Abstract
In the visual thalamus, retinal afferents activate both local interneurons and excitatory thalamocortical relay neurons, leading to robust feedforward inhibition that regulates the transmission of sensory information from retina to neocortex. Peculiarly, this feedforward inhibitory pathway is dominated by presynaptic dendrites. Previous work has shown that the output of dendritic terminals of interneurons, also known as F2 terminals, are regulated by both ionotropic and metabotropic glutamate receptors. However, it is unclear whether both classes of glutamate receptors regulate output from the same or distinct dendritic terminals. Here, we used focal glutamate uncaging and whole cell recordings to reveal two types of F2 responses in rat visual thalamus. The first response, which we are calling a Type-A response, was mediated exclusively by ionotropic glutamate receptors (i.e., AMPA and NMDA). In contrast, the second response, which we are calling a Type-B response, was mediated by a combination of ionotropic and type 5 metabotropic glutamate receptors (i.e., mGluR5). In addition, we demonstrate that both F2 responses are evoked in the same postsynaptic neurons, which are morphologically distinct from neurons in which no F2 responses are observed. Since photostimulation was relatively focal and small in magnitude, these results suggest distinct F2 terminals, or small clusters of terminals, could be responsible for generating the two inhibitory responses observed. Because of the nature of ionotropic and metabotropic glutamate receptors, we predict the efficacy by which the retina communicates with the thalamus would be strongly regulated by 1) the activity level of a given retinogeniculate axon, and 2) the specific type of F2 terminals activated.
Keywords: dorsal lateral geniculate nucleus, presynaptic dendrite, glutamate uncaging, two-photon imaging, interneuron
the thalamus is the entry point through which sensory information must pass before entering the neocortex. In the visual thalamus (i.e., dorsal lateral geniculate nucleus, dLGN), there are two classes of neurons excited by retinal afferents: excitatory relay neurons that project to layer IV visual cortex and inhibitory interneurons that project to relay neurons and each other (Sherman and Guillery 1996). The GABAergic connections made by thalamic interneurons, in turn, form a retinogeniculate-driven feedforward inhibitory circuit, which is thought to enhance stimulus selectivity, improve sensory coding, and ensure temporal precision of spiking (Wang et al. 2011).
Peculiarly, this local inhibitory circuit is dominated by dendrodendritic synapses formed between dendrites of interneurons and relay neurons (Famiglietti and Peters 1972; Guillery 1969; Hamos et al. 1985; Lieberman 1973; Montero 1986; Ohara et al. 1983; Ralston 1971). These dendritic terminals (i.e., F2 terminals) are often found in triads, a synaptic arrangement in which an interneuron dendrite is presynaptic to a relay neuron dendrite and postsynaptic to an excitatory retinogeniculate terminal that, in turn, targets the same postsynaptic relay dendrite (Sherman 2004). Since F2 terminals are located on distal dendrites of interneurons and are in close proximity to excitatory input, these terminals are thought to integrate local subthreshold synaptic information independently of the axon as well as each other (Bloomfield and Sherman 1989; Cox et al. 1998; Crandall and Cox 2012). In theory, this would allow thalamic interneurons to operate as multiplexors, containing numerous independently operating input-output devices (i.e., F2 terminals).
Considering F2 terminals are postsynaptic to excitatory afferents (Hamos et al. 1985), glutamate must play a major role in regulating dendrodendritic communication in thalamus. Currently, there is substantial evidence suggesting GABA release from F2 terminals is regulated by the activation of metabotropic glutamate receptors (mGluRs; Cox and Sherman 2000; Cox et al. 1998; Godwin et al. 1996; Govindaiah and Cox 2004, 2006) and ionotropic glutamate receptors [iGluRs; dl-α-amino-3-hydroxy-5-methylisoxazole-propionic acid (AMPA) and N-methyl-d-aspartate (NMDA) receptors; Acuna-Goycolea et al. 2008; Blitz and Regehr 2005; Cox and Sherman 2000; Crandall and Cox 2012]. However, it is unclear whether F2 terminals vary in their complement of receptors. Addressing this question is a critical step toward understanding how thalamic interneurons process and modulate visual information before entering the neocortex.
In this study, we used focal glutamate uncaging to study local synaptic connections between presynaptic dendrites of rat thalamic inhibitory interneurons and relay neurons (Crandall and Cox 2012). Combining this approach with in vitro whole cell recordings and two-photon laser-scanning microscopy, we identified two types of F2 responses based on their glutamate receptor regulation. Our data suggest that the efficacy, by which the retina communicates with the thalamus and subsequent information transfer to the neocortex, would be strongly regulated by the activity level of retinal ganglion cells and the specific feedforward inhibitory pathway engaged by the retinogeniculate afferents.
MATERIALS AND METHODS
Slice preparation.
All experimental procedures were carried out in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and approved by the University of Illinois Animal Care and Use Committee. Tissue sections were prepared from young Sprague-Dawley rats (postnatal age: 13–20 days) of either sex as previously described (Crandall et al. 2010). Briefly, rats were deeply anesthetized with pentobarbital sodium (50 mg/kg) and perfused with cold, oxygenated slicing solution before being decapitated. The brains were quickly removed and immediately placed in cold (4°C), oxygenated (5% CO2-95% O2) slicing solution containing (in mM): 2.5 KCl, 1.25 NaH2PO4, 10.0 MgSO4, 0.5 CaCl2, 26.0 NaHCO3, 10.0 glucose, and 234.0 sucrose. Coronal slices (∼300 μm) containing dLGN were obtained using a vibrating tissue slicer. Once sliced, tissue sections were immediately transferred to a holding chamber containing oxygenated, physiological saline solution and incubated for 15–20 min at 32 ± 1°C and an additional 60 min at room temperature. The physiological solution was continuously oxygenated (5% CO2-95% O2, pH 7.4) and contained (in mM): 126.0 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2.0 MgCl2, 2.0 CaCl2, 26.0 NaHCO3, and 10.0 glucose.
Whole cell recording procedures.
For recording, individual tissue sections were transferred to a submersion type recording chamber maintained at 32 ± 1°C and continuously superfused (2.5–3 ml/min) with recirculating oxygenated physiological saline. Individual neurons were visualized using a BX51WI fixed-stage microscope equipped with Dodt contrast optics and a water-immersion objective (×60, 0.9-numerical aperture; Olympus). Electrophysiological data were acquired using a MultiClamp 700A amplifier filtered at 2–3 kHz and digitized at 10 kHz using a Digidata 1440A digitizer in combination with pCLAMP 10 software (Molecular Devices). For voltage-clamp recordings, patch pipettes had tip resistances of 3–6 MΩ when filled with a cesium-based internal solution containing (in mM): 117.0 cesium gluconate, 13.0 CsCl, 1.0 MgCl2, 0.07 CaCl2, 0.1 EGTA, 10 HEPES, 2.0 Na2-ATP, and 0.4 Na-GTP (pH 7.3, 290 mOsm). To isolate inhibitory postsynaptic currents (IPSCs) better, all voltage-clamp experiments were performed at a command voltage of ∼0 mV after correcting for a liquid junction potential (∼10 mV). For current-clamp recordings, pipettes contained (in mM): 117.0 potassium gluconate, 13.0 KCl, 1.0 MgCl2, 0.07 CaCl2, 0.1 EGTA, 10.0 HEPES, 2.0 Na2-ATP, and 0.4 Na-GTP (pH 7.3, 290 mOsm). Pipettes also contained Alexa Fluor 594 (50 μM) to allow for imaging. During recordings, the pipette capacitance was neutralized, and access resistance was continually monitored.
Pharmacological agents were prepared and stored as recommended by the manufacturer and subsequently diluted in physiological saline just before use. All pharmacological agents were bath-applied at least 10 min before subsequent experimental tests. TTX, 6,7-dinitroquinoxaline-2,3-dione (DNQX), 3-((R)-2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP), and 2-methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP) were all purchased from Tocris Bioscience. cis-[Ru(bpy)2(PMe3)GluH2](PF6)2 [with bpy = 2,2′-bipyridine and PMe3 = trimethylphosphine (RuBi-Glutamate)], 2-(3-carboxypropyl)-3-amino-6-(4-methoxyphenyl)-pyridazinium bromide (SR-95531), and (S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid (LY-367385) were purchased from Tocris Bioscience and Ascent Scientific.
Two-photon imaging and single-photon glutamate uncaging.
