Abstract
G protein-coupled receptor/adenylyl cyclase (AC)/cAMP signaling is crucial for all cellular responses to physiological and pathophysiological stimuli. There are nine isoforms of membrane-bound AC, with type 5 being one of the two major isoforms in the heart. Since the role of AC in the heart in regulating cAMP and acute changes in inotropic and chronotropic state are well known, this review will address our current understanding of the distinct regulatory role of the AC5 isoform in response to chronic stress. Transgenic overexpression of AC5 in cardiomyocytes of the heart (AC5-Tg) improves baseline cardiac function but impairs the ability of the heart to withstand stress. For example, chronic catecholamine stimulation induces cardiomyopathy, which is more severe in AC5-Tg mice, mediated through the AC5/sirtuin 1/forkhead box O3a pathway. Conversely, disrupting AC5, i.e., AC5 knockout, protects the heart from chronic catecholamine cardiomyopathy as well as the cardiomyopathies resulting from chronic pressure overload or aging. Moreover, AC5 knockout results in a 30% increase in a healthy life span, resembling the most widely studied model of longevity, i.e., calorie restriction. These two models of longevity share similar gene regulation in the heart, muscle, liver, and brain in that they are both protected against diabetes, obesity, and diabetic and aging cardiomyopathy. A pharmacological inhibitor of AC5 also provides protection against cardiac stress, diabetes, and obesity. Thus AC5 inhibition has novel, potential therapeutic applicability to several diseases not only in the heart but also in aging, diabetes, and obesity.
Keywords: adenylyl cyclase type 5, cardiomyopathy, aging, metabolism, AC5 inhibitor
this article is part of a collection on G Protein Kinase A Signaling in Cardiovascular Physiology and Disease. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
Adenylyl cyclase (AC) is the enzyme that catalyzes the conversion of ATP to adenosine 3′,5′-cyclic monophosphate (cAMP), a key intracellular second messenger, which in the heart mediates inotropy and chronotropy. Since the pioneering work 55 years ago by Sutherland (73), it has been known that AC-cAMP signaling plays crucial roles in normal biological function, for example, lipolysis (53), gluconeogenesis (54), respiration (29) and cytoskeletal organization (25), and its dysregulation in pathophysiological states including memory (52) and neurodegenerative disorders (69), tumorigenesis (66), and heart disease (13, 19). The diverse actions of cAMP are mediated through the cAMP-dependent activation of protein kinase A (PKA), cyclic nucleotide-gated ion channels, and cAMP-activated exchange proteins. Ten different isoforms of ACs have been identified in mammalian tissues; nine are G protein-regulated transmembrane ACs, and one is a soluble form of AC (9). Each of the various isoforms has a unique chromosomal distribution (28), indicating that there is a significant heterogeneity in the distribution and biochemical properties within AC isoforms. The overall amino acid similarity between the different AC isoforms is ∼60%. Although all the AC isoforms are ubiquitously expressed, each is characterized by distinct biochemical properties, differential regulatory roles, and tissue-specific distribution throughout the body. Local increases in cAMP derived from tissue-specific isoforms of ACs can selectively regulate closely associated proteins, providing possibilities for different cells to respond diversely to similar stimuli. Thus the AC isoforms are subclassified according to their regulation by various endogenous modulators such as calcium/calmodulin and protein kinase C (PKC) and PKA feedback phosphorylation.
The membrane-bound ACs (AC1–AC9) are large proteins (∼120–140 kDa) that share a common structure consisting of an intracellular NH2-terminus, two repeats of six transmembrane domains (M1 and M2) and two cytoplasmic catalytic domains of ∼40 kDa each (C1 and C2). Crystal structure-coupled with biochemical data indicate that two cytosolic domains form the catalytic core pocket, and ATP binds at one of two pseudosymmetric binding sites at the C1-C2 interface (10, 75). Forskolin binds in two almost equivalent pockets at either end of C1 and C2 domains (87). For the isoforms of AC1, AC2, and AC5, expression of either the α-half (M1/C1) or the β-half (M2/C2) of the molecule alone is insufficient to generate enzymatic activity. The specificity of AC response likely depends on the creation of intracellular microdomains containing signaling molecules. In the submicromolar range of Ca2+, the sensitivity of ACs for Ca2+ is coupled with distinct subcellular localization of Ca2+-sensitive AC isoforms (82, 83), suggesting a temporally and spatially distinct pattern of cAMP signaling, depending on the localization of ACs in Ca2+ microdomains within the plasma membrane or cytoplasm. For instance, studies in overexpression models suggested that AC8 may augment cardiac contractility by preferentially activating Ca2+ loading of sarcoplasmic reticulum through cAMP compartmentation, rather than enhancing Ca2+ influx via L-type Ca2+ channels (21). Dyachok et al. (12) suggested that oscillations of cAMP lead to selective target activation by restricting the spatial redistribution of PKA (12). β-Adrenergic receptors (β-AR) are selectively located in plasma membrane lipid raft microdomains, resulting in more efficient coupling to AC compared with nonlipid raft microdomain receptors, such as the E-prostanoid-2 receptor. Signaling modules that include AC isoforms also contain A kinase anchoring proteins (AKAPs), PKA, and anchored phosphodiesterases to provide microdomains of cAMP production and signaling (2, 34, 82, 86).
Since AC signaling in general and AC5 signaling in particular have been extensively reviewed (31, 56, 67), this review will focus on AC5 and its regulation of responses to chronic stress and disease. We will also provide a brief overview of the potential translational direction of this work, discussing some of our recent findings with a pharmacological AC5 inhibitor.
