Abstract
Dopamine (DA) is a neuromodulator that in the retina adjusts the circuitry for visual processing in dim and bright light conditions. It is synthesized and released from retinal interneurons called dopaminergic amacrine cells (DACs), whose basic physiology is not yet been fully characterized. To investigate their cellular and input properties as well as light responses, DACs were targeted for whole cell recording in isolated retina using two-photon fluorescence microscopy in a mouse line where the dopamine receptor 2 promoter drives green fluorescent protein (GFP) expression. Differences in membrane properties gave rise to cell-to-cell variation in the pattern of resting spontaneous spike activity ranging from silent to rhythmic to periodic burst discharge. All recorded DACs were light sensitive and generated responses that varied with intensity. The threshold response to light onset was a hyperpolarizing potential change initiated by rod photoreceptors that was blocked by strychnine, indicating a glycinergic amacrine input onto DACs at light onset. With increasing light intensity, the ON response acquired an excitatory component that grew to dominate the response to the strongest stimuli. Responses to bright light (photopic) stimuli also included an inhibitory OFF response mediated by GABAergic amacrine cells driven by the cone OFF pathway. DACs expressed GABA (GABAAα1 and GABAAα3) and glycine (α2) receptor clusters on soma, axon, and dendrites consistent with the light response being shaped by dual inhibitory inputs that may serve to tune spike discharge for optimal DA release.
Keywords: amacrine cell, dopaminergic neuron, electrophysiology, immunocytochemistry, retina
dopamine (DA) is a modulatory neurotransmitter that is present in the peripheral and central nervous system of all vertebrates. In the retina, it is synthesized and released from a subset of amacrine interneurons called dopaminergic amacrine cells (DACs) and plays a key role in numerous events that accompany the switch from rod (dim light)- to cone (bright light)-mediated vision as night turns to day (Witkovsky 2004). Despite the importance of DACs in orchestrating the diurnal changes in the performance of the visual system, in situ functional studies of DACs have been hindered by the fact that they are sparsely distributed, 20–30 per mm2, ∼450/mouse retina (Gustincich et al. 1997), and are indistinguishable among the myriad of other similar-looking neurons in the intact retina. They can, however, be labeled and specifically targeted for electrical recording using transgenic methods in which the promoter for tyrosine hydroxylase (TH), the rate-limiting enzyme for DA biosynthesis, directs the selective expression of a reporter, either alkaline phosphatase (Gustincich et al. 1997) or fluorescent protein (Zhang et al. 2004).
Such methods have been used most commonly to identify DACs isolated from enzymatically dissociated retina for studies of their biophysical properties and repertoire of neurotransmitter receptors using pharmacology and immunohistochemistry (Feigenspan et al. 1998, 2000; Gustincich et al. 1997, 1999; Steffen et al. 2003; Xiao et al. 2004). This approach has also been used to fluorescently tag DACs in whole mount retina for recording purposes (Zhang et al. 2007, 2008). These studies were based, however, on extracellularly recorded spike activity and used only intensely saturating light stimuli. For these reasons they provide an incomplete description of the cellular physiology of DACs and the stimulus dependence of their light responses. In this study, we use whole cell current- and voltage-clamp recording to examine the functional properties of DACs in the intact retina and the synaptic inputs that give rise to their responses to a full range of light intensities.
Amacrine cells were targeted for whole cell recording by using two-photon laser scanning fluorescence microscopy in a BAC transgenic mouse line in which the promoter for dopamine receptor 2 (Drd2) drove expression of green fluorescent protein (GFP). In this line, two distinct populations of amacrine cells express GFP. One population comprised exclusively DACs, which could be identified on the basis of characteristic morphological features and colocalization with TH labeling. The DAC population was morphologically homogeneous but physiologically diverse. DACs exhibited spontaneous activity with a stable spike pattern that ranged from nearly silent, to rhythmic firing at a steady rate, to firing in periodic bursts. These differences were accompanied by cell-to-cell differences in membrane properties. All of the DACs in the recorded sample generated light responses that depended on stimulus strength. At light onset, threshold stimuli evoked glycinergic inhibition, whereas stronger stimuli evoked a net excitatory response that was followed by a GABAergic inhibitory response at light offset. Furthermore, the postsynaptic receptor composition of these inhibitory inputs and their distribution across the DAC were identified by immunolabeling.
METHODS
All experiments were conducted in accordance with institutional and national guidelines for animal care using procedures and protocols that were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Washington. We used postnatal 21- to 50-day-old Gensat BAC transgenic mice (RP23-161H15) crossed into C57/B6 background. In this line, GFP transgene was inserted immediately after the ATG start codon of the Drd2 promoter. Animals were housed in University of Washington-approved facilities on a 12:12-h light-dark cycle with ad libitum access to food and water.
Tissue Preparation
Experiments began during the animals' subjective day, ∼5 h into their daily light cycle. After 2–3 h of dark adaptation, mice were killed by cervical dislocation, and eyes were removed in the dark using infrared illumination with image converters, placed in carbogenated (95% O2 and 5% CO2) Ames' medium (Sigma, St. Louis, MO) at room temperature, and hemisected. The posterior half of the eyecup was cut into three to five smaller pieces. Retina was isolated from each of the pieces as needed, adhered photoreceptor side down to a translucent Anodisc filter (Whatman, Florham Park, NJ) by wicking away excess solution, and transferred to a recording chamber fixed to the stage of a custom-built two-photon laser scanning fluorescence microscope. The mounted retina was perfused with warmed (30–34°C) carbogenated Ames' medium at a rate of 5–7 ml/min and viewed with a charge-coupled device camera using infrared illumination.
Cell Identification
In the Drd2-GFP BAC transgenic mouse line, GFP expression was visualized in whole mount retina using two-photon microscopy (Denk et al. 1990; Denk and Detwiler 1999; Euler et al. 2009). The light source for two-photon fluorescence excitation was a pumped laser (Mira; Coherent) that delivered ∼100-fs laser pulses of 930-nm light at 100 MHz with estimated average power at the retina of 4–8 mW. Fluorescence emission was collected by a ×40, 1.0-NA water-immersion objective (Nikon). Custom bandpass (BP) filters (Chroma Technology) directed green (535 nm, BP 50 nm) and red (622 nm, BP 36 nm) fluorescence to two independent photomultiplier tubes (Hamamatsu). The green channel was used to visualize GFP-positive cells in the inner nuclear layer (INL), and the red channel was used to visualize the recording pipette filled with an intracellular recording solution containing 100 μM Alexa Fluor 594 (Invitrogen).
Retinal photoreceptors are not blind to the pulses of long-wavelength (930 nm) light used to excite fluorescence by two-photon absorption in laser scanning microscopy (Euler et al. 2009). The light sensitivity of alpha retinal ganglion cells (RGCs) was used to assess the effect of laser exposure on retinal function. All recordings were done in the presence of 2 Rh*·rod−1·s−1 background illumination. After a 2- to 3-min period of laser scanning that mimicked the conditions used to target DACs using GFP fluorescence, the threshold intensity (500-ms step of 440-nm light) for a just-detectible change in extracellularly recorded spike activity (loose cell-attached patch) was increased by about 1–1.5 log unit for 20–40 s before returning to a control step sensitivity of ∼1 Rh*/rod per step. At the onset of laser scanning, with the focal plane in the inner plexiform layer (IPL), alpha RGCs were initially sensitive to the excitation light and fired spikes in synchrony with the rate of image scanning as the laser spot swept across the receptive field of the cell. With continued scanning, the retina adapted to the stimulus and the laser-evoked spike activity disappeared. Spike response to test flashes as well as laser exposure returned with the recovery of light sensitivity following the termination of laser scanning. These observations indicate that the two-photon imaging methods we have used to target DACs for whole cell recording did not have a persistent effect on retinal function as appraised by light sensitivity.
Electrophysiology
GFP-labeled cells in the INL were accessed on a diagonal trajectory from a microdissected hole in the internal limiting membrane, 50–75 μm from the targeted cell (Margolis and Detwiler 2007). Patch-clamp recordings were obtained using 3- to 5-MΩ electrodes, and signals were amplified using an Axopatch 200B amplifier (Axon Instruments). For current-clamp recordings, the standard internal solution contained (in mM) 122 K-gluconate, 10 Na-HEPES, 6 KCl, 6 K-EGTA, 3 Mg-ATP, and 0.2 Tris-GTP, brought to pH 7.4 with NaOH. The internal solution used for voltage-clamp recordings contained (in mM) 105 cesium methanesulfonate, 10 tetraethylammonium chloride, 10 K-EGTA, 2 QX-314, 5 Mg-ATP, and 0.5 Tris-GTP, brought to pH 7.3 with ∼35 mM CsOH. Voltages were corrected for a −10-mV liquid junction potential that was determined experimentally (Neher 1992). Series resistances (RS), as measured from the average response to trains of −5-mV steps, were 10–20 MΩ, and holding potentials (Vhold) were corrected offline by subtracting the product of leakage current and RS from the applied voltage.
Voltage and current signals were filtered at 2 kHz and digitized at a sampling interval of 0.1 ms via an ITC-16 interface (Instratech) using custom software written in Igor Pro (WaveMetrics) by Fred Rieke (University of Washington, Seattle, WA).
Receptor Antagonists
To block synaptic transmission, neurotransmitter receptor antagonists were added to the extracellular solution. These included (in μM) 40 gabazine (SR-95531), 50–75 l-(+)-2-amino-4-phosphonobutyric acid (l-APB), 20–40 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX), 50 (1,2,5,6-tetrahydropyridin-4-yl)methylphosphinic acid hydrate (TPMPA), and 1 strychnine. Chemicals were purchased from Sigma or Tocris (Ellisville, MO).
Light Stimuli
An optical bench (Baylor and Hodgkin 1973) with a quartz-halogen light source was used with a substage condenser to deliver focused visual stimuli to the photoreceptor layer of the retina. Unless otherwise noted, stimuli were 500-ms steps of 720-μm-diameter (full field) circular spots of 440-nm light centered on the receptive field of the cell with an unattenuated intensity of 2.1 × 106 photons·μm−2·s−1, which corresponds to 9 × 105 Rh*·rod−1·0.5 s−1 step using an effective collecting area of 0.85 μm2 for mouse rods (Lyubarsky et al. 2004). All experiments were done in the presence of a background light intensity equivalent to 2 Rh*·rod−1·s−1 to provide a defined and reproducible baseline level of illumination and consistent light-adaptational state.