Individual relay neurons were filled via recording pipette with Alexa Fluor 594 (50 μM; Molecular Probes) and imaged by laser excitation (820 nm) using a two-photon laser-scanning microscopy system (Ultima; Prairie Technologies) coupled with a Ti:Sapphire laser (Mai Tai HP; Spectra-Physics). To photorelease glutamate, a single-photon visible laser (405 nm) was coupled into the scan head with a photoactivation module and focused at a relay neuron dendrite using a second set of galvanometers (Prairie Technologies). A short-duration laser pulse (0.1–3.0 ms) was used, and the laser intensity was adjusted to just above response threshold. Bath application of RuBi-Glutamate (100 μM) was chosen because of its high quantum efficiency and reduced antagonistic effects on GABAergic transmission compared with other caged-glutamate compounds (Fino et al. 2009). The extent of glutamate diffusion following photorelease was determined by varying the lateral distance between the dendrite and the center of the light beam using basal dendrites of layer V pyramidal neurons. The relationship between lateral position and the magnitude of the glutamate response was described by a single Gaussian function, and the half-width was used to determine spread.
Data analyses.
Detection and analysis of IPSC activity were performed offline using Mini Analysis software (Synaptosoft). All events were detected automatically by the software and verified post hoc by visual analysis. The amplitude threshold (8–12 pA) was adjusted above baseline noise level recorded in the presence of a GABAA receptor antagonist (SR-95531; 10 μM). It should be noted that any frequency analysis of miniature IPSC (mIPSC) activity is confounded by the background release arising from F1 terminals and therefore will have impacted both the calculated baseline activity and peak F2-mediated response. Calculating the change in IPSC activity in response to (R,S)-3,5-dihydroxyphenylglycine (DHPG) application was accomplished using two different measures: IPSC frequency and the root mean square (RMS) of the current recording. The change in IPSC frequency in response to DHPG was determined by subtracting the average baseline frequency, over a 2-min period, from the peak DHPG response, over a 5-s period. The change in RMS, which is a measure of the power of IPSC activity, was calculated using pCLAMP 10 software. Briefly, the RMS was calculated across a single 10-s sweep, and the change in response to DHPG was determined by subtracting the average baseline activity (2 min; 12 sweeps) from the peak DHPG response over a 10-s period (1 sweep). The net change in charge during the late phase of both Type-A and Type-B responses was calculated from baseline by integrating the current signal over a 5-s period beginning 1 s after the onset of the stimulation. To classify a given relay neuron as iGluR-sensitive or iGluR-insensitive, proximal dendritic locations were stimulated with different laser powers and durations. We have previously shown the vast majority of F2 responses will be found on proximal dendrites (Crandall and Cox 2012). For a cell to be classified as iGluR-sensitive, just a single location needed to generate a glutamate-evoked increase in IPSC activity (gluIPSC). In contrast, a cell was classified as iGluR-insensitive if it did not produce a single detectable gluIPSC response after stimulating 30–50 different locations.
For morphological analyses, we acquired stacked images of the cell (5.12 pixels/μm; 0.25-μm steps in z-axis) and reconstructed it using NIH ImageJ Software. Dendritic orientation was quantified using a Sholl ring analysis (Friedlander et al. 1981; Krahe et al. 2011). For each neuron, five concentric rings at 20-μm intervals were centered on the soma. The outermost ring (100 μm) was large enough to encompass all but the tips of the longest dendrites. To determine dendritic orientation, dendritic arbors were divided into four quadrants by two perpendicular lines passing through the center of the soma. Relay neurons were then oriented so that the maximum number of dendritic intersections (quantified by Sholl analyses) occurred along the a-axial plane (Krahe et al. 2011). The ratio of dendritic intersections in the b-plane vs. the a-plane (i.e., minimum/maximum) was then taken as an index of dendritic orientation (DOi). Thus a purely symmetrical neuron would have a DOi of 1.0, and a purely bipolar neuron would be 0.0 (Krahe et al. 2011). When counting branch points, only primary branches that appeared intact (i.e., not cut during the sectioning process) were included. Unless otherwise indicated, population data are expressed as means ± standard deviation and significance defined as P < 0.05.
RESULTS
To explore the functional regulation of inhibitory dendrodendritic synapses, we used single-photon glutamate uncaging to evoke local GABA release from dendrites of rat thalamic interneurons (Crandall and Cox 2012). Since caged-glutamate compounds antagonize inhibitory activity, we used RuBi-Glutamate because it produces the weakest block of GABAergic transmission and has been used previously to study inhibitory synaptic connections (Crandall and Cox 2012; Fino et al. 2009; Fino and Yuste 2011; Packer and Yuste 2011). At the concentration used in this study, RuBi-Glutamate (100 μM) attenuated the frequency and amplitude of mIPSCs by 30.9 ± 11.3 and 16.7 ± 9.0%, respectively (Control TTX: 9.2 ± 2.6 Hz, 22.2 ± 4.2 pA; +RuBi-Glutamate: 6.4 ± 2.1 Hz, 18.3 ± 2.9 pA, n = 6).
To locate a dendrodendritic connection between an inhibitory F2 terminal and postsynaptic relay neuron, we photoreleased RuBi-Glutamate (0.1–3.0 ms; λ = 405 nm) near a dendrite of a dLGN relay neuron filled with Alexa Fluor 594 (50 μM) and recorded inhibitory activity in the same relay neuron (Fig. 1A). Because previous anatomic and physiological studies have shown F2 terminals preferentially target the proximal dendrites of relay neurons (Crandall and Cox 2012; Hamos et al. 1987; Wilson et al. 1984), we focused our search to dendrites located within 10–50 μm of the soma. To eliminate glutamate-generated action potentials in interneurons and thus axonal output (F1 terminals) from these cells, the voltage-gated sodium channel blocker TTX (1 μM) was present in all experiments. Since our previous work has shown glutamate does not act directly on F1 terminals (Cox et al. 1998; Crandall and Cox 2012), any change in mIPSC frequency will result from the local and selective activation of dendritic F2 terminals.
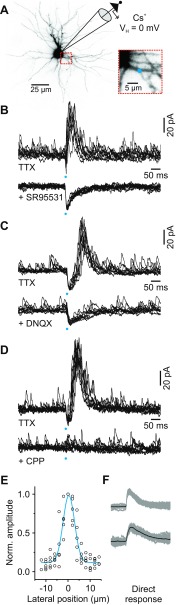
Fig. 1.
Glutamate uncaging evokes ionotropic glutamate receptor (iGluR)-mediated GABA release from the presynaptic dendrites of thalamic interneurons. A: image of rat thalamic relay neuron filled with Alexa Fluor 594 (50 μM). Inhibitory activity in the cell was monitored using a cesium (Cs+) pipette and a command voltage (VH) of 0 mV. Inset: an image showing the dendrite targeted for single-photon glutamate uncaging (blue dot). B, top: in TTX (1 μM), 10 control responses produced by uncaging glutamate at the location shown in A. Bottom: 10 responses obtained from the same location after adding the GABAA receptor antagonist SR-95531 (10 μM). C, top: in TTX (1 μM), 10 control responses from a different relay neuron produced by uncaging glutamate. Bottom: 10 responses obtained from the same location after adding the dl-α-amino-3-hydroxy-5-methylisoxazole-propionic acid (AMPA) receptor antagonist 6,7-dinitroquinoxaline-2,3-dione (DNQX; 20 μM). D, top: in TTX (1 μM), 10 control responses from a different relay neuron produced by uncaging glutamate. Bottom: 10 responses obtained from the same location after adding the N-methyl-d-aspartate (NMDA) receptor antagonist 3-((R)-2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP; 10 μM). E: plot showing the relationship between the lateral position of the laser beam and the normalized (Norm.) amplitude of the glutamate-evoked response. The blue line is a single Gaussian function fit. F: in TTX (1 μM), direct excitatory responses recorded from 3 different relay neurons. The black line indicates the average of 20 responses (gray traces).