β-AR-G Protein-AC-cAMP Signaling Pathway
The β-AR-G protein-AC-cAMP signaling pathway is one of the major pathophysiological mechanisms for regulation of cardiac function (31, 45, 47, 78). By targeting Ca2+ handling proteins, it provides strong inotropic and chronotropic response in times of need, such as in fight or flight (22, 48, 70, 72). Throughout much of the 20th century, it was believed that stimulation of this pathway could provide inotropic support and should be used in heart failure therapy. It was shown that transgenic (Tg) mice with up to 60-fold overexpression of β2-AR had enhanced cardiac function without signs of cardiac pathology (46, 51). Furthermore, β2-AR transgene experiments showed improvement in function in failing rabbit hearts (76). More recent work with adenoviral-mediated β2-AR transgene overexpression demonstrated enhanced cardiac function in a rat model of heart failure (65). However, the concept of treating heart failure with chronically enhanced β-AR stimulation became controversial when patients responded positively to acute β-AR inotropic therapy, particularly with dopamine and dobutamine, but had poor outcomes when on prolonged inotropic therapy (14, 44, 55). An experimental study that first highlighted the adverse effects of chronic β-AR signaling was shown in Gsα Tg mice (36). Although these animals had higher responsiveness to isoproterenol (Iso) when young, a picture of cardiomyopathy developed as they aged, including myocardial hypertrophy, fibrosis and necrosis, and depression of cardiac function (1, 36, 37). Later studies using β1-AR (15, 16, 63)- and β2-AR (11, 63)-overexpressed models confirmed these findings, i.e., hyperfunction at young age and deterioration of function with aging. These studies (1, 11, 15, 16, 36, 37, 63) in combination with clinical studies showing poor outcomes in patients on β-AR agonists (14, 44, 55) and Bristow's classical study in The New England Journal of Medicine demonstrating desensitization of the β-AR in patients with heart failure (4) changed the paradigm from treating patients with heart failure with β-AR agonists to antagonists (7, 8, 30, 60, 68). Heart failure still remains as the leading cause of mortality and morbidity in the United States. For this reason, targeting components distal to the β-AR signaling, such as ACs, will be important for the development of future treatment of heart failure.
AC in the Heart
Whereas AC2, -3, -4, -5/6, and -7 are detected in rat cardiac fibroblasts (59), AC5 and AC6 are the two major isoforms expressed in the adult mammalian heart (23, 35). Both AC5 and AC6 regulate heart rate and contractility, but AC6 plays a more significant role at baseline in view of the relatively minor reduction in AC content and corresponding reductions in cardiac contractility observed in AC5 knockout (AC5-KO) hearts (58). However, the role of these two major isoforms in the heart in mediating the response to cardiac stress is controversial. In this article, we first review the studies demonstrating an adverse effect of overexpression of AC5 and beneficial effects of disrupting AC5 on cardiomyopathies induced by chronic Iso stimulation, aging, and pressure overload in either AC5-Tg or AC5-KO mice. This leads to a discussion of other factors involved in AC5 protection against aging, e.g., metabolism and diabetes. Since not all studies are in agreement, we then discuss those with an opposite point of view and reconcile the differences. The controversial studies on AC6 overexpression and disruption are beyond the scope of this review, which focuses on AC5.
Regulation of Cardiomyopathy by AC5
Chronic catecholamine cardiomyopathy.
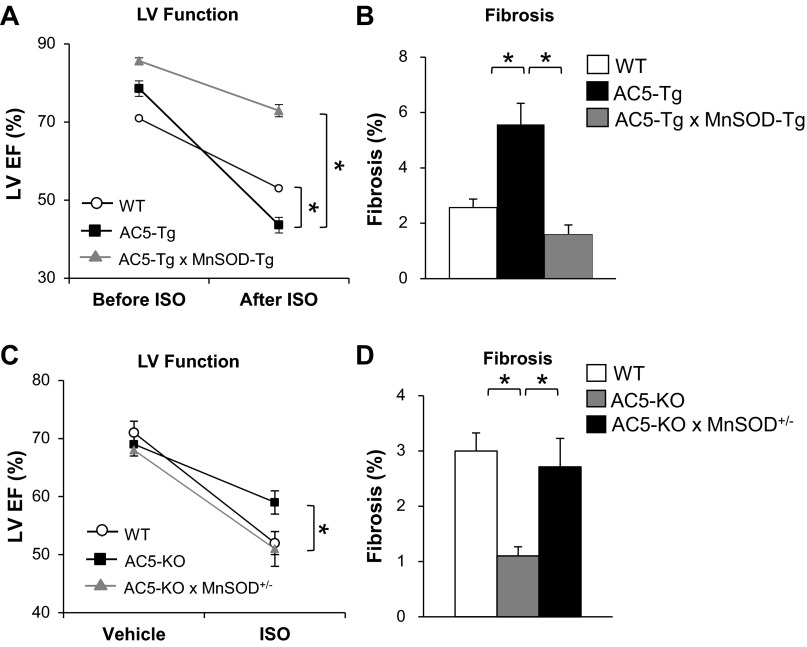
Chronic Iso increased oxidative stress and induced a more severe cardiomyopathy in AC5-Tg compared with wild-type (WT) mice, as reflected by a greater impairment of left ventricular (LV) ejection fraction (EF) along with greater LV dilation and increased fibrosis, apoptosis, and hypertrophy (41) (Fig. 1, A and B). LV EF fell more (P < 0.05) in AC5-Tg than WT mice (−35 ± 2 vs. −18 ± 1%). Oxidative stress induced by chronic Iso was greater in AC5-Tg hearts, whereas protein expression of manganese superoxide dismutase (MnSOD), which protects against oxidative stress, was reduced by 36%, suggesting that the increased severity of the cardiomyopathy in AC5-Tg may have resulted as a consequence of decreased MnSOD expression. This was confirmed by mating AC5-Tg with MnSOD-Tg mice. These bigenic mice no longer responded to chronic Iso with more severe cardiomyopathy than WT mice. In fact, LV EF fell less in AC5-Tg × MnSOD-Tg (−13 ± 1%) versus either AC5-Tg or WT mice. LV EF fell similarly in MnSOD-Tg alone (−13 ± 2%). Conversely, AC5-KO mice are protected from the cardiomyopathy induced by chronic Iso treatment (58), as reflected by less of a reduction with chronic Iso (P < 0.05) in AC5-KO than WT (−10 ± 2 vs. −19 ± 2%) mice, and this protection was lost in bigenic AC5-KO mice mated with MnSOD heterozygous KO mice, where LV EF fell by −18 ± 3% (Fig. 1, C and D). The decrease in LV EF with chronic Iso in the bigenic AC5-KO × MnSOD heterozygous mouse was similar to that in the MnSOD heterozygous alone (−18 ± 3 vs. −18 ± 4%). The decreases in LV EF must be interpreted with the histological changes in the heart consistent with chronic cardiomyopathy, e.g., fibrosis and apoptosis. When the data are all taken together, the picture of intensification of cardiomyopathy with AC5-Tg and protection with MnSOD-Tg or AC5-KO becomes even more apparent. We also demonstrated that AC5, but not AC6, regulates MnSOD at the transcriptional level via the sirtuin 1/forkhead box O3a pathway (Fig. 2). Thus the cardiomyopathy induced by chronic catecholamine stress is intensified in AC5-Tg by inhibiting sirtuin 1/forkhead box O3a, which downregulates MnSOD transcription, resulting in oxidative stress intolerance (41).
