Abstract
This study characterizes tonic and phasic stretch reflex and stiffness and viscosity changes associated with spastic hemiparesis. Perturbations were applied to the ankle of 27 hemiparetic and 36 healthy subjects under relaxed or active contracting conditions. A nonlinear delay differential equation model characterized phasic and tonic stretch reflex gains, elastic stiffness, and viscous damping. Tendon reflex was characterized with reflex gain and threshold. Reflexively, tonic reflex gain was increased in spastic ankles at rest (P < 0.038) and was not regulated with muscle contraction, indicating impaired tonic stretch reflex. Phasic-reflex gain in spastic plantar flexors was higher and increased faster with plantar flexor contraction (P < 0.012) than controls (P < 0.023) and higher in dorsi-flexors at lower torques (P < 0.038), primarily because of its increase at rest (P = 0.045), indicating exaggerated phasic stretch reflex especially in more spastic plantar flexors, which showed higher phasic stretch reflex gain than dorsi-flexors (P < 0.032). Spasticity was associated with increased tendon reflex gain (P = 0.002) and decreased threshold (P < 0.001). Mechanically, stiffness in spastic ankles was higher than that in controls across plantar flexion/dorsi-flexion torque levels (P < 0.032), and the more spastic plantar flexors were stiffer than dorsi-flexors at comparable torques (P < 0.031). Increased stiffness in spastic ankles was mainly due to passive stiffness increase (P < 0.001), indicating increased connective tissues/shortened fascicles. Viscous damping in spastic ankles was increased across the plantar flexion torque levels and at lower dorsi-flexion torques, reflecting increased passive viscous damping (P = 0.033). The more spastic plantar flexors showed higher viscous damping than dorsi-flexors at comparable torque levels (P < 0.047). Simultaneous characterizations of reflex and nonreflex changes in spastic hemiparesis may help to evaluate and treat them more effectively.
Keywords: reflexes, stiffness, spasticity, rehabilitation, stroke
spastic hypertonia (Lance 1980) is associated with functional limitations and is a major source of disability in many neurological disorders, including stroke, traumatic brain injury, cerebral palsy, multiple sclerosis, and spinal cord injury (Kim and Park 2011; Sunnerhagen et al. 2013). Despite the clinical significance of spastic hypertonia, the underlying mechanisms are often not clear (Dietz 2000; Rymer and Katz 1994; Sinkjær and Magnussen 1994; Young 1994). The increased mechanical resistance to passive movement can be due to hyperactive reflexes and/or to nonreflex changes in muscles and connective tissues like contracture (Rymer and Katz 1994). The reflex changes have both phasic (dynamic) and tonic (static) components, and the nonreflex contributions include the dynamic component of viscous damping (also called viscosity, dashpotlike property with resistance proportional to velocity) and the static component of elastic stiffness (springlike property with resistance proportional to displacement). All of these components may contribute simultaneously to the increased resistance in passive movement of spastic limbs, whether each of these components (tonic stretch reflex, phasic stretch reflex, elastic stiffness, and viscous damping) is enhanced in spastic limbs or not is not always clear, and there is a lack of methods to characterize all these components simultaneously (Dietz and Berger 1983; Galiana et al. 2005; Gottlieb et al. 1978; Lorentzen et al. 2010; Ludvig et al. 2011; O'Dwyer et al. 1996; Rymer and Katz 1994; Sinkjær and Magnussen 1994; Thilmann et al. 1991; Young 1998).
On one hand, some investigators have shown that the increased resistance in spastic limb movement is mainly due to hyperactive reflexes, as shown in exaggerated tendon jerks and the increased H-reflex response (Galiana et al. 2005; Gottlieb et al. 1978; Levin and Hui-Chan 1993; Meinders et al. 1996; Pierrot-Deseilligny and Mazieres 1985; Rack et al. 1984; Thilmann et al. 1991). However, it is not clear whether reflex hyperexcitability is due to an increase in reflex gain or a decrease in reflex threshold (Levin and Hui-Chan 1993; Powers et al. 1988, 1989; Rack et al. 1984; Thilmann et al. 1991; Zhang et al. 2000). Thilmann et al. argued that while changes in passive mechanical properties may play a role when spasticity has been established for more than a year, the major cause of spastic muscle hypertonus is hyperactive reflex activity. Furthermore, they suggested that spastic hypertonus is not due to a reduction in reflex threshold but due to a pathological increase in stretch reflex gain (Thilmann et al. 1991). In contrast, Powers et al. showed that spastic hypertonia results primarily from a reduction in stretch reflex threshold (measured by joint angle) rather than an increase in reflex gain (Powers et al. 1988, 1989). Levin and Feldman reported that static and dynamic stretch reflex thresholds were decreased in spastic hemiparetic compared with normal subjects and the thresholds depended on velocity (Levin and Feldman 1994). With the use of isometric tests to minimize intrinsic (muscle contracting) and passive (muscle relaxed) contributions to joint torque, it was shown that spastic hypertonia in multiple sclerosis is associated with both increases in reflex gain and contraction rate and a decrease in reflex threshold (Zhang et al. 2000).
On the other hand, other investigators believe that muscle hypertonia is independent of hyperactive reflex and that mechanical changes of muscles are the main reasons for the increased muscle tone in spasticity (Dietz and Berger 1983; Dietz et al. 1991; Lee et al. 1987; O'Dwyer and Ada 1996; O'Dwyer et al. 1996; Sinkjær et al. 1993, 1996; Sinkjær and Magnussen 1994). Dietz et al. suggested that changes in mechanical muscle properties were mainly responsible for muscle hypertonia (Dietz and Berger 1983; Dietz et al. 1991). Lee et al. reported that for voluntarily activated muscles of spastic hemiparetic patients the stretch reflex gains of spastic and contralateral limbs are not significantly different (Lee et al. 1987). O'Dwyer et al. reported that hypertonia in the upper limbs of stroke patients within 13 mo of their stroke was associated with contracture but not with reflex hyperexcitability (O'Dwyer and Ada 1996; O'Dwyer et al. 1996). Sinkjær et al. reported that spastic muscles in stroke patients had an increased nonreflex stiffness but reflex-mediated stiffness during sustained voluntary contraction was not significantly different from normal subjects (Sinkjær et al. 1993, 1996; Sinkjær and Magnussen 1994). Since spasticity involves both reflex and nonreflex actions and both dynamic (phasic) and static (tonic) components (Katz et al. 2000; Lance 1980; Rymer and Katz 1994), it is important to characterize the different components simultaneously in studying spasticity. Most studies generally characterize only some of the components or do not separate different components. For example, although both tonic and phasic stretch reflex components were included in the most widely used definition of spasticity (Lance 1980), the two components have not been evaluated quantitatively and simultaneously with a separate measure for each of them. There is a great need to simultaneously evaluate the various reflex and nonreflex contributions to increased muscle tone in spasticity/contracture, which may help provide appropriate treatments for the different conditions (Dietz and Sinkjær 2007; Rymer and Katz 1994; Sinkjær and Magnussen 1994).
The objectives of this study were to evaluate simultaneously both reflex (dynamic and static stretch reflex gains corresponding to phasic and tonic reflex actions, respectively) and nonreflex (joint elastic stiffness and viscous damping characterizing displacement- and velocity-dependent resistances, respectively) changes in ankles of spastic hemiparetic patients through in vivo experiments under both passive (relaxed) and active (muscle contracting) conditions. Furthermore, tendon jerk, a key element of spasticity (Lance 1980), was evaluated specifically by tapping the Achilles tendon under isometric conditions, which minimized nonreflex actions associated with joint movement and manifested tendon reflexes. The hypotheses of the study were as follows. 1) Dynamic and static stretch reflex gains and intrinsic and passive joint stiffness and viscous damping are changed significantly in spastic hemiparesis, with plantar flexor and dorsi-flexor muscles affected differently. 2) Reflex hypertonia is associated with both increases in reflex gains (tendon reflex gain, dynamic and static stretch reflex gains) and decrease in threshold (measured in tendon tapping force). Investigation of the various components contributing to spastic hypertonia and impairments helps us gain insights into the mechanisms underlying spastic hypertonia, conduct impairment-specific rehabilitation, and evaluate pathological changes and treatment outcome more accurately.
MATERIALS AND METHODS
Subjects.
In total, 63 subjects participated in the study, including 27 patients with neurological disorders and 36 healthy subjects. The 27 hemiparetic patients (17 men and 10 women, 25 with stroke and 2 with traumatic brain injury) had unilateral brain damage at least 1 yr before the experiments. Causes of this brain damage were ischemic, hemorrhagic, and unilaterally traumatic in 10, 15, and 2 subjects, respectively. The time since the initial brain damage was 9.1 ± 5.6 yr (mean ± SD). All patients showed spasticity at the ankle on the impaired side. The 36 (19 men and 17 women) healthy subjects with no prior history of neurological disorder and muscular injury were recruited as normal control subjects. The ages of the spastic hemiparetic patients and healthy subjects were 54.8 ± 10.3 and 47.5 ± 19.9 yr, respectively (Table 1). The study was approved by the Institutional Review Board. All subjects gave informed consent before participating in the study.
Table 1.
