Abstract
The biosynthesis of nanoparticles has been proposed as a cost effective environmental friendly alternative to chemical and physical methods. Microbial synthesis of nanoparticles is under exploration due to wide biomedical applications, research interest in nanotechnology and microbial biotechnology. In the present study, an ecofriendly process for the synthesis of nanoparticles using a novel Nocardiopsis sp. MBRC-1 has been attempted. We used culture supernatant of Nocardiopsis sp. MBRC-1 for the simple and cost effective green synthesis of silver nanoparticles. The reduction of silver ions occurred when silver nitrate solution was treated with the Nocardiopsis sp. MBRC-1 culture supernatant at room temperature. The nanoparticles were characterized by UV-visible, TEM, FE-SEM, EDX, FTIR, and XRD spectroscopy. The nanoparticles exhibited an absorption peak around 420 nm, a characteristic surface plasmon resonance band of silver nanoparticles. They were spherical in shape with an average particle size of 45 ± 0.15 nm. The EDX analysis showed the presence of elemental silver signal in the synthesized nanoparticles. The FTIR analysis revealed that the protein component in the form of enzyme nitrate reductase produced by the isolate in the culture supernatant may be responsible for reduction and as capping agents. The XRD spectrum showed the characteristic Bragg peaks of 1 2 3, 2 0 4, 0 4 3, 1 4 4, and 3 1 1 facets of the face centered cubic silver nanoparticles and confirms that these nanoparticles are crystalline in nature. The prepared silver nanoparticles exhibited strong antimicrobial activity against bacteria and fungi. Cytotoxicity of biosynthesized AgNPs against in vitro human cervical cancer cell line (HeLa) showed a dose-response activity. IC50 value was found to be 200 μg/mL of AgNPs against HeLa cancer cells. Further studies are needed to elucidate the toxicity and the mechanism involved with antimicrobial and anticancer activity of the synthesized AgNPs as nanomedicine.
1. Introduction
Nanotechnology is emerging as a rapidly growing field with its application in science and technology [1]. Noble metal nanoparticles such as gold, silver, and platinum are widely applied in medicinal applications. Marine actinobacteria are high Guanine+Cytosine content Gram-positive bacteria with an unparalleled ability to produce diverse secondary metabolites, such as antibiotics, immunosuppressors, and many other biologically active compounds [2]. Exploitation of marine actinobacteria in nanotechnology has recently received considerable attention [3, 4]. Nanotechnology holds promising application in biosensing, drug delivery, and cancer therapy [5–7]. The expensive and extensive use of toxic solvents and hazardous reducing agents in chemical procedures to synthesize nanoparticles has augmented the necessity in view of ecofriendly and green chemistry approach. Hence, a well established nontoxic and ecofriendly potent methodology for the synthesis of nanoparticles has mounted to a level of supreme importance [8–11]. An alternative approach for the synthesis of metal nanoparticles is to apply biomaterials such as plants, microorganisms encompassing groups such as bacteria, fungi, and actinobacteria as nanofactories [12–14]. Emerging multidrug resistant (MDR) bacteria has raised a demand for the urgent need to identify novel antimicrobial agents. It was reported that silver had been used as antimicrobial agents since ancient times [3]. With the advancements in nanotechnology, AgNPs have found its significant applications as antimicrobial agents, in fields of microelectronics, catalysis, and biomolecular detection [15–17]. Although the antibacterial activity of AgNPs has been proved in the recent years, the actual mechanism of action is not yet clear. They may inactivate microorganisms by interacting with their enzymes, proteins, or DNA to inhibit cell proliferation [18]. It is also evident that the increased antimicrobial activity of AgNPs may be attributed to its special characteristics of small size and high surface area to volume ratio [19]. The advantage of adapting biosynthesis of AgNPs is the simplicity of extracellular synthesis and downstream processing [20, 21].
Nanoparticles have a wide range of applications, as in combating microbes [22], biolabelling [23], and in the treatment of cancer [24]. The antibacterial activity of silver species is known since ancient times [25] and it has been demonstrated that, at low concentrations, silver is nontoxic to human cells [26]. It has also been reported that Ag+ ions uncouple the respiratory chain from oxidative phosphorylation or collapse the proton-motive force across the cytoplasmic membrane [27]. The interaction of Ag+ with bacteria is directly related to the size and shape of the nanoparticles [26, 28].
Sastry et al. [29] reported on the biosynthesis of metal nanoparticles using the mycelial extract of fungi and actinobacteria [29]. In addition, the time required for completion of the reaction using both bacteria and fungi ranges between approximately 24 hrs and 120 hrs, whereas maximum synthesis of AgNPs can be achieved after 24 hrs of incubation. Moreover, metal accumulation is dependent on the growth phase of the cells [30]. Sadhasivam et al. [3] reported on the extracellular biosynthesis of NPs by Streptomyces hygroscopicus and antimicrobial activity against medically important pathogenic micro-organisms [3]. Sivalingam et al. [31] reported on the biosynthesis of bactericidal silver nanoparticles (AgNPs) using a novel Streptomyces sp. BDUKAS10, an isolated mangrove sediment [31]. Though the mechanism of silver resistance offered by bacteria using the silver binding protein is well documented, their extraction and purification need to be elucidated further for large-scale production. However, only a few studies have examined the components of marine actinobacteria that mediated the reduction of silver ions into AgNPs. In this study, we examined and characterized the extracellular biosynthesis of AgNPs using a novel Nocardiopsis sp. MBRC-1, which is a very important micro-organism to the production of several antibiotics and enzymes of commercial value. To the best of our knowledge, this marine actinobacterium (Nocardiopsis sp. MBRC-1) has never been used for nanoparticles biosynthesis.
2. Materials and Methods
2.1. Chemicals
All analytical reagents and media components were purchased from Sigma-Aldrich (St. Louis, USA).
2.2. Microbial Synthesis of AgNPs
The Nocardiopsis sp. MBRC-1 strain was isolated from the marine sediment samples from the Busan coast (Lat 35°09′ N; Long 129°07′ E), South Korea. Their partial 16S rRNA gene sequences were deposited in GenBank under the accession number KC179785. For the synthesis of silver nanoparticles, the active Nocardiopsis sp. MBRC-1 culture was freshly inoculated on sterile starch casein medium and the flasks were incubated at 25–28°C and 180 rpm for 96 hrs (pH 7.0). After the incubation period was complete, the culture was centrifuged at 5000 rpm for 30 min and the supernatant was used for the biosynthesis of AgNPs. Deionized water was used as a solvent in the synthesis of AgNPs. The collected supernatant (pH 7.0) was added separately to the reaction vessel containing silver nitrate at a concentration of 10−3 M (1% (v/v)) and incubated on an orbital shaker (dark condition) for 96 hrs at 30°C. The reaction was carried out in the dark after the addition of the AgNO3, and color change appeared transparent. It confirmed the synthesis of AgNPs. The formation of the AgNPs was monitored by UV-vis spectroscopy using Shimadzu (Model No-UV 1800) double beam UV-vis spectrophotometer [3]. All the experiments were carried out in triplicate and average values have been reported.