When systematically photostimulating the dendrites of relay neurons, we identified a dendrodendritic connection as a gluIPSC that was associated with the direct photostimulation of the postsynaptic relay neuron (Fig. 1B). GluIPSCs were completely blocked by antagonist SR-95531 (10 μM, n = 6), indicating that the responses were mediated by GABAA receptors on the postsynaptic relay neuron (Fig. 1B). GluIPSCs were also blocked by the AMPA receptor antagonist DNQX (20–40 μM, n = 7) and the NMDA receptor antagonist CPP (10–20 μM, n = 4; Fig. 1, C and D). These results are consistent with a feedforward inhibitory mechanism that depends on fast glutamatergic excitation of a presynaptic interneuron dendrite via iGluRs and are qualitatively similar to the TTX-insensitive responses we have previously shown to be F2 terminal-mediated (Cox and Sherman 1999, 2000; Crandall and Cox 2012). To determine the size of the active area during photostimulation, we performed additional experiments in which we measured the relationship between the peak amplitude of a glutamate-induced response and the lateral position of the light beam relative to an isolated dendrite (Fig. 1E). From these experiments, we estimated that the lateral full-width-at-half-maximal (FWHM) diameter of the stimulation was 5.8 ± 1.2 μm (n = 5). To gain insight into the magnitude of excitation with photostimulation, we also examined the direct excitatory response on primary dendrites (10–20 μm from soma) of relay neurons (Fig. 1F). While in TTX (1 μM) and at a membrane potential of −65 mV, photostimulation resulted in a mean amplitude of 1.4 ± 0.4 mV (n = 6). Thus photostimulating local GABA release from a presynaptic dendrite was focal, small in amplitude, and reliable.
iGluRs and mGluRs mediate dendrodendritic inhibitory activity in the same relay neuron.
Since previous studies have demonstrated dendritic release is regulated by both iGluRs and mGluRs (Acuna-Goycolea et al. 2008; Cox et al. 1998; Crandall and Cox 2012; Govindaiah and Cox 2006), we asked whether both classes of glutamate receptors regulated dendritic output onto the same or distinct populations of relay neurons. To address this issue, we combined the one-photon photostimulation method with a previously established agonist application method to selectively activate F2 terminals regulated by different classes of glutamate receptors (Cox and Sherman 2000; Cox et al. 1998). Since glutamate-mediated changes in inhibitory activity are not observed in all relay neurons (Cox et al. 1998; Crandall and Cox 2012), we initially identified relay neurons as iGluR-sensitive or iGluR-insensitive based on the presence or absence of gluIPSCs, respectively. Once identified, we then bath-applied the group I mGluR agonist DHPG (25 μM; 15–25 s) to test whether the same cell received input from F2 terminals regulated by mGluRs. Previous work has demonstrated that many F2 terminals contain type 5 mGluRs (mGluR5) and that DHPG application will activate these terminals, leading to a robust TTX-insensitive increase in IPSC activity (Godwin et al. 1996; Govindaiah and Cox 2006).
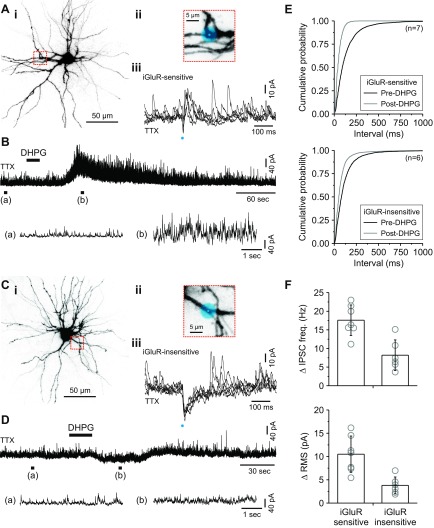
For relay neurons identified as iGluR-sensitive, DHPG application consistently produced a robust increase in IPSC activity as demonstrated by a significant decrease in the inter-IPSC interval (P < 0.001, Kolmogorov-Smirnov test; Fig. 2, A, B, and E). In iGluR-insensitive relay neurons, DHPG application produced a weaker but statistically significant reduction in the inter-IPSC interval (P < 0.001, Kolmogorov-Smirnov test; Fig. 2, C, D, and E). To test whether the magnitude of change was different between identified relay neurons, we compared the peak change in IPSC frequency after DHPG application. On average, DHPG produced a significantly larger change in IPSC frequency in the iGluR-sensitive population than in the iGluR-insensitive population (iGluR-sensitive: 17.6 ± 4.1 Hz, n = 7; iGluR-insensitive: 8.2 ± 4.1 Hz, n = 6; P < 0.01, Student's t-test; Fig. 2F). Similar results were found when calculating the change in the RMS of the current recording, which is a measure of the power of IPSC activity (iGluR-sensitive: 10.5 ± 3.9 pA, n = 7; iGluR-insensitive: 3.8 ± 1.8 pA, n = 6; P < 0.01, Student's t-test; Fig. 2F). These results indicate that the same postsynaptic relay neuron receives inhibitory input from both iGluR- and mGluR-mediated F2 terminals (i.e., fast-transient and slow-longer-lasting, respectively).
Fig. 2.
Both iGluRs and metabotropic glutamate receptors (mGluRs) mediate dendrodendritic activity in the same relay neuron. A, i: image of a thalamic relay neuron. ii, Inset: image of the dendrite targeted for glutamate uncaging (blue dot). iii: In TTX (1 μM), 5 control responses produced by uncaging at the location shown. The glutamate-evoked increase in inhibitory postsynaptic current (IPSC) activity (gluIPSC) indicates that the cell received iGluR-sensitive F2 input. B, top: subsequent application of the group I mGluR agonist (R,S)-3,5-dihydroxyphenylglycine (DHPG; 25 μM) to the same neuron produced a robust increase in F2-mediated IPSC activity. Bottom: expanded traces before (a) and after (b) DHPG application are shown and correspond to the region indicated. C, i: image of a thalamic relay neuron. ii, Inset: image of the dendrite targeted for glutamate uncaging (blue dot). iii: In TTX (1 μM), 5 control responses produced by uncaging at the location shown. The lack of a gluIPSC indicates that the cell did not receive iGluR-sensitive F2 input. D, top: subsequent application of DHPG did not produce a robust change in F2-mediated IPSC activity. Bottom: expanded traces before (a) and after (b) DHPG application are shown and correspond to the region indicated. E: cumulative probability plots showing a significant decrease in the interevent interval with DHPG application for both cell types. F: summary of the peak change in IPSC frequency (freq.) and root mean square (RMS) for both populations after DHPG application.
Morphological correlates.