Immunohistochemistry
Retinas were isolated in cold oxygenated mouse artificial cerebrospinal fluid (pH 7.4) containing (in mM) 119 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgCl2, 1 NaH2PO4, 11 glucose, and 20 HEPES and mounted RGC side up on black membrane filters (HABP013; Millipore). The retina and filter paper were then immersed for 20 min in 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS), rinsed with PBS, preincubated in PBS with 5% donkey serum and 0.5% Triton overnight, and then incubated for 3 nights with primary antibodies. For vibratome sections, 60-μm slices were made postfixation. Antibodies used were directed against TH (mouse monoclonal, 1:500; Chemicon), GFP (rabbit polyclonal, 1:1,000; Chemicon), GABAA receptor α3-subunit (guinea pig polyclonal, 1:3,000; kindly provided by J. M. Fritschy), GABAA receptor α1-subunit (guinea pig polyclonal, 1:5,000; kindly provided by J. M. Fritschy), GABAC receptor ρ-subunit (rabbit polyclonal, 1:500; kindly provided by R. Enz), glycine receptor α2-subunit (goat polyclonal, 1:300; Santa Cruz), and vesicular inhibitory amino acid transporter (VIAAT; rabbit polyclonal, 1:1,000; Synaptic Systems). Secondary antibodies utilized were either anti-isotypic Alexa Fluor conjugates (1:1,000; Invitrogen) or DyLight conjugates (Jackson ImmunoResearch). Secondary antibody incubation was carried out in PBS overnight, and the retinas were subsequently mounted in Vectashield (Vector Labs).
For DAC fills, 5 mM neurobiotin was used and retinas were postfixed for 20 min and processed thereafter for receptor labeling. To amplify the neurobiotin signal, streptavidin conjugated to Alexa Fluor 568 (1:200; Invitrogen) was included with the secondary antibody.
Image Analysis
Images were acquired on an Olympus FV1000 laser scanning confocal microscope using a ×60, 1.35-NA oil objective. Raw image stacks were processed using MetaMorph (Universal Imaging) and Amira (Mercury Computer Systems) software. For volume estimation, the neurobiotin signal was used to mask in three dimensions (3-D) the DAC dendrites or axon, using the “label field” function in Amira. Alternatively, the TH signal was used to mask the DAC soma in 3-D. Thereafter, the DAC mask was multiplied to the receptor channel to isolate the receptor signal exclusively within the mask. A constant threshold, chosen subjectively to exclude background (average fluorescence intensity of nonclustered pixels), was then applied to the receptor-labeled channel to isolate receptors within the mask. The volume of the pixels above this threshold was expressed as a percentage of the total volume occupied by the pixels within the mask. To determine whether or not immunolabeled receptor clusters represent inhibitory synaptic sites, we quantified the extent to which GABAAα3 receptor clusters (most abundant on DAC processes) were apposed to inhibitory presynaptic terminals. To do so, we performed triple immunostaining for the receptor (α3)-, VIAAT-, and TH-positive process in retinal slices. Receptor puncta within the TH mask were isolated, the VIAAT signal thresholded, and apposition of both signals was judged by eye on rotation of the image volumes in 3-D. Each GABA receptor cluster was considered to be apposed to a VIAAT-positive terminal if the two signals showed overlap of pixels (>1 pixel overlap) at all angles of the 3-D rotation.
RESULTS
Cell Identification and Targeted Recording
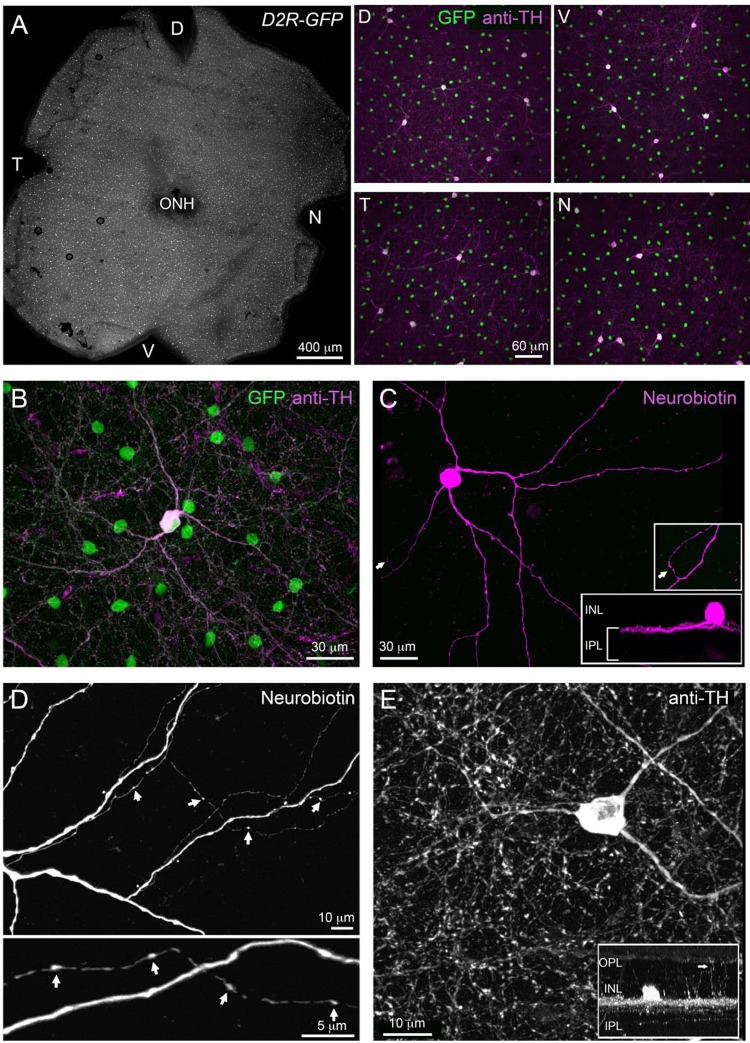
In the Drd2-GFP transgenic mouse line, the promoter sequence for dopamine receptor 2 (DR2) drives selective GFP expression in two separate populations of retinal amacrine cells that can be distinguished on the basis of soma size and density (Fig. 1A). The smaller cells, with soma diameters of 7–9 μm, were distributed numerously in the inner quarter of the INL. They had dendrites that branched out first as a narrow band extending laterally along the outer boundary of the IPL and then diffusely in the middle third of the IPL. These cells are the subject of an ongoing investigation and will not be discussed further. The second population of Drd2-GFP amacrine cells had large (13–18 μm in diameter) spherical somas that were distributed sparsely along the inner boundary of the INL. In all regions (dorsal, ventral, temporal, and nasal) of the Drd2-GFP retina, cells that were immunolabeled with TH antibodies also expressed GFP (n = 76 cells, n = 4 retinas; Fig. 1, A and B). Images of neurobiotin-filled cells (Fig. 1C) showed somas with two to three monostratified primary dendrites that spread out tangentially in the outer most (S1) strata of the IPL over an area roughly 600 μm in diameter. The dendrites gave rise to two different kinds of secondary extensions. One was a small number (1 to 3) of axon-like processes that were beaded with varicosities and coursed laterally for long distances (1–2 mm) across the retina (Fig. 1D). The primary dendrites also gave rise to a number of faint interplexiform processes that projected vertically into the outer plexiform layer (OPL) (Fig. 1E). There were no apparent differences in the gross morphology of DACs targeted for intracellular dye filling at randomly selected retinal eccentricities.
Fig. 1.
Morphology of dopaminergic amacrine cells (DACs) in dopamine receptor 2-green fluorescent protein (Drd2-GFP) transgenic mice. A: distribution of GFP-expressing cells across a Drd2-GFP retina (left) and higher magnification of 4 representative regions immunolabeled for tyrosine hydroxylase (TH) in different parts of the Drd2-GFP retina (right). D, dorsal; V, ventral; T, temporal, N, nasal, ONH, optic nerve head. All GFP-expressing cells with large somata are TH positive. B: higher magnification view showing a large TH-positive, GFP-expressing cell. C: morphology of a large GFP-positive cell as revealed by intracellular filling with neurobiotin. Its cell body lies in the inner nuclear layer (INL), and processes stratify in the outermost part of the inner plexiform layer (IPL; bottom inset). An axonal process is highlighted by an arrow and is shown at higher magnification (top inset). D: a neurobiotin-filled DAC with varicosities along its axonal process (arrows). E: TH immunolabeling in retinal whole-mounts shows a fraction of dopaminergic amacrine processes (arrow in inset) reaching the outer plexiform layer (OPL).
On the basis of these anatomic features (Dacey 1990; Gustincich et al. 1997; Kolb et al. 1991; Versaux-Botteri et al. 1984), along with the fact that these cells (unlike the GFP-positive cells with small somas) colocalized with TH labeling (Fig. 1), the large soma Drd2-GFP cells were classified as interplexiform DACs; also referred to as type 1 catecholamine cells. Our observations suggest that in the transgenic mouse used in our study, fluorescent protein expression in cells with large somata represents a single population of dopaminergic neurons based on their morphology.
Resting Properties of DACs
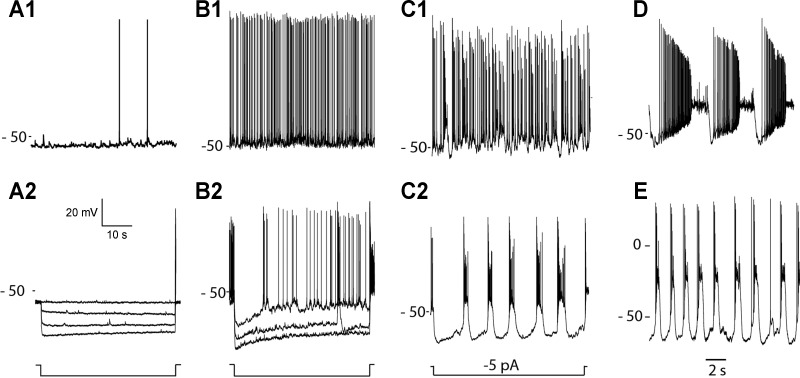
In whole cell current-clamp recordings, DACs had resting potentials in the vicinity of −30 to −65 mV (mean 54 ± 1.04 mV, n = 81) and generated action potentials spontaneously (in such cells resting potential was defined as the baseline voltage at spike threshold). The pattern of spontaneous spike activity was not the same in all cells (Fig. 2). In a set of experiments on 59 DACs, in which the analysis focused on spontaneous activity in darkness, only 5 (8%) were quiet cells that generated spikes infrequently (<0.1 Hz) at irregular intervals (Fig. 2A1). In all other cells in this sample, spontaneous spike discharge occurred more frequently, either at a steady rate of ∼2–8 Hz (in 25% of the cells) (Fig. 2B1) or in bursts (in 62% of the cells) that were intermingled with a more regular rate of ongoing spike activity (Fig. 2C1). In 5% (3/59) of the recorded cells, discrete high-frequency bursts of action potentials were generated at regular (∼1–5 s) intervals on an otherwise quiet background of spike activity (Fig. 2D). The cell-to-cell variation in the pattern of spontaneous spike activity was not associated with observable differences in DAC gross morphology or cellular health, as judged by the stability of resting membrane potential and the presence of action potentials. There were no significant differences in the resting potentials of cells that exhibited the different categories of activity, with mean values that ranged from −52 to −48 mV in quiet and periodic bursting DACs, respectively. The characteristics of the spontaneous activity in a particular cell did not change over the course of a typical whole cell recording lasting 30–45 min. In cells having a mixture of single spikes and bursts of activity, hyperpolarizing current injection accentuated the bursting component of the activity and revealed an underlying “pacemaker-like” periodicity (Fig. 2, C1 and C2).
Fig. 2.
