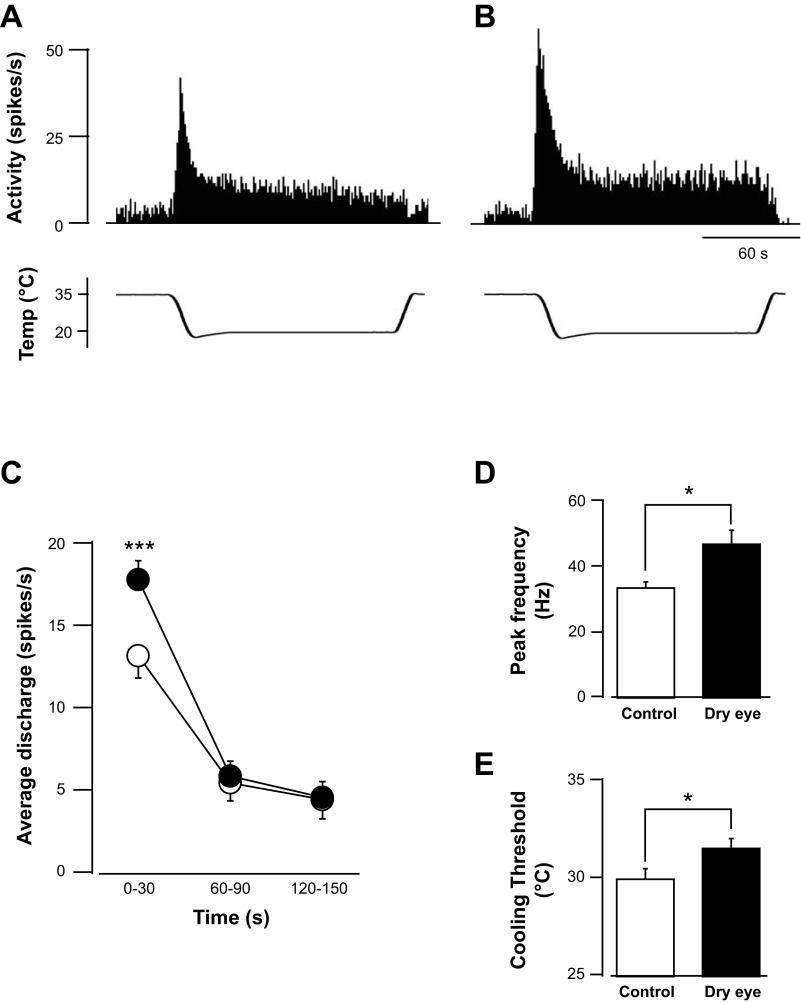
All neurons responded to cooling of the receptive field with an increase in activity. Cold-evoked activity consisted of a robust dynamic phase during the change in temperature (phasic period) followed by a relatively lower degree of activity during the temperature plateau (static period). Neurons recorded in both control and dry eye animals displayed a similar temporal pattern of activity to the cooling stimulus (Fig. 2, A and B). The dynamic and static phases were examined by analyzing activity at three different time periods from the start of the cooling stimulus: 0–30, 60–90, and 120–150 s. A comparison of activity in dry eye and control animals indicated a difference only during the first epoch (Fig. 2C). The average number of spikes during the dynamic phase of cooling was significantly greater in dry eye animals compared with controls (17.7 ± 1.1 vs. 13.6 ± 1.1 spikes/s, respectively, P < 0.05, 2-way ANOVA with repeated measures). No difference in evoked activity during the static phase of cooling was found (Fig. 2C).
Fig. 2.
Effect of the dry eye condition on cold-evoked activity. Examples of cold-evoked discharge in a control (A) and dry eye animal (B) are shown. Similar temporal patterns of activity were found in both groups of animals. Activity was greatest during the change in temperature (phasic period) and decreased to a stable level at the temperature plateau (static period). C: average discharge evoked during the cooling stimulus, divided into 3 epochs: 0–30, 60–90, and 120–150 s. Cold-evoked activity was increased in dry eye animals (filled circles) compared with controls (open circles) only during the initial, phasic period of cooling. D: group average peak frequency of activation, which occurred during the phasic period of the cooling stimulus, in control and dry eye animals. E: average temperature threshold for activating cool cells in control and dry eye animals (n = 27 units in control animals and 22 units in dry eye animals). *P < 0.05; ***P < 0.001.
Additional analysis was consistent in demonstrating an increased sensitivity to the cooling stimulus in dry eye animals. The peak frequency of activity, which occurred during the dynamic phase of cooling, was 1.4 times greater in dry eye animals compared with controls (Fig. 2D, P < 0.05, Mann-Whitney rank sum test). Furthermore, the cooling threshold in dry eye animals of 31.5 ± 0.5°C was significantly higher than the threshold of 29.9 ± 0.5°C recorded in controls (Fig. 2E, P < 0.05, Mann-Whitney rank sum test).
