Abstract
The impact of regional hippocampal interactions and GABAergic transmission on ictogenesis remain unclear. Cortico-hippocampal slices from pilocarpine-treated epileptic rats were compared with controls to investigate associations between seizurelike events (SLE), GABAergic transmission, and neuronal synchrony within and between cortico-hippocampal regions. Multielectrode array recordings revealed more prevalent hippocampal SLE in epileptic tissue when excitatory transmission was enhanced and GABAergic transmission was intact [removal of Mg2+ (0Mg)] than when GABAergic transmission was blocked [removal of Mg2+ + bicuculline methiodide (0Mg+BMI)]. When activity within individual regions was analyzed, spectral and temporal slow oscillation/SLE correlations and cross-correlations were highest within the hilus of epileptic tissue during SLE but were similar in 0Mg and 0Mg+BMI. GABAergic facilitation of spectral “slow” oscillation and ripple correlations was most prominent within CA3 of epileptic tissue during SLE. When activity between regions was analyzed, slow oscillation and ripple coherence was highest between the hilus and dentate gyrus as well as between the hilus and CA3 of epileptic tissue during SLE and was significantly higher in 0Mg than 0Mg+BMI. High 0Mg-induced SLE cross-correlations between the hilus and dentate gyrus as well as between the hilus and CA3 were reduced or abolished in 0Mg+BMI. SLE cross-correlation lag measurements provided evidence for a monosynaptic connection from the hilus to the dentate gyrus during SLE. Findings implicate the hilus as an oscillation generator, whose impact on other cortico-hippocampal regions is mediated by GABAergic transmission. Data also suggest that GABAA receptor-mediated transmission facilitates back-propagation from CA3/hilus to the dentate gyrus and that this back-propagation augments SLE in epileptic hippocampus.
Keywords: CA1, entorhinal cortex, epilepsy, hilus, hippocampus, multielectrode array, electrophysiology
temporal lobe epilepsy (TLE) is a common neurological disorder, but the mechanisms underlying seizure generation remain uncertain. The hippocampal formation clearly plays a prominent role in limbic seizure generation (ictogenesis) (Engel et al. 1993) and is often described as a trisynaptic structure with activity from entorhinal cortex propagating first to dentate granule cells, then to CA3 pyramidal cells, and finally to CA1 pyramidal cells (Amaral and Witter 1989). In normal brain, CA3 generates oscillations (Csicsvari et al. 2000; Ylinen et al. 1995) and is the most excitable and seizure-prone hippocampal region (Miles and Wong 1986), possibly because of abundant recurrent excitatory synapses between CA3 pyramidal cells. Conversely, the dentate gyrus is the least excitable region and is equipped with strong GABAergic inhibition onto and sparse recurrent excitatory synapses between granule cells. In normal brain, the dentate gyrus limits abnormal activity transmission into the hippocampus. Attenuation of this “dentate gate” may promote limbic epileptogenesis and subsequent ictogenesis (Behr et al. 1996, 1998; Collins et al. 1983; Pathak et al. 2007). Increased recurrent granule cell synapses associated with granule cell mossy fiber axon sprouting in epileptic brain are postulated to promote seizure initiation and/or propagation through hippocampal pathways (Molnar and Nadler 1999; Wuarin and Dudek 1996) and thereby attenuate the dentate gate. However, activity does not always flow unidirectionally through classical hippocampal trisynaptic pathways. Back-projections from CA3 pyramidal cells to dentate gyrus via the hilus exist in normal hippocampus and are more robust in epileptic brain (Li et al. 1994; Scharfman 1993, 1994a, 1994b; Scharfman et al. 2000, 2001; Zhang et al. 2012). Given the role of CA3 in governing hippocampal excitability, increased excitatory feedback from CA3 to the hilus and dentate gyrus may dramatically affect granule cell excitability and the ability of the dentate gyrus to act as a “gate,” and thereby promote hippocampal ictogenesis.
The role of GABAA receptor-mediated transmission in cortico-hippocampal interactions in the epileptic brain is also unresolved. TLE is associated with partial loss and partial deafferentation of GABAergic neurons (Obenaus et al. 1993; Sloviter 1987, 1994), suggesting that decreased GABAergic inhibition contributes to ictogenesis. Supporting this idea, neuronal firing increases when GABAA receptor-mediated inhibition is decreased (Sloviter 1987; Williamson et al. 1999) and many clinically efficacious antiseizure drugs increase GABAergic neurotransmission. However, GABAergic transmission also depolarizes neurons during epochs of elevated activity (Khirug et al. 2008), plays a key role in neuronal synchronization (Cobb et al. 1995), and facilitates seizurelike events (SLE) in in vitro seizure models (Barbarosie et al. 2002; Khazipov and Holmes 2003; Köhling et al. 2000). Thus, while GABAA receptor-mediated transmission can restrain neuronal activity, it also may promote pathological synchronous oscillatory firing that defines seizures (Staley et al. 1998).
Given these findings, we hypothesized that when excitatory transmission is increased in the epileptic brain, GABAergic transmission facilitates SLE and this SLE augmentation is associated with increased neuronal oscillation synchrony and back-propagation from CA3 to the hilus and dentate gyrus. We further postulated that this back-propagation is driven by CA3 and facilitated by GABAA receptor-mediated transmission. To test these hypotheses, we used microelectrode arrays (MEA) to simultaneously record field potentials in five distinct cortico-hippocampal regions under conditions that manipulated activity in excitatory and inhibitory circuits in slices prepared from control and pilocarpine-treated epileptic rats. We focused on relationships between electrographic SLE generation and correlated activity in space, time, and frequency domains.
MATERIALS AND METHODS
Treatment of Animals
All treatment of animals was conducted according to National Institutes of Health, Department of Defense, and institutional guidelines and was approved by the Uniformed Services University Institutional Animal Care and Use Committee.
Pilocarpine-Induced Epilepsy
The pilocarpine model was chosen because it recapitulates the chronic recurrent spontaneous seizures and hippocampal pathology associated with human TLE and CA3 remains intact (Mello et al. 1993). Adult male Sprague-Dawley rats (175–200 g; Taconic, Germantown, NY) were injected with pilocarpine hydrochloride (350–380 mg/kg in sterile 0.1% saline ip; Sigma, St. Louis, MO) 30 min after pretreatment with methylscopolamine (1 mg/kg in sterile 0.1% saline ip; Sigma) as described previously (Bausch and Chavkin 1997; Turski et al. 1983). Control rats received methylscopolamine but were injected with saline instead of pilocarpine. After 1 h of continuous status epilepticus (SE), diazepam (4 mg/kg ip) was administered every hour until behavioral seizures ceased. All animals were given rat chow soaked in Gatorade and glucose for 2 days after injection. To document that pilocarpine-treated rats were epileptic (i.e., exhibited spontaneous recurrent behavioral seizures) prior to electrophysiological recordings, rats were video monitored for 3 h/day beginning at 3–4 wk after injections. Rats exhibiting at least three spontaneous seizures were defined as epileptic. Once rats were deemed epileptic, time-intensive video monitoring was discontinued. None of the control rats and 95% of the rats that exhibited ≥1 h of sustained SE immediately after pilocarpine injection were confirmed epileptic by the seventh week after injection. Pilocarpine-treated rats that were not deemed epileptic by the end of the seventh week after injection were not used in the study. Rats were euthanized for electrophysiological recordings ∼4–6 mo (5.4 ± 0.4 mo) after saline or pilocarpine injection. Rats were age matched for different treatment groups and recording conditions (t-test P > 0.05).
Slice Preparation
Rats were anesthetized with isoflurane and decapitated. Brains were removed quickly and immersed in cold (0–2°C) dissecting buffer composed of (in mM) 128 NaCl, 3.5 KCl, 2.0 CaCl2, 3 MgSO4, 25 NaHCO3, 1.3 NaH2PO4, and 10 glucose saturated with 5% CO2-95% O2. Transverse 400-μm cortico-hippocampal slices from the middle portion of hippocampus were cut with a vibratome (TPI, St. Louis, MO). Slices were incubated for 30 min at 37°C in physiological artificial cerebrospinal fluid (aCSF; dissecting buffer with 1.3 MgSO4) and then stored at room temperature in aCSF for at least 30 min before recording.
Extracellular MEA Recordings
We simultaneously recorded extracellular field potentials from the entorhinal cortex, dentate gyrus, hilus, CA3, and CA1 in cortico-hippocampal slices prepared from control and pilocarpine-treated epileptic rats with custom three-dimensional MEA (60 electrodes, 6 × 10 layout, 500-μm spacing, 100-μm height, 100-kΩ resistance; Ayanda Biosystems, Lausanne, Switzerland). Cortico-hippocampal slices were placed onto the MEA, which was inserted into a MEA-60 amplifier attached to a Nikon Diaphot inverted microscope, and digitally photographed with a Nikon Coolpix 5000 camera (Nikon USA, Melville, NY) to document electrode locations relative to cortico-hippocampal subregions (Fig. 1A). Slices were superfused (2 ml/min) at 30°C with physiological aCSF for 10 min followed by an additional 110 min with 1) aCSF without MgCl2 (designated 0Mg) to enhance NMDA receptor (NMDAR)-mediated excitatory glutamatergic transmission onto both excitatory and inhibitory neurons or 2) 0 mM Mg2+ aCSF + 10 μM bicuculline methiodide (BMI; Sigma) (designated 0Mg+BMI) to elevate NMDAR-mediated excitatory glutamatergic transmission and block GABAA receptor-mediated inhibition. All recording buffers were equilibrated with 95% O2-5% CO2. This approach was chosen because removal of Mg2+ from the recording solution can elicit SLE in the CA1 and/or CA3 regions in hippocampal slices from normal rats (Anderson et al. 1986; Rafiq et al. 1993) and these SLE are facilitated by GABAA receptor-mediated oscillations (Köhling et al. 2000). Recording solutions were optimized to maximize differences in SLE between epileptic and control tissue. Raw unfiltered field potentials were acquired and digitized continuously from all 60 electrodes for the full 2 h with a Multi Channel filter amplifier (MEA-60, Multi Channel Systems, Reutlingen, Germany) with 10 Hz-3 kHz frequency limits, ±0.4 V voltage limits, and 1,200× gain. Sampling rate was 5 kHz, which was well above the 2-kHz Nyquist sampling frequency required to adequately detect oscillations up to 1 kHz. All data were analyzed off-line with MATLAB 7.3 (The MathWorks, Natick, MA). Electrode localization within cortico-hippocampal subregions was confirmed by cresyl violet stain of slices after recordings. No electrical activity was detected in physiological aCSF, so only data recorded in 0Mg or 0Mg+BMI are presented.
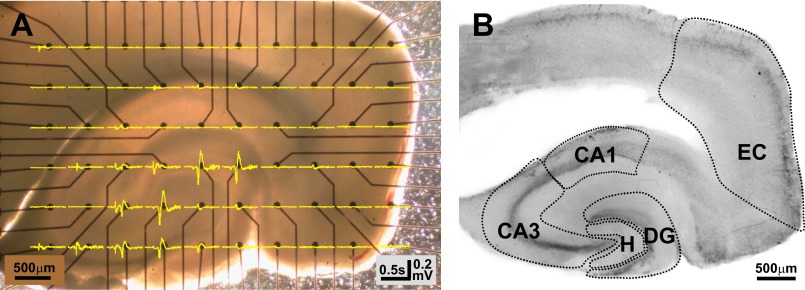
Fig. 1.
Electrical signals were assigned to distinct cortico-hippocampal subregions. Microelectrode array (MEA) electrodes were mapped and associated field potential recordings grouped into 5 distinct cortico-hippocampal subregions as described in materials and methods. A: representative photograph of a cortico-hippocampal slice from a control rat on a 6 × 10 MEA [electrodes appear as black dots with leads (black lines)]. Field potentials (yellow lines) recorded after superfusion with 0Mg at all 60 microelectrodes were overlaid onto the slice/MEA. B: the same 400-μm-thick slice depicted in A after staining with cresyl violet. Dotted lines delineate regions used to map electrodes and group field potential recordings. Since CA2 is ill-defined in rat, the region marked CA3 includes CA3 and CA2, as described by Amaral and Lavenex (2007). DG, dentate gyrus; EC, entorhinal cortex; H, hilus.
