Full text
PDF
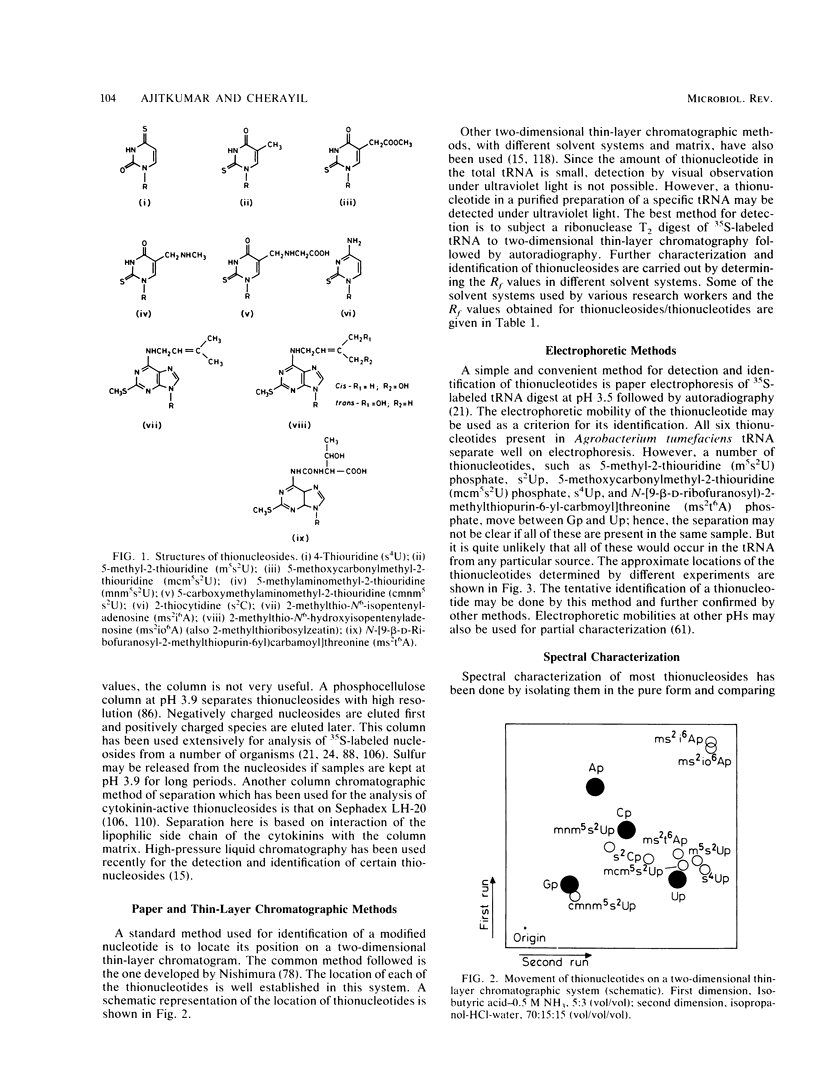
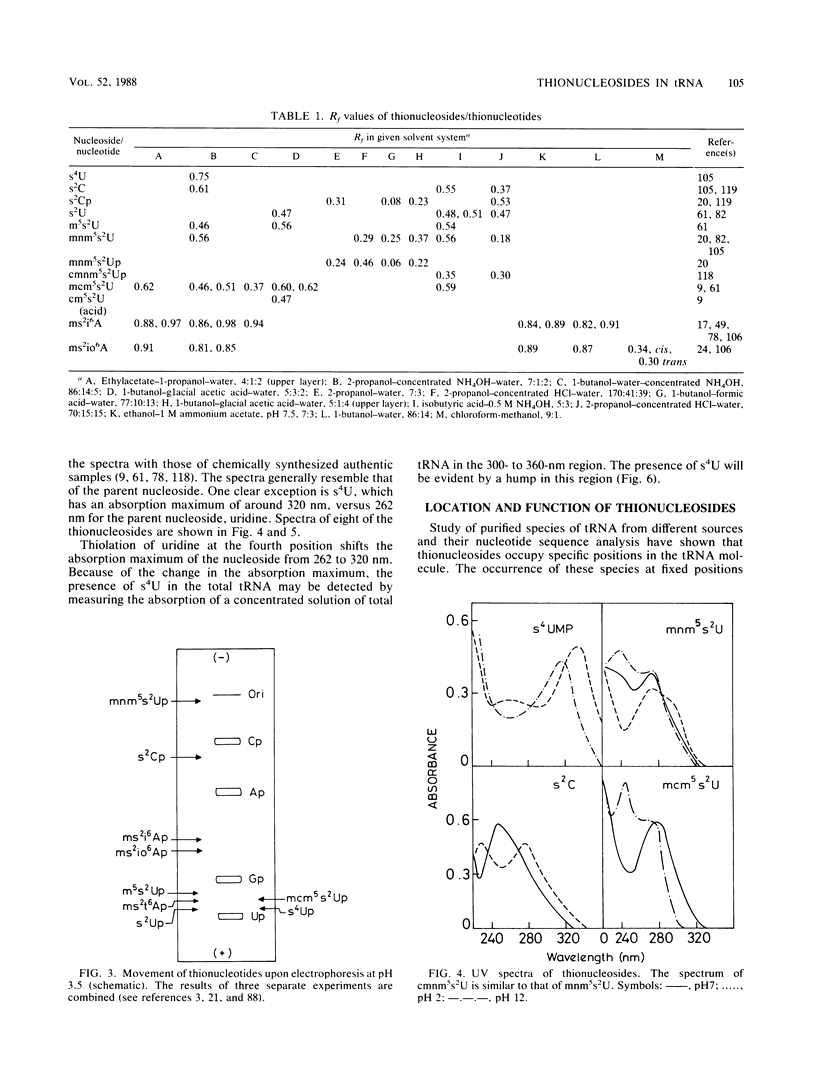
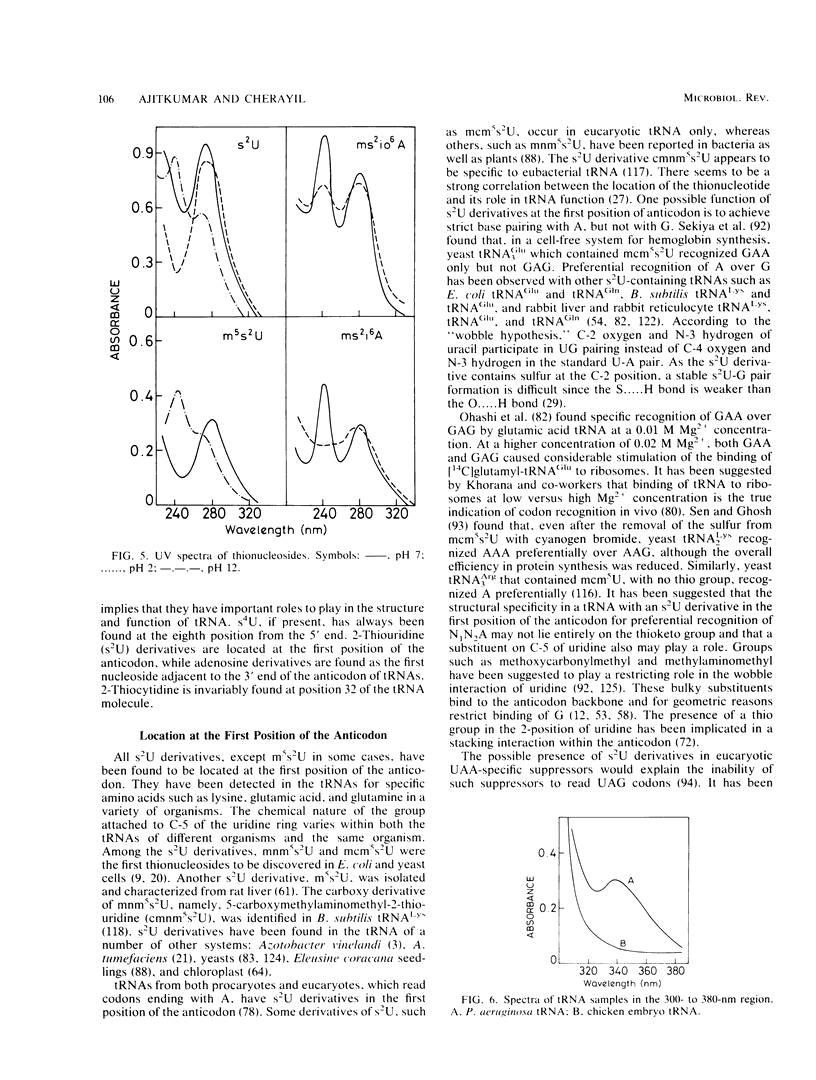







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agris P. F., Armstrong D. J., Schäfer K. P., Söll D. Maturation of a hypermodified nucleoside in transfer RNA. Nucleic Acids Res. 1975 May;2(5):691–698. doi: 10.1093/nar/2.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajitkumar P., Cherayil J. D. Presence of 2-methylthioribosyl-trans-zeatin in Azotobacter vinelandii tRNA. J Bacteriol. 1985 May;162(2):752–755. doi: 10.1128/jb.162.2.752-755.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. J., Burrows W. J., Skoog F., Roy K. L., Söll D. Cytokinins: distribution in transfer RNA species of Escherichia coli. Proc Natl Acad Sci U S A. 1969 Jul;63(3):834–841. doi: 10.1073/pnas.63.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. J., Evans P. K., Burrows W. J., Skoog F., Petit J. F., Dahl J. L., Steward T., Strominger J. L., Leonard N. J., Hecht S. M. Cytokinins. Activity and identification in Staphylococcus epidermidis transfer ribonucleic acid. J Biol Chem. 1970 Jun 10;245(11):2922–2926. [PubMed] [Google Scholar]
- Arnold H. H., Raettig R. Isoaccepting phenylalanine tRNAs from Bacillus subtilis as a function of growth conditions. Differences in the content of modified nucleosides. FEBS Lett. 1977 Feb 1;73(2):210–214. doi: 10.1016/0014-5793(77)80983-6. [DOI] [PubMed] [Google Scholar]
- Baczynskyj L., Biemann K., Hall R. H. Sulfur-containing nucleoside from yeast transfer ribonucleic acid: 2-thio-5(or 6)-uridine acetic acid methyl ester. Science. 1968 Mar 29;159(3822):1481–1483. doi: 10.1126/science.159.3822.1481. [DOI] [PubMed] [Google Scholar]
- Bartz J. K., Söll D. N 6 -( 2 -isopentenyl) adenosine: biosynthesis in vitro in transfer RNA by an enzyme purified from Escherichia coli. Biochimie. 1972;54(1):31–39. doi: 10.1016/s0300-9084(72)80035-x. [DOI] [PubMed] [Google Scholar]
- Bartz J., Söll D., Burrows W. J., Skoog F. Identification of the cytokinin-active ribonucleosides in pure Escherichia coli tRNA species. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1448–1453. doi: 10.1073/pnas.67.3.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman H. M., Marcu D., Narayanan P. Modified bases in tRNA: the structures of 5-carbamoylmethyl- and 5-carboxymethyl uridine. Nucleic Acids Res. 1978 Mar;5(3):893–903. doi: 10.1093/nar/5.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck M., Ames B. N. A modified nucleotide in tRNA as a possible regulator of aerobiosis: synthesis of cis-2-methyl-thioribosylzeatin in the tRNA of Salmonella. Cell. 1984 Feb;36(2):523–531. doi: 10.1016/0092-8674(84)90245-9. [DOI] [PubMed] [Google Scholar]
- Buck M., Griffiths E. Regulation of aromatic amino acid transport by tRNA: role of 2-methylthio-N6-(delta2-isopentenyl)-adenosine. Nucleic Acids Res. 1981 Jan 24;9(2):401–414. doi: 10.1093/nar/9.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck M., McCloskey J. A., Basile B., Ames B. N. cis 2-Methylthio-ribosylzeatin (ms2io6A) is present in the transfer RNA of Salmonella typhimurium, but not Escherichia coli. Nucleic Acids Res. 1982 Sep 25;10(18):5649–5662. doi: 10.1093/nar/10.18.5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows W. J., Armstrong D. J., Kaminek M., Skoog F., Bock R. M., Hecht S. M., Dammann L. G., Leonard N. J., Occolowitz J. Isolation and identification of four cytokinins from wheat germ transfer ribonucleic acid. Biochemistry. 1970 Apr 28;9(9):1867–1872. doi: 10.1021/bi00811a001. [DOI] [PubMed] [Google Scholar]