Cellular properties of DACs. Resting spontaneous spike activities in 4 different cells provide examples of DACs that are nearly silent (A1), continuously active (B1), or exhibit steady discharge with intermixed bursts of spikes (C1) or periodic bursts (D). Resting membrane potentials (in mV) are indicated at left of each trace. The voltage responses evoked by constant current steps in the same cells are shown by traces in A2, B2, and C2, respectively. Negative current steps in A2 and B2 were 20, 30, and 40 pA. Periodic burst firing persists in the presence of a mixture of excitatory and inhibitory synaptic blockers: l-(+)-2-amino-4-phosphonobutyric acid (l-APB; 50 μM), AP5 (50 μM), CNQX (50 μM), picrotoxin (50 μM), and strychnine (1 μM). Labels at left of each recording identify the resting potential (in mV) relative to the voltage trace using the voltage scale provided.
Bursting spike activity and the unmasking effect of hyperpolarization persisted in the presence of a combination of excitatory and inhibitory synaptic blockers (Fig. 2E), consistent with generation by an intrinsic mechanism. In quiet cells that displayed little to no spontaneous activity, the voltage response to a hyperpolarizing step of current was flat and showed no evidence of relaxing from an initial peak hyperpolarization (Fig. 2A2). In contrast, the hyperpolarizing step response in cells with regular and/or bursts of spike activity exhibited a prominent sag as voltage relaxed from an initial negative peak back toward a less negative steady-state potential with a time constant of 1–2 s (Fig. 2B2). This suggests a hyperpolarization-activated inward current (IH) may play a role in the biophysical mechanism that gives rise to the periodic bursts of action potentials, similar to what has been reported for several other types of neurons exhibiting bursting behavior (Alonso and Llinas 1989; McCormick and Pape 1990a; Mercuri et al. 1995; Yung et al. 1991).
Light Response Properties of DACs
Over the course of this study, we have recorded from more than 300 DACs and have yet to find one that did not respond to light. This result differs sharply from earlier reports where a large fraction (40%) of DACs did not respond to light (Zhang et al. 2007, 2008).
The minimally detectable full-field (720-μm diameter) scotopic stimulus evoked a hyperpolarizing potential change and a transient suppression of spontaneous spike activity at the onset of steps of 440-nm light with intensities that ranged from 9 to 70 Rh*/rod across cells (Fig. 3, A and B). In many but not all cases, the recovery from the hyperpolarizing response evoked by dim light included a brief increase in spike activity caused by a delayed excitatory after potential (see Fig. 5A). This is a feature of responses evoked at the end of hyperpolarizing voltage steps in neurons that express IH (Robinson and Siegelbaum 2003).
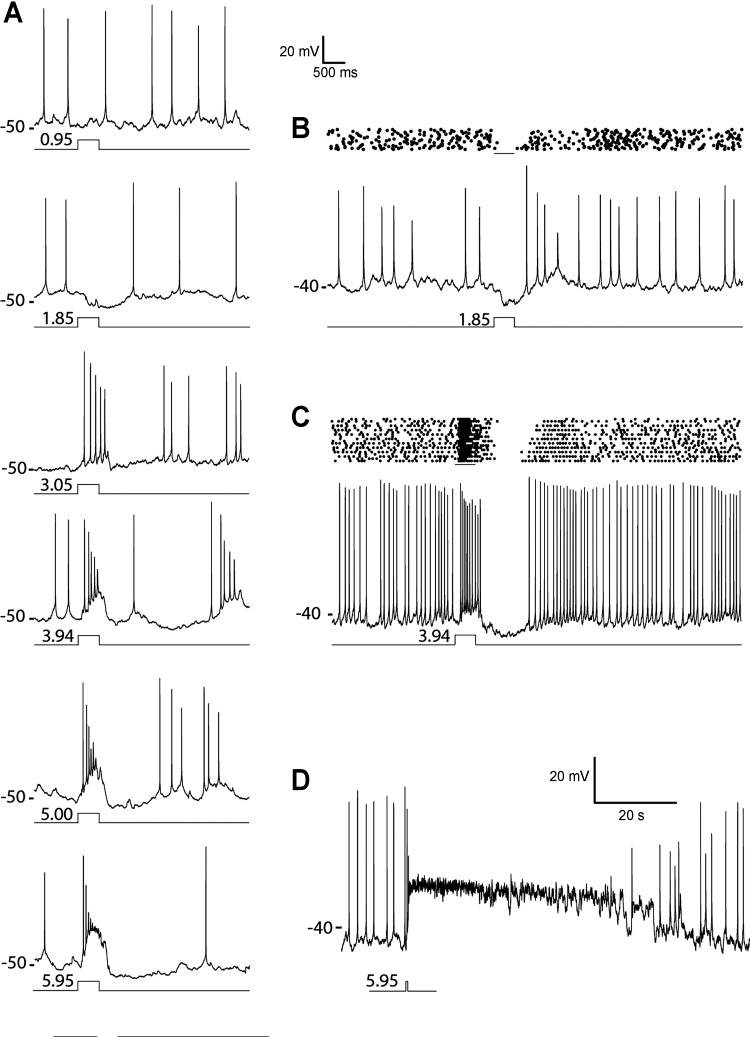
Fig. 3.
DAC light response properties. A: DAC light response evoked by 500-ms steps of full-field (700-μm-diameter spot) 440-nm light of indicated intensities (log10 Rh*/rod per step). B and C: spike rasters and example trace showing responses to light steps in different cells. D: an example of DAC that exhibited a prolonged response to a bright full-field step of 440-nm light (9 × 105 Rh*·rod−1·0.5 s−1). Labels at left of each recording indicate the resting potentials (in mV) relative to the voltage trace using the voltage scales provided.
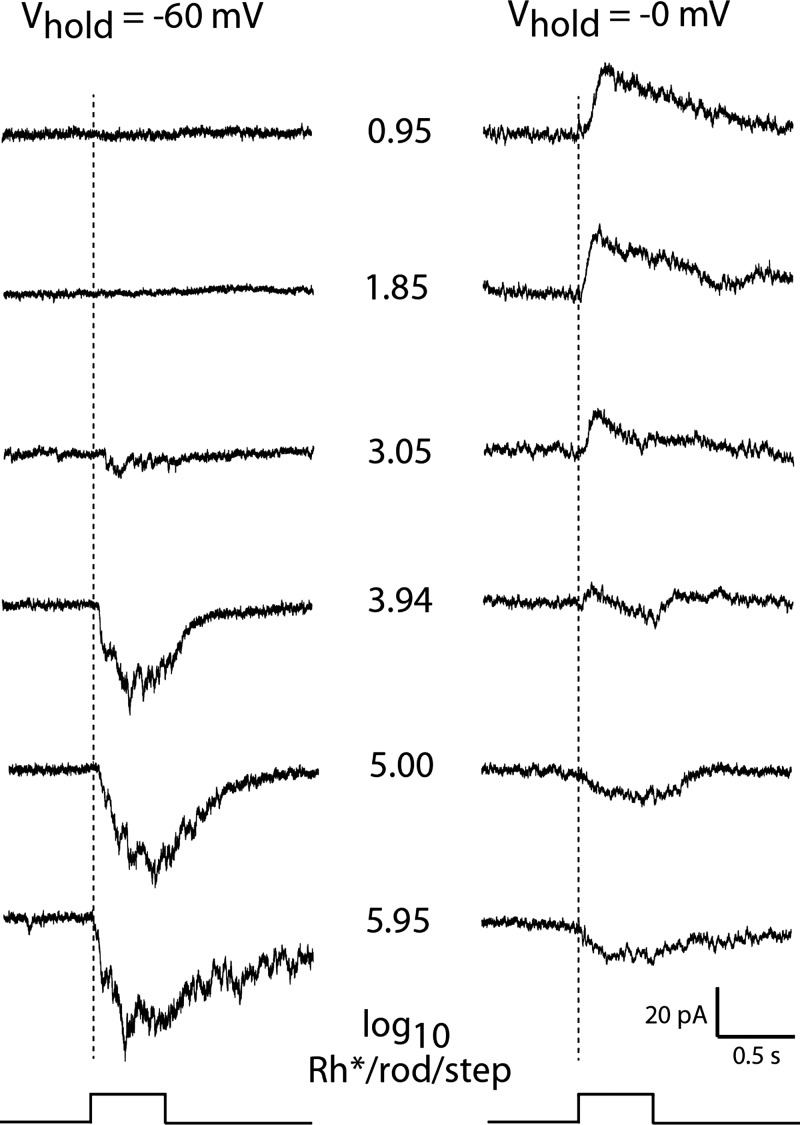
Fig. 5.
Light response intensity series in voltage clamp. Responses evoked by 500-ms steps of full-field 440-nm light at the intensities (middle) expressed as log10 Rh*·rod−1·0.5 s−1 in a representative DAC held at a voltage (Vhold) close to the reversal potential for inhibitory synaptic input (−60 mV; left) and excitatory synaptic input (0 mV; right) in Ames' solution.
With increasing step intensity, the DAC light response grew to become dominated by excitatory input at light onset and a strong hyperpolarizing response at light offset (Fig. 3, A and C). The ON and OFF components of the DAC response to a bright stimuli are apparent in a raster plot as an initial burst of spikes followed by an inhibition of spike activity at light offset (Fig. 3C). Small-diameter (35 μm) spots of bright light evoked similar light responses when centered on the soma as when displaced from it by up to 300 μm (data not shown), suggesting that DACs do not receive antagonistic synaptic input from a surround that extends over that area.
In a minority of cells, the strongest stimuli recruited an additional excitatory input that overcame the inhibitory OFF response and extended the step response well beyond the duration of the stimulus (Fig. 3D). The prolongation of the response grew with further increase in light intensity, resulting in responses to the brightest light that typically lasted 15–20 s, 7–10 times longer than the responses evoked by the same stimulus in the majority of DACs. Long-lasting responses such as these have been reported previously in extracellular recordings of DACs and attributed to there being a subset of DACs that receive excitatory synaptic input from intrinsically photosensitive retinal ganglion cells (ipRGCs) (Zhang et al. 2008). Recordings from these types of cells were infrequent (∼12% of recorded DACs), and since they obscured the inhibitory response evoked at light offset, our experiments concentrated on GFP-labeled DACs that did not exhibit any evidence of ipRGC input.
As the amplitude of the depolarizing ON response grew with increasing light intensity, spike threshold was exceeded and action potentials were triggered that declined in amplitude over the course of the excitatory response (Fig. 3A). With further increase in stimulus strength, the frequency of the evoked spike discharge increased whereas the accompanying decline in spike amplitude accelerated, resulting in the failure of action potential generation (Fig. 3A). The rapid inactivation of spike production by depolarization block is a characteristic feature of DAC electrophysiology as well as an established attribute of midbrain dopaminergic neurons (Bunney and Grace 1978; Grace and Bunney 1986). It limits the response evoked by intense stimuli to an initial transient burst of spikes at the onset of a strongly depolarizing synaptic potential (Fig. 3, A and D).