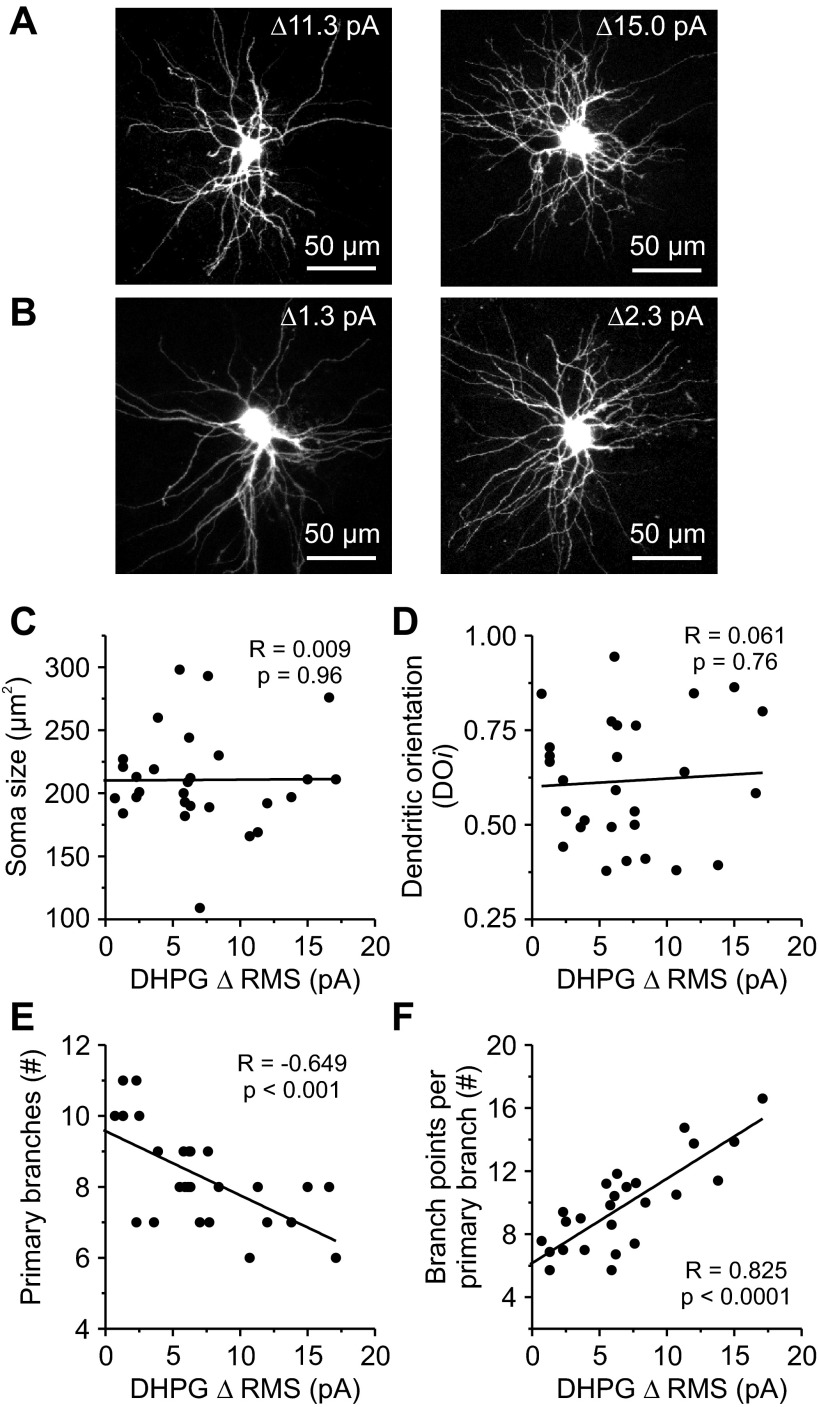
Given the results of the previous experiment and the use of bath-applied mGluR agonist, we sought to determine whether relay neurons could be grouped into morphologically distinct classes based on inhibitory input from F2 terminals. This is of interest because, for many mammals such as cats and nonhuman primates, the visual system consists of separate but parallel retino-geniculo-cortical pathways (Nassi and Callaway 2009; Sherman and Spear 1982). To approximate the strength of F2 terminal inhibition onto an individual relay neuron, we measured the peak change in IPSC activity after brief bath application of DHPG (25 μM; 15–25 s) in the presence of TTX. In total, 30 thalamocortical relay neurons from animals ranging in age from postnatal day 13 to 17 were tested and then reconstructed from serial images of the recorded neuron obtained from the 2-photon microscope (Fig. 3, A and B). Overall, the change in IPSC activity in response to DHPG, as measured by the RMS of the current recording, was normally distributed (P = 0.07, Shapiro-Wilk test; range: minimum = −0.5 pA, maximum = 17.1 pA, n = 30). Similar results were obtained when measuring the change in frequency in response to DHPG (P = 0.23, Shapiro-Wilk test; range: minimum = 3.7 Hz, maximum = 24.8 Hz, n = 30). When we calculated the soma size, we found no significant correlation with DHPG sensitivity (r = 0.009, P = 0.96; n = 29; Fig. 3C). To assess whether dendritic geometry was related to DHPG sensitivity, we used a modified Sholl ring analysis to quantify DOi (see materials and methods; Friedlander et al. 1981; Krahe et al. 2011). Using this method, a purely symmetrical neuron would have a DOi of 1.0, and a purely bipolar neuron would be 0.0 (Krahe et al. 2011). Overall, the DOi of rat dLGN neurons was not significantly correlated with DHPG sensitivity (r = 0.061, P = 0.76; n = 28; Fig. 3D). Although there was no relationship between DOi and DHPG sensitivity, we did find that the number of primary branches a relay neuron had was negatively correlated with DHPG sensitivity (r = −0.649, P < 0.001; n = 28; Fig. 3E). Considering the number of dendritic intersections of a given relay neuron (quantified by Sholl analyses) did not correlate with DHPG sensitivity (r = −0.019, P = 0.92; n = 28; data not shown), these results suggested that relay neurons with strong DHPG sensitivity could have more branch points per primary branch. Therefore, we proceeded to calculate the average number of branch points per primary branch for each relay neuron. As shown in Fig. 3F, the average number of branch points per primary branch was strongly and significantly correlated with DHPG sensitivity (r = 0.825, P < 0.0001; n = 26; Fig. 3F). These results suggest that arbor complexity, as measured by branch points and not dendritic orientation, is a key morphological feature associated with rat relay neurons innervated by inhibitory F2 terminals.
Fig. 3.
The relationship between morphology and F2 innervation for rat thalamic relay neurons. A: images of thalamic relay neurons with “strong” F2 innervation as determined by their DHPG sensitivity (i.e., ΔRMS ≥ 10 pA). B: images of thalamic relay neurons with “weak” F2 innervation as determined by their DHPG sensitivity (i.e., ΔRMS ≤ 5 pA). C–F: group data showing morphological relationships with F2 innervation characterized by a DHPG change in inhibitory activity (i.e., ΔRMS). C: soma size was not correlated with DHPG sensitivity. D: dendritic orientation (DOi) was not correlated with DHPG sensitivity. E: primary branches decreased significantly with DHPG sensitivity. #, Number of. F: branch points per primary branch increased significantly with DHPG sensitivity.
Two distinct types of F2 terminals mediate dendrodendritic inhibition in thalamus.
From our earlier studies, we found that not only do iGluR- and mGluR-mediated output from F2 terminals presynaptic to the same cell, but also these dendritic terminals preferentially target a morphologically distinct population of rat relay neurons. We next asked whether iGluRs and mGluRs regulate output from the same or distinct F2 terminals. To address this, we wanted to photostimulate local iGluR- and mGluR-mediated responses from the same dendrodendritic connection. Considering mGluR-mediated responses in dLGN are evoked following high-frequency, tetanic electrical stimulation of afferent fibers (Govindaiah and Cox 2004; McCormick and von Krosigk 1992), we first determined whether photoreleased glutamate could repeatedly and reliably evoke a local mGluR response in our slice preparation. This was done by directly stimulating a relay neuron dendrite with tetanic photostimulation (10 pulses, 1.5-ms duration, 100 Hz; Fig. 4A). To isolate the response better, experiments were performed with SR-95531 (10 μM) and TTX (1 μM) in the bath to eliminate any glutamate-evoked GABAergic activity or action potentials, respectively. Since previous anatomic and physiological studies have shown that mGluRs on relay neurons are opposite corticothalamic terminals located along intermediate-distal dendrites (Godwin et al. 1996; McCormick and von Krosigk 1992; Wilson et al. 1984), photostimulation was limited to second-order branches (25–60 μm from soma).
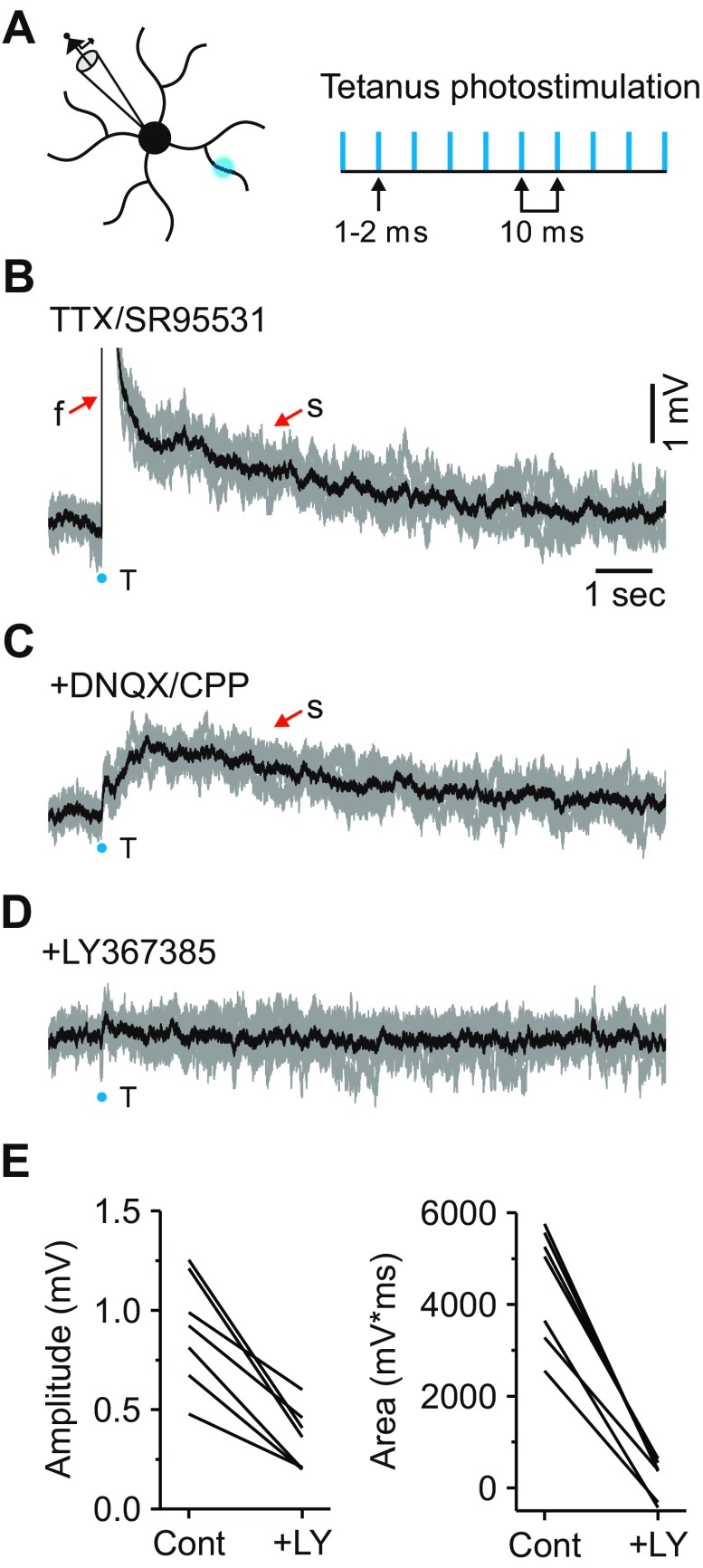
Fig. 4.