2.3. Characterization of AgNPs
The synthesized AgNPs were freeze dried, powdered, and used for XRD analysis. The spectra were evaluated using an X-ray diffractometer (PHILIPS X'Pert-MPD diffractometer, The Netherlands) and Cu-Kαradiation 1.5405 Å over an angular range of 5 to 80°, a step size of 0.02, a scan speed of 4° m−1 at a 40 kV voltage, and a 30 mA current. The dried powder was diluted with potassium bromide in the ratio of 1 : 100 and recorded the Fourier transform infrared spectroscopy (FTIR) (Perkin Elmer Inc., USA) and spectrum GX spectrometry within the range of 400 to 4000 cm−1. Synthesized AgNPs were mounted on specimen stubs with double-sided adhesive tape coated with platinum in a sputter coater and examined under field emission scanning electron microscopy (FE-SEM) (JSM-6700, JEOL, Japan). For transmission electron microscopy (TEM) imaging, a drop of aqueous solution containing the AgNPs was placed on carbon coated copper grids and dried under an infrared lamp (JEM 1010 JEOL, Japan) (AC voltage −80.0 kV). In addition, the presence of silver metals in the sample was analyzed by energy dispersive X-ray analysis (EDX) combined with FE-SEM. Finally, the size distribution of the nanoparticles was evaluated using dynamic light scattering measurements conducted with a Malvern Zetasizer ZS compact scattering spectrometer (Malvern Instruments Ltd., Malvern, UK).
2.4. Particle-Size Distribution of AgNPs
Particle-size distribution analysis was carried out after treatment of a 1 mM solution of AgNO3 with the culture supernatant of Nocardiopsis sp. MBRC-1 at room temperature for 98 hrs. The organism was grown in starch casein broth under incubation at 30°C for 98 hrs. After the incubation period, the culture was centrifuged at 10,000 rpm and the supernatant was used to reduce the AgNO3 solution. For the DLS measurements, the supernatant thus obtained was a clear brown homogenous suspension of AgNPs diluted 10-fold for all experiments involving measurement of DLS. The solutions were then filtered through syringe membrane filters with pores less than 0.4 μm, then centrifuged at 5000 rpm for 30 min.
2.5. Antimicrobial Activity of the AgNPs
The antimicrobial activity of the microbiologically synthesized AgNPs against pathogenic organisms such as bacteria (Escherichia coli, Bacillus subtilis, Enterococcus hirae, Pseudomonas aeruginosa, Shigella flexneri and Staphylococcus aureus) and fungi (Aspergillus niger, A. brasiliensis, A. fumigates and Candida albicans) was measured using the well-diffusion method [26]. Pure cultures of bacteria and fungi were grown in Mueller-Hinton broth (Sigma, USA) for bacteria and Sabouraud-broth for fungi at 35°C and 30°C, respectively, on a rotary shaker at 180 rpm. Wells that were 6 mm in diameter were made on the Mueller-Hinton agar and Sabouraud agar plates using a gel puncture and each well was inoculated with individual cultures. The AgNPs in various concentrations (10, 20, 30, 40, and 50 μg/mL) were loaded in each well. The positive and negative controls were also maintained, and the plates (triplicates) were incubated at 35°C and 30°C for 24 and 48 hrs. Simultaneously, the synergistic effects of different commercial antibiotics (Amoxicillin and Nystatin, Sigma, USA) with AgNPs against multidrug resistant pathogens were also checked in well diffusion method. After incubation, the susceptibility pattern of the test organisms was determined by measuring the diameter of the zone of inhibition for well diffusion method.
2.6. Determination of Minimum Inhibitory Concentration
The synthesized silver nanoparticles were tested (triplicates) for minimum inhibitory concentration by microtiter broth dilution method [32]. Muller-Hinton broth was used as diluents for bacterial strains and Sabouraud broth for fungal species. About 106 CFU/mL cells were inoculated, and the final volume in each microtiter plate well was 0.1 mL. After incubation for 24 h, at 35°C for bacterial strains and 30°C for fungal strains, the microtiter plates were read at 450 nm using TRIAD multimode reader prior to and after incubation to determine the minimum inhibitory concentration (MIC) values. The MIC is defined as the lowest concentration of compound, which inhibited 90% of the growth when compared with that of the growth control.
2.7. Cell Culture
Human cervical cancer cell line (HeLa) was cultured in Dulbecco's Modified Eagle Medium (DMEM). Culture media were supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic and antimycotic (Penicillin-Streptomycin cocktail) solution. The cells were grown in a humidified atmosphere containing 5% CO2 at 37°C and subcultured by detaching with trypsin-EDTA solution at about 70–80% confluent.
2.8. Cytotoxic Activity
Cell viability was evaluated by the MTT colorimetric technique. Human HeLa cancer cell lines (5000 cells/well) were seeded in 96 well tissue culture plates. Stock solutions of nanoparticles (5 mg/mL) were prepared in sterile distilled water and diluted to the required concentrations (50, 100, 150, 200, and 250 μg/mL) using the cell culture medium. Appropriate concentrations of AgNPs stock solution were added to the cultures to obtain respective concentration of AgNPs and incubated for 24 hrs at 37°C. Nontreated cells were used as control. After 24 hrs, cells were washed with PBS and then 100 μL of the yellow tetrazolium MTT solution (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) without phenol red (0.5 mg/mL in phosphate buffer solution) was added to each well. The plates were incubated for 3-4 hrs at 37°C, for reduction of MTT by metabolically active cells, in part by the action of dehydrogenase enzymes, to generate reducing equivalents such as NADH and NADPH. For solubilization of the MTT crystals, 100 μL of DMSO was added to the wells. The plates were placed on a shaker for 15 min to complete solubilization of crystals, and then the optical density of each well was determined. The quantity of formazan product as measured by the amount of 545 nm absorbance is directly proportional to the number of living cells in culture. Each experiment was done in triplicate. The relative cell viability (%) related to control wells containing cell culture medium without nanoparticles as a vehicle was calculated as follows: Percentage of cell viability (%) = Sample absorbance/control absorbance × 100.