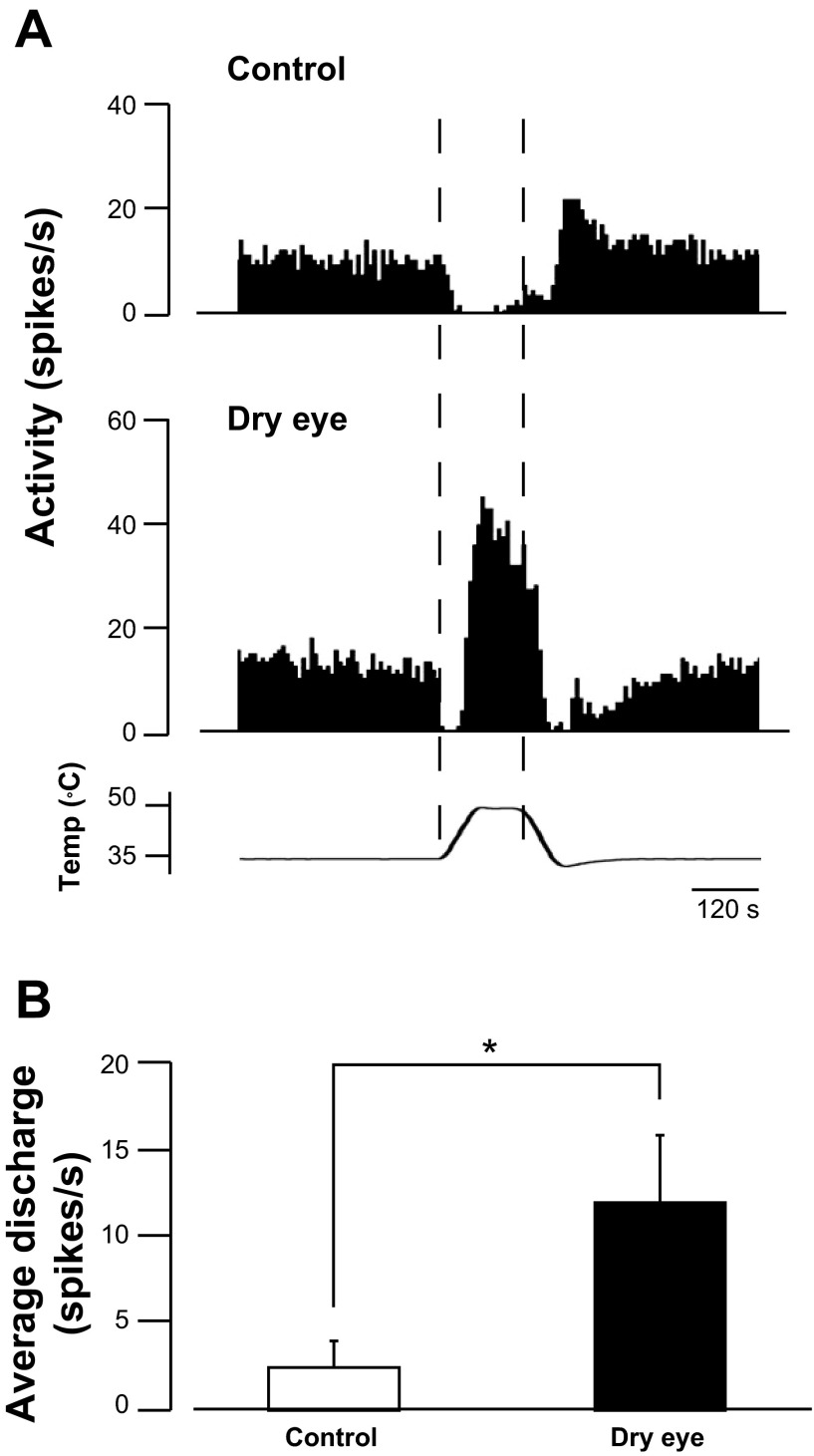
The response of cool cells to noxious heat was compared in dry eye and control animals. Although cool cells are often inhibited by noxious heat (Fig. 3A, top), they are sometimes activated by noxious heat after a brief period of inhibition, often referred to as the “paradoxical” heat response (Long 1973, 1977). Neurons recorded in dry eye animals were significantly more likely to be activated by noxious heat, which was defined as a positive Rmag value (P < 0.01, χ2 analysis). In control animals, 18.5% (5/27) of the neurons were activated by noxious heat, whereas 54.5% (12/22) of the neurons recorded in dry eye animals were activated by the same stimulus. Neurons recorded in dry eye animals, however, still displayed a brief period of inhibition before their activation (Fig. 3A, bottom). Overall, the mean frequency of the heat response in dry eye animals was significantly greater than the heat response in control animals (11.9 ± 3.9 vs. 2.5 ± 1.5 spikes/s, respectively, P < 0.05, Mann-Whitney rank sum test, Fig. 3B). The cooling temperature thresholds did not differ between heat-sensitive and heat-insensitive neurons. Cooling temperature thresholds in heat-insensitive and heat-sensitive neurons recording from control animals were 29.9 ± 0.6 and 30.0 ± 0.8°C, respectively, whereas these thresholds in heat-insensitive and heat-sensitive neurons in dry eye animals were 31.9 ± 0.6 and 31.1 ± 0.8°C, respectively.
Fig. 3.
Responses to noxious heat in control and dry eye animals. A: example of an inhibitory response to noxious heat recorded in a control animal (top) and an excitatory response by the same stimulus in a dry eye animal (bottom). B: overall, mean discharge evoked during heating was greater in dry eye animals compared with control animals (n = 27 units in control animals and 22 units in dry eye animals). *P < 0.05.
Menthol responses in corneal cool cells.
