Abstract
Infant mammalian feeding consists of rhythmic suck cycles and reflexive pharyngeal swallows. Although we know how oropharyngeal sensation influences the initiation and frequency of suck and swallow cycles, the role of palatal sensation is unknown. We implanted EMG electrodes into the mylohyoid muscle, a muscle active during suckling, and the thyrohyoid muscle, a muscle active during swallowing, in eight infant pigs. Pigs were then bottle-fed while lateral videofluoroscopy was simultaneously recorded from the electrodes. Two treatments were administered prior to feeding and compared with control feedings: 1) palatal anesthesia (0.5% bupivacaine hydrochloride), and 2) palatal saline. Using the timing of mylohyoid muscle and thyrohyoid muscle activity, we tested for differences between treatment and control feedings for swallowing frequency and suck cycle duration. Following palatal anesthesia, four pigs could not suck and exhibited excessive jaw movement. We categorized the four pigs that could suck after palatal anesthesia as group A, and those who could not as group B. Group A had no significant change in suck cycle duration and a higher swallowing frequency after palatal saline (P = 0.021). Group B had significantly longer suck cycles after palatal anesthesia (P < 0.001) and a slower swallowing frequency (P < 0.001). Swallowing frequency may be a way to predict group membership, since it was different in control feedings between groups (P < 0.001). The qualitative and bimodal group response to palatal anesthesia may reflect a developmental difference. This study demonstrates that palatal sensation is involved in the initiation and frequency of suck and swallow cycles in infant feeding.
Keywords: electromyography, swallowing, sucking, bupivacaine, palate
infant mammalian feeding is a complex sensorimotor behavior with both rhythmic and reflexive components (German et al. 2009; Thexton et al. 2012). Because it is relatively simpler than adult feeding, infant mammalian feeding is a good model for understanding how the sensory and motor aspects of feeding interact (German and Crompton 2000; German et al. 2004b). The rhythmic component consists of the pure suck cycles that both extract milk from the nipple and transport it through the oropharynx prior to a swallow. The reflexive component is the pharyngeal swallow where the bolus is transported into the esophagus. This reflexive component is inserted into a suck cycle, and collectively that cycle is termed a suck-swallow cycle (Thexton et al. 2012). Throughout a feeding session, sucking is an ongoing rhythmic behavior, and suck-swallow cycles occur every one to three suck cycles early during a feeding session and are less frequent toward the end. Although we know some of the sensory mechanisms that elicit the swallow in adults, including bolus volume, temperature, taste, and carbonation (Butler et al. 2011; Michou et al. 2012; Yamamura et al. 2010), it is unknown how specific sensory information gathered during the oral transport process impacts the onset of the reflexive pharyngeal swallow in infants.
During sucking, sensory information about the bolus is sent to the sucking pattern generator in the brain stem that regulates the motor output necessary to transport the bolus to the oropharynx (Barlow 2009; Steele and Miller 2010). Sensory information from the oropharynx synapses in the swallowing pattern generator in the brain stem to initiate the motor function necessary for a pharyngeal swallow (Barlow 2009; Steele and Miller 2010). Although oral sensation from the trigeminal nerve projects to the region of the brain stem where the swallowing pattern generator is located, its role in the triggering of the pharyngeal swallow is not clear. During sucking, three regions of the oral cavity, supplied by branches of the trigeminal nerve, are either in contact with the nipple, or deform as a result of the negative pressure: the hard palate, the tongue, and the lips. To what extent sensation carried by the trigeminal nerve, from these regions in the oral cavity, is involved in sucking, swallowing, and their coordination is unknown.
Taste and mechanical sensation from the oral cavity and oropharynx impact the threshold for stimulating the initiation of an oral transport cycle in adults (Steele and Miller 2010) and of a suck cycle in infants (German et al. 1997). Based on past studies in infant pigs, the initiation of a suck cycle is dependent on milk being present in the nipple and on the frequency of milk delivery when using an automated feeding system (German et al. 1997). Clinically, oral stimulation helps infants struggling to feed to develop stronger sucking (Finan and Barlow 1998). Regulation of swallowing frequency is better known. Swallowing frequency relies on sensation from the superior laryngeal nerve (CNX; cranial nerve ten), as well as the glossopharyngeal and facial nerves, that is relayed to the brain stem (Steele and Miller 2010). There is also descending cortical input to the brain stem that regulates swallowing frequency. In infant pigs, the frequency of delivery of a bolus, from an automated feeding system, can change the frequency of swallowing, indicating that swallowing may also be dependent on trigeminal sensation (German et al. 2004a).
The central pattern generator (CPG) for sucking and the CPG for swallowing appear to communicate in infant pigs (Thexton et al. 2012). After a pharyngeal swallow, there is a set amount of time before the next suck cycle begins that is dependent on the duration of the oral transport cycle (Thexton et al. 2012). In that study, a suck cycle that contained a swallow (suck-swallow cycle) was divided into two predictable phases: phase 1 was from the start of the suck-swallow cycle to the start of the pharyngeal swallow, and phase 2 was from the start of the pharyngeal swallow to the end of the suck-swallow cycle. Suck-swallow cycle length was correlated with phase 2 length, but not phase 1; however, it is not known what role oral sensation plays in maintaining this temporal relationship. Understanding these relationships in the periphery will provide data for the functional significance of central connections.
We evaluated the impact of palatal sensation on sucking and swallowing frequencies in an infant pig model (German et al. 2004b) by anesthetizing the palate and evaluating how that altered or reduced sensation affected 1) swallowing frequency, 2) suck cycle duration, and 3) suck cycles per swallow. Additionally, we included a saline injection treatment, intended as a sham treatment. The study utilized a repeated-measures model. We hypothesized that, with palatal anesthesia, sucking and swallowing frequencies would be reduced because of the reduced sensation. We also evaluated if the suck-swallow cycle length was correlated with the time from the start of the suck-swallow to the pharyngeal swallow (phase 1) or with the time from the pharyngeal swallow to the end of the suck-swallow cycle (phase 2) when the hard palate was anesthetized.
MATERIALS AND METHODS
Animal model.
This study included eight infant pigs obtained from Tom Morris Farms (Reisterstown, MD) that were 2–3 wk old and weighed 3.0–5.5 kg. This was based on power calculations from previous studies (Ding et al. 2013a, 2013b; Thexton et al. 2009). At this age the animals were comparable to 6-mo to 1-yr-old human infants as judged by tooth eruption, weaning status, and skeletal development (Book and Bustad 1974; Weaver et al. 1969). Although pigs are precocious relative to humans, the developmental patterns of feeding infants are remarkably similar across most species of mammals (German and Crompton 2000). Infant pigs have been used in previous studies of normal feeding and swallowing neurophysiology, and thus a large body of comparable data exists for comparison with the results of this study (Campbell-Malone et al. 2011; Ding et al. 2013a; German et al. 2004b; Thexton et al. 2007, 2009). The pigs were trained to feed from a bottle for 4–6 days prior to the start of the study. All procedures were approved by the Johns Hopkins University Institutional Animal Care and Use Committee (SW10M212).
Electromyographic electrode implantation.
