Abstract
Cerebellar damage impairs the control of complex dynamics during reaching movements. It also impairs learning of predictable dynamic perturbations through an error-based process. Prior work suggests that there are distinct neural mechanisms involved in error-based learning that depend on the size of error experienced. This is based, in part, on the observation that people with cerebellar degeneration may have an intact ability to learn from small errors. Here we studied the relative effect of specific dynamic perturbations and error size on motor learning of a reaching movement in patients with cerebellar damage. We also studied generalization of learning within different coordinate systems (hand vs. joint space). Contrary to our expectation, we found that error size did not alter cerebellar patients' ability to learn the force field. Instead, the direction of the force field affected patients' ability to learn, regardless of whether the force perturbations were introduced gradually (small error) or abruptly (large error). Patients performed best in fields that helped them compensate for movement dynamics associated with reaching. However, they showed much more limited generalization patterns than control subjects, indicating that patients rely on a different learning mechanism. We suggest that patients typically use a compensatory strategy to counteract movement dynamics. They may learn to relax this compensatory strategy when the external perturbation is favorable to counteracting their movement dynamics, and improve reaching performance. Altogether, these findings show that dynamics affect learning in cerebellar patients more than error size.
Keywords: cerebellar ataxia, motor learning, dynamics, error size
people with cerebellar damage exhibit the neurological symptom of ataxia, i.e., poor movement coordination. Reaching movements made by cerebellar patients characteristically exhibit directional errors, dysmetria (under- or overshooting of the target), and increased variability (Bastian et al. 1996; Topka et al. 1998). The cerebellum is hypothesized to be responsible for storing neural representations of body movement dynamics, i.e., internal models (Wolpert et al. 1998). Damage to the cerebellum may disrupt such models, resulting in an impaired ability to properly predict and produce motor commands to counteract the kinematic effects of dynamic forces during movement (Bastian et al. 1996, 2000; Schweighofer et al. 1998).
In addition to storing internal models of limb dynamics, the cerebellum is also thought to update these models based on experienced error to facilitate changes in motor commands and adapt to new dynamics. Accordingly, damage to the cerebellum has been shown to critically impair motor adaptation in response to new dynamics and predictable perturbing forces (Maschke et al. 2004; Smith and Shadmehr 2005).
However, we recently reported that cerebellar patients are capable of adapting their reaching movements when new dynamics are gradually introduced over time, resulting in small movement errors, rather than large, abrupt ones (Criscimagna-Hemminger et al. 2010). Other studies with healthy subjects have shown differences between abruptly and gradually introduced perturbations regarding the amount of adaptation (Torres-Oviedo and Bastian 2012; Wong and Shelhamer 2011), duration of aftereffects (Hatada et al. 2006), retention (Huang and Shadmehr 2009; Klassen et al. 2005), and generalization (Kluzik et al. 2008; Malfait and Ostry 2004; Torres-Oviedo and Bastian 2012). Generalization, the transfer of information learned in one state to another state that was not previously practiced, has been hypothesized to reveal the coordinate system in which the learned representation is stored (Shadmehr 2004). Such distinctions between abrupt and gradual adaptation and subsequent generalization suggest distinct neural mechanisms for learning based on error size.
This study was originally designed to examine the generalization of motor commands after reaching in an abruptly versus gradually introduced force field. We expected that cerebellar patients would perform better in the gradual field, and that the comparison of their generalization pattern to that of healthy subjects would determine whether the two groups used the same neural mechanism to learn. However, the counterbalancing of field direction in this experiment revealed an unexpected effect of clockwise (CW) versus counterclockwise (CCW) perturbing forces on cerebellar patient performance. For the reaches that we trained, patients could learn the CW field much better than the CCW field (the opposite was true for left-handed subjects). Thus the study was expanded to examine the effects of both dynamics and error size on motor learning. The findings show a larger effect of dynamics (i.e., force field direction) than error size (i.e., force field introduction) on learning in cerebellar patients. When patients did learn, generalization was limited, suggesting that they use a strategy different from control subjects.
MATERIALS AND METHODS
Subjects
Nineteen patients with cerebellar ataxia (Table 1) and fifteen healthy subjects, matched for age, handedness, and sex, participated in the study. The severity of patients' ataxia was rated with the International Cooperative Ataxia Rating Scale (ICARS) (Trouillas et al. 1997). An additional neurological exam, which included tests for reflexes, proprioception, and fine touch using monofilaments, was performed to ensure that patients' symptoms were purely cerebellar. The experimental protocol was approved by the Johns Hopkins University School of Medicine Institutional Review Board, and all subjects signed a consent form prior to participating.
Table 1.
Characteristics of patients with cerebellar damage
| Patient ID | Age, yr | Sex | Handedness | Diagnosis | ICARS Total | ICARS Kinetic |
|---|---|---|---|---|---|---|
| 1 | 39 | M | L | SCA8 | 43 | 16 |
| 2 | 57 | M | R | SCA6 | 64 | 23 |
| 3 | 69 | F | R | ADCA | 20 | 11 |
| 4 | 57 | M | L | Sporadic | 66 | 25 |
| 5 | 57 | M | R | Sporadic | 48 | 26 |
| 6 | 66 | M | R | ADCA | 79 | 34 |
| 7 | 42 | F | R | SCA6 | 50 | 25 |
| 8 | 70 | F | R | ADCA | 62 | 22 |
| 9 | 58 | M | R | Sporadic | 61 | 26 |
| 10 | 49 | M | R | Sporadic | 45 | 25 |
| 11 | 61 | F | R | SCA6 | 31 | 12 |
| 12 | 59 | M | L | SCA6 | 12 | 5 |
| 13 | 63 | F | R | ADCA | 41 | 20 |
| 14 | 59 | F | R | SCA6 | 43 | 21 |
| 15 | 74 | M | R | SCA6 | 52 | 20 |
| 16 | 64 | F | L | Sporadic | 47 | 17 |
| 17 | 59 | F | R | Sporadic | 43 | 23 |
| 18 | 79 | M | R | Sporadic | 38 | 21 |
| 19 | 74 | M | R | Sporadic | 48 | 17 |
ICARS, International Cooperative Ataxia Rating Scale; SCA, spinocerebellar ataxia; ADCA, autosomal dominant cerebellar ataxia.
Patients 1–15 and the matched control subjects participated in the main experiment, in which they adapted reaching in separate sessions to gradual and abrupt force perturbations, where one was a CW and the other a CCW force field. The order and pairing of these factors were counterbalanced across subjects. Patient 1 performed the main experiment twice (separated by 10 mo), receiving four conditions, i.e., abrupt and gradual perturbations each in CW and CCW directions. All other subjects received only two of four possible conditions. Patients 1 and 8, and two matched control subjects, participated in supplemental experiment 1. Patients 1, 8, and 16–19 participated in supplemental experiment 2.
Experimental Task
Experiments were conducted with the KINARM Exoskeleton Lab (BKIN Technologies, Kingston, ON, Canada), an exoskeleton robotic device that allows planar arm movement of the shoulder and elbow joints. Subjects were seated with their dominant arm positioned in the robot. The visual display showed virtual targets and hand position (1-cm-diameter circles) in the same plane as the arm, while visual feedback of the actual arm was hidden. The system recorded arm kinematics and applied joint torques at a rate of 1 kHz.
Each trial began with the subject's hand at the gray start target (Fig. 1). After 0.7 s, a white target appeared 10 cm away. Subjects were instructed to make a fast, continuous reach and “shoot” through the target without stopping. Visual feedback of the hand cursor was displayed throughout the reaching movement. After the hand passed the target, the robot generated a soft virtual wall (with a stiffness of 125 N/m and damping of 4 Ns/m) to bring the subject's arm to rest and then returned the subject's hand to the start target. Cerebellar patients, who typically exhibit slower movement speeds than control subjects, have been shown to perform this shooting task at speeds comparable to those of control subjects (Criscimagna-Hemminger et al. 2010; Tseng et al. 2007). The shooting movement, rather than a point-to-point movement, was chosen to minimize the effect of intention tremor characteristic of cerebellar patients.