Depolarization block shapes the response to steady illumination in a similar manner (Fig. 4A). Action potentials evoked at the onset of a step of bright light decline rapidly in amplitude to the point of spike failure after a short-lived burst of activity. The absence of spike activity persists until the underlying depolarizing potential decays for sufficient relief of inactivation for the generation of spikes that first appear as miniature (∼5-mV peak amplitude) spikes and grow to full amplitude (∼90 mV) at an irregular firing rate. Depolarization block also plays a role in shaping the responses evoked by a train of repetitive flashes of light (Fig. 4B). Stimuli that flicker ON and OFF at a low rate (1–3 Hz) evoke a brief burst of spikes at the onset of the periods of light exposure that are quickly silenced by depolarization block. Under these conditions the hyperpolarizing (inhibitory) OFF response accelerates the recovery from depolarization block and serves to remove spike inactivation in time for the next flash in the stimulus train.
Fig. 4.
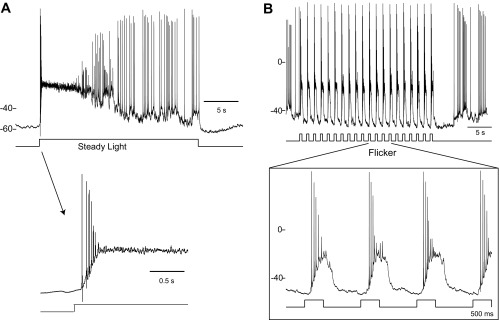
DAC responses to steady and flickering light. A: 20-s step of light evoked a transient burst of spikes at light onset that gave way to irregular lower frequency spike discharge as the cell adapted to the steady light (n = 4). The termination of action potential discharge at the leading edge of the response by depolarization spike block is shown on an expanded time base in the insert (arrow). B: traces show the entrainment of DAC spike responses to periodic 500-ms stimulus (3.94 log10 Rh*·rod−1·0.5 s−1) that flickers ON and OFF at 0.7 Hz on 2 different time scales. Labels at left of each recording indicate the resting potentials (in mV) relative to the voltage trace using the voltage scales provided.
Light-Evoked Synaptic Currents
The underlying excitatory and inhibitory synaptic currents that gave rise to the DAC light response were revealed by recording stimulus-activated currents in voltage-clamped cells held at either −60 or 0 mV, i.e., respectively at potentials where net current flow through inhibitory or excitatory transmitter gated ion channels would be expected to be eliminated (Fig. 5). Dim light evoked a net outward current when the cell was held at 0 mV, consistent with the DAC threshold light response being a hyperpolarizing voltage change in current-clamp recordings. In cells held at −60 mV, stimuli in the same intensity range had no effect on the recorded current, whereas stronger stimuli evoked an inward current response that grew in amplitude and duration with further increase in intensity. In cells held at 0 mV, the amplitude of the outward current evoked by dim stimuli remained constant until the stimuli reached the intensity level that had first evoked an inward current response when the cell was held at −60 mV. From that point on, the outward response declined with increasing light intensity to become a net inward current response when stimulated with the brightest light (Fig. 5). These changes in the amplitude and polarity of the response suggest that the responses to all but the weakest stimuli are the sum of inhibitory and excitatory inputs with relative strengths that change with light intensity. This may arise as a consequence of voltage-clamp error due to the well-established inability to control dendritic voltage by clamping somatic voltage (Armstrong and Gilly 1992; Williams and Mitchell 2008), a particularly acute problem in cells, such as DACs, with extensive dendritic arbors. Under these conditions setting the somatic voltage at the expected reversal potential for excitatory synaptic current would not uniformly nullify the current throughout the dendritic tree. This would allow the contribution of inward currents from excitatory dendritic inputs to the net stimulus-activated current recorded in the soma to increase with stimulus intensity. This can also account for the fact that the hyperpolarizing potential change that is associated with the offset of steps of bright light in current-clamp recording is not apparent in voltage clamp as an outward current at Vhold = 0 mV. The presence of the inhibitory OFF response is, however, apparent as an outward current when the contamination by light-activated inward current has been eliminated by blocking excitatory input with l-APB (see Figs. 7B and 8C).
Fig. 7.
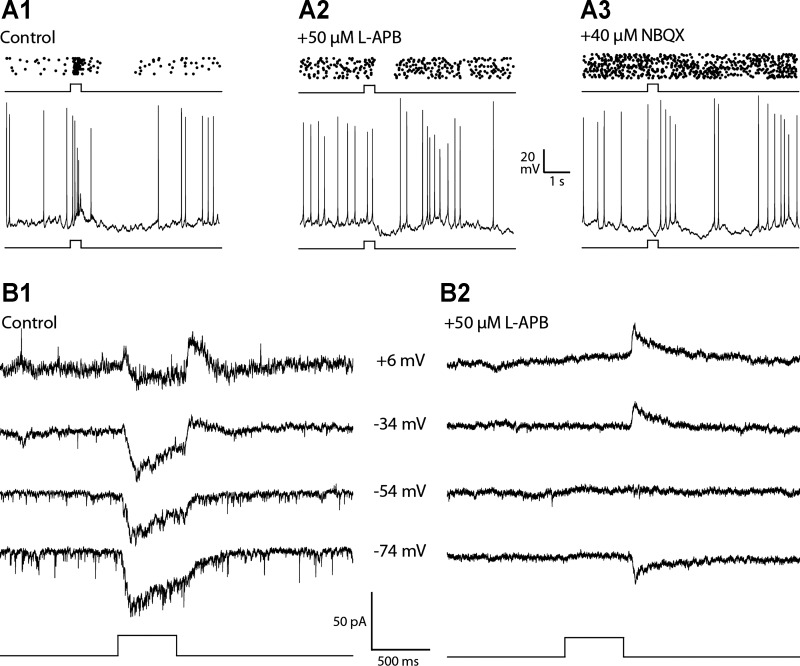
Synaptic origin of the excitatory ON response evoked by bright light. Spike raster plots (top) and example single traces (bottom) show the response evoked by a bright step of light in the absence of glutamatergic reagents (A1) and in the presence of l-APB, a metabotropic glutamate receptor agonist that quenches the excitatory light response in ON bipolar cells, alone (A2) and with the addition of 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX;A3). The results show that the control response had an excitatory ON response that was eliminated by l-APB and an inhibitory OFF response that was blocked by the addition of NBQX (n = 6). B1 and B2 show the currents evoked by bright light in voltage-clamp recordings at the indicated holding potentials (middle) in the absence (control; B1) and presence of l-APB (B2). The ON pathway antagonist eliminated the inward current evoked at light onset but not the outward current at light offset (n = 4). Light stimuli were 500-ms full-field steps of 440-nm light equivalent to 5.0 log10 Rh*·rod−1·0.5 s−1.
Fig. 8.
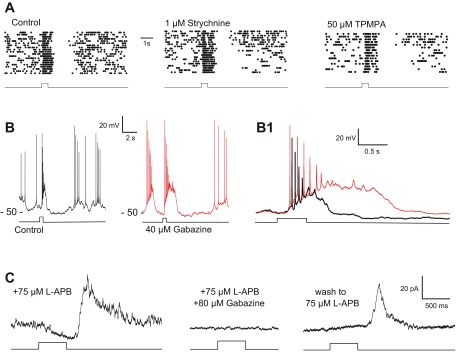
GABAergic inhibitory OFF response evoked by bright light. A: spike rasters showing the persistence of the inhibitory OFF response in the presence of strychnine (n = 4), a glycine receptor antagonist, and (1,2,5,6-tetrahydropyridin-4-yl)methylphosphinic acid hydrate (TPMPA; n = 3), a GABAC receptor antagonist. B: bath application of gabazine (red trace) prolonged the depolarizing response to light, shown on an expanded time base (B1) that compares superimposed responses in the absence (black) and presence (red) of gabazine (n = 9). Labels at left of each recording indicate the resting potentials (in mV) relative to the voltage trace using the voltage scales provided. C: in voltage-clamp recordings (Vhold = 0 mV) in the presence of l-APB there was a slowly increasing negative (inward) current during the light step that was supplanted by a large positive (outward) current at light offset. Both components of the step response were eliminated by gabazine and partially reversed on washout (n = 3). Light stimuli were 500-ms full-field steps of 440-nm light equivalent to 3.94 (A and B) and 5.95 log10 Rh*·rod−1·0.5 s−1 (C).
Taking these findings together, there are three distinguishing components to the DAC light response: a dim step ON inhibition, a moderate-to-bright step ON excitation, and an OFF inhibition. These intensity-dependent features of the light response were observed in DACs that had a low rate of resting spike activity (quiet cells) as well as in DACs that spontaneously discharged spikes at either a steady rate or intermingled with bursts of activity. The intensity dependence of the light responses in the relatively small number (∼5%) of DACs that fired spikes in discrete periodic bursts (Fig. 2D) could not be clearly demonstrated on the background of the pacemaker-like fluctuations in cell voltage that generated the periodic bursts of activity.
Synaptic Origin of Light Response Components
To investigate the synaptic inputs that underlie the DAC light responses, we next examined the three light response components with the help of pharmacological agents.
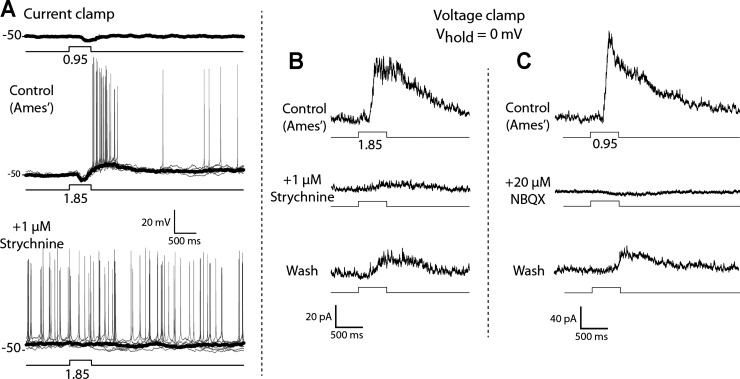
Dim step ON inhibition.
Bath application of 1 μM strychnine eliminated the hyperpolarizing voltage response as well as the outward current response evoked by dim light in current-clamp (resting potential at zero current) and voltage-clamp (Vhold = 0 mV) recordings (Fig. 6; n = 7 in current-clamp recordings, n = 3 in voltage-clamp recordings). The inhibitory input stimulated by low-intensity steps persists in the presence of gabazine (40 μM) and TPMPA (50 μM) and is blocked by either l-APB (50 μM) or NBQX (40 μM), indicating that DACs receive light-driven glycinergic input from an amacrine cell type that is excited by ON rod bipolar cells. The glycinergic input to the DACs at light onset does not appear to be mediated by AII amacrine cells, which are an established source of glycinergic inhibition under scotopic conditions (Bloomfield and Dacheux 2001). This is because the inhibitory input evoked by bright light is blocked by NBQX (Fig. 6C), showing that glycinergic input is not able to be driven by electrical coupling with ON cone bipolar cells (CBCs), as would be the case if the amacrine in question were an AII (Pang et al. 2007; Veruki and Hartveit 2002; Xin and Bloomfield 1999).
Fig. 6.