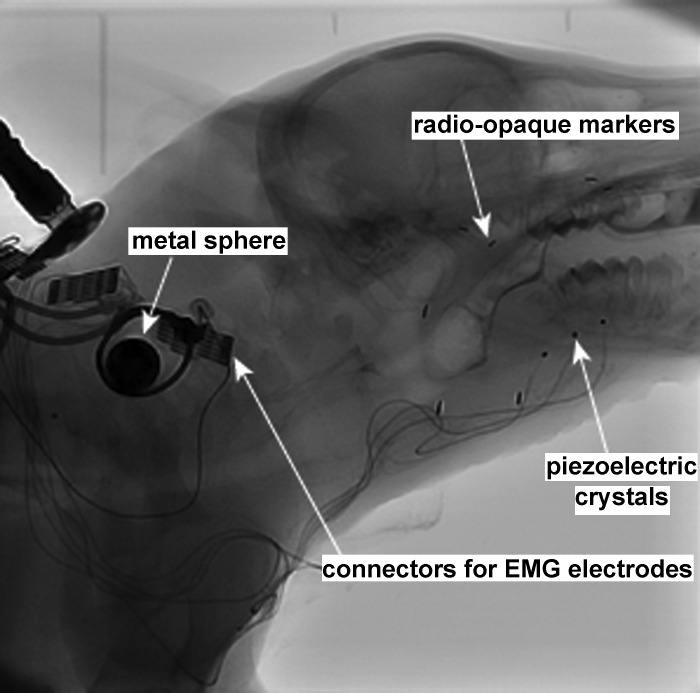
After trained to feed from a bottle, the pigs underwent surgery to implant fine-wire bipolar electromyographic (EMG) electrodes into several oropharyngeal and hyolaryngeal muscles (Fig. 1). The EMG electrodes recorded from motor units in the vicinity of that implanted electrode (Loeb and Gans 1986). Animals were intubated and anesthetized with 2–3% isoflurane until they were in Stage III, Plane III anesthesia. During surgery, electrodes were implanted into the thyrohyoid (TH) muscle. Additionally, patch EMG electrodes were placed on the ventral surface of the anterior mylohyoid (MH) muscle. Lastly, three 2-mm piezoelectric crystals (Sonometrics, London, ON) were inserted into the midline of the genioglossus muscle (Holman et al. 2012; Konow et al. 2010) (Fig. 1). All muscles were accessed through a midline incision, and the electrodes exited through a window on the left lateral neck. In previous studies where the electrodes exited the midline incision, the wires often broke due to the movement in that region during head flexion. One radio-opaque marker was sutured to the hyoid bone, and another one was sutured to the thyroid cartilage. The same surgeons performed all surgeries. After surgery, the suture lines were wrapped in VetWrap (3M, St. Paul, MN). A small metal sphere (12.69 mm in diameter) was placed on the lateral neck under the VetWrap which would later be used to correct for absolute size of the images during videofluoroscopy data analysis. The electrodes were plugged into a connector that exited the bandage on the back of the animal. The procedures followed for EMG surgery and analysis are those used in several previous studies (German et al. 2009; Konow et al. 2010; Thexton et al. 2012). Analyses of kinematics as well as animal discomfort show that these procedures and instrumentation have minimal, if any, impact on feeding ability in mammals (Dutra et al. 2010; Thexton et al. 2007).
Fig. 1.
Videofluoroscopy image. One frame from the 60 frames/s videofluoroscopy recording shows the infant pig with EMG electrodes and radio-opaque markers.
After the electrodes were implanted, the animal had several intraoral radio-opaque markers (<0.5 cm long) placed into the tongue and other soft tissue structures (Fig. 1). The markers were inserted in the midline of the anterior hard palate, the soft and hard palate junction, the tongue ∼10 mm deep to the foramen cecum, and the right and left mucogingival junction by the maxillary molar. Previous studies have demonstrated that these markers are not harmful to the animals and that they do not affect their oral motor function (German and Franks 1991; Thexton 1981). After the markers were placed, the animals were taken off the general anesthesia and quickly recovered in ∼20 min. The entire surgical procedure took 2–4 h. Once the animals were standing and vocalizing, following surgery, they were offered milk every hour until they were able to feed normally. They were subsequently fed every 4 h. The following medications were administered postoperatively and were continued for the duration of the experiment: ampicillin (0.16 ml of 250 mg/ml) and buprenorphine (0.17 ml of 0.3 mg/ml) twice daily and meloxicam (0.1 ml of 5 mg/ml) once daily. The implantation of radio-opaque markers has been previously shown to not impact feeding kinematics (Thexton et al. 1998, 2007).
Treatments.
The feeding study began the day after electrodes were implanted and lasted for 4 days. Each day, the animal was placed under stage III, plane III, or deep/surgical anesthesia for 20 min. A laryngoscope was used to visualize the epiglottis, and a Weck Hemoclip Ligating Clip (Pilling Weck, Research Triangle Park, NC) was placed in the midline on the tip of the epiglottis. The epiglottis marker typically falls off after 2 days; in these cases it was replaced. After this, either no treatment was given (control), or one of two treatments was administered. The two treatments were as follows: 1) saline and 2) anesthesia nerve blocks bilaterally to the greater palatine nerves and to the nasopalatine nerve, for three total injections per animal. The term “nerve block” describes the dental technique where a nerve is anesthetized by injecting local anesthesia at the foramen where that nerve exits the cranium, therefore blocking action potentials from the branches of that nerve from being transmitted beyond that point. Both the nasopalatine and greater palatine nerves are branches of the maxillary branch of the trigeminal nerve (cranial nerve V). The nasopalatine nerve provides sensation to the anterior one-third of the hard palate, and the two greater palatine nerves provide sensation to the posterior two-thirds of the hard palate. The local anesthetic used was 0.5% bupivacaine hydrochloride (Marcaine, 5 mg/ml, Hospira, Lake Forest, IL). Injections were administered using standard veterinary dental techniques (Reuss-Lamky 2007). One-half milliliter was injected to each site as a nerve block (Jonnavithula et al. 2010; Reuss-Lamky 2007). Nerve blocks to the hard palate have a high rate of success, since there are no alternative spaces for the anesthetic to travel without affecting the sensory nerves and their branches. There are also no muscles in the area, ensuring that only sensory nerves are blocked. The same experimenter administered all injections. The duration of anesthesia with these nerve blocks is from 1 to 3 h in anesthetized infant pigs before oral reflexes are observed (Holman et al. 2013). The order of the treatments and control was randomized for each pig using a random number generator. On either the second or third day of the study, there was no treatment given. This would be on the day after an injection (anesthesia or saline) is given to rule out a lingering effect of treatment. The last day of the study was always a treatment day (anesthesia or saline). After the position of the epiglottis marker was verified, and the treatment given, if necessary, the pig was removed from the general anesthesia and typically recovered in 5–10 min.
Feeding sessions.
Each pig was freely fed milk containing barium from a pig nipple in a plastic feeding box 30–60 min posttreatment. During the feeding session we recorded lateral videofluoroscopy at 60 frames/s (Allura FD20, Philips Healthcare, Best, The Netherlands) equipped with a high-resolution digital flat-panel detector (154 × 154 micron pixel-pitch, 30 × 40 cm) and EMG (10 kHz) recorded with a synchronization signal on a Powerlab 30/16 (AD Instruments, Colorado Springs, CO). The milk was made using eight ounces of prepared pig milk replacer (Land O Lakes Solustart pig milk replacer, St. Paul, MN) with one-third cup of barium powder. The bottle was warmed in a hot water bath for ∼2 min to ensure that each bottle was the same temperature. Temperature and the amount of barium in the bottles was standardized since both taste, texture, and temperature have been shown to alter swallowing kinematics (Cichero et al. 2010; Ebihara et al. 2011; Lee et al. 2013). The pig was fed until 20 swallows were recorded or until five 15-s videos were recorded. At the conclusion of the study, the animals were euthanized, and the location of the electrodes was verified during a postmortem dissection conducted by one of the experimenters not involved in electrode placement.
Data analysis.