Fig. 1.
A and B: chronic isoproterenol (Iso) exacerbated cardiomyopathy in transgenic overexpression of adenylyl cyclase 5 in cardiomyocytes of the heart (AC5-Tg) compared with wild-type (WT), and the cardiomyopathy was rescued by mating the AC5-Tg mice with MnSOD-Tg (AC5-Tg × MnSOD-Tg) mice (41). C and D: downregulation of MnSOD eliminated the protective effects of AC5-knockout (KO) with chronic Iso. LV, left ventricular; EF, ejection fraction. *P < 0.05 (41). Figures used are modified with permission from Lai et al. (41).
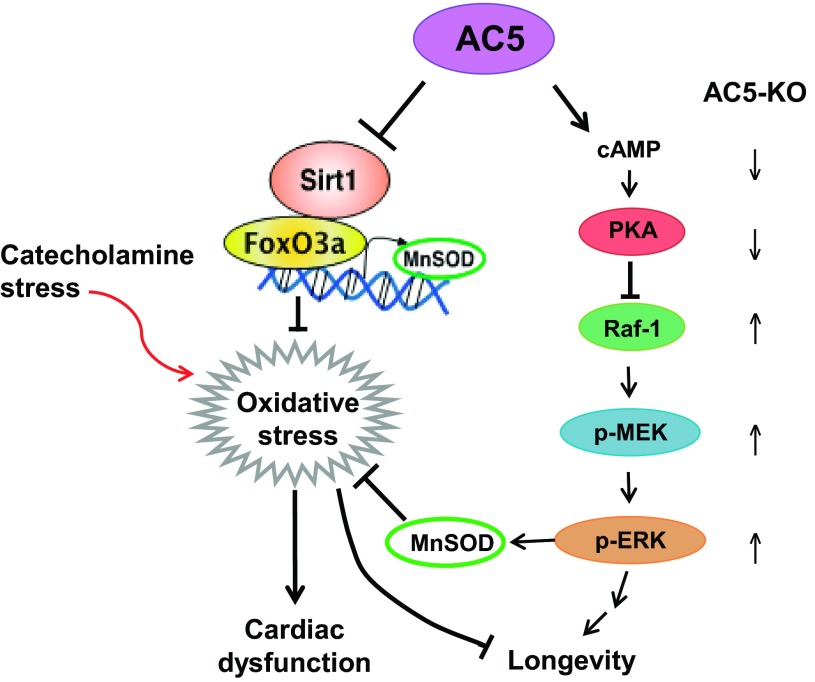
Fig. 2.
Signaling diagram for AC5 mediating cardiac stress and longevity. Left: cardiac dysfunction: signaling diagram for AC5 regulation of MnSOD transcriptionally through the sirtuin 1/forkhead box O3a (SIRT1/FoxO3a) pathway is shown. Imbalance between reactive oxygen species production and the intracellular antioxidant system results in the intolerance of AC5-Tg to stress (41). Right: longevity: disruption of AC5 activates the Raf/MEK/ERK signaling pathway. The activation of ERK activates antioxidative stress and cell survival mechanism, which leads to longevity in AC5-KO mice (85). Arrows indicate the direction of signaling. Figures used are modified with permission from Lai et al. (41) and Yan et al. (85).
Chronic pressure-overload cardiomyopathy.
Cardiac hypertrophy in response to pressure overload is a double- edged sword; on the one-hand it compensates for the pressure overload, whereas on the other hand LV hypertrophy impairs LV function (26, 40), eventually leading to heart failure. AC5-KO mice tolerated chronic pressure overload better than WT, with improved LV function, less fibrosis, and apoptosis in the heart (57).
We previously showed that AC5 and AC6 have opposite protein expression levels in response to pressure overload LV hypertrophy, e.g., an upregulation of AC5 and a downregulation of AC6 (33), suggesting unique regulatory pathways for AC5 in response to chronic pressure overload cardiomyopathy. In addition, it was reported that myocardial AC5 mRNA expression was increased from 5–12 wk in spontaneously hypertensive rats, which was accompanied by development of LV hypertrophy and hypertension (20). Recently, from microarray analysis we found several genes in AC5-Tg hearts related to LV hypertrophy, which was similar to those in a public data set for pressure overload LV hypertrophy and that the transcription factor binding site of nuclear factor of activated t-cells (NFAT), a key prohypertrophic pathway (3, 81), is enriched in AC5-Tg hearts even at baseline, suggesting that cardiac overexpression of AC5 predisposes the heart to LV hypertrophy (61), which is not observed in AC6-Tg hearts (27). Another mechanism mediating the role of AC5 and hypertrophy is the muscle protein AKAP (mAKAPβ), which is required for the cAMP second messenger controlling cardiac myocyte hypertrophy. AC5 binds selectively and directly to a unique NH2-terminal site on mAKAPβ, but not AC6 (39).
Aging cardiomyopathy.