- Burrows W. J., Armstrong D. J., Skoog F., Hecht S. M., Boyle J. T., Leonard N. J., Occolowitz J. The isolation and identification of two cytokinins from Escherichia coli transfer ribonucleic acids. Biochemistry. 1969 Jul;8(7):3071–3076. doi: 10.1021/bi00835a057. [DOI] [PubMed] [Google Scholar]
- Buu A., Menichi B., Heyman T. Thiomethylation of tyrosine transfer ribonucleic acid is associated with initiation of sporulation in Bacillus subtilis: effect of phosphate concentration. J Bacteriol. 1981 May;146(2):819–822. doi: 10.1128/jb.146.2.819-822.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon J., David H., Studier M. H. Thiobases in Escherchia coli Transfer RNA: 2-Thiocytosine and 5-Methylaminomethyl-2-thiouracil. Science. 1968 Sep 13;161(3846):1146–1147. doi: 10.1126/science.161.3846.1146. [DOI] [PubMed] [Google Scholar]
- Chapman R. W., Morris R. O., Zaerr J. B. Occurrence of trans-ribosylzeatin in Agrobacterium tumefaciens tRNA. Nature. 1976 Jul 8;262(5564):153–154. doi: 10.1038/262153a0. [DOI] [PubMed] [Google Scholar]
- Cherayil J. D., Lipsett M. N. Zeatin ribonucleosides in the transfer ribonucleic acid of Rhizobium leguminosarum, Agrobacterium tumefaciens, Corynebacterium fascians, and Erwinia amylovora. J Bacteriol. 1977 Sep;131(3):741–744. doi: 10.1128/jb.131.3.741-744.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chheda G. B., Hall R. H., Mozejko J., Magrath D. I., Schweizer M. P., Stasiuk L., Taylor P. R. Aminoacyl nucleosides. VI. Isolation and preliminary characterization of threonyladenine derivatives from transfer ribonucleic acid. Biochemistry. 1969 Aug;8(8):3278–3282. doi: 10.1021/bi00836a022. [DOI] [PubMed] [Google Scholar]
- Chheda G. B., Hong C. I., Piskorz C. F., Harmon G. A. Biosynthesis of N-(purin-6-ylcarbamoyl)-L-threonine riboside. Incorporation of L-threonine in vivo into modified nucleoside of transfer ribonucleic acid. Biochem J. 1972 Apr;127(3):515–519. doi: 10.1042/bj1270515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark B. F. Correlation of biological activities with structural features of transfer RNA. Prog Nucleic Acid Res Mol Biol. 1977;20:1–19. doi: 10.1016/s0079-6603(08)60468-7. [DOI] [PubMed] [Google Scholar]
- Cortese R., Landsberg R., Haar R. A., Umbarger H. E., Ames B. N. Pleiotropy of hisT mutants blocked in pseudouridine synthesis in tRNA: leucine and isoleucine-valine operons. Proc Natl Acad Sci U S A. 1974 May;71(5):1857–1861. doi: 10.1073/pnas.71.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue J. On N-H--S hydrogen bonds. J Mol Biol. 1969 Oct 28;45(2):231–235. doi: 10.1016/0022-2836(69)90102-8. [DOI] [PubMed] [Google Scholar]
- Edwards C. A., Armstrong D. J. Cytokinin-Active Ribonucleosides in Phaseolus RNA: I. IDENTIFICATION IN tRNA FROM ETIOLATED PHASEOLUS VULGARIS L. SEEDLINGS. Plant Physiol. 1981 Jun;67(6):1181–1184. doi: 10.1104/pp.67.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C. A., Armstrong D. J. Cytokinin-Active Ribonucleosides in Phaseolus RNA: II. DISTRIBUTION IN tRNA SPECIES FROM ETIOLATED P. VULGARIS L. SEEDLINGS. Plant Physiol. 1981 Jun;67(6):1185–1189. doi: 10.1104/pp.67.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einset J. W., Skoog F. K. Isolation and identification of ribosyl-cis-zeatin from transfer RNA of Corynebacterium fascians. Biochem Biophys Res Commun. 1977 Dec 21;79(4):1117–1121. doi: 10.1016/0006-291x(77)91121-4. [DOI] [PubMed] [Google Scholar]