Inhibitory ON response evoked by a dim step of light is blocked by strychnine. A: current-clamp recordings of responses to weak steps of light in the absence and presence of 1 μM strychnine (n = 7). Average response is shown in black, and individual responses are displayed in gray. Labels at left of each recording indicate the resting potentials (in mV) relative to the voltage trace using the voltage scale provided. B: voltage-clamp recordings of outward current response at 0-mV holding potential evoked by dim steps in the absence and presence of 1 μM strychnine. Stimulus intensities are reported as log10 Rh*·rod−1·0.5 s−1 for full-field 440-nm light and are associated with traces showing the timing of the light step.
Bright step ON excitation.
Bath application of l-APB blocked the transient spike discharge triggered by the onset of a bright step of light in current-clamp recordings (Fig. 7, A1 and A2; n = 6) as well as an inward current response in voltage-clamped cells at light onset (Fig. 7, B1 and B2; n = 4). That the DAC excitatory ON response is also blocked by NBQX alone (data not shown) indicates that it is mediated by glutamate release from ON bipolar cells acting on postsynaptic ionotropic glutamate receptors. This is in agreement with experiments showing that solitary DACs express AMPA receptors activated by either glutamate or kainate (Gustincich et al. 1997). It appears that the excitatory input to the DAC maybe driven by light activation of both cone and rod pathways. The switch of the DAC light response from net inhibition at light onset to net excitation occurs when the step intensity is sufficiently bright to excite cones, suggests that DACs receive excitatory input from ON CBCs. The threshold response in cells held at 0 mV is an outward current that decreases with increasing stimulus intensity before reaching photopic (bright) light levels, which suggests that scotopic (dim light) stimuli are too weak to directly excite cones and instead give rise to stimulus-evoked currents that are the sum of rod-mediated excitatory and inhibitory synaptic currents (Fig. 5).
Bright step OFF inhibition.
In current-clamp recordings, the inhibitory OFF response causes a transient suppression of spike activity at light offset that was not affected by bath application of strychnine or TPMPA (Fig. 8A; n = 4 for strychnine, n = 3 for TPMPA), ruling out mediation by glycinergic input or by GABAergic drive mediated by GABAC receptors. The duration of the depolarizing light response evoked by moderate to bright light was increased by more than twofold (Fig. 8, B and B1; n = 9) in the presence of gabazine, a selective GABAA antagonist. This is consistent with the elimination of inhibitory synaptic input at light offset that would normally curtail the light response by opposing the excitatory ON response. Gabazine treatment was associated with the generation of a spontaneous voltage oscillation that triggered bursts of spikes that were followed by a silent period of suppressed spike activity. This made it difficult to use light-evoked spike activity to evaluate the effect of gabazine on the inhibitory OFF response as in the experiments with strychnine and TPMPA (Fig. 8A). To circumvent this problem, we tested the effect of gabazine on the inhibitory OFF response in voltage clamp (Fig. 8C; n = 3). In the presence of a high concentration (75 μM) of l-APB, a step of bright light evoked an inward current (Vhold = 0 mV) that developed slowly during the step and was followed by a larger and faster outward current at light offset (Fig. 8C). Both components of the response were eliminated by gabazine, suggesting that the DAC receives tonic GABAergic input in darkness from an unidentified amacrine cell (AC) that is excited by OFF CBCs, as postulated previously (Critz and Marc 1992; Gustincich et al. 1997; see however, Zhang et al. 2007). Light, by inhibiting OFF bipolar cells, extinguishes the GABAergic input to the DAC, causing a reduction of the outward current that appears as a slow activation of a net inward current during the step of light. At light offset the OFF pathway is disinhibited, which excites the GABAergic AC in the circuit, causing in turn an outward current response at the termination of the stimulus (Fig. 8C).
These results can be summarized by a synaptic wiring diagram in which the DAC receives excitatory and inhibitory input via the ON pathway and inhibition via the OFF pathway (Fig. 9). In the proposed circuit the threshold response to scotopic stimuli is generated by inhibitory input at light onset from an unidentified glycinergic AC that is activated by excitatory input from rod-driven ON bipolar cells. Photopic stimuli evoke a net depolarizing ON response that is produced by direct excitatory input to the DAC from ON CBCs. The inhibitory OFF response that is a characteristic feature of the response to photopic stimuli includes GABAergic input to the DAC from an unidentified AC that is excited by OFF CBCs. This limb of the circuit provides tonic GABAergic inhibition to the DAC in the dark that is suppressed by light and re-excited at light offset. Although the glycinergic and GABAergic ACs in the proposed diagram are unidentified, they are likely to have narrow- and wide-field arbor, respectively, based on the type of neurotransmitter they release (Wassle et al. 1998).
Fig. 9.
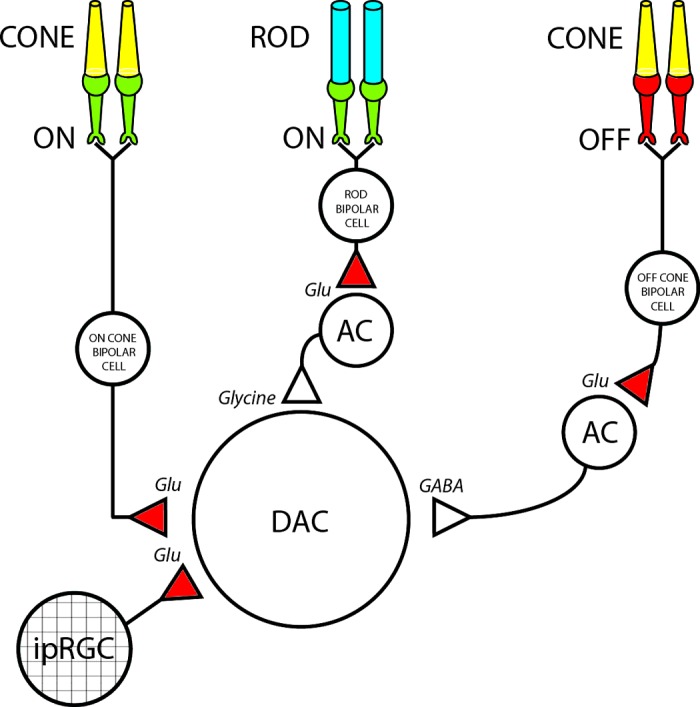
DAC synaptic wiring diagram. In the proposed schematic DACs receive excitatory glutamatergic input from ON cone bipolar cells, as well as from intrinsically photosensitive retinal ganglion cells (ipRGCs) in a subset (∼15%) of DACs. In addition, DACs received inhibitory input from 2 sources: one is an unidentified glycinergic amacrine cell (AC) that is driven by excitatory input from rod bipolar cells, and the other is an unidentified GABAergic AC that is driven by excitatory input from OFF cone bipolar cells. Red and green terminals represent ionotropic (AMPA/kainate) and metabotropic (mGluR) glutamatergic synapses, respectively.
Inhibitory Receptor Subtypes Expressed on Mouse DACs
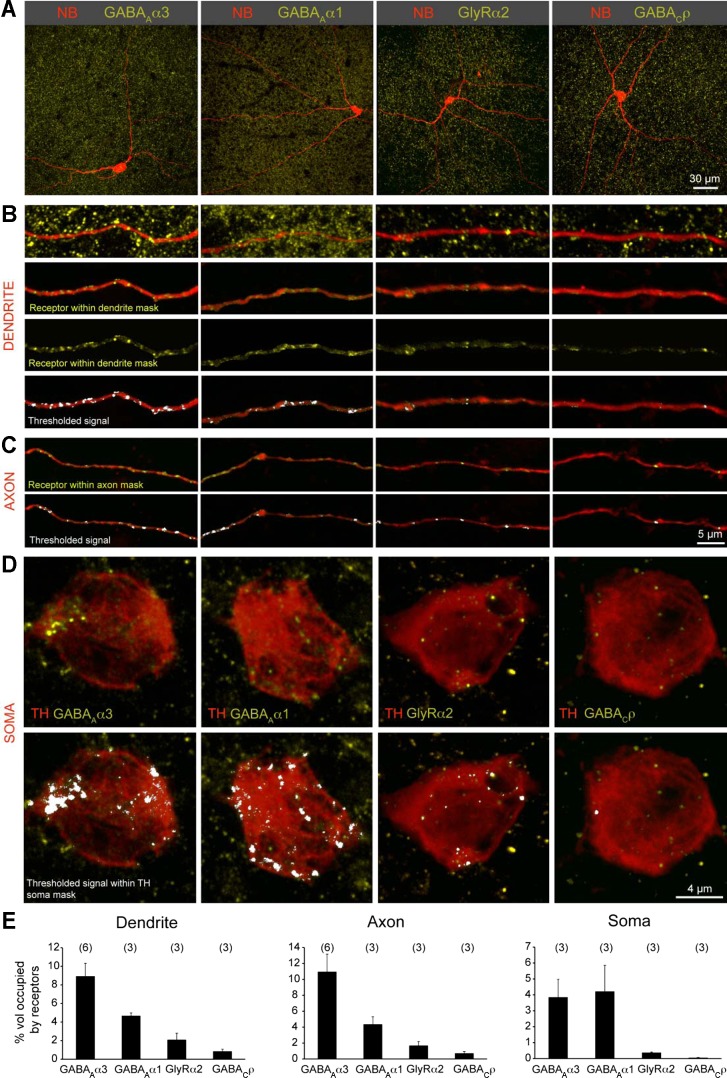
Our pharmacological analysis suggests that distinct inhibitory inputs shape the DAC response to light onset and light offset (ON glycinergic, OFF GABAergic) and that different GABA receptor subtypes (GABAA and not GABAC) are involved. To correlate the physiological observations with expression of GABA and glycine receptors on DACs, we performed immunolabeling using antibodies directed against specific GABA and glycine receptor subunits. In the IPL of the rodent retina, individual GABAA and glycine receptor clusters contain specific α-subunits (α1–α3) (Koulen et al. 1996; Wassle et al. 1998, 2009). TH-immunopositive DAC processes exhibited numerous GABAAα1 and GABAAα3 receptor clusters, some glycine receptor α2 (GlyRα2) clusters, but few, if any, GABAC receptor clusters. To quantify the relative expression levels of the various GABA and glycine receptor types, DACs in the Drd2-GFP whole mount retina were intracellularly filled with neurobiotin and afterward immunolabeled for GABAAα3, GABAAα1, GlyRα2, and GABACρ receptor subunits (Fig. 10A). Three-dimensional masks of the dendritic and axonal processes were generated to exclude receptor staining outside the DAC processes of interest (Fig. 10, B and C) and to estimate the percent volume occupied by a particular receptor type (Fig. 10E). This was then used to compare the relative abundance of each receptor type on DAC axonal and dendritic processes. GABAAα3 receptors were the most abundant inhibitory receptor type expressed on both DAC dendrites and axonal processes (Fig. 10E), followed by GABAAα1 and, to a much lesser extent, GlyRα2 receptor clusters. GABAC receptors were minimally expressed on both the axonal and dendritic compartments of the DAC.
Fig. 10.