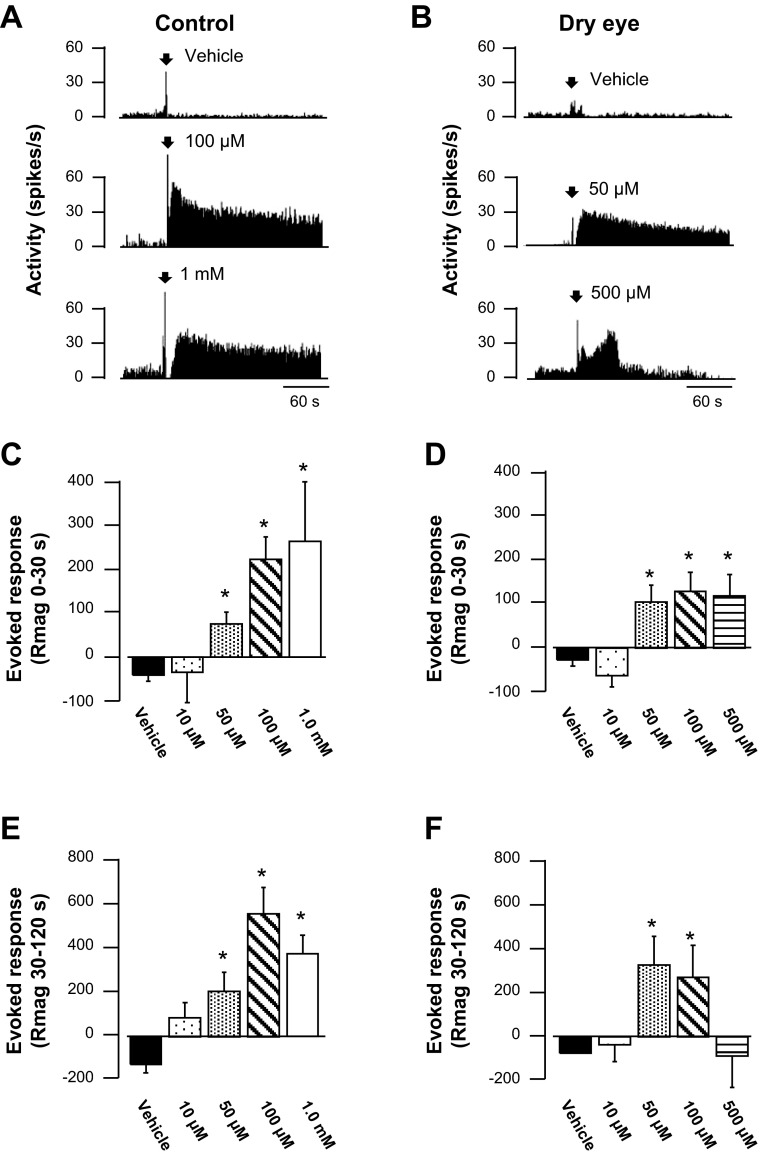
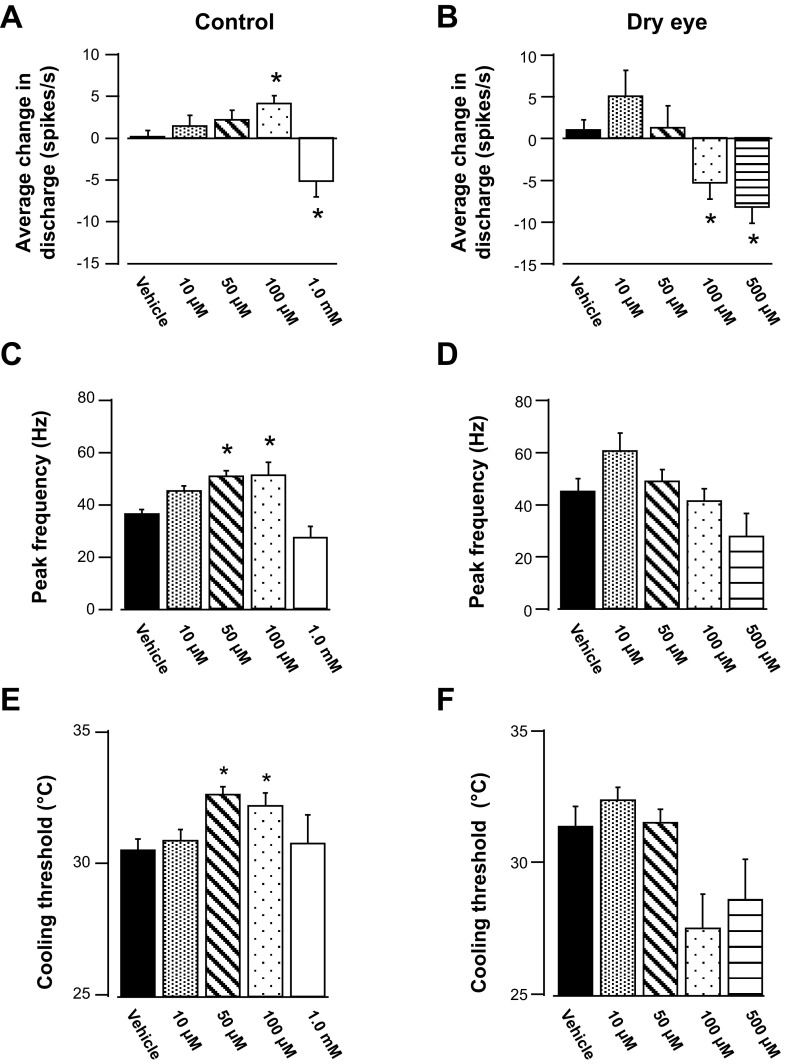
The effect of topical menthol application on the ongoing activity was compared in dry eye and control animals. In control animals, menthol concentrations at or above 50 μM evoked sustained discharge for several minutes (Fig. 4A). Menthol produced a similar effect in dry eye animals at lower concentrations (Fig. 4B); however, at the highest concentration (500 μM), menthol increased discharge for a brief period of time, followed by a longer period of inhibition (Fig. 4B, bottom). This difference between control and dry eye animals in their response to menthol was examined by analyzing the response magnitude from 0–30 and 30–120 s after menthol application. In control animals, menthol produced a concentration-dependent increase in activity during the initial 30-s period compared with vehicle (Fig. 4C, P < 0.05, Kruskal-Wallis). The initial response to menthol in dry eye animals also consisted of increased discharge beginning at the 50 μM concentration, although the responses to higher concentrations were blunted (Fig. 4D, P < 0.05, Kruskal-Wallis). The increase in response magnitude was sustained in control animals, even at the highest menthol concentrations tested, as determined by analysis of the time period 30–120 s after menthol application (Fig. 4E, P < 0.05, Kruskal-Wallis). In contrast, discharge in dry eye animals was maintained only for the lower concentrations of menthol (50 and 100 μM, P < 0.05, Kruskal-Wallis), whereas discharge after the highest concentration of menthol was not greater than vehicle during the time period 30–120 s (Fig. 4F, P > 0.05, Kruskal-Wallis).