- Eisenberg S. P., Yarus M., Soll L. The effect of an Escherichia coli regulatory mutation on transfer RNA structure. J Mol Biol. 1979 Nov 25;135(1):111–126. doi: 10.1016/0022-2836(79)90343-7. [DOI] [PubMed] [Google Scholar]
- Eliceiri G. L. Incorporation of 35S into mammalian 4-S RNA. Biochim Biophys Acta. 1970;209(2):387–395. doi: 10.1016/0005-2787(70)90736-7. [DOI] [PubMed] [Google Scholar]
- Elkins B. N., Keller E. B. The enzymatic synthesis of N-(purin-6-ylcarbamoyl)threonine, an anticodon-adjacent base in transfer ribonucleic acid. Biochemistry. 1974 Oct 22;13(22):4622–4628. doi: 10.1021/bi00719a024. [DOI] [PubMed] [Google Scholar]
- Feldmann H., Falter H. Transfer ribonucleic acid from Mycoplasma laidlawii A. Eur J Biochem. 1971 Feb;18(4):573–581. doi: 10.1111/j.1432-1033.1971.tb01278.x. [DOI] [PubMed] [Google Scholar]
- Fittler F., Kline L. K., Hall R. H. N6-(Delta 2-isopentenyl)adenosine: biosynthesis in vitro by an enzyme extract from yeast and rat liver. Biochem Biophys Res Commun. 1968 May 23;31(4):571–576. doi: 10.1016/0006-291x(68)90516-0. [DOI] [PubMed] [Google Scholar]
- Gauss D. H., Sprinzl M. Compilation of tRNA sequences. Nucleic Acids Res. 1983 Jan 11;11(1):r1–53. [PMC free article] [PubMed] [Google Scholar]
- Gefter M. L., Russell R. L. Role modifications in tyrosine transfer RNA: a modified base affecting ribosome binding. J Mol Biol. 1969 Jan 14;39(1):145–157. doi: 10.1016/0022-2836(69)90339-8. [DOI] [PubMed] [Google Scholar]
- Gefter M. L. The in vitro synthesis of 2'-omethylguanosine and 2-methylthio 6N (gamma,gamma, dimethylallyl) adenosine in transfer RNA of Escherichia coli. Biochem Biophys Res Commun. 1969 Aug 7;36(3):435–441. doi: 10.1016/0006-291x(69)90583-x. [DOI] [PubMed] [Google Scholar]
- Goehler B., Doi R. H. Presence and function of sulur-containing transfer ribonucleic acid of Bacillus subtilis. J Bacteriol. 1968 Mar;95(3):793–800. doi: 10.1128/jb.95.3.793-800.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman H. M., Abelson J., Landy A., Brenner S., Smith J. D. Amber suppression: a nucleotide change in the anticodon of a tyrosine transfer RNA. Nature. 1968 Mar 16;217(5133):1019–1024. doi: 10.1038/2171019a0. [DOI] [PubMed] [Google Scholar]
- Guillemaut P., Martin R., Weil J. H. Purification and base composition of a chloroplastic tRANphe from Phaseolus vulgaris. FEBS Lett. 1976 Apr 1;63(2):273–277. doi: 10.1016/0014-5793(76)80110-x. [DOI] [PubMed] [Google Scholar]
- Hall R. H. N6-(delta 2-isopentenyl)adenosine: chemical reactions, biosynthesis, metabolism, and significance to the structure and function of tRNA. Prog Nucleic Acid Res Mol Biol. 1970;10:57–86. doi: 10.1016/s0079-6603(08)60561-9. [DOI] [PubMed] [Google Scholar]
- Harada F., Gross H. J., Kimura F., Chang S. H., Nishimura S., RajBhandary U. L. 2-Methylthio N6-(delta 2-isopentenyl) adenosine: a component of E. coli tyrosine transfer RNA. Biochem Biophys Res Commun. 1968 Oct 24;33(2):299–306. doi: 10.1016/0006-291x(68)90784-5. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Fisher H., Söll D. Transfer ribonucleic acid from Mycoplasma. Biochemistry. 1969 Sep;8(9):3680–3686. doi: 10.1021/bi00837a028. [DOI] [PubMed] [Google Scholar]