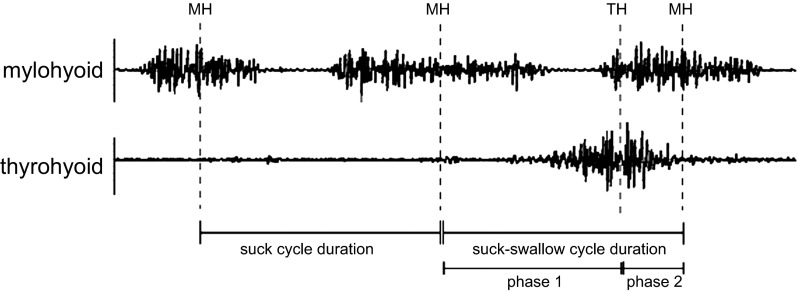
We analyzed EMG recordings from the MH and TM to address our aims. Based on the results of the postmortem dissection and the quality of the EMG recordings, two EMG signals (from one MH and one TH electrode per pig) were selected for data analysis. The timing of MH and TH EMG activity in each feeding session was used to identify suck and swallow cycles (Fig. 2). A pharyngeal swallow was identified by the midpoint of activity in the TH, and a suck cycle was identified by the midpoint activity in the MH (Thexton et al. 2012). The pigs were latching onto and suckling from the bottle throughout the entire feeding sequence. We were simultaneously recording lateral videofluoroscopy at 60 frames/s where we could clearly see the sucking and swallowing cycles. There were two types of suck cycles (Fig. 2): 1) the pure suck cycle, and 2) the suck-swallow cycle (Thexton et al. 2012). The MH activity immediately before the TH activity was identified as the start of the suck-swallow cycle. The next MH activity was the end of the suck-swallow cycle. The MH activity observed before the suck-swallow defined the start of the pure suck cycle. Midpoint activity was assigned to the middle of the signal burst of MH and TH, even if the burst was skewed in any way (Fig. 2). This method represents a departure of the methods used in Thexton et al. (2012) since the feedings in this study were recorded over several days and the quality of the signal changed over this time period. All labeling was done in LabChart 7 (AD Instruments, Colorado Springs, CO).
Fig. 2.
Electromyographic recording of mylohyoid (MH) and thyrohyoid (TH) with cycles labeled. Electromyographic recordings are shown with labeled midpoints of MH and TH. Also labeled are the suck cycle duration and suck-swallow cycle durations, as well as phase 1 and 2 lengths.
Swallow frequency was measured as the inverse of the time between pharyngeal swallows, or TH activity (Thexton et al. 2012). The number of sucking, or oral transport cycles per pharyngeal swallow, was calculated by counting the number of suck cycles (MH activity) between the start of a pharyngeal swallow and the start of the next pharyngeal swallow. The MH activity that was the start of a suck-swallow cycles was not included, since they always occurred with a pharyngeal swallow. We began counting after the first complete swallow.
Differences between treatments for each of these dependent variables were evaluated using a General Linear Model ANOVA with the independent, repeated-measures variable being anesthetic treatment (control, anesthesia, saline), and individual as a random variable. The unit of analysis is a cycle. The inclusion of individual as a random factor accounted for the differences within individuals. This model is less susceptible to extreme outliers than one using averages over individuals as the unit of analysis (German et al. 2008). A post hoc Tukey's Honestly Significant Difference test evaluated for specific differences. All statistical analysis was performed using SYSTAT 13 (Systat Software, Chicago, IL).
We also tested whether the suck-swallow cycle and phase relationships described by Thexton et al. (2012) in control animals was modified after palatal saline and palatal anesthesia. They defined a cycle as the suck-swallow cycle described previously (Fig. 2). They divided the cycle into two phases. Phase 1 was from the start of the suck-swallow cycle to the swallow, as defined by the midpoint of TH activity (Fig. 2). Phase 2 was the time from the swallow, as defined by TH, to the end of the suck-swallow cycle (Fig. 2). Following Thexton et al., we used a linear regression test for relationships between phase duration and cycle length. This was done for each treatment. Thexton et al. found that cycle length was correlated with phase 2 duration. Linear regression determined if the slope of that line was statistically significantly different from zero, or was a flat line (Thexton et al. 2012). If it was not a flat line, then phase length is independent of cycle length, and if it was significantly different from zero, there was a significant relationship between those variables.
Testing blockade of the greater palatine nerve.
In a study of three euthanized infant pigs, we tested if the block of the greater palatine nerve also anesthetized the lesser palatine nerve. The goal of a nerve block, often used in dentistry, is to deposit the local anesthetic into the foramen that the sensory nerve exits. The local anesthetic will block the propagation of an action potential to the central nervous system. By depositing the anesthetic at the foramen, before it branches, the operator has effectively blocked all sensory information from that nerve's branches to the central nervous system. The lesser palatine nerve is very small relative to the greater palatine nerve and provides sensation to the anterior soft palate. Immediately following euthanasia (within 20 min), we injected a mixture of 0.1 ml India ink with 0.9 ml 0.5% bupivacaine hydrochloride as a nerve block bilaterally to the greater palatine nerves. After injection, the mucosa was lifted from the hard palate using a scalpel to expose the greater and lesser palatine nerves bilaterally. The ink sticks to the nerve, and its distribution was used to assess the distribution of the anesthetic in vivo. By confirming that the local anesthetic, with the ink, was able to enter the foramen that the nerve exits, we can conclude that no sensation could be transmitted from that sensory nerve, or its branches on the palate, to the brain stem.
RESULTS
Qualitative difference between individuals.
With palatal anesthesia, four of the eight pigs could not suck normally. These pigs were still able to collect a bolus in the valleculae, after multiple oral transport cycles, and swallow; however, their method of transport was qualitatively distinct, characterized by very little surface deformation of the tongue and masticatory-like jaw movements. Sucking is a very well-described behavior that is rhythmic with vertical tongue movement that either strips milk out of the nipple or creates pressure changes within the oral cavity. There is very little, if any, rhythmic jaw movement (da Costa et al. 2010; German et al. 1992, 2004b).
This difference in gross feeding behavior was immediately obvious during the feeding session and was an all-or-none occurrence. Normally, infants have little or no jaw movement, latch onto the nipple, and show significant surface deformation. There were three characteristics that defined this behavioral alternative, all of which always appeared in an individual: excessive jaw movement, failure to latch onto the nipple, and little surface deformation of the tongue. Since such a distinct, qualitative difference in a normally consistent behavior was observed, the eight pigs were divided into two groups. Pigs in group A could suck with normal characteristics after palatal anesthesia. Pigs in group B showed all three of the alternative features described above during feeding after palatal anesthesia. No individual had only a subset of these characteristics, and there were no intermediate forms of these characteristics. This particular behavior did not ever occur with the palatal saline treatment. For the statistical analysis of the dependent variables measured in this study, in addition to having treatment (control, anesthesia, saline) as a fixed factor, group (A or B) was also evaluated for effects. The interaction between treatment and group was also tested. The individual (pig) was tested in the model nested within group to account for the random variation between animals.
Swallow frequency.
There was a difference overall between treatments (control, anesthesia, and saline) among the eight pigs (P < 0.001, Table 1); however, there was no statistical difference in swallowing frequency between group A and group B (Table 1). The specific tests revealed significant differences among all three treatments, with P < 0.001 for each comparison. The frequency was lowest after palatal anesthesia, followed by control and then palatal saline (Table 1).
Table 1.