2.9. Cytomorphological Changes in HeLa Cells by AgNPs
HeLa cells (1 × 105 cells/well) were seeded in a 6 well plate for 24 hrs. After 24 hrs, they were treated with 100 and 200 μg/mL of synthesized AgNPs and incubated for 24 hrs at 37°C in 5% CO2 atmosphere. After the incubation, the cells were washed twice with PBS, and morphological changes in the cells were visualized and photographed under phase contrast microscope (CTR 6000; Leica, Wetzlar, Germany).
2.10. Statistical Analysis
The grouped data were statistically evaluated using ANOVA with SPSS/14 software. Values are presented as the mean ± SD of the three replicates of each experiment.
3. Results and Discussion
3.1. Isolation and Identification of Marine Actinobacteria
A marine actinobacterium MBRC-1 strain was isolated from the marine sediment samples from the Busan coast, South Korea, and was used for the synthesis of silver nanoparticles. The marine actinobacterium MBRC-1 shows that the presence of meso-diaminopimelic acid as the amino acid in the cell wall and arabinose and galactose as whole cell sugars and the absence of characteristic glycine in their cell credibly categorized the cell wall of this strain belonged to the cell wall type-IV [33]. This isolate was identified as Nocardiopsis sp. MBRC-1 based on the morphological, physiological, and biochemical characteristics, and it was confirmed by the 16S rDNA sequencing (Figure 1). The sequence was submitted to GenBank in NCBI (http://www.ncbi.nlm.nih.gov/nuccore/443501390/) with the accession number KC179785.
Figure 1.
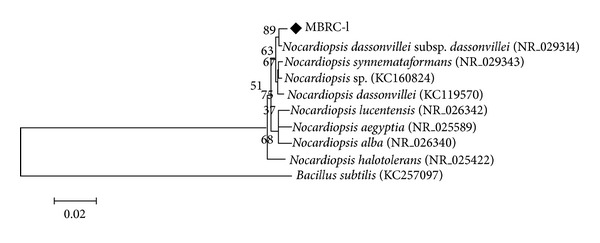
Phylogenetic tree of the 16S rDNA sequence of strain Nocardiopsis sp. MBRC-1 and related strains.
3.2. UV-Vis Analysis of AgNPs
In this study, AgNPs were successfully synthesized in the culture supernatant of Nocardiopsis sp. MBRC-1. Interestingly, the culture supernatant incubated with the silver nitrate mediated the biosynthesizing of AgNPs within 24 hrs of incubation. During the experiment, the pH of the sample was adjusted to 7.0. The appearance of a yellowish brown color in the silver nitrate treated flask indicated the formation of silver nanoparticles, whereas no color change was observed in either the culture supernatant without silver nitrate or the silver nitrate control experiments. Notably, the intensity of the brown color increased dramatically up to 24 hrs and was maintained throughout the experiment. This may have been due to the excitation of surface plasmon resonance (SPR) and the reduction of AgNO3. In the UV-visible spectrum, a strong and broad peak was observed between 420 nm, indicating the presence of AgNPs. This may have occurred due to the reduction of metal ions by secondary metabolites present in the cells. The 24, 48, 72, and 96 hrs peaks indicate the absorption spectra of biosynthesized AgNPs at different incubation times (Figure 2). Numerous reports have discussed the biosynthesis of silver nanoparticles [3, 31, 34], but to the best of knowledge, this was the first report on biosynthesis of silver nanoparticles using a novel Nocardiopsis sp. MBRC-1.
Figure 2.
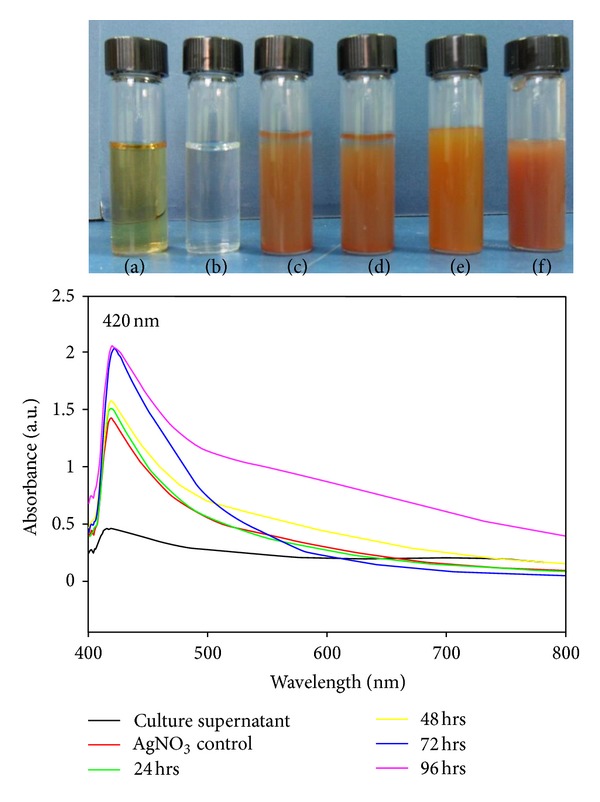
UV-Vis spectra of AgNPs synthesized using cell free supernatant of Nocardiopsis sp. MBRC-1. (a) Culture supernatant; (b) AgNO3 control; ((c)–(f)) correspond to the AgNO3 treated with culture supernatant incubated for 24, 48, 72, and 96 hrs, respectively.
3.3. FTIR Analysis of AgNPs
FTIR spectrum analysis of AgNPs showed intense absorption bands at 3440, 2923, 2853, 1655, 1460, and 685 cm−1. The intense broad absorbance at 3440 cm−1 (O–H stretch) is the characteristic of the H-bonded functional group in alcohols and phenolic compounds. The band at 2923 and 2853 cm−1 (C–H stretch) can be assigned to the alkanes group. The intense medium absorbance at 1655 cm−1 (–C=C– stretch) is the characteristic of the alkenes group. The intense medium absorbance at 1460 cm−1 (C–H bend) is the characteristic of the alkanes group. The intense broad absorbance at 685 cm−1 (–C=C–H: C–H bend) is the characteristic of the alkynes group. A previous report reveals that the alcohols, phenolic, alkynes, and alkanes groups have a strong ability to interact with nanoparticles [31, 35, 36].