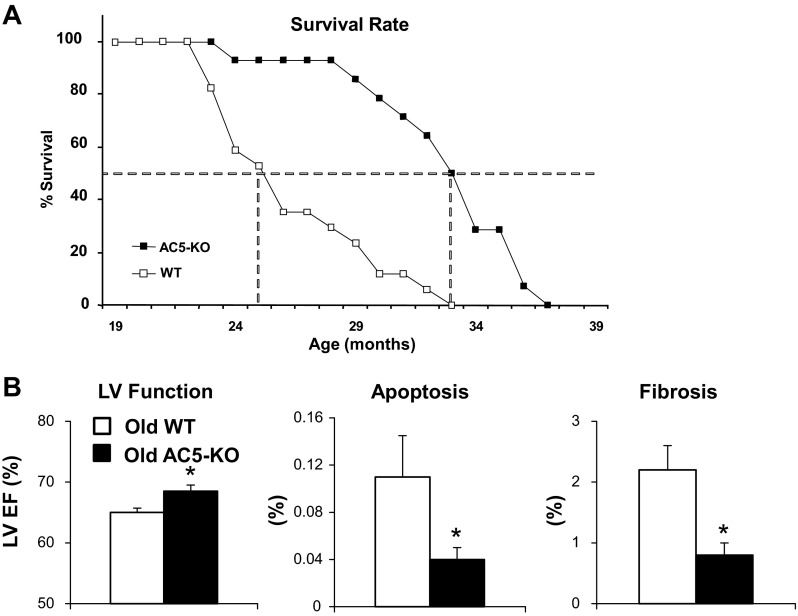
The genetically engineered mouse model in which type 5 AC was knocked out, i.e., AC5-KO mice, have increased median life span of ∼30% (Fig. 3A) and are protected from aging-induced cardiomyopathy (85), including decreased LV hypertrophy, decreased fibrosis, and decreased apoptosis compared with WT as they age (Fig. 3B). Using a proteomic-based approach, we demonstrated a significant activation of the Raf/MEK/ERK signaling pathway, which results in protection from oxidative stress, leading to longevity in AC5-KO mice (Fig. 2). In addition to the prolonged life and protection against aging cardiomyopathy in AC5-KO mice, this model also exhibits protection against the osteoporosis of aging (85). Furthermore, both young and old AC5-KO mice had better exercise endurance than WT mice of the corresponding age. These beneficial effects of AC5 disruption on aging are synergistic in clinical relevance of AC5 inhibition, since elderly patients have an increased prevalence of heart failure (42, 43).
Fig. 3.
Disruption of AC5 protects against cardiac stress. A: effects of AC5-KO on longevity (85). B: effects of AC5-KO on aging cardiomyopathy. LV EF, apoptosis, and fibrosis were significantly different in old AC5-KO vs. old WT. *P < 0.05. Figures used are modified with permission from Yan et al. (85).
AC5-KO model vs. calorie restriction models of longevity.
Calorie restriction (CR) is the most widely studied model of longevity (5, 50, 71). Our hypothesis was that superimposing CR on the AC5-KO would combine their potentially different mechanisms mediating longevity resulting in a superlongevity model. This hypothesis was not correct, and superimposing CR on the AC5-KO was uniformly lethal within a month (79, 84). AC5-KO mice on CR developed a syndrome similar to starvation, as evidenced by greater decrease in body weight, blood glucose, fat and glycogen storage, and greater increase in ketone bodies than either AC5-KO or CR alone. Accordingly, we adopted the converse hypothesis that the longevity mechanisms were similar in the two models. To test this, we recently compared AC5-KO model with CR in terms of physical phenotype as well as metabolic and gene expression profiles (84). Similar to the mice on CR, AC5-KO mice exhibit a lower body weight, reduced fat accumulation (Fig. 4B) and glycogen stores, and lower fasting blood glucose levels. However, in contrast to CR with restricted food intake, AC5-KO mice eat more compared with their WT littermates. Microarray analysis revealed a remarkable similarity of gene profiles between AC5-KO and CR mice in the heart, skeletal muscle, and brain (Fig. 4A). Many tissue-specific pathways in the regulation of metabolism, longevity, and stress resistance overlap in the AC5-KO and CR mouse models, including sensory perception in heart and brain, muscle function in skeletal muscle, and lipid metabolism in liver (Fig. 4C). Importantly, the similarly regulated genes and pathways for AC5-KO and CR will begin to establish a unified theory for longevity, stress resistance, and potentially for diabetes and obesity.
Fig. 4.

Common mechanisms for calorie restriction (CR) and AC5-KO models. A: microarray analysis revealed a similarity between the changes in gene expression between AC5-KO and CR mice. Con, control diet; DN, down; UP, up (84). B: adiposity index showed that AC5-KO mice have less fat accumulation. WT/Con, WT on control diet; WT/CR, WT on calorie restriction; AC5-KO/CON, AC5-KO on control diet. *P < 0.05 (84). BW, body weight. C: differences and similarities between AC5-KO and CR mice are shown in the metabolic phenotypes and the common mechanisms regarding resistance to aging and stress (84). D: fasting glucose level and insulin resistance of AC5-KO and WT following 6 h fasting. ; BW, body weight; HOMA IR, homeostasis model of assessment-insulin resistance. *P < 0.05 (84). Figure used is modified with permission from Yan et al. (84).
Diabetic cardiomyopathy.
A key extrapolation from the above study comparing AC5-KO and CR is that both models of longevity protect against glucose intolerance and insulin resistance (24, 32, 80, 84) and, taken together with AC5-KO's ability to protect against pressure overload and catecholamine cardiomyopathy, raises the likely probability that AC5-KO also might protect against diabetic cardiomyopathy. Even at baseline, in the absence of a high-fat diet, levels of fasting glucose and insulin resistance were lower in AC5-KO (Fig. 4D). Our preliminary results suggest that AC5-KO protects against diabetic cardiomyopathy (32). When the AC5-KO and their WT were placed on a high-fat diet, the WT rapidly developed a reduction in cardiac function with histopathological evidence of cardiomyopathy, as typically reported in the literature (6, 18, 62). However, the AC5-KO was protected against high-fat diet-induced cardiomyopathy (32). These observations underlie several important and clinical relevant questions. For example, is the protection of the cardiomyopathy due to an action of AC5-KO on the heart, i.e., the target organ of the cardiomyopathy, or is it indirectly due to an action on metabolism, i.e., the initiating cause of the cardiomyopathy? These and other related investigations are currently underway.
Controversy in role of AC5 in the heart.
Not all studies have found that overexpression of AC5 is deleterious or that its disruption is salutary. For example, when AC5 is overexpressed in the heart, LV function is improved as well as the response to exercise (17). This is not particularly surprising since increasing any component of the β-AR signaling pathway, even at the level of the β-receptor, improves cardiac performance at baseline and in the response to exercise, as we have also observed in our AC5-Tg models. The adverse effects appear much later with chronically enhanced β-AR signaling. The bottom line is that patients with heart failure respond favorably to β-AR blockade over the long haul but have increased mortality with chronically enhanced β-AR stimulation. A more controversial finding is that AC5-Tg was able to rescue Gαq overexpression-induced cardiomyopathy (74) but not cardiomyopathy induced by cardiac overexpression of β-AR (64). Conversely, AC5-KO mice were not able to rescue Gαq overexpression-induced cardiomyopathy (77). These seemingly different results from rescue of cardiomyopathy (57, 58, 77) are not likely due to different backgrounds in the KO mice, but rather reconciliation of the differences in these studies is more apparent when understanding the signaling pathways. For example, Tg mice with cardiac-specific overexpression of Gαq showed that the cardiomyopathy was mediated by PKC with a significant reduction in AC5. Therefore, it is logical that replacing AC5 in this situation would be beneficial and that reducing it further, as with the AC5-KO, would not be beneficial. However, β1-AR or chronic Iso-stimulated cardiomyopathy is mediated by PKA with increased levels of AC5 (58). These results, taken together, support our hypothesis that chronically elevated levels of AC activation, like β-AR (11, 16, 63) or Gsα (1, 36, 37), are deleterious and facilitate development of cardiomyopathy. In contrast, when a cardiomyopathy develops associated with reduced levels of AC5, restoration of AC5 expression may be beneficial for normal cardiac function under these conditions.