Swallowing frequency
| Treatment | n | Mean ± SD | Range |
|---|---|---|---|
| Swallow frequency by treatment | |||
| Control | 150 | 1.17 ± 0.94a | 0.33–6.54 |
| Palatal anesthesia | 149 | 2.71 ± 3.40b | 0.26–26.52 |
| Palatal saline | 151 | 0.91 ± 0.59c | 0.23–3.96 |
| Swallow frequency by group | |||
| Group A | 226 | 1.23 ± 0.80 | 0.41–6.54 |
| Group B | 224 | 1.96 ± 2.98 | 0.23–26.52 |
| Group A swallow frequency | |||
| Control | 75 | 0.69 ± 0.93d | 0.15–2.25 |
| Palatal anesthesia | 76 | 0.82 ± 1.45 | 0.32–2.46 |
| Palatal saline | 75 | 0.98 ± 2.38e | 0.48–2.35 |
| Group B swallow frequency | |||
| Control | 75 | 1.13 ± 1.52f | 0.27–3.08 |
| Palatal anesthesia | 73 | 0.26 ± 0.23g | 0.04–3.79 |
| Palatal saline | 76 | 1.26 ± 1.41f | 0.25–4.29 |
Values are means ± SD and range in swallows per second; n= sample size. Shown are swallowing frequency by treatment (control, palatal anesthesia, palatal saline), by group, for group A by treatment, and for group B by treatment.
Groups with different letters next to the means ± SD are statistically significantly different from each other (P < 0.05). Significant differences across categories are not shown.
There were also significant interactions between treatment and group. Among group A, there was a higher swallowing frequency in the pigs with palatal saline compared with control (P = 0.021, Table 1). There was no significant difference with palatal anesthesia compared with control or palatal saline. In the group B, swallow frequency was lower with palatal anesthesia compared with control (P < 0.001) and compared with palatal saline (P = 0.001, Table 1). There was no difference between control and palatal saline. The control feedings were significantly different in group A and group B (P < 0.001). There was a significant difference between the groups after palatal anesthesia (P < 0.001) and palatal saline (P < 0.001).
Suck cycle and suck-swallow cycle durations.
For suck cycle duration, there was a significant difference overall between treatments (P < 0.001, Table 2). There was no significant difference between groups (Table 2). After palatal anesthesia, suck cycle duration was longer compared with control (P < 0.001). After palatal saline, suck cycle duration was longer compared with control (P = 0.050). Suck cycles after palatal anesthesia were longer than those after palatal saline, with P < 0.001.
Table 2.
Suck cycle durations
| Treatment | n | Mean ± SD | Range |
|---|---|---|---|
| Suck cycle | |||
| Duration by treatment | |||
| Control | 160 | 0.24 ± 0.05a | 0.15–0.40 |
| Palatal anesthesia | 156 | 0.29 ± 0.11b | 0.19–0.86 |
| Palatal saline | 160 | 0.25 ± 0.05c | 0.18–0.47 |
| Duration by group | |||
| Group A | 240 | 0.26 ± 0.05 | 0.16–0.47 |
| Group B | 236 | 0.26 ± 0.09 | 0.15–0.86 |
| Group A duration | |||
| Control | 80 | 0.24 ± 0.04 | 0.16–0.38 |
| Palatal anesthesia | 80 | 0.26 ± 0.04 | 0.19–0.42 |
| Palatal saline | 80 | 0.26 ± 0.06 | 0.19–0.47 |
| Group B duration | |||
| Control | 80 | 0.23 ± 0.05d | 0.15–0.40 |
| Palatal anesthesia | 76 | 0.36 ± 0.14e | 0.19–0.86 |
| Palatal saline | 80 | 0.23 ± 0.03d | 0.18–0.33 |
| Suck-swallow cycle | |||
| Duration by treatment | |||
| Control | 160 | 0.24 ± 0.04a | 0.15–0.36 |
| Palatal anesthesia | 159 | 0.29 ± 0.10a,b | 0.18–0.98 |
| Palatal saline | 160 | 0.25 ± 0.05b | 0.17–0.52 |
| Duration by group | |||
| Group A | 240 | 0.25 ± 0.05 | 0.17–0.52 |
| Group B | 239 | 0.26 ± 0.09 | 0.15–0.98 |
| Group A duration | |||
| Control | 80 | 0.24 ± 0.04 | 0.17–0.36 |
| Palatal anesthesia | 80 | 0.25 ± 0.03e | 0.18–0.34 |
| Palatal saline | 80 | 0.26 ± 0.06f | 0.17–0.52 |
| Group B duration | |||
| Control | 80 | 0.23 ± 0.05c | 0.15–0.36 |
| Palatal anesthesia | 79 | 0.32 ± 0.14c,d,e | 0.19–0.98 |
| Palatal saline | 80 | 0.23 ± 0.03d,f | 0.19–0.33 |
Values are means ± SD and range in seconds; n= sample size. Shown are suck cycle and suck-swallow cycle duration by treatment (control, palatal anesthesia, palatal saline), by group, for group A by treatment, and for group B by treatment.
Groups with different letters next to the means ± SD are statistically significantly different from each other (P < 0.05). Significant differences across categories are not shown.
There was also a significant interaction between group and treatment (P < 0.001, Table 2). There were no significant differences between treatments in group A (Table 2). There was significantly longer suck cycles in group B after palatal anesthesia compared with control (P < 0.001) and to palatal saline (P < 0.001, Table 2). There was no significant difference between control and palatal saline. There was also no difference between control suck cycle durations in the two groups. There was a significant difference between the reaction to palatal anesthesia in group A and group B with longer cycles in group B (P < 0.001). Likewise, there was a significantly longer cycle duration in response to palatal saline in group A compared with group B (P = 0.001).
For suck-swallow cycle duration, overall there was a significant difference between treatments (P < 0.001, Table 2), and there was no significant difference between groups (P = 0.281, Table 2). There was no significant difference between palatal saline and control (P = 0.104); however, cycles were longer with palatal anesthesia relative to both control (P < 0.001) and palatal saline (P < 0.001).
There was a significant interaction between treatment and group (P < 0.001, Table 2). There were no significant differences in suck-swallow cycle duration between treatments in group A. In group B the suck-swallow cycles were longer with palatal anesthesia relative to control (P < 0.001). There was also a significant difference between palatal saline and palatal anesthesia cycle durations (P < 0.001). There was no difference between control and palatal saline cycle durations. There was no significant difference in cycle durations during control feedings between group A and group B. There was, however, a significant difference between cycle durations after palatal anesthesia between group A and group B (P < 0.001) and palatal saline (P = 0.012).
Oral transport cycles per swallow.
Overall there was a significant difference in the number of oral transport cycles between all of the factors tested. There was also a difference overall between treatments (P < 0.001, Table 3). After palatal anesthesia, there were more cycles per swallow relative to control (P < 0.001) and palatal saline (P < 0.001). There were also more following control relative to palatal saline (P = 0.022). Overall there was a significant difference between groups (P < 0.001) with group B having more oral transport cycles per swallow (Table 3).
Table 3.