- Hecht S. M., Leonard N. J., Burrows W. J., Armstrong D. J., Skoog F., Occolowitz J. Cytokinin of wheat germ transfer RNA: 6-(4-hydroxy-3-methyl-2-butenylamino)-2-methylthio-9-beta-D-ribofuranosylpurine. Science. 1969 Dec 5;166(3910):1272–1274. doi: 10.1126/science.166.3910.1272. [DOI] [PubMed] [Google Scholar]
- Hecker L. I., Uziel M., Barnett W. E. Comparative base compositions of chloroplast and cytoplasmic tRNAPhe's from Euglena gracilis. Nucleic Acids Res. 1976 Feb;3(2):371–380. doi: 10.1093/nar/3.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillen W., Egert E., Lindner H. J., Gassen H. G. Crystal and molecular structure of 2-thio-5 carboxymethyluridine and its methyl ester: helix terminator nucleosides in the first position of some anticodons. Biochemistry. 1978 Nov 28;17(24):5314–5320. doi: 10.1021/bi00617a036. [DOI] [PubMed] [Google Scholar]
- Hilse K., Rudloff E. Glutamine cognate codons in rabbit haemoglobin mRNAs. FEBS Lett. 1975 Dec 15;60(2):380–383. doi: 10.1016/0014-5793(75)80753-8. [DOI] [PubMed] [Google Scholar]
- Hoburg A., Aschhoff H. J., Kersten H., Manderschied U., Gassen H. G. Function of modified nucleosides 7-methylguanosine, ribothymidine, and 2-thiomethyl-N6-(isopentenyl)adenosine in procaryotic transfer ribonucleic acid. J Bacteriol. 1979 Nov;140(2):408–414. doi: 10.1128/jb.140.2.408-414.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikura H., Nishimura S. Fractionation of serine transfer ribonucleic acids from Escherichia coli and their coding properties. Biochim Biophys Acta. 1968 Jan 29;155(1):72–81. doi: 10.1016/0005-2787(68)90336-5. [DOI] [PubMed] [Google Scholar]
- Janzer J. J., Raney J. P., McLennan B. D. The transfer RNA of certain Enterobacteriacae contain 2-methylthiozeatin riboside (ms2io6A) an isopentenyl adenosine derivative. Nucleic Acids Res. 1982 Sep 25;10(18):5663–5672. doi: 10.1093/nar/10.18.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Nishimura S., Vorbrüggen H., Iitaka Y. Crystal and molecular structure of the acetonide of 5-methylaminomethyl-2-thiouridine: a minor constituent of Escherichia coli tRNAs. FEBS Lett. 1979 Jul 15;103(2):270–273. doi: 10.1016/0014-5793(79)81343-5. [DOI] [PubMed] [Google Scholar]
- Keith G., Rogg H., Dirheimer G., Menichi B., Heyham T. Post-transcriptional modification of tyrosine tRNA as a function of growth in Bacillus subtilis. FEBS Lett. 1976 Jan 15;61(2):120–123. doi: 10.1016/0014-5793(76)81017-4. [DOI] [PubMed] [Google Scholar]
- Kim S. H. Three-dimensional structure of transfer RNA and its functional implications. Adv Enzymol Relat Areas Mol Biol. 1978;46:279–315. doi: 10.1002/9780470122914.ch4. [DOI] [PubMed] [Google Scholar]
- Kimura-Harada F., Saneyoshi M., Nishimura S. 5-methyl-2-thiouridine: A new sulfur-containing minor constituent from rat liver glutamic acid and lysine tRNAs. FEBS Lett. 1971 Apr 2;13(6):335–338. doi: 10.1016/0014-5793(71)80254-5. [DOI] [PubMed] [Google Scholar]