Clinical Relevance of AC5
Although hundreds, if not thousands, of novel and exciting discoveries have been made by alterations in genes in genetically engineered mice, relatively few have translated into improving clinical care. One reason for the lack of success is that it is difficult to overexpress or delete a gene in patients. Therefore, the goal becomes to have a pharmacological analog of the altered gene that can be safely delivered to patients. A current goal of our laboratory is to translate the beneficial effects of the AC5-KO model to clinical therapy. In this connection, while screening for a commercially available drug for the AC5 inhibition, adenine-9-β-d-arabinofuranoside (Ara-A; Vidarabine) showed a selective inhibition of AC5. Recent studies in our laboratory demonstrated that Ara-A selectively inhibits AC5 activity in AC5-Tg mice, but not in AC6-Tg mice. In cardiac membrane preparations with Iso stimulation, Ara-A (10 μM) reduced cAMP production much more in AC5-Tg (49%) than in WT and not at all in AC5-KO (38). Ara-A was originally developed as an antiviral drug, which was approved by the United States Food and Drug Administration. It has been clinically used for treatment of herpes virus infection, but it was found to be less efficient for viral therapy than the newer drug, acyclovir. We also found that this pharmacological AC5 inhibitor recapitulates the favorable effects of AC5 disruption and ameliorated the development of cardiomyopathy and heart failure induced by either permanent coronary artery occlusion or chronic catecholamine infusion (38). Ara-A significantly improved the survival rate and LV function compared with vehicle after 3 wk of coronary artery occlusion, and these beneficial effects of Ara-A were abolished by U0126, a MEK inhibitor, suggesting the involvement of the downstream MEK-ERK pathway of AC5 (38). This is significant since the same signaling pathway was found mediating the longevity in AC5-KO (85). In heart failure, Ara-A has also been shown to reduce autophagy by inhibition of AMPK (49). Since toxicology for the drug has found little to be contraindicated in heart failure and since adverse effects were only manifest with very high chronic doses, low dose Ara-A is a strong candidate for a clinical trial for heart failure since it selectively inhibits AC5, has been shown to protect against heart failure without adverse effects, and has been already approved by the United States Food and Drug Administration. One potential limitation to this drug is that only an intravenous formulation is currently available. Accordingly, drug discovery studies will have to be pursued for oral delivery and optimizing the compound for heart failure applications.
Conclusions
There are several take-home messages. First, although AC5 and AC6 are the two major isoforms in the heart, they mediate dramatically different functions, particularly in response to stress. Second, although AC5 is one of the major cardiac isoforms of AC, potentially its most important role will be in mediating diabetes, obesity, and longevity, even more so than in cardiac protection. Finally, it may be possible to translate the beneficial effects of the AC5-KO to the bedside, by using a pharmacological analog, which preferentially inhibits AC5.
GRANTS
This study was supported by National Institutes of Health Grants HL-093481, HL-106511, HL-033107, HL-095888, HL-069020, and AG-027211.
DISCLOSURES
S. F. Vatner and D. E. Vatner both work for Vasade Biosciences, which also has research interests in AC5 but has no intellectual property and did not provide support for this study. All research was funded by the NIH.
AUTHOR CONTRIBUTIONS
S.F.V. and D.E.V. conception and design of research; S.F.V., M.P., and L.Y. drafted manuscript; S.F.V., M.P., G.J.A.L., L.L., K.I., Y.I., J.E.P., and D.E.V. edited and revised manuscript; S.F.V. and D.E.V. approved final version of manuscript; M.P. prepared figures.
REFERENCES
- 1. Asai K, Yang GP, Geng YJ, Takagi G, Bishop S, Ishikawa Y, Shannon RP, Wagner TE, Vatner DE, Homcy CJ, Vatner SF. Beta-adrenergic receptor blockade arrests myocyte damage and preserves cardiac function in the transgenic G(salpha) mouse. J Clin Invest 104: 551–558, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bauman AL, Soughayer J, Nguyen BT, Willoughby D, Carnegie GK, Wong W, Hoshi N, Langeberg LK, Cooper DM, Dessauer CW, Scott JD. Dynamic regulation of cAMP synthesis through anchored PKA-adenylyl cyclase V/VI complexes. Mol Cell 23: 925–931, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bourajjaj M, Armand AS, da Costa Martins P.A, Weijts B, van der Nagel R, Heeneman S, Wehrens XH, De Windt LJ. NFATc2 is a necessary mediator of calcineurin-dependent cardiac hypertrophy and heart failure. J Biol Chem 283: 22295–22303, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med 307: 205–211, 1982 [DOI] [PubMed] [Google Scholar]
- 5. Canto C, Auwerx J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab 20: 325–331, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cao J, Sodhi K, Puri N, Monu SR, Rezzani R, Abraham NG. High fat diet enhances cardiac abnormalities in SHR rats: protective role of heme oxygenase-adiponectin axis. Diabetol Metab Syndr 3: 37, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CIBIS Investigators and Committees. A randomized trial of beta-blockade in heart failure. The Cardiac Insufficiency Bisoprolol Study (CIBIS). Circulation 90: 1765–1773, 1994 [DOI] [PubMed] [Google Scholar]
- 8.CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study I.I. (CIBIS-II): a randomized trial. Lancet 353: 9–13, 1999 [PubMed] [Google Scholar]