Clinical measures of hemiparetic and healthy subjects
| Patient | Age, yr | Sex | MAS (0–4) | DTR (0–4) | Diagnosis | Plegic Side | AFO | Duration, yr | Clonus | Ambulation |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 55.2 | M | 0 | 3 | Ischemic | R | Y | 5.8 | − | Y |
| 2 | 55.4 | M | 1 | 3 | Hemorr | L | N | 6.5 | − | Cane |
| 3 | 45.1 | M | 3 | 3 | Hemorr | R | Y | 6.7 | + | Cane |
| 4 | 57.6 | M | 2 | 3 | Hemorr | L | Y | 16.7 | − | Y |
| 5 | 44.1 | M | 3 | 3 | TBI | R | N | 9.3 | + | WC |
| 6 | 62.9 | M | 1 | 3 | Ischemic | R | N | 6.5 | − | Y |
| 7 | 58.1 | F | 2 | 3 | Ischemic | L | Y | 6.5 | + | Y |
| 8 | 65.6 | M | 3 | 4 | Ischemic | R | Y | 10.4 | + | WC |
| 9 | 48.6 | M | 3 | 4 | Ischemic | L | Y | 4.6 | + | WC |
| 10 | 40.2 | F | 4 | 4 | Hemorr | L | Y | 7.9 | + | Y |
| 11 | 62.0 | M | 3 | 4 | Hemorr | L | Y | 14.1 | + | Y |
| 12 | 37.3 | F | 3 | 3 | Hemorr | R | Y | 7.5 | + | Y |
| 13 | 49.5 | F | 1 | 2 | Hemorr | R | N | 13.9 | − | Y |
| 14 | 71.9 | M | 4 | 3 | Ischemic | R | Y | 7.2 | + | Y |
| 15 | 51.8 | F | 2 | 3 | Hemorr | L | N | 5.4 | + | Y |
| 16 | 62.7 | F | 2 | 3 | Ischemic | R | N | 7.7 | − | Y |
| 17 | 57.9 | F | 3 | 4 | Hemorr | L | Y | 4.2 | + | Cane |
| 18 | 65.1 | F | 3 | 3 | Ischemic | R | Y | 16.3 | + | WC |
| 19 | 47.0 | M | 3 | 4 | Ischemic | R | Y | 10.4 | + | cane |
| 20 | 75.8 | F | 3 | 2 | Hemorr | L | Y | 1.2 | − | WC |
| 21 | 46.4 | M | 3 | 4 | Hemorr | L | N | 13.6 | + | Cane |
| 22 | 45.2 | M | 3 | 4 | Hemorr | R | Y | 1.9 | + | Y |
| 23 | 65.0 | M | 2 | 3 | Ischemic | R | Y | 9.2 | + | Walker |
| 24 | 46.0 | M | 3 | 3 | Hemorr | R | Y | 6.8 | + | Cane |
| 25 | 44.1 | M | 1 | 2 | TBI | L | N | 2.8 | − | Y |
| 26 | 43.8 | F | 1 | 1 | Hemorr | R | Y | 20.3 | − | WC |
| 27 | 69.5 | M | 1 | 2 | Hemorr | L | Y | 12.4 | − | WC |
| Mean ± σ | 54.8 ± 10.3 | 17 M, 10 F | 2.3 ± 0.9 | 3.0 ± 0.9 | 10 Isch, 15 Hemo, 2 TBI | 14 R, 13 L | 9.1 ± 5.6 | |||
| Control mean ± σ | 47.5 ± 19.9 | 19 M, 17 F | 0.0 ± 0.0 | 2.0 ± 0.0 | 35 R, 1 L |
Modified Ashworth scale (MAS, 0–4) and deep tendon reflex (DTR, 0–4) are Ashworth scale and deep tendon reflex scale evaluated for the plantar flexor muscles, both in the range of 0 to 4. Duration gives the number of years since a patient had the first injury. AFO, use of ankle-foot orthosis; TBI, traumatic brain injury; Hemorr, hemorrhagic. Cane, walker, and wheelchair (WC) indicate the tools used by the patients in ambulation.
Each patient was examined initially for spasticity with the modified Ashworth scale (in the range of 0 to 4) and the tendon reflex scale (0 to 4) (Bates 1991; Bohannon and Smith 1987; Meythaler et al. 1996). Passive limb movement in the modified Ashworth scale evaluation was done about three times before the modified Ashworth scale was determined (Bates 1991; Meythaler et al. 1996). The clinical tendon reflex scale was evaluated by tapping the Achilles tendon (scores 2 and 1 correspond to average normal and low normal, respectively). The ankle passive range of motion (ROM) was measured manually with a goniometer under light push for both plantar flexions (PROMPF) and dorsi-flexions (PROMDF), as an evaluation of contracture. More strict evaluation of ROMs under servomotor-controlled moment of spastic ankles is reported elsewhere (Chung et al. 2004). Other physical impairments that could be related to the reflex and nonreflex changes such as clonus were also noted. If a hemiparetic patient had a zero Ashworth scale, a tendon reflex score no more than 2 (average normal response), and normal ankle ROM, he/she was considered not spastic and was not included in the study.
Experimental setup.
With the subject seated upright, a joint-driving device was used to perturb the ankle under both passive (muscles relaxed) and active (muscles contracting in plantar flexion or dorsi-flexion) conditions (Fig. 1). The seat could be moved in the horizontal plane along a set of X-Y tracks in the anteroposterior and mediolateral directions. It could also be rotated to an appropriate orientation and raised to different heights to align the ankle flexion axis with the motor shaft of the joint-driving device. After the leg and foot were positioned properly, the seat was locked in all degrees of freedom to form a solid base and the leg was strapped to a leg support. The foot was fixed to a footplate with adjustable clamp, heel block, and ankle straps. The footplate was mounted onto the motor shaft through a six-axis force sensor (JR3, Woodland, CA). The footplate could be adjusted in the medial-lateral, anterior-posterior, and proximal-distal directions of the foot for proper foot mounting and alignment. The knee and hip were flexed at 60° and 85° flexion, respectively. The ankle was positioned at 0° plantar flexion during the experiment, except for three patients with severe contracture of the triceps surae muscles. In these three cases, the ankle was positioned at a position as close to 0° plantar flexion as possible, at 5°, 10°, and 20° plantar flexion.
Fig. 1.
Experimental setup: the seat is adjusted in 4 degrees of freedom to align the ankle flexion axis with the motor shaft. In addition to other protective measures, the safety screws are used as mechanical stops to restrict the motor range of motion during the perturbation. The foot is fixed to the attachment through a clamp, and the foot attachment can be adjusted and locked in 4 degrees of freedom to achieve appropriate alignment. A 6-axis force sensor is mounted between the foot attachment and the motor shaft. The leg and thigh are strapped to the leg support and seat, respectively.
Control of perturbation.
To characterize reflex and nonreflex properties of the ankle neuromuscular system, the joint was perturbed by a band-limited white noise sequence with appropriate amplitude (amplitude standard deviation σ = 1.5°) and bandwidth (7 Hz) to manifest both reflex and nonreflex properties (Fig. 2) (Zhang and Rymer 1997, 2001). The joint-driving device was driven by a servomotor (Kollmorgen Goldline B806) controlled by a digital controller using a Texas Instruments TMS320 digital signal processor (Zhang and Rymer 1997). On one hand, the bandwidth of the perturbation signal needs to be high so that the joint neuromuscular system receives persistent excitation (Söderström and Stoica 1989) and the subjects can maintain a steady mean muscle torque during the random perturbations. On the other hand, the bandwidth should be low enough to avoid potential vibratory suppression of reflex muscle activation or impaired muscle contractility (Stein and Kearney 1995; Zhang and Rymer 1997, 2001). As a safety precaution, the controller checked the joint position and torque signals at 2 kHz and shut down the system if they were out of prespecified ranges.
Fig. 2.

Representative mechanical and EMG signals during small-amplitude random perturbations. Each column corresponds to a trial. Left and right: trials during which the subject maintained steady dorsi-flexion and plantar flexion background muscle torques, respectively. Center: trial during which the subject was relaxed. First row: ankle dorsi-flexion (DF) torque with dorsi-flexor muscle contraction generating positive torque. Second row: ankle dorsi-flexion angle. Third and fourth rows: ankle plantar flexor (soleus) and dorsi-flexor [tibialis anterior (TA)] EMG signals (normalized to maximum values during experiment), respectively.
Tapping Achilles tendon.
To manifest reflex action and evaluate it more reliably, an instrumented tendon tapper with a force sensor at its tip was used to tap the Achilles tendon with the ankle locked at 0° plantar flexion. The isometric condition manifested reflex actions and minimized nonreflex actions associated with joint movement, including torques generated by the inertial, viscous, and elastic components during joint movement. First, the most sensitive spot on the Achilles tendon with the strongest reflex response was located and a self-adhesive rubber bumper was mounted at the spot, which made the tapping more consistent (Zhang et al. 1999). Second, the tapping force was increased gradually until obvious triceps surae muscle contractions were evoked. The Achilles tendon was then tapped slightly above the force level, which was also taken as a measure of the reflex threshold in tapping force (Zhang et al. 2000).
Protocol.
Ankle flexion angle θ(t), moment T(t), and EMG signals from the soleus, medial and lateral gastrocnemius, and tibialis anterior muscles were recorded. The signals were low-pass filtered with eighth-order Butterworth filters (230-Hz cutoff frequency), and the EMG signals were also high-pass filtered with a 20-Hz cutoff frequency. All signals were sampled at 500 Hz. At first, all signals were recorded at quiescent level while the subject remained relaxed. Next, at 0° plantar flexion, isometric maximum voluntary contractions (MVCs) in dorsi-flexion and plantar flexion were measured three times each and the average MVC values over the trials were taken as the corresponding dorsi-flexion and plantar flexion MVC torques.