3.4. XRD Analysis of AgNPs
The XRD pattern of the silver nitrate-treated sample (Figure 3) corresponds to that of silver nanoparticles. The XRD pattern shows five intense peaks in the whole spectrum of 2θ values ranging from 30 to 80. It is important to know the exact nature of the silver particles formed and this can be deduced from the XRD spectrum of the sample. XRD spectra of pure nanoparticles silver structures and pure silver nitrate have been published by the Joint Committee on Powder Diffraction Standards (file no. 04-0783). A comparison of our XRD spectrum with the standard confirmed that the silver particles formed in our experiments were in the form of nanoparticles, as evidenced by the peaks at 2θ values of 38.44°, 44.38°, 56.77°, 64.38°, and 77.50°, corresponding to 1 2 3, 2 0 4, 0 4 3, 1 4 4, and 3 1 1 planes for silver, respectively. The full width at half maximum (FWHM) values measured for 1 2 3, 2 0 4, 0 4 3, 1 4 4, and 3 1 1 planes of reflection was used with the Debye-Scherrer equation to calculate the size of the nanoparticles. The particle sizes obtained from XRD line broadening agreed well with those obtained from SEM. From these, the average particle size was found to be around 45 ± 0.05 nm.
Figure 3.
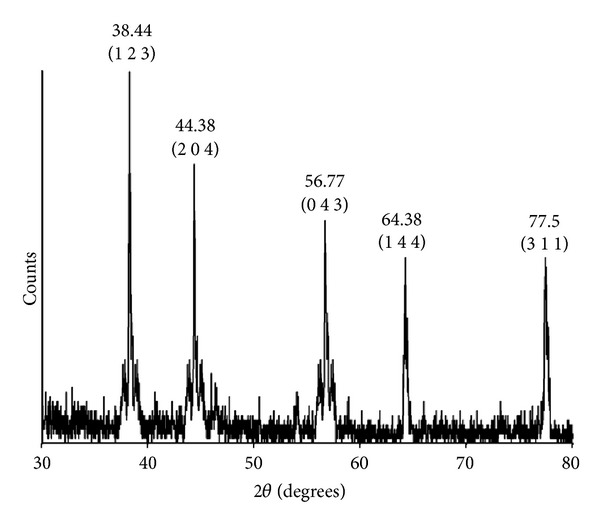
X-ray diffraction pattern of the AgNPs obtained from Nocardiopsis sp. MBRC-1.
3.5. FE-SEM Analysis of AgNPs
FE-SEM determinations of the above-mentioned sample showed the formation of nanoparticles, which were confirmed to be of silver by EDX. As shown in Figures 4(a) and 4(b), well-dispersed nanoparticles could be seen in the samples treated with silver nitrate. EDX analysis also showed a peak in the silver region, confirming the formation of silver nanoparticles (Figure 4(c)). The optical absorption peak is observed approximately at 3 keV, which is typical for the absorption of metallic silver nanoparticles due to surface Plasmon resonance [37]. In addition, other peaks for Cl and O were observed which are possibly due to emissions from proteins or enzymes present in the culture supernatant [30].
Figure 4.
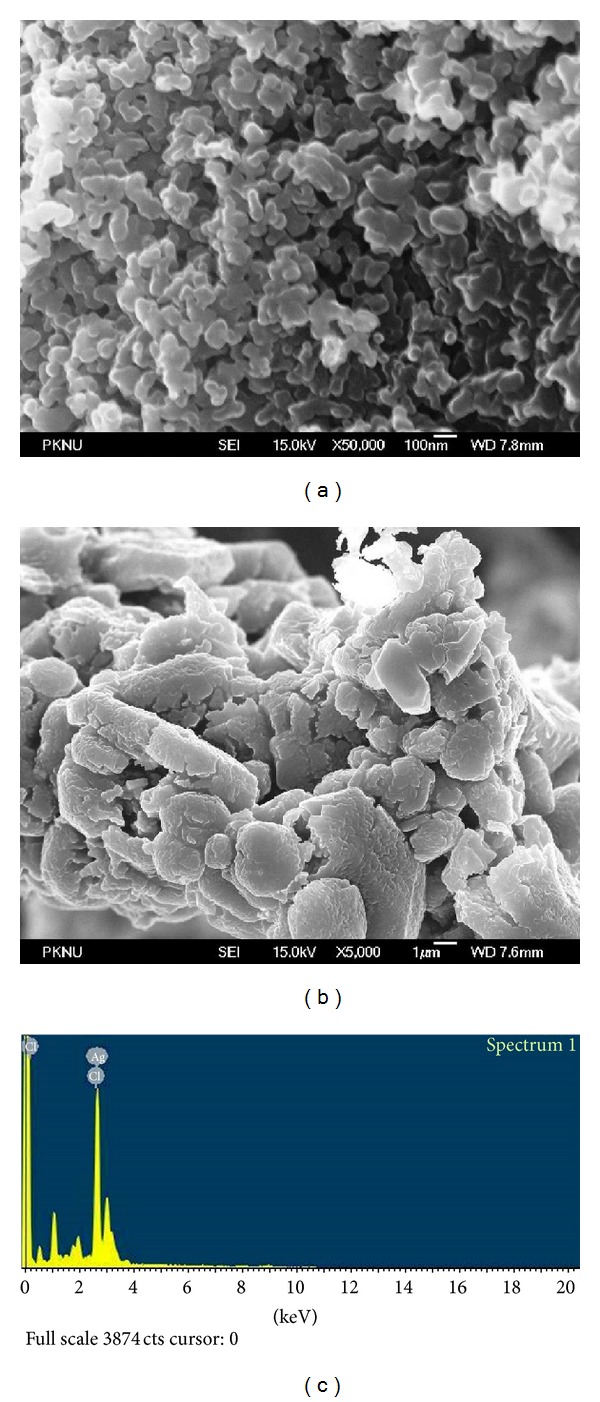
((a) and (b)) FE-SEM images of AgNPs synthesized by Nocardiopsis sp. MBRC-1. (a) 100 nm scale, (b) 1 µm scale, and (c) EDX analysis of AgNPs synthesized by Nocardiopsis sp. MBRC-1.
3.6. TEM Analysis of AgNPs
The TEM image analysis (Figures 5(a) and 5(b)) revealed that silver nanoparticles were spherical in shape. The micrograph showed NPs with variable shape; most of them present in spherical in nature. The TEM micrograph also confirmed the size of NPs, which were in the range of 30–90 nm with an average particle size of 45 ± 0.15 nm. Majority of the AgNPs were aggregates with only a few of them showing scattering of varying sizes as observed under TEM. The particle size distribution histogram plot constructed from the TEM micrograph is shown in Figure 5(c). Synthesis of AgNPs by treating AgNO3 solution with the culture supernatant of K. pneumonia (belonging to the family Enterobacteriaceae) has also been reported, in which the particles range in size from 28.2 to 122 nm and possess an average size of 52.5 nm [14]. A study on synthesis of AgNPs using Morganella sp. (belonging to the family Enterobacteriaceae) reported spherical nanoparticles of ~20 nm size [38].