- 9. Defer N, Best-Belpomme M, Hanoune J. Tissue specificity and physiological relevance of various isoforms of adenylyl cyclase. Am J Physiol Renal Physiol 279: F400–F416, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Dessauer CW, Gilman AG. The catalytic mechanism of mammalian adenylyl cyclase. Equilibrium binding and kinetic analysis of P-site inhibition. J Biol Chem 272: 27787–27795, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Du XJ, Gao XM, Wang B, Jennings GL, Woodcock EA, Dart AM. Age-dependent cardiomyopathy and heart failure phenotype in mice overexpressing beta(2)-adrenergic receptors in the heart. Cardiovasc Res 48: 448–454, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Dyachok O, Isakov Y, Sagetorp J, Tengholm A. Oscillations of cyclic AMP in hormone-stimulated insulin-secreting beta-cells. Nature 439: 349–352, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Efendiev R, Dessauer CW. A kinase-anchoring proteins and adenylyl cyclase in cardiovascular physiology and pathology. J Cardiovasc Pharmacol 58: 339–344, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elkayam U, Tasissa G, Binanay C, Stevenson LW, Gheorghiade M, Warnica JW, Young JB, Rayburn BK, Rogers JG, DeMarco T, Leier CV. Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure. Am Heart J 153: 98–104, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Engelhardt S, Hein L, Dyachenkow V, Kranias EG, Isenberg G, Lohse MJ. Altered calcium handling is critically involved in the cardiotoxic effects of chronic beta-adrenergic stimulation. Circulation 109: 1154–1160, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice. Proc Natl Acad Sci USA 96: 7059–7064, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Esposito G, Perrino C, Ozaki T, Takaoka H, Defer N, Petretta MP, De Angelis MC, Mao L, Hanoune J, Rockman HA, Chiariello M. Increased myocardial contractility and enhanced exercise function in transgenic mice overexpressing either adenylyl cyclase 5 or 8. Basic Res Cardiol 103: 22–30, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Fang CX, Dong F, Thomas DP, Ma H, He L, Ren J. Hypertrophic cardiomyopathy in high-fat diet-induced obesity: role of suppression of forkhead transcription factor and atrophy gene transcription. Am J Physiol Heart Circ Physiol 295: H1206–H1215, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feldman AM. Adenylyl cyclase: a new target for heart failure therapeutics. Circulation 105: 1876–1878, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Fujino T, Hasebe N, Kawabe J, Fujita M, Fukuzawa J, Tobise K, Kikuchi K. Effect of beta-adrenoceptor antagonist and angiotensin-converting enzyme inhibitor on hypertension-associated changes in adenylyl cyclase type V messenger RNA expression in spontaneously hypertensive rats. J Cardiovasc Pharmacol 41: 720–725, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Georget M, Mateo P, Vandecasteele G, Jurevicius J, Lipskaia L, Defer N, Hanoune J, Hoerter J, Fischmeister R. Augmentation of cardiac contractility with no change in L-type Ca2+ current in transgenic mice with a cardiac-directed expression of the human adenylyl cyclase type 8 (AC8). FASEB J 16: 1636–1638, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Gerhardstein BL, Puri TS, Chien AJ, Hosey MM. Identification of the sites phosphorylated by cyclic AMP-dependent protein kinase on the beta 2 subunit of L-type voltage-dependent calcium channels. Biochemistry 38: 10361–10370, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Gottle M, Geduhn J, Konig B, Gille A, Hocherl K, Seifert R. Characterization of mouse heart adenylyl cyclase. J Pharmacol Exp Ther 329: 1156–1165, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Gresl TA, Colman RJ, Roecker EB, Havighurst TC, Huang Z, Allison DB, Bergman RN, Kemnitz JW. Dietary restriction and glucose regulation in aging rhesus monkeys: a follow-up report at 8.5 yr. Am J Physiol Endocrinol Metab 281: E757–E765, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Gros R, Ding Q, Chorazyczewski J, Pickering JG, Limbird LE, Feldman RD. Adenylyl cyclase isoform-selective regulation of vascular smooth muscle proliferation and cytoskeletal reorganization. Circ Res 99: 845–852, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest 56: 56–64, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guellich A, Gao S, Hong C, Yan L, Wagner TE, Dhar SK, Ghaleh B, Hittinger L, Iwatsubo K, Ishikawa Y, Vatner SF, Vatner DE. Effects of cardiac overexpression of type 6 adenylyl cyclase affects on the response to chronic pressure overload. Am J Physiol Heart Circ Physiol 299: H707–H712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haber N, Stengel D, Defer N, Roeckel N, Mattei MG, Hanoune J. Chromosomal mapping of human adenylyl cyclase genes type III, type V and type VI. Hum Genet 94: 69–73, 1994 [DOI] [PubMed] [Google Scholar]
- 29. Hakonarson H, Grunstein MM. Regulation of second messengers associated with airway smooth muscle contraction and relaxation. Am J Respir Crit Care Med 158: S115–S122, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Hjalmarson A, Goldstein S, Fagerberg B, Wedel H, Waagstein F, Kjekshus J, Wikstrand J, El Allaf D, Vitovec J, Aldershvile J, Halinen M, Dietz R, Neuhaus KL, Janosi A, Thorgeirsson G, Dunselman PH, Gullestad L, Kuch J, Herlitz J, Rickenbacher P, Ball S, Gottlieb S, Deedwania P. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). MERIT-HF Study Group. JAMA 283: 1295–1302, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Ho D, Yan L, Iwatsubo K, Vatner DE, Vatner SF. Modulation of beta-adrenergic receptor signaling in heart failure and longevity: targeting adenylyl cyclase type 5. Heart Fail Rev 15: 495–512, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ho D, Yan L, Zhao X, Bravo C, Stanley W, Vatner DE, Pessin J, Vatner SF. Disruption of adenylyl cyclase type 5, a novel target for obesity, diabetes and diabetic cardiomyopathy (Abstract). Circulation 126: A19323 2012 [Google Scholar]