- Kline L. K., Fittler F., Hall R. H. N6-(delta-2-isopentenyl) adenosine. Biosynthesis in transfer ribonucleic acid in vitro. Biochemistry. 1969 Nov;8(11):4361–4371. doi: 10.1021/bi00839a021. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Seno T., Nishimura S. Fragmented E. coli methionine tRNA f as methyl acceptor for rat liver tRNA methylase: alteration of the site of methylation by the conformational change of tRNA structure resulting from fragmentation. Biochem Biophys Res Commun. 1971 May 7;43(3):476–483. doi: 10.1016/0006-291x(71)90638-3. [DOI] [PubMed] [Google Scholar]
- Körner A., Söll D. N-(purin-6-ylcarbamoyl)threonine: biosynthesis in vitro in transfer RNA by an enzyme purified from Escherichia coli. FEBS Lett. 1974 Mar 1;39(3):301–306. doi: 10.1016/0014-5793(74)80135-3. [DOI] [PubMed] [Google Scholar]
- Laten H., Gorman J., Bock R. M. Isopentenyladenosine deficient tRNA from an antisuppressor mutant of Saccharomyces cerevisiae. Nucleic Acids Res. 1978 Nov;5(11):4329–4342. doi: 10.1093/nar/5.11.4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsett M. N., Doctor B. P. Studies on tyrosine transfer ribonucleic acid, a sulfur-rich species from Escherichia coli. J Biol Chem. 1967 Sep 25;242(18):4072–4077. [PubMed] [Google Scholar]
- Lipsett M. N. Enzymes producing 4-thiouridine in Escherichia coli tRNA: approximate chromosomal locations of the genes and enzyme activities in a 4-thiouridine-deficient mutant. J Bacteriol. 1978 Sep;135(3):993–997. doi: 10.1128/jb.135.3.993-997.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsett M. N. The isolation of 4-thiouridylic acid from the soluble ribonucleic acid of Escherichia coli. J Biol Chem. 1965 Oct;240(10):3975–3978. [PubMed] [Google Scholar]
- Litwack M. D., Peterkofsky A. Transfer ribonucleic acid deficient in N6-(delta 2-isopentenyl)adenosine due to mevalonic acid limitation. Biochemistry. 1971 Mar 16;10(6):994–1001. doi: 10.1021/bi00782a010. [DOI] [PubMed] [Google Scholar]
- MacLennan B. D. Enzymatic demodification of transfer RNA species containing N-6-(delta-2-isopentenyl)adenosine. Biochem Biophys Res Commun. 1975 Jul 8;65(1):345–351. doi: 10.1016/s0006-291x(75)80099-4. [DOI] [PubMed] [Google Scholar]
- Mazumdar S. K., Saenger W. Molecular structure of poly-2-thiouridylic acid, a double helix with non-equivalent polynucleotide chains. J Mol Biol. 1974 May 15;85(2):213–219. doi: 10.1016/0022-2836(74)90361-1. [DOI] [PubMed] [Google Scholar]
- Menichi B., Heyman T. Study of tyrosine transfer ribonucleic acid modification in relation to sporulation in Bacillus subtilis. J Bacteriol. 1976 Jul;127(1):268–280. doi: 10.1128/jb.127.1.268-280.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. P., Hussain Z., Schweizer M. P. The involvement of the anticodon adjacent modified nucleoside N-(9-(BETA-D-ribofuranosyl) purine-6-ylcarbamoyl)-threonine in the biological function of E. coli tRNAile. Nucleic Acids Res. 1976 May;3(5):1185–1201. doi: 10.1093/nar/3.5.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. O., Regier D. A., Olson R. M., Jr, Struxness L. A., Armstrong D. J. Distribution of cytokinin-active nucleosides in isoaccepting transfer ribonucleic acids from Agrobacterium tumefaciens. Biochemistry. 1981 Oct 13;20(21):6012–6017. doi: 10.1021/bi00524a014. [DOI] [PubMed] [Google Scholar]