Fig. 1.
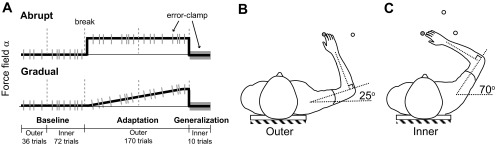
Main experiment setup and protocol. A: subjects participated in 2 sessions, counterbalanced for curl field introduction (abrupt/gradual) and curl field direction [clockwise (CW)/counterclockwise (CCW)]. Using an exoskeleton robot, subjects made reaching movements in a null field (baseline), a curl field that perturbed the hand perpendicular to the direction of motion (adaptation), and an error-clamp force channel (gray bars throughout all blocks; generalization). Dashed lines represent breaks between blocks. B: reach adaptation from the gray start target to a single white target was tested in the outer region of the workspace. C: generalization to 2 different targets (equivalent to the adaptation target in either hand or joint space) was tested in the inner region of the workspace.
Once the subject's hand passed the target, a secondary circle appeared where the hand crossed a boundary of radius 10 cm. If the movement was within the desired duration of 200–400 ms, this circle was green. Movements that were too slow or too fast resulted in a circle that was blue or red, respectively. Target accuracy was rewarded by an “exploding” target, but only if the movement timing was acceptable. The endpoint accuracy (within 2.5° or 5° of the target) determined the size of the explosion (big or small). Subjects were instructed to make timing their first priority, followed by target accuracy.
In null field trials, the robot did not generate any forces. In force field trials, a “curl field” was applied that provided forces perpendicular to the hand motion such that f = Bv, where f is the force applied at the hand, v is the hand velocity, and B = α [0 −13; 13 0] Ns/m. A positive α value corresponds to a CCW curl field, whereas a negative value corresponds to a CW curl field. During the abrupt field introduction α = 1, whereas α linearly increased from 0 to 1 during the gradual field introduction. In error-clamp trials, movement was constrained to a straight path to the target via a force channel (with a wall stiffness of 2,500 N/m). These trials allowed for the measurement of forces subjects produced perpendicular to the direction of movement, which are indicative of changes in their motor output.
Experimental Sessions
Main experiment.
Subjects were tested in two sessions, counterbalanced for both introduction of force (abrupt/gradual) and curl field direction (CW/CCW), separated by at least 1 h to minimize carryover of aftereffects (Fig. 1A). For example, if the first session was in a CW abrupt field, the second session was in a CCW gradual field. Learning of the curl field was assessed during reaches to a single target in the outer region of the workspace (Fig. 1B). The location of the start position was consistent across subjects in joint space (shoulder angle = 25°, elbow angle = 90°). To test how learning generalized to movements made when the arm was in a different configuration (shoulder angle = 70°, elbow angle = 90°), subjects made reaches to targets in the inner region of the workspace (Fig. 1C).
Each session began with a familiarization phase (not recorded), during which subjects became accustomed to completing the task in a null field with the robot. The first recorded block consisted of baseline reaches in the outer region of the workspace (Fig. 1B). Thirty-six reaches were made to a target at 0° (for left-handed subjects, targets were mirrored across the vertical axis), consisting of 32 null field and 4 error-clamp trials (pseudorandomly presented). Next, subjects performed a baseline block in the inner region of the workspace (Fig. 1C). The two targets in the inner region, at 0° and 45°, were equivalent to the outer region target in extrinsic (i.e., equal displacement of the hand in Cartesian space) and intrinsic (i.e., equal displacement of the shoulder and elbow in joint space) coordinates, respectively. The target angles and initial joint configurations were selected to ensure sufficient separation of the two targets in both extrinsic and intrinsic space. A total of 72 reaches were made during the inner baseline block, consisting of 32 null field and 4 error-clamp trials to each of the two targets. Subjects then performed two adaptation blocks, separated by a short break, with the 0° target in the outer region. The first adaptation block began with 5 null field reaches (not recorded), followed by 72 curl field and 8 error-clamp trials. The second adaptation block consisted of 81 curl field and 9 error-clamp trials. The curl field was always at full strength for the abrupt condition. For the gradual condition, the strength of the curl field increased over the two adaptation blocks, where the last 10 trials were at full strength. The last block tested for generalization to the two targets in the inner region, consisting of five error-clamp trials to each target.
Supplemental experiment 1.
Our findings from the main experiment suggested that patients were impaired at making straight reaches in a null field, and their baseline movement bias may have influenced their ability to learn in the CW and CCW fields. This supplemental experiment was performed to examine control subjects' and patients' baseline movement biases while making reaches to different targets. Subjects performed two blocks in the outer region, each consisting of four reaches to 16 different targets (equally spaced by 22.5°) in a null field.
Supplemental experiment 2.
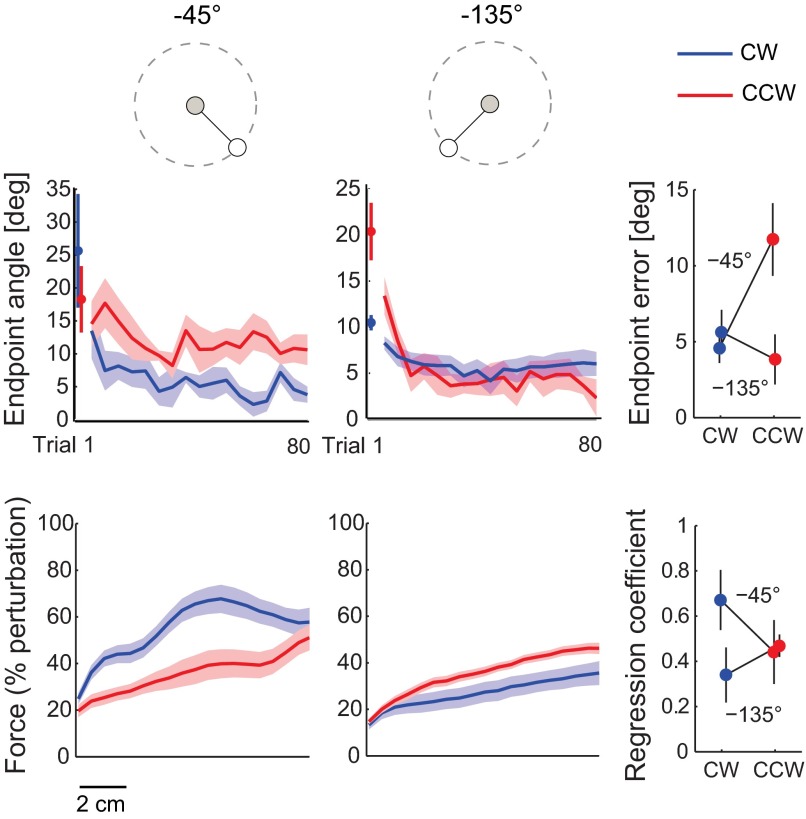
This experiment tested how curl field direction affects patient learning of reaches to target directions where they have different baseline movement biases (supplemental experiment 1). This was a shorter version of the main experiment without the inner region blocks, and patients reached to two different targets (−45° and −135°; see Fig. 9) in the abrupt curl field (CW and CCW). Subjects were tested in four sessions, one for each combination of target angle and field direction, with a 1-h break separating two back-to-back sessions. The back-to-back sessions tested different targets and different field directions. Each session contained a baseline, an adaptation, and a washout block. The baseline block consisted of 28 null field and 6 error-clamp trials. The adaptation block consisted of 5 null field trials (not recorded), followed by 72 curl field and 8 error-clamp trials. Last, null field trials (18 for 3 of the subjects and 30 for the other subjects to ensure complete washout) and six error-clamp trials were performed in the washout block.
Fig. 9.
Patient learning in different directions. Learning to the −45° (left) and −135° (center) targets varies with field direction. Trial-by-trial endpoint angular error (mean ± SE) is different for the CW and CCW force fields (top left and center). The first abrupt trial is plotted separately, and a bin size of 4 trials is subsequently used. The average baseline value was subtracted for each subject. Endpoint angular error relative to baseline during the last 10 adaptation trials (baseline subtracted) reveals an interaction between target and field direction (top right). The force measured during error-clamp trials represented as % of the perturbation (mean ± SE) also supports unequal learning (bottom left and center). The last 3 error-clamp trials during adaptation were sampled every 1 mm, baseline subtracted, averaged for each subject, and normalized by the peak ideal force needed to compensate for the curl field (as calculated from the measured hand velocity during late adaptation). The regression coefficient (mean ± SE) also suggests greater learning in the CW field for the −45° direction but in the CCW field for the −135° direction (bottom right).
Data Analysis
The main kinematic measure was angular error, i.e., the angle between the actual and desired movement direction to the target. We first determined movement onset as the first time the hand speed was above a threshold of 10% of the maximum speed. We then calculated the initial and endpoint angular error (i.e., early and late in the movement). In null and curl field trials, the initial angle was measured between a vector from the hand position at movement onset to the hand position after 150 ms and a vector from the hand position at movement onset to the target. A CCW angle was defined as positive. The endpoint angle was measured between the target and the position where the hand crossed the 10 cm boundary. Data from left-handed subjects were mirrored over the midline for equivalence to those of right-handed subjects in joint space. Importantly, all curl field errors are reported below with the average null field error subtracted, in order to emphasize the changes from baseline performance.
Error-clamp trials were used to quantify the extent to which subjects produced forces that would counter the curl field. In these trials, the force that subjects exerted against the force channel was measured and compared to the ideal force necessary to compensate for the curl field tested during that session by calculating a regression coefficient k, i.e., factual = k × fideal. The ideal force was calculated from the hand velocity in the force channel. To remove the baseline bias, the average force profile from corresponding baseline error-clamp trials was sampled every 1 mm and subtracted from force profiles measured during adaptation and generalization blocks.
Statistical analysis by ANOVA tested factors of diagnosis (control subject or patient), field direction (CW or CCW), and field introduction (abrupt or gradual). When ANOVA was performed on only the abrupt or gradual data from the adaptation blocks, the tested factor was group (CCW: control subjects, CW; CCCW: control subjects, CCW; PCW: patients, CW; PCCW: patients, CCW).
RESULTS
We first tested whether patients who received the CW field paired with gradual introduction (and thus CCW abrupt) differed in ataxia severity from those who received the opposite pairings (CW abrupt, CCW gradual). The ICARS scores (means ± SD) were 43 ± 19 and 52 ± 15, and ANOVA showed that the two groups were not significantly different in terms of severity (P = 0.27).
Directional Bias in the Null Field
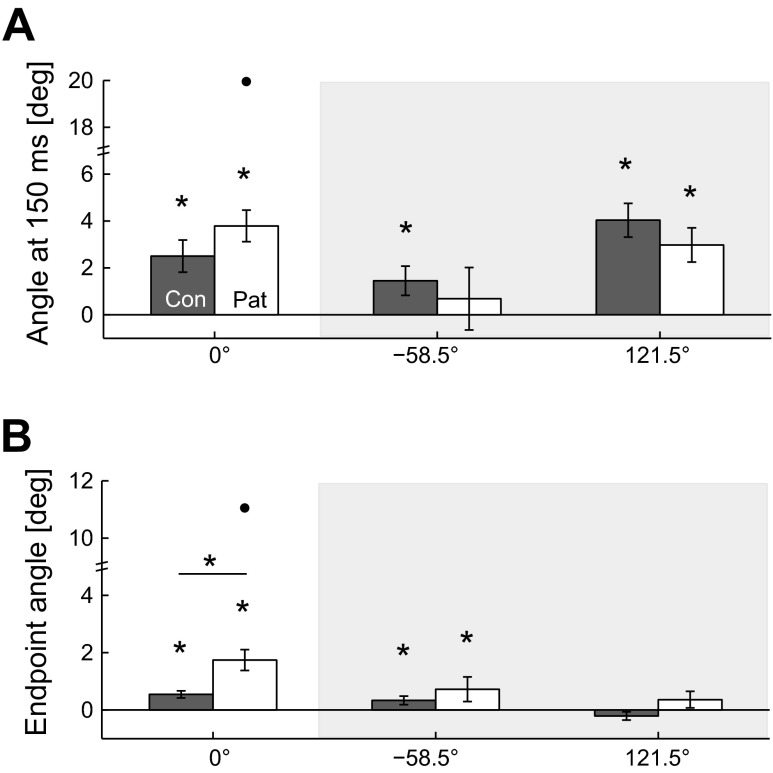
All subjects showed a CCW bias to the 0° target at baseline (Fig. 2). Both control subjects and patients were significantly biased from zero at 150 ms and at the end of the reach (Fig. 2, A and B, left; all t-tests P < 0.005). Patients were more biased than control subjects at the end of the reach (P = 0.018), although there was no statistical significance early in the reach at 150 ms (P = 0.11). We also show a reanalysis of the baseline reaching data for the two targets studied in Criscimagna-Hemminger et al. (2010). Here too, we see CCW biases that are larger during early movement, particularly for the 121.5° target. The implications of this are addressed in discussion.
Fig. 2.
Baseline bias in the null field. Reaches from the present experiment (0° target, white background) and from the Criscimagna-Hemminger et al. (2010) experiment (−58.5° and 121.5°, gray background) were analyzed. For the present experiment, both control subjects (Con) and patients (Pat) have a consistent bias in the CCW direction (positive angle), present early in the reach (A) and at the end of the reach (B). Values are means ± SE of the last 20 null field reaches. A CCW bias is also present in our reanalysis of the reaches of the Criscimagna-Hemminger et al. (2010) study, although not always in both the early and late stages of movement. Values are means ± SE of null field reaches from the second baseline block (32 null field reaches for each target). Note that different robotic systems and initial arm configurations were used in this experiment and the 2010 study. Dots represent outliers (>2 SD from the mean of all subjects). *P < 0.05.
Patients Perform Better in the CW Field, Regardless of Abrupt or Gradual Introduction
During the adaptation blocks of the main experiment, we were surprised to find that patients' performance was affected more by the curl field direction (better with CW vs. worse with CCW) than by the introduction protocol (abrupt vs. gradual). Average hand trajectories for the different groups are shown in Fig. 3 for the beginning, middle, and end of the adaptation blocks (average baseline trajectory subtracted). In the CW field, the adaptation of patients' trajectories is similar to that of control subjects for both the abrupt and gradual conditions. In contrast, patients did not adapt to the CCW field, showing large errors even in late adaptation. This was the case in both the abrupt and gradual conditions.
Fig. 3.
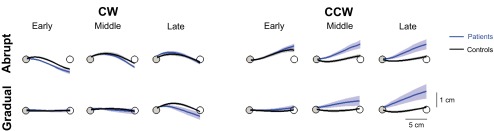
Average hand trajectories. The first 10, middle 20, and last 20 reaches of the adaptation blocks were averaged for the early, middle, and late trajectories (means ± SE), respectively. Trajectories were sampled every 1 mm from the gray start position to the white target (targets not drawn to scale). The average baseline reach was subtracted for each subject.