To excite the reflex and nonreflex properties involved, the ankle joint was perturbed with the small-amplitude and band-limited white noise perturbation. The subject was asked to either relax or maintain a steady background muscle torque by matching a target torque during the perturbation trials of 10-s length (Fig. 2). The target torque was specified by the experimenter for each trial, and the target-torque matching error was calculated and displayed on the computer monitor screen in real time. The subject was instructed to keep the average level of this error at zero, whatever the target torque level was. Generally, background dorsi-flexion and plantar flexion muscle torques were generated alternately, and a rest period of 30 s was taken between trials to minimize muscle fatigue.
With the ankle fixed isometrically at 0° plantar flexion, the Achilles tendon was tapped at the selected force level ∼10 times during each 30-s-long trial, with random interstimulus intervals of ∼2.5 s. Three trials were collected. The subject was asked to relax during the tapping. If the subject needed to move during the experiment, the examiner would wait until the subject settled down again. Tendon tapping force, triceps surae and tibialis anterior EMG signals, and ankle joint torque were sampled at 500 Hz.
Simultaneous identification of reflex and nonreflex properties.
The measured joint flexion angle and torque were used to identify the following nonlinear delay differential equation model, which was simplified from a previous model by omitting the dynamic stretch reflex gain for muscle shortening as well as setting the stretch reflex threshold in movement velocity to zero (Zhang and Rymer 1993, 1997, 2001). Considering that reflex and nonreflex contributions to increased muscle tone at the ankle were expressed as a summation of the corresponding torque components, the physiological model characterized ankle reflex and nonreflex properties by summing the torque components associated with dynamic (phasic) stretch reflex gain Bd(λ), static (tonic) stretch reflex gain Kd(λ), elastic stiffness K(λ) (also called stiffness for simplicity), viscosity B(λ), and foot inertia I(λ) as follows:
| 1 |
where Δθ(t) and ΔT(t) were the deviations of the ankle dorsi-flexion angle θ(t) and external dorsi-flexion perturbing torque T(t) from their mean values during the small-amplitude perturbations, respectively. λ was the operating state that included the mean background muscle torque, mean ankle flexion angle, and perturbation bandwidth (λ is omitted in the following for simplicity). e(t) was the modeling error. Rd(·) represented half-wave rectification. For plantar flexor and dorsi-flexor contractions during the perturbations, Rd(·) rectified the dorsi-flexion (which stretched contracting plantar flexor muscles) and plantar flexion (stretching contracting dorsi-flexors) movements, respectively. δd was the threshold under which the dynamic movement caused virtually no reflexive joint torque. It was taken as zero in this study for simplicity. Gravity force on the foot was assumed to be constant under the small-amplitude perturbations.
For physiological interpretations, the above model was represented by components with physical or physiological meanings (Fig. 3). Figure 3, top, characterizes the passive and intrinsic muscle-joint properties, where K and B are the joint elastic stiffness and viscous damping coefficient, respectively. Figure 3, bottom, describes the reflex feedback properties of muscles crossing the joint. In addition to the intrinsic (with muscle contracting) and passive (muscle relaxed) muscle-joint properties, the reflex parameters, Bd and Kd, characterize gains of the dynamic and static stretch reflex actions caused by angular velocity and position perturbations occurring td1 seconds ago, respectively. To simplify the identification and reduce the number of parameters estimated simultaneously, td1 was estimated separately in the tendon tapping tests as the delay from the onset of the tapping force to the start of the reflex-mediated torque response (Zhang and Rymer 1997, 2001). Bd corresponds to unidirectional velocity of muscle stretching (dorsi-flexion and plantar flexion for contracting plantar flexors and dorsi-flexors, respectively).
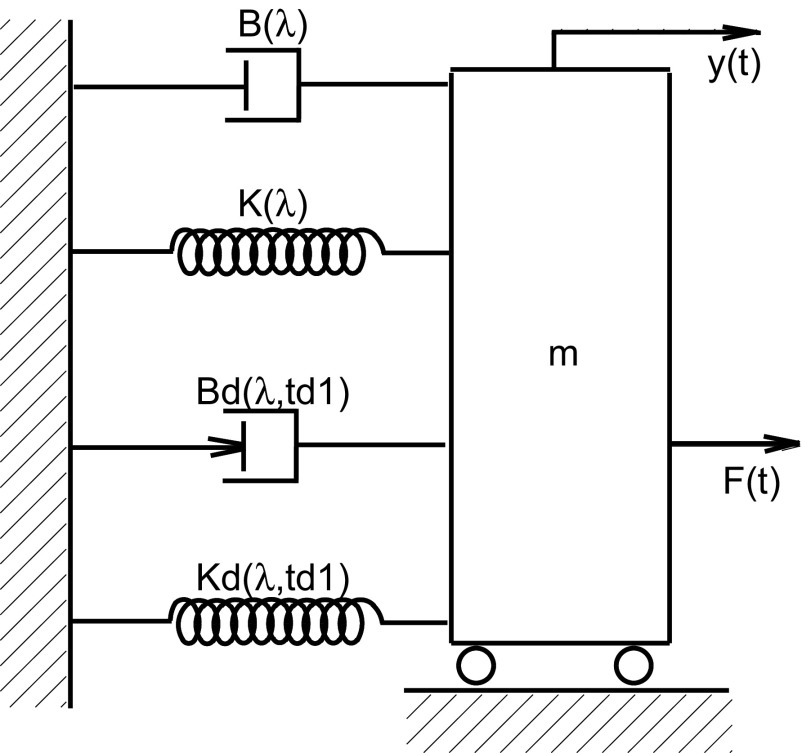
Fig. 3.
Nonlinear delay differential equation model represented by physical components of spring [with elastic stiffness K(λ)], dashpot [with coefficient of viscous damping B(λ)], mass (m), dynamic (phasic) stretch reflex gain Bd(λ,td1), and static (tonic) stretch reflex gain Kd(λ,td1). When the cart is pulled horizontally, the resistance force F(t) is generated by all 5 components, K, B, m, Bd, and Kd. In the corresponding rotational case, K(λ), B(λ), and m correspond to joint elastic stiffness, joint viscous damping, and foot inertia, respectively. The lower part represents the reflex feedback properties of the ankle joint and muscles crossing it. λ represents the system operating state and td1 the reflex-loop delay. Different from the nonreflex muscle-joint properties, the two reflex parameters, Bd(λ,td1) and Kd(λ,td1), characterize stretch reflex actions that are caused by the angular perturbation that occurred td1 seconds ago. Note that Bd(λ,td1) corresponds to unidirectional velocity of muscle stretching.
Data analysis.
The reflex/nonreflex and dynamic/static parameters (K, B, Bd, and Kd) in Eq. 1 were estimated with system identification techniques for each trial during which the subject maintained a steady level of plantar flexor or dorsi-flexor muscle contraction (Zhang and Rymer 1997, 2001). The same estimations were repeated for different levels of plantar flexion and dorsi-flexion torque for each subject (see Fig. 5). Since different subjects did not perform the plantar flexion or dorsi-flexion at exactly the same torque levels, a second-order polynomial was used to fit the parameters estimated across different torque levels for each subject (see Fig. 5). The same estimation procedure was repeated for each subject, and mean and variance of the parameters were then calculated across multiple subjects.
Fig. 5.
Reflex and nonreflex properties of a representative healthy subject. Top left: joint elastic stiffness K as a function of the background muscle contraction. x-Axis represents the background muscle torque, and its positive direction corresponds to plantar flexor contraction. Each “+” corresponds to parameters estimated from a trial in which the subject maintained a steady level of contraction. Top right: joint viscous damping coefficient B as a function of the background muscle contraction. Bottom left: dynamic stretch reflex gain Bd as a function of the background muscle contraction. Bottom right: static stretch reflex gain Kd as a function of the background muscle contraction.
The joint stiffness K of the ankle joint was composed of two components: the passive stiffness (muscles relaxed or no voluntary muscle contraction) and the intrinsic stiffness (muscles contracting). K estimated in the zero background muscle torque (muscle relaxed) condition was taken as the passive stiffness, and the intrinsic component (varying with muscle contraction) of the joint elastic stiffness was the difference between the total joint elastic stiffness (varying with muscle contraction) and passive stiffness (independent of muscle contraction). Similarly, viscous damping was decomposed into passive viscous damping (muscles relaxed or without voluntary muscle contraction) and intrinsic viscous damping (with muscles contracting) components.
For the Achilles tendon reflex, tendon tapping force and reflex-mediated plantar flexion torque were taken as the system input and output, respectively. A system identification technique was used to estimate the tendon reflex gain (Gs) based on the relationship between the tapping force and reflex torque response, which minimized the variations caused by changes in the tapping force (Zhang et al. 1999). The tendon reflex gain characterized the brisk phasic contraction triggered by the dynamic stretching of the triceps surae muscles and spindles inside during the quick tapping of the Achilles tendon (Gordon and Ghez 1991). In addition, reflex threshold in tapping force ft was determined by the averaged peak tapping force considering that the tendon was tapped repeatedly in the experiment just above the threshold.