- 33. Hu CL, Chandra R, Ge H, Pain J, Yan L, Babu G, Depre C, Iwatsubo K, Ishikawa Y, Sadoshima J, Vatner SF, Vatner DE. Adenylyl cyclase type 5 protein expression during cardiac development and stress. Am J Physiol Heart Circ Physiol 297: H1776–H1782, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Iancu RV, Jones SW, Harvey RD. Compartmentation of cAMP signaling in cardiac myocytes: a computational study. Biophys J 92: 3317–3331, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ishikawa Y, Katsushika S, Chen L, Halnon NJ, Kawabe J, Homcy CJ. Isolation and characterization of a novel cardiac adenylylcyclase cDNA. J Biol Chem 267: 13553–13557, 1992 [PubMed] [Google Scholar]
- 36. Iwase M, Bishop SP, Uechi M, Vatner DE, Shannon RP, Kudej RK, Wight DC, Wagner TE, Ishikawa Y, Homcy CJ, Vatner SF. Adverse effects of chronic endogenous sympathetic drive induced by cardiac GS alpha overexpression. Circ Res 78: 517–524, 1996 [DOI] [PubMed] [Google Scholar]
- 37. Iwase M, Uechi M, Vatner DE, Asai K, Shannon RP, Kudej RK, Wagner TE, Wight DC, Patrick TA, Ishikawa Y, Homcy CJ, Vatner SF. Cardiomyopathy induced by cardiac Gsα overexpression. Am J Physiol Heart Circ Physiol 272: H585–H589, 1997 [DOI] [PubMed] [Google Scholar]
- 38. Iwatsubo K, Bravo C, Uechi M, Baljinnyam E, Nakamura T, Umemura M, Lai L, Gao S, Yan L, Zhao X, Park M, Qiu H, Okumura S, Iwatsubo M, Vatner DE, Vatner SF, Ishikawa Y. Prevention of heart failure in mice by an antiviral agent that inhibits type 5 cardiac adenylyl cyclase. Am J Physiol Heart Circ Physiol 302: H2622–H2628, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kapiloff MS, Piggott LA, Sadana R, Li J, Heredia LA, Henson E, Efendiev R, Dessauer CW. An adenylyl cyclase-mAKAPbeta signaling complex regulates cAMP levels in cardiac myocytes. J Biol Chem 284: 23540–23546, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med 114: 345–352, 1991 [DOI] [PubMed] [Google Scholar]
- 41. Lai L, Yan L, Gao S, Hu CL, Ge H, Davidow A, Park M, Bravo C, Iwatsubo K, Ishikawa Y, Auwerx J, Sinclair DA, Vatner SF, Vatner DE. Type 5 adenylyl cyclases increases oxidative stress by transcriptional regulation of MnSOD via the SIRT1/FoxO3a pathway. Circulation 127: 1692–1701, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lakatta EG. Age-associated cardiovascular changes in health: impact on cardiovascular disease in older persons. Heart Fail Rev 7: 29–49, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation 107: 490–497, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Lambertz H, Meyer J, Erbel R. Long-term hemodynamic effects of prenalterol in patients with severe congestive heart failure. Circulation 69: 298–305, 1984 [DOI] [PubMed] [Google Scholar]
- 45. Lee GJ, Yan L, Vater DE, Vatner SF. β-Adrenergic receptor signaling in heart failure. In: Cardiac Remodeling: Molecular Mechanisms, edited by Jugdutt BI, Dhalla NS. New York: Springer, 2013 [Google Scholar]
- 46. Liggett SB, Tepe NM, Lorenz JN, Canning AM, Jantz TD, Mitarai S, Yatani A, Dorn GW., 2nd Early and delayed consequences of beta(2)-adrenergic receptor overexpression in mouse hearts: critical role for expression level. Circulation 101: 1707–1714, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res 93: 896–906, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101: 365–376, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res 100: 914–922, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 489: 318–321, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Milano CA, Allen LF, Rockman HA, Dolber PC, McMinn TR, Chien KR, Johnson TD, Bond RA, Lefkowitz RJ. Enhanced myocardial function in transgenic mice overexpressing the beta 2-adrenergic receptor. Science 264: 582–586, 1994 [DOI] [PubMed] [Google Scholar]
- 52. Mons N, Guillou JL, Jaffard R. The role of Ca2+/calmodulin-stimulable adenylyl cyclases as molecular coincidence detectors in memory formation. Cell Mol Life Sci 55: 525–533, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Muller G, Wied S, Over S, Frick W. Inhibition of lipolysis by palmitate, H2O2 and the sulfonylurea drug, glimepiride, in rat adipocytes depends on cAMP degradation by lipid droplets. Biochemistry 47: 1259–1273, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Neves MJ, Terenzi HF. In vivo control of gluconeogenesis in wild-type Neurospora crassa and in the adenylate cyclase-deficient cr-1 (crisp) mutant. J Bacteriol 171: 1767–1771, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. O'Connor CM, Gattis WA, Uretsky BF, Adams KF, Jr, McNulty SE, Grossman SH, McKenna WJ, Zannad F, Swedberg K, Gheorghiade M, Califf RM. Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: insights from the Flolan International Randomized Survival Trial (FIRST). Am Heart J 138: 78–86, 1999 [DOI] [PubMed] [Google Scholar]
- 56. Okumura S, Suzuki S, Ishikawa Y. New aspects for the treatment of cardiac diseases based on the diversity of functional controls on cardiac muscles: effects of targeted disruption of the type 5 adenylyl cyclase gene. J Pharm Sci 109: 354–359, 2009 [DOI] [PubMed] [Google Scholar]
- 57. Okumura S, Takagi G, Kawabe J, Yang G, Lee MC, Hong C, Liu J, Vatner DE, Sadoshima J, Vatner SF, Ishikawa Y. Disruption of type 5 adenylyl cyclase gene preserves cardiac function against pressure overload. Proc Natl Acad Sci USA 100: 9986–9990, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Okumura S, Vatner DE, Kurotani R, Bai Y, Gao S, Yuan Z, Iwatsubo K, Ulucan C, Kawabe J, Ghosh K, Vatner SF, Ishikawa Y. Disruption of type 5 adenylyl cyclase enhances desensitization of cyclic adenosine monophosphate signal and increases Akt signal with chronic catecholamine stress. Circulation 116: 1776–1783, 2007 [DOI] [PubMed] [Google Scholar]
- 59. Ostrom RS, Naugle JE, Hase M, Gregorian C, Swaney JS, Insel PA, Brunton LL, Meszaros JG. Angiotensin II enhances adenylyl cyclase signaling via Ca2+/calmodulin. Gq-Gs cross-talk regulates collagen production in cardiac fibroblasts. J Biol Chem 278: 24461–24468, 2003 [DOI] [PubMed] [Google Scholar]