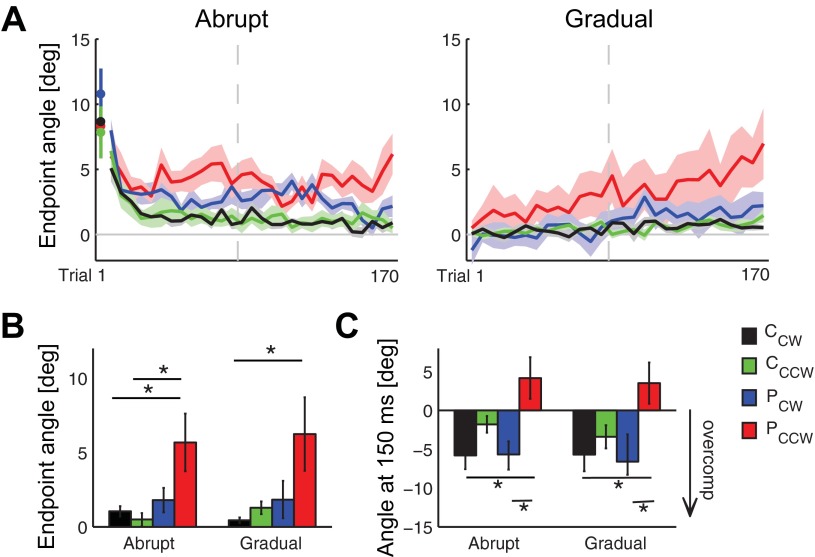
Figure 4 shows group kinematics during adaptation across the different conditions. Figure 4A shows trial-by-trial plots of the endpoint angular error relative to baseline averaged for the different groups. Note that the patients moving in the CCW field have large angular errors (i.e., adapt poorly), regardless of whether they are in the abrupt or gradual group. The patients moving in the CW field performed better, and the control subjects performed best. Figure 4B shows the endpoint error during late adaptation (last 10 trials with average baseline value subtracted). A three-way ANOVA revealed a main effect of diagnosis (P = 0.0015), with patients learning less than control subjects, but no effect of field introduction (abrupt vs. gradual). There was also a main effect of field direction (P = 0.023), with subjects learning more in the CW field than the CCW field, and a diagnosis × field direction interaction (P = 0.034), indicating that patients, more so than control subjects, were affected by the field direction.
Fig. 4.
Kinematic metrics. A: trial-by-trial endpoint angular error (mean ± SE) during abrupt and gradual adaptation is comparable for control subjects in the CW (CCW) and CCW field (CCCW) and patients in the CW field (PCW). Data from the CW field was inverted such that perturbation from the field is positive and overcompensation is negative. The first abrupt trial is plotted separately, and a bin size of 6 trials is subsequently used. The average baseline value was subtracted. The gray dashed line indicates the break between adaptation blocks. Angular error late (B) and early (C) in the movement during the last 10 adaptation trials (mean ± SE) shows that patients in the CCW field (PCCW) have larger errors and fail to overcompensate. The average baseline value was subtracted. Statistics indicate the results of post hoc Tukey's tests (*P < 0.05).
These results were not due to any systematic difference in the perturbation amount or speed of movement. In Fig. 4A, the endpoint error in the first trial of the abrupt curl field is plotted separately for each group. A two-way ANOVA on the magnitude of endpoint error in the first abrupt trial (average baseline endpoint error subtracted) showed no main effects of diagnosis or field direction and no interaction effect (all P > 0.16). Although patients initially showed a CCW bias in the null field, these errors were small compared with the errors induced by the curl field. Thus the curl field perturbation did not immediately straighten out the reaches of the patients. Movement speed, which affected the strength of the velocity-dependent curl field, was also comparable at the end of the adaptation blocks. A three-way ANOVA using the last 10 adaptation trials showed no significant main effects of diagnosis, field direction, or field introduction and no significant interaction effects (all P > 0.08).
Early angular error yielded results similar to the endpoint error—patients performed better when the field was CW and worse when the field was CCW, regardless of whether the presentation was abrupt or gradual. Figure 4C shows the initial angular error at 150 ms during late adaptation (last 10 trials with average baseline value subtracted). Note that the errors for the control subjects are negative in these plots in both the CW and CCW fields. This means that control subjects overcompensate for the field early in movement when the curl field forces are small. This has been suggested to be the optimal strategy to minimize effort during adaptation to a velocity-dependent curl field (Izawa et al. 2008). Similar to control subjects, patients also overcompensate for the CW field as early as 150 ms. However, patients do not overcompensate in the CCW direction, and are instead perturbed by the field in both the abrupt and gradual conditions.
For the early angular error, a three-way ANOVA revealed a main effect of diagnosis (P = 0.019), with patients overcompensating less than control subjects, a main effect of field direction (P < 0.001), with CW better than CCW, and a diagnosis × field direction interaction (P = 0.048), demonstrating that patients compensate less in the CCW field. There was no main effect of field introduction early in the movement, again suggesting that gradual versus abrupt introduction was not the most important factor. These results also suggest that the improved patient performance in the CW field cannot be solely explained by feedback correction.
The distinction between patient performance in the CW and CCW fields is further confirmed by force data from the error-clamp trials (Fig. 5). Throughout the CW adaptation blocks, control subjects and patients both learned to produce similar levels of force against the channel to counteract the perturbation. These results suggest that improved patient performance in the CW field is not simply due to increased cocontraction or correction of their baseline bias by the field perturbation, neither of which would yield force production in error-clamp trials. Thus the patients altered their motor output in order to improve their reaching performance in the CW field. Alternatively, patients produced significantly less force in the opposing direction to counteract the CCW force field than control subjects.
Fig. 5.
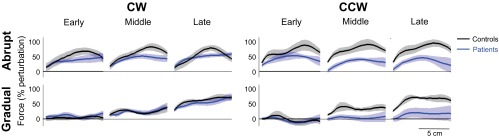
Motor output during error-clamp trials. The measured force is represented as % of the perturbation (normalized by the peak ideal force needed to compensate for the full strength curl field, as calculated from the measured hand velocity during late adaptation). The first 2, middle 3, and last 3 error-clamp trials of the adaptation blocks were averaged for the early, middle, and late force profiles (means ± SE), respectively. Forces applied against the force channel by control subjects and patients were sampled every 1 mm. The average baseline force was subtracted for each subject.
We quantified how well the actual force applied by the subjects matched the ideal force by computing the average regression coefficient from the last three error-clamp trials during adaptation (perfect correspondence results in a value of 1). Regardless of the field conditions, control subjects have regression coefficients of ∼0.8 (Fig. 6A). For patients, the regression coefficient is larger for the CW field, regardless of how the field was introduced. A three-way ANOVA on the regression coefficient revealed a main effect of diagnosis (P = 0.0046), with patients showing lower coefficients than control subjects, but no effect of field introduction. There was also a main effect of field direction (P = 0.020), with CW fields showing higher regression coefficients than CCW, and a diagnosis × field direction interaction (P = 0.024) showing that patients performed better in the CW than the CCW field. Figure 6B shows that patients who were more severely impaired had lower adaptation regression coefficients (R2 = 0.34, P = 0.018) in the abrupt and gradual CCW fields. However, ataxia severity was not a good predictor of the regression coefficient in the CW field because all patients performed well (R2 = 0.0015, P = 0.89).
Fig. 6.