Fig. 4.
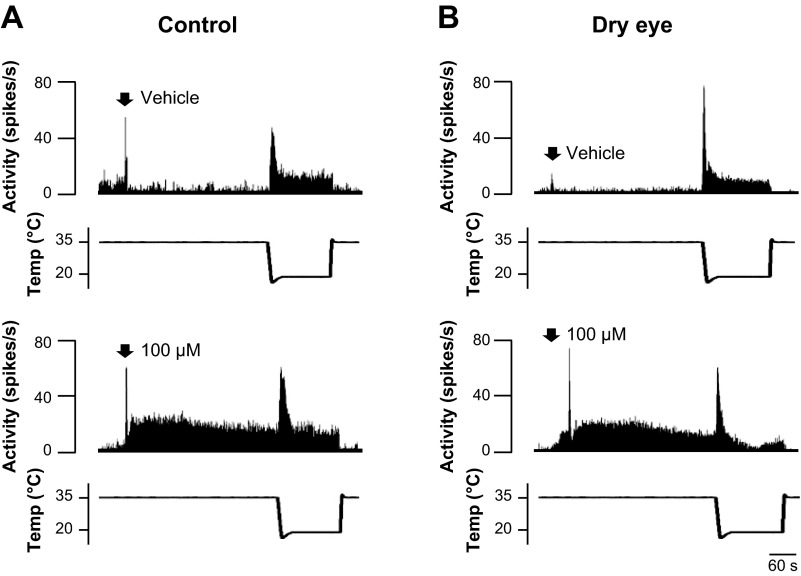
Menthol-evoked discharge in control and dry eye animals. A: example of neuronal activity following application of vehicle, 100 μM menthol, and 1.0 mM menthol in a control animal. B: neuronal activity in a corneal unit following vehicle, 50 μM menthol, and 500 μM menthol in a dry eye animal. C: average response magnitude (Rmag) of units recorded in control animals calculated 0–30 s after the application of menthol. D: average Rmag 0–30 s after menthol application in dry eye animals. E: average Rmag recorded 30–120 s following menthol application in control animals. F: average Rmag calculated 30–120 s following menthol application in dry eye animals. In control animals, n = 27, 8, 11, 19, and 6 for vehicle, 10 μM, 50 μM, 100 μM, and 1 mM menthol, respectively. In dry eye animals, n = 22, 8, 17, 20, and 9 for vehicle, 10 μM, 50 μM, 100 μM, and 500 μM menthol, respectively. *P < 0.05 vs. vehicle.
Cold-evoked discharge after menthol.
The response to corneal cooling was examined 5 min after menthol application in dry eye and control animals (Fig. 5). Breaking down the cooling response pattern into the initial phasic (0–30 s) and static (120–150 s) periods revealed significant differences between control and dry eye animals after menthol application. During the phasic period of cooling, 100 μM menthol produced a significant increase in activity in control animals (Fig. 6A, P < 0.05, Kruskal-Wallis, see Fig. 5A for example). In contrast, this same concentration of menthol in dry eye animals produced a decrease in discharge (Fig. 6B, P < 0.05, Kruskal-Wallis, see Fig. 5B for example). Decreased discharge was also the result after application of the highest concentration of menthol in both control and dry eye animals (Fig. 5, A and B, P < 0.05, Kruskal-Wallis). Menthol-induced changes in peak frequency discharges were generally consistent with those changes observed during the phasic period of cooling (Fig. 6, C and D). During the static period, menthol did not significantly affect cold-evoked activity in control animals (Table 1, P > 0.05, Kruskal-Wallis). However, in dry eye animals the highest concentration of menthol decreased cold-evoked activity (Table 1, P < 0.05, Kruskal-Wallis).
Fig. 5.
Examples of the response to cooling applied 5 min after the application of vehicle (top) and 100 μM menthol (bottom). A: in a cool cell recorded from a control animal, menthol produced an increase in cold-evoked activity during the initial phasic period of cooling. B: in the dry eye animal, the same concentration of menthol inhibited the response to cooling.
Fig. 6.
Effect of menthol on the response to cooling in control (A, C, E) and dry eye animals (B, D, F). A: average discharge during the initial 30 s of cooling (phasic period) after vehicle and increasing concentrations of menthol in control animals. B: average discharge during the initial 30 s of cooling in dry eye animals. C: average peak frequency responses to cooling after vehicle and increasing concentrations of menthol in control animals. D: average peak frequency responses in dry eye animals. E: average cooling thresholds in control animals after vehicle or menthol application to the cornea. F: average cooling thresholds in dry eye animals after vehicle or menthol application. The number of neurons for each group is the same as in Fig. 4. *P < 0.05 vs. vehicle.
Table 1.
Effect of menthol on cold-evoked responses during the static period of cooling in control and dry eye animals
| Menthol Concentration |
||||||
|---|---|---|---|---|---|---|
| Vehicle | 10 μM | 50 μM | 100 μM | 500 μM | 1.0 mM | |
| Control | 0.67 ± 0.53 | 0.39 ± 1.63 | −0.22 ± 1.00 | 0.21 ± 0.88 | −2.91 ± 2.23 | |
| Dry eye | 0.55 ± 0.51 | 5.20 ± 2.35 | 0.57 ± 1.77 | −2.66 ± 1.01 | −5.09 ± 1.40* | |
Values represent average discharge (spikes/s) evoked during the final 30 s of the cooling stimulus, calculated by taking the difference in cold-evoked discharge during a baseline stimulation trial from cold discharge evoked after either vehicle or menthol application (see materials and methods for details).