Tetanic photostimulation reliably activates local mGluRs. A: schematic illustrating how mGluRs were locally activated using tetanic photostimulation protocol (10 pulses, 1–2 ms, 100 Hz). B: in TTX (1 μM) and SR-95531 (10 μM), 5 individual responses produced by tetanic photostimulation (gray traces). The onset of the stimulus is shown by the blue dot, and average response in black. Under these conditions, tetanic photostimulation (T) resulted in both a fast (f; truncated) and slow (s) excitatory potential. C: subsequent application of iGluR blockers (DNQX: 20–40 μM; CPP: 10–20 μM) eliminated the fast potential while leaving the slow potential unaffected. D: the slow potential was attenuated with the addition of the mGluR1 antagonist LY-367385 (100 μM). E: summary of the effects of LY-367385 (LY) on the slow excitatory potential isolated with iGluR blockers (Cont).
At a membrane potential of −65 mV, tetanic photostimulation of a relay neuron dendrite resulted in a fast depolarization that was followed by a slow depolarizing response in 13 of 16 neurons examined (fast depolarization: peak: 8.6 ± 4.5 mV; latency to peak: 69.9 ± 22.8 ms, n = 13; Fig. 4B). These responses were noticeably different in both magnitude and duration compared with those observed after a single-laser photostimulation (Fig. 1F). Subsequent application of the AMPA receptor antagonist DNQX (20–40 μM) and NMDA receptor antagonist CPP (10–20 μM) blocked the fast potential but did not eliminate the slow depolarization (peak: 1.1 ± 0.5 mV; latency to peak: 1.2 ± 0.5 s; area: 5,040 ± 2,053 mV*ms, n = 8; Fig. 4C). The long-lasting duration of the slow response (duration: 11.5 ± 2.9 s; range: 8–17 s) is consistent with mGluR activation and is qualitatively similar to responses observed after a high-frequency stimulation of corticothalamic fibers (McCormick and von Krosigk 1992). Subsequent application of the mGluR1 antagonist LY-367385 (100 μM) resulted in a significant attenuation of the amplitude and area of the slow depolarization (peak: 0.4 ± 0.2 mV, n = 7, P < 0.001; area: 228 ± 422 mV*ms, n = 7, P < 0.0001, paired t-test; Fig. 4, D and E). These results demonstrate that local tetanic photostimulation can repeatedly and reliably activate local mGluRs in the slice preparation.
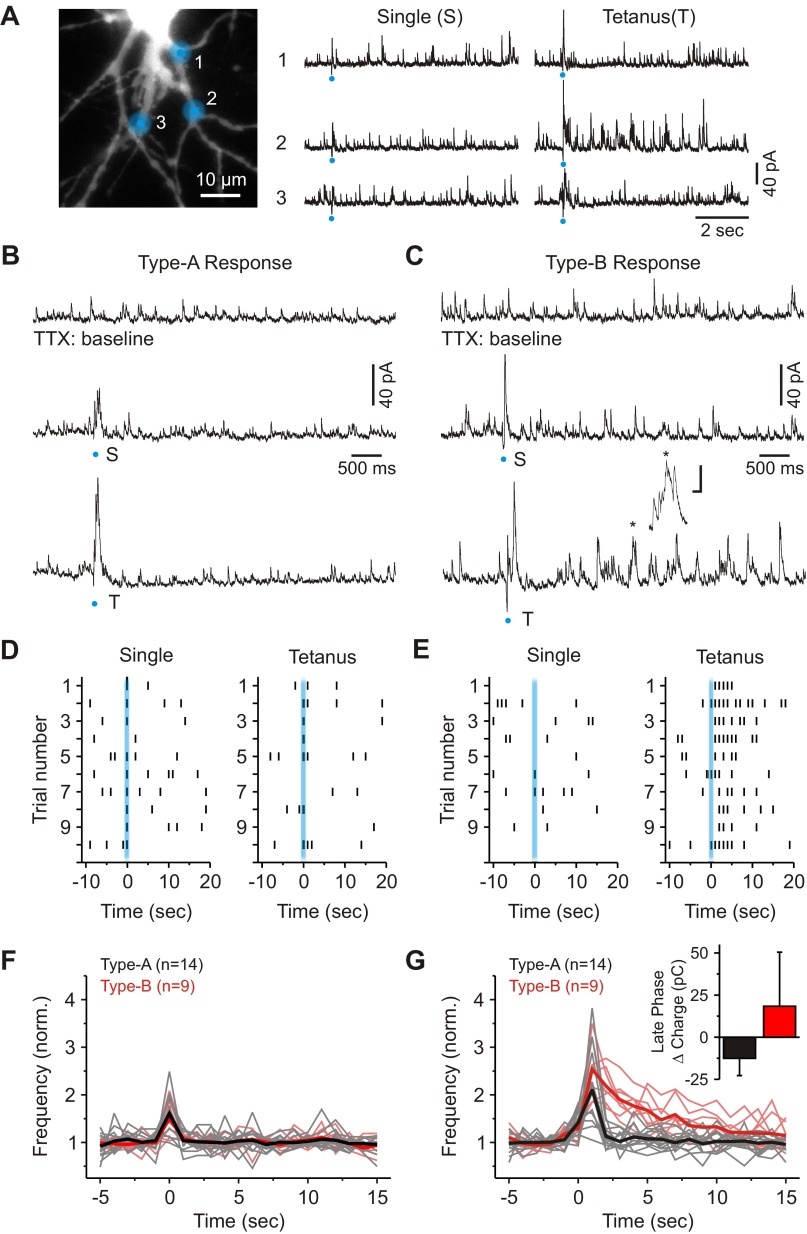
To evoke a local mGluR-mediated inhibitory output from interneuron dendrites, we first located a dendrodendritic connection by photostimulating with a single laser pulse (iGluR-mediated gluIPSC; Fig. 5A). We subsequently stimulated the same location with a tetanic photostimulation (10 pulses, 1.0–3.0 ms, 100 Hz). As illustrated in Fig. 5, B and C, tetanic photostimulation produced two distinct types of responses. The first, which we are calling a “Type-A F2 response,” was characterized by a transient increase in gluIPSC activity (Fig. 5, B and D). The second response, which we are calling a “Type-B F2 response,” was characterized by a transient increase in gluIPSC activity and a delayed increase in IPSC activity that lasted many seconds (8.9 ± 2.6 s; range: 5–13 s, n = 9; Fig. 5, C and E). Overall, the average change in charge recorded at the soma during the late phase of the Type-B response was significantly larger than that observed after the Type-A response (Type-B: 18.4 ± 32.1 pC, n = 9; Type-A: −12.5 ± 10.2 pC, n = 14; P < 0.001, Mann-Whitney Test; Fig. 5G). In the end, we observed Type-B responses in 12 of 38 dendrodendritic connections tested (30.8%: from 34 different neurons).
Fig. 5.