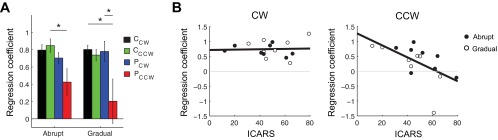
Force metrics during late adaptation. A: group regression coefficient (mean ± SE) showed decreased performance for the PCCW group. Statistics indicate the results of post hoc Tukey's tests (*P < 0.05). B: CW and CCW regression coefficients plotted as a function of ataxia severity [International Cooperative Ataxia Rating Scale (ICARS)]. The regression coefficient was calculated with the last 3 error-clamp trials from the adaptation blocks, sampled every 1 mm.
Patients Use a Different Strategy than Control Subjects to Improve Performance in CW Field
Generalization patterns, which are thought to reflect the structure of the representation acquired during learning, can also provide insight into the process by which improved performance occurs. We used error-clamp trials to two targets in the inner region of the workspace to test whether changes in motor commands transferred in intrinsic or extrinsic coordinates. CW and CCW data were combined for control subjects, as this factor did not make a difference with these subjects.
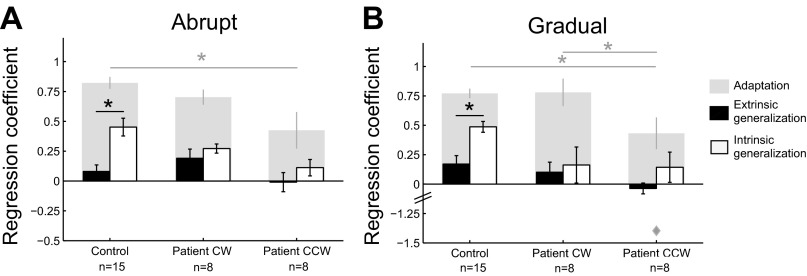
With this scheme, a one-way ANOVA showed a main effect of group for the adaptation regression coefficient in the abrupt (Fig. 7A; P = 0.009) and gradual (Fig. 7B; P = 0.009) conditions (regression coefficients calculated as in Fig. 6). Post hoc Tukey's tests confirmed that patients in the CCW field performed worse than the other groups.
Fig. 7.
Generalization in extrinsic and intrinsic coordinates. For abrupt (A) and gradual (B) adaptation, regression coefficients (means ± SE) are similar for control subjects and patients in the CW field (gray bars, adaptation regression coefficients calculated as in Fig. 6). An outlier in the patient CCW group for gradual adaptation is shown as the gray diamond. Generalization regression coefficients reveal that control subjects generalize more in intrinsic (open bars) than extrinsic (filled bars) coordinates (means ± SE), whereas patients do not show the same generalization pattern. Five error-clamp trials to each target in the generalization block were used to calculate the generalization regression coefficient. *P < 0.05.
Generalization was quantified with a regression coefficient computed from the five error-clamp trials to each of the two generalization targets. Consistent with previous studies, Fig. 7 shows that control subjects had stronger generalization in intrinsic than extrinsic coordinates after both abrupt and gradual adaptation (t-test, both P < 0.0001). The change in motor commands observed during reaches to the intrinsic target suggests that control subjects learned a joint space representation relating torque and joint movement, rather than a relationship between endpoint force and Cartesian hand movement. Importantly, patients did not show the same generalization pattern in the CW field (i.e., generalization to the intrinsic target was not significantly greater than generalization to the extrinsic target) as control subjects in either the abrupt (P = 0.19) or the gradual (P = 0.37) condition, despite performance comparable to control subjects during the adaptation blocks. A two-way ANOVA on the generalization regression coefficient confirmed a significant interaction between group (control subjects vs. patients in the CW field) and coordinate system (extrinsic vs. intrinsic) for the abrupt condition (P = 0.046) but not the gradual condition (P = 0.14). In the CCW field, patients also failed to generalize like the control subjects (abrupt, P = 0.16; gradual, P = 0.14), which was expected since they showed minimal learning.
Supplemental Experiment 1
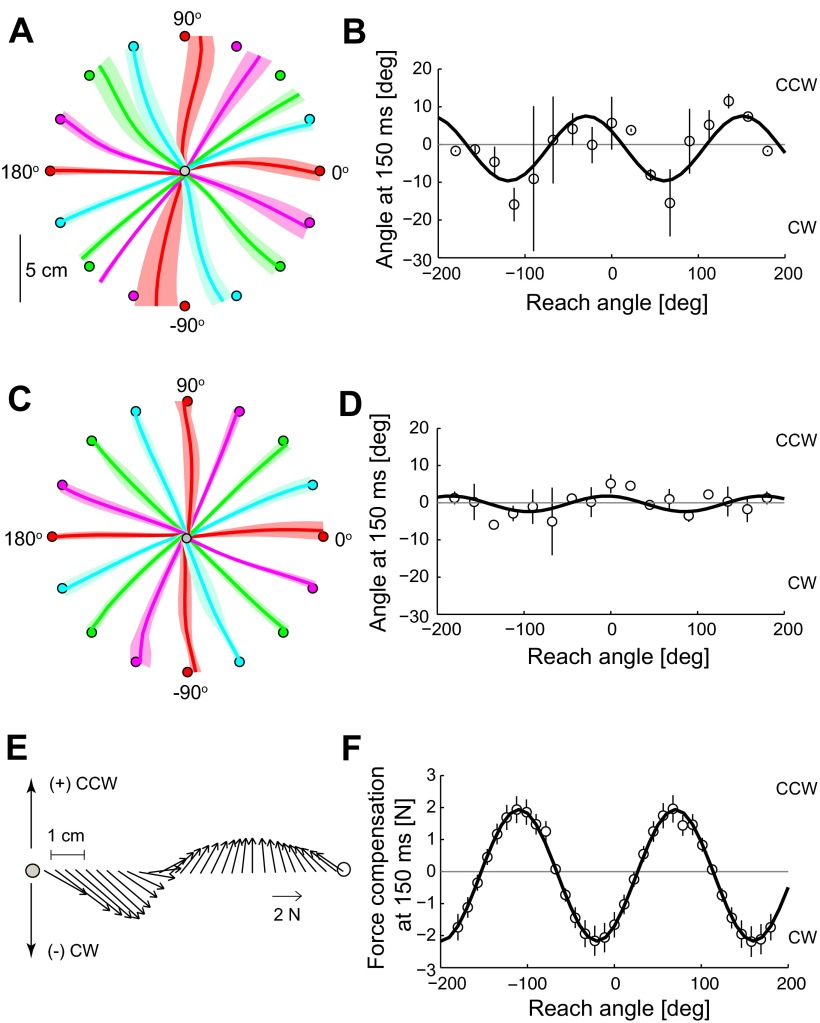
In this experiment, we asked how subjects' baseline movement bias varied with the reach direction, as we suspected that this bias influenced patient performance in different force field directions. The patient in Fig. 8A shows systematic biases that depend on the reach direction. Note that the reach to the 0° target (i.e., 3 o'clock) had a CCW bias; this was the target that was used for adaptation in the main experiment. Figure 8B shows that the bias during early movement, determined by the angular error at 150 ms, can be CW or CCW, depending on the movement direction. The data were fit with a sinusoid with period constrained to 180° (R2 = 0.50). The movements of the control subject shown in Fig. 8C are straighter and more accurate than those of the patients. Figure 8D shows the average control subject biases, noticeably smaller in magnitude than patient biases, fitted with a sinusoid (R2 = 0.25).
Fig. 8.
Movement bias in different directions. A and C: reaches (means ± SD) made by patient 8 (A) and a matched control subject (C) in the null field. Colors match corresponding reaches and targets. B and D: angle at 150 ms (mean ± SD) in different movement directions for 2 patients (B) and 2 control subjects (D), fit with a sinusoid function. A CCW bias is positive. E: an example of the forces required to compensate for arm/robot dynamics and make a straight reach to the 0° target. Initially, CW forces are needed to counteract movement dynamics. F: average force during the initial 150 ms (mean ± SD) required to compensate for dynamic forces perpendicular to movement in different directions, fit with a sinusoid function. Force in the CCW direction is positive.