Oral transport cycles per swallow
| Treatment | n | Mean ± SD | Range |
|---|---|---|---|
| Oral transport cycles per swallow by treatment | |||
| Control | 160 | 4.21 ± 3.75a | 1–21 |
| Palatal anesthesia | 160 | 8.56 ± 10.10b | 0–63 |
| Palatal saline | 160 | 2.79 ± 2.44c | 0–16 |
| Oral transport cycles per swallow by group | |||
| Group A | 240 | 4.03 ± 2.98d | 1–21 |
| Group B | 240 | 6.35 ± 9.04e | 0–63 |
| Group A oral transport cycles per swallow | |||
| Control | 80 | 5.1 ± 3.9 | 1–21 |
| Palatal anesthesia | 80 | 3.8 ± 2.5 | 1–12 |
| Palatal saline | 80 | 3.2 ± 1.8 | 1–7 |
| Group B oral transport cycles per swallow | |||
| Control | 80 | 3.3 ± 3.3f | 1–18 |
| Palatal anesthesia | 80 | 13.4 ± 12.4g | 0–63 |
| Palatal saline | 80 | 2.4 ± 2.9f | 0–16 |
Values are means ± SD and range; n= sample size. Shown are oral transport cycles per swallow by treatment, by group, for group A by treatment (control, palatal anesthesia, palatal saline), and for group B by treatment.
Groups with different letters next to the means ± SD are statistically significantly different from each other (P < 0.05). Significant differences across categories are not shown.
There was also a significant difference among the interactions between group and treatment (P < 0.001, Table 3). In group A there was no difference in number of oral transport cycles per pharyngeal swallow among the treatments. In group B, after palatal anesthesia there were more oral transport cycles per swallow compared with both control (P < 0.001) and palatal saline (P < 0.001). This difference was extreme with a wide range of values for the pigs with palatal anesthesia, often with over 20 transport cycles. There was no difference between palatal saline and control. There was no difference in sucks per swallow between group A and group B response to control or palatal saline. There was a significant difference between groups A and B after palatal anesthesia, with group B pigs having more oral transport cycles per swallow.
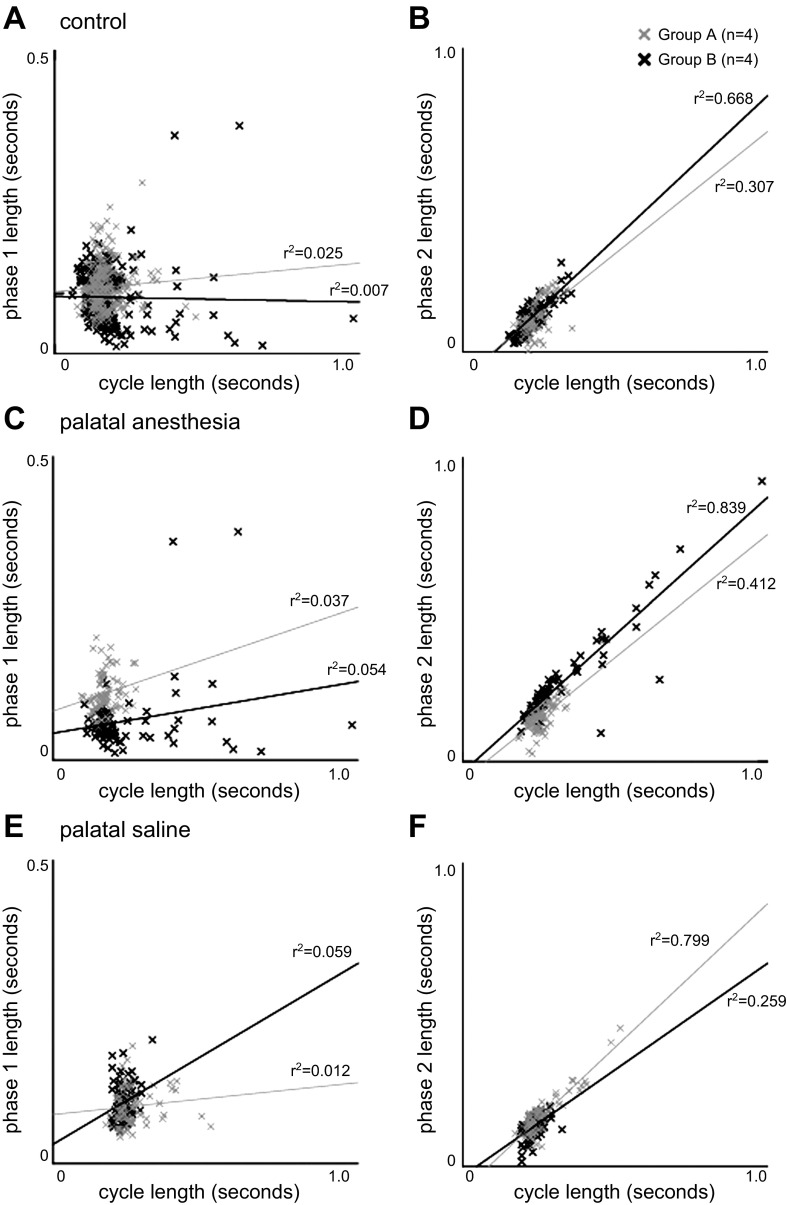
Cycle and phase relationships.
For phase 1, there was no significant relationship between phase length and cycle duration (Fig. 3). In group A, the time between the suck-swallow cycles (cycle duration) was not correlated for phase 1 length for control (r2 = 0.025, P = 0.159, Fig. 3A), palatal anesthesia (r2 = 0.037, P = 0.086, Fig. 3C), or palatal saline (r2 = 0.012, P = 0.330, Fig. 3E). For group B, no correlation was seen for control (r2 = 0.007, P = 0.457, Fig. 3A), but with palatal anesthesia (r2 = 0.054, P = 0.039, Fig. 3C) and palatal saline (r2 = 0.059, P = 0.029, Fig. 3E) there was a minimal correlation that was significantly different from zero. The group B with palatal anesthesia had a wider range of durations for cycle length and a couple of outliers showing extremely large phase 1 lengths.
Fig. 3.
Correlation between cycle and phase length. A: correlation between the time from suck-swallow cycle to postswallow suck cycle (cycle) and the suck-swallow cycle to pharyngeal swallow (phase 1) for the control feedings. B: correlation between cycle length and the time from pharyngeal swallow to postswallow suck cycle (phase 2) for the control feedings. C: correlation between phase 1 and cycle duration for palatal local anesthesia. D: correlation between phase 2 and cycle duration for palatal local anesthesia. E: correlation between phase 1 and cycle duration for palatal saline. F: correlation between phase 2 and cycle duration for palatal saline.
For phase 2, there were significant relationships for all treatments in both groups between phase length and cycle duration. For group A there was a statistically significantly relationship between cycle duration and phase 2 length that was different from zero for control (r2 = 0.307, P < 0.001, Fig. 3B), palatal anesthesia (r2 = 0.412, P < 0.001, Fig. 3D), and palatal saline (r2 = 0.799, P < 0.001, Fig. 3F). For group B this relationship was also observed between cycle duration and phase 2 for control (r2 = 0.668, P < 0.001, Fig. 3B), palatal anesthesia (r2 = 0.839, P < 0.001, Fig. 3D), and palatal saline (r2 = 0.259, P < 0.001, Fig. 3F). The group B with palatal anesthesia had a wider range of values for cycle duration and phase 2 length that maintained the same linear relationship seen with the control and palatal saline treatments. It also had a greater average slope.
Blockade of the lesser palatine nerve.
In two of six cases (3 infant pigs with 2 greater palatine nerve blocks, bilaterally) the India ink reached the lesser palatine nerve and foramen following the greater palatine nerve block.
DISCUSSION
Two distinct reactions to palatal local anesthesia.
All individuals suckled normally during control and saline treatments; but with a reduction of palatal sensation only half of the infants had normal function. We hypothesize that the extreme jaw movement seen in dysfunctional suckling was a less efficient compensation to reestablish some form of intraoral transport. The obliteration of the typical vertical, wave-like tongue movement is consistent with the documented link between palatal sensation and tongue rhythmicity (Thexton 1973). Because jaw movement, vertical tongue movement, and latching were either all affected or all normal suggests a central link amongst them.