Cresyl Violet Stain and Cortico-Hippocampal Subregion Definitions
To more precisely delineate cortico-hippocampal regions, slices used for recordings were immersion fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4) for 24 h immediately after recordings and mounted onto subbed glass slides. Slide-mounted sections were put through an ascending ethanol series, stained with cresyl violet, dehydrated, cleared, and coverslipped as described previously (Bausch and Chavkin 1997). Images were collected with a dissecting microscope (Leica MZ12, Wetzlar, Germany) and a Nikon Coolpix 5000 camera.
Electrodes were assigned to five distinct cortico-hippocampal subregions: entorhinal cortex, dentate gyrus, hilus, CA3, and CA1 (Fig. 1B) based upon Paxinos and Watson (1998) and Amaral and Lavenex (2007). The dentate gyrus was defined as the dentate granule cell and molecular layers. The hilus was defined as the region between the two blades of the granule cell layer, excluding the CA3 pyramidal cell layer, and was delimited at the open end by a straight line drawn between the two blades of the granule cell layer. The CA3 region was defined as the tightly packed CA3 pyramidal cell layer, including CA3a,b,c as well as strata oriens, lucida, and the proximal half of stratum radiatum. Since CA2 is ill-defined in rat, CA3 included CA2 as described in Amaral and Lavenex (2007). The CA1 region was defined as the tightly packed CA1 pyramidal cell layer as well as stratum oriens and the proximal half of stratum radiatum. The entorhinal cortex consisted of medial and lateral entorhinal cortices and was defined as the multilaminate cortex between the pre-/parasubiculum and the rhinal sulcus. Electrodes that could not be assigned unequivocally to a single region were excluded from analyses. Electrode placement was not more precisely defined by regional sublayers (i.e., strata oriens, pyramidale, radiatum, moleculare, etc.) because the number of electrodes in each sublayer per recording was variable given the MEA layout/electrode spacing as well as the difficulty in precisely and reproducibly positioning the slice on the MEA. Because of variable electrode placement in regional sublayers and the influence of electrode placement relative to current sinks and sources on event/oscillation phase (Niedermeyer and Lopez da Silva 2005), phase components were not incorporated into subsequent analyses.
Detection and Analyses of Interictal Spikes, Epileptiform Events, and Electrographic SLE
A custom program written in MATLAB 7.3 was used to detect interictal spikes, epileptiform events, and SLE and sort their locations according to defined cortico-hippocampal regions. Briefly, the threshold for event detection was set at >3 standard deviations (±3σ) of the mean baseline noise. Putative events > 1 kHz were excluded. If the interval between the end of one event and the start of the next was <0.5 s, then the two events were considered as one event. Interictal spikes were defined as events ≤ 80 ms, epileptiform events > 80 ms but ≤ 3 s, and SLE >3 s in duration (Fig. 2A), as described previously (Bausch and McNamara 2000). Further constraints for SLE included an abrupt onset and termination, a waveform that evolved over time, a 3-s postictal silent period (Bausch and McNamara 2000), and a mean 2-Hz event cycle (White et al. 2006). All recordings were scanned manually by investigators blinded to experimental conditions/preconceived criteria/definitions to document the reliability of our detection algorithms. Manual detection of all 1,175 SLE revealed a 3% false positive rate and a 5% false negative rate, which is slightly better than the 4–5% false positive rate of existing algorithms used to detect seizures in vivo (White et al. 2006). Manual detection of all interictal spikes and epileptiform events was not feasible because of their very high number. However, manual detection of 746 interictal spikes and 792 epileptiform events in 90-s epochs from all 5 regions in 3 experiments/experimental group revealed 5% false positive and 3% false negative rates for interictal spikes and 2% false positive and 8% false negative rates for epileptiform events. For all events, our detection algorithm exhibited a 91% accuracy rate.
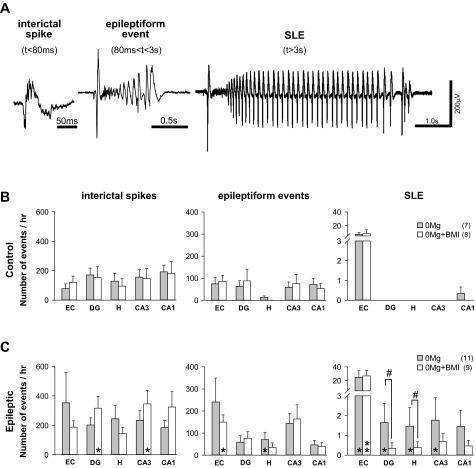
Fig. 2.
Seizurelike event (SLE) incidence was greater in slices from epileptic compared with control rats and was highest when excitatory transmission was enhanced and GABAergic transmission was intact. Electrographic events induced by 0Mg or 0Mg+BMI were detected and defined as described in materials and methods. A: representative interictal spike, epileptiform event, and SLE induced by 0Mg in a slice from an epileptic rat. B: in slices from control rats, interictal spikes (left) and epileptiform events (center) were detected in all cortico-hippocampal regions while SLE (right) occurred almost exclusively in the entorhinal cortex [entorhinal cortex vs. all hippocampal regions, P < 0.05, ANOVA on ranks with least significant difference (LSD) post hoc comparison], with rare SLE recorded in CA1. C: in slices from epileptic rats, interictal spikes (left), epileptiform events (center), and SLE (right) were detected in all cortico-hippocampal regions. SLE were more prevalent in 0Mg than 0Mg+BMI in hippocampus but not entorhinal cortex. Bars indicate means ± SE; no. of slices/rats is indicated in parentheses. Different from control, Mann-Whitney test: *P < 0.05, **P < 0.01. Different from 0Mg, ANOVA on ranks with LSD post hoc comparison: #P < 0.05. Vertical scale bar in A applies to all traces.
Detection and Analyses of Oscillations
Oscillations were defined as relatively “slow” oscillations [10–100 Hz, which included beta and fast gamma oscillations (β, 10–30 and γ, 30–100 Hz, respectively)], ripples (101–200 Hz), and fast ripples (201 Hz–1 kHz) (see Fig. 4A) as described previously (Bragin et al. 2002; Khosravani et al. 2005; Staba et al. 2002). Delta, theta, and alpha oscillations were not documented because the frequency of these oscillations fell below the lower 10-Hz cutoff frequency of our amplifier. A Butterworth filter was used originally to detect oscillations, but these filtered data were not used in analyses because of the propensity of such digital filters to introduce spectral distortions/delays. Instead, raw, unfiltered electrical signals from each electrode were first subjected to frequency-domain filtering [fast Fourier transform (FFT)–filter–inverse FFT (iFFT)] as described by Smith (1997a) and Grandmaison et al. (2004). Briefly, this approach measured oscillation frequency in sliding 0.5-s windows of recording data to generate 10 Hz–1 kHz frequency spectra. Frequency spectra were then binned into “slow” oscillations (10–100 Hz), ripples (101–200 Hz), and fast ripples (201 Hz–1 kHz) with unitary square filter functions. Finally, individual oscillations were resynthesized point by point using the sliding center point of the sliding 0.5-s windows, which minimized the possibility of “ringing” (Smith 1997c). Any aliases associated with our FFT calculations would not occur in the 10 Hz–1 kHz range because the Nyquist criterion was met (Smith 1997b). Detection threshold was set at greater than ±3σ of mean baseline noise. Putative events of ≤10-ms duration were excluded, which minimized contamination of our slow oscillations with stand-alone population spikes (∼7 ms in duration). If the interval between the end of one event and the start of the next was <0.5 s, then the two events were considered as one. A custom program written in MATLAB 7.3 was used to measure oscillation duration. Manual detection of all oscillations was not possible because of their high incidence. However, manual detection of 137 slow oscillations, 134 ripples, and 129 fast ripples in 30-s windows from all 5 regions in 3 experiments/experimental group yielded 4% false positive and 3% false negative rates for “slow” oscillations, 3% false positive and 0% false negative rates for ripples, and 8% false positive and 0% false negative rates for fast ripples.
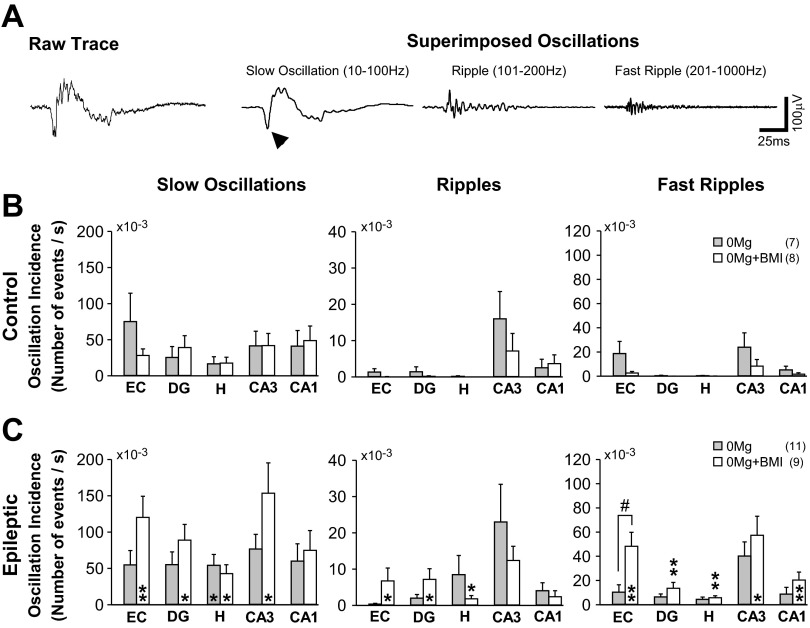
Fig. 4.
Incidence of hippocampal oscillations in individual regions was not significantly affected by manipulations of excitatory and inhibitory activity. Slow oscillations, ripples, and fast ripples were detected and defined as described in materials and methods. A, left: representative raw recording of a 0Mg-induced interictal spike in CA3 of a slice from an epileptic rat. Right: the same trace separated into the superimposed slow oscillation [with overlying suprathreshold population spike (arrowhead; spike and wave complex), which forms the “backbone” of the interictal spike], ripple, and fast ripple. Similar superimposition of ripples and fast ripples on the slow oscillations/spike and wave complexes were evident during SLE (not shown). Potential contamination of slow oscillation measures with stand-alone population spikes (∼7 ms in duration) was minimized by exclusion of putative events ≤10 ms in duration. B and C: the incidence of slow oscillations (left), ripples (center), and fast ripples (right) in individual hippocampal regions for the entire duration of the recording was not significantly different in 0Mg and 0Mg+BMI but was significantly higher in slices from epileptic (C) compared with control (B) rats in 0Mg+BMI. Bars indicate means ± SE; no. of slices/rats is indicated in parentheses. *Different from control, t-test, P < 0.05. **Different from control, t-test, P < 0.01. #Different from other recording conditions, ANOVA with Sidak post hoc comparison, P < 0.05. Scale bars in A, right, apply to all traces.
Spectral Oscillation Analyses
Running 0.5-s windows of data from each electrode were subjected to FFT to enable quantitative spectral analyses of the entire recording duration (Gabor 1946). The 0.5-s temporal window is well above the 50-ms window required to detect 10-Hz oscillations [F = 1/(2 × T), where F is frequency, T is temporal window, and 10 Hz is the lower cutoff frequency of the MEA hardware]. For each 0.5-s window, spectra < 10 Hz (lower cutoff frequency of the amplifier) and > 1 kHz were discarded, resultant frequency data were smoothed with a running 5-point average (Sasaki 2001), and every other spectral point for each electrode was used in subsequent analyses to conserve memory and ensure simultaneous data processing from all 60 channels with MATLAB 7.3. Frequency spectra for each individual electrode within a defined region were calculated for each individual 0.5-s temporal window for the entire duration of each individual experiment [S(f)k] with the following equation and stored for further analyses:
| 1 |
where S(f)k is a complex variable, f = 10 Hz… 1 kHz, F(t1, t2) is the field potential data, t1 = 10, t2 = 120 min, Δt = 0.5 s (the temporal window), k is a sequential integer from 1 to M, and M = (t2 − t1)/Δt = 13,200 (the total number of 0.5-s temporal windows). Since all experimental time frames and window sizes were identical across experimental groupings, Bartlett's method of normalization (Bartlett 1948) was not applied. While the population spike is not a slow oscillation per se and stand-alone population spikes were excluded from analysis, population spikes superimposed on suprathreshold “slow” oscillations may contaminate our spectral analyses for slow oscillations at ∼71 Hz (marked with arrowheads in Figs. 5 and 6).