This systematic bias appears to be related to inadequate compensation of dynamics during movement. We calculated the forces required to make a straight reach in different movement directions, using the dynamic equation of motion for the human and KINARM (see appendix). The dynamic parameters were obtained from manufacturer specifications, direct measurements, and human anthropometric tables. The example in Fig. 8E is a reach to the 0° target. Note that the forces required for subjects to make a straight movement include a component orthogonal to the direction of motion, initially in the CW direction, to counteract the effect of arm and robot dynamics. If this compensation were not present, a CCW kinematic error would occur, as it did for the patient in Fig. 8A. The average perpendicular force within the first 150 ms fluctuates between CW and CCW, depending on the direction of movement (Fig. 8F). A sinusoid with period constrained to 180° was fit to the data (R2 = 0.99). Finally, we noted that the two sinusoidal functions in Fig. 8, B and D, are out of phase with the sinusoidal function in Fig. 8F (with the peaks in Fig. 8, B and D, roughly aligning with the troughs in Fig. 8F), which suggests that the kinematic errors noted were due to a lack of the perpendicular force component for a given reach direction.
Supplemental Experiment 2
Patient performance for various movement directions and field directions appears to be related to how well the curl field counteracts arm/robot dynamics early in movement. For the reach directions in which CW forces are initially required to counteract the effect of arm/robot dynamics (Fig. 8F), we predict that patients would learn better in a CW field. Similarly, we expect better learning in a CCW field for reach directions that require CCW force compensation. We used targets at −45° and −135° (Fig. 9) because the initial forces to counteract arm/robot dynamics are in opposite directions (Fig. 8F). Patients with severe ataxia (ICARS 49 ± 8) were chosen to highlight the effect of field direction on their ability to learn.
As in the main experiment, patients showed unequal learning in the abrupt CW and CCW force fields. However, the direction of the force field in which the patients performed better varied with the reach direction; the CW field was not always better for learning. We used the same endpoint angular error and error-clamp trial analysis as the main experiment. Figure 9, top, shows the trial-by-trial error at the end of the movement, relative to baseline, during the adaptation session. On average, patients were able to decrease their kinematic error more in the CW field than in the CCW field when making reaches toward the −45° target. For the −135° target, patients were initially perturbed more by the CCW field, yet they showed a greater reduction in endpoint error for the CCW than the CW condition. A two-way ANOVA on the endpoint error during late adaptation revealed an interaction between target (−45° vs. −135°) and field direction (CW vs. CCW) (P < 0.001), signifying that the effect of the force field direction varied with the reach direction. There were no significant main effects of target (P = 0.08) or field direction (P = 0.12).
Figure 9, bottom, shows the average force profiles for the group during late adaptation. At the −45° target, patients applied greater forces to counteract the CW perturbation than the CCW perturbation. The opposite is true for the −135° target. As in the main experiment, an adaptation regression coefficient was calculated to quantify how well patients modified their motor commands to compensate for the force field. In a two-way ANOVA on the regression coefficient values, the interaction between target and field direction did not reach significance (P = 0.08). Nevertheless, the trends seen in the force data complement the kinematic result that patients exhibited greater learning in the CW and CCW fields for the −45° and −135° targets, respectively.
DISCUSSION
We have shown that environment dynamics strongly impact how well cerebellar patients can improve their movements in a force field adaptation paradigm. This was an unexpected finding but is intuitive in hindsight. When the force field helps patients compensate for their baseline reaching deficits, they perform better. When the force field adds to their deficits, they perform worse. We were surprised to find no benefit of the gradual over the abrupt introduction of the force field, particularly in light of our previous results. Thus, given favorable environment dynamics, regardless of error size, patients may be able to take advantage of other compensatory strategies to improve their movement.
Arm and Robot Dynamics Affect Patient Movement Performance in Null and Curl Fields
We observed kinematic biases of reaches made in the null field that may be related to inadequate compensation of movement dynamics. The forces required to make a straight reach consist of a component parallel to movement, to accelerate the arm toward the target, and a component perpendicular to movement, to counteract motion resulting from arm/robot dynamics (e.g., inertial and interaction torques). The cerebellum has been hypothesized to house internal models of body dynamics (Wolpert et al. 1998), allowing for accurate prediction and compensation of such dynamic forces. Our findings suggest that cerebellar patients are unable to properly compensate for dynamics during early movement, thus creating biases in predictable directions (supplemental experiment 1). Similar to our observations, Smith and Shadmehr (2005) attributed cerebellar patient biases to deficient compensation of passive robot dynamics. While other studies have reported cerebellar patient movement errors due to both under (Bastian et al. 1996)- and over (Bastian et al. 2000)-compensation of interaction torques, such discrepancies may depend on the task at hand and whether or not a robot was used. The bias of control subjects, albeit smaller than that of patients, may result from incomplete compensation for robot dynamics or simply reflect a strategy that leads to slightly curved reaching (e.g., minimum torque; Uno et al. 1989).
Our patients' ability to counteract the curl field depended on the direction in which dynamics biased their arm movement. While a previous study by Darainy et al. (2008) examined the effect of arm dynamics in different reach directions on adaptation and generalization in healthy subjects, the effect of field direction has not been systematically studied. The field direction that enables patient learning is predicted by the direction in which dynamics compensation is required. For particular reach directions, CW forces are necessary to counteract the kinematic effects of arm/robot dynamics early in movement. Accordingly, patients showed better performance in the CW field in these reach directions (0° and −45° targets). The opposite effect was observed for a reach direction that required CCW force compensation (−135° target). The magnitude of the difference between learning in the CW and CCW fields also seemed to vary with the target, which could reflect the varying complexity of joint coordination and dynamics compensation associated with different movement directions. Moreover, field direction affected adaptation less for control subjects than for patients.
How Does Patients' Performance Improve?
Cerebellar damage impairs the prediction and counteraction of limb dynamics (Bastian et al. 1996). As such, cerebellar patients likely use alternative, less optimal compensatory strategies than control subjects to reduce movement errors. Such strategies, including slowed movement, joint movement decomposition, or the locking of joints, are thought to minimize the effect of dynamics (Baker et al. 2006; Bastian et al. 1996). We previously found that cerebellar patients could not stabilize the shoulder properly when making rapid elbow movements, resulting in large overshooting errors (Bastian et al. 2000). In that study, patients produced shoulder muscle activity that overcompensated for interaction torques caused by elbow motions. When the shoulder was mechanically stabilized, thus eliminating the need for compensation of interaction torques, patients performed better and, importantly, did not activate shoulder muscles. This was in contrast to control subjects, who continued to activate shoulder muscles even when the shoulder was restrained. We hypothesized that the cerebellar patients adopted a strategy of volitional muscle activation when the shoulder was unrestrained, which was easily turned off when it was not needed. In contrast, control subjects may have used a more automatic mechanism at the shoulder that is not easily disengaged when the shoulder was restrained (Debicki and Gribble 2005). While the nervous system is capable of reducing motor commands when additional support from the environment provides stability, e.g., during the maintenance of postural equilibrium (Cordo and Nashner 1982), compensation for arm interaction torques in healthy individuals may be less adjustable.
This finding may help to explain our present results. During null field reaches, patients could have used a similar volitional strategy in an attempt to reduce the effects of arm/robot dynamics that drive the hand off course. When they are exposed to a curl field that helps to offset the effects of limb dynamics, they may relax their compensatory strategy. During error-clamp trials, if patients continue to relax their compensatory strategy, we would observe a force pattern in the same direction as that of control subjects. We speculate that this is different from the adaptive motor output of control subjects, which is thought to reflect an updated cerebellum-dependent internal model of the mapping between motor commands and motion. Finally, it is worth noting that patients' steady performance during the gradual condition and their improved performance throughout the abrupt condition suggest that they can scale and modify the amount of strategic compensation; it is not simply all-or-nothing.