Inhibitory receptor density on DACs. A: neurobiotin fills (NB) of DACs in the Drd2-GFP line colabeled for specific inhibitory receptor subsets: GABAA receptors (α3- or α1-subunit), glycine receptor (α2-subunit), and GABAC receptors (ρ-subunit). B: higher magnification of a stretch of dendrite and receptor labeling. Receptors localized within the volume of the dendrite are visualized by first creating a 3-dimensional (3-D) “dendrite mask” of the NB signal and then digitally excluding signal of the receptor immunolabeling outside this mask. A threshold is imposed above which receptor signal intensities are quantified. C: receptor labeling within the axon mask. D: receptor labeling within the soma mask. Somata were labeled by anti-TH. E: quantification of the percentage of volume of the dendrite, axon, or soma occupied by receptors containing different subunits.
Receptor expression on DAC soma was estimated in a similar way, but after a 3-D mask of TH-immunopositive somata was generated (Fig. 10D), rather than using neurobiotin-filled cells in which the soma was stained strongly and generated a saturating fluorescence signal. GABAAα1 and GABAAα3 were equally abundant on the soma (Fig. 10E) in contrast to DAC processes that express higher levels of receptors with α3- than α1-subunits. This suggests that the DAC uses different GABAA receptor subsets to process dendritic/axonal vs. somatic inhibition. The soma, like the DAC dendrites and axons, exhibited very few receptor clusters that were GABACρ positive (Fig. 10E). These results are consistent with the persistence of the inhibitory OFF response in the presence of bath-applied TPMPA, a selective GABAC receptor antagonist (Fig. 8).
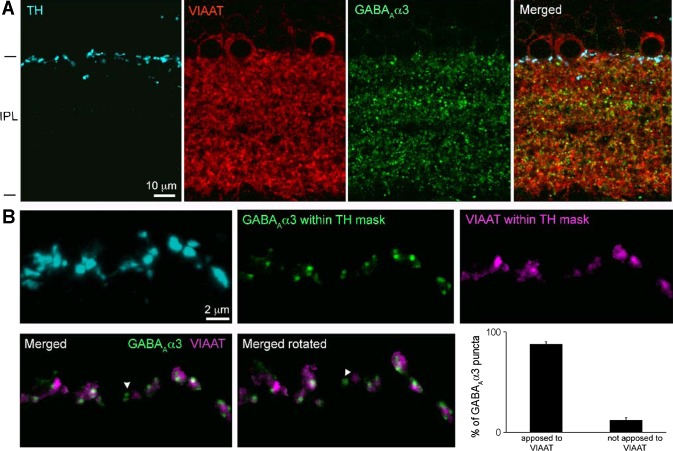
Because of the dense labeling of receptors in the IPL, two strategies were used to evaluate the specificity of receptor labeling within DAC processes. First, DACs were filled with neurobiotin and the retina double-labeled with an antibody against the COOH-terminal binding protein 2 (CtBP2). We chose CtBP2 because it is densely expressed throughout the IPL. Also, CtBP2 is localized at synaptic ribbons of excitatory glutamatergic presynaptic terminals in the retina (Schmitz et al. 2000; Soto et al. 2011) and thus should not be within DAC processes. Upon quantification, the percent occupancy of CtBP2 puncta on DAC dendritic and axonal processes was found to be only 0.98 ± 0.17% and 0.64 ± 0.15% (n = 2 cells) of the available dendritic and axonal volume, respectively. This suggests that the probability of random association of receptor clusters with DAC processes is less than 1 in 100. Second, we determined the percentage of GABAAα3 (most abundant receptor type on DACs) clusters on TH-immunopositive processes that were apposed to terminals labeled with antibodies directed against VIAAT (Sagne et al. 1997; Soto et al. 2011) (Fig. 11A). TH-positive processes were masked to isolate GABAAα3 receptor clusters specifically within the DAC neurite; we found that 87.9 ± 2.4% (n = 152 GABAAα3 puncta from 2 animals) of the GABAAα3 clusters were apposed to VIAAT-positive presynaptic terminals (Fig. 11B). Thus we conclude that the vast majority of immunolabeled receptor puncta on DAC processes represent sites of inhibitory synaptic input.
Fig. 11.
GABA receptor puncta on TH-immunopositive processes are apposed to inhibitory presynaptic terminals. A: dopaminergic amacrine processes labeled with TH in retinal slices, together with labeling for GABAAα3 receptors and the inhibitory presynaptic marker vesicular inhibitory amino acid transporter (VIAAT) in IPL. Maximum intensity projection of confocal image stack represents 0.9-μm thicknesses. B: to quantify GABAAα3-VIAAT appositions, TH-labeled processes were masked in thicker image stacks (4.8 μm). GABAAα3 and VIAAT signal within the masks were isolated to reveal appositions in 3-D. The majority of GABAAα3 receptor puncta were apposed to VIAAT (exception: arrowhead in merged images). Plots show quantification of the appositions identified by 3-D rotation of the merged images.
DISCUSSION
Functional Diversity of DACs
DACs appear to be a morphologically homogeneous population of cells but exhibit cell-to-cell differences in their functional properties. These are apparent in the cell-to-cell variation of their spontaneous activity in darkness. The characteristics of their spike activity fell into four categories: quiet cells that generated spikes infrequently at random intervals, rhythmic cells that fired spikes at a maintained rate, and cells that generated bursts that were either mixed with single spikes or occurred in discrete bursts at regular intervals (Fig. 2). The existence of two categories of DAC bursting activity agrees with the report of Zhang et al. (2007), as does the presence of a minority of DACs that are not spontaneously active. The presence of cells in the DAC population that exhibit the observed categories of spiking activity is also consistent with the range of cell-to-cell differences in spontaneous spike discharge exhibited by midbrain dopaminergic neurons (Grace and Bunney 1984a, 1984b). The differences in spike patterns ranging from infrequent discharge of single spikes to periodic bursts may participate in setting the basal tone of DA release, the only function that DACs and dopaminergic midbrain neurons have in common. A diversity in resting DA release across the DAC population might have the advantage of offering a richer selection of options for regulation than if all the cells had the same level and mode of DA release. In any case, the variability in spike activity appears to be a feature of DACs in the intact retina, since solitary DACs isolated from enzymatically dissociated mouse retina generate spikes rhythmically at a steady rate (3–9 Hz) that is remarkably uniform from one cell to the next and devoid of periodic burst firing (Feigenspan et al. 1998; Gustincich et al. 1997; Steffen et al. 2003; Xiao et al. 2004).
The difference between quiet and active DACs is not due to differences in cell health, recording time, or experimental conditions but it is associated with differences in their responses to hyperpolarizing current injection (Fig. 2). Active, but not quiet, DACs express a voltage-dependent cation conductance that is turned on by hyperpolarization. Since IH is a known contributor to pacemaking and oscillatory spike discharge in a variety of cell types, including midbrain dopaminergic neurons (Alonso and Llinas 1989; McCormick and Pape 1990a; Mercuri et al. 1995; Yung et al. 1991), it reasonable to suggest that it plays a role in the mechanism that generates burst firing in active DACs. In this regard it is relevant to note that spontaneous spike activity in solitary DACs is silenced by a 5- to 10-mV hyperpolarizing step that does not exhibit a voltage sag (IH) or unmask pacemaker activity as it does with DACs in the intact retina (Feigenspan et al. 1998; Gustincich et al. 1997; Steffen et al. 2003; Xiao et al. 2004). The reason for the differences between the intrinsic properties of solitary DACs and DACs in the intact retina is not clear. It could be that the ion conductances that are the source of biophysical differences between solitary and intact DAC reside in the neurites that are shorn off in the dissociation process and constitute the bulk of surface area of the DAC. It is also possible that the retina milieu includes neuromodulators that are absent from the saline solution in which solitary cells are maintained.
The explanation for the cell-to-cell differences in membrane properties of in vivo DACs is not known. It is possible that the DAC population is divided into physiologically discrete subtypes with fixed differences in their intrinsic properties. It is also possible that DACs exhibit physiological differences depending on the strength of neuromodulatory control and/or circadian regulation. Neuromodulators, such as DA, are known to influence the repertoire of ion channel expression in a variety of neuronal cell types and affect their pattern of spontaneous spike activity (Lacey et al. 1987; McCormick and Pape 1990b; Pape and McCormick 1989; Stefani et al. 1995). Furthermore, DACs contain clock genes (Dorenbos et al. 2007), and DA release oscillates in tune with the circadian cycle (Mangel and Dowling 1987; Ribelayga et al. 2004; Storch et al. 2007; Weiler et al. 1997). Although our present observations do not discriminate between these possibilities, our findings do raise the need for future experiments to correlate the spontaneous spike patterns of DACs with their release of dopamine.
DAC Light Responses and Distinct Modulatory Roles for GABA and Glycine
The DAC light response depends on stimulus intensity. Onset of dim light evoked a hyperpolarizing response that was initiated by rods and mediated by inhibitory synaptic input from an unidentified glycinergic AC that appears not to be an AII amacrine (Fig. 6C). At brighter light intensities the DAC ON response switched from being inhibitory to net excitatory. This was attributed to the recruitment of cones and direct excitatory input to DACs from ON CBCs. Although we have assumed that the glycinergic AC responsible for the inhibitory response evoked by dim light is excited by direct synaptic input from ON rod bipolar cells (Fig. 9), we cannot exclude the possibility that rod signals evoked by weak stimuli can electrically spread to cones via gap junctions. Such coupling would allow glycinergic ACs to be excited by ON CBCs. Since inhibition evoked by dim light is eliminated by strychnine without unmasking a residual depolarizing response, the ON CBCs that are excited by cones coupled to rods would have different properties than the ON CBCs providing direct excitatory input to DACs in response to bright light. The segregation of ON CBC signals evoked by rod-to-cone coupling from those evoked by light-activated cones could be based on differences in the ON CBCs such that weak signals in the cone stimulate ON CBCs that excite glycinergic AC, whereas strong signals stimulate ON CBC that excite DACs directly.
The DAC OFF response is evoked by stimuli in the same intensity range as the stimuli associated with the switch in the ON response from being inhibitory to net excitatory at light levels that begin to activate cones as well as rods. The OFF response evoked by moderate-to-bright light is generated by strong inhibitory synaptic inputs from unidentified GABAergic ACs that are excited by cone-driven OFF CBCs. The synaptic circuit inferred from the intensity dependence and pharmacology of the DAC light response (Fig. 9) is consistent with immunohistochemistry showing the expression of GABAAα1, GABAAα3, and GlyRα2 receptor clusters on DAC processes. The distinct receptor subtypes were not confined to specific cellular compartments; each receptor subtype was present on the soma, axon, and dendrites. This indicates that glycinergic inhibition evoked by dim light onset and GABAergic inhibition at bright light offset are not correlated with a differential distribution of GABA and glycine receptors. Whereas DAC somata possess similar amounts of GABAAα1 and α3 receptor clusters, GABAAα3 receptors were relatively more abundant in the dendritic and axonal processes. That gating kinetics are slower and the agonist affinity higher in GABAA receptors with α3- than with α1-subunits (Gingrich et al. 1995) suggests that GABAergic regulation of DAC activity is complex and tuned by a variety of factors. The presence of inhibitory input on dendrites may modulate excitatory synaptic input, whereas the inhibitory input on the axonal processes, which have not been described previously in retina, may serve to modulate the fidelity of spike-mediated communication between the DAC soma and its wide-field synaptic targets. The exclusive expression of glycine receptors with α2 receptors contrasts with previous observations in the primate retina showing that DACs preferentially express GlyRα3 (Jusuf et al. 2005). The functional significance of the species difference in GlyRα subunit expression in the same type of AC is not known.