P < 0.05 vs. vehicle treatment, Kruskal- Wallis 1-way ANOVA on ranks and Dunn's methods.
Menthol also produced concentration-dependent changes in temperature thresholds. In control animals, both 50 and 100 μM menthol produced a decrease in the cooling threshold compared with vehicle, because neurons responded to the cooling stimulus at warmer temperatures (Fig. 6E, P < 0.05, Kruskal-Wallis). However, no change in threshold occurred after the application of either the lowest (10 μM) or highest (1.0 mM) menthol concentrations. In dry eye animals, menthol failed to produce significant changes in cooling thresholds, although a trend toward an increase in threshold after 10 μM menthol and a decrease in threshold after 100 and 500 μM menthol was observed (Fig. 6F, P > 0.05, Kruskal-Wallis).
DISCUSSION
In this study, we examined the effect of a dry eye condition on the response properties of corneal cool cells, a set of afferent neurons that mediate a tearing reflex in response to evaporation of the tear film. As expected, and consistent with previous reports (Fujihara et al. 2001; Higuchi et al. 2010, 2012; Kaminer et al. 2011), removal of the lacrimal glands reduced tear production, increased blinking, and caused an increase in epithelial cell fluorescein staining. The chronic dry eye condition also sensitized corneal cool cells, evidenced by an increase in cold-evoked responses and an increase in the temperature activation threshold. The response to menthol was also altered in dry eye animals in a manner consistent with an increase in sensitivity to TRPM8 activation. In control animals, menthol caused a dose-dependent change in cold-evoked responses, increasing activity at lower concentrations but decreasing activity at the highest concentrations. A similar trend was observed in dry eye animals; however, under the dry eye condition menthol decreased cold-evoked responses at concentrations 10 times lower than those that decreased responses in control animals.
Previous studies have used lacrimal gland removal to produce a dry condition on the ocular surface (Fujihara et al. 2001; Higuchi et al. 2010, 2012; Kaminer et al. 2011). Using this dry eye model, investigators have examined the central circuitry underlying spontaneous blinking, which involves processing in the spinal trigeminal nucleus and input from the basal ganglia (Kaminer et al. 2011). This model has also been used to assess the ability of eye drop treatments to reduce corneal fluorescein staining and other indicators of corneal epithelial barrier disruption (Fujihara et al. 2001; Higuchi et al. 2010, 2012). Alterations in corneal epithelial cells in dry eye, however, may not be indicative of changes in the properties of corneal primary afferent neurons. In the present study, characterization of corneal cool cells under these conditions has revealed significant alterations, including an increase in the sensitivity to cooling.
The mechanism of sensitization in corneal cool cells in dry eye animals remains unknown. Cold allodynia has been reported in nerve injury-induced chronic pain models, yet the sensitization of primary afferent cool cells under these conditions has not been described (Cha et al. 2012; Chichorro et al. 2006; Rossi et al. 2012). In one study that examined the properties of cultured trigeminal ganglion neurons after infraorbital nerve injury, both cold- and menthol-evoked responses were diminished after nerve injury, which was in contrast to the increase in heat responses found under similar conditions (Schmid et al. 2011). Dry eye, however, may have more in common with a chronic inflammatory condition on the ocular surface than a nerve injury state.
Induction of dry eye in rodents, produced by either environmentally controlled arid conditions or the injection of choleratoxin B into the lacrimal gland, increased the corneal expression of inflammatory cytokines (Dursun et al. 2002; Lee et al. 2012; Okanobo et al. 2012; Shim et al. 2012; Stevenson et al. 2012; Zhu et al. 2009, 2012). However, just as nerve injury reduced cold- and menthol-evoked activity, inflammation also appears to inhibit TRPM8-mediated responses. In cultured dorsal root ganglion (DRG) neurons, the inflammatory mediators bradykinin and prostaglandin E2 initially increased calcium influx in a subset of cold- and menthol-sensitive neurons (Linte et al. 2007). However, these neurons were subsequently less sensitive to cooling, demonstrating a reduction in the overall amplitude in response to cooling as well as lower cold temperature thresholds.
Inflammatory mediators have also been shown to produce inhibitory effects on cold responses recorded from corneal primary afferent terminals (Zhang et al. 2012). Furthermore, in this same study, carrageenan-induced inflammation of the tongue inhibited cold-evoked activity in lingual afferent nerve fibers. Based on the results from these studies, inflammatory mediators appear unlikely to account for the increase in cold-evoked responses recorded in dry eye animals. Instead, other mediators, such as neuronal growth factors, may be involved in sensitizing corneal cool cells in the dry eye condition. Nerve growth factor (NGF) levels are increased in dry eye disease, and NGF increased cold sensitivity and the percentage of menthol-sensitive DRG neurons in culture (Babes et al. 2004; Lambiase et al. 2011).