Consistent with our hypothesis regarding different learning mechanisms, we saw very different generalization patterns in control subjects and patients. Control subjects generalize robustly in intrinsic coordinates, which is consistent with previous work and suggests that they are learning and storing an internal model of dynamics in joint space (Shadmehr 2004). The patients, on the other hand, did not show consistent generalization in intrinsic or extrinsic coordinates. This could suggest that their change in behavior was context specific to the target where the curl field was presented, or perhaps a more cognitive strategy with different generalization properties was used (Malfait and Ostry 2004). Thus it seems evident that patients did not learn in the same manner as control subjects.
The level of learning by the cerebellar patients in our study is surprisingly high compared with previous studies (Martin et al. 1996; Morton and Bastian 2006; Smith and Shadmehr 2005). However, the reduced complexity of the single-target protocol is likely to have enabled better patient performance. Similarly, other studies have observed higher levels of cerebellar patient learning during movements that required minimal multijoint coordination, thus simplifying the motor complexity (Maschke et al. 2004; Richter et al. 2004). Previous findings of impaired adaptation in patients included reaches to multiple targets, whereas this tactic of reducing a compensatory strategy does not generally work for all reach directions for a given curl field direction.
Akin to our findings, other adaptation experiments in which cerebellar patients showed improved performance can be related to their cerebellar deficits. Golla et al. (2008) showed asymmetric saccadic adaptation in patients when the location of the visual target was repetitively shifted during the saccade. Patients appeared to adapt to an inward target shift (requiring a decrease in saccade amplitude) but not an outward shift (requiring an increase in saccade amplitude); control subjects adapted to both directions. The patients' apparent inward adaptation was explained by uncompensated oculomotor fatigue, whereas outward adaptation requires active cerebellar compensation of this deterioration. Taylor et al. (2010) showed that patients were able to effectively use an explicit strategy to consistently counteract a visual perturbation during reaching. For control subjects, however, the initial benefit of using an explicit strategy decreased over time, because of interference by an implicit adaptive process based on sensorimotor errors computed by the cerebellum.
Negligible Effect of Error Size on Patient Movement Performance
Contrary to the initial premise of our study, patient performance was not affected by error size, as determined by the abrupt versus gradual curl field introduction. Similarly, there is conflicting evidence for enhanced gradual adaptation in cerebellar patients during visuomotor adaptation. During adaptation to a gradual CCW visuomotor rotation, Izawa et al. (2012) showed comparable learning for control subjects and patients at a single target. Schlerf et al. (2013), however, used the same rotation direction with eight targets and observed impaired patient learning in both the abrupt and gradual conditions. It is not clear, however, how our hypothesis extends to learning of a visuomotor rotation.
We previously reported that severely impaired cerebellar patients (ICARS > 40) improved their performance in the gradually introduced curl field (Criscimagna-Hemminger et al. 2010). However, field direction in that study was not counterbalanced; the gradual and abrupt conditions were always performed in the CW and CCW directions, respectively. We reanalyzed subjects' reaches (from the second baseline block of Criscimagna-Hemminger et al. 2010) to their two targets, using our metrics of angular error at 150 ms and endpoint error. Similar to our present results, we find some evidence of CCW biases at baseline (Fig. 2). For the target at −58.5° (toward the shoulder), reaches were slightly, yet consistently, biased at the endpoint for all subjects, although this bias was not present early in movement for the patients. For the target at 121.5°, all subjects showed a larger CCW bias early in movement, which disappeared by the end of the reach, possibly because of feedback corrections. Although the two experiments used different robots and initial arm configurations, the magnitudes of the early movement bias at the 0° and 121.5° targets were comparable in size. Thus the apparent effect of error size in the earlier study may have been confounded by an effect of dynamics.
Alternatively, differences in the experimental protocols may account for the discrepancy in our results. The smaller endpoint error of baseline reaches in the previous study (Fig. 2B) could be attributed to the longer baseline block and smaller target, effectively placing more emphasis on achieving endpoint accuracy. This focus on accuracy during late movement may have enabled cerebellar patients to use feedback mechanisms to enhance learning in the gradual condition. Another possibility is that the two-target protocol may have been more conducive to revealing the advantage of a gradual perturbation, since learning in multiple directions introduces additional motor control complexity.
Analogous to our present findings, we previously suggested different learning mechanisms for severe patients and control subjects in the gradual field (Criscimagna-Hemminger et al. 2010). Severe patients maintained a persistent aftereffect following curl field learning. (Our present experiment was not designed to test aftereffect duration, though we counterbalanced the sessions to minimize potential effects of interference.) Additionally, severe patients did not overcompensate for the curl field early in movement, despite improved endpoint performance. In the main experiment of our present study, overcompensation (quantified by the angular error at 150 ms) was present during learning for both control subjects and patients (Fig. 4C). As previously explained, patients may have learned to relax a compensatory strategy while exposed to the curl field. Since limb dynamics vary with reach direction, the curl field may not counteract the arm/robot dynamics of all reaches equally, resulting in overcompensation in some directions but not others.
While the effect of error size on learning in cerebellar patients is unclear, its effect on adaptation and generalization in control subjects may be the result of differences in the credit assignment of errors (Berniker and Kording 2008; Torres-Oviedo and Bastian 2012) or the involvement of separate neural mechanisms (Boyden et al. 2006; Robertson and Miall 1999). However, error size does not seem to affect generalization to a different region of the workspace, since control subjects generalized in intrinsic coordinates after both abrupt and gradual adaptation. Although we did not observe a difference between abrupt and gradual force field learning in patients with cerebellar damage, this does not refute the idea that error size affects adaptation in healthy individuals. In conclusion, our results support the notion that adaptation to both small and large errors is based on cerebellum-dependent neural mechanisms, as both are fundamentally disrupted in cerebellar patients.
GRANTS
This work was supported by National Institutes of Health Grants R21 NS-061189 (to A. M. Okamura) and R01 HD-040289 (to A. J. Bastian) and a Link Foundation Fellowship (to T. L. Gibo).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.L.G. conception and design of research; T.L.G. and S.E.C.-H. performed experiments; T.L.G. analyzed data; T.L.G., A.M.O., and A.J.B. interpreted results of experiments; T.L.G. prepared figures; T.L.G. drafted manuscript; T.L.G., S.E.C.-H., A.M.O., and A.J.B. edited and revised manuscript; T.L.G., S.E.C.-H., A.M.O., and A.J.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the cerebellar patients and their families for participating in this study. We thank David I. Grow and Nasir H. Bhapuri for consultation on robot modeling and experiment design. We also thank Reza Shadmehr for helpful discussion of this article and allowing us to reanalyze data from the previous study.
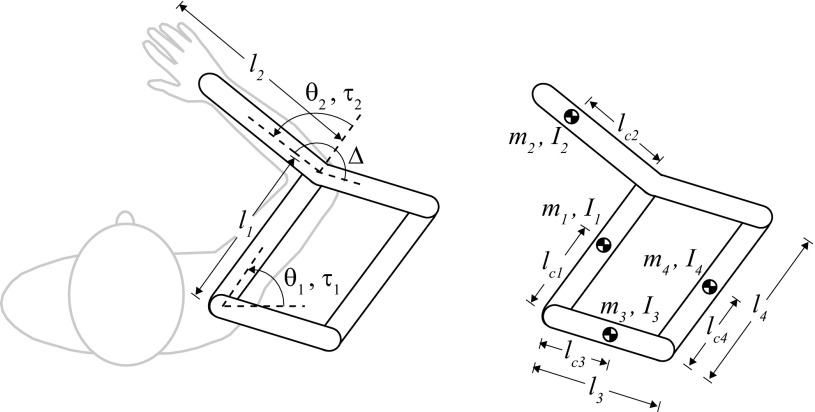
APPENDIX
We used the equation of motion for the human and KINARM robot (derived in Grow 2011) in an inverse dynamics analysis to compute the shoulder and elbow torques necessary to make straight shooting movements toward various target directions. Kinematics (joint position, velocity, and acceleration) from reaches made in error-clamp trials were used as “ideal” shooting movements, as these reaches were nearly perfectly straight. The general equation of motion for the human and KINARM (Fig. 10) is
where Δ = 166° and
The parameters of the robot and arm trays were obtained from manufacturer specifications and direct measurements. The human parameters were estimated with anthropometric tables.