- Murai N., Armstrong D. J., Skoog F. Incorporation of mevalonic Acid into ribosylzeatin in tobacco callus ribonucleic Acid preparations. Plant Physiol. 1975 May;55(5):853–858. doi: 10.1104/pp.55.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S. Minor components in transfer RNA: their characterization, location, and function. Prog Nucleic Acid Res Mol Biol. 1972;12:49–85. [PubMed] [Google Scholar]
- Nishimura S., Yamada Y., Ishikura H. The presence of 2-methylthio-N6-(delta-2-isopentenyl) adenosine in serine and phenylalanine transfer RNA's from Escherichia coli. Biochim Biophys Acta. 1969 Apr 22;179(2):517–520. doi: 10.1016/0005-2787(69)90065-3. [DOI] [PubMed] [Google Scholar]
- Oashi Z., Saneyoshi M., Harada F., Hara H., Nishimura S. Presumed anticodon structure of glutamic acid tRNA from E. coli: a possible location of a 2-thiouridine derivative in the first position of the anticodon. Biochem Biophys Res Commun. 1970 Aug 24;40(4):866–872. doi: 10.1016/0006-291x(70)90983-6. [DOI] [PubMed] [Google Scholar]
- Patwardhan S., Cherayil J. D. 5-Methyl-2-thiouridine in the tRNA of Candida tropicalis and its localization in lysine tRNA. J Bacteriol. 1985 Apr;162(1):55–60. doi: 10.1128/jb.162.1.55-60.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky A. The incorporation of mevalonic acid into the N6-(delta 2-isopentenyl) adenosine of transfer ribonucleic acid in Lactobacillus acidophilus. Biochemistry. 1968 Jan;7(1):472–482. doi: 10.1021/bi00841a059. [DOI] [PubMed] [Google Scholar]
- Powers D. M., Peterkofsky A. Biosynthesis and specific labeling of N-(purin-6-ylcarbamoyl)threonine of Escherichia coli transfer RNA. Biochem Biophys Res Commun. 1972 Jan 31;46(2):831–838. doi: 10.1016/s0006-291x(72)80216-x. [DOI] [PubMed] [Google Scholar]
- Rajbhandary U. L., Chang S. H., Stuart A., Faulkner R. D., Hoskinson R. M., Khorana H. G. Studies on polynucleotides, lxviii the primary structure of yeast phenylalanine transfer RNA. Proc Natl Acad Sci U S A. 1967 Mar;57(3):751–758. doi: 10.1073/pnas.57.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao Y. S., Cherayil J. D. Separation of 35 S-labeled thionucleosides of Escherichia coli and Pseudomonas aeruginosa transfer RNAs on a phosphocellulose column. Biochim Biophys Acta. 1973 Feb 23;299(1):1–7. doi: 10.1016/0005-2787(73)90391-2. [DOI] [PubMed] [Google Scholar]
- Schaefer K. P., Altman S., Söll D. Nucleotide modification in vitro of the precursor of transfer RNA of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3626–3630. doi: 10.1073/pnas.70.12.3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmel P. R. Understanding the recognition of transfer RNAs by aminoacyl transfer RNA synthetases. Adv Enzymol Relat Areas Mol Biol. 1979;49:187–222. doi: 10.1002/9780470122945.ch5. [DOI] [PubMed] [Google Scholar]
- Schweizer M. P., Chheda G. B., Baczynskyj L., Hall R. H. Aminoacyl nucleosides. VII. N-(Purin-6-ylcarbamoyl)threonine. A new component of transfer ribonucleic acid. Biochemistry. 1969 Aug;8(8):3283–3289. doi: 10.1021/bi00836a023. [DOI] [PubMed] [Google Scholar]
- Schön A., Krupp G., Gough S., Berry-Lowe S., Kannangara C. G., Söll D. The RNA required in the first step of chlorophyll biosynthesis is a chloroplast glutamate tRNA. Nature. 1986 Jul 17;322(6076):281–284. doi: 10.1038/322281a0. [DOI] [PubMed] [Google Scholar]
- Sekiya T., Takeishi K., Ukita T. Specificity of yeast glutamic acid transfer RNA for codon recognition. Biochim Biophys Acta. 1969 Jun 17;182(2):411–426. doi: 10.1016/0005-2787(69)90192-0. [DOI] [PubMed] [Google Scholar]