Inflammatory mediators may play more of a role in increasing the heat-evoked responses observed in dry eye animals. Several studies have demonstrated inflammation-induced sensitization of heat-evoked activity in polymodal nociceptive neurons (LaMotte et al. 1992; Liang et al. 2001; Szolcsanyi 1987) through the phosphorylation of noxious heat-sensitive TRPV1 channels (Mizumura et al. 2009; Moriyama et al. 2005; Sugiura et al. 2002). Similarly, an inflammatory soup applied to the cornea has been demonstrated to increase heat-evoked responses in cool afferent fibers even while inhibiting cold-evoked responses (Zhang et al. 2012). These apparently disparate results may be due to the fact that a number of signaling pathways that sensitize the heat-activated TRPV1 channels desensitize TRPM8 channels (reviewed by Patapoutian et al. 2009). Alternatively, the increase in heat-evoked responses may be caused by changes in the properties of TRPV1-positive polymodal nociceptors that leave them responsive to cooling. Along these lines, an increase in TRPM8 expression and menthol sensitivity in capsaicin-sensitive neurons has been reported in acutely dissociated DRG neurons following chronic constriction nerve injury (Xing et al. 2007).
In addition to altering thermal stimulation-evoked responses, the dry eye condition also shifted the concentration response to menthol. A biphasic response to menthol has previously been described (Klein et al. 2010, 2012). In these studies, low concentrations of menthol produced an increase in sensitivity to cold stimuli and enhanced cold-evoked neuronal discharge from spinal cord dorsal horn neurons, whereas high concentrations caused a decrease in cold sensitivity and inhibited cold-evoked discharge from dorsal horn neurons. In the dry eye animal, the desensitization following 500 μM menthol is similar to an effect previously described after 50 mM menthol in control animals (Robbins et al. 2012). This effect is also reminiscent of the capsaicin-induced desensitization via TRPV1 in polymodal nociceptors (Koplas et al. 1997; Mohapatra and Nau 2003). The shift in sensitivity to menthol concentration in the dry eye condition was also reflected by alterations in cold-evoked activity following menthol application. Whereas lower concentrations of menthol increased cold-evoked activity and cooling thresholds in control animals, similar concentrations of menthol produced the opposite effect, causing a decrease in cold-evoked responses and thresholds.
The increased sensitivity of cool cells to the desensitizing effects of menthol may be the result of increased TRPM8 channel expression, which has been reported after nerve injury (Xing et al. 2007). These results may also reflect changes in the regulation of TRPM8 channels in the dry eye condition. The regulation of TRPM8 channels, a calcium-dependent process, has been suggested to play a role in the rapid adaptation to prolonged cooling stimulation and involves several factors (Abe et al. 2006; Daniels et al. 2009; Sarria and Gu 2010; Sarria et al. 2011; Thut et al. 2003). TRPM8 activity is increased by the membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) and reduced by phospholipase C-mediated hydrolysis of PIP2 (Daniels et al. 2009; Rohacs et al. 2005; Yudin et al. 2011; Yudin and Rohacs 2012). Of note, these studies have all been performed in vitro, often in DRG cultured neurons, and the involvement of similar factors in TRPM8 regulation in vivo still requires verification. Additionally, alterations in the properties of other cold-sensitive channels, including members of the KCNK channel family or TRPA1, may play a role in the sensitization of cool cells in the dry eye condition (Thut et al. 2003).
Activation of TRPM8 channels has been proposed as a treatment for dry eye, offering potential benefits over current treatments that involve the repeated application of lubricating artificial tears (Hirata and Meng 2010; Parra et al. 2010; Robbins et al. 2012). Although the application of menthol under control conditions may increase tear secretions without producing unwanted side effects, our results suggest that this may not be the case in the dry eye condition. Instead, TRPM8 activation in dry eye could result in the desensitization of neurons involved in the production of tears. Thus menthol, contained in some over-the-counter eye drops, may have detrimental effects in dry eye sufferers.
GRANTS
Funding for these studies was provided by National Institutes of Health Grants R01 EY021230 (to I. D. Meng) and P20 GM103643.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.K. and I.D.M. conception and design of research; M.K. performed experiments; M.K. analyzed data; M.K. and I.D.M. interpreted results of experiments; M.K. and I.D.M. prepared figures; M.K. and I.D.M. edited and revised manuscript; M.K. and I.D.M. approved final version of manuscript; I.D.M. drafted manuscript.
ACKNOWLEDGMENTS
We thank Christine Graham for helpful comments on an earlier version of this manuscript.
REFERENCES
- Abe J, Hosokawa H, Sawada Y, Matsumura K, Kobayashi S. Ca2+-dependent PKC activation mediates menthol-induced desensitization of transient receptor potential M8. Neurosci Lett 397: 140–144, 2006 [DOI] [PubMed] [Google Scholar]
- Abelson MB, Ousler GW, 3rd, Maffei C. Dry eye in 2008. Curr Opin Ophthalmol 20: 282–286, 2009 [DOI] [PubMed] [Google Scholar]
- Acosta MC, Belmonte C, Gallar J. Sensory experiences in humans and single-unit activity in cats evoked by polymodal stimulation of the cornea. J Physiol 534: 511–525, 2001a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta MC, Peral A, Luna C, Pintor J, Belmonte C, Gallar J. Tear secretion induced by selective stimulation of corneal and conjunctival sensory nerve fibers. Invest Ophthalmol Vis Sci 45: 2333–2336, 2004 [DOI] [PubMed] [Google Scholar]
- Acosta MC, Tan ME, Belmonte C, Gallar J. Sensations evoked by selective mechanical, chemical, and thermal stimulation of the conjunctiva and cornea. Invest Ophthalmol Vis Sci 42: 2063–2067, 2001b [PubMed] [Google Scholar]