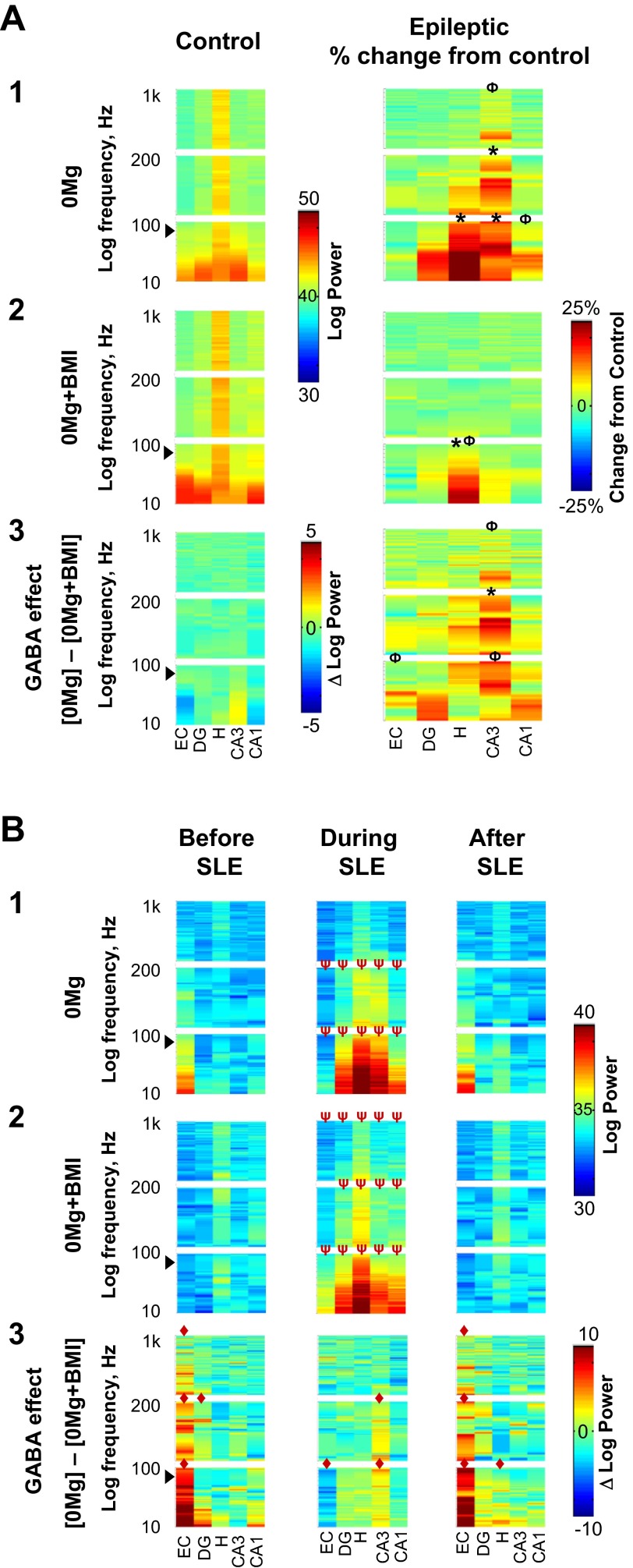
Fig. 5.
When excitatory transmission was enhanced, hilar spectral slow oscillation correlation was elevated and GABAergic transmission facilitated CA3 spectral slow oscillation and ripple correlation. Correlation of oscillation frequencies in individual 0.5-s windows arising from different electrodes within individual regions were calculated for slices from control (A, left) and epileptic (A, right and B) rats; correlations were then compiled and compilations plotted as power spectra as described in materials and methods. Although stand-alone population spikes (∼7 ms in duration) were excluded from analysis, population spikes superimposed on suprathreshold slow oscillations may contaminate slow oscillation results at ∼71 Hz (arrowheads). The y-axis is stretched to yield equal height panels for each frequency range. A: correlations of each 0.5-s window recorded from different electrodes within an individual region were compiled for all 0.5-s windows in the entire duration of recording. A1: in 0Mg, spectral slow oscillation correlations within the hilus, CA3, and CA1 as well as spectral ripple and fast ripple correlations in CA3 were greater in slices from epileptic (n = 11) compared with control (n = 7) rats. A2: in 0Mg+BMI, only hilar spectral slow oscillation correlation was higher in epileptic (n = 9) compared with control (n = 8) rats. A3: subtraction of power spectra in A1 and A2 revealed that when excitatory transmission was enhanced, GABAergic transmission (right) facilitated spectral slow oscillation and ripple correlations within CA3 to a greater degree in slices from epileptic rats, which was significantly greater in epileptic compared with control rats. B: correlations were calculated for all 0.5-s windows in the 3 s immediately before SLE (left), the first 3 s of SLE (center), and the first 3 s after SLE termination (right) in slices from epileptic rats. The start of SLE was defined as described in materials and methods. B1: in 0Mg, spectral slow oscillation and ripple correlations were decreased in the entorhinal cortex and increased in the dentate gyrus, hilus, CA3, and CA1 during SLE compared with before and after SLE. B2: in 0Mg+BMI, spectral slow oscillation, ripple, and fast ripple correlations were increased in all hippocampal regions during compared with before and after SLE. B3: subtraction of power spectra in B1 and B2 revealed that when excitatory transmission was enhanced GABAergic transmission facilitated spectral slow oscillation, ripple, and fast ripple correlations within the entorhinal cortex immediately before and after SLE (left and right) and decreased entorhinal spectral slow oscillation correlation and facilitated spectral slow oscillation and ripple correlations within CA3 during SLE (center). *Overall range different from control, Φat least 1 peak wider than 5 Hz different from control, ◆overall range difference between 0Mg and 0Mg+BMI, ψoverall range different from before and after SLE, P < 0.05, 2-way ANOVA with Sidak post hoc comparison. Scale bar in A1 and 2, left, applies to both left panels in A1 and 2; scale bar in A2, right, applies to all right panels in A1–3; scale bar in A3, left, applies only to that panel. Scale bar in B1 and 2, right, applies to all panels in B1 and 2; scale bar in B3, right, applies to all panels in B3.
Fig. 6.
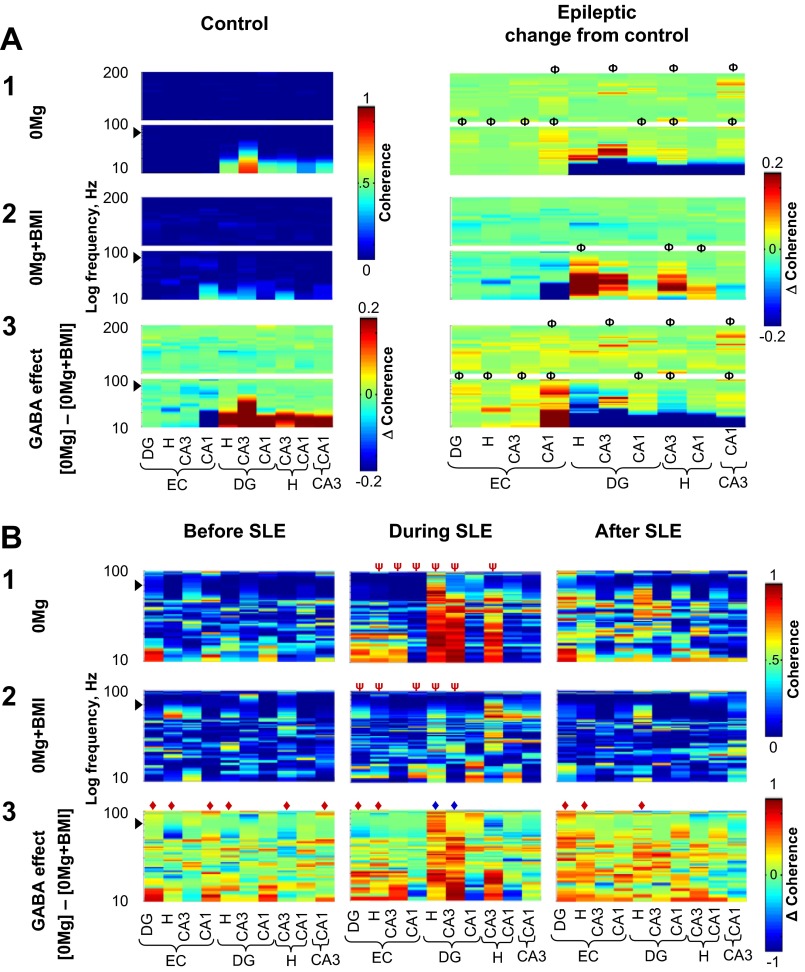
When excitatory transmission was enhanced, slow oscillation coherence was highest between the hilus, dentate gyrus, and CA3 and GABAergic transmission facilitated slow oscillation coherence between CA3, hilus, and dentate gyrus during SLE. Oscillation coherence in individual 0.5-s windows recorded from electrodes in different regions was calculated for slices from control (A, left) and epileptic (A, right, and B) rats; coherence measures from experimental groups were then averaged and plotted as described in materials and methods. Although stand-alone population spikes (∼7 ms in duration) were excluded from analysis, population spikes superimposed on suprathreshold slow oscillations may contaminate slow oscillation results at ∼71 Hz (arrowheads). The y-axis is stretched to yield equal height panels for each frequency range. Coherence measures for fast ripples (201 Hz–1 kHz) in A and B and ripples (101–200 Hz) in B are not shown because there were no dramatic changes in these oscillation ranges across experimental groups. A: coherence measures of each 0.5-s window recorded from different electrodes across different regions were compiled for all 0.5-s windows in the entire duration of recording. In both 0Mg (A1) and 0Mg+BMI (A2), slow oscillation coherence was highest between the dentate gyrus, hilus, and CA3 and this coherence was shifted to higher slow oscillation frequency bands in epileptic (0Mg, n = 11; 0Mg+BMI, n = 9) compared with control (0Mg, n = 7; 0Mg+BMI, n = 8) tissue. In 0Mg (A1), but not 0Mg+BMI (A2), bands of ripple coherence between CA3 and other hippocampal regions were increased in slices from epileptic compared with control rats. A3: subtraction of coherences in A1 and A2 revealed that in slices from control rats (left), when excitatory transmission was enhanced GABAergic transmission facilitated slow oscillation coherence between hippocampal regions and reduced slow oscillation coherence between the entorhinal cortex and CA1. Right: when excitatory transmission was enhanced, GABAergic transmission facilitated slow oscillation coherence between CA3 and the dentate gyrus and reduced slow oscillation coherence between the hilus, dentate gyrus, and CA3 to a greater degree in slices from epileptic compared with control rats. GABAergic transmission also facilitated ripple coherence between CA3 and other hippocampal subregions to a greater degree in epileptic rats. B: coherences were calculated for all 0.5-s windows in the 3 s immediately before SLE (left), the first 3 s of SLE (center), and the first 3 s after SLE termination (right) in slices from epileptic rats. The start of SLE was defined as described in materials and methods. In 0Mg (B1), but not 0Mg+BMI (B2), slow oscillation coherence between the dentate gyrus, hilus, and CA3 was increased during SLE compared with before and after SLE. B3: subtraction of coherence in B1 and B2 revealed that when excitatory transmission was enhanced GABAergic transmission facilitated oscillation coherence between the dentate gyrus, hilus and CA3 during SLE (center, blue ◆) compared with before and after SLE. ΦAt least 1 peak wider than 5 Hz different from control, ◆overall range difference between 0Mg and 0Mg+BMI, ψoverall range different than before and after SLE, P < 0.05, 2-way ANOVA with Sidak post hoc comparison. Scale bar in A1 and 2, left, applies to both left panels in A1 and 2; scale bar in A2, right, applies to all right panels in A1–3; scale bar in A3, left, applies only to that panel. Scale bar in B1 and 2, right, applies to all panels in B1 and 2; scale bar in B3, right, applies to all panels in B3.