This movement is not part of normal infant or adult behavior. Adult pig drinking involves suction, with the tongue remaining in their mouth, and significant surface deformation of the tongue (Herring and Scapino 1973; Thexton et al. 1998). Mastication includes jaw movement in all planes (Herring and Scapino 1973). Although the amount of jaw opening after palatal anesthesia was similar to mastication, they were limited to the sagittal plane (Herring and Scapino 1973).
The difference between the two behavioral groups is similar to a difference observed in two sets of same-aged infant opossums. In these animals, two distinct oral transport mechanisms were described (German and Crompton 1996). In infants that were younger (45 days old), they had a sucking mechanism similar to infant pigs. The infants that were older (65 days) and separated from their mother transitioned to a feeding mechanism where they protruded their tongue to feed from the nipple, in a manner similar to lapping. Even though lapping is only seen in adult opossums, they made that transition when separated from their mothers. Adult pigs drink using a sucking mechanism, not a lapping one, where the tongue remains in the oral cavity and the head is down and snout immersed in milk (Herring and Scapino 1973). The nonsucking behavior we observed after palatal anesthesia is consistent with previous findings of two different feeding mechanisms existing simultaneously, prior to the adult mechanism, that is not exclusively dependent on maturation, but also environmental or, in this case, a sensory change.
The reason for the existence of two distinct groups of animals with different feeding mechanisms after palatal anesthesia is unclear. One explanation is that one-half of the infants had the anterior soft palate anesthetized due to the lesser palatine nerve being anesthetized. We reject this explanation because this sensory nerve is relatively very small and only provides sensation to the anterior region of the soft palate. Another explanation is that there is a developmental difference and that the feeding mechanism exhibited in group B after anesthesia could be an alternative feeding strategy seen in more developed animals. The variation in feeding kinematics in normal pigs, even after decerebration (German et al. 2009; Thexton et al. 2007), is large and well documented. Another potential explanation is that the rate of adaptation was different between animals and that, in the time between the injection and feeding session, some animals were able to adapt and start sucking quicker than others. There was no correlation between the order of treatments and whether the pigs had a group A or B response to anesthesia.
One explanation for the different responses to anesthesia could be their rate of development. Developmental differences in the role of oropharyngeal sensation during respiration have been noted in previous studies, and the same may be seen for the swallowing reflex as well (Litmanovitz et al. 1994; Penatti et al. 2006). Additionally, early studies of swallowing in decerebrate cats showed substantial variation in recordings from motor nerves following different oropharyngeal sensory stimulations that resulted in swallows in infants (Sumi 1967). That study showed that the brain stem developed postnatally for the first 3 mo of life, and that there was a large range of variation between animals. This could be due to developmental changes in myelination as is seen with other oropharyngeal sensory nerves (Miller and Dunmire 1976). In the present study, the variable response to anesthesia could reflect a varying stage of development of the reflex, as was seen in the previous studies.
An early study of rabbit chewing and swallowing found that, in response to lingual nerve stimulation, there were two distinct responses from hypoglossal nerves: either excitatory or inhibitory (Sumi 1970). The complexity of the hypoglossal nerve and its functional linkage to sensation from the trigeminal nerve branches in the tongue could explain why the tongue reacted so differently to anesthesia in the infant pigs. In one-half of the pigs (group B), the tongue was moving significantly more and was unable to latch to the nipple. Like sensation from the tongue, sensation of the palate is also linked to motor output from the tongue. More studies are needed to further understand the changing myelination of the trigeminal nerve and relative role of the sensory and motor nerves during swallowing throughout early postnatal development.
Sucking and swallowing after palatal local anesthesia.
All infants were able to adapt or compensate to reduced palatal sensation and elicit swallows. The reduction in swallowing frequency after palatal anesthesia in group B was most likely due to the inefficient oral transport cycles. Alternatively, palatal stimulation may influence the threshold to initiate the swallow. With anesthesia it was more difficult to reach that threshold. We found that the only parameter that was different in group A and group B during control feedings was swallowing frequency. Group B pigs had a slower swallowing frequency during control feedings. This could be a factor that could predict whether a pig will have a group A or group B response to anesthesia. Further testing of this hypothesis is needed.
The vomeronasal organ, which detects pheromones, was also most likely affected by the nasopalatine nerve block. In a study of opposums, they found that blocking the nasopalatine canal resulted in a change of food preference, but did not report any change in mastication or feeding ability (Poran 1998). It is unknown if the same would be seen in infant pigs; however, we did not notice any less willingness to feed after palatal anesthesia.
Sucking and swallowing following palatal saline.
Palatal saline could potentially provide analgesia due to pressure on the nerve, or pain due to the expansion of the soft tissues covering the palate. Although originally intended as a sham treatment, the possibility exists that it was another palatal sensory disruption. Little information exists to clarify these results. A study evaluating palatal sensory nerve blocks following palatoplasty in children found that a palatal saline injection produced variable results that were different from untreated patients, demonstrating that the saline treatment disrupted sensation (Jonnavithula et al. 2010). It is important to note that there was no way to know if the effects of both palatal saline and anesthesia were due to volume of fluid or the sensory effects that both most likely had.
The palatal saline treatment results were variable both between groups A and B and over the different outcome measures. Swallowing frequency increased only in group A with palatal saline, which indicates that there could have been a painful reaction that caused heightened sensory input that triggered swallow cycles more frequently. There was a statistically significant difference between group A and group B swallow frequency after palatal saline and after control feedings which points to a physiologically significant difference in their reactions.
The results indicate that palatal saline caused a sensory disruption that affected the initiation of suck cycles and did so in all pigs, regardless of group, causing them to be less frequent. Further studies are needed to determine if the palatal saline treatment caused pain or anesthesia and to determine how long that effect would have remained.
Cycle and phase relationships after palatal sensory disruption.
Thexton et al. postulated that a suck-swallow cycle could be divided into two phases in a manner similar to other rhythmic activities in mammals, one of which was stable in length, the other of which is function of the length of the entire cycle (Frigon and Gossard 2009; Thexton et al. 2012). The relationship between suck-swallow cycle and both phase lengths in the control and treatments was that described by Thexton et al. (2012) and Ding et al. (2013a). Our results support and refine these hypotheses. We found a stable phase 1 and a linear relationship between phase 2 and cycle length. The only exception was an extremely small slope that is marginally from zero.
This pattern of phase relationships exists even without sensory feedback in other models (Frigon and Gossard 2009; Gossard et al. 2011). While we found this pattern in the palatal saline and control, we did find one interesting difference in the response to palatal anesthesia. Group B with palatal anesthesia had a higher slope than group A with palatal anesthesia, indicating longer phase 2 lengths. Despite significant differences in their oral transport cycle lengths, the same linear, temporal relationship existed between cycle and phase lengths. Thus, while palatal anesthesia lengthened cycles and phase 2 for some animals, it did not change the basic pattern of phase relationships. This is in contrary to the finding of Ding et al. (2013a) that the relationship between cycle and phase 1 length changed after a unilateral superior laryngeal nerve lesion in infant pigs. It is evident that the pattern generator for the pharyngeal swallow must communicate with the pattern generator for the oral transport or suck cycles so it delays the onset of that next suck cycle in a predictable way. This relationship is mediated by the superior laryngeal nerve, and possibly other sensory nerves, but not the greater palatine or nasopalatine nerves. While one neurophysiology study indicated this network may exist and may indeed influence the coordination of sucking and swallowing, the interactions between the sucking pattern generator and the swallowing pattern generators in the brain stem need to be further investigated (Sessle and Storey 1972).