Figure 5.
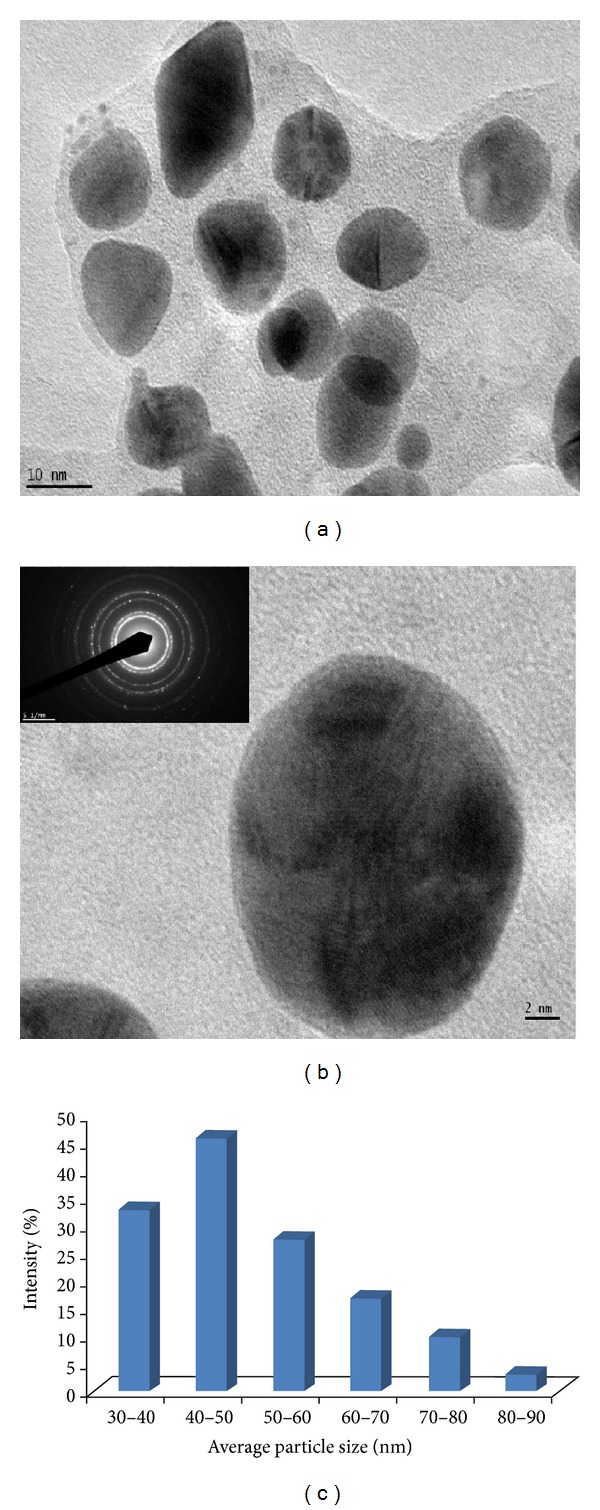
HR-TEM images of AgNPs formed by Nocardiopsis sp. MBRC-1. (a) 10 nm scale, (b) 2 nm scale and selected area diffraction pattern. (c) Particle-size distribution under unoptimized conditions. The particle-size distribution revealed that the particles ranging from 30 to 90 nm had the maximum intensity, and thereafter the intensity was reduced. The average particle size was found to be 45 ± 0.15 nm.
3.7. Antimicrobial Activity of the AgNPs
In this study, the antimicrobial activity of AgNPs using a novel biosynthetic method was evaluated. In this analysis, the AgNPs displayed antimicrobial activity against a range of different pathogenic microorganisms (Table 1). The mean of three replicates of the diameter of the zone of inhibition (30 μg/mL) for each microorganism was determined to be about 18.8 ± 0.30, 22.5 ± 0.10, 17.2 ± 0.15, 19.4 ± 0.20, 15.4 ± 0.15, 19.1 ± 0.20, 17.3 ± 0.25, 14.6 ± 0.20, 19.3 ± 0.20, and 22.4 ± 0.25 mm, respectively, for Escherichia coli, Bacillus subtilis, Enterococcus hirae, Pseudomonas aeruginosa, Shigella flexneri, Staphylococcus aureus, Aspergillus niger, A. brasiliensis, A. fumigates, and Candida albicans. The highest antimicrobial activity was observed against Bacillus subtilis, Pseudomonas aeruginosa, and Candida albicans. These findings are in agreement with previous studies that examined the antimicrobial activity of AgNPs against Bacillus subtilis and Candida albicans [3]. The antimicrobial activity of silver nanoparticles was reported to be due to the penetration into the bacteria, damage of cell membrane, and release of cell contents [39]. Another possibility suggested that [40, 41] was the release of silver ions from the nanoparticles, which may contribute to the bactericidal properties of silver nanoparticles.
Table 1.
Antimicrobial activity of the AgNPs against various pathogenic micro-organisms. The data is presented as the mean ± value standard deviation of three replicates.
| Micro-organisms | Zone of inhibition (mm in diameter) | |||||
|---|---|---|---|---|---|---|
| 10 µg/mL | 20 µg/mL | 30 µg/mL | 40 µg/mL | 50 µg/mL | Antibiotics 30 µg/mL | |
| Bacteria | Amoxicillin | |||||
| Escherichia coli ATCC 10536 | 7.5 ± 0.35 | 15.2 ± 0.31 | 18.8 ± 0.30 | 23.3 ± 0.20 | 27.3 ± 0.15 | 19.3 ± 0.10 |
| Bacillus subtilis ATCC 6633 | 11.2 ± 0.35 | 19.4 ± 0.25 | 22.5 ± 0.10 | 28.1 ± 0.20 | 33.2 ± 0.20 | 23.8 ± 0.25 |
| Enterococcus hirae ATCC 10541 | 6.3 ± 0.20 | 13.3 ± 0.14 | 17.2 ± 0.15 | 21.8 ± 0.30 | 25.4 ± 0.25 | 19.5 ± 0.10 |
| Pseudomonas aeruginosa ATCC 27853 | 9.1 ± 0.15 | 17.7 ± 0.30 | 19.4 ± 0.20 | 23.6 ± 0.35 | 28.3 ± 0.20 | 21.3 ± 0.30 |
| Shigella flexneri ATCC 12022 | 5.2 ± 0.20 | 11.2 ± 0.21 | 15.4 ± 0.15 | 19.3 ± 0.25 | 22.5 ± 0.10 | 17.5 ± 0.30 |
| Staphylococcus aureus ATCC 6538 | 7.8 ± 0.25 | 15.1 ± 0.32 | 19.1 ± 0.20 | 24.2 ± 0.20 | 27.1 ± 0.15 | 21.3 ± 0.10 |
|
| ||||||
| Fungi | Nystatin | |||||
| Aspergillus niger ATCC 1015 | 6.7 ± 0.32 | 13.6 ± 0.22 | 17.3 ± 0.25 | 21.4 ± 0.20 | 25.3 ± 0.15 | 18.1 ± 0.10 |
| A. brasiliensis ATCC 16404 | 4.8 ± 0.25 | 10.2 ± 0.15 | 14.6 ± 0.20 | 19.4 ± 0.10 | 23.4 ± 0.15 | 15.8 ± 0.30 |
| A. fumigates ATCC 1022 | 7.2 ± 0.35 | 15.4 ± 0.22 | 19.3 ± 0.20 | 24.3 ± 0.10 | 26.3 ± 0.30 | 21.4 ± 0.15 |
| Candida albicans ATCC 10231 | 9.5 ± 0.20 | 18.1 ± 0.21 | 22.4 ± 0.25 | 25.2 ± 0.25 | 28.4 ± 0.25 | 24.5 ± 0.20 |
3.8. Determination of Minimum Inhibitory Concentration
Minimum inhibitory concentration of AgNPs (Table 2) was evaluated against various pathogenic bacteria and fungi. The silver nanoparticles exhibited lowest minimum inhibitory concentration (MIC) against Bacillus subtilis at 7 μg/mL, Bacillus subtilis 10 μg/mL, and Candida albicans at 10 μg/mL, suggesting the broad spectrum nature of their minimum inhibitory concentration. Kumar and Mamidyala [35] reported the minimum inhibitory concentration of AgNPs against Gram-positive, Gram-negative, and different Candida species at concentrations ranging between 4 and 32 μg/mL.
Table 2.
Minimum inhibitory concentration of the AgNPs against various bacterial and fungal strains. The data is presented as the mean ± value standard deviation of three replicates.
| Micro-organisms |
Minimum inhibitory concentration |
|
|---|---|---|
| AgNPs (µg/mL) | Antibiotics (µg/mL) | |
| Bacteria | Amoxicillin | |
| Escherichia coli ATCC 10536 | 13 | 11 |
| Bacillus subtilis ATCC 6633 | 7 | 6 |
| Enterococcus hirae ATCC 10541 | 16 | 14 |
| Pseudomonas aeruginosa ATCC 27853 | 10 | 9 |
| Shigella flexneri ATCC 12022 | 18 | 15 |
| Staphylococcus aureus ATCC 6538 | 14 | 12 |
|
| ||
| Fungi | Nystatin | |
| Aspergillus niger ATCC 1015 | 16 | 14 |
| A. brasiliensis ATCC 16404 | 18 | 16 |
| A. fumigates ATCC 1022 | 13 | 12 |
| Candida albicans ATCC 10231 | 10 | 7 |
3.9. Cytotoxic Activity
The in vitro potential cytotoxic activity of AgNPs against cervical cancer cell lines HeLa. The use of synthetic AgNPs, there are only a few studies to determine that the cytotoxic effects of biologically synthesized AgNPs. MTT assay was used to assess the effect of AgNPs on the cytotoxicity of cancer cells. This study to evaluate the marine sediment samples isolated species Nocardiopsis sp. MBRC-1 derived AgNPs cytotoxicity against HeLa cancer cell lines. AgNPs inhibit the viability of the HeLa cancer cell lines in dose dependent manner. The IC50 value of biosynthesized AgNPs against HeLa cells at 200 μg/mL concentrations (Figure 6(a)). Previously, synthesized AgNPs inducing cytotoxicity were discussed by Sriram et al. [42] and Safaepour et al. [43].
Figure 6.
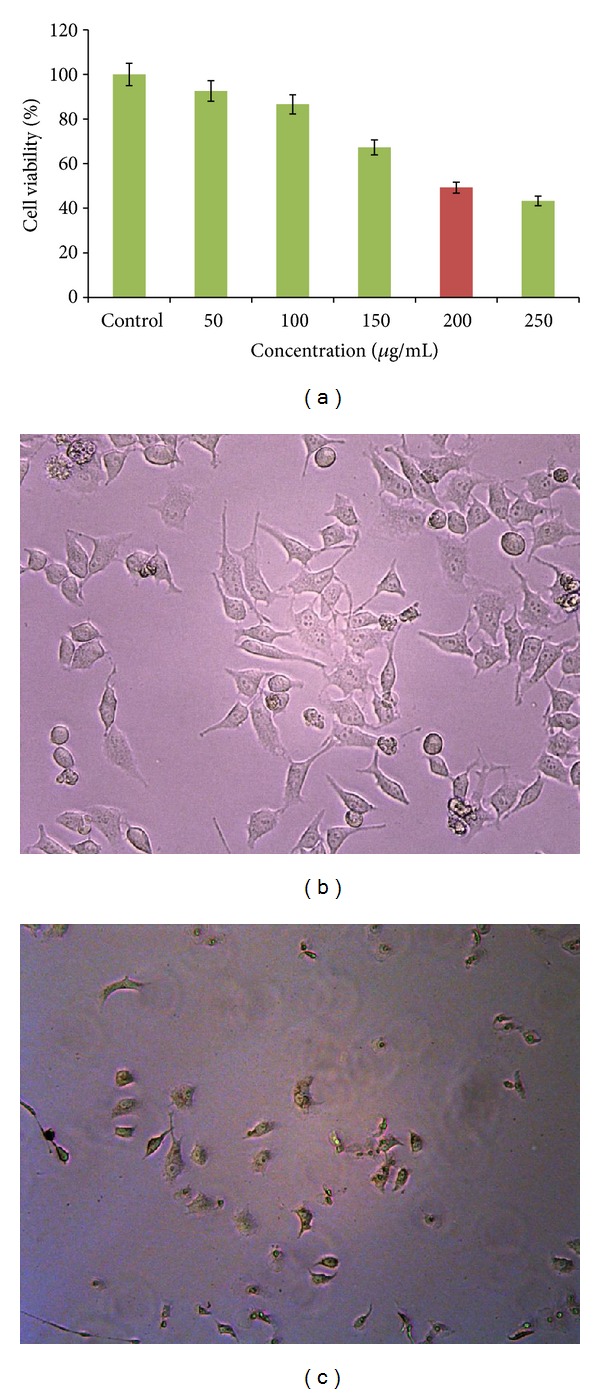
(a) MTT assay results confirming the in vitro cytotoxicity of AgNPs against HeLa cell lines. ((b) and (c)) Morphology of control and AgNPs treated HeLa cell lines (10x magnification). (b) Control. (c) IC50 concentration (200 µg/mL).