Local glutamate uncaging reveals distinct types of F2 responses in the visual thalamus. A, left: image of a thalamic relay neuron showing the 3 dendritic locations targeted for glutamate uncaging. Right: inhibitory activity evoked from the 3 different locations after delivering a single and tetanic photostimulation. B: characterization of a Type-A response. Top: shown is baseline IPSC activity in the presence of TTX (1 μM). Middle: photostimulation with a single laser pulse produced a fast change in IPSC activity. Bottom: tetanic photostimulation at the same location did not increase the duration of IPSC activity. C: characterization of a Type-B F2 response. Top: shown is baseline IPSC activity in the presence of TTX. Middle: photostimulation with a single laser pulse produced a fast change in IPSC activity. Bottom: tetanic photostimulation at the same location increased IPSC activity over many seconds. A cluster of bursts of IPSCs is identified by the asterisk in the trace. Scale = 20 pA and 50 ms. D: raster plot showing the responses to a single and subsequent train of laser pulses for the cell shown in B. Marks indicate IPSC activity 2 SD above the level of baseline activity. Bins = 1 s. E: raster plot showing the responses to a single and subsequent train of laser pulses for the cell shown in C. F: population data illustrating IPSC frequency over time for a single laser pulse. The thick black and red lines represent averages. Bins = 1 s. G: population data illustrating IPSC frequency over time following tetanic photostimulation. The thick black and red lines represent averages. Bins = 1 s. Inset: a plot illustrating the average change in charge during the late phase of the Type-A and Type-B responses.
Curiously, many of the gluIPSCs during the late phase of the Type-B response appeared in clusters or bursts (Fig. 5C, inset). In total, 7 of the 12 dendrodendritic connections identified as producing a Type-B response also produced clear clusters of IPSCs. Of these dendrodendritic connections, clusters began appearing 0.5–1.0 s following the initial response, and an average of 8.1 ± 4.2 clusters were observed per stimulation, which was significantly more than those produced after a single laser pulse (1.0 ± 0.9 bursts, n = 7; P < 0.01, paired t-test). Clusters of IPSCs had a mean peak-to-peak interval of 411 ± 212 ms or occurred at a frequency of 3.0 ± 1.5 Hz (n = 209 bursts from 7 neurons).
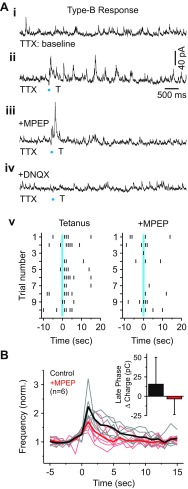
Overall, the longer time course of the inhibitory output generated during the Type-B response is suggestive of mGluR-mediated activation (Govindaiah and Cox 2004; McCormick and von Krosigk 1992). To test the potential contribution of mGluRs, we used the selective mGluR5 antagonist MPEP because it has been shown to attenuate dendrodendritic activity in thalamus (Govindaiah and Cox 2006). In MPEP (50 μM), the evoked increase in IPSC activity during the late phase was strongly attenuated (Fig. 6A). The initial response, which was unaffected by MPEP, was subsequently blocked by DNQX (20 μM, n = 6; Fig. 6A, bottom trace). In Fig. 6B, the time course of IPSC activity before and after MPEP application is summarized for 6 of 8 terminals (8 neurons) that maintained stable baseline activity throughout the recording. Consistent with the observed attenuation in IPSC activity, we found that the average change in charge during the late phase of the Type-B response was significantly reduced with MPEP application (Control: 15.5 ± 34.6 pC; +MPEP: −3.3 ± 20.3 pC, n = 6; P < 0.05, Wilcoxon signed-ranks test; Fig. 6B). These results indicate that the inhibitory output produced during the late phase of the Type-B response is dependent on the activation of mGluRs.
Fig. 6.
mGluRs regulate prolonged inhibitory output from sites producing the Type-B F2 response. A, i: baseline IPSC activity in TTX (1 μM). ii: Tetanic photostimulation at increased IPSC activity over many seconds. iii: Application of mGluR5 antagonist 2-methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP; 50 μM) attenuated the duration of IPSC activity. iv: Subsequent application of DNQX (20–40 μM) blocked the initial fast change in IPSC activity. v: Raster plot showing the responses to tetanic photostimulation before and after MPEP application. B: population data illustrating IPSC frequency over time for the 2 conditions (1-s bins). The thick black and red lines represent averages. Inset: a plot illustrating the average change in charge during the late phase of the Type-B response before and after MPEP application.
DISCUSSION
In this study, we used focal glutamate uncaging and whole cell recordings to investigate the local regulation of feedforward inhibitory microcircuits involving presynaptic dendrites of interneurons in the visual thalamus (i.e., F2 terminals). Here, we describe two distinct types of F2-mediated responses in rat visual thalamus based on glutamate receptor regulation and physiological output. The most frequently encountered response was the Type-A response, which was mediated exclusively by iGluRs (i.e., AMPA and NMDA). In contrast, the less frequently encountered Type-B response was mediated by a combination of iGluRs and mGluR5. We also demonstrate that iGluR- and mGluR-mediated F2 responses are commonly observed in the same postsynaptic neurons, which are morphologically distinct from those neurons in which no F2-mediated responses were observed. Since photostimulation was relatively focal (∼6 μm at FWHM) and resulted in small direct excitatory responses (∼1.5 mV), these results strongly suggest distinct types of F2 terminals could be responsible for generating the responses described. However, it is worth noting that the responses described are likely to have resulted from direct activation of more than one F2 terminal as well as nonsynaptic receptors, since F2 terminals are small (∼1 μm) and typically found in clusters (Montero 1986).
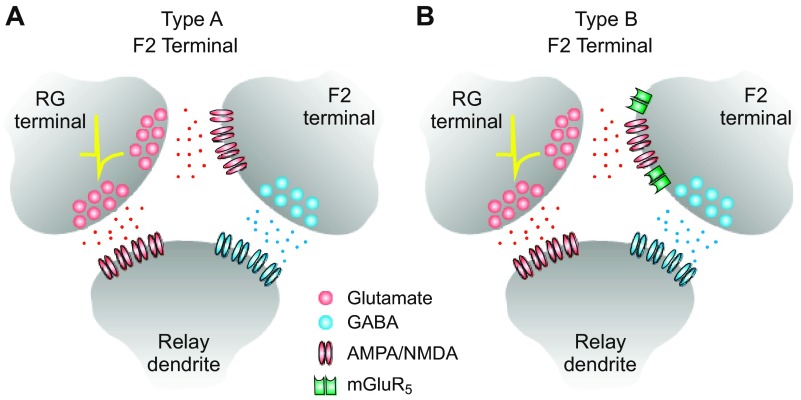
The Type-A and Type-B F2 terminal.
Figure 7 depicts a putative Type-A and Type-B F2 terminal participating in a triadic synaptic arrangement. Based on the physiological data presented in this study, the Type-A F2 terminal would be exclusively regulated by iGluRs (i.e., AMPA and NMDA receptors), whereas the Type-B F2 terminals would be regulated by both iGluRs and mGluRs (i.e., AMPA, NMDA, and mGluR5 receptors). Interestingly, Type-A terminals were encountered more often than the Type-B terminals (∼2:1). However, since both Type-A and Type-B responses were first identified by their iGluR-mediated output, we cannot rule out the possibility we missed a population of F2 responses/terminals exclusively mediated by mGluRs.
Fig. 7.
Schematic diagram of 2 putative types of F2 terminal located in the rat visual thalamus. A: inhibitory output from Type-A F2 terminals is exclusively regulated by AMPA/NMDA (ionotropic) glutamate receptors. B: inhibitory output from Type-B F2 terminals is regulated by both AMPA/NMDA (ionotropic) and mGluR5. RG, retinogeniculate.
The finding that most F2 responses evoked with local glutamate uncaging were mediated exclusively by iGluRs was surprising given previous physiological data that supported a significant role for mGluRs and to a lesser extent iGluRs in regulating GABA output from the dendrites of interneurons (Cox and Sherman 2000; Cox et al. 1998; Govindaiah and Cox 2004, 2006). In these previous studies, GABA release from dendrites was primarily achieved through 1) the pharmacological isolation of iGluR- and mGluR-mediated responses by bath-applying receptor agonists in the presence of TTX (i.e., mGluRs: ACPD or DHPG; iGluRs: AMPA), and 2) high-intensity stimulation of retinogeniculate axons while in the presence of iGluR antagonists so to isolate mGluR-mediated responses. Although GABA release from interneuron dendrites occurred independently of activity at the soma/axon, it could only be inferred that individual F2 terminals were regulated by both iGluRs and mGluRs. This is because both experimental approaches would have activated the entire dendritic arbor and thus numerous F2 terminals. Only recently have experiments with caged-glutamate been able to examine the local regulation of F2 terminals physiologically (Crandall and Cox 2012). Previous immunocytochemical work has demonstrated the localization of mGluR5 on F2 terminals in the adult cat (Godwin et al. 1996), however, we are unaware of a study examining the localization of iGluRs on F2 terminals.