Implications for feeding neurophysiology and the role of the central nervous system.
This study provides evidence that trigeminal sensation directly affects the frequency of swallowing, oral transport, and the coordination of relevant pattern generators in the brain stem. Oropharyngeal sensory nerves synapse in and around the nucleus tractus solitarius (NTS) in the brain stem which is part of the CPG for swallowing. The motor program for the swallow originates in and around the nucleus ambiguus (NA) in the brain stem, which is also part of the CPG for swallowing, and is influenced by both the NTS and descending cortical input. Trigeminal afferents synapse in the trigeminal sensory nucleus in the brain stem; however, it is known that it also sends information to the NTS, which means it could also influence swallowing centers within the NTS or NA (Capra 1995; Sweazey and Bradley 1989). Alternatively, trigeminal sensation could influence the NA by altering descending cortical input. There is evidence from functional MRI that stimulating oral sensory receptors activates primary and secondary somatosensory cortex and thalamus (Lowell et al. 2008). It is clear that these interactions are not simple, and that feeding has complex regulation. Further investigation is needed to understand the exact connectivity between these nuclei in the brain stem and the cortex.
The relative importance of the hard palate sensation for initiating oral transport, or suck, cycles is a key finding since most feeding therapies for infants having trouble sucking are aimed at the perioral region. This study also showed that not only was initiation of the suck cycle affected in all animals, but in one-half of the animals, palatal anesthesia led to the inability to suck due to pathologically altered jaw and tongue movements.
The coordination between the swallowing and sucking pattern generators is fundamental to protecting the airway from aspiration (Barlow 2009; Lau and Hurst 1999). Our findings suggest that the swallowing and sucking pattern generators communicate to coordinate the start of the suck cycle that follows a pharyngeal swallow, and that this communication occurs even after sensory disruption to the hard palate.
Palatal sensation has a clear impact on normal oral and pharyngeal function in infant pigs. What requires further study are the causes of the individual differences that produced group A and group B. A developmental difference could be the reason why two distinct feeding mechanisms are seen after palatal anesthesia. Further evaluation of this data will help us understand how the motor pattern of the swallow, kinematics, and airway protection is affected by palatal anesthesia. Additional experiments as a function of developmental time could test this hypothesis.
GRANTS
This project was funded by a 2012 American Association for Dental Research Student Research Fellowship to S. D. Holman; National Institutes of Health (NIH) Grant T32 HD-007414 to support the training of R. Campbell-Malone; United Negro College Fund/Merck Science Initiative Postdoctoral Fellowship to R. Campbell-Malone; NIH Grant F30 DE-021944 to S. D. Holman; NIH Grant T32 DE-07309 to support the training of S. D. Holman; and NIH Grant R01 DC-03604 to R. Z. German.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.D.H. and R.Z.G. conception and design of research; S.D.H., R.C.-M., P.D., E.M.G.-N., and S.L.L. performed experiments; S.D.H. and D.R.W. analyzed data; S.D.H., D.R.W., and R.Z.G. interpreted results of experiments; S.D.H. prepared figures; S.D.H. drafted manuscript; S.D.H., D.R.W., R.C.-M., P.D., E.M.G.-N., S.L.L., and R.Z.G. edited and revised manuscript; S.D.H., D.R.W., R.C.-M., P.D., E.M.G.-N., S.L.L., and R.Z.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Melanie Albano, Kristy Koenig, and Drs. Rachel Cohen, Eric Hutchinson, and Dawn Ruben for veterinary assistance during these procedures. We also thank Anne Griffioen for assistance in animal care. Additionally, we thank Drs. Ianessa Humbert, Radi Masri, Jin Ro, and Maureen Stone for thoughtful discussions of the data.
Present addresses: S. L. Lukasik, Dept. of Pharmacology, University of Maryland School of Medicine, 655 W. Baltimore St., Baltimore, MD 21201; R. Z. German, Dept. of Anatomy and Neurobiology, Northeast Ohio Medical University, Rootstown, OH 44272.
REFERENCES
- Barlow SM. Central pattern generation involved in oral and respiratory control for feeding in the term infant. Curr Opin Otolaryngol Head Neck Surg 17: 187–193, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Book SA, Bustad LK. The fetal and neonatal pig in biomedical research. J Anim Sci 38: 997–1002, 1974 [DOI] [PubMed] [Google Scholar]
- Butler SG, Stuart A, Case LD, Rees C, Vitolins M, Kritchevsky SB. Effects of liquid type, delivery method, and bolus volume on penetration-aspiration scores in healthy older adults during flexible endoscopic evaluation of swallowing. Ann Otol Rhinol Laryngol 120: 288–295, 2011 [DOI] [PubMed] [Google Scholar]
- Campbell-Malone R, Crompton AW, Thexton AJ, German RZ. Ontogenetic changes in mammalian feeding: insights from electromyographic data. Integr Comp Biol 51: 282–288, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capra N. Mechanisms of oral sensation. Dysphagia 10: 235–247, 1995 [DOI] [PubMed] [Google Scholar]
- Cichero J, Nicholson T, Dodrill P. Liquid barium is not representative of infant formula: characterisation of rheological and material properties. Dysphagia 26: 264–271, 2011 [DOI] [PubMed] [Google Scholar]
- da Costa SP, van der Schans CP, Boelema SR, van der Meij E, Boerman MA, Bos AF. Sucking patterns in fullterm infants between birth and 10 weeks of age. Infant Behav Dev 33: 61–67, 2010 [DOI] [PubMed] [Google Scholar]
- Ding P, Campbell-Malone R, Holman S, Lukasik S, Fukuhara T, Gierbolini-Norat E, Thexton A, German R. Unilateral superior laryngeal nerve lesion in an animal model of dyphagia and its effect on suckling and swallowing. Dysphagia. In press, 2013a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding P, Campbell-Malone R, Holman SD, Lukasik SL, Gierbolini-Norat EM, Thexton AJ, German RZ. The effect of unilateral superior laryngeal nerve lesion on swallowing threshold volume. Laryngoscope. In press, 2013b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra EH, Caria PH, Rafferty KL, Herring SW. The buccinator during mastication: a function and anatomical evaluation in minipigs. Arch Oral Biol 55: 627–638, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara S, Kohzuki M, Sumi Y, Ebihara T. Sensory stimulation to improve swallowing reflex and prevent aspiration pneumonia in elderly dysphagic people. J Pharm Sci 115: 99–104, 2011 [DOI] [PubMed] [Google Scholar]
- Finan DS, Barlow SM. Intrinsic dynamics and mechanosensory modulation of non-nutritive sucking in human infants. Early Hum Dev 52: 181–197, 1998 [DOI] [PubMed] [Google Scholar]
- Frigon A, Gossard JP. Asymmetric control of cycle period by the spinal locomotor rhythm generator in the adult cat. J Physiol 587: 4617–4627, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- German RZ, Crompton AW. The ontogeny of feeding in mammals. In: Feeding: Form, Function and Evolution in Tetrapod Vertebrates, edited by Schwenk K. San Diego, CA: Academic, 2000, p. 449–457 [Google Scholar]