3.10. Cytomorphological Changes of HeLa Cells Induced by AgNPs
The morphological examinations of the HeLa cancer cells were observed and photographed using phase contrast microscope. The morphological alteration was observed in control and AgNPs treated HeLa cancer cells. The HeLa cells were treated with AgNPs at 100 and 200 μg/mL concentrations for 24 hrs showing that significant morphological changes, which are characteristic features of apoptotic cells, such as loss of membrane integrity, cell shrinkage, and reduced cell density (Figures 6(b) and 6(c)).
4. Conclusions
In conclusion, silver nanoparticles are synthesized by the biomass of the marine actinobacterium, Nocardiopsis sp. MBRC-1. Marine actinobacteria are easy to handle and can be manipulated genetically without much difficulty. Considering these advantages, a bacterial system could prove to be an excellent alternative for synthesis of AgNPs. Nocardiopsis sp. MBRC-1 can be a good candidate for the synthesis of the AgNPs using silver nitrate of average size 45 ± 0.15 nm. Nocardiopsis sp. MBRC-1 genetics and enzymatic activities, sophisticated molecular breeding can produce strains and biotechnological processes, which could eliminate many types of contaminants in an economical, efficient, and simple process and environmentally friendly manner. The biosynthesized silver nanoparticles showed excellent antimicrobial activity and possessed considerable cytotoxic effect against in vitro HeLa cancer cell lines. IC50 value was found to be 200 μg/mL of AgNPs against HeLa cell lines. The data represented in our study contribute to a novel and unexplored area of nanomaterials as alternative medicine. Furthermore, the biosynthesized AgNPs displayed a pronounced antimicrobial and cytotoxicity activity against clinical pathogenic microorganisms and HeLa cancer cell lines. Taken together, the data collected in this study suggests that it would be important to understand the mode of action of the biosynthesized nanoparticles prior to their use in nanomedicine applications.
Supplementary Material
Supplemental Figure 1. Biosynthesis, characterization and biomedical applications of silver nanoparticles by marine actinobacterium Nocardiopsis sp. MBRC-1.
Acknowledgment
This research was supported by a grant from Marine Bioprocess Research Center of the Marine Biotechnology Program funded by the Ministry of Oceans and Fisheries, Republic of Korea. One of the authors Kannan Sivakumar expresses his thanks to the Director, Centre of Advanced Study in Marine Biology, Faculty of Marine Sciences and Annamalai University authorities for facilities and encouragement.
References
- 1.Albrecht MA, Evans CW, Raston CL. Green chemistry and the health implications of nanoparticles. Green Chemistry. 2006;8(5):417–432. [Google Scholar]
- 2.Chater KF. Genetics of differentiation in Streptomyces. Annual Review of Microbiology. 1993;47:685–713. doi: 10.1146/annurev.mi.47.100193.003345. [DOI] [PubMed] [Google Scholar]
- 3.Sadhasivam S, Shanmugam P, Yun K. Biosynthesis of silver nanoparticles by Streptomyces hygroscopicus and antimicrobial activity against medically important pathogenic microorganisms. Colloids and Surfaces B. 2010;81(1):358–362. doi: 10.1016/j.colsurfb.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 4.Sadhasivam S, Shanmugam P, Veerapandian M, Subbiah R, Yun K. Biogenic synthesis of multidimensional gold nanoparticles assisted by Streptomyces hygroscopicus and its electrochemical and antibacterial properties. BioMetals. 2011;25(2):351–360. doi: 10.1007/s10534-011-9506-6. [DOI] [PubMed] [Google Scholar]
- 5.Willets KA, Van Duyne RP. Localized surface plasmon resonance spectroscopy and sensing. Annual Review of Physical Chemistry. 2007;58:267–297. doi: 10.1146/annurev.physchem.58.032806.104607. [DOI] [PubMed] [Google Scholar]
- 6.Gittins DI, Bethell D, Schiffrin DJ, Nichols RJ. A nanometre-scale electronic switch consisting of a metal cluster and redox-addressable groups. Nature. 2000;408(6808):67–69. doi: 10.1038/35040518. [DOI] [PubMed] [Google Scholar]
- 7.Jain PK, ElSayed IH, El-Sayed MA. Au nanoparticles target cancer. Nano Today. 2007;2(1):18–29. [Google Scholar]
- 8.Yu D. Formation of colloidal silver nanoparticles stabilized by Na+-poly(γ-glutamic acid)-silver nitrate complex via chemical reduction process. Colloids and Surfaces B. 2007;59(2):171–178. doi: 10.1016/j.colsurfb.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Mallick K, Witcomb MJ, Scurrell MS. Self-assembly of silver nanoparticles in a polymer solvent: Formation of a nanochain through nanoscale soldering. Materials Chemistry and Physics. 2005;90(2-3):221–224. [Google Scholar]
- 10.Liu Y-C, Lin L-H. New pathway for the synthesis of ultrafine silver nanoparticles from bulk silver substrates in aqueous solutions by sonoelectrochemical methods. Electrochemistry Communications. 2004;6(11):1163–1168. [Google Scholar]
- 11.Smetana AB, Klabunde KJ, Sorensen CM. Synthesis of spherical silver nanoparticles by digestive ripening, stabilization with various agents, and their 3-D and 2-D superlattice formation. Journal of Colloid and Interface Science. 2005;284(2):521–526. doi: 10.1016/j.jcis.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 12.Kowshik M, Ashtaputre S, Kharrazi S, et al. Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology. 2003;14(1):95–100. [Google Scholar]
- 13.Senapati S, Ahmad A, Khan MI, Sastry M, Kumar R. Extracellular biosynthesis of bimetallic Au–Ag alloy nanoparticles. Small. 2005;1(5):517–520. doi: 10.1002/smll.200400053. [DOI] [PubMed] [Google Scholar]
- 14.Shahverdi AR, Minaeian S, Shahverdi HR, Jamalifar H, Nohi A. Rapid synthesis of silver nanoparticles using culture supernatants of Enterobacteria: a novel biological approach. Process Biochemistry. 2007;42(5):919–923. [Google Scholar]
- 15.Liong M, France B, Bradley KA, Zink JI. Antimicrobial activity of silver nanocrystals encapsulated in mesoporous silica nanoparticles. Advanced Materials. 2009;21(17):1684–1689. [Google Scholar]
- 16.Cho K-H, Park J-E, Osaka T, Park S-G. The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochimica Acta. 2005;51(5):956–960. [Google Scholar]