Correlation/Coherence Analyses
Results from Eq. 1 were used to calculate correlations and coherence as described previously (Bendat and Piersol 1971; Niedermeyer and Lopez da Silva 2005).
Correlation spectra.
Correlation spectra, which correlated oscillation frequencies in individual 0.5-s windows recorded from different electrodes within an individual region, were calculated as
| 2 |
where R is the correlation, I is the cortico-hippocampal subregion, S is S(f) from Eq. 1, S′ denotes a complex conjugation, k is a sequential integer from 1 to M, and M =(t2 − t1)/Δt = 13,200 (the total number of temporal windows). Correlations (R) from individual experiments were converted to power spectra to avoid phase interactions across experiments. Power spectra from individual experiments were then averaged for plotting purposes as two-dimensional (2D) color-coded images.
Coherence function.
We first correlated oscillation frequencies in individual 0.5-s windows recorded from different electrodes across different regions, using the following equation in a 5 × 5 matrix:
| 3 |
where C is the cross spectrum, S is S(f) from Eq. 1, S′ denotes a complex conjugated spectrum, I and J represent different cortico-hippocampal subregions (I ≠ J), k is a sequential integer from 1 to M, and M = (t2 − t1)/Δt = 13,200 (the total number of temporal windows). We then assessed coherences between regions at each frequency as follows:
| 4 |
where C is the correlation spectrum from Eq. 3, R is the correlation spectrum from Eq. 2, I and J represent different cortico-hippocampal regions (I ≠ J), and f is a frequency. Coherence functions (CF) from individual experiments were then averaged and plotted as 2D color-coded images.
Temporal SLE cross-correlations.
Temporal SLE cross-correlations were calculated with the MATLAB 7.3 cross-correlation function xcorr(x), where x was a 5 × N matrix composed of field potential recordings from the 5 cortico-hippocampal regions and N was the number of points associated with a single event. Correlation coefficients for each SLE were averaged to yield a single correlation coefficient vs. lag for each experiment/recording, and then correlation coefficients were compiled and represented as color-coded 2D image plots, similar to power spectra. Because cross-correlations can arise from altered correlations and/or autocorrelations, we also applied the autoregressive moving average (ARMA) model prior to cross-correlations across hippocampal subregions to more precisely define time lags (Granger and Newbold 1977). In brief, raw SLE waveforms were processed first through the MATLAB ARMAX function using 5 points of history for both the autoregressive and moving average. The prediction error between the model and raw data was calculated by using the MATLAB PE function to condition/prewhiten the data before cross-correlation.
Whole cell current-clamp recordings.
Slices were placed into a submerged recording chamber mounted to a BX50WI Olympus microscope equipped with IR-DIC optics and a DAGE-MTI CCD camera (Michigan City, IN) and perfused (2 ml/min) at 30°C with physiological aCSF. Recording pipettes (∼6 MΩ) were filled with (in mM) 125 K-gluconate, 13 KCl, 10 EGTA, 2 Mg-ATP, and 10 HEPES, pH 7.2 with KOH and 280 mosM. Granule cells and CA3 pyramidal cells were visualized by IR-DIC, and their identity was confirmed by filling individual cells with Neurobiotin (0.4%, Vector, Burlingame, CA) and subsequent histological visualization, as described previously (Bausch and McNamara 2000; Bausch et al. 2006). Electrophysiological signals were recorded with a MultiClamp 700A amplifier, CV-7A headstage, Digidata 1322A interface, and MultiClamp 700A and pCLAMP software (Axon Instruments, Foster City, CA). Criteria for acceptable recordings were resting membrane potential immediately after establishing whole cell configuration more negative than −55 mV; current needed to maintain the cell at −70 mV < 100 pA; and series resistance (<20 MΩ) varying < 20%. The resting membrane potential was determined with MultiClamp software in I=0 mode immediately after establishing whole cell configuration. Cells were then maintained at −70 mV with constant current injection in current-clamp mode. After sequential 30-min superfusion with 0Mg and 0Mg+BMI, action potentials were generated with a series of 1-s, 100-pA steps (100 pA–2 nA or sufficient to depolarize the neuron to ∼20 mV) with 4-s resting intervals between steps. Total time required for each series was ≤2 min. The number of action potentials/voltage step was counted off-line with pCLAMP 9 software.
Statistics.
Most statistical analyses were performed with SPSS (SPSS, Chicago, IL). Power spectra were tested for significance with MATLAB 7.3. Data are represented as means ± SE. Data fitting a parametric distribution were tested for significance with an ANOVA with Sidak post hoc comparison for multiple groups and t-test for two groups. Data fitting a nonparametric distribution were tested with an ANOVA on ranks with least significant difference (LSD) post hoc comparison for multiple groups or Mann-Whitney test for two groups. Firing rate distributions were tested for differences with the Kolmogorov-Smirnov test after being fit with a log normal peak function (not shown) in SigmaPlot 7.01 (Systat Software, Chicago, IL). Correlations were tested with Spearman rank order correlation. Significance was defined as P < 0.05.
RESULTS
SLE Incidence and Augmentation by GABAergic Transmission
We first sought to document differences in SLE generation between epileptic and control tissue and to replicate previous reports of GABAA receptor-mediated facilitation of hippocampal 0Mg-induced SLE generation (Köhling et al. 2000) under our recording conditions. In slices from control rats, 0Mg-induced SLE were seen occasionally in CA1 but were not observed in other hippocampal regions (Fig. 2B, right). In slices from epileptic rats, SLE were recorded in all hippocampal regions but were more prevalent in 0Mg than 0Mg+BMI (Fig. 2C, right). Hippocampal SLE number was significantly higher in epileptic compared with control tissue in 0Mg but not 0Mg+BMI. SLE incidence in the entorhinal cortex was higher in epileptic versus control tissue but was similar in 0Mg and 0Mg+BMI. SLE duration was similar in 0Mg and 0Mg+BMI in all regions except dentate gyrus, where it was only 21% longer in 0Mg than in 0Mg+BMI (P < 0.05, ANOVA on ranks with LSD post hoc comparison; data not shown). These data show increased SLE generation in epileptic compared with control tissue and replicate and extend previous findings of GABAergic augmentation of hippocampal SLE incidence when excitatory transmission is enhanced with 0Mg in epileptic tissue.
Potential Depolarizing Block of Principal Cell Firing
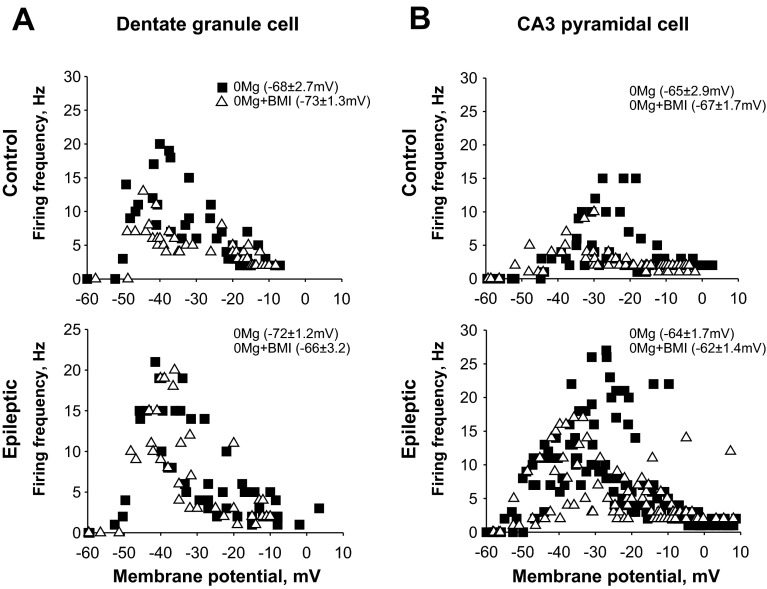
Both potentiation of depolarizing NMDAR-mediated cation currents with 0Mg and blockade of hyperpolarizing GABAA receptor-mediated Cl− currents with BMI can depolarize neurons and therefore reduce relative action potential threshold. Additive effects of concurrent 0Mg and BMI application could promote depolarization block of action potential firing. However, we showed that SLE durations were similar in 0Mg and 0Mg+BMI and epileptiform event incidence was similar in 0Mg and 0Mg+BMI for all cortico-hippocampal regions in slices from both control (Fig. 2B, center) and epileptic (Fig. 2C, center) rats. Furthermore, whole cell current-clamp recordings in a limited number of cells revealed only a slight depolarization of granule cell (Fig. 3A; 5–6 mV) and CA3 pyramidal cell (Fig. 3B; 2 mV) resting membrane potential and significant overlap in maximal action potential firing in 0Mg compared with subsequent 0Mg+BMI perfusion in slices from either control (Fig. 3, A and B, top) or epileptic (Fig. 3, A and B, bottom) rats. These findings suggest that GABAergic inhibition, rather than depolarization block of hippocampal principal cell firing, may augment hippocampal SLE generation.
Fig. 3.
When excitatory transmission was enhanced with 0Mg, GABAergic transmission did not significantly affect dentate granule cell or CA3 pyramidal cell firing frequency. Firing frequencies of dentate granule cells (A) and CA3 pyramidal cells (B) in slices isolated from control (top) and epileptic (bottom) rats were measured after current steps with whole cell current-clamp recordings as described in materials and methods. All data points are depicted for individual cells in each experimental and recording condition. Although the resting membrane potential (in parentheses ± SE) was slightly more positive in 0Mg+BMI than in 0Mg, this shift was not significant (t-test, P > 0.05). Similarly, there was significant overlap in the distribution of firing in 0Mg and in 0Mg+BMI [best fit (r2 ≥ 0.9) with log-normal peak function, Kolmogorov-Smirnov test, P > 0.05], but there was a trend toward higher firing frequency in the −40 to −30 mV range in dentate granule cells from control rats (A, top) and in the −25 to −20 mV range for CA3 pyramidal cells from control (B, top) and epileptic (B, bottom) rats in 0Mg compared with 0Mg+BMI. n = 4–6 cells/slices/rats in each group.
Slow, Ripple, and Fast Ripple Oscillations and Modulation by GABAergic Transmission
Prior to interrogating oscillation synchrony within and between cortico-hippocampal regions, we first measured the overall prevalence of oscillatory activity in slices from control and epileptic rats. Oscillations were split into three broad frequency ranges: relatively “slow” oscillations [including beta (β) and gamma (γ) activity, 10–30 and 30–100 Hz, respectively], ripples (101–200 Hz oscillations), and fast ripples (>200 Hz oscillations) (Fig. 4A), which are thought to arise from synchronous activity within distinct neuronal populations. Delta (δ), theta (θ), and alpha (α) oscillations were not documented because the frequency of these oscillations falls below the lower 10-Hz cutoff frequency of our amplifier. “Slow” oscillations represent relatively long-range activity propagation in excitatory circuits (Salinas and Sejnowski 2001). When slow oscillations are suprathreshold for action potential generation, they often present with a superimposed population spike (spike and wave complex; Fig. 4A). Ripples are generated predominantly in CA3, propagate to downstream synaptic targets (Buhl and Buzsáki 2005; Csicsvari et al. 2000; Ylinen et al. 1995), and reflect coordinated circuit activity driven by 1) interneurons that facilitate neuronal synchronization and/or 2) gap junction-mediated electrical coupling (Draguhn et al. 1998; Klausberger et al. 2003, 2004; Le Van Quyen et al. 2008; Traub and Bibbig 2000). Fast ripples are thought to arise from highly synchronous firing in small principal cell islands and may reflect pathological ripple desynchronization in seizure-prone brain regions (Bragin et al. 1999, 2002, 2004; Foffani et al. 2007; Traub and Wong 1982). No attempt was made to differentiate between normal and pathological high-frequency oscillations (101 Hz–1 kHz; see Engel et al. 2009).