- Sen G. C., Ghosh H. P. Role of modified nucleosides in tRNA: effect of modification of the 2-thiouridine derivative located at the 5'-end of the anticodon of yeast transfer RNA Lys2. Nucleic Acids Res. 1976 Mar;3(3):523–535. doi: 10.1093/nar/3.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal R. P., Vold B. Changes in transfer ribonucleic acids of Bacillus subtilis during different growth phases. Nucleic Acids Res. 1976 May;3(5):1249–1262. doi: 10.1093/nar/3.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog F., Armstrong D. J., Cherayil J. D., Hampel A. E., Bock R. M. Cytokinin activity: localization in transfer RNA preparations. Science. 1966 Dec 9;154(3754):1354–1356. doi: 10.1126/science.154.3754.1354. [DOI] [PubMed] [Google Scholar]
- Stewart T. S., Roberts R. J., Strominger J. L. Novel species of tRNA. Nature. 1971 Mar 5;230(5288):36–38. doi: 10.1038/230036a0. [DOI] [PubMed] [Google Scholar]
- Struxness L. A., Armstrong D. J. Distribution of Cytokinin-active Ribonucleosides in Wheat Germ tRNA Species. Plant Physiol. 1979 Jan;63(1):35–41. doi: 10.1104/pp.63.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundharadas G., Katze J. R., Söll D., Konigsberg W., Lengyel P. On the recognition of serine transfer RNA's specific for unrelated codons by the same seryl-transfer RNA synthetase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):693–700. doi: 10.1073/pnas.61.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan S., Bock R. M. Subcellular localization of cytokinins in transfer ribonucleic Acid. Plant Physiol. 1977 Apr;59(4):558–563. doi: 10.1104/pp.59.4.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söll D. Enzymatic modification of transfer RNA. Science. 1971 Jul 23;173(3994):293–299. doi: 10.1126/science.173.3994.293. [DOI] [PubMed] [Google Scholar]
- Taya Y., Nishimura S. Biosynthesis of 5-methylaminomethyl-2-thiouridylate. I. Isolation of a new tRNA-methylase specific for 5-methylaminomethyl-2-thiouridylate. Biochem Biophys Res Commun. 1973 Apr 16;51(4):1062–1068. doi: 10.1016/0006-291x(73)90035-1. [DOI] [PubMed] [Google Scholar]
- Taya Y., Tanaka Y., Nishimura S. 5'-AMP is a direct precursor of cytokinin in Dictyostelium discoideum. Nature. 1978 Feb 9;271(5645):545–547. doi: 10.1038/271545a0. [DOI] [PubMed] [Google Scholar]
- Thimmappaya B., Cherayil J. D. Unique presence of 2-methylthio-ribosylzeatin in the transfer ribonucleic acid of the bacterium Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1974 Sep 23;60(2):665–672. doi: 10.1016/0006-291x(74)90292-7. [DOI] [PubMed] [Google Scholar]
- Vold B. S., Lazar J. M., Gray A. M. Characterization of a deficiency of N6-(delta 2-isopentenyl)-2-methylthioadenosine in the Escherichia coli mutant trpX by use of antibodies to N6-(delta 2-isopentenyl)adenosine. J Biol Chem. 1979 Aug 10;254(15):7362–7367. [PubMed] [Google Scholar]