- 17.Wei H, Chen C, Han B, Wang E. Enzyme colorimetric assay using unmodified silver nanoparticles. Analytical Chemistry. 2008;80(18):7051–7055. doi: 10.1021/ac801144t. [DOI] [PubMed] [Google Scholar]
- 18.Singh AK, Talat M, Singh DP, Srivastava ON. Biosynthesis of gold and silver nanoparticles by natural precursor clove and their functionalization with amine group. Journal of Nanoparticle Research. 2010;12(5):1667–1675. [Google Scholar]
- 19.Shahverdi AR, Fakhimi A, Shahverdi HR, Minaian S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli . Nanomedicine. 2007;3(2):168–171. doi: 10.1016/j.nano.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Ingle A, Rai M, Gade A, Bawaskar M. Fusarium solani: a novel biological agent for the extracellular synthesis of silver nanoparticles. Journal of Nanoparticle Research. 2009;11(8):2079–2085. [Google Scholar]
- 21.Bai H, Yang B, Chai C, Yang G, Jia W, Yi Z. Green synthesis of silver nanoparticles using Rhodobacter sphaeroides . World Journal of Microbiology and Biotechnology. 2011;27(11):2723–2728. [Google Scholar]
- 22.Durán N, Marcato PD, Alves OL, De Souza GIH, Esposito E. Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. Journal of Nanobiotechnology. 2005;3, article 8 doi: 10.1186/1477-3155-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klaus T, Joerger R, Olsson E, Granqvist C. Silver-based crystalline nanoparticles, microbially fabricated. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(24):13611–13614. doi: 10.1073/pnas.96.24.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arora S, Jain J, Rajwade JM, Paknikar KM. Cellular responses induced by silver nanoparticles: in vitro studies. Toxicology Letters. 2008;179(2):93–100. doi: 10.1016/j.toxlet.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Gurunathan S, Kalishwaralal K, Vaidyanathan R, et al. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli . Colloids and Surfaces B. 2009;74(1):328–335. doi: 10.1016/j.colsurfb.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 26.Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli . Applied and Environmental Microbiology. 2007;73(6):1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holt KB, Bard AJ. Interaction of silver(I) ions with the respiratory chain of Escherichia coli: an electrochemical and scanning electrochemical microscopy study of the antimicrobial mechanism of micromolar Ag+ . Biochemistry. 2005;44(39):13214–13223. doi: 10.1021/bi0508542. [DOI] [PubMed] [Google Scholar]
- 28.Morones JR, Elechiguerra JL, Camacho A, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16(10):2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 29.Sastry M, Ahmad A, Islam Khan M, Kumar R. Biosynthesis of metal nanoparticles using fungi and actinomycete. Current Science. 2003;85(2):162–170. [Google Scholar]
- 30.Mukherjee P, Ahmad A, Mandal D, et al. Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesis. Nano Letters. 2001;1(10):515–519. [Google Scholar]
- 31.Sivalingam P, Antony JJ, Siva D, Achiraman S, Anbarasu K. Mangrove Streptomyces sp. BDUKAS10 as nanofactory for fabrication of bactericidal silver nanoparticles. Colloids and Surfaces A. 2012;98:12–17. doi: 10.1016/j.colsurfb.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 32.Sarker SD, Nahar L, Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods. 2007;42(4):321–324. doi: 10.1016/j.ymeth.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lechevalier MP, Lechevalier H. Chemical composition as a criterion in the classification of aerobic actinomycetes. International Journal of Systematic Bacteriology. 1970;20(4):435–443. [Google Scholar]
- 34.Kirthi AV, Rahuman AA, Jayaseelan C, et al. Novel approach to synthesis silver nanoparticles using plant pathogenic fungi, Puccinia graminis . Materials Letters. 2013;81:61–72. [Google Scholar]
- 35.Kumar CG, Mamidyala SK. Extracellular synthesis of silver nanoparticles using culture supernatant of Pseudomonas aeruginosa . Colloids and Surfaces B. 2011;84(2):462–466. doi: 10.1016/j.colsurfb.2011.01.042. [DOI] [PubMed] [Google Scholar]
- 36.Krishnaraj C, Jagan EG, Rajasekar S, Selvakumar P, Kalaichelvan PT, Mohan N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids and Surfaces B. 2010;76(1):50–56. doi: 10.1016/j.colsurfb.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Magudapathy P, Gangopadhyay P, Panigrahi BK, Nair KGM, Dhara S. Electrical transport studies of Ag nanoclusters embedded in glass matrix. Physica B: Condensed Matter. 2001;299(1-2):142–146. [Google Scholar]
- 38.Kalishwaralal K, Deepak V, Ramkumarpandian S, Nellaiah H, Sangiliyandi G. Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis . Materials Letters. 2008;62(29):4411–4413. [Google Scholar]
- 39.Panáček A, Kvítek L, Prucek R, et al. Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. Journal of Physical Chemistry B. 2006;110(33):16248–16253. doi: 10.1021/jp063826h. [DOI] [PubMed] [Google Scholar]
- 40.Kim KJ, Sung WS, Suh BK, et al. Antifungal activity and mode of action of silver nano-particles on Candida albicans . BioMetals. 2009;22(2):235–242. doi: 10.1007/s10534-008-9159-2. [DOI] [PubMed] [Google Scholar]
- 41.Li W-R, Xie X-B, Shi Q-S, Zeng H-Y, Ou-Yang Y, Chen Y-B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli . Applied Microbiology and Biotechnology. 2010;85(4):1115–1122. doi: 10.1007/s00253-009-2159-5. [DOI] [PubMed] [Google Scholar]
- 42.Sriram MI, Kanth SBM, Kalishwaralal K, Gurunathan S. Antitumor activity of silver nanoparticles in Dalton’s lymphoma ascites tumor model. International Journal of Nanomedicine. 2010;5(1):753–762. doi: 10.2147/IJN.S11727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Safaepour M, Shahverdi AR, Shahverdi HR, Khorramizadeh MR, Gohari AR. Green synthesis of small silver nanoparticles using geraniol and its cytotoxicity against Fibrosarcoma-Wehi 164. Avicenna Journal of Medical Biotechnology. 2009;1(2):111–115. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Biosynthesis, characterization and biomedical applications of silver nanoparticles by marine actinobacterium Nocardiopsis sp. MBRC-1.