- Babes A, Zorzon D, Reid G. Two populations of cold-sensitive neurons in rat dorsal root ganglia and their modulation by nerve growth factor. Eur J Neurosci 20: 2276–2282, 2004 [DOI] [PubMed] [Google Scholar]
- Barabino S, Dana MR. Animal models of dry eye: a critical assessment of opportunities and limitations. Invest Ophthalmol Vis Sci 45: 1641–1646, 2004 [DOI] [PubMed] [Google Scholar]
- Barabino S, Dana MR. Dry eye syndromes. Chem Immunol Allergy 92: 176–184, 2007 [DOI] [PubMed] [Google Scholar]
- Belmonte C, Giraldez F. Responses of cat corneal sensory receptors to mechanical and thermal stimulation. J Physiol 321: 355–368, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock JA, Pianova S, Belmonte C. Differences between nerve terminal impulses of polymodal nociceptors and cold sensory receptors of the guinea-pig cornea. J Physiol 533: 493–501, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha M, Kohan KJ, Zuo X, Ling JX, Gu JG. Assessment of chronic trigeminal neuropathic pain by the orofacial operant test in rats. Behav Brain Res 234: 82–90, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Gallar J, Pozo MA, Baeza M, Belmonte C. CO2 stimulation of the cornea: a comparison between human sensation and nerve activity in polymodal nociceptive afferents of the cat. Eur J Neurosci 7: 1154–1163, 1995 [DOI] [PubMed] [Google Scholar]
- Chichorro JG, Zampronio AR, Souza GE, Rae GA. Orofacial cold hyperalgesia due to infraorbital nerve constriction injury in rats: reversal by endothelin receptor antagonists but not non-steroidal anti-inflammatory drugs. Pain 123: 64–74, 2006 [DOI] [PubMed] [Google Scholar]
- Daniels RL, Takashima Y, McKemy DD. Activity of the neuronal cold sensor TRPM8 is regulated by phospholipase C via the phospholipid phosphoinositol 4,5-bisphosphate. J Biol Chem 284: 1570–1582, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartt DA. Dysfunctional neural regulation of lacrimal gland secretion and its role in the pathogenesis of dry eye syndromes. Ocul Surf 2: 76–91, 2004 [DOI] [PubMed] [Google Scholar]
- Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res 28: 155–177, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dursun D, Wang M, Monroy D, Li DQ, Lokeshwar BL, Stern M, Pflugfelder SC. Experimentally induced dry eye produces ocular surface inflammation and epithelial disease. Adv Exp Med Biol 506: 647–655, 2002 [DOI] [PubMed] [Google Scholar]
- Fabiani C, Barabino S, Rashid S, Dana MR. Corneal epithelial proliferation and thickness in a mouse model of dry eye. Exp Eye Res 89: 166–171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihara T, Murakami T, Fujita H, Nakamura M, Nakata K. Improvement of corneal barrier function by the P2Y2 agonist INS365 in a rat dry eye model. Invest Ophthalmol Vis Sci 42: 96–100, 2001 [PubMed] [Google Scholar]
- Gallar J, Pozo MA, Tuckett RP, Belmonte C. Response of sensory units with unmyelinated fibres to mechanical, thermal and chemical stimulation of the cat's cornea. J Physiol 468: 609–622, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi A, Inoue H, Kawakita T, Ogishima T, Tsubota K. Selenium compound protects corneal epithelium against oxidative stress. PLoS One 7: e45612, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi A, Takahashi K, Hirashima M, Kawakita T, Tsubota K. Selenoprotein P controls oxidative stress in cornea. PLoS One 5: e9911, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Meng ID. Cold-sensitive corneal afferents respond to a variety of ocular stimuli central to tear production: implications for dry eye disease. Invest Ophthalmol Vis Sci 51: 3969–3976, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminer J, Powers AS, Horn KG, Hui C, Evinger C. Characterizing the spontaneous blink generator: an animal model. J Neurosci 31: 11256–11267, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AH, Sawyer CM, Carstens MI, Tsagareli MG, Tsiklauri N, Carstens E. Topical application of l-menthol induces heat analgesia, mechanical allodynia, and a biphasic effect on cold sensitivity in rats. Behav Brain Res 212: 179–186, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AH, Sawyer CM, Takechi K, Davoodi A, Ivanov MA, Carstens MI, Carstens E. Topical hindpaw application of l-menthol decreases responsiveness to heat with biphasic effects on cold sensitivity of rat lumbar dorsal horn neurons. Neuroscience 219: 234–242, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koplas PA, Rosenberg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J Neurosci 17: 3525–3537, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurose M, Meng ID. Corneal dry-responsive neurons in the spinal trigeminal nucleus respond to innocuous cooling in the rat. J Neurophysiol 109: 2517–2522, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambiase A, Micera A, Sacchetti M, Cortes M, Mantelli F, Bonini S. Alterations of tear neuromediators in dry eye disease. Arch Ophthalmol 129: 981–986, 2011 [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Lundberg LE, Torebjork HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol 448: 749–764, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Hattori T, Park EY, Stevenson W, Chauhan SK, Dana R. Expression of toll-like receptor 4 contributes to corneal inflammation in experimental dry eye disease. Invest Ophthalmol Vis Sci 53: 5632–5640, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemp MA. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J 21: 221–232, 1995 [PubMed] [Google Scholar]
- Liang YF, Haake B, Reeh PW. Sustained sensitization and recruitment of rat cutaneous nociceptors by bradykinin and a novel theory of its excitatory action. J Physiol 532: 229–239, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linte RM, Ciobanu C, Reid G, Babes A. Desensitization of cold- and menthol-sensitive rat dorsal root ganglion neurones by inflammatory mediators. Exp Brain Res 178: 89–98, 2007 [DOI] [PubMed] [Google Scholar]