In slices from control rats, “slow” oscillations were fairly evenly distributed across all cortico-hippocampal regions and ripples and fast ripples occurred more frequently in CA3 than in other hippocampal regions (Fig. 4B, center and right), consistent with previous reports of CA3 as an oscillation generator (Csicsvari et al. 2000; Ylinen et al. 1995). In slices from epileptic rats, the incidence of 0Mg-induced oscillations was similar to control, except for hilar slow oscillations, which were more prevalent in epileptic compared with control tissue. In contrast, slow oscillations, ripples, and fast ripples were significantly more prevalent in epileptic compared with control tissue in 0Mg+BMI (Fig. 4C). However, within each hippocampal region the incidence of oscillations was not statistically different in 0Mg and 0Mg+BMI for both control and epileptic tissue (Fig. 4C). Given the higher incidence of seizures in 0Mg than 0Mg+BMI in epileptic tissue, these data suggest that oscillation number per se was not a good predictor of SLE incidence under our recording conditions.
Spectral Correlations Within Cortico-Hippocampal Regions and Modulation by GABAergic Transmission
To expose regions whose neurons tend to oscillate at a similar frequency in the same 0.5-s recording window (spectral correlation) and gauge each region's propensity to generate/drive oscillations, we next correlated individual oscillation frequencies in running individual 0.5-s windows of data recorded from different electrodes within an individual region. Correlations from individual experiments were converted to power spectra, and finally power spectra from individual experiments were averaged. Although oscillation incidence within each hippocampal region was similar in 0Mg and 0Mg+BMI for both control and epileptic tissue, this stepwise approach further minimized potential confounds of overall differences in oscillation prevalence. Correlations between different electrodes within an individual region for each 0.5-s recording window were first compiled for the entire recording (termed “entire recording correlations”). This analysis showed the highest spectral correlation in the hilus across most frequency ranges for both 0Mg and 0Mg+BMI in both control and epileptic tissue (Fig. 5A, 1 and 2, left), consistent with the hilus acting as an oscillation generator. When slices from epileptic rats were compared with controls, hilar slow oscillations showed the largest overall increase in spectral correlation, which was similar in 0Mg and 0Mg+BMI (Fig. 5A, 1 and 2, right). Next, correlations between different electrodes within an individual region for each 0.5-s recording window were binned and compiled according to the 3 s immediately prior to SLE, the first 3 s of SLE, and the 3 s immediately after SLE termination (termed “correlations relative to SLE”). This analysis showed that hilar spectral correlation was particularly strong during SLE compared with either immediately before or after SLE in epileptic tissue (Fig. 5B, 1 and 2) and was similar in 0Mg and 0Mg+BMI. Increased SLE-associated spectral correlation also was apparent within other hippocampal regions and frequencies, albeit to a lesser degree. These findings are consistent with an increased propensity of the hilus to act as a “slow” oscillation generator in epileptic tissue and a relative lack of GABAergic control over hilar spectral oscillation correlation.
We then further examined the role of GABAergic transmission on spectral correlation ([0Mg] − [0Mg+BMI]) within each region and how it was altered in the epileptic brain. The most striking finding for entire recording correlations was increased GABAergic facilitation of spectral “slow” oscillation and ripple correlation within CA3 of epileptic compared with control tissue (Fig. 5A3). This GABAergic facilitation of spectral correlation in CA3 was more prominent during SLE compared with immediately before and after SLE in epileptic tissue (Fig. 5B3). GABAergic facilitation of spectral correlation in entorhinal cortex was apparent in all frequency ranges immediately before and after, but not during, SLE. These data predict a role for GABAergic transmission in spectral CA3 correlation, which may facilitate hippocampal SLE generation.
Oscillation Coherence Between Cortico-Hippocampal Regions and Modulation by GABAergic Transmission
To examine spectral oscillation coherence between different cortico-hippocampal regions, we next correlated individual oscillations at each frequency (oscillation coherence function) across different regions in running individual 0.5-s windows of data. Coherences from individual experiments were then averaged by group. Entire recording coherences showed that in both 0Mg and 0Mg+BMI “slow” oscillation coherence was highest between the dentate gyrus, hilus, and CA3, and this coherence was shifted to higher “slow” oscillation frequency bands (fast γ activity) in epileptic compared with control tissue (Fig. 6A, 1 and 2).
We then further examined the role of GABAergic transmission ([0Mg] − [0Mg+BMI]) on oscillation coherence between regions and how it was altered in the epileptic brain. The most striking findings for entire recording coherences were 1) reduced GABAergic facilitation of low-frequency “slow” oscillation coherence between hippocampal regions (Fig. 6A3) and 2) increased GABAergic facilitation in bands of ripple coherence between CA3 and other hippocampal regions in slices from epileptic compared with control rats (Fig. 6A3). When coherences relative to SLE in epileptic tissue were computed, GABAergic facilitation of “slow” oscillation coherence between the dentate gyrus, hilus, and CA3 were increased during SLE compared with immediately before and after SLE (Fig. 6B). Taken together, these data suggest that GABAergic transmission facilitated oscillation coherence between CA3 and other subregions in epileptic tissue, which may augment SLE.
SLE Cross-Correlation Within and Between Cortico-Hippocampal Regions and Modulation by GABAergic Transmission
While spectral correlation and coherence data provide compelling evidence for oscillation generators and synchrony within and between cortico-hippocampal regions, these measures only approximate coexisting spectral bands in individual 0.5-s recording windows without regard to temporal synchrony. While the Morlet transform (Grossmann and Morlet 1984) would definitively document temporal synchrony in individual spectral bands, this approach is impractical for determining and compiling temporal synchrony of different event types and the large oscillation numbers recorded in each experiment and then averaging these data across multiple experiments. Therefore, to extend our spectral correlation and coherence measures during SLE, we examined SLE cross-correlation vs. lag within and between cortico-hippocampal regions. Cross-correlation analyses were used to document spatio-temporal relationships of SLE waveforms recorded by individual electrodes within and between cortico-hippocampal regions in slices from epileptic rats. Cross-correlations were calculated on each individual SLE recorded from different electrodes within and across individual regions. Correlations from individual experiments were then averaged. This stepwise approach minimized potential confounds of differences in waveform shape between different experiments and recording conditions.
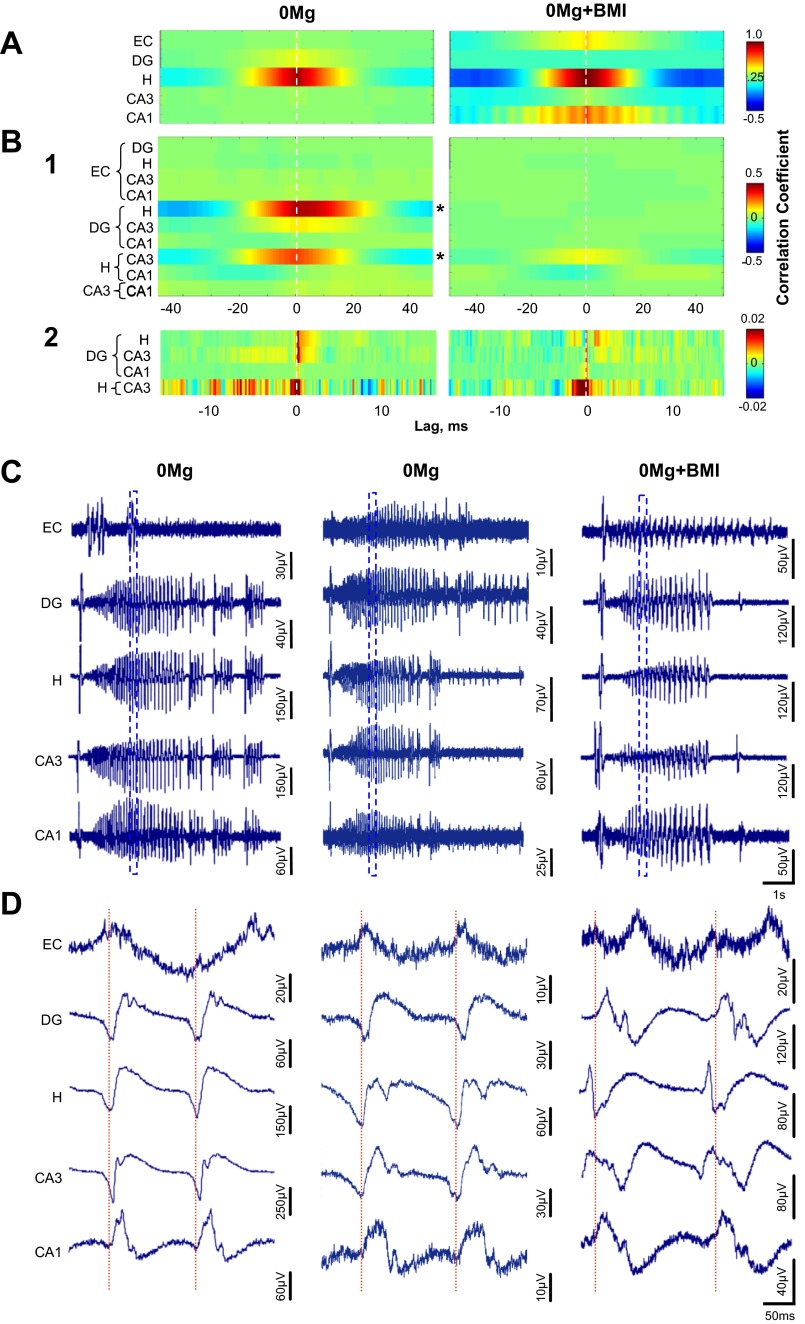
We first documented cross-correlations of SLE waveforms recorded by different electrodes within an individual region. Surprisingly, the hilus, but not CA3 or the dentate gyrus, exhibited a strong SLE cross-correlation, which was similar in 0Mg and 0Mg+BMI (Fig. 7A, left and right, respectively). The strong cross-correlation within the hilus in both 0Mg and 0Mg+BMI suggests that the waveform peaks and troughs show a consistent 0-ms lag (latency) and that differences in waveform shape between 0Mg and 0Mg+BMI do not preclude strong cross-correlations. The high cross-correlation within the hilus also suggests that its neurons fire in unison during SLE. These data were consistent with our spectral coherence findings and provided further evidence for an increased propensity of the hilus to act as an oscillation/SLE generator in the epileptic brain and a relative lack of GABAergic control over hilar oscillation/SLE coherence.
Fig. 7.
When excitatory transmission was enhanced, GABAergic transmission was not required for SLE cross-correlation within the hilus but was required for SLE cross-correlation between the hilus and other hippocampal regions. Cross-correlations vs. lag were calculated for 0Mg (left, n = 11 slices/rats)- and 0Mg+BMI (right, n = 9 slices/rats)-induced SLE in slices from epileptic rats as described in materials and methods. A: cross-correlations of waveforms recorded by different electrodes within an individual region were high in the hilus and were similar in 0Mg (left) and 0Mg+BMI (right). Right: weaker cross-correlations within the entorhinal cortex and CA1 were apparent in 0Mg+BMI, but they were not significantly different from 0Mg. B1: cross-correlations of waveforms recorded from electrodes in different regions were high between the hilus and the dentate gyrus and between the hilus and CA3 in 0Mg (left) and were significantly lower in 0Mg+BMI (right). B2: similar results were seen with the autoregressive moving average (ARMA) model prior to performing cross-correlations. Exceptions were a more prominent cross-correlation between CA3 and the dentate gyrus and a second series of cross-correlation peaks between CA3 and the hilus in 0Mg (left) but not 0Mg+BMI (right). Reference regions for the cross-correlation lags are on right. C: representative SLE recorded simultaneously from all cortico-hippocampal regions in 0Mg (left and center) and 0Mg+BMI (right). D: expanded time scale of traces enclosed by boxes in C illustrates slight time lags between the hilus, CA3, and dentate gyrus (left) and time lock between the hilus and CA3, with a slight time lag with the dentate gyrus in 0Mg (center), and time lock between CA3 and the hilus in 0Mg+BMI (right). *Difference between 0Mg and 0Mg+BMI, P < 0.05, 2-way ANOVA. Horizontal scale bar in C, bottom right, applies to all traces in C; horizontal scale bar in D, bottom right, applies to all traces D.