Between the spastic hemiparetic patients and normal control subjects, Student's t-test was used to test whether each of the response variables was different, including the reflex (dynamic stretch reflex gain Bd and static stretch reflex gain Kd), intrinsic, and passive (joint elastic stiffness K and viscous damping coefficient B under relaxed and muscle contracting conditions, respectively) variables calculated over various contraction levels for each subject. The test was done similarly at different background muscle contraction levels. The response variables also include Achilles tendon reflex gain and reflex threshold in tapping force tested under the passive condition. The null hypothesis was that the two group means were equal. To evaluate whether each of the reflex and nonreflex parameters increased/decreased with muscle contraction, dependence of the parameter on muscle contraction was characterized by the slope of the relationship between them (parameter as a function of the joint torque). The slope was calculated separately for plantar flexor and dorsi-flexor contractions. Relationships among the multiple reflex and nonreflex variables were analyzed by calculating the correlation coefficients between each pair of variables. The significance level was set at 0.05.
RESULTS
General features.
The modified Ashworth scale of the triceps surae muscles in the hemiparetic patients was 2.2 ± 1.1 (mean ± SD) on the 0 to 4 scale (Table 1). Their dorsi-flexor muscles were much less spastic and the Ashworth scales were <1. Their Achilles tendon reflex scale was 3.1 ± 0.8 on the scale of 0 to 4. Their plantar flexion and dorsi-flexion MVC torques were 10.4 ± 10.7 and 10.9 ± 7.7 N·m, respectively. The Achilles tendon reflex scale of the healthy subjects was 2.0 ± 0.0. The hemiparetic patients with spastic ankle had a significantly lower passive ROM in dorsi-flexion (P < 0.001) than that of normal control subjects. The average dorsi-flexion ROM was −17.0 ± 6.4° for the control group (dorsi-flexion was measured as negative angle) and −1.3 ± 11.4° for the hemiparetic patients. The plantar flexion ROM of the spastic hemiparetic group (39.3 ± 7.4°) was also smaller than that of the control group (43.9 ± 10.8°) (P = 0.008).
Simulations based on Eq. 1 with parameters identified from experiments on individual subjects showed that the model (Eq. 1) characterized the neuromuscular system dynamics closely. In a representative case (a stroke survivor with spastic plantar flexors and a modified Ashworth scale of 3), the model accounted for 92% of the variance in the torque signal in a trial during which the subject maintained a strong level of plantar flexor contraction (Fig. 4). Over the various levels of background muscle contraction and across the subjects, the variance accounted for (VAF) was 87.6 ± 5.2% (mean ± SD). Representative reflex (dynamic and static stretch reflex gains) and nonreflex (elastic stiffness and coefficient of viscous damping) properties from a healthy subject are shown in Fig. 5.
Fig. 4.
Measured (solid line) and simulated (dashed line) ankle plantar flexion torques. The simulation was based on the model in Eq. 1. Data were from a male stroke patient with severe plantar flexor spasticity.
Changes in passive and intrinsic joint stiffness in spastic hemiparesis.
The joint elastic stiffness in spastic ankles of spastic hemiparetic patients was significantly higher than that in normal control subjects across the range of background muscle torque, including both background plantar flexor (P < 0.002) and dorsi-flexor (P < 0.032) contractions (Fig. 6). For both spastic hemiparetic patients and healthy subjects, joint stiffness K increased with the background muscle torque in both plantar flexion (P < 0.001) and dorsi-flexion (P < 0.001) (Fig. 6). At comparable levels of background muscle torque, there was no significant difference between the stiffness generated by the plantar flexors and dorsi-flexors in normal control subjects (P > 0.057). However, the stiffness generated by the plantar flexors was greater than that generated by the dorsi-flexors in the spastic hemiparetic patients at torque levels of <7 N·m (P < 0.031) (Fig. 6).
Fig. 6.
Reflex (C and D) and nonreflex (A and B) properties in 36 normal subjects and 27 spastic hemiparetic patients. A: joint elastic stiffness K as a function of the background muscle contraction. x-Axis represents the background muscle torque, and its positive and negative directions correspond to plantar flexor and dorsi-flexor muscle contractions, respectively. Vertical lines give the SE of the mean across subjects in each group. △ and * correspond to spastic hemiparetic and healthy subjects, respectively. B: joint viscosity B as a function of the background muscle contraction. C: dynamic stretch reflex gain Bd as a function of the background muscle contraction. D: static stretch reflex gain Kd as a function of the background muscle contraction.
Between the passive and intrinsic components of the total joint stiffness, the passive joint stiffness in spastic ankles of the hemiparetic patients was significantly higher than that of normal control subjects (P < 0.001). However, the intrinsic joint stiffness was not significantly different between the spastic hemiparetic and normal groups at any torque level in either dorsi-flexor contraction (P > 0.500) or plantar flexor contraction (P > 0.187).
Changes in passive and intrinsic joint viscous damping in spastic hemiparesis.
Joint viscous damping coefficient B in the spastic ankles of hemiparetic patients was significantly higher than that in normal control subjects (Fig. 6). The difference was significant across the plantar flexor torque levels (P < 0.024) and for <2 N·m dorsi-flexor contraction (P = 0.033). For both normal and spastic hemiparetic groups, ankle joint viscous damping coefficient B(λ) increased monotonically with increasing plantar flexor (P < 0.001) and dorsi-flexor (P < 0.009) muscle torque (Fig. 6). At comparable levels of background muscle torque there was no significant difference between viscous damping of the plantar flexors and that of the dorsi-flexors in normal control subjects (P > 0.052), and beyond 16 N·m viscous damping of the plantar flexors became greater than that of the dorsi-flexors in normal control subjects (P < 0.047). In contrast, across the torque levels tested, viscous damping of the plantar flexors was significantly higher than that of the dorsi-flexors in the spastic hemiparetic patients (P < 0.048) (Fig. 6).
Between the passive and intrinsic components of the total joint viscous damping, the passive viscous damping in the spastic ankles of the hemiparetic patients was significantly higher than that of normal control subjects (P = 0.033). However, intrinsic viscous damping was not significantly different between the spastic hemiparetic and normal groups at any torque level in either dorsi-flexor contraction (P > 0.500) or plantar flexor contraction (P > 0.219).
Changes in dynamic stretch reflex gain in spastic hemiparesis.
Across the range of plantar flexor contraction, dynamic stretch reflex gain Bd for stretching the contracting plantar flexor muscles in the spastic hemiparetic patients was higher than that in normal control subjects at the same levels of torque (P < 0.023) (Fig. 6). Similarly, dynamic stretch reflex gain Bd for stretching dorsi-flexor muscles in the spastic hemiparetic group was higher than that in normal control subjects (P < 0.038). At the passive state (muscle relaxed), dynamic stretch reflex gain Bd was significantly higher than that in normal control subjects (P = 0.045).
As the background muscle torque increased, the dynamic stretch reflex gain Bd increased with both ankle plantar flexor (P < 0.001) and dorsi-flexor (P < 0.017) contractions for both normal control subjects and spastic hemiparetic patients (Fig. 6). For the dorsi-flexor muscles, Bd increase due to muscle contraction in spastic hemiparetic patients was not higher than similar Bd increase in normal control subjects. For the more spastic plantar flexor muscle, however, there was more significant Bd increase with muscle contraction in the spastic hemiparetic patients than in normal control subjects for plantar flexor torque >10 N·m (P < 0.012).
In the spastic ankles, dynamic stretch reflex gain Bd associated with the more spastic plantar flexors was significantly higher than that associated with the dorsi-flexors at all comparable levels of torque (P < 0.032) (Fig. 6). In contrast, there was no significant difference between the dynamic stretch reflex gain Bd of the plantar flexors and that of the dorsi-flexors in normal control subjects at comparable levels of muscle torque across the range of 0 to 17 N·m (P > 0.101).
Changes in static stretch reflex gain in spastic hemiparesis.
At the passive state (muscle relaxed), static stretch reflex gain Kd in spastic hemiparetic ankles was significantly higher than that in normal controls (P = 0.038) and Kd in spastic hemiparetic ankles remained significantly higher than that in normal controls for plantar flexor torques <4 N·m (P < 0.037) and for dorsi-flexor torques <3 N·m (P ≤ 0.038) (Fig. 6).
As the background muscle torque increased, the static stretch reflex gain Kd increased with both ankle plantar flexor (P < 0.001) and dorsi-flexor (P < 0.001) contractions in normal control subjects (Fig. 6). However, it did not increase with either plantar flexor (P = 0.233) or dorsi-flexor (P = 0.952) contraction in spastic hemiparetic patients.
Changes in Achilles tendon reflex gain in spastic hemiparesis.