- Long RR. Cold fiber heat sensitivity: dependency of “paradoxical” discharge on body temperature. Brain Res 63: 389–392, 1973 [DOI] [PubMed] [Google Scholar]
- Long RR. Sensitivity of cutaneous cold fibers to noxious heat: paradoxical cold discharge. J Neurophysiol 40: 489–502, 1977 [DOI] [PubMed] [Google Scholar]
- Madrid R, Donovan-Rodriguez T, Meseguer V, Acosta MC, Belmonte C, Viana F. Contribution of TRPM8 channels to cold transduction in primary sensory neurons and peripheral nerve terminals. J Neurosci 26: 12512–12525, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers WD. Why the eye becomes dry: a cornea and lacrimal gland feedback model. CLAO J 26: 159–165, 2000 [PubMed] [Google Scholar]
- Mizumura K, Sugiura T, Katanosaka K, Banik RK, Kozaki Y. Excitation and sensitization of nociceptors by bradykinin: what do we know? Exp Brain Res 196: 53–65, 2009 [DOI] [PubMed] [Google Scholar]
- Mohapatra DP, Nau C. Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J Biol Chem 278: 50080–50090, 2003 [DOI] [PubMed] [Google Scholar]
- Moriyama T, Higashi T, Togashi K, Iida T, Segi E, Sugimoto Y, Tominaga T, Narumiya S, Tominaga M. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol Pain 1: 3, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Shibuya M, Nakashima H, Imagawa T, Uehara M, Tsubota K. d-β-Hydroxybutyrate protects against corneal epithelial disorders in a rat dry eye model with jogging board. Invest Ophthalmol Vis Sci 46: 2379–2387, 2005 [DOI] [PubMed] [Google Scholar]
- Okanobo A, Chauhan SK, Dastjerdi MH, Kodati S, Dana R. Efficacy of topical blockade of interleukin-1 in experimental dry eye disease. Am J Ophthalmol 154: 63–71, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra A, Madrid R, Echevarria D, del Olmo S, Morenilla-Palao C, Acosta MC, Gallar J, Dhaka A, Viana F, Belmonte C. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat Med 16: 1396–1399, 2010 [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source. Nat Rev Drug Discov 8: 55–68, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A, Kurose M, Winterson BJ, Meng ID. Menthol activation of corneal cool cells induces TRPM8-mediated lacrimation but not nociceptive responses in rodents. Invest Ophthalmol Vis Sci 53: 7034–7042, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohacs T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci 8: 626–634, 2005 [DOI] [PubMed] [Google Scholar]
- Rossi HL, Jenkins AC, Kaufman J, Bhattacharyya I, Caudle RM, Neubert JK. Characterization of bilateral trigeminal constriction injury using an operant facial pain assay. Neuroscience 224: 294–306, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarria I, Gu J. Menthol response and adaptation in nociceptive-like and nonnociceptive-like neurons: role of protein kinases. Mol Pain 6: 47, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarria I, Ling J, Zhu MX, Gu JG. TRPM8 acute desensitization is mediated by calmodulin and requires PIP(2): distinction from tachyphylaxis. J Neurophysiol 106: 3056–3066, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid D, Messlinger K, Belmonte C, Fischer MJ. Altered thermal sensitivity in neurons injured by infraorbital nerve lesion. Neurosci Lett 488: 168–172, 2011 [DOI] [PubMed] [Google Scholar]
- Shim J, Park C, Lee HS, Park MS, Lim HT, Chauhan S, Dana R, Lee H, Lee HK. Change in prostaglandin expression levels and synthesizing activities in dry eye disease. Ophthalmology 119: 2211–2219, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol 130: 90–100, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Tominaga M, Katsuya H, Mizumura K. Bradykinin lowers the threshold temperature for heat activation of vanilloid receptor 1. J Neurophysiol 88: 544–548, 2002 [DOI] [PubMed] [Google Scholar]
- Suwan-apichon O, Rizen M, Rangsin R, Herretes S, Reyes JM, Lekhanont K, Chuck RS. Botulinum toxin B-induced mouse model of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci 47: 133–139, 2006 [DOI] [PubMed] [Google Scholar]
- Szolcsanyi J. Selective responsiveness of polymodal nociceptors of the rabbit ear to capsaicin, bradykinin and ultra-violet irradiation. J Physiol 388: 9–23, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut PD, Wrigley D, Gold MS. Cold transduction in rat trigeminal ganglia neurons in vitro. Neuroscience 119: 1071–1083, 2003 [DOI] [PubMed] [Google Scholar]
- van Bijsterveld OP, Kruize AA, Bleys RL. Central nervous system mechanisms in Sjogren's syndrome. Br J Ophthalmol 87: 128–130, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venable JH, Grafflin AL. Gross anatomy of the orbital glands in the albino rat. J Mammal 21: 66–71, 1940 [Google Scholar]
- Xing H, Chen M, Ling J, Tan W, Gu JG. TRPM8 mechanism of cold allodynia after chronic nerve injury. J Neurosci 27: 13680–13690, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudin Y, Lukacs V, Cao C, Rohacs T. Decrease in phosphatidylinositol 4,5-bisphosphate levels mediates desensitization of the cold sensor TRPM8 channels. J Physiol 589: 6007–6027, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudin Y, Rohacs T. Regulation of TRPM8 channel activity. Mol Cell Endocrinol 353: 68–74, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Mak S, Li L, Parra A, Denlinger B, Belmonte C, McNaughton PA. Direct inhibition of the cold-activated TRPM8 ion channel by Gαq. Nat Cell Biol 14: 851–858, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Shen J, Zhang C, Park CY, Kohanim S, Yew M, Parker JS, Chuck RS. Inflammatory cytokine expression on the ocular surface in the Botulinum toxin B induced murine dry eye model. Mol Vis 15: 250–258, 2009 [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Zhang C, Chuck RS. Topical steroid and non-steroidal anti-inflammatory drugs inhibit inflammatory cytokine expression on the ocular surface in the botulinum toxin B-induced murine dry eye model. Mol Vis 18: 1803–1812, 2012 [PMC free article] [PubMed] [Google Scholar]