- German RZ, Crompton AW. Ontogeny of suckling mechanisms in opossums (Didelphis virginiana). Brain Behav Evol 48: 157–164, 1996 [DOI] [PubMed] [Google Scholar]
- German RZ, Crompton AW, Hertweck DW, Thexton AJ. Determinants of rhythm and rate in suckling. J Exp Zool 278: 1–8, 1997 [DOI] [PubMed] [Google Scholar]
- German RZ, Crompton AW, Levitch LC, Thexton AJ. The mechanism of suckling in two species of infant mammal: miniature pigs and long-tailed macaques. J Exp Zool 261: 322–330, 1992 [DOI] [PubMed] [Google Scholar]
- German RZ, Crompton AW, Owerkowicz T, Thexton AJ. Volume and rate of milk delivery as determinants of swallowing in an infant model (Sus scrofia). Dysphagia 19: 147–154, 2004a [DOI] [PubMed] [Google Scholar]
- German RZ, Crompton AW, Thexton AJ. Integration of the reflex pharyngeal swallow into rhythmic oral activity in a neurologically intact pig model. J Neurophysiol 102: 1017–1025, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- German RZ, Crompton AW, Thexton AJ. The role of animal models in understanding feeding behavior in infants. Int J Orofacial Myology 30: 20–30, 2004b [PubMed] [Google Scholar]
- German RZ, Crompton AW, Thexton AJ. Variation in EMG activity: a hierarchical approach. Integr Comp Biol 48: 272–282, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- German RZ, Franks HA. Timing in the movement of jaws, tongue and hyoid during feeding in the hyrax, Procavia syriacus. J Exp Zool 257: 34–42, 1991 [DOI] [PubMed] [Google Scholar]
- Gossard JP, Sirois J, Noue P, Cote MP, Menard A, Leblond H, Frigon A. Chapter 2: The spinal generation of phases and cycle duration. Prog Brain Res 188: 15–29, 2011 [DOI] [PubMed] [Google Scholar]
- Herring SW, Scapino RP. Physiology of feeding in miniature pigs. J Morphol 141: 427–460, 1973 [DOI] [PubMed] [Google Scholar]
- Holman SD, Gierbolini-Norat EM, Lukasik SL, Campbell-Malone R, Ding P, German RZ. Duration of action of bupivacaine hydrochloride used for palatal sensory nerve block in infant pigs. J Vet Dent. In press, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman SD, Konow N, Lukasik SL, German RZ. Regional variation in geniohyoid muscle strain during suckling in the infant pig. J Exp Zool 317: 359–370, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonnavithula N, Durga P, Madduri V, Ramachandran G, Nuvvula R, Srikanth R, Damalcheruvu MR. Efficacy of palatal block for analgesia following palatoplasty in children with cleft palate. Pediatr Ann 20: 727–733, 2010 [DOI] [PubMed] [Google Scholar]
- Konow N, Thexton AJ, Crompton AW, German RZ. Regional differences in length-change and electromyographic heterogeneity in the sternohyoid muscle during infant mammalian swallowing. J Appl Physiol 109: 439–448, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C, Hurst N. Oral feeding in infants. Curr Probl Pediatr 29: 105–124, 1999 [DOI] [PubMed] [Google Scholar]
- Lee SI, Yoo JY, Kim M, Ryu JS. Changes of timing variables in swallowing of boluses with different viscosities in patients with dysphagia. Arch Phys Med Rehabil 94: 120–126, 2013 [DOI] [PubMed] [Google Scholar]
- Litmanovitz I, Dreshaj I, Miller MJ, Haxhiu MA, Martin RJ. Central chemosensitivity affects respiratory muscle responses to laryngeal stimulation in the piglet. J Appl Physiol 76: 403–408, 1994 [DOI] [PubMed] [Google Scholar]
- Loeb G, Gans C. Electromyography for Experimentalists. Chicago, IL: University of Chicago Press, 1986, p. 119–120 [Google Scholar]
- Lowell S, Poletto C, Knorr-Chung B, Reynolds R, Simonyan K, Ludlow C. Sensory stimulation activates both motor and sensory components of the swallowing system. Neuroimage 42: 285–295, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michou E, Mastan A, Ahmed S, Mistry S, Hamdy S. Examining the role of carbonation and temperature on water swallowing performance: a swallowing reaction-time study. Chem Senses 37: 799–807, 2012 [DOI] [PubMed] [Google Scholar]
- Miller AJ, Dunmire CR. Characterization of the postnatal development of superior laryngela nerve fibers in the postnatal kitten. J Neurobiol 7: 483–494, 1976 [DOI] [PubMed] [Google Scholar]
- Penatti EM, Berniker AV, Kereshi B, Cafaro C, Kelly ML, Niblock MM, Gao HG, Kinney HC, Li A, Nattie EE. Ventilatory response to hypercapnia and hypoxia after extensive lesion of medullary serotonergic neurons in newborn conscious piglets. J Appl Physiol 101: 1177–1188, 2006 [DOI] [PubMed] [Google Scholar]
- Poran NS. Vomeronasal organ and its associated structures in the opossum Monodelphis domestica. Microsc Res Tech 43: 500–510, 1998 [DOI] [PubMed] [Google Scholar]
- Reuss-Lamky H. Administering dental nerve blocks. J Am Anim Hosp Assoc 43: 298–305, 2007 [DOI] [PubMed] [Google Scholar]
- Sessle B, Storey A. Periodontal and facial influences on the laryngeal input to the brain stem of the cat. Arch Oral Biol 17: 1583–1595, 1972 [DOI] [PubMed] [Google Scholar]
- Steele CM, Miller AJ. Sensory input pathways and mechanisms in swallowing: a review. Dysphagia 25: 323–333, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi T. Changes of hypoglossal nerve activity during inhibition of chewing and swallowing by lingual nerve stimulation. Pflügers Arch 317: 303–309, 1970 [DOI] [PubMed] [Google Scholar]
- Sumi T. The nature and postnatal development of reflex deglutition in the kitten. Jpn J Physiol 17: 200–210, 1967 [DOI] [PubMed] [Google Scholar]
- Sweazey RD, Bradley RM. Responses of neurons in the lamb nucleus tractus solitarius to stimulation of the caudal oral cavity and epiglottis with different stimulus modalities. Brain Res 480: 133–150, 1989 [DOI] [PubMed] [Google Scholar]
- Thexton A. Oral reflexes elicited by mechanical stimulation of palatal mucosa in the ca. Arch Oral Biol 18: 971–980, 1973 [PubMed] [Google Scholar]
- Thexton A, Crompton A, German R. EMG activity in hyoid muscles during pig suckling. J Appl Physiol 112: 1512–1519, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thexton AJ. Tongue and Hyoid Movement in the Cat. Tokyo: Quintessence, 1981, p. 301–312 [Google Scholar]
- Thexton AJ, Crompton AW, German RZ. Electromyographic activity during the reflex pharyngeal swallow in the pig: Doty and Bosma (1956) revisited. J Appl Physiol 102: 587–600, 2007 [DOI] [PubMed] [Google Scholar]
- Thexton AJ, Crompton AW, German RZ. Transition from suckling to drinking at weaning: a kinematic and electromyographic study in miniature pigs. J Exp Zool 280: 327–343, 1998 [DOI] [PubMed] [Google Scholar]
- Thexton AJ, Crompton AW, Owerkowicz T, German RZ. Impact of rhythmic oral activity on the timing of muscle activation in the swallow of the decerebrate pig. J Neurophysiol 101: 1386–1393, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver ME, Jump EB, McKean CF. The eruption pattern of permanent teeth in miniature swine. Arch Oral Biol 14: 323-IN312, 1969 [DOI] [PubMed] [Google Scholar]
- Yamamura K, Kitagawa J, Kurose M, Sugino S, Takatsuji H, Mostafeezur RM, Zakir HM, Yamada Y. Neural mechanisms of swallowing and effects of taste and other stimuli on swallow initiation. Biol Pharm Bull 33: 1786–1790, 2010 [DOI] [PubMed] [Google Scholar]