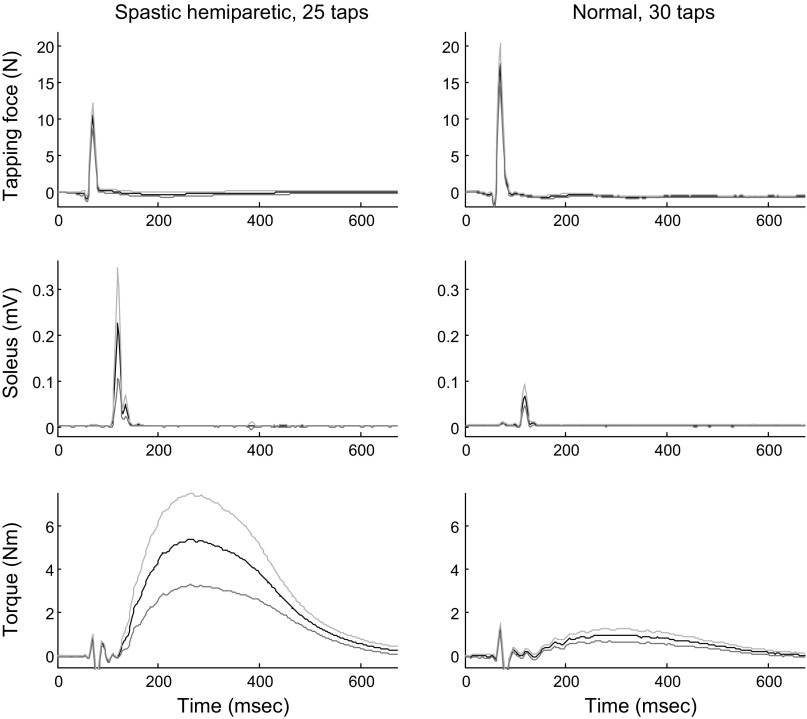
In general, stronger Achilles tendon reflex responses in reflex-mediated torque and EMG signals were induced in spastic ankles than in normal controls (Fig. 7). With the muscles relaxed (the passive state), the Achilles tendon reflex gain Gs of spastic ankles was significantly higher than that of healthy controls (P = 0.002), with the tendon reflex gain for the spastic hemiparetic and healthy groups being 7.4 ± 5.9 and 3.4 ± 2.5 cm·ms, respectively.
Fig. 7.
Achilles tendon tapping results from a male stroke patient's spastic ankle (left) and from a normal male subject's ankle (right). The reflex threshold and tendon reflex gain for the stroke patient were 11.0 N and 12.8 cm, respectively. They were 16.8 N and 1.2 cm, respectively, for the normal subject. From top to bottom, tendon tapping force, soleus muscle EMG signal, and reflex-mediated plantar flexor ankle joint torque are shown. Subjects were asked to relax during the tapping trials. The ankle was fixed isometrically at 0° plantar flexion. Means ± SD over the different taps (25 taps for the spastic hemiparetic patient and 30 taps for the normal subject) are shown for each signal. The initial spike in the reflex torque signal (at ∼80 ms) was due to the mechanical impact to the torque sensor caused by the tapping.
On the other hand, much weaker taps were needed to induce reflex responses in spastic triceps surae muscles than in normal controls (Fig. 7). The threshold in tapping force ft was 13.6 ± 3.5 and 20.2 ± 6.4 N for the spastic hemiparetic and healthy groups, respectively. The reflex threshold ft was significantly lower for the spastic hemiparetic population than for normal control subjects (P < 0.001).
Correlations between reflex and nonreflex changes.
Table 2 summarizes Pearson product-moment correlation coefficients (r) between each pair of the reflex (Bd, Kd, Gs, and ft) and nonreflex (PROMDF, K, B, plus plantar flexor MVC) measures, calculated over the patients after stroke. The subscript “passive” was added for their values under the passive muscle relaxed conditions. The passive ROM in dorsi-flexion (DF ROM) of the patients was correlated negatively with the stiffness Kpassive (r = −0.462 and P = 0.015) and viscosity Bpassive (r = −0.485 and P = 0.010) of the passive ankle and with the tendon reflex gain Gs (r = −0.428 and P = 0.037). The passive stiffness Kpassive was strongly correlated with most of the nonreflex and reflex measures, especially the passive viscosity Bpassive (r = 0.943 and P < 0.001), dynamic reflex gain Bd_passive (r = 0.759 and P < 0.001) and static reflex gain Kd_passive (r = 0.614 and P = 0.001), and tendon reflex gain Gs (r = 0.714 and P < 0.001). The passive viscosity Bpassive was also strongly correlated with the dynamic reflex gain Bd_passive (r = 0.783) and static reflex gain Kd_passive (r = 0.640) and tendon reflex gain Gs (r = 0.671), with P < 0.001 for all (Table 2).
Table 2.
Correlations between reflex and nonreflex measures in spastic hemiparesis
| DF ROM, ° | Kpassive, N•m/rad | Bpassive, Nm•S/rad | Bd_passive, Nm•S/rad | Kd_passive, N•m/rad | Gs, cm•ms | ft, N | PF MVC, N•m | |
|---|---|---|---|---|---|---|---|---|
| DF ROM | 1 | −0.462* | −0.485* | −0.194 | −0.162 | −0.428* | 0.090 | −0.169 |
| P | 0.015 | 0.010 | 0.332 | 0.419 | 0.037 | 0.675 | 0.400 | |
| Kpassive | — | 1 | 0.943† | 0.759† | 0.614† | 0.714† | 0.082 | 0.158 |
| P | 0.462* | 0.000 | 0.000 | 0.001 | 0.000 | 0.704 | 0.430 | |
| 0.015 | ||||||||
| Bpassive | — | 0.943† | 1 | 0.783† | 0.640† | 0.671† | 0.150 | 0.271 |
| P | 0.485* | 0.000 | 0.000 | 0.000 | 0.000 | 0.485 | 0.172 | |
| 0.010 | ||||||||
| Bd_passive | −0.194 | 0.759† | 0.783† | 1 | 0.522† | 0.479* | 0.183 | 0.177 |
| P | 0.332 | 0.000 | 0.000 | 0.005 | 0.018 | 0.392 | 0.377 | |
| Kd_passive | −0.162 | 0.614† | 0.640† | 0.522† | 1 | 0.710† | −0.011 | −0.005 |
| P | 0.419 | 0.001 | 0.000 | 0.005 | 0.000 | 0.960 | 0.982 | |
| Gs | — | 0.714† | 0.671† | 0.479* | 0.710† | 1 | −0.291 | 0.089 |
| P | 0.428* | 0.000 | 0.000 | 0.018 | 0.000 | 0.168 | 0.678 | |
| 0.037 | ||||||||
| ft | 0.090 | 0.082 | 0.150 | 0.183 | −0.011 | −0.291 | 1 | 0.219 |
| P | 0.675 | 0.704 | 0.485 | 0.392 | 0.960 | 0.168 | 0.303 | |
| PF MVC | −0.169 | 0.158 | 0.271 | 0.177 | −0.005 | 0.089 | 0.219 | 1 |
| P | 0.400 | 0.430 | 0.172 | 0.377 | 0.982 | 0.678 | 0.303 |
Values are Pearson's product-moment correlation coefficients (upper triangle), calculated with data from all 27 spastic hemiparetic patients. DF ROM, mean dorsiflexion range of motion; PF MVC, plantar flexor maximal voluntary contraction torque; Kpassive, Bpassive, Bd_passive, Kd_passive, K(λ), B(λ), Bd(λ), Kd(λ) in the passive condition, respectively; Gs and ft, tendon reflex gain and reflex threshold in tapping force, respectively.
and †, significance at 0.05 and 0.01 level (2-tailed), respectively.
The dynamic reflex gain Bd_passive was correlated with the tendon reflex gain Gs (r = 0.479 and P = 0.018) and static reflex gain Kd_passive (r = 0.522 and P = 0.005). The static reflex gain Kd_passive was also correlated with tendon reflex gain Gs (r = 0.710 and P < 0.001). The threshold in tapping force ft had low correlations with all other variables. The plantar flexor muscle strength (PF MVC) did not have significant correlations with the other measures (Table 2).
DISCUSSION
It is not clear how much spastic hypertonia is due to hyperactive stretch reflexes (Gottlieb et al. 1978; Levin and Hui-Chan 1993; Meinders et al. 1996; Mirbagheri et al. 2001; Pierrot-Deseilligny and Mazieres 1985; Powers et al. 1988; Rack et al. 1984; Thilmann et al. 1991; Zhang et al. 2000) and how much is due to the nonreflex part of muscle stiffness (Chung et al. 2004; Dietz and Berger 1983; Galiana et al. 2005; Lee et al. 1987; O'Dwyer and Ada 1996; O'Dwyer et al. 1996; Sinkjær and Magnussen 1994). A difficulty involved is that the reflex and nonreflex dynamic and static components contributing to spastic hypertonia coexist in spastic muscles/joints and evaluating some components without considering the others appropriately cannot differentiate their contributions. The above approach provides us with a useful tool to simultaneously identify reflex and nonreflex changes associated with spastic limbs through in vivo experiments. The study showed that spastic hemiparesis was associated with a number of reflex and nonreflex changes simultaneously: 1) Dynamic stretch reflex was hyperactive in spastic ankles, and the more spastic plantar flexors showed higher dynamic stretch reflex gain than the dorsi-flexors. The hyperexcitability of phasic stretch reflex was corroborated by significantly higher tendon reflex gain in relaxed muscle. 2) Hyperactive static stretch reflex was associated with spastic hemiparesis at the passive and low levels of muscle torque. Furthermore, spastic hemiparetic patients did not regulate their static stretch reflex gain with muscle contraction as the normal control subjects did. 3) Elastic stiffness in the passive state (muscle relaxed) was significantly higher in spastic ankles than in normal controls, and intrinsic stiffness was not different between spastic and normal groups. 4) Joint viscous damping in the passive state was higher in spastic hemiparesis than in normal control subjects. 5) Together with increases in the reflex gains, reflex threshold measured in tapping force was significantly lower in spastic hemiparesis than in normal control subjects. These findings on reflex and nonreflex and dynamic and static changes help us understand mechanisms underlying spastic hypertonia and allow us to evaluate pathological changes and treatment outcomes more accurately.