- 60. Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. US Carvedilol Heart Failure Study Group. N Engl J Med 334: 1349–1355, 1996 [DOI] [PubMed] [Google Scholar]
- 61. Park M, Park J, Lee J, Tian B, Lai L, Iwatsubo K, Ishikawa Y, Sadoshima J, Vatner DE, Vatner SF. Cardiac overexpression of adenylyl cyclase type 5 induces left ventricular hypertrophy potentially by activating calcineurin-NFAT signaling (Abstract). FASEB J 365–311, 2011 [Google Scholar]
- 62. Park SY, Cho YR, Kim HJ, Higashimori T, Danton C, Lee MK, Dey A, Rothermel B, Kim YB, Kalinowski A, Russell KS, Kim JK. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes 54: 3530–3540, 2005 [DOI] [PubMed] [Google Scholar]
- 63. Peter PS, Brady JE, Yan L, Chen W, Engelhardt S, Wang Y, Sadoshima J, Vatner SF, Vatner DE. Inhibition of p38 alpha MAPK rescues cardiomyopathy induced by overexpressed beta 2-adrenergic receptor, but not beta 1-adrenergic receptor. J Clin Invest 117: 1335–1343, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Petrashevskaya N, Gaume BR, Mihlbachler KA, Dorn GW, 2nd, Liggett SB. Bitransgenesis with beta(2)-adrenergic receptors or adenylyl cyclase fails to improve beta(1)-adrenergic receptor cardiomyopathy. Clin Transl Sci 1: 221–227, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rengo G, Zincarelli C, Femminella GD, Liccardo D, Pagano G, de Lucia C, Altobelli GG, Cimini V, Ruggiero D, Perrone-Filardi P, Gao E, Ferrara N, Lymperopoulos A, Koch WJ, Leosco D. Myocardial beta(2)-adrenoceptor gene delivery promotes coordinated cardiac adaptive remodelling and angiogenesis in heart failure. Br J Pharmacol 166: 2348–2361, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rosenberg D, Groussin L, Bertagna X, Bertherat J. cAMP pathway alterations from the cell surface to the nucleus in adrenocortical tumors. Endocr Res 28: 765–775, 2002 [DOI] [PubMed] [Google Scholar]
- 67. Sadana R, Dessauer CW. Physiological roles for G protein-regulated adenylyl cyclase isoforms: insights from knockout and overexpression studies. Neurosignals 17: 5–22, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Segev A, Mekori YA. The Cardiac Insufficiency Bisoprolol Study II. Lancet 353: 1361, 1999 [DOI] [PubMed] [Google Scholar]
- 69. Shah RS, Lee HG, Xiongwei Z, Perry G, Smith MA, Castellani RJ. Current approaches in the treatment of Alzheimer's disease. Biomed Pharmacother 62: 199–207, 2008 [DOI] [PubMed] [Google Scholar]
- 70. Simmerman HK, Jones LR. Phospholamban: protein structure, mechanism of action, and role in cardiac function. Physiol Rev 78: 921–947, 1998 [DOI] [PubMed] [Google Scholar]
- 71. Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev 126: 987–1002, 2005 [DOI] [PubMed] [Google Scholar]
- 72. Sulakhe PV, Vo XT. Regulation of phospholamban and troponin-I phosphorylation in the intact rat cardiomyocytes by adrenergic and cholinergic stimuli: roles of cyclic nucleotides, calcium, protein kinases and phosphatases and depolarization. Mol Cell Biochem 149–150: 103–126, 1995 [DOI] [PubMed] [Google Scholar]
- 73. Sutherland EW, Rall TW. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J Biol Chem 232: 1077–1091, 1958 [PubMed] [Google Scholar]
- 74. Tepe NM, Liggett SB. Transgenic replacement of type V adenylyl cyclase identifies a critical mechanism of beta-adrenergic receptor dysfunction in the G alpha q overexpressing mouse. FEBS Lett 458: 236–240, 1999 [DOI] [PubMed] [Google Scholar]
- 75. Tesmer JJ, Sunahara RK, Gilman AG, Sprang SR. Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsalpha.GTPgammaS. Science 278: 1907–1916, 1997 [DOI] [PubMed] [Google Scholar]
- 76. Tevaearai HT, Eckhart AD, Walton GB, Keys JR, Wilson K, Koch WJ. Myocardial gene transfer and overexpression of beta2-adrenergic receptors potentiates the functional recovery of unloaded failing hearts. Circulation 106: 124–129, 2002 [DOI] [PubMed] [Google Scholar]
- 77. Timofeyev V, Porter CA, Tuteja D, Qiu H, Li N, Tang T, Singapuri A, Han PL, Lopez JE, Hammond HK, Chiamvimonvat N. Disruption of adenylyl cyclase type V does not rescue the phenotype of cardiac-specific overexpression of Galphaq protein-induced cardiomyopathy. Am J Physiol Heart Circ Physiol 299: H1459–H1467, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Vatner DE, Sato N, Ishikawa Y, Kiuchi K, Shannon RP, Vatner SF. Beta-adrenoceptor desensitization during the development of canine pacing-induced heart failure. Clin Exp Pharmacol Physiol 23: 688–692, 1996 [DOI] [PubMed] [Google Scholar]
- 79. Vatner SF, Vatner DE, Yan L. Models of longevity (calorie restriction and AC5 KO): result of three bad hypotheses. Aging (Albany NY) 4: 662–663, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr 84: 1033–1042, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res 94: 110–118, 2004 [DOI] [PubMed] [Google Scholar]
- 82. Willoughby D, Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev 87: 965–1010, 2007 [DOI] [PubMed] [Google Scholar]
- 83. Willoughby D, Wachten S, Masada N, Cooper DM. Direct demonstration of discrete Ca2+ microdomains associated with different isoforms of adenylyl cyclase. J Cell Sci 123: 107–117, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yan L, Park JY, Dillinger JG, De Lorenzo MS, Yuan C, Lai L, Wang C, Ho D, Tian B, Stanley WC, Auwerx J, Vatner DE, Vatner SF. Common mechanisms for calorie restriction and adenylyl cyclase type 5 knockout models of longevity. Aging Cell 11: 1110–1120, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yan L, Vatner DE, O'Connor JP, Ivessa A, Ge H, Chen W, Hirotani S, Ishikawa Y, Sadoshima J, Vatner SF. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell 130: 247–258, 2007 [DOI] [PubMed] [Google Scholar]
- 86. Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science 295: 1711–1715, 2002 [DOI] [PubMed] [Google Scholar]
- 87. Zhang G, Liu Y, Ruoho AE, Hurley JH. Structure of the adenylyl cyclase catalytic core. Nature 386: 247–253, 1997 [DOI] [PubMed] [Google Scholar]