When we next calculated SLE cross-correlations across different cortico-hippocampal regions, 0Mg induced high SLE cross-correlation between the hilus and CA3 and between the hilus and dentate gyrus as well as a weak relationship between CA3 and dentate gyrus (Fig. 7, B1 and C and D, left). These data are consistent with our findings of increased spectral coherence between these regions in epileptic tissue. Blockade of GABAA receptor-mediated transmission in 0Mg (0Mg+BMI) abolished the hilus-dentate gyrus and dentate gyrus-CA3 SLE cross-correlation and reduced the hilus-CA3 SLE cross-correlation, compared with 0Mg alone (Fig. 7B1). These data are consistent with our spectral coherence findings in 0Mg compared with 0Mg+BMI in epileptic tissue. To more precisely define cross-correlation lags, we “prewhitened” the data with the ARMA model prior to calculating the cross-correlation. The primary 0-ms lag between CA3 and the hilus in both 0Mg (Fig. 7B2, left) and 0Mg+BMI (Fig. 7B2, right) suggests synchronous activity between CA3 and the hilus during the majority of the SLE time course or most experiments. That said, less prominent peaks centered around −7-ms as well as +7- and +10-ms lag for 0Mg (Fig. 7B2, left) suggest additional dynamic interplay between CA3 and the hilus over the evolving SLE time course or differences between experiments or SLE events. For CA3-dentate gyrus in 0Mg, the smear of low correlation peaks at −7 to −2 ms is consistent with the expected propagation of activity from the dentate gyrus to CA3. However, the primary peaks from 0 to +2 ms suggest more prominent propagation from CA3 to the dentate gyrus (Fig. 7B2, left). For the hilus as a reference and the dentate gyrus, in 0Mg, the 0- to +2-ms lag (Fig. 7B2, left) is consistent with monosynaptic connections from the hilus to the dentate gyrus. The absence of many of these prominent peaks in 0 Mg+BMI (Fig. 7B2, right) suggests strong GABAergic control over the interplay between the hilus, CA3, and the dentate gyrus during SLE. Our method of averaging SLE cross-correlations across multiple events and experiments may have minimized events with varying lag and thus underestimated less frequent and more variable pathways that may be involved in SLE synchronization. However, consistent with our hypothesis, these data strongly implicate the hilus and back-propagation from CA3/hilus to the dentate gyrus as dominant mediators of synchronization during hippocampal SLE and GABAA receptor-mediated transmission as a key facilitator of oscillation and SLE synchrony across, but not within, hippocampal regions.
DISCUSSION
This study used a 60-microelectrode array to simultaneously record field potentials in five distinct cortico-hippocampal regions under conditions that manipulated excitatory and inhibitory circuit activity and maximized differences in SLE generation between control and epileptic tissue. Field potentials recorded from slices isolated from pilocarpine-treated epileptic rats were compared with controls. The principal findings can be summarized as follows. First, hippocampal SLE were more prevalent in slices from epileptic rats when excitatory transmission was enhanced and GABAergic transmission was intact (0Mg) than when excitatory transmission was enhanced and GABAergic transmission was blocked (0Mg+BMI). These data, together with results from control experiments, confirm earlier reports of GABAA receptor-mediated facilitation of hippocampal SLE in slices from normal rats when excitatory transmission is elevated with 0Mg (Köhling et al. 2000) or other chemical agents (Barbarosie et al. 2002; Khazipov and Holmes 2003) and extend this finding to epileptic tissue. Second, high spectral and temporal slow oscillation/SLE correlations and cross-correlations, respectively, within the hilus were 1) greater in epileptic compared with control tissue; 2) highest during SLE compared with either immediately before or after SLE; and 3) similar in 0Mg and 0Mg+BMI. These findings are consistent with an increased propensity of excitatory circuits within the hilus to act as a slow oscillation/SLE generator in epileptic tissue and a relative lack of GABAergic control over this oscillation/SLE generator. Third, GABAergic facilitation of spectral “slow” oscillation and ripple correlations within CA3 was increased in epileptic compared with control tissue and was more prominent during compared with immediately before and after SLE. These data suggest a potential role for GABAergic facilitation of CA3 synchrony in augmenting hippocampal SLE generation in epileptic tissue. Fourth, slow oscillation and ripple coherence between the hilus, dentate gyrus, and CA3 was increased in epileptic compared with control tissue, particularly during SLE, and was significantly higher in 0Mg than 0Mg+BMI. High 0Mg-induced SLE coherence between 1) hilus and CA3, 2) hilus and dentate gyrus, and 3) CA3 and dentate gyrus was reduced or abolished in 0Mg+BMI. These data predict a role for GABAA receptor-mediated transmission in promoting synchrony between the hilus, dentate gyrus, and CA3 in epileptic tissue, which may facilitate SLE generation. Finally, SLE cross-correlation lag measurements suggested that CA3 and the hilus fired in unison in a majority of SLE/experiment and provided evidence for a monosynaptic connection from the hilus to the dentate gyrus during SLE. These data strongly implicate the hilus and back-propagation from CA3/hilus to the dentate gyrus as dominant mediators of slow oscillation synchronization during hippocampal SLE and GABAA receptor-mediated transmission as a key facilitator of this back-propagation. Taken together, these data implicate 1) an excitatory circuit within the hilus as an oscillation/SLE generator and 2) GABAA receptor-mediated transmission and CA3 synchrony as facilitators of back-propagation from CA3 to the dentate gyrus, which may augment SLE in epileptic hippocampus.
Caveats
The primary caveat for interpretation of our electrophysiological results in the context of human TLE is that slices isolated from animal models may not reflect pathophysiology in patients. Studies utilizing slices prepared from resected human epileptic brain have yielded important information but historically have been limited to pharmacoresistant, late-stage TLE (e.g., Köhling et al. 1998). Therefore, many investigations focused on basic ictogenesis mechanisms in early-stage TLE utilize animal models. In vivo animal model studies are restricted predominantly to electrical recordings from large cortical regions with surface electrodes or from smaller, deeper brain areas with-low density depth electrodes that minimize confounding lesions. These relatively low-resolution approaches have begun to yield intriguing insights into population activity within and across brain regions in epileptic brain (e.g., Bragin et al. 2002; Le Van Quyen et al. 2008). However, higher spatial resolution of electrical activity is required to better understand regional circuit interactions. Although in vitro slice preparation severs extrahippocampal inputs and intrinsic longitudinal hippocampal connections, hippocampal slices remain quite popular in epilepsy research because the high achievable resolution affords investigation into cellular correlates of ictogenesis. Another caveat is our global application of SLE-inducing agents. However, the relatively short brain slice “life span” coupled with low spontaneous SLE frequency makes spontaneous SLE detection in slices impractical. Perfusion with 0 mM Mg2+ aCSF is often used to model ictogenesis in slices from normal animals because 0Mg-induced activity closely resembles that observed in human epilepsy (Babb et al. 1987; Wyler et al. 1982). Mg2+ removal also unmasks upregulated NMDAR in epileptic brain (Kohr et al. 1993; Kraus et al. 1994; Mody et al. 1988). Despite these caveats, our results provide important new information on the hilus as an oscillation generator and potential conduit for hippocampal synchrony between CA3 and the dentate gyrus as well as GABAergic modulation of oscillation synchrony and SLE coherence within and between hippocampal regions in the epileptic brain.
Correlated Activity Within Cortico-Hippocampal Regions
When epileptic tissue was compared with control tissue, our study revealed the most dramatic increase in spectral slow oscillation correlation and temporal SLE cross-correlation within the hilus. This finding was surprising because we expected that recurrent excitatory connections between CA3 in normal and epileptic tissue and dentate granule cells following seizure-induced axonal sprouting (Bausch and Chavkin 1997; Houser et al. 1990; Siddiqui and Joseph 2005; Sutula et al. 1989; Tauck and Nadler 1985) would lead to a higher incidence of correlated activity than other hippocampal regions. The neurons/circuits mediating hilar synchrony are not definitively clear because the hilus is made up of a heterogeneous group of neurons including mossy cells, an extremely diverse population of interneurons (Amaral 1978; Freund and Buzsaki 1996), and, in the epileptic brain, ectopic granule cells. The most likely mediator is a recurrent excitatory glutamatergic circuit involving surviving, hyperexcitable mossy cells (Ratzliff et al. 2002; Scharfman et al. 2001) and/or ectopic granule cells, which arise from abnormal migration of newly born granule cells (Parent et al. 1997; Scharfman et al. 2000). The absence of significant impact of the GABAA receptor antagonist BMI on hilar oscillation/SLE correlations suggests that TLE-associated changes in GABAergic interneuron survival/structure/function did not contribute directly to the elevated hilar synchrony in the epileptic brain.
Our spectral analyses also showed a more modestly increased spectral “slow” oscillation and ripple correlations within CA3 in epileptic compared with control tissue when excitatory transmission was elevated and GABAA receptor-mediated transmission was intact. However, SLE cross-correlation vs. lag measures did not identify CA3 as a SLE generator under either of our recording conditions. Thus, contrary to our hypothesis, CA3 does not appear to be a primary driver of SLE generation but could act in concert with other mechanisms underlying GABAA receptor-mediated facilitation of SLE generation.
Correlated Activity Between Dentate Gyrus, Hilus, and CA3
The dramatically increased spectral slow oscillation coherence and cross-correlation as well as high SLE coherence between the hilus, CA3, and dentate gyrus in epileptic compared with control tissue, which was reduced after GABAA receptor blockade, is consistent with a polysynaptic excitatory circuit whose throughput is upregulated by GABAergic transmission. In normal tissue, anatomical and electrophysiological studies have documented back-projections from CA3 pyramidal cells to the hilus and dentate gyrus (Li et al. 1994) and functional polysynaptic back-projections from CA3 pyramidal cells to dentate granule cells (Gonzalez-Sulser et al. 2011; Scharfman 1993, 1994a, 1994b) via a hilar mossy cell intermediate (Scharfman 1993, 1994a, 1994b). Our SLE cross-correlation lag measures are consistent with previous work showing synchronized spontaneous discharges between CA3 pyramidal cells and hilar mossy cells in the epileptic brain (Scharfman 1993, 1994a, 1994b; Scharfman et al. 2000, 2001) and a profound hilar influence on granule cell function (Deller et al. 1999; Dougherty and Milner 1999; Gulyás et al. 1999). In TLE, an excitatory circuit is formed between hyperexcitable mossy cells (Pierce et al. 2007; Ratzliff et al. 2002; Scharfman et al. 2001) and ectopic hilar granule cells, which in turn synapse onto other ectopic hilar granule cells, normotopic granule cells, CA3 pyramidal cells, and surviving hilar mossy cells (Cameron et al. 2011; Scharfman et al. 2000, 2001). This excitatory circuit together with our data showing TLE-associated increased SLE and slow oscillation synchrony within the hilus and between the hilus, CA3, and dentate gyrus further suggests hilar amplification of back-propagating CA3 to granule cell activity and a hyperexcitable, potentially ictogenic hilar focus that could modify the dentate gate (Scharfman 2007).
GABAergic Modulation of Correlated Activity and SLE Generation
Our findings suggest a role for GABAA receptor-mediated transmission in facilitating slow oscillation and SLE synchrony measures and raise the questions of potential GABAA receptor-mediated depolarization block and putative source(s) of GABAergic control. In regard to depolarization block, previous work by Aradi and Maccaferri (2004) showed that elevation of extracellular K+ produced network-driven spontaneous bursts in CA3 that were analogous in shape and duration to the epileptiform events described in the present study. Addition of the GABAA receptor antagonist gabazine to the high-K+ recording buffer significantly reduced epileptiform burst incidence, increased burst amplitude, and reduced repetitive bursting of CA3 pyramidal cells. On the basis of these findings they postulated that depolarization block of CA3 pyramidal cell firing may have led to the reduced incidence of epileptiform bursting. However, we showed that addition of the GABAA receptor antagonist BMI to the 0Mg recording buffer had little effect on SLE durations or epileptiform event incidence. Furthermore, Borck and Jefferys (1999) showed that waveforms similar to those presented by Aradi and Maccaferri (2004) formed the tertiary bursts of elevated K++BMI-induced SLE in CA3, and Ziburkus et al. (2006) reported no depolarization block of action potential firing in CA1 pyramidal cells during 4-aminopyridine (4-AP)-induced SLE. Patrylo and Dudek (1998) also showed SLE in the dentate gyrus from epileptic rats in high K++BMI, suggesting that dentate granule cells do not undergo depolarization block under these depolarizing conditions. Our whole cell current-clamp data are consistent with these previous reports. Thus, while we did not conduct current-clamp recordings in all hippocampal cell types during SLE, all the findings together suggest that GABAergic inhibition, rather than depolarization block of hippocampal principal cell firing, may augment hippocampal SLE generation.
In regard to putative source(s) of GABAergic control, GABAergic influence on network function is governed partially by subcellular synapse location. Dendritic GABAergic synapses normally control synaptic input and plasticity but seldom elicit effective coincident inhibition in large neuronal populations (Miles et al. 1996). That said, dendritic GABAergic synapses can modulate slow network oscillations (Gloveli et al. 2005; Szabadics et al. 2001) and are increased in TLE (Thind et al. 2010; Zhang et al. 2009). Perisomatic GABAergic synapses normally limit action potential firing, control neuronal output, and facilitate oscillations and synchrony (Buzsaki et al. 2004; DeFelipe 1999; Freund 2003; Howard et al. 2005; Miles et al. 1996) and can influence the full spectrum of oscillation frequencies (Gulyas et al. 2010; Losonczy et al. 2010; Mann et al. 2005). Coincident GABAA receptor-mediated transmission through either set of synapses could facilitate synchrony by simultaneously “resetting” neuronal membrane potential via inhibitory Cl− influx and/or dendritic shunting (Bormann et al. 1987; Staley and Mody 1992) or by depolarizing GABAA receptor-mediated responses via altered Cl− equilibrium potentials and K+-Cl− cotransporter activity following prolonged GABAA receptor activation (Ben-Ari and Holmes 2005; Cobb et al. 1995; Dzhala and Staley 2003; Fujiwara-Tsukamoto et al. 2006, 2010; Khirug et al. 2008; Michelson and Wong 1991; Perrault and Avoli 1992). Either mechanism could contribute to synchrony in our study.
GRANTS
Work was supported by the American Epilepsy Society (B. Gafurov), Congressionally Directed Medical Research Programs Award W81XWH-04-1-0065/PR030035 (S. B. Bausch), and National Institute of Neurological Disorders and Stroke Grant NS-045964 (S. B. Bausch).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.G. and S.B.B. conception and design of research; B.G. performed experiments; B.G. analyzed data; B.G. and S.B.B. interpreted results of experiments; B.G. prepared figures; B.G. drafted manuscript; B.G. and S.B.B. edited and revised manuscript; B.G. and S.B.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Li-Rong Shao for helpful discussions. The opinions or assertions contained herein are the private ones of the authors and not to be construed as official or reflecting the views of the Department of Defense or the Uniformed Services University.
REFERENCES
- Amaral D, Lavenex P. Hippocampal neuroanatomy. In: The Hippocampus Book, edited by Andersen P, Morris R, Amaral D, Bliss T, O'Keefe J. Oxford, UK: Oxford Univ. Press, 2007 [Google Scholar]
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 31: 571–591, 1989 [DOI] [PubMed] [Google Scholar]
- Amaral DG. A Golgi study of cell types in the hilar region of the hippocampus in the rat. J Comp Neurol 182: 851–914, 1978 [DOI] [PubMed] [Google Scholar]
- Anderson WW, Lewis DV, Swartzwelder HS, Wilson WA. Magnesium-free medium activates seizure-like events in the rat hippocampal slice. Brain Res 398: 215–219, 1986 [DOI] [PubMed] [Google Scholar]
- Aradi I, Maccaferri G. Cell type-specific synaptic dynamics of synchronized bursting in the juvenile CA3 rat hippocampus. J Neurosci 24: 9681–9692, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb TL, Wilson CL, Isokawa-Akesson M. Firing patterns of human limbic neurons during stereoencephalography (SEEG) and clinical temporal lobe seizures. Electroencephalogr Clin Neurophysiol 66: 467–482, 1987 [DOI] [PubMed] [Google Scholar]
- Barbarosie M, Louvel J, D'Antuono M, Kurcewicz I, Avoli M. Masking synchronous GABA-mediated potentials controls limbic seizures. Epilepsia 43: 1469–1479, 2002 [DOI] [PubMed] [Google Scholar]
- Bartlett MS. Smoothing periodograms from time-series with continuous spectra. Nature 161: 686–687, 1948 [Google Scholar]
- Bausch SB, Chavkin C. Changes in hippocampal circuitry following pilocarpine-induced seizures as revealed by opioid receptor distribution and activation. J Neurosci 17: 477–492, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bausch SB, He S, Petrova Y, Wang XM, McNamara JO. Plasticity of both excitatory and inhibitory synapses is associated with seizures induced by removal of chronic blockade of activity in cultured hippocampus. J Neurophysiol 96: 2151–2167, 2006 [DOI] [PubMed] [Google Scholar]
- Bausch SB, McNamara JO. Synaptic connections from multiple hippocampal subfields contribute to hyperexcitability of dentate granule cells in long-term organotypic hippocampal slice cultures. J Neurophysiol 84: 2918–2932, 2000 [DOI] [PubMed] [Google Scholar]
- Behr J, Empson RM, Schmitz D, Gloveli T, Heinemann U. Electrophysiological properties of rat subicular neurons in vitro. Neurosci Lett 220: 41–44, 1996 [DOI] [PubMed] [Google Scholar]
- Behr J, Gloveli T, Heinemann U. The perforant path projection from the medial entorhinal cortex layer III to the subiculum in the rat combined hippocampal-entorhinal cortex slice. Eur J Neurosci 10: 1011–1018, 1998 [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Holmes GL. The multiple facets of gamma-aminobutyric acid dysfunction in epilepsy. Curr Opin Neurol 18: 141–145, 2005 [DOI] [PubMed] [Google Scholar]
- Bendat J, Piersol A. (Editors). Stationary random processes, basic concepts. In: Random Data: Analysis and Measurement Procedures. New York: Wiley-Interscience, 1971 [Google Scholar]
- Borck C, Jefferys JG. Seizure-like events in disinhibited ventral slices of adult rat hippocampus. J Neurophysiol 82: 2130–2142, 1999 [DOI] [PubMed] [Google Scholar]
- Bormann J, Hamill OP, Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurons. J Physiol 385: 243–286, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Buzsaki G. High frequency oscillations in human brain. Hippocampus 9: 137–142, 1999 [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Almajano J, Mody I, Engel J., Jr High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia 45: 1017–1023, 2004 [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Staba RJ, Reddick M, Fried I, Engel J., Jr Interictal high-frequency oscillations (80–500 Hz) in the human epileptic brain: entorhinal cortex. Ann Neurol 52: 407–415, 2002 [DOI] [PubMed] [Google Scholar]
- Buhl DL, Buzsáki G. Developmental emergence of hippocampal fast-field “ripple” oscillations in the behaving rat pups. Neuroscience 134: 1423–1430, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Geisler C, Henze DA, Wang XJ. Interneuron Diversity series: Circuit complexity and axon wiring economy of cortical interneurons. Trends Neurol Sci 27: 186–193, 2004 [DOI] [PubMed] [Google Scholar]
- Cameron MC, Zhan RZ, Nadler JV. Morphologic integration of hilar ectopic granule cells into dentate gyrus circuitry in the pilocarpine model of temporal lobe epilepsy. J Comp Neurol 519: 2175–2192, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature 378: 75–78, 1995 [DOI] [PubMed] [Google Scholar]
- Collins RC, Tearse RG, Lothman EW. Functional anatomy of limbic seizures: focal discharges from medial entorhinal cortex in rat. Brain Res 280: 25–40, 1983 [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Mamiya A, Buzsaki G. Ensemble patterns of hippocampal CA3-CA1 neurons during sharp wave-associated population events. Neuron 28: 585–594, 2000 [DOI] [PubMed] [Google Scholar]
- DeFelipe J. Chandelier cells and epilepsy. Brain 122: 1807–1822, 1999 [DOI] [PubMed] [Google Scholar]
- Deller T, Katona I, Cozzari C, Frotscher M, Freund TF. Cholinergic innervation of mossy cells in the rat fascia dentata. Hippocampus 9: 314–320, 1999 [DOI] [PubMed] [Google Scholar]
- Dougherty KD, Milner TA. Cholinergic septal afferent terminals preferentially contact neuropeptide Y-containing interneurons compared to parvalbumin-containing interneurons in the rat dentate gyrus. J Neurosci 19: 10140–10152, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draguhn A, Traub RD, Schmitz D, Jefferys JR. Electrical coupling underlies high-frequency oscillations in the hippocampus in vitro. Nature 394: 189–192, 1998 [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Staley KJ. Excitatory actions of endogenously released GABA contribute to initiation of ictal epileptiform activity in the developing hippocampus. J Neurosci 23: 1840–1846, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J, Jr, Bragin A, Staba R, Mody I. High-frequency oscillations: what is normal and what is not? Epilepsia 50: 598–604, 2009 [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, Van Ness P, Rasmussen TB, Ojemann LM. Outcome with respect to epileptic seizures. In: Surgical Treatment of the Epilepsies, edited by Engel J., Jr. New York: Raven, 1993 [Google Scholar]
- Foffani G, Uzcategui YG, Gal B, Menendez de la Prida L. Reduced spike-timing reliability correlates with the emergence of fast ripples in the rat epileptic hippocampus. Neuron 55: 930–941, 2007 [DOI] [PubMed] [Google Scholar]
- Freund TF. Interneuron diversity series: rhythm and mood in perisomatic inhibition. Trends Neurosci 26: 489–495, 2003 [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus 6: 347–470, 1996 [DOI] [PubMed] [Google Scholar]
- Fujiwara-Tsukamoto Y, Isomura Y, Takada M. Comparable GABAergic mechanisms of hippocampal seizurelike activity in posttetanic and low-Mg2+ conditions. J Neurophysiol 95: 2013–2019, 2006 [DOI] [PubMed] [Google Scholar]
- Fujiwara-Tsukamoto Y, Isomura Y, Imanishi M, Ninomiya T, Tsukada M, Yanagawa Y, Fukai T, Takada M. Prototypic seizure activity driven by mature hippocampal fast-spiking interneurons. J Neurosci 30: 13679–13689, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabor D. Theory of communication. JIEE 93: 429–457, 1946 [Google Scholar]
- Gloveli T, Dugladze T, Saha S, Monyer H, Heinemann U, Traub RD, Whittington MA, Buhl EH. Differential involvement of oriens/pyramidale interneurones in hippocampal network oscillations in vitro. J Physiol 562: 131–147, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Susler A, Wang J, Motamedi GK, Avoli M, Vicini S, Dzakpasu R. The 4-aminopyridine in vitro epilepsy model analyzed with a perforated multi-electrode array. Neuropharmacology 60: 1142–1153, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandmaison M, Belzile J, Thibeault C, Gagnon F. Frequency domain filter using an accurate reconfigurable FFT/IFFT core. In: Second Annual IEEE Northeast Workshop on Circuits and Systems. Washington DC: IEEE, 2004, p. 165–168 [Google Scholar]
- Granger CW, Newbold P. Forecasting Economic Time Series. New York: Academic, 1977 [Google Scholar]
- Grossmann A, Morlet J. Decomposition of Hardy functions into square integrable wavelets of constant shape. SIAM J Math Analysis 15: 723–736, 1984 [Google Scholar]
- Gulyás AI, Megıyas M, Emri Z, Freund TF. Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J Neurosci 19: 10082–10097, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Szabo GG, Ulbert I, Holderith N, Monyer H, Erdelyi F, Szabo G, Freund TF, Hajos N. Parvalbumin-containing fast-spiking basket cells generate the field potential oscillations induced by cholinergic receptor activation in the hippocampus. J Neurosci 30: 15134–15145, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser CR, Miyashiro JE, Swartz BE, Walsh GO, Rich JR, Delgado-Escueta AV. Altered patterns of dynorphin immunoreactivity suggest mossy fiber reorganization in human hippocampal epilepsy. J Neurosci 10: 267–282, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard A, Tamas G, Soltesz I. Lighting the chandelier: new vistas for axo-axonic cells. Trends Neurosci 28: 310–316, 2005 [DOI] [PubMed] [Google Scholar]
- Khazipov R, Holmes GL. Synchronization of kainate-induced epileptic activity via GABAergic inhibition in the superfused rat hippocampus in vivo. J Neurosci 23: 5337–5341, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khirug S, Yamada J, Afzalov R, Voipio J, Khiroug L, Kaila K. GABAergic depolarization of the axon initial segment in cortical principal neurons is caused by the Na-K-2Cl cotransporter NKCC1. Neuroscience 28: 4635–4639, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravani H, Pinnegar CR, Mitchell JR. Increased high-frequency oscillations precede in vitro low-Mg2+ seizures. Epilepsia 46: 1188–1197, 2005 [DOI] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Márton LF, Roberts JD, Cobden PM, Buzsáki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature 421: 844–848, 2003 [DOI] [PubMed] [Google Scholar]
- Klausberger T, Márton LF, Baude A, Roberts JD, Magill PJ, Somogyi P. Spike timing of dendrite-targeting bistratified cells during hippocampal network oscillations in vivo. Nat Neurosci 7: 41–47, 2004 [DOI] [PubMed] [Google Scholar]
- Köhling R, Lücke A, Straub H, Speckmann EJ, Tuxhorn I, Wolf P, Pannek H, Oppel F. Spontaneous sharp waves in human neocortical slices excised from epileptic patients. Brain 121: 1073–1087, 1998 [DOI] [PubMed] [Google Scholar]
- Köhling R, Vreugdenhil M, Bracci E, Jefferys JG. Ictal epileptiform activity is facilitated by hippocampal GABAA receptor-mediated oscillations. J Neurosci 20: 6820–6829, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohr G, De Koninck Y, Mody I. Properties of NMDA receptor channels in neurons acutely isolated from epileptic (kindled) rats. J Neurosci 13: 3612–3627, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus JE, Yeh GC, Bonhaus DW, Nadler JV, McNamara JO. Kindling induces the long-lasting expression of a novel population of NMDA receptors in hippocampal region CA3. J Neurosci 14: 4196–4205, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Van Quyen M, Bragin A, Staba R, Crépon B, Wilson CL, Engel J., Jr Cell type-specific firing during ripple oscillations in the hippocampal formation of humans. J Neurosci 28: 6104–6110, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XG, Somogyi P, Ylinen A, Buzsáki G. The hippocampal CA3 network: an in vivo intracellular labeling study. J Comp Neurol 339: 181–208, 1994 [DOI] [PubMed] [Google Scholar]
- Losonczy A, Zemelman BV, Magee JC. Network mechanisms of theta related neuronal activity in hippocampal CA1 pyramidal neurons. Nat Neurosci 13: 967–972, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EO, Suckling JM, Hajos N, Greenfield SA, Paulsen O. Perisomatic feedback inhibition underlies cholinergically induced fast network oscillations in the rat hippocampus in vitro. Neuron 45: 105–117, 2005 [DOI] [PubMed] [Google Scholar]
- Mello LE, Cavalheiro EA, Tan AM, Kupfer WR, Pretorius JK, Babb TL, Finch DM. Circuit mechanisms of seizures in the pilocarpine model of chronic epilepsy: cell loss and mossy fiber sprouting. Epilepsia 34: 985–995, 1993 [DOI] [PubMed] [Google Scholar]
- Michelson HB, Wong RK. Excitatory synaptic responses mediated by GABAA receptors in the hippocampus. Science 253: 1420–1423, 1991 [DOI] [PubMed] [Google Scholar]
- Miles R, Toth K, Gulyas AI, Hajos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron 16: 815–823, 1996 [DOI] [PubMed] [Google Scholar]
- Miles R, Wong RK. Excitatory synaptic interactions between CA3 neurons in the guinea pig hippocampus. J Physiol 373: 397–418, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I, Stanton PK, Heinemann U. Activation of N-methyl-d-aspartate receptors parallels changes in cellular and synaptic properties of dentate gyrus granule cells after kindling. J Neurophysiol 59: 1033–1054, 1988 [DOI] [PubMed] [Google Scholar]
- Molnar P, Nadler JV. Mossy fiber-granule cell synapses in the normal and epileptic rat dentate gyrus studied with minimal laser photostimulation. J Neurophysiol 82: 1883–1894, 1999 [DOI] [PubMed] [Google Scholar]
- Niedermeyer E, Lopez da Silva F. (Editors). EEG analyses: theory and practice. In: Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. Philadelphia, PA: Lippincott Williams & Wilkins, 2005 [Google Scholar]
- Obenaus A, Esclapez M, Houser C. Loss of glutamate decarboxylase mRNA-containing neurons in the rat dentate gyrus following pilocarpine-induced seizures. J Neurosci 13: 4470–4485, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci 17: 3727–3738, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak HR, Weissinger F, Terunuma M, Carlson GC, Hsu FC, Moss SJ, Coulter DA. Disrupted dentate granule cell chloride regulation enhances synaptic excitability during development of temporal lobe epilepsy. J Neurosci 27: 14012–14022, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrylo PR, Dudek FE. Physiological unmasking of new glutamatergic pathways in the dentate gyrus of hippocampal slices from kainate-induced epileptic rats. J Neurophysiol 79: 418–429, 1998 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (4th ed.). San Diego, CA: Academic, 1998 [Google Scholar]
- Perreault P, Avoli M. 4-Aminopyridine-induced epileptiform activity and a GABA-mediated long-lasting depolarization in the rat hippocampus. J Neurosci 12: 104–115, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JP, Punsoni M, McCloskey DP, Scharfman HE. Mossy cell axon synaptic contacts on ectopic granule cells that are born following pilocarpine-induced seizures. Neurosci Lett 422: 136–140, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiq A, DeLorenzo RJ, Coulter DA. Generation and propagation of epileptiform discharges in a combined entorhinal cortex/hippocampal slice. J Neurophysiol 70: 1962–1974, 1993 [DOI] [PubMed] [Google Scholar]
- Ratzliff AH, Santhakumar V, Howard A, Soltesz I. Mossy cells in epilepsy: rigor mortis or vigor mortis? Trends Neurosci 25: 140–144, 2002 [DOI] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci 2: 539–550, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K. An automatic determination of smoothing bandwidth in B-T method for power spectral estimation. J Sound Vibration 247: 165–173, 2001 [Google Scholar]
- Scharfman HE. Activation of dentate hilar neurons by stimulation of the fimbria in rat hippocampal slices. Neurosci Lett 156: 61–66, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. Evidence from simultaneous intracellular recordings in rat hippocampal slices that area CA3 pyramidal cells innervate dentate hilar mossy cells. J Neurophysiol 72: 2167–2180, 1994a [DOI] [PubMed] [Google Scholar]
- Scharfman HE. EPSPs of dentate gyrus granule cells during epileptiform bursts of dentate hilar “mossy” cells and area CA3 pyramidal cells in disinhibited rat hippocampal slices. J Neurosci 14: 6041–6057, 1994b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. The CA3 “backprojection” to the dentate gyrus. Prog Brain Res 163: 627–637, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Sollas AL. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J Neurosci 20: 6144–6158, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Smith KL, Goodman JH, Sollas AL. Survival of dentate hilar mossy cells after pilocarpine-induced seizures and their synchronized burst discharges with area CA3 pyramidal cells. Neuroscience 104: 741–759, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui AH, Joseph SA. CA3 axonal sprouting in kainate-induced chronic epilepsy. Brain Res 1066: 129–146, 2005 [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science 235: 73–76, 1987 [DOI] [PubMed] [Google Scholar]
- Sloviter RS. The functional organization of the hippocampal dentate gyrus and its relevance to the pathogenesis of temporal lobe epilepsy. Ann Neurol 35: 640–654, 1994 [DOI] [PubMed] [Google Scholar]
- Smith SW. (Editor). FFT filter. In: The Scientist and Engineer's Guide to Digital Signal Processing. San Diego, CA: California Technical, 1997a [Google Scholar]
- Smith SW. (Editor). Sampling theorem. In: The Scientist and Engineer's Guide to Digital Signal Processing. San Diego, CA: California Technical, 1997b [Google Scholar]
- Smith SW. (Editor). Gibbs effect. In: The Scientist and Engineer's Guide to Digital Signal Processing. San Diego, CA: California Technical, 1997c [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Fried I, Engel J., Jr Quantitative analysis of high-frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol 88: 1743–1752, 2002 [DOI] [PubMed] [Google Scholar]
- Staley KJ, Longacher M, Bains JS, Yee A. Presynaptic modulation of CA3 network activity. Nat Neurosci 1: 201–209, 1998 [DOI] [PubMed] [Google Scholar]
- Staley KJ, Mody I. Shunting of excitatory input to dentate gyrus granule cells by a depolarizing GABAA receptor-mediated postsynaptic conductance. J Neurophysiol 68: 197–212, 1992 [DOI] [PubMed] [Google Scholar]
- Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol 26: 321–330, 1989 [DOI] [PubMed] [Google Scholar]
- Szabadics J, Lorincz A, Tamas G. Beta and gamma frequency synchronization by dendritic GABAergic synapses and gap junctions in a network of cortical neurons. J Neurosci 21: 5824–5831, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauck DL, Nadler JV. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J Neurosci 5: 1016–1022, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thind KK, Yamawaki R, Phanwar I, Zhang G, Wen X, Buckmaster PS. Initial loss but later excess of GABAergic synapses with dentate granule cells in a rat model of temporal lobe epilepsy. J Comp Neurol 518: 647–667, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Bibbig A. A model of high-frequency ripples in the hippocampus based on synaptic coupling plus axon-axon gap junctions between pyramidal neurons. J Neurosci 20: 2086–2093, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Wong RK. Cellular mechanism of neuronal synchronization in epilepsy. Science 216: 745–747, 1982 [DOI] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L. Limbic seizures produced by pilocarpine in rats: behavioural, electroencephalographic and neuropathological study. Behav Brain Res 9: 315–335, 1983 [DOI] [PubMed] [Google Scholar]
- White AM, Williams PA, Ferraro DJ, Clark S, Kadam SD, Dudek FE, Staley KJ. Efficient unsupervised algorithms for the detection of seizures in continuous EEG recordings from rats after brain injury. J Neurosci Methods 152: 255–266, 2006 [DOI] [PubMed] [Google Scholar]
- Williamson A, Patrylo PR, Spencer DD. Decrease in inhibition in dentate granule cells from patients with medial temporal lobe epilepsy. Ann Neurol 45: 92–99, 1999 [DOI] [PubMed] [Google Scholar]
- Wuarin JP, Dudek FE. Electrographic seizures and new recurrent excitatory circuits in the dentate gyrus of hippocampal slices from kainate-treated epileptic rats. J Neurosci 16: 4438–4448, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyler AR, Ojemann GA, Ward AA., Jr Neurons in human epileptic cortex: correlation between unit and EEG activity. Ann Neurol 11: 301–308, 1982 [DOI] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nadasdy Z, Jando G, Szabo I, Sik A, Buzsaki G. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci 15: 30–46, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Huguenard JR, Buckmaster PS. Increased excitatory synaptic input to granule cells from hilar and CA3 regions in a model of temporal lobe epilepsy. J Neurosci 32: 1183–1196, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yamawaki R, Wen X, Uhl J, Diaz J, Prince DA, Buckmaster PS. Surviving hilar somatostatin interneurons enlarge, sprout axons, and form new synapses with granule cells in a mouse model of temporal lobe epilepsy. J Neurosci 29: 14247–14256, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziburkus J, Cressman JR, Barreto E, Schiff SJ. Interneuron and pyramidal cell interplay during in vitro seizure-like events. J Neurophysiol 95: 3948–3954, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]