- Vold B. S. Post-transcriptional modifications of the anticodon loop region: alterations in isoaccepting species of tRNA's during development in Bacillus subtilis. J Bacteriol. 1978 Jul;135(1):124–132. doi: 10.1128/jb.135.1.124-132.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreman H. J., Skoog F. Cytokinins in pisum transfer ribonucleic Acid. Plant Physiol. 1972 May;49(5):848–851. doi: 10.1104/pp.49.5.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron C., Cox B. S., Wills N., Gesteland R. F., Piper P. W., Colby D., Guthrie C. Yeast ochre suppressor SUQ5-ol is an altered tRNA Ser UCA. Nucleic Acids Res. 1981 Jul 10;9(13):3077–3088. doi: 10.1093/nar/9.13.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Oshima T., Iijima K., Yamaizumi Z., Nishimura S. Purification and thermal stability of several amino acid-specific tRNAs from an extreme thermophile, Thermus thermophilus HB8. J Biochem. 1980 Jan;87(1):1–13. doi: 10.1093/oxfordjournals.jbchem.a132713. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Oshima T., Saneyoshi M., Nishimura S. Replacement of ribothymidine by 5-methyl-2-thiouridine in sequence GT psi C in tRNA of an extreme thermophile. FEBS Lett. 1974 Jul 1;43(1):59–63. doi: 10.1016/0014-5793(74)81105-1. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Shinma M., Oshima T., Nishimura S. Heat-induced stability of tRNA from an extreme thermophile, Thermus thermophilus. Biochem Biophys Res Commun. 1976 Oct 4;72(3):1137–1144. doi: 10.1016/s0006-291x(76)80250-1. [DOI] [PubMed] [Google Scholar]
- Weissenbach J., Dirheimer G. Pairing properties of the methylester of 5-carboxymethyl uridine in the wobble position of yeast tRNA3Arg. Biochim Biophys Acta. 1978 May 23;518(3):530–534. doi: 10.1016/0005-2787(78)90171-5. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Ishikura H. Nucleotide sequence of a lysine tRNA from Bacillus subtilis. Nucleic Acids Res. 1977 Dec;4(12):4291–4303. doi: 10.1093/nar/4.12.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Murao K., Ishikura H. 5-(carboxymethylaminomethyl)-2-thiouridine, a new modified nucleoside found at the first letter position of the anticodon. Nucleic Acids Res. 1981 Apr 24;9(8):1933–1939. doi: 10.1093/nar/9.8.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Saneyoshi M., Nishimura S., Ishikura H. Isolation and characterization of 2-thiocytidine from a serine transfer ribonucleic acid of Escherichia coli. FEBS Lett. 1970 Apr 16;7(3):207–210. doi: 10.1016/0014-5793(70)80161-2. [DOI] [PubMed] [Google Scholar]
- Yamaizumi Z., Kuchino Y., Harada F., Nishimura S., McCloskey J. A. Primary structure of Escherichia coli tRNA UUR Leu. Presence of an unknown adenosine derivative in the first position of the anticodon which recognizes the UU codon series. J Biol Chem. 1980 Mar 10;255(5):2220–2225. [PubMed] [Google Scholar]
- Yaniv M., Folk W. R. The nucleotide sequences of the two glutamine transfer ribonucleic acids from Escherichia coli. J Biol Chem. 1975 May 10;250(9):3243–3253. [PubMed] [Google Scholar]
- Yanofsky C. Mutations affecting tRNATrp and its charging and their effect on regulation of transcription termination at the attenuator of the tryptophan operon. J Mol Biol. 1977 Jul 15;113(4):663–677. doi: 10.1016/0022-2836(77)90229-7. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Takeishi K., Ukita T. Anticodon structure of GAA-specific glutamic acid tRNA from yeast. Biochem Biophys Res Commun. 1970 Jun 5;39(5):852–857. doi: 10.1016/0006-291x(70)90401-8. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Takeishi K., Ukita T. Structural studies on a yeast glutamic acid tRNA specific to GAA codon. Biochim Biophys Acta. 1971 Jan 1;228(1):153–166. doi: 10.1016/0005-2787(71)90555-7. [DOI] [PubMed] [Google Scholar]