The relative contributions of the reflex and nonreflex components were dependent on the experimental conditions (perturbation bandwidths, amplitudes, muscle activation, etc.) at the ankle joint, and the reflex and nonreflex parameters estimated may vary considerably with the conditions. Reflex contributions, for example, could vary from 0% as in the case of slow passive movement with only nonreflex-mediated resistance and no reflex actions induced as in previous studies (Chung et al. 2004) to almost 100% as in the case of tendon reflexes induced by tapping the Achilles tendon with the ankle at a fixed position (Zhang et al. 2000). In general, reflex and nonreflex contributions estimated in different studies using different protocols could be quite different from each other. However, what is important is to use the same experimental protocol across different subjects and compare their reflex and nonreflex contributions at comparable conditions, which was done in the present study.
The significantly higher dynamic stretch reflex gain [Bd(λ)] in spastic ankles than in normal controls indicates hyperactive phasic stretch reflex in spasticity. Furthermore, the larger increase in dynamic stretch reflex gain (in comparison with normal controls) over the torque range of the more spastic plantar flexors and the smaller increase in dynamic stretch reflex gain of the less spastic dorsi-flexors indicate that the increase in dynamic (phasic) stretch reflex gain is associated with severity of spasticity. Similarly, the higher dynamic stretch reflex gain in the more spastic plantar flexors than in the less spastic dorsi-flexors at comparable torque levels also supports the positive correlation between severity of spasticity and hyperactive phasic stretch reflex.
It was found that a major component of the increased dynamic stretch reflex gain was due to its increase in the passive state (muscle resting/relaxed), indicating hyperexcitability of motoneurons associated with resting spastic muscles crossing the ankle. Furthermore, after subtraction of the gain in the passive state, the dynamic stretch reflex gain in the more spastic plantar flexors was still significantly higher than that in normal controls in the torque range of >10 N·m. This indicates that dynamic stretch reflex gain in strongly activated spastic muscles was further increased in the more spastic plantar flexor muscles.
Increased phasic stretch reflex in spastic ankles as reflected by the increased dynamic stretch reflex gain was corroborated by the increased tendon reflex gain and significant correlation between the two measures (Table 2), with the latter determined isometrically and thus minimizing nonreflex contributions to the joint torque. This was consistent with the pathological increase in reflex gain in the biceps muscle EMG response observed in 19 hemiparetic patients (Thilmann et al. 1991). It was also in agreement with the increase in reflex stiffness gain reported by Galiana et al. (2005) and Mirbagheri et al. (2001), although their reflex stiffness gain measure was not exactly the same as our measures of dynamic stretch reflex gain because of the different model structures used.
By tapping the Achilles tendon under an isometric condition that minimized nonreflex actions associated with joint movement and thus manifested reflex actions, we found that reflex threshold measured in tapping force was significantly reduced in spastic hemiparetic ankles and a weaker stimulus was needed to induce reflex responses in the spastic plantar flexor muscles than that needed for normal controls. Our emphasis was to minimize nonreflex contributions associated with joint movement with focus on reflex excitability. It was found that even with a lighter tap the spastic plantar flexor muscles responded reflexively with higher gain than normal controls. The decreased reflex threshold and increased tendon reflex gain indicated that spastic hypertonia involved reflex changes in both gain (Gottlieb et al. 1978; Rack et al. 1984; Thilmann et al. 1991) and threshold (Levin and Feldman 1994; O'Sullivan et al. 1998; Powers et al. 1988; Rymer and Katz 1994). Of note is that the reflex threshold in tapping force characterized in the present study is different from the reflex threshold in joint angle measured during passive movement (Levin and Feldman 1994; Rymer and Katz 1994), although the two threshold measures are related. Increase in tendon reflex gain and decrease in threshold coexisted in spastic plantar flexor muscles and reflected a common phenomenon of hyperactive reflex, which could be due to increased motoneuron excitability and/or tighter mechanical transmission along the path from the tapping of the Achilles tendon to the stretching of spindles in the triceps surae muscles and from the contraction of the triceps surae muscles to the production of ankle plantar flexion torque (Zhang et al. 2000). Such an increased mechanical transmission in spastic muscles was corroborated by the significant correlations between the increased stiffness/viscosity and the increased tendon reflex gain in the hemiparetic patients (Table 2).
Muscle spindles contain dynamic nuclear bag intrafusal fibers and static nuclear bag and nuclear chain intrafusal fibers with differential responses to dynamic and static phases of muscle length changes (Gordon and Ghez 1991). The dynamic stretch reflex gain [Bd(λ)] and static stretch reflex gain [Kd(λ)] were used in this study to characterize the corresponding dynamic (phasic) and static (tonic) reflex actions, respectively. We found that static stretch reflex gain Kd(λ) in spastic hemiparetic ankles was significantly higher than that in normal controls at the passive state (resting muscles with no voluntary contraction) and at low levels of plantar flexor and dorsi-flexor contractions. This was consistent with the increased tonic stretch reflex in spasticity (Lance 1980). Lieber and Fridén (2002) measured the sarcomere length in flexor carpi ulnaris (FCU) muscles from patients with severely spastic wrist flexion contractures and found that spastic FCU muscles had extremely long sarcomere lengths even with the wrist fully flexed. It is conceivable that elongated sarcomeres may be associated with elongated static nuclear bag and nuclear chain fibers in spindles, which would likely result in increased tonic stretch reflex gain. Furthermore, the static stretch reflex gain Kd(λ) was not regulated with either plantar flexor or dorsi-flexor contraction in spastic hemiparetic patients as was the case in normal control subjects, indicating an impaired reflex control mechanism associated with elongations of sarcomeres and chain and static bag fibers in spasticity.
It was found that both plantar flexors and dorsi-flexors in spastic ankles were significantly stiffer (higher elastic stiffness) than their counterparts in normal controls across the range of contractions, and the more spastic plantar flexors in spastic ankles were stiffer than the less spastic dorsi-flexors at comparable levels of torque. This indicates that elastic stiffness is closely related to spasticity and is positively correlated with spasticity. Among the various correlations in Table 2, passive stiffness showed very strong correlations with other reflex and nonreflex measures (viscosity, dynamic and static reflex gains, and tendon reflex gain), suggesting the clinical need for reducing passive stiffness to treat spasticity and motor impairment. Furthermore, it was shown that passive stiffness was a major contributor to the increased total joint stiffness in spastic ankles while intrinsic stiffness was not significantly increased, indicating that changes in connective tissues, tendons, and passive muscle fascicle properties are likely to be major contributors to the increased stiffness in spastic ankles. It is conceivable that decreased fascicle length after stroke (Gao et al. 2009; Gao and Zhang 2008) would result in increased passive tension and passive elastic stiffness. In addition, many of the spastic hemiparetic patients in this study (19 of 27) used an ankle-foot orthosis (AFO), which helped keep the ankle in place but also reduced the movement of muscles across the ankle. It was shown that immobilization of muscles caused considerable increase in collagen and loss of sarcomeres, and both quantitative increase and qualitative rearrangement of the connective tissue contributed to increase in muscle stiffness (Williams and Goldspink 1981, 1984). Booth et al. (2001) showed that connective tissue, and more specifically the structural protein collagen, accumulated within spastic muscles of children with cerebral palsy and the amount of collagen had a high significant correlation with severity of spasticity quantified by the Ashworth scale. Increases in connective tissues, shortening of muscle fascicles, and fiber atrophy fibers could contribute to the increase in passive stiffness in spastic muscles.
Viscous damping is involved in all dynamic movement, and appropriate damping is important in efficient and smooth limb movement (Cavagna 1970; Martin et al. 1994; Niku and Henderson 1989). The viscous damping coefficient [B(λ)] in spastic ankles was significantly higher than that in normal controls, especially in the more spastic plantar flexor muscles, reflecting an increase in the velocity-dependent resistance in spastic muscles/joints during dynamic movement. This indicates that viscous damping as well as hyperactive reflexes contribute to the increased velocity-dependent resistance associated with spastic limb movement. Furthermore, viscous resistance at the ankle joint can be generated by both muscle fibers (contracting or relaxed) and connective tissues (Zuurbier and Huijing 1992). We found that the increased viscous damping in spastic hemiparetic ankles was due to an increase in passive viscous damping and intrinsic viscous damping associated with muscle contraction was not different between the spastic and control groups. This indicates that the increased viscous damping was likely due to an increase in connective tissues and/or changes in passive muscle fiber properties (reduction in fiber size and loss of muscle fibers) in spastic muscles. It is conceivable that, similar to the increase in passive elastic stiffness, the increase in passive viscous damping may also be related to the elongated sarcomeres (Lieber and Fridén 2002), accumulation of collagen (Booth et al. 2001), and change in the aponeurosis within spastic muscles.