Despite observing far fewer Type-B responses in the present study, our data demonstrate these putative F2 terminals can exert a powerful inhibitory control over relay neuron excitability through the activation of presynaptic mGluRs. Since mGluRs typically require higher levels of synaptic activity to activate (Govindaiah and Cox 2004; McCormick and von Krosigk 1992), this suggests that feedforward inhibitory microcircuits involving Type-A and Type-B F2 terminals would serve distinct roles in modulating retinogeniculate communication and subsequent transfer of sensory information to the neocortex. We also speculate that our results might represent the synaptic basis for differential processing of sensory information in thalamus across species and modalities.
Are Type-A and Type-B F2 terminals directly involved in the retinogeniculate pathway?
Anatomic and physiological studies have demonstrated thalamic interneurons receive glutamatergic input from a number of sources, including retinal, cortical, and axon collaterals of neighboring relay neurons (Acuna-Goycolea et al. 2008; Augustinaite et al. 2011; Bickford et al. 2008; Errington et al. 2011; Govindaiah and Cox 2004; Hamos et al. 1985; Williams et al. 1996). Since glutamate uncaging serves only to mimic the function of the presynaptic terminal, we cannot be certain of the endogenous source of glutamate for the two types of F2 terminal described in this study. However, we can presume that many if not all of the F2 terminals in this study receive endogenous input from excitatory retinogeniculate terminals. Previous studies indicate that retinal synapses are the abundant source of excitatory afferents to thalamic interneurons (Erisir et al. 1998; Montero 1991; Van Horn et al. 2000) with the vast majority targeting F2-terminal-rich dendritic appendages rather than the main dendritic trunks (Hamos et al. 1985; Montero 1986, 1991). Moreover, anatomic studies indicate that most F2 terminals are postsynaptic to excitatory retinogeniculate terminals (Guillery 1969; Hamos et al. 1985; Montero 1986; Ohara et al. 1983). Finally, our data obtained with glutamate uncaging is consistent with previous physiological studies in which optic tract stimulation evokes either iGluR- or mGluR-mediated inhibitory output from interneuron dendrites (Acuna-Goycolea et al. 2008; Govindaiah and Cox 2004). It is therefore likely that both types of F2 terminals would be under retinal control.
Similarities between rat and cat dLGN relay neurons.
The visual system of many mammals can be divided into several functionally distinct, parallel pathways from the retina to neocortex responsible for processing different forms of visual information (Lennie 1980; Livingstone and Hubel 1987; Nassi and Callaway 2009; Sherman and Spear 1982). In the cat, the W-, X-, and Y-pathways target three physiologically and morphologically distinct relay cell types in the dLGN (Lennie 1980; Sherman and Spear 1982). Although all three cell types receive input from axons of thalamic interneurons, the dendrites of interneurons preferentially contact X-type thalamocortical neurons (Friedlander et al. 1981; Wilson et al. 1984). Unlike the cat, such a functional organization has not been well-established in the rat dLGN (Lam et al. 2005). Limited rat studies have differentiated X- and Y-like physiology from in vivo recordings (Davidowa et al. 1993; Gabriel et al. 1996; Lennie and Perry 1981; Reese 1988), but it remains unknown whether distinct morphologies are associated with distinct physiological properties.
In the cat, X-cells tend to be bipolar in shape with dendrites that are elongated along projection lines, whereas Y-cells tend to be more radially symmetrical (Friedlander et al. 1981). We found that dendritic orientation is not a great indicator of F2 innervation of rat dLGN relay neurons. However, relay neurons with strong F2 innervation did have more complex dendritic arborizations than those with weak innervation as defined by the number of branch points per primary dendritic branch. This finding correlates well with our anecdotal evidence that F2 terminals are commonly found at or near branch points. The complex arborization of rat relay neurons is also qualitatively similar to those morphological features described in the cat. In that, Y-cell dendrites tend to be large and fairly straight and possess few simple appendages, whereas X-cell dendrites tend to be thin and sinuous with many grapelike appendages (Friedlander et al. 1981). These grapelike dendritic appendages are frequently found near branch points and often participate in triadic arrangements (Friedlander et al. 1981; Wilson et al. 1984). Although we do not see many dendritic appendages in rat dLGN relay neurons, the association between branch points and F2 terminals could represent a functional similarity with the cat X-like cells.
Functional significance of two types of F2 terminals.
If we presume that both Type-A and Type-B F2 terminals are predominately under retinal control, it is unclear whether one or both classes of terminals are involved in the synaptic triad. The triad is a unique synaptic arrangement in which an interneuron dendrite (i.e., F2 terminal) is presynaptic to a relay neuron dendrite and postsynaptic to an excitatory retinogeniculate terminal that, in turn, targets the same postsynaptic relay dendrite (Hamos et al. 1985; Sherman 2004). If both Type-A and Type-B terminals are found to participate in the triadic arrangement, fast glutamate release from a presynaptic retinogeniculate terminal would result in a monosynaptic excitation of both interneuron and relay neuron dendrite, leading to a fast disynaptic inhibition via the F2 terminal. Because the tight spatial relationship of the triad, fast inhibitory output from the F2 terminal would function to regulate the temporal window for a single retinogeniculate excitatory event to integrate with other excitatory inputs (Cruikshank et al. 2007; Pouille and Scanziani 2001). Such temporal modulation could strongly influence spike output (Rathbun et al. 2010; Sincich et al. 2007; Usrey et al. 1998).
Alternatively, during states of high retinal activity, only Type-B terminals would be engaged, given sufficient activation of mGluRs. In theory, the long-lasting output from Type-B terminals would modulate the balance between excitation and inhibition within the triad and thus regulate the gain of an individual retinogeniculate synapse. The increase in inhibitory tone would ultimately reduce the overall strength of the synapse and therefore decrease the probability of the involved retinogeniculate terminals to contribute to relay neuron spiking. Clearly the contributions of iGluRs and/or mGluRs to regulating the output of F2 terminals likely give rise to distinct functional significance. The iGluR control of F2 terminals could shape the afferent excitation of thalamocortical relay neurons, thereby having important consequences on frequency integration of retinogeniculate activity. On the other hand, the role of mGluR-mediated regulation would produce a longer-lasting dampening or elimination of retinogeniculate excitation of the thalamocortical neurons. Future studies are required to determine how these different forms of regulation influence and thereby regulate thalamic gating of bottom-up information transfer.
GRANTS
This work was supported by the NIH National Eye Institute (Grant EY-014024).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.R.C. and C.L.C. conception and design of research; S.R.C. performed experiments; S.R.C. analyzed data; S.R.C. and C.L.C. interpreted results of experiments; S.R.C. and C.L.C. prepared figures; S.R.C. drafted manuscript; S.R.C. and C.L.C. edited and revised manuscript; S.R.C. and C.L.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. G. Govindaiah for helpful comments on the manuscript.
Present address of S. R. Crandall: Dept. of Neuroscience, Brown Univ., Providence, RI 02912.