The DAC is thus a novel example of a retinal neuron that receives inhibitory input at light onset and offset using different transmitters. The physiological consequences of this arrangement are not clear, but it is reasonable to consider it in the context of DA release, which is the central DAC function. Our results show that DACs exhibit diverse intrinsic properties and receive a repertoire of synaptic contacts that regulate the strength of excitatory input and axonal output. These cellular attributes of DAC physiology may act together to fine-tune spike-triggered DA release in a manner that would not be possible if the cell behaved as a simple on/off switch. Numerous studies based on quantitative chemical analysis of DA levels in retina superfusate have established that DA release is increased by light (Ishita et al. 1988; Iuvone et al. 1978; Kamp and Morgan, 1981; Kirsch and Wagner 1989; Kramer 1971; Morgan and Kamp 1980; Nichols et al. 1967; O'Connor et al. 1987), in which case rod-mediated glycinergic inhibition may act to suppress spike activity and DA release in darkness. Previous studies have also demonstrated that DA release is increased more strongly by light that flickers ON and OFF at a low frequency (1–3 Hz) than by steady illumination (Dowling and Watling 1981; Kirsch and Wagner 1989; Kolbinger and Weiler 1993; Weiler et al. 1997). Our results, along with evidence that DA release from isolated DACs is spike dependent (Puopolo et al. 2001), provide an explanation for the difference in the effectiveness of these two stimulus procedures. Light that flickers ON and OFF triggered strong bursts of spikes in phase with periods of light exposure (Fig. 4B), whereas steady light evoked an initial transient burst of spikes at light onset that was rapidly silenced by the depolarizing block and remained silent until the depolarizing excitatory response declined to a level that relieved spike block, at which point spike generation resumed at an irregular rate not that different from the cell's rate in darkness (Fig. 4A), consistent with reports that DA release is not increased over its level in darkness by continuous light exposure (Godley and Wurtman 1988; Kolbinger and Weiler 1993; Weiler et al. 1997). The inhibitory OFF response participates in the response to flicker by hyperpolarizing the cell during periods of darkness, thereby relieving depolarizing spike block in time for the generation of action potentials at the onset of the next period of light exposure.
The reason for the difference in the effectiveness of flicker and steady light in triggering DA release is not known but seems likely to be the result of facilitated vesicular release that in other neuronal cell types is commonly associated with repetitive firing and increased intracellular Ca2+ due to summated influx (Fioravante and Regehr 2011). This is in agreement with evidence that neuromodulator release is evoked more strongly by phasic than tonic spike generation (Dutton and Dyball 1979; Floresco et al. 2003; Gonon 1988). The inhibitory OFF response evoked by moderate to bright light rapidly terminates spike discharge after a period of light exposure and thus benefits the generation of responses characterized by transient increases in spike discharge. In the midbrain DA system tonic and phasic spike activity control different higher level behaviors (Floresco et al. 2003; Tsai et al. 2009; Zweifel et al. 2008) and have been modeled in terms of changes in the balance between DR1 and DR2 signaling pathways (Dreyer et al. 2010). It is possible that DA signaling in the retina is influenced in a similar manner and that a mixture of inhibitory inputs are important controlling factors in this process, a suggestion that remains to be explored.
Comparison With Previous Studies
The results presented in this article differ in several significant ways from the results presented in the only two previous articles on in situ DACs that were identified and targeted for single-cell recording in intact retina using fluorescence protein expression in a transgenic mouse line (Zhang et al. 2007, 2008). The earlier studies reported that 40% of DACs did not respond to light and that the remaining light-sensitive cells belonged to two distinct classes in which light onset evoked either a transient burst or a sustained train of spikes with no evidence of an OFF response in either. The ON-sustained response, which was recorded from 44% of the subpopulation of light-sensitive DACs, was attributed to excitatory input from ipRGCs.
In contrast, over the course of our experiments we found that 100% of the recorded DACs responded to light and that whether they generated a sustained or transient spike response depended on stimulus intensity (Fig. 3A). Sustained spike trains were evoked by weak stimuli, whereas depolarization block limited the ON response evoked by bright light to an initial transient burst of spikes at response onset. This was also the case in a minority of DACs in our sample that generated exceptionally prolonged large depolarizing responses to strong stimuli, which we considered to be the result of direct excitatory input from ipRGCs (Fig. 3D). In addition, our results show clear evidence of inhibitory ON and OFF responses, which contradicts previous reports that neither GABAergic nor glycinergic OFF-channel signals were involved in DAC light responses (Zhang et al. 2007, 2008).
Whereas both the earlier studies and our study were based on recordings from DACs in different transgenic mouse lines, this seems unlikely to be an explanation for the difference in the results since in the Drd2-GFP Gensat line used presently, all ACs that were labeled with antibodies to TH also express GFP. This would presumably encompass the population of DACs that were targeted by using the TH promoter to drive red fluorescent protein expression in the studies by Zhang and colleagues. It seems more likely that discrepancies between our results and those of Zhang et al. (2007, 2008) are related to important differences in methodology. In the earlier studies DACs were targeted using fluorescence excited by one-photon absorption of short-wavelength light. Although no specific information is given about the intensity or illumination area of the excitation light, it was presumed to have reduced the light sensitivity of the retina (Zhang et al. 2008). Changes in the adaptational state of the retina were avoided in the present study by exciting fluorescence using two-photon absorption of infrared light (see methods). Furthermore, the results of the earlier studies were based on extracellularly recorded spike activity evoked using only intense photopic stimuli that would saturate the photoresponses of both rods and cones. The use of saturating light exposure to evoke responses that can only be detected if they are superthreshold for action potential production provides a severely limited description of the light response properties and fails to fully characterize the dynamic range of DAC light sensitivity and the subthreshold synaptic inputs that give rise to the intensity-dependent properties of the DAC light response.
In the studies by Zhang et al., a reduction in the light sensitivity from the use of short-wavelength light to excite fluorescence for cell targeting and from repeated exposure of the retina to steps of saturating light could account for the presence of cells that did not generate a strong enough response to evoke spikes and thus were classified as nonresponsive (null) DACs. In any case, the absence of light-evoked spike activity in a retinal neuron is not sufficient evidence to conclude that it does not respond to light. Light-adaptational changes in the retina might also serve to reduce the amplitude of the depolarizing response evoked by excitatory input to a level that is not sufficient to cause a depolarizing spike block, accounting for the presence of a subpopulation of DACs in which the response to saturating light is a sustained spike train rather than a transient burst as seen in our recording.
In view of the fundamental differences in the experimental methods used in this and the earlier studies, it is not surprising that there is limited agreement of the results. The main points of accordance include cell-to-cell variation in the pattern of spontaneous spike activity (with disagreement about the extent of the differences) and the elimination of the transient excitatory ON response by l-APB.
In summary, our experiments on in situ DACs characterize the intensity dependence of the synaptic inputs that give rise to their response to light. The onset of dim stimuli evokes an inhibitory response that with increasing light intensity becomes a net depolarizing response that is truncated at light offset by GABAergic inhibition via the OFF pathway. The interplay between light intensity and the strength and timing of multiple excitatory and inhibitory inputs determines the DAC response to light and the role it plays in modulating retina function through the release of dopamine.
GRANTS
This work was supported by National Eye Institute Grants Vision Research Center EY07031 (to G. S. Newkirk), EY10699 (to R. O. Wong), and EY02048 (to P. B. Detwiler).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.S.N., M.H., R.O.W., and P.B.D. conception and design of research; G.S.N. and M.H. performed experiments; G.S.N., M.H., R.O.W., and P.B.D. analyzed data; G.S.N., M.H., R.O.W., and P.B.D. interpreted results of experiments; G.S.N., M.H., R.O.W., and P.B.D. prepared figures; G.S.N., M.H., R.O.W., and P.B.D. drafted manuscript; G.S.N., M.H., R.O.W., and P.B.D. edited and revised manuscript; G.S.N., M.H., R.O.W., and P.B.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Joshua H. Singer for introducing us to the Drd2-GFP BAC transgenic mouse line, providing animals at the start of the project, and being a continuous source of advice and encouragement throughout the study. We also thank Fred Rieke for many useful suggestions and insightful discussions, Winfried Denk for assistance with two-photon microscopy, Paul Newman for technical support, and J. M. Fritschy and R. Enz for antibodies directed against GABA receptors.