By evaluating the passive, intrinsic, and reflex-mediated mechanical responses to a 4° stretch of the ankle extensors at 200 ms after the stretch, Sinkjær and Magnussen (1994) reported increased passive stiffness and unchanged intrinsic stiffness in spastic ankle extensors in nine hemiparetic patients. Our results on passive and intrinsic elastic stiffness were consistent with their results. Furthermore, we evaluated viscous damping and static stretch reflex gain, which also contributed to the ankle joint torque during the applied position perturbations. On the other hand, they reported that reflex stiffness of the spastic and contralateral legs was within the normal range of the reflex stiffness in healthy subjects, which was different from the increased dynamic stretch reflex gain [Bd(λ)] observed in our study. Several factors might have contributed to the differences. First, our patients had rather severe spasticity, with average modified Ashworth scale and tendon reflex scale of 2.3 and 3.0, respectively. Second, the number of subjects involved in our study was larger (27 hemiparetic patients and 36 control subjects) than theirs (9 spastic hemiparetic patients tested bilaterally and 8 control subjects), which increased the statistical power. Third, the patients in our study had rather long durations of brain damage, averaging 9.1 ± 5.6 yr since the first incident (compared to 31 ± 24 mo in their study). The type of injury (ischemic or hemorrhagic) and plegic side of the patients in the two studies were also quite different.
The patients investigated in this study were chronic, averaging 9.1 ± 5.6 yr post brain injury. The long duration after injury may have played a major role in the significant changes in the reflex and nonreflex properties observed in this study. Over time, reduced joint ROM, muscle fiber atrophy, and collagen accumulation may occur, and joint contracture may be developed (Gao et al. 2009; Selles et al. 2005), which may contribute to the substantial increase of passive elastic stiffness, for example. Significant correlations among the reflex and nonreflex variables may also be affected by the long duration after stroke of the patients in this study. Different changes in reflex and nonreflex properties and different correlations may be observed with acute and subacute patients after stroke, which need to be investigated in future studies.
Different perturbations with different bandwidths, amplitudes, etc. applied to the ankle joint may manifest or suppress different components to be determined. For example, wide-bandwidth perturbations tend to suppress reflex contributions and promote intrinsic/passive contributions, while narrow-bandwidth perturbations or perturbations with well-separated stretching and shortening movement may do the opposite (Stein and Kearney 1995; Zhang and Rymer 1997, 2001). The perturbations used in this study might not manifest reflex and nonreflex and dynamic and static properties optimally. The small-amplitude band-limited “white noise” perturbations used in this study made it easier for the subjects to maintain steady contractions and thus steady reflex and nonreflex actions during the perturbations. The reflex and nonreflex parameters [B(λ), K(λ), Bd(λ), and Kd(λ)] thus remained constant within the trials, making it easier to estimate them more reliably. Still, it was difficult for some patients to generate steady contractions at higher torque levels, which made the range of contraction torque during the small-amplitude random perturbations relatively small.
The low correlations of the reflex threshold in tapping force with the other reflex and nonreflex measures (see Table 2) might be related to the way the threshold was determined. During the experiment, the examiner tapped the Achilles tendon slightly above the threshold level and the averaged peak tapping force was used as the reflex threshold. The exact threshold point beyond which reflex responses were elicited should be slightly lower than the threshold determined in our experiment. Further work needs to be done to investigate different methods of threshold determination and to tap the tendon below as well as above the threshold to determine the reflex threshold accurately.
Spasticity involves both reflex and nonreflex and both dynamic and static changes, which made its evaluation difficult. The significant correlations among the reflex and nonreflex changes in spastic hemiparesis indicated the need for evaluations of the reflex and nonreflex changes through an integrated approach. The method in this study provided us a tool to evaluate the multiaspect pathological changes simultaneously. Further study needs to be done to improve the method and corroborate it further with related investigations such as evaluations of changes of spastic muscle fascicles and tendons (Gao et al. 2009; Gao and Zhang 2008; Zhao et al. 2009).
Clinically, further work should be done to potentially implement the method as part of clinical diagnosis, which can be used to guide rehabilitation of spastic limbs more accurately in an impairment-specific way, and to evaluate the outcome comprehensively and quantitatively (Chung et al. 2008; Gao et al. 2009; Gao and Zhang 2008; Selles et al. 2005; Waldman et al. 2013; Zhang et al. 2002; Zhao et al. 2009). For example, if increased passive stiffness with reduced ROM is the major contributing factor, passive stretching may be chosen as the focused treatment (Gao et al. 2011; Selles et al. 2005). If it is predominantly impairment under active plantar flexor contraction, active movement training of the target calf muscles should be focused on (Waldman et al. 2013; Wu et al. 2011). We expect that in many patients the impairments are contributed by multiple reflex and nonreflex factors, and thus combined treatment and evaluation are needed (Waldman et al. 2013; Wu et al. 2011). Practically, an implementation of a more convenient and portable setup should be considered to make it suitable for clinical settings.
GRANTS
The authors gratefully acknowledge the support of the National Institutes of Health, the National Institute on Disability and Rehabilitation Research, and the Buehler Center on Aging at Northwestern University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.-Q.Z., S.G.C., and W.Z.R. conception and design of research; L.-Q.Z., S.G.C., Y.R., and L.L. performed experiments; L.-Q.Z., S.G.C., Y.R., and L.L. analyzed data; L.-Q.Z., S.G.C., Y.R., L.L., E.J.R., and W.Z.R. interpreted results of experiments; L.-Q.Z., S.G.C., Y.R., and L.L. prepared figures; L.-Q.Z. drafted manuscript; L.-Q.Z., S.G.C., E.J.R., and W.Z.R. edited and revised manuscript; L.-Q.Z., S.G.C., E.J.R., and W.Z.R. approved final version of manuscript.
REFERENCES
- Bates B. A Guide to Physical Examination and History Taking. Philadelphia, PA: Lippincott, 1991 [Google Scholar]
- Bohannon RW, Smith MB. Interrater reliability of modified Ashworth scale of muscle spasticity. Phys Ther 67: 206–207, 1987 [DOI] [PubMed] [Google Scholar]
- Booth CM, Cortina-Borja MJ, Theologis TN. Collagen accumulation in muscles of children with cerebral palsy and correlation with severity of spasticity. Dev Med Child Neurol 43: 314–320, 2001 [DOI] [PubMed] [Google Scholar]
- Cavagna GA. Elastic bounce of the body. J Appl Physiol 29: 279–282, 1970 [DOI] [PubMed] [Google Scholar]
- Chung SG, van Rey E, Bai Z, Rymer WZ, Roth EJ, Zhang LQ. Separate quantification of reflex and non-reflex components of spastic hypertonia in chronic hemiparesis. Arch Phys Med Rehabil 89: 700–710, 2008 [DOI] [PubMed] [Google Scholar]
- Chung SG, van Rey EM, Bai Z, Roth EJ, Zhang LQ. Biomechanic changes in passive properties of hemiplegic ankles with spastic hypertonia. Arch Phys Med Rehabil 85: 1638–1646, 2004 [DOI] [PubMed] [Google Scholar]
- Dietz V. Spastic movement disorder. Spinal Cord 38: 389–393, 2000 [DOI] [PubMed] [Google Scholar]
- Dietz V, Berger W. Normal and impaired regulation of muscle stiffness in gait: a new hypothesis about muscle hypertonia. Exp Neurol 79: 680–687, 1983 [DOI] [PubMed] [Google Scholar]
- Dietz V, Sinkjær T. Spastic movement disorder: impaired reflex function and altered muscle mechanics. Lancet Neurol 6: 725–733, 2007 [DOI] [PubMed] [Google Scholar]
- Dietz V, Trippel M, Berger W. Reflex activity and muscle tone during elbow movements in patients with spastic paresis. Ann Neurol 30: 767–779, 1991 [DOI] [PubMed] [Google Scholar]
- Galiana L, Fung J, Kearney R. Identification of intrinsic and reflex ankle stiffness components in stroke patients. Exp Brain Res 165: 422–434, 2005 [DOI] [PubMed] [Google Scholar]
- Gao F, Grant TH, Roth EJ, Zhang LQ. Changes in passive mechanical properties of the gastrocnemius muscle at the muscle fascicle and joint levels in stroke survivors. Arch Phys Med Rehabil 90: 819–826, 2009 [DOI] [PubMed] [Google Scholar]