Fig. 10.
Human arm and robot parameters used in the equation of motion.
With the “ideal” shooting movement kinematics and the dynamic equation of motion, the necessary shoulder and elbow torques were calculated. At each time step, the torques were converted to endpoint (hand) forces with the Jacobian
where Fx and Fy are forces at the hand in the x- and y-directions, respectively.
REFERENCES
- Baker M, Allum JH, Visser JE, Gruneberg C, van de Warrenburg BP, Kremer BH, Bloem BR. Postural responses to multidirectional stance perturbations in cerebellar ataxia. Exp Neurol 202: 21–35, 2006 [DOI] [PubMed] [Google Scholar]
- Bastian AJ, Martin TA, Keating JG, Thach WT. Cerebellar ataxia: abnormal control of interaction torques across multiple joints. J Neurophysiol 76: 492–509, 1996 [DOI] [PubMed] [Google Scholar]
- Bastian AJ, Zackowski KM, Thach WT. Cerebellar ataxia: torque deficiency or torque mismatch between joints? J Neurophysiol 83: 3019–3030, 2000 [DOI] [PubMed] [Google Scholar]
- Berniker M, Kording K. Estimating the sources of motor errors for adaptation and generalization. Nat Neurosci 11: 1454–1461, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Katoh A, Pyle JL, Chatila TA, Tsien RW, Raymond JL. Selective engagement of plasticity mechanisms for motor memory storage. Neuron 51: 823–834, 2006 [DOI] [PubMed] [Google Scholar]
- Cordo PJ, Nashner LM. Properties of postural adjustments associated with rapid arm movements. J Neuropysiol 47: 287–302, 1982 [DOI] [PubMed] [Google Scholar]
- Criscimagna-Hemminger SE, Bastian AJ, Shadmehr R. Size of error affects cerebellar contributions to motor learning. J Neurophysiol 103: 2275–2284, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darainy M, Mattar AA, Ostry DJ. Effects of human arm impedance on dynamics learning and generalization. J Neurophysiol 101: 3158–3168, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debicki DB, Gribble P. Persistence of inter-joint coupling during single-joint elbow flexions after shoulder fixation. Exp Brain Res 163: 252–257, 2005 [DOI] [PubMed] [Google Scholar]
- Golla H, Tziridis K, Haarmeier T, Catz N, Barash S, Their P. Reduced saccadic resilience and impaired saccadic adaptation due to cerebellar disease. Eur J Neurosci 27: 132–144, 2008 [DOI] [PubMed] [Google Scholar]
- Grow DI. Robotic Assistance for Rehabilitation of Coordination Deficits (Dissertation) Baltimore, MD: Johns Hopkins University, 2011. Ann Arbor, MI: ProQuest/UMI, 2011 [Google Scholar]
- Hatada Y, Miall RC, Rossetti Y. Two waves of a long-lasting aftereffect of prism adaptation measured over 7 days. Exp Brain Res 169: 417–426, 2006 [DOI] [PubMed] [Google Scholar]
- Huang VS, Shadmehr R. Persistence of motor memories reflects statistics of the learning event. J Neurophysiol 102: 931–940, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa J, Criscimagna-Hemminger SE, Shadmehr R. Cerebellar contributions to reach adaptation and learning sensory consequences of action. J Neurosci 32: 4230–4239, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa J, Rane T, Donchin O, Shadmehr R. Motor adaptation as a process of reoptimization. J Neurosci 28: 2883–2891, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen J, Tong C, Flanagan JR. Learning and recall of incremental kinematic and dynamic sensorimotor transformations. Exp Brain Res 164: 250–259, 2005 [DOI] [PubMed] [Google Scholar]
- Kluzik J, Diedrichsen J, Shadmehr R, Bastian AJ. Reach adaptation: what determines whether we learn an internal model of the tool or adapt the model of our arm? J Neurophysiol 100: 1455–1464, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfait N, Ostry DJ. Is interlimb transfer of force-field adaptation a cognitive response to the sudden introduction of load? J Neurosci 24: 8084–8089, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Keathing JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain 119: 1183–1198, 1996 [DOI] [PubMed] [Google Scholar]
- Maschke M, Gomez CM, Ebner TJ, Konczak J. Hereditary cerebellar ataxia progressively impairs force adaptation during goal-directed arm movements. J Neurophysiol 91: 230–238, 2004 [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci 26: 9107–9116, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter S, Maschke M, Timmann D, Konczak J, Kalenscher T, Illenberger AR, Kalveram KT. Adaptive motor behavior of cerebellar patients during exposure to unfamiliar external forces. J Motor Behav 36: 28–38, 2004 [DOI] [PubMed] [Google Scholar]
- Robertson EM, Miall RC. Visuomotor adaptation during inactivation of the dentate nucleus. Neuroreport 10: 1029–1034, 1999 [DOI] [PubMed] [Google Scholar]
- Schlerf JE, Xu J, Klemfuss NM, Griffiths TL, Ivry RB. Individuals with cerebellar degeneration show similar adaptation deficits with large and small visuomotor errors. J Neurophysiol 109: 1164–1173, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighofer N, Arbib MA, Kawato M. Role of the cerebellum in reaching movements in humans. I. Distributed inverse dynamics control. Eur J Neurosci 10: 86–94, 1998 [DOI] [PubMed] [Google Scholar]
- Shadmehr R. Generalization as a behavioral window to the neural mechanisms of learning internal models. Hum Mov Sci 23: 543–568, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington's disease but not cerebellar degeneration. J Neurophysiol 93: 2809–2821, 2005 [DOI] [PubMed] [Google Scholar]
- Taylor JA, Klemfuss NM, Ivry RB. An explicit strategy prevails when the cerebellum fails to compute movement errors. Cerebellum 9: 580–586, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topka H, Konczak J, Dichgans J. Coordination of multi-joint arm movements in cerebellar ataxia: analysis of hand and angular kinematics. Exp Brain Res 119: 483–492, 1998 [DOI] [PubMed] [Google Scholar]
- Torres-Oviedo G, Bastian AJ. Natural error patterns enable transfer of motor learning to novel contexts. J Neurophyiol 107: 346–356, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, Bryer A, Diener HC, Massaquoi S, Gomez CM, Coutinho P, Ben Hamida M, Campanella G, Filla A, Schut L, Timann D, Honnorat J, Nighoghossian N, Manyam B. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci 145: 205–211, 1997 [DOI] [PubMed] [Google Scholar]
- Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol 98: 54–62, 2007 [DOI] [PubMed] [Google Scholar]
- Uno Y, Kawato M, Suzuki R. Formation and control of optimal trajectory in human multijoint arm movement. Biol Cybern 61: 89–101, 1989 [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci 2: 338–347, 1998 [DOI] [PubMed] [Google Scholar]
- Wong AL, Shelhamer M. Saccade adaptation improves in response to a gradually introduced stimulus perturbation. Neurosci Lett 500: 207–211, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]