REFERENCES
- Alonso A, Llinas RR. Subthreshold Na+-dependent theta-like rhythmicity in stellate cells of entorhinal cortex layer II. Nature 342: 175–177, 1989 [DOI] [PubMed] [Google Scholar]
- Armstrong CM, Gilly WF. Access resistance and space clamp problems associated with whole-cell patch clamping. Methods Enzymol 207: 100–122, 1992 [DOI] [PubMed] [Google Scholar]
- Baylor DA, Hodgkin AL. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol 234: 163–198, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA, Dacheux RF. Rod vision: pathways and processing in the mammalian retina. Prog Retin Eye Res 20: 351–384, 2001 [DOI] [PubMed] [Google Scholar]
- Bunney BS, Grace AA. Acute and chronic haloperidol treatment: comparison of effects on nigral dopaminergic cell activity. Life Sci 23: 1715–1727, 1978 [DOI] [PubMed] [Google Scholar]
- Critz SD, Marc RE. Glutamate antagonists that block hyperpolarizing bipolar cells increase the release of dopamine from turtle retina. Vis Neurosci 9: 271–278, 1992 [DOI] [PubMed] [Google Scholar]
- Dacey DM. The dopaminergic amacrine cell. J Comp Neurol 301: 461–489, 1990 [DOI] [PubMed] [Google Scholar]
- Denk W, Detwiler PB. Optical recording of light-evoked calcium signals in the functionally intact retina. Proc Natl Acad Sci USA 96: 7035–7040, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science 248: 73–76, 1990 [DOI] [PubMed] [Google Scholar]
- Dorenbos R, Contini M, Hirasawa H, Gustincich S, Raviola E. Expression of circadian clock genes in retinal dopaminergic cells. Vis Neurosci 24: 573–580, 2007 [DOI] [PubMed] [Google Scholar]
- Dowling JE, Watling KJ. Dopaminergic mechanisms in the teleost retina. II. Factors affecting the accumulation of cyclic AMP in pieces of intact carp retina. J Neurochem 36: 569–579, 1981 [DOI] [PubMed] [Google Scholar]
- Dreyer JK, Herrik KF, Berg RW, Hounsgaard JD. Influence of phasic and tonic dopamine release on receptor activation. J Neurosci 30: 14273–14283, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton A, Dyball RE. Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol 290: 433–440, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Hausselt SE, Margolis DJ, Breuninger T, Castell X, Detwiler PB, Denk W. Eyecup scope–optical recordings of light stimulus-evoked fluorescence signals in the retina. Pflügers Arch 457: 1393–1414, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A, Gustincich S, Bean BP, Raviola E. Spontaneous activity of solitary dopaminergic cells of the retina. J Neurosci 18: 6776–6789, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A, Gustincich S, Raviola E. Pharmacology of GABAA receptors of retinal dopaminergic neurons. J Neurophysiol 84: 1697–1707, 2000 [DOI] [PubMed] [Google Scholar]
- Fioravante D, Regehr WG. Short-term forms of presynaptic plasticity. Curr Opin Neurobiol 21: 269–274, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci 6: 968–973, 2003 [DOI] [PubMed] [Google Scholar]
- Gingrich KJ, Roberts WA, Kass RS. Dependence of the GABAA receptor gating kinetics on the alpha-subunit isoform: implications for structure-function relations, and synaptic transmission. J Physiol 489: 529–543, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godley BF, Wurtman RJ. Release of endogenous dopamine from the superfused rabbit retina in vitro: effect of light stimulation. Brain Res 452: 393–395, 1988 [DOI] [PubMed] [Google Scholar]
- Gonon FG. Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience 24: 19–28, 1988 [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci 4: 2877–2890, 1984a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci 4: 2866–2876, 1984b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Induction of depolarization block in midbrain dopamine neurons by repeated administration of haloperidol: analysis using in vivo intracellular recording. J Pharmacol Exp Ther 238: 1092–1100, 1986 [PubMed] [Google Scholar]
- Gustincich S, Feigenspan A, Sieghart W, Raviola E. Composition of the GABAA receptors of retinal dopaminergic neurons. J Neurosci 19: 7812–7822, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustincich S, Feigenspan A, Wu DK, Koopman LJ, Raviola E. Control of dopamine release in the retina: a transgenic approach to neural networks. Neuron 18: 723–736, 1997 [DOI] [PubMed] [Google Scholar]
- Ishita S, Negishi K, Teranishi T, Shimada Y, Kato S. GABAergic inhibition on dopamine cells of the fish retina: a [3H]dopamine release study with isolated fractions. J Neurochem 50: 1–6, 1988 [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Galli CL, Garrison-Gund CK, Neff NH. Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons. Science 202: 901–902, 1978 [DOI] [PubMed] [Google Scholar]
- Jusuf PR, Haverkamp S, Grunert U. Localization of glycine receptor alpha subunits on bipolar and amacrine cells in primate retina. J Comp Neurol 488: 113–128, 2005 [DOI] [PubMed] [Google Scholar]
- Kamp CW, Morgan WW. GABA antagonists enhance dopamine turnover in the rat retina in vivo. Eur J Pharmacol 69: 273–279, 1981 [DOI] [PubMed] [Google Scholar]
- Kirsch M, Wagner HJ. Release pattern of endogenous dopamine in teleost retinae during light adaptation and pharmacological stimulation. Vision Res 29: 147–154, 1989 [DOI] [PubMed] [Google Scholar]
- Kolb H, Cuenca N, Dekorver L. Postembedding immunocytochemistry for GABA and glycine reveals the synaptic relationships of the dopaminergic amacrine cell of the cat retina. J Comp Neurol 310: 267–284, 1991 [DOI] [PubMed] [Google Scholar]
- Kolbinger W, Weiler R. Modulation of endogenous dopamine release in the turtle retina: effects of light, calcium, and neurotransmitters. Vis Neurosci 10: 1035–1041, 1993 [DOI] [PubMed] [Google Scholar]
- Koulen P, Sassoe-Pognetto M, Grunert U, Wassle H. Selective clustering of GABAA and glycine receptors in the mammalian retina. J Neurosci 16: 2127–2140, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer SG. Dopamine: a retinal neurotransmitter. I. Retinal uptake, storage, and light-stimulated release of H3-dopamine in vivo. Invest Ophthalmol 10: 438–452, 1971 [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol 392: 397–416, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubarsky AL, Daniele LL, Pugh EN., Jr From candelas to photoisomerizations in the mouse eye by rhodopsin bleaching in situ and the light-rearing dependence of the major components of the mouse ERG. Vision Res 44: 3235–3251, 2004 [DOI] [PubMed] [Google Scholar]
- Mangel SC, Dowling JE. The interplexiform-horizontal cell system of the fish retina: effects of dopamine, light stimulation, and time in the dark. Proc R Soc Lond B Biol Sci 231: 91–121, 1987 [DOI] [PubMed] [Google Scholar]
- Margolis DJ, Detwiler PB. Different mechanisms generate maintained activity in ON and OFF retinal ganglion cells. J Neurosci 27: 5994–6005, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol 431: 291–318, 1990a [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurones. J Physiol 431: 319–342, 1990b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuri NB, Bonci A, Calabresi P, Stefani A, Bernardi G. Properties of the hyperpolarization-activated cation current Ih in rat midbrain dopaminergic neurons. Eur J Neurosci 7: 462–469, 1995 [DOI] [PubMed] [Google Scholar]
- Morgan WW, Kamp CW. A GABAergic influence on the light-induced increase in dopamine turnover in the dark-adapted rat retina in vivo. J Neurochem 34: 1082–1086, 1980 [DOI] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol 207: 123–131, 1992 [DOI] [PubMed] [Google Scholar]
- Nichols CW, Jacobowitz D, Hottenstein M. The influence of light and dark on the catecholamine content of the retina and choroid. Invest Ophthalmol 6: 642–646, 1967 [PubMed] [Google Scholar]
- O'Connor PM, Zucker CL, Dowling JE. Regulation of dopamine release from interplexiform cell processes in the outer plexiform layer of the carp retina. J Neurochem 49: 916–920, 1987 [DOI] [PubMed] [Google Scholar]
- Pang JJ, Abd-El-Barr MM, Gao F, Bramblett DE, Paul DL, Wu SM. Relative contributions of rod and cone bipolar cell inputs to AII amacrine cell light responses in the mouse retina. J Physiol 580: 397–410, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, McCormick DA. Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature 340: 715–718, 1989 [DOI] [PubMed] [Google Scholar]
- Puopolo M, Hochstetler SE, Gustincich S, Wightman RM, Raviola E. Extrasynaptic release of dopamine in a retinal neuron: activity dependence and transmitter modulation. Neuron 30: 211–225, 2001 [DOI] [PubMed] [Google Scholar]
- Ribelayga C, Wang Y, Mangel SC. A circadian clock in the fish retina regulates dopamine release via activation of melatonin receptors. J Physiol 554: 467–482, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol 65: 453–480, 2003 [DOI] [PubMed] [Google Scholar]
- Sagne C, El Mestikawy S, Isambert MF, Hamon M, Henry JP, Giros B, Gasnier B. Cloning of a functional vesicular GABA and glycine transporter by screening of genome databases. FEBS Lett 417: 177–183, 1997 [DOI] [PubMed] [Google Scholar]
- Schmitz F, Konigstorfer A, Sudhof TC. RIBEYE, a component of synaptic ribbons: a protein's journey through evolution provides insight into synaptic ribbon function. Neuron 28: 857–872, 2000 [DOI] [PubMed] [Google Scholar]
- Soto F, Bleckert A, Lewis R, Kang Y, Kerschensteiner D, Craig AM, Wong RO. Coordinated increase in inhibitory and excitatory synapses onto retinal ganglion cells during development. Neural Dev 6: 31, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani A, Pisani A, Bernardi G, Bonci A, Mercuri NB, Stratta F, Calabresi P. The modulation of dopamine receptors in rat striatum. J Neural Transm Suppl 45: 61–66, 1995 [PubMed] [Google Scholar]
- Steffen MA, Seay CA, Amini B, Cai Y, Feigenspan A, Baxter DA, Marshak DW. Spontaneous activity of dopaminergic retinal neurons. Biophys J 85: 2158–2169, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch KF, Paz C, Signorovitch J, Raviola E, Pawlyk B, Li T, Weitz CJ. Intrinsic circadian clock of the mammalian retina: importance for retinal processing of visual information. Cell 130: 730–741, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324: 1080–1084, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versaux-Botteri C, Nguyen-Legros J, Vigny A, Raoux N. Morphology, density, and distribution of tyrosine hydroxylase-like immunoreactive cells in the retina of mice. Brain Res 301: 192–197, 1984 [DOI] [PubMed] [Google Scholar]
- Veruki ML, Hartveit E. Electrical synapses mediate signal transmission in the rod pathway of the mammalian retina. J Neurosci 22: 10558–10566, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassle H, Koulen P, Brandstatter JH, Fletcher EL, Becker CM. Glycine and GABA receptors in the mammalian retina. Vision Res 38: 1411–1430, 1998 [DOI] [PubMed] [Google Scholar]
- Wassle H, Heinze L, Ivanova E, Majumdar S, Weiss J, Harvey RJ, Haverkamp S. Glycinergic transmission in the Mammalian retina. Front Mol Neurosci 2: 6, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler R, Baldridge WH, Mangel SC, Dowling JE. Modulation of endogenous dopamine release in the fish retina by light and prolonged darkness. Vis Neurosci 14: 351–356, 1997 [DOI] [PubMed] [Google Scholar]
- Williams SR, Mitchell SJ. Direct measurement of somatic voltage clamp errors in central neurons. Nat Neurosci 11: 790–798, 2008 [DOI] [PubMed] [Google Scholar]
- Witkovsky P. Dopamine and retinal function. Doc Ophthalmol 108: 17–40, 2004 [DOI] [PubMed] [Google Scholar]
- Xiao J, Cai Y, Yen J, Steffen M, Baxter DA, Feigenspan A, Marshak D. Voltage-clamp analysis and computational model of dopaminergic neurons from mouse retina. Vis Neurosci 21: 835–849, 2004 [DOI] [PubMed] [Google Scholar]
- Xin D, Bloomfield SA. Comparison of the responses of AII amacrine cells in the dark- and light-adapted rabbit retina. Vis Neurosci 16: 653–665, 1999 [DOI] [PubMed] [Google Scholar]
- Yung WH, Hausser MA, Jack JJ. Electrophysiology of dopaminergic and non-dopaminergic neurones of the guinea-pig substantia nigra pars compacta in vitro. J Physiol 436: 643–667, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DQ, Stone JF, Zhou T, Ohta H, McMahon DG. Characterization of genetically labeled catecholamine neurons in the mouse retina. Neuroreport 15: 1761–1765, 2004 [DOI] [PubMed] [Google Scholar]
- Zhang DQ, Wong KY, Sollars PJ, Berson DM, Pickard GE, McMahon DG. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc Natl Acad Sci USA 105: 14181–14186, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DQ, Zhou TR, McMahon DG. Functional heterogeneity of retinal dopaminergic neurons underlying their multiple roles in vision. J Neurosci 27: 692–699, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Argilli E, Bonci A, Palmiter RD. Role of NMDA receptors in dopamine neurons for plasticity and addictive behaviors. Neuron 59: 486–496, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]