REFERENCES
- Acuna-Goycolea C, Brenowitz SD, Regehr WG. Active dendritic conductances dynamically regulate GABA release from thalamic interneurons. Neuron 57: 420–431, 2008 [DOI] [PubMed] [Google Scholar]
- Augustinaite S, Yanagawa Y, Heggelund P. Cortical feedback regulation of input to visual cortex: role of intrageniculate interneurons. J Physiol 589: 2963–2977, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford ME, Wei H, Eisenback MA, Chomsung RD, Slusarczyk AS, Dankowsi AB. Synaptic organization of thalamocortical axon collaterals in the perigeniculate nucleus and dorsal lateral geniculate nucleus. J Comp Neurol 508: 264–285, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Regehr WG. Timing and specificity of feed-forward inhibition within the LGN. Neuron 45: 917–928, 2005 [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Sherman SM. Dendritic current flow in relay cells and interneurons of the cat's lateral geniculate nucleus. Proc Natl Acad Sci USA 86: 3911–3914, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CL, Sherman SM. Control of dendritic outputs of inhibitory interneurons in the lateral geniculate nucleus. Neuron 27: 597–610, 2000 [DOI] [PubMed] [Google Scholar]
- Cox CL, Sherman SM. Glutamate inhibits thalamic reticular neurons. J Neurosci 19: 6694–6699, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CL, Zhou Q, Sherman SM. Glutamate locally activates dendritic outputs of thalamic interneurons. Nature 394: 478–482, 1998 [DOI] [PubMed] [Google Scholar]
- Crandall SR, Cox CL. Local dendrodendritic inhibition regulates fast synaptic transmission in visual thalamus. J Neurosci 32: 2513–2522, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall SR, Govindaiah G, Cox CL. Low-threshold Ca2+ current amplifies distal dendritic signaling in thalamic reticular neurons. J Neurosci 30: 15419–15429, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci 10: 462–468, 2007 [DOI] [PubMed] [Google Scholar]
- Davidowa H, Albrecht D, Gabriel HJ, Zippel U. SLOW and FAST lateral geniculate neurons are differently influenced by acetylcholine. Brain Res Bull 31: 455–461, 1993 [DOI] [PubMed] [Google Scholar]
- Erisir A, Van Horn SC, Sherman SM. Distribution of synapses in the lateral geniculate nucleus of the cat: differences between laminae A and A1 and between relay cells and interneurons. J Comp Neurol 390: 247–255, 1998 [PubMed] [Google Scholar]
- Errington AC, Di Giovanni G, Crunelli V, Cope DW. mGluR control of interneuron output regulates feedforward tonic GABAA inhibition in the visual thalamus. J Neurosci 31: 8669–8680, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti EV, Jr, Peters A. The synaptic glomerulus and the intrinsic neuron in the dorsal lateral geniculate nucleus of the cat. J Comp Neurol 144: 285–334, 1972 [DOI] [PubMed] [Google Scholar]
- Fino E, Araya R, Peterka DS, Salierno M, Etchenique R, Yuste R. RuBi-Glutamate: two-photon and visible-light photoactivation of neurons and dendritic spines. Front Neural Circuits 3: 2, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Yuste R. Dense inhibitory connectivity in neocortex. Neuron 69: 1188–1203, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander MJ, Lin CS, Stanford LR, Sherman SM. Morphology of functionally identified neurons in lateral geniculate nucleus of the cat. J Neurophysiol 46: 80–129, 1981 [DOI] [PubMed] [Google Scholar]
- Gabriel S, Gabriel HJ, Grutzmann R, Berlin K, Davidowa H. Effects of cholecystokinin on Y, X, and W cells in the dorsal lateral geniculate nucleus of rats. Exp Brain Res 109: 43–55, 1996 [DOI] [PubMed] [Google Scholar]
- Godwin DW, Van Horn SC, Eriir A, Sesma M, Romano C, Sherman SM. Ultrastructural localization suggests that retinal and cortical inputs access different metabotropic glutamate receptors in the lateral geniculate nucleus. J Neurosci 16: 8181–8192, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindaiah G, Cox CL. Metabotropic glutamate receptors differentially regulate GABAergic inhibition in thalamus. J Neurosci 26: 13443–13453, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindaiah G, Cox CL. Synaptic activation of metabotropic glutamate receptors regulates dendritic outputs of thalamic interneurons. Neuron 41: 611–623, 2004 [DOI] [PubMed] [Google Scholar]
- Guillery RW. The organization of synaptic interconnections in the laminae of the dorsal lateral geniculate nucleus of the cat. Z Zellforsch Mikrosk Anat 96: 1–38, 1969 [DOI] [PubMed] [Google Scholar]
- Hamos JE, Van Horn SC, Raczkowski D, Sherman SM. Synaptic circuits involving an individual retinogeniculate axon in the cat. J Comp Neurol 259: 165–192, 1987 [DOI] [PubMed] [Google Scholar]
- Hamos JE, Van Horn SC, Raczkowski D, Uhlrich DJ, Sherman SM. Synaptic connectivity of a local circuit neurone in lateral geniculate nucleus of the cat. Nature 317: 618–621, 1985 [DOI] [PubMed] [Google Scholar]
- Krahe TE, El-Danaf RN, Dilger EK, Henderson SC, Guido W. Morphologically distinct classes of relay cells exhibit regional preferences in the dorsal lateral geniculate nucleus of the mouse. J Neurosci 31: 17437–17448, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam YW, Cox CL, Varela C, Sherman SM. Morphological correlates of triadic circuitry in the lateral geniculate nucleus of cats and rats. J Neurophysiol 93: 748–757, 2005 [DOI] [PubMed] [Google Scholar]
- Lennie P. Parallel visual pathways: a review. Vision Res 20: 561–594, 1980 [DOI] [PubMed] [Google Scholar]
- Lennie P, Perry VH. Spatial contrast sensitivity of cells in the lateral geniculate nucleus of the rat. J Physiol 315: 69–79, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman AR. Neurons with presynaptic perikarya and presynaptic dendrites in the rat lateral geniculate nucleus. Brain Res 59: 35–59, 1973 [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. Psychophysical evidence for separate channels for the perception of form, color, movement, and depth. J Neurosci 7: 3416–3468, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, von Krosigk M. Corticothalamic activation modulates thalamic firing through glutamate “metabotropic” receptors. Proc Natl Acad Sci USA 89: 2774–2778, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero VM. A quantitative study of synaptic contacts on interneurons and relay cells of the cat lateral geniculate nucleus. Exp Brain Res 86: 257–270, 1991 [DOI] [PubMed] [Google Scholar]
- Montero VM. Localization of gamma-aminobutyric acid (GABA) in type 3 cells and demonstration of their source to F2 terminals in the cat lateral geniculate nucleus: a Golgi-electron-microscopic GABA-immunocytochemical study. J Comp Neurol 254: 228–245, 1986 [DOI] [PubMed] [Google Scholar]
- Nassi JJ, Callaway EM. Parallel processing strategies of the primate visual system. Nat Rev Neurosci 10: 360–372, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara PT, Lieberman AR, Hunt SP, Wu JY. Neural elements containing glutamic acid decarboxylase (GAD) in the dorsal lateral geniculate nucleus of the rat; immunohistochemical studies by light and electron microscopy. Neuroscience 8: 189–211, 1983 [DOI] [PubMed] [Google Scholar]
- Packer AM, Yuste R. Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? J Neurosci 31: 13260–13271, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science 293: 1159–1163, 2001 [DOI] [PubMed] [Google Scholar]
- Ralston HJ., 3rd Evidence for presynaptic dendrites and a proposal for their mechanism of action. Nature 230: 585–587, 1971 [DOI] [PubMed] [Google Scholar]
- Rathbun DL, Warland DK, Usrey WM. Spike timing and information transmission at retinogeniculate synapses. J Neurosci 30: 13558–13566, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese BE. ‘Hidden lamination’ in the dorsal lateral geniculate nucleus: the functional organization of this thalamic region in the rat. Brain Res 472: 119–137, 1988 [DOI] [PubMed] [Google Scholar]
- Sherman SM. Interneurons and triadic circuitry of the thalamus. Trends Neurosci 27: 670–675, 2004 [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Functional organization of thalamocortical relays. J Neurophysiol 76: 1367–1395, 1996 [DOI] [PubMed] [Google Scholar]
- Sherman SM, Spear PD. Organization of visual pathways in normal and visually deprived cats. Physiol Rev 62: 738–855, 1982 [DOI] [PubMed] [Google Scholar]
- Sincich LC, Adams DL, Economides JR, Horton JC. Transmission of spike trains at the retinogeniculate synapse. J Neurosci 27: 2683–2692, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usrey WM, Reppas JB, Reid RC. Paired-spike interactions and synaptic efficacy of retinal inputs to the thalamus. Nature 395: 384–387, 1998 [DOI] [PubMed] [Google Scholar]
- Van Horn SC, Erisir A, Sherman SM. Relative distribution of synapses in the A-laminae of the lateral geniculate nucleus of the cat. J Comp Neurol 416: 509–520, 2000 [PubMed] [Google Scholar]
- Wang X, Sommer FT, Hirsch JA. Inhibitory circuits for visual processing in thalamus. Curr Opin Neurobiol 21: 726–733, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR, Turner JP, Anderson CM, Crunelli V. Electrophysiological and morphological properties of interneurones in the rat dorsal lateral geniculate nucleus in vitro. J Physiol 490: 129–147, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JR, Friedlander MJ, Sherman SM. Fine structural morphology of identified X- and Y-cells in the cat's lateral geniculate nucleus. Proc R Soc Lond B Biol Sci 221: 411–436, 1984 [DOI] [PubMed] [Google Scholar]