- Gao F, Ren Y, Roth EJ, Harvey R, Zhang LQ. Effects of repeated ankle stretching on calf muscle-tendon and ankle biomechanical properties in stroke survivors. Clin Biomech 26: 516–522, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Zhang LQ. Altered contractile properties of the gastrocnemius muscle poststroke. J Appl Physiol 105: 1802–1808, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J, Ghez C. Muscle receptors and spinal reflexes: the stretch reflex. In: Principles of Neural Science, edited by Kandel ER, Schwartz JH, Jessell TS. New York: Elsevier, 1991, p. 564–580 [Google Scholar]
- Gottlieb GL, Agarwal GC, Penn R. Sinusoidal oscillation of the ankle as a means of evaluating the spastic patients. J Neurol Neurosurg Psychiatry 41: 32–39, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz RT, Dewald JP, Schmit BD. Spasticity. In: Physical Medicine and Rehabilitation, edited by Braddom RL. Philadelphia, PA: W. B. Saunders, 2000, p. 592–615 [Google Scholar]
- Kim WH, Park EY. Causal relation between spasticity, strength, gross motor function, and functional outcome in children with cerebral palsy: a path analysis. Dev Med Child Neurol 53: 68–73, 2011 [DOI] [PubMed] [Google Scholar]
- Lance JW. Symposium synopsis. In: Spasticity: Disordered Motor Control, edited by Feldman RG, Young RR, Koella WP. Chicago, IL: Year Book Medical, 1980, p. 485–494 [Google Scholar]
- Lee WA, Boughton A, Rymer WZ. Absence of stretch reflex gain enhancement in voluntarily activated spastic muscle. Exp Neurol 98: 317–335, 1987 [DOI] [PubMed] [Google Scholar]
- Levin MF, Feldman AG. The role of stretch reflex threshold regulation in normal and impaired motor control. Brain Res 657: 23–30, 1994 [DOI] [PubMed] [Google Scholar]
- Levin MF, Hui-Chan C. Are H and stretch reflexes in hemiparesis reproducible and correlated with spasticity? J Neurol 240: 63–71, 1993 [DOI] [PubMed] [Google Scholar]
- Lieber RL, Fridén J. Spasticity causes a fundamental rearrangement of muscle-joint interaction. Muscle Nerve 25: 265–270, 2002 [DOI] [PubMed] [Google Scholar]
- Lorentzen J, Grey MJ, Crone C, Mazevet D, Biering-Sørensen F, Nielsen JB. Distinguishing active from passive components of ankle plantar flexor stiffness in stroke, spinal cord injury and multiple sclerosis. Clin Neurophysiol 121: 1939–1951, 2010 [DOI] [PubMed] [Google Scholar]
- Ludvig D, Visser TS, Giesbrecht H, Kearney RE. Identification of time-varying intrinsic and reflex joint stiffness. IEEE Trans Biomed Eng 58: 1715–1723, 2011 [DOI] [PubMed] [Google Scholar]
- Martin A, Martin L, Morlon B. Theoretical and experimental behaviour of the muscle viscosity coefficient during maximal concentric actions. Eur J Appl Physiol 69: 539–544, 1994 [DOI] [PubMed] [Google Scholar]
- Meinders M, Price R, Lehmann JF, Questad KA. The ankle reflex response in the normal and spastic ankle: effect of ankle position. Arch Phys Med Rehabil 77: 487–492, 1996 [DOI] [PubMed] [Google Scholar]
- Meythaler JM, DeVivo MJ, Hadley M. Prospective study on the use of bolus intrathecal baclofen for spastic hypotonia due to acquired brain injury. Arch Phys Med Rehabil 77: 461–466, 1996 [DOI] [PubMed] [Google Scholar]
- Mirbagheri MM, Barbeau H, Ladouceur M, Kearney RE. Intrinsic and reflex stiffness in normal and spastic, spinal cord injured subjects. Exp Brain Res 141: 446–459, 2001 [DOI] [PubMed] [Google Scholar]
- Niku S, Henderson JM. Viscosity of the flexor muscles of the elbow joint under maximum contraction condition. J Biomech 22: 523–527, 1989 [DOI] [PubMed] [Google Scholar]
- O'Dwyer NJ, Ada L. Reflex hyperexcitability and muscle contracture in relation to spastic hypertonia. Curr Opin Neurol 9: 451–455, 1996 [DOI] [PubMed] [Google Scholar]
- O'Dwyer NJ, Ada L, Neilson PD. Spasticity and muscle contracture following stroke. Brain 119: 1737–1749, 1996 [DOI] [PubMed] [Google Scholar]
- O'Sullivan MC, Miller S, Ramesh V, Conway E, Gilfillan K, McDonough S, Eyre JA. Abnormal development of biceps brachii phasic stretch reflex and persistence of short latency heteronymous reflexes from biceps to triceps brachii in spastic cerebral palsy. Brain 121: 2381–2395, 1998 [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Mazieres L. Spinal mechanisms underlying spasticity. In: Clinical Neurophysiology in Spasticity, edited by Delwaide PJ, Young RR. Amsterdam: Elsevier Science, 1985, p. 63–76 [Google Scholar]
- Powers RK, Campbell DL, Rymer WZ. Stretch reflex dynamics in spastic elbow flexor muscles. Ann Neurol 25: 32–42, 1989 [DOI] [PubMed] [Google Scholar]
- Powers RK, Marder-Meyer J, Rymer WZ. Quantitative relations between hypertonia and stretch reflex threshold in spastic hemiparesis. Ann Neurol 23: 115–124, 1988 [DOI] [PubMed] [Google Scholar]
- Rack PM, Ross HF, Thilmann AF. The ankle stretch reflexes in normal and spastic subjects. Brain 107: 637–654, 1984 [DOI] [PubMed] [Google Scholar]
- Rymer WZ, Katz RT. Mechanism of spastic hypertonia. Phys Med Rehabil 8: 441–454, 1994 [PubMed] [Google Scholar]
- Selles RW, Li X, Lin F, Chung SG, Roth EJ, Zhang LQ. Feedback-controlled and programmed stretching of the ankle plantarflexors and dorsiflexors in stroke: effects of a 4-week intervention program. Arch Phys Med Rehabil 86: 2330–2336, 2005 [DOI] [PubMed] [Google Scholar]
- Sinkjær T, Andersen JB, Nielsen JF. Impaired stretch reflex and joint torque modulation during spastic gait in multiple sclerosis patients. J Neurol 243: 566–574, 1996 [DOI] [PubMed] [Google Scholar]
- Sinkjær T, Magnussen I. Passive, intrinsic and reflex-mediated stiffness in the ankle extensors of hemiparetic patients. Brain 117: 355–363, 1994 [DOI] [PubMed] [Google Scholar]
- Sinkjær T, Toft E, Larsen K, Andreassen S, Hansen HJ. Non-reflex and reflex mediated ankle joint stiffness in multiple sclerosis patients with spasticity. Muscle Nerve 16: 69–76, 1993 [DOI] [PubMed] [Google Scholar]
- Söderström T, Stoica P. System Identification. London: Prentice-Hall International, 1989 [Google Scholar]
- Stein RB, Kearney RE. Nonlinear behavior of muscle reflexes at the human ankle joint. J Neurophysiol 73: 65–72, 1995 [DOI] [PubMed] [Google Scholar]
- Sunnerhagen KS, Olver J, Francisco GE. Assessing and treating functional impairment in poststroke spasticity. Neurology 80: S35–S44, 2013 [DOI] [PubMed] [Google Scholar]
- Thilmann AF, Fellows SJ, Garms E. The mechanism of spastic muscle hypertonus: variation in reflex gain over the time course of spasticity. Brain 114: 233–244, 1991 [PubMed] [Google Scholar]
- Waldman G, Yang CY, Ren Y, Liu L, Guo X, Harvey RL, Roth EJ, Zhang LQ. Effects of robot-assisted passive stretching and active movement training of ankle and mobility impairments in stroke. NeuroRehabilitation 32: 625–634, 2013 [DOI] [PubMed] [Google Scholar]
- Williams PE, Goldspink G. Changes in the connective tissue component of muscle during periods of decreased or increased activity. J Anat 133: 133, 1981 [Google Scholar]
- Williams PE, Goldspink G. Connective tissue changes in immobilised muscle. J Anat 138: 343–350, 1984 [PMC free article] [PubMed] [Google Scholar]
- Wu YN, Hwang M, Ren Y, Gaebler-Spira DJ, Zhang LQ. Combined passive stretching and active movement rehabilitation of lower-limb impairments in children with cerebral palsy using a portable robot. Neurorehabil Neural Repair 25: 378–385, 2011 [DOI] [PubMed] [Google Scholar]
- Young RR. Hypertonia: diagnosis and management. In: Principles of Neurologic Rehabilitation, edited by Lazar RB. New York: McGraw-Hill, 1998, p. 329–336 [Google Scholar]
- Young RR. Spasticity: a review. Neurology 44: S12–S20, 1994 [PubMed] [Google Scholar]
- Zhang L, Rymer WZ. Modeling of muscle mechanical and reflex properties using nonlinear delay differential equations. In: Proc 15th Annu Int Conf IEEE EMBS San Diego, CA: 1993, p. 1165–1166 [Google Scholar]
- Zhang LQ, Chung SG, Bai Z, van Rey EM, Rogers MW, Johnson ME, Roth EJ. Intelligent stretching for ankle joints with contracture/spasticity. IEEE Trans Neural Syst Rehabil Eng 10: 149–157, 2002 [DOI] [PubMed] [Google Scholar]
- Zhang LQ, Huang H, Sliwa JA, Rymer WZ. System identification of tendon reflex dynamics. IEEE Trans Rehabil Eng 7: 193–203, 1999 [DOI] [PubMed] [Google Scholar]
- Zhang LQ, Rymer WZ. Reflex and intrinsic changes induced by fatigue of human elbow extensor muscles. J Neurophysiol 86: 1086–1094, 2001 [DOI] [PubMed] [Google Scholar]
- Zhang LQ, Rymer WZ. Simultaneous and nonlinear identification of mechanical and reflex properties of human elbow joint muscles. IEEE Trans Biomed Eng 44: 1192–1209, 1997 [DOI] [PubMed] [Google Scholar]
- Zhang LQ, Wang G, Nishida T, Xu D, Sliwa JA, Rymer WZ. Hyperactive tendon reflexes in spastic multiple sclerosis: measures and mechanisms of action. Arch Phys Med Rehabil 81: 901–909, 2000 [DOI] [PubMed] [Google Scholar]
- Zhao H, Ren Y, Wu YN, Liu SQ, Zhang LQ. Ultrasonic evaluations of Achilles tendon mechanical properties poststroke. J Appl Physiol 106: 843–849, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuurbier CJ, Huijing PA. Influence of muscle geometry on shortening speed of fibre, aponeurosis and muscle. J Biomech 25: 1017–1026, 1992 [DOI] [PubMed] [Google Scholar]