Abstract
The cardiac ryanodine receptor (RyR2) is inhibited by calmodulin (CaM) and S100A1. Simultaneous substitution of three amino acid residues (W3587A, L3591D, F3603A; RyR2ADA) in the CaM binding domain of RyR2 results in loss of CaM inhibition at submicromolar (diastolic) and micromolar (systolic) Ca2+, cardiac hypertrophy, and heart failure in Ryr2ADA/ADA mice. To address whether cardiac hypertrophy results from the elimination of CaM and S100A1 inhibition at diastolic or systolic Ca2+, a mutant mouse was generated with a single RyR2 amino acid substitution (L3591D; RyR2D). Here we report that in single-channel measurements RyR2-L3591D isolated from Ryr2D/D hearts lost CaM inhibition at diastolic Ca2+ only, whereas S100A1 regulation was eliminated at both diastolic and systolic Ca2+. In contrast to the ∼2-wk life span of Ryr2ADA/ADA mice, Ryr2D/D mice lived longer than 1 yr. Six-month-old Ryr2D/D mice showed a 9% increase in heart weight-to-body weight ratio, modest changes in cardiac morphology, and a twofold increase in atrial natriuretic peptide mRNA levels compared with wild type. After 4-wk pressure overload with transverse aortic constriction, heart weight-to-body weight ratio and atrial natriuretic peptide mRNA levels increased and echocardiography showed changes in heart morphology of Ryr2D/D mice compared with sham-operated mice. Collectively, the findings indicate that the single RyR2-L3591D mutation, which distinguishes the effects of diastolic and systolic Ca2+, alters heart size and cardiac function to a lesser extent in Ryr2D/D mice than the triple mutation in Ryr2ADA/ADA mice. They further suggest that CaM inhibition of RyR2 at systolic Ca2+ is important for maintaining normal cardiac function.
Keywords: cardiac ryanodine receptor, calmodulin, S100A1, cardiac hypertrophy
cardiac muscle ryanodine receptors (RyR2s) are large, ligand-gated ion channels composed of four 560-kDa RyR2 subunits, four 12.6-kDa FK506 binding protein subunits, and various associated proteins that include protein kinases, protein phosphatases, S100A1, and calmodulin (CaM) (11, 17, 19, 30). Binding of CaM to RyR2 inhibits the release of Ca2+ from sarcoplasmic reticulum (SR) at diastolic and systolic Ca2+ (2, 8, 19, 31). Deletion of RyR2 amino acid residues 3583–3603 and mutations in this region eliminate CaM binding and inhibition of the recombinant RyR2 by CaM in vitro (36). An in vivo role of RyR2 regulation by CaM was established by preparing mutant mice with three amino acid substitutions in the CaM binding domain of RyR2 (W3587A, L3591D, F3603A; RyR2ADA). Ryr2ADA/ADA mice die within ∼2 wk after birth because of the rapid development of cardiac hypertrophy and heart failure (35). Their early death is associated with abnormal SR Ca2+ release and altered gene regulation (33, 35).
S100A1 is a small Ca2+ binding protein expressed at high levels in cardiomyocytes (13). S100A1 targets multiple sites that include sarcomeric proteins, mitochondrial proteins, and Ca2+ handling proteins Cav1.2, RyR2, and sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA)2a and SERCA2a regulatory protein phospholamban (PLN) (10, 21, 24, 29). S100A1 also regulates skeletal muscle RyR1 and binds to the conserved CaM binding domain of RyR1 (25, 27). S100A1−/− mice have normal life span and cardiac function under normal conditions (7). However, transgenic overexpression of S100A1 increases SR Ca2+ content and Ca2+ release and improves myocardial contractile performance (21). More recent studies have shown that molecular targeting of S100A1 to myocardially infarcted animals (23) and human failing cardiomyocytes (4) restores cardiac function by improving mitochondrial function, SR Ca2+ handling, and contractile performance. This suggested that S100A1 gene targeting may be a promising strategy to treat heart failure.
To further define the role of RyR2 and Ca2+ in cardiac development and function, a second mouse model was prepared with a single amino acid substitution in the CaM and S100A1 binding domain of RyR2 (L3591D; RyR2D). This mutation was selected on the basis of earlier studies showing that the RyR2-L3591D mutant is inhibited by CaM at micromolar Ca2+ but not submicromolar Ca2+ concentrations when expressed in human embryonic kidney 293 (HEK293) cells (36). Here we report that, in contrast to Ryr2ADA/ADA mice, Ryr2D/D mice live longer than 1 yr and develop a less severe impairment of cardiac function than Ryr2ADA/ADA mice.
MATERIALS AND METHODS
Ethics statement.
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocols were approved by the University of North Carolina at Chapel Hill and the Medical University of South Carolina Institutional Animal Care and Use Committees. Animals were anesthetized and killed with 1–4% isoflurane (drop method) or intraperitoneal injection of Avertin (160 mg/kg) followed by physical euthanasia.
Materials.
[3H]ryanodine was obtained from PerkinElmer and unlabeled ryanodine from EMD Biosciences. Complete protease inhibitors were from Roche Applied Science and phospholipids from Avanti Polar Lipids. Unlabeled CaM was prepared as described previously (2). Rabbit polyclonal antibody RyR2-F9221 was prepared by New England Peptide (Gardner, MA). Unless otherwise specified, all other chemicals were obtained from Sigma-Aldrich.
Preparation of mutant mice.
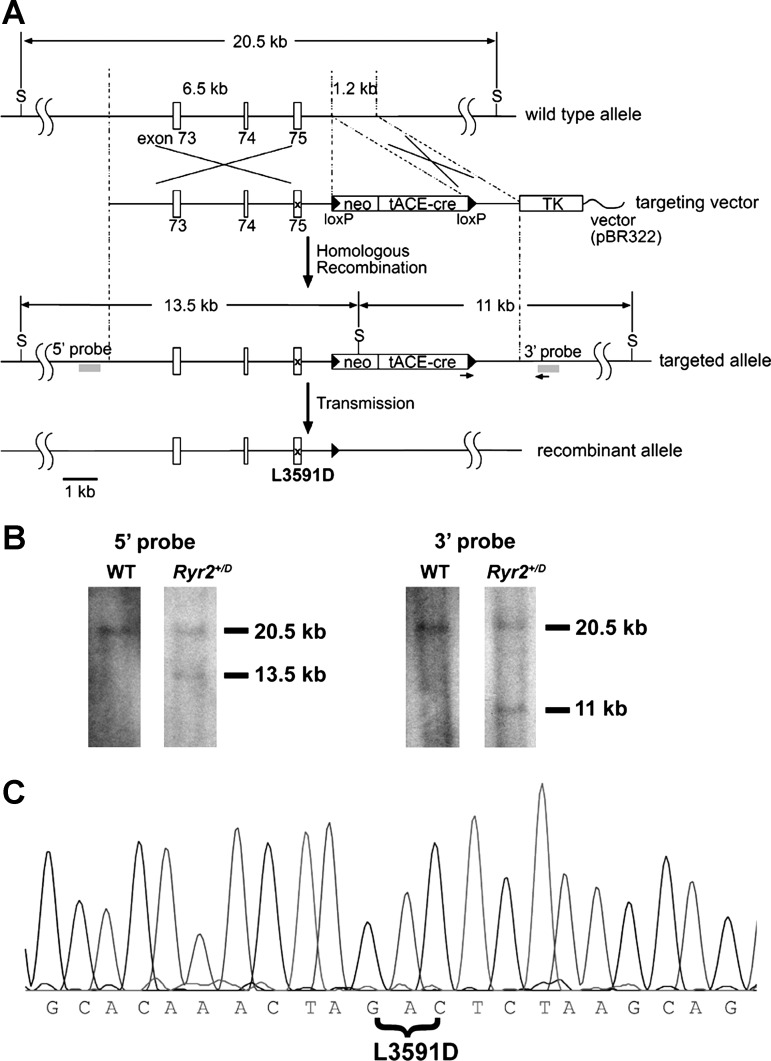
Mutant mice carrying the RyR2-L3591D mutation were prepared with established methods (35). Briefly, linearized targeting vector was transfected into E14 embryonic stem (ES) cells by electroporation. Cells were selected in the presence of G418 and gancyclovir and screened by both PCR and Southern blot analysis. Mutations were confirmed by DNA sequencing. Targeted ES cells were injected into the blastocysts of C57BL/6J mice in the Animal Models Core Facility of the University of North Carolina at Chapel Hill. A male chimera mouse carrying the mutation in the Ryr2 gene was mated with a 129/SvEv female mouse to obtain heterozygous offspring. The neomycin-resistant gene flanked by loxP sequences was removed by Cre recombinase driven by the testis-specific angiotensin-converting enzyme promoter (Fig. 1). Germ line transmission of the mutation to heterozygous offspring caused removal of a gene cassette flanked by loxP sequences (5).
Fig. 1.
Generation of mice with RyR2-L3591D mutation. A: schematic representation of the mouse Ryr2 gene and targeting construct. S represents SphI restriction site. Neomycin-resistant gene (neo), Cre recombinase gene driven by testis-specific angiotensin-converting enzyme promoter (tACE-cre), and thymidine kinase gene (TK) are indicated. Arrows and x indicate the position of primers for screening and a mutation site, respectively. B: Southern blot of genomic DNA. After restriction enzyme digestion of genomic DNA with SphI, 5′ and 3′ probes identify 13.5-kb and 11-kb fragments in the targeted allele, respectively. Both probes hybridize with 20.5-kb fragments in the wild-type (WT) allele. C: DNA sequence analysis of RyR2-L3591D. cDNA encoding the CaM binding site of RyR2 was amplified from total RNA from homozygous (hybrid genetic background) mouse cardiac muscle and sequenced. The RyR2-L3591D mutation was confirmed. A HinfI site created by the L3591D mutation (GACTC) was used for screening the mutant allele.
Homozygous gene-targeted animals (Ryr2D/D) were obtained by mating heterozygous mice (Ryr2+/D). Previous experiments with Ryr2ADA/ADA mice (35) and experiments with Ryr2D/D mice were performed with animals that were backcrossed at least five times into the 129/SvEv genetic background. Offspring were genotyped by PCR followed by restriction digestion with HinfI.
Morphological analyses.
Hearts from 6- to 7-mo-old mice were fixed with 4% (wt/vol) paraformaldehyde in PBS (pH 7.4) and dehydrated with increasing concentrations of ethanol in water. Paraffin-embedded hearts were sectioned into 6- to 9-μm thickness and stained with hematoxylin and eosin. Tetramethylrhodamine isothiocyanate (TRITC)-conjugated wheat germ agglutinin was used to visualize the cell membranes in the sections (35). TRITC fluorescence was captured by confocal microscopy (Leica TCS SP5), and cross-sectional areas of individual cells in left and right ventricles and interventricular septum were calculated with ImageJ software. Thirty cells were randomly selected from wild-type and mutant mice.
Echocardiography.
Left ventricular cardiac parameters were determined by transthoracic M-mode echocardiography on restrained mice with a Vevo 2100 high-resolution imaging system (VisualSonics) with a 40-MHz probe. Unanesthetized 4- and 6-mo-old mice were restrained on a warmed mouse board (Indus Instruments for VisualSonics).
Transverse aortic constriction.
Pressure overload in the left ventricle was induced by transverse aortic constriction (TAC) of 3-mo-old wild-type and Ryr2D/D mice as described previously (3). The aorta was ligated between the innominate and left common carotid arteries with a 7-10 silk suture and a tapered 27-gauge needle placed on top of the aorta. The tapered needle was removed, leaving the suture to produce a defined stenosis of the vessel. Control mice were subjected to a sham operation in which a band was twined around the aorta, but not ligated, and subsequently removed. The skin was closed with separate sutures, and buprenorphine was administered for analgesia. Echocardiography was performed after 4 wk of constriction.
Quantitative RT-PCR.
Gene expression was measured by quantitative RT-PCR with the ABI Prism 7700 Sequence Detector (Applied Biosystems) (15). RNA was isolated from the left ventricle of 4- and 6-mo-old mice with the ABI Prism 6700 Automated Nucleic Acid Workstation according to the manufacturer's protocol. Forward and reverse primers and corresponding fluorogenic probes were β-actin: AAGAGCTATGAGCTGCCTGA, ACGGATGTCAACGTCACACT, FTCACTATTGGCAACGAGCGGTTCGCQ; α-myosin heavy chain (α-MHC): AGCTCAGCCAGGCCAATAGA, TGGGTGTCCTTCAAGTGAGC, FAGAATTCTTCAGGTGTTTCTGTGCCTCAGQ; β-MHC: AGCTCAGCCATGCCAACCGT, TGAGTGTCCTTCAGCAGACT, FAGGCTCTTCACTTGTTTCTGGGCCTCAQ; atrial natriuretic peptide (ANP): GAGAAGATGCCGGTAGAAGA, AAGCACTGCCGTCTCTCAGA, FATGCCCCCGCAGGCCCGGQ; brain natriuretic peptide (BNP): CTGCTGGAGCTGATAAGAGA, TGCCCAAAGCAGCTTGAGCT, FCTCAAGGCAGCACCCTCCGGGQ; Cav1.2: AGACATCTCTCAGAAGACAGC, TCAAGCCTGCCACTGCCAAT, FTGCCCTTGCATCTGGTTCATCACCAGQ; RyR2: CTGTCCCTGAGGTACAAGAA, AGCTTTCTCTGGTTCCGACT, FTTCAGGAGCAGAAGGCGAAAGAGGAQ; inositol trisphosphate receptor (IP3R): TCCTCAAGACAACCTGCTTC, CTGACTTGATGTGCTCCTCA, FCTGTGGCCTGGAGAGAGACAAGTTTGQ; calsequestrin (CSQ2): AAGAGTCACCCAGATGGCTA, ATGCTCAAGTCAGGATTGTCA, FCCTAGAGATCCTGAAACAGGTTGCCCQ; SERCA2a: TCCTCTACGTGGAACCTTTG, AAGGAGATTTTCAGCACCATC, FCCAGATCACACCGCTGAATCTGACCCQ; and sodium-calcium exchanger (NCX)1: AAGCAGCCACTGACCAGCA, TGGTGTGCTCGCCTAGGAT, FCCCATTTCCGCAATGCGCCTCTCCQ [F is 5′-fluorescein (FAM) and Q is quencher (TAMRA)]. Levels of gene expression were quantitated relative to β-actin.
Preparation of heart homogenates and crude membrane fractions.
Hearts were homogenized in 20 mM imidazole, pH 7.0, 0.3 M sucrose, 0.15 M NaCl, 0.1 mM EGTA, protease inhibitors (Complete; Roche Applied Science), and 1 mM glutathione (oxidized form) with a Tekmar Tissumizer twice for 5 s at 13,500 rpm (35). Homogenates were centrifuged for 45 min at 100,000 g in a type 75Ti rotor (Beckman). Pellets were suspended in the aforementioned buffer without EGTA and glutathione to obtain a crude membrane fraction. Homogenates and membranes were stored in small aliquots at −80°C.
Immunoblot analyses.
Homogenates (10 μg protein/lane) were separated by 3–12% gradient SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blotted with 2% ECL Advance blocking reagent (Amersham Biosciences) in 0.5% Tween 20-Tris-buffered saline (TBS), pH 7.4 at 24°C for 1 h and probed with primary rabbit polyclonal antibody RyR2-F9221 and secondary peroxidase-conjugated anti-rabbit IgG antibody. Immunoblots were developed with enhanced chemiluminescence and quantified with ImageQuantTL Analysis Software. Glyceraldehyde-3-phosphate dehydrogenase was the loading control.
Single-channel recordings.
Single-channel measurements of wild-type and mutant channels were performed in planar lipid bilayers (32). Measurements were made with symmetrical 0.25 M CsCl, 20 mM Cs HEPES, pH 7.4 with the indicated concentrations of Ca2+. A strong dependence of single-channel activities on the cis Ca2+ concentration indicated that the large cytosolic “foot” region faced the cis chamber of the bilayers. The trans SR luminal side of the bilayer was defined as ground. Exogenous CaM and S100A1 were added to the cis, cytosolic solution. Electrical signals were filtered at 2 kHz, digitized at 10 kHz, and analyzed as described previously (32). Channel open probability (Po) was obtained from 2-min recordings by setting the threshold level at 50% of the current amplitude between the closed and open channel states.
[3H]ryanodine binding.
Ryanodine binds specifically to RyRs. Hence, [3H]ryanodine binding can be used as a probe of channel activity and concentration (26). Ca2+ dependence of [3H]ryanodine binding was determined by incubating homogenates with a relatively low [3H]ryanodine concentration (3 nM) at 24°C for 20 h in 0.25 M KCl, 20 mM imidazole, pH 7.0, protease inhibitors, and indicated free Ca2+ concentrations. Maximum binding capacity (Bmax) of [3H]ryanodine binding was determined by incubating homogenates for 4–5 h at 24°C with a near-saturating concentration of 20 nM [3H]ryanodine in 20 mM imidazole, pH 7.0, 0.6 M KCl, protease inhibitors, and 0.1 mM Ca2+. Nonspecific binding was determined with a 1,000-fold excess of unlabeled ryanodine. Aliquots of samples were diluted ninefold with ice-cold water and placed on Whatman GF/B filters saturated with 2% polyethyleneimine. Filters were washed with ice-cold 0.1 M KCl, 1 mM K-PIPES, pH 7.0, and radioactivity remaining on the filters was determined by liquid scintillation counting to obtain bound [3H]ryanodine.
45Ca2+ uptake rate.
ATP-dependent 45Ca2+ uptake rates by homogenates were determined by a filtration assay (33). 45Ca2+ uptake rates were determined in 0.15 M KCl, 20 mM imidazole, pH 7.0 solution containing 5 mM ATP, 8 mM Mg2+, and 5 mM potassium oxalate, a Ca2+ precipitating agent to increase Ca2+ uptake capacity, 10 μM ruthenium red to inhibit RyR2, 5 mM NaN3 to inhibit mitochondrial Ca2+ uptake, 1 mM EGTA, and Ca2+ and 45Ca2+ to yield a free Ca2+ concentration of 0.5 μM.
Biochemical assays and data analysis.
Free Ca2+ concentrations were measured with a Ca2+-selective electrode. Results are expressed as means ± SE. Differences between wild-type and mutant samples were determined by one-way ANOVA followed by Tukey's test. P < 0.05 was considered significant.
RESULTS
Mice carrying three mutations in the CaM binding domain of RyR2 (W3587A, L3591D, F3603A; RyR2ADA) rapidly develop cardiac hypertrophy and die at ∼2 wk of age (35). The triple mutation eliminated CaM-dependent inhibition of RyR2 activity at both diastolic and systolic Ca2+. To determine whether the phenotype was caused by loss of CaM inhibition at diastolic or systolic Ca2+, another genetically modified mouse carrying a single amino acid mutation in Ryr2 gene (L3591D) was prepared by gene targeting (Fig. 1). This mutation was hypothesized to eliminate CaM inhibition of RyR2 at diastolic Ca2+ only (36).
Single-channel measurements indicate loss of CaM and S100A1 regulation of RyR2-L3591D in mice at diastolic Ca2+.
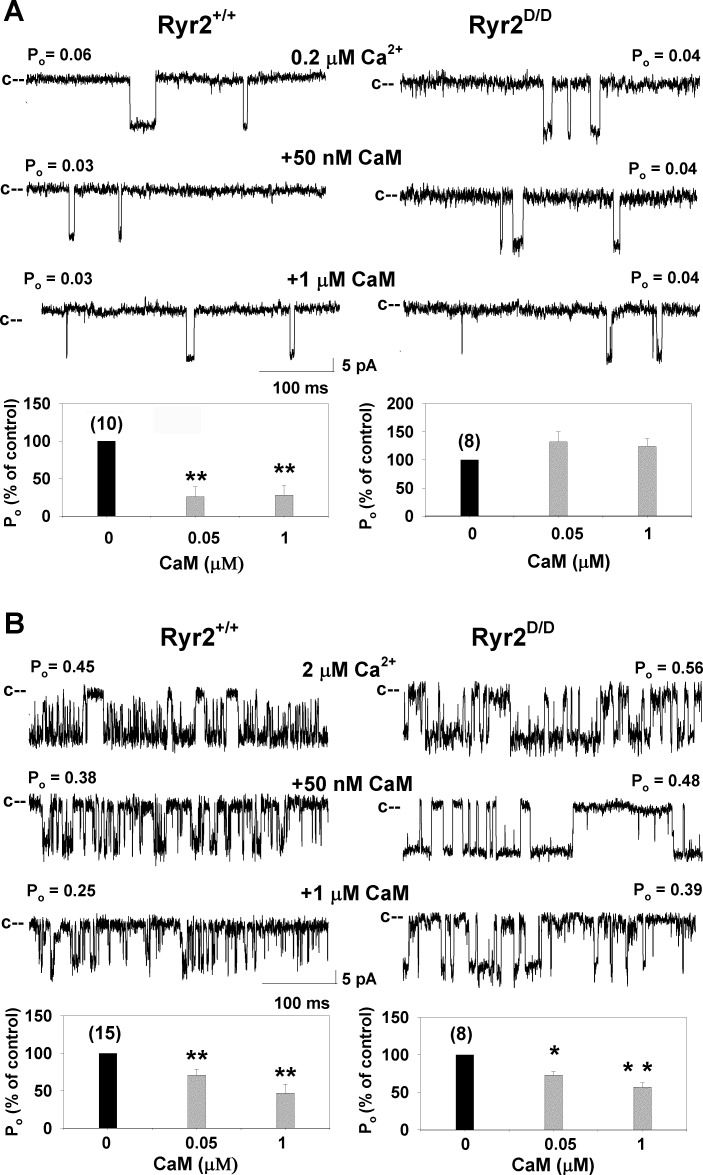
We previously showed that the RyR2-L3591D mutation caused loss of CaM-dependent regulation of channel activity at submicromolar (diastolic) but not micromolar (systolic) Ca2+ when expressed in HEK293 cells (36). To determine whether CaM regulation of the RyR2-L3591D channel was maintained in mice, membranes isolated from hearts of wild-type and Ryr2D/D mice were fused with a lipid bilayer (Fig. 2). RyR2 regulation by CaM, determined by changes in channel Po, was recorded in the presence of 0.2 μM or 2 μM free cis cytosolic Ca2+ in the absence and on the subsequent addition of cis cytosolic CaM. At 0.2 μM cytosolic Ca2+, addition of 50 nM and 1 μM cytosolic CaM resulted in significant inhibition of channel Po of wild-type RyR2, with no changes in Po for RyR2-L3591D (Fig. 2A). In contrast, in the presence of 2 μM Ca2+, Po of both wild type and RyR2-L3591D decreased at 50 nM and 1 μM CaM (Fig. 2B). Thus CaM regulation of RyR2-L3591D was lost at low Ca2+ but maintained at higher Ca2+ levels.
Fig. 2.
Effects of calmodulin (CaM) on wild-type and RyR2-L3591D single-channel activities. Membranes isolated from the hearts of wild-type and Ryr2D/D mice were fused with a lipid bilayer. Top: single-channel currents were recorded at −35 mV in the presence of 0.2 μM (A) and 2 μM (B) cis cytosolic Ca2+ as described in materials and methods in the absence of CaM (top) and on the subsequent addition of 50 nM (middle) and 1 μM (bottom) cis CaM. Po, open channel probability. Bottom: mean ± SE Po of number of recordings indicated in parentheses, normalized to control. *P < 0.05, **P < 0.001 compared with control (no CaM added). Mean Po in absence of CaM were 0.08 ± 0.02 (n = 10) and 0.10 ± 0.07 (n = 8) at 0.2 μM Ca2+ and 0.55 ± 0.05 (n = 15) and 0.47 ± 0.12 (n = 8) at 2 μM Ca2+ for wild-type and RyR2-L3591D channels, respectively.
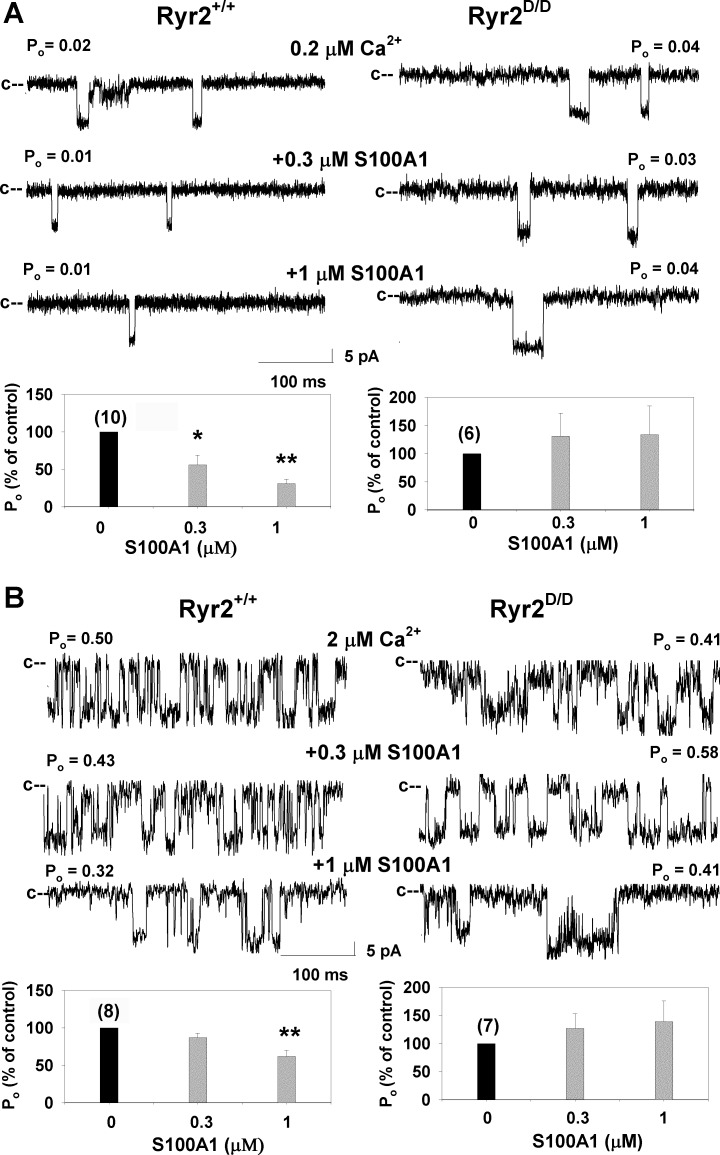
S100A1 is a Ca2+ binding protein reported to bind to the conserved CaM binding region of RyR1 (25) and regulate RyR2 activity (10, 21, 24, 29). Single-channel measurements in Fig. 3A show that at 0.2 μM Ca2+ the addition of 0.3 μM and 1 μM cytosolic S100A1 reduced Po of wild type. At 2 μM Ca2+, addition of 1 μM S100A1 was required for significant wild-type inhibition (Fig. 3B). No inhibition of RyR2-L3591D channels by S100A1 was observed under any of these conditions. The single-channel data suggest that the L3591D mutation impairs CaM and S100A1 regulation of RyR2 at 0.2 μM Ca2+. At 2 μM Ca2+, RyR2-L3591D inhibition by CaM was retained whereas that by S100A1 was lost. One possible explanation is that, in contrast to RyR1 (34), S100A1 and CaM do not compete for the same CaM binding site in RyR2. Nitu et al. (22), using fluorescence resonance energy transfer, recently reported that S100A1 likely interacts allosterically with the CaM binding domain on RyR2 rather than by a direct interaction.
Fig. 3.
Effects of S100A1 on wild-type and RyR2-L3591D single-channel activities. Top: single RyR2 channels isolated from hearts were recorded in the presence of 0.2 μM (A) and 2 μM (B) cis cytosolic Ca2+ as described in Fig. 2 in the absence of S100A1 (top) and on the subsequent addition of 0.3 μM (middle) and 1 μM (bottom) cis S100A1. Bottom: mean ± SE Po of number of recordings indicated in parentheses, normalized to control (−S100A1). *P < 0.05, **P < 0.001 compared with control (no S100A1 added).
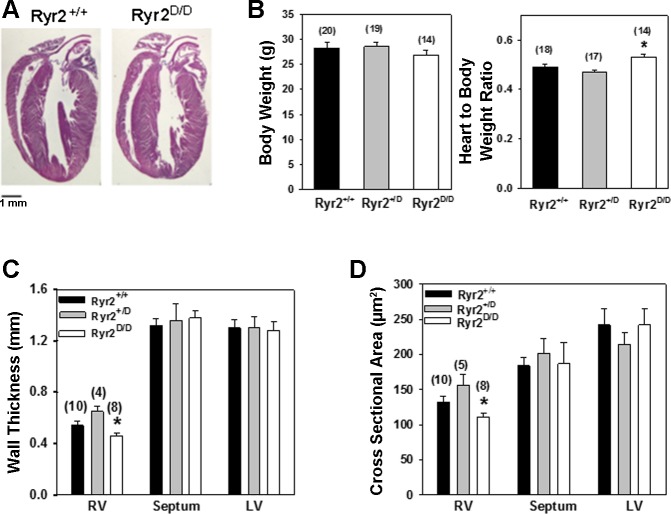
Ryr2D/D mice show modest signs of cardiac hypertrophy.
Unlike Ryr2ADA/ADA mice (35), Ryr2D/D mice live longer than 1 yr without a significant difference in cardiac performance compared with Ryr2+/+ mice, as determined by echocardiography (n = 3, not shown). No major changes in cardiac gross morphology were observed for 6- to 7-mo-old homozygous mutant mice (Fig. 4A). In homozygous mice, heart weight-to-body weight ratio increased by 11% (P < 0.003) compared with Ryr2+/D mice and by 9% (P < 0.02) compared with wild-type mice (Fig. 4B). Histological examination showed modest but significant changes in right ventricular wall thickness and cross-sectional area in homozygous mutant mice compared with heterozygous mice (Fig. 4, C and D). However, the three groups of mice showed no significant changes in septal and left ventricular wall thickness and cross-sectional area.
Fig. 4.
Phenotypic characterization of wild-type and mutant hearts. A: hematoxylin- and eosin-stained heart sections from 6- to 7-mo-old wild-type and Ryr2D/D mice. B: body weights and heart weight-to-body weight ratios of wild-type and mutant mice. Data are means ± SE for number of mice shown in parentheses. *P < 0.05 compared with Ryr2+/+ and Ryr2+/D mice. C: wall thickness of ventricles of 6- to 7-mo-old wild-type and mutant mice. RV, right ventricle; LV, left ventricle. D: cross-sectional areas of individual cardiomyocytes in ventricles were measured as described in materials and methods. C and D: data are means ± SE for number of mice shown in parentheses. *P < 0.05 compared with Ryr2+/D mice.
To determine the effect of the RyR2-L3591D mutation on cardiac function, echocardiography was performed on conscious 6-mo-old animals (Table 1). Significant increases in left ventricular end-diastolic and end-systolic dimensions were observed in heterozygous mice compared with wild-type mice. Left ventricular posterior diastolic and systolic wall thicknesses were increased in homozygous mice compared with heterozygous mice. Heart rate, fractional shortening, interventricular septum, and left ventricular diastolic and systolic posterior wall thicknesses were all similar among the three genotypes, suggesting limited alterations in cardiac morphology and contractility of the mutant mice.
Table 1.
Echocardiography in 6-mo-old wild-type and mutant mice
| Parameters | Ryr2+/+ (n = 13) | Ryr2+/D (n = 15) | Ryr2D/D (n = 11) |
|---|---|---|---|
| HR, beats/min | 542 ± 30 | 449 ± 30 | 491 ± 23 |
| LVEDD, mm | 2.82 ± 0.12* | 3.34 ± 0.08 | 3.00 ± 0.12 |
| LVESD, mm | 1.40 ± 0.10* | 1.90 ± 0.09 | 1.54 ± 0.12 |
| FS, % | 50.6 ± 2.6 | 43.2 ± 1.8 | 49.3 ± 2.4 |
| IVSD, mm | 1.23 ± 0.07 | 1.06 ± 0.06 | 1.22 ± 0.07 |
| IVSS, mm | 1.77 ± 0.10 | 1.54 ± 0.06 | 1.83 ± 0.10 |
| LVPWD, mm | 1.10 ± 0.07 | 0.97 ± 0.04 | 1.34 ± 0.11* |
| LVPWS, mm | 1.69 ± 0.09 | 1.47 ± 0.04 | 1.79 ± 0.13* |
Data are means ± SE for n mice.
HR, heart rate; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension; FS, fractional shortening [LVEDD − LVESD)/LVEDD]; IVSD, interventricular septum diastolic thickness; IVSS, interventricular septum systolic thickness; LVPWD, left ventricular posterior wall diastolic thickness; LVPWS, left ventricular posterior wall systolic thickness.
P < 0.05 compared with heterozygous mice.
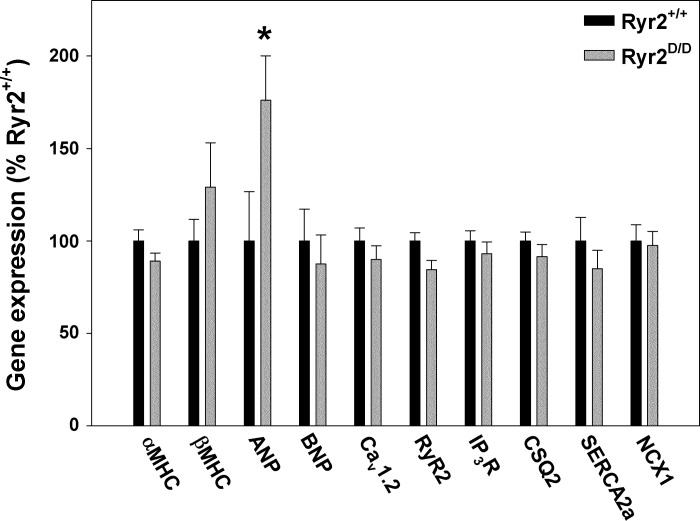
Relevant gene expression in 6-mo-old wild-type and Ryr2D/D mice was determined by quantitative RT-PCR. ANP mRNA increased 1.8-fold in the hearts of homozygous mice compared with wild type (Fig. 5). This suggests that the RyR2-L3591D mutation elicited a modest hypertrophic response in Ryr2D/D mice (16). However, mRNA levels of other genes associated with cardiac remodeling and Ca2+ handling were not altered compared with wild-type (Fig. 5). These included cardiac hypertrophy-associated genes β-MHC and BNP and genes for Ca2+ handling proteins Cav1.2, RyR2, IP3R, CSQ2, SERCA2a, and NCX1.
Fig. 5.
Quantitative RT-PCR of 6-mo-old wild-type and Ryr2D/D mice. RT-PCR was performed with total RNA from left ventricles of 6-mo-old mice. RNA levels were normalized to those of wild type for each gene. α-MHC, α-myosin heavy chain; β-MHC, β-myosin heavy chain; ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; Cav1.2, L-type Ca2+ channel; RyR2, cardiac ryanodine receptor; IP3R, inositol trisphosphate receptor; CSQ2, calsequestrin; SERCA2a, sarco(endo)plasmic reticulum Ca2+-ATPase 2a; NCX1, sodium-calcium exchanger 1. Data are means ± SE of 9–16 samples. *P < 0.05 compared with Ryr2+/+.
Cardiac muscle SR Ca2+ handling.
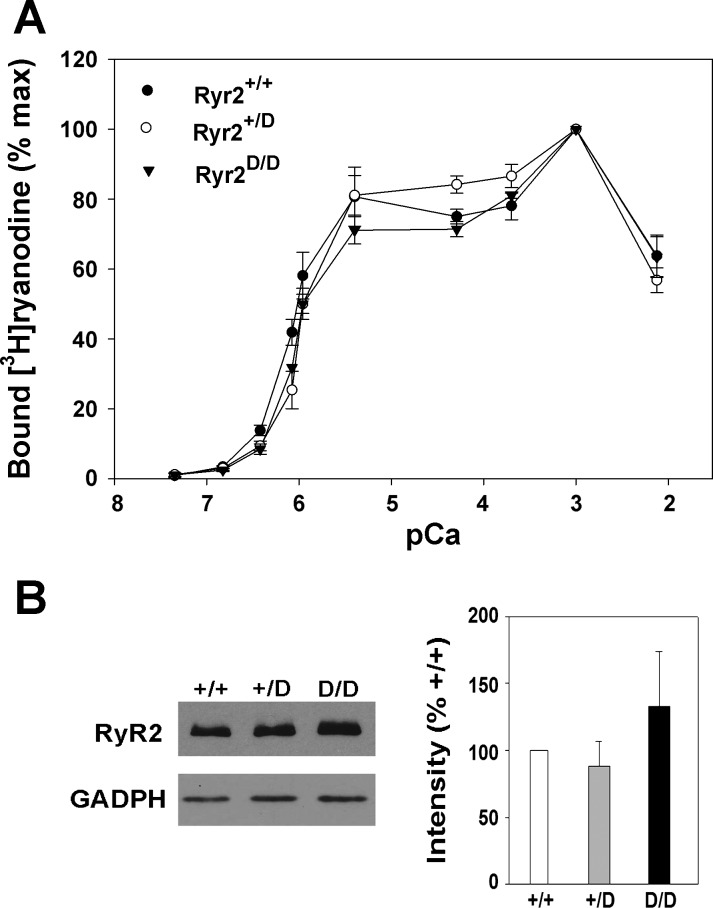
The SR Ca2+ handling properties of wild-type and Ryr2D/D mice were compared in heart homogenates of 6-mo-old mice. In Fig. 6A, a similar Ca2+ dependence of [3H]ryanodine binding suggested that the leucine-to-aspartic acid substitution in the CaM binding domain did not alter the Ca2+ sensitivity of the receptor in Ryr2+/D and Ryr2D/D mice. Bmax of [3H]ryanodine binding, a measure of RyR protein concentration, indicated a modest decrease (not significant) in RyR2 concentration in hearts of homozygous mice compared with heterozygous and wild-type mice (Table 2). Likewise, immunoblot analysis did not reveal significant differences in RyR2 expression among the three genotypes (Fig. 6B). 45Ca2+ uptake rates, a measure of SERCA activity by cardiac muscle isolates, were not altered (Table 2).
Fig. 6.
Ca2+ dependence of [3H]ryanodine binding and RyR2 expression in hearts of wild-type and mutant mice. A: specific [3H]ryanodine binding to homogenates containing wild-type and mutant RyR2s was carried out at the indicated free Ca2+ concentrations as described in materials and methods. Data are means ± SE of 4 samples each. B, left: immunoblots of RyR2 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) of heart homogenates from 6-mo-old Ryr2+/+, Ryr2+/D, and Ryr2D/D mice. Right: levels of RyR2 expression, quantitated relative to GAPDH, were normalized to RyR2+/+. Data are means ± SE of 3 preparations done in triplicate.
Table 2.
Calcium handling by heart homogenates of 6-mo-old wild-type and Ryr2D/D mice
| Genotype | Bmax of [3H]Ryanodine Binding, pmol/mg protein | 45Ca2+ Uptake Rate. pmol•mg protein−1•min−1 |
|---|---|---|
| Ryr2+/+ | 0.16 ± 0.02 (10) | 3.67 ± 0.26 (6) |
| Ryr2+/D | 0.17 ± 0.02 (6) | 3.44 ± 0.49 (4) |
| Ryr2D/D | 0.13 ± 0.02 (13) | 3.76 ± 0.34 (6) |
Values are means ± SE for number of heart preparations indicated in parentheses.
Bmax, maximum binding capacity.
Pressure overload induces a modest hypertrophic response in Ryr2D/D mice.
To determine whether Ryr2D/D mice are capable of a cardiac hypertrophic response under conditions of pressure overload, 3-mo-old Ryr2D/D and wild-type mice were subjected to TAC for 4 wk. TAC induced a significant 1.4-fold increase in heart weight-to-body weight ratio in Ryr2D/D mice compared with 1.1-fold increase in wild-type mice (Table 3). Echocardiography indicated that TAC increased interventricular septum dimensions and left ventricular posterior wall diastolic thickness in homozygous mice compared with sham-operated wild-type mice, without significantly affecting overall cardiac performance.
Table 3.
Echocardiography of Ryr2+/+ and Ryr2D/D mice after TAC
| Sham |
TAC |
|||
|---|---|---|---|---|
| Parameters | Ryr2+/+ (n = 8) | Ryr2D/D (n = 8) | Ryr2+/+ (n = 6) | Ryr2D/D (n = 8) |
| HW/BW, % | 0.55 ± 0.01 | 0.56 ± 0.01 | 0.63 ± 0.03 | 0.79 ± 0.07*† |
| HR, beats/min | 583 ± 33 | 573 ± 27 | 650 ± 10 | 587 ± 19 |
| LVEDD, mm | 2.67 ± 0.15 | 2.18 ± 0.09* | 2.59 ± 0.10 | 2.42 ± 0.08 |
| LVESD, mm | 1.25 ± 0.17 | 0.67 ± 0.07* | 1.12 ± 0.09 | 0.96 ± 0.15 |
| FS, % | 55.0 ± 5.1 | 69.4 ± 2.4 | 57.1 ± 2.1 | 60.4 ± 5.6 |
| IVSD, mm | 1.12 ± 0.13 | 1.37 ± 0.06 | 1.36 ± 0.08 | 1.59 ± 0.14* |
| IVSS, mm | 1.75 ± 0.18 | 2.10 ± 0.07 | 2.04 ± 0.09 | 2.33 ± 0.11* |
| LVPWD, mm | 1.07 ± 0.14 | 1.32 ± 0.06 | 1.39 ± 0.11 | 1.67 ± 0.13* |
| LVPWS, mm | 1.62 ± 0.21 | 1.89 ± 0.09 | 1.88 ± 0.13 | 2.22 ± 0.13 |
Data are means ± SE for n mice.
HW/BW, heart weight-to-body weight ratio; Sham, sham operated; TAC, transverse aortic constriction.
P < 0.05 compared with Sham Ryr2+/+;
P < 0.05 compared with Sham Ryr2D/D.
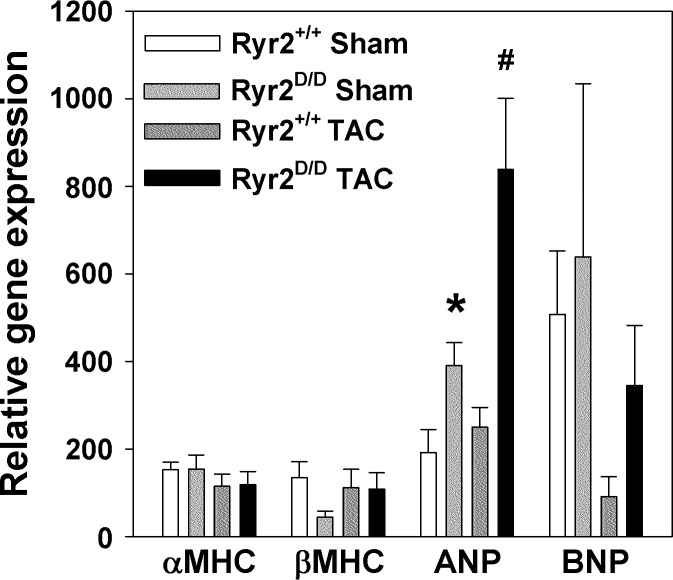
To further investigate the response of Ryr2D/D mice to pressure overload, mRNA levels of cardiac hypertrophy-related genes were measured by quantitative RT-PCR. No significant changes in α-MHC, β-MHC, and BNP mRNA levels were observed (Fig. 7). However, there was a significant two- to threefold increase in ANP levels of sham-operated Ryr2D/D animals compared with sham-operated wild-type mice. In pressure-overloaded mice, 1.3- and 2.1-fold increases in ANP mRNA levels were observed in wild-type and homozygous mutant mice, respectively, compared with sham-operated animals. Together, the data suggest that small differences between mutant and wild-type mice under baseline conditions become more prominent during pressure overload of Ryr2D/D mice.
Fig. 7.
Quantitative RT-PCR analysis of marker genes for cardiac hypertrophy in mice subjected to transverse aortic constriction (TAC). RT-PCR was performed with total RNA from left ventricles of 4-mo-old mice. RNA levels were normalized to β-actin. Abbreviations are as in Fig. 5. Data are means ± SE of 6–8 samples. *P < 0.05 compared with sham-operated (Sham) wild type; #P < 0.05 compared with Sham Ryr2D/D.
DISCUSSION
Accumulating evidence suggests that a dysfunctional RyR2 calcium channel has a major role in the genesis of cardiomyopathy. Aberrant phosphorylation, oxidation, and S-nitrosylation of RyR2, which cause leaky Ca2+ channels with an increased activity at diastolic Ca2+, have been implicated in arrhythmias and heart failure (1, 6, 18, 30, 37). Three simultaneous mutations in the CaM binding site of RyR2 (W3587A, L3591D, F3603A; RyR2ADA) attenuated CaM regulation of RyR2 at diastolic and systolic Ca2+ concentrations (35). Ryr2ADA/ADA knockin mice carrying the three mutations have severe cardiac hypertrophy and early death. Cardiomyocytes derived from homozygous Ryr2ADA/ADA mice showed prolonged Ca2+ release. This suggests that CaM inhibition of RyR2 likely contributes to the termination of intracellular Ca2+ transients. To determine at which Ca2+ concentration, at diastolic or systolic Ca2+, CaM inhibition of RyR2 is of physiological significance, an additional knockin mouse carrying a single mutation (L3591D; RyR2D) was generated. In contrast to Ryr2ADA/ADA mice, Ryr2D/D mice showed only modest changes in heart size and function. Single RyR2ADA and RyR2D channels lost CaM inhibition at diastolic Ca2+; however, unlike RyR2ADA channels, RyR2D channels maintained CaM inhibition at systolic Ca2+. The results suggest that loss of CaM regulation does not have as much an impact in Ryr2D/D mice as in Ryr2ADA/ADA mice, hence implying that CaM inhibition of RyR2 at systolic Ca2+ is necessary for normal cardiac growth and function.
Ryr2D/D mice showed modest changes in heart contractility, morphology, and gene expression compared with Ryr2ADA/ADA mice. Heart weight-to-body weight ratio of 6-mo-old Ryr2D/D mice increased 9% compared with wild-type mice. In comparison, a more than twofold increase in heart weight-to-body weight ratio was observed for 10-day-old Ryr2ADA/ADA mice compared with wild-type mice (35). ANP gene expression increased 1.8-fold in 6-mo-old Ryr2D/D mice compared with ∼40-fold increase in 10-day-old Ryr2ADA/ADA mice. In [3H]ryanodine binding experiments, functional RyR2 protein content decreased moderately by ∼20% (not significant) in hearts of 6-mo-old Ryr2D/D mice compared with an ∼60% (significant) decrease in 10-day-old Ryr2ADA/ADA mice.
Some differences were noted between the two mutant genotypes as well. Heterozygous mice had a lower heart weight-to-body weight ratio but increased right ventricular wall thickness and cross-sectional area and reduced posterior wall thickness as determined by echocardiography compared with homozygous mice. RyR2 is composed of four identical subunits, and, assuming a random distribution, 15 of 16 RyR2 have at least one wild-type subunit in heterozygous mice. Since the binding of about one CaM is sufficient to inhibit the tetrameric RyR (2), a majority of RyR2s in heterozygous mice were likely inhibited by CaM at systolic Ca2+. Additional studies are required to learn how the changes in CaM occupancy of heterozygous and homozygous mutant RyR2s affect cardiac morphology.
Stimulation of neonatal cardiomyocytes with endothelin-1 (9) and pacing-induced heart failure in canines (12) resulted in partial dissociation of CaM from RyR2. Expression of CaM isoform with higher binding affinity restored normal SR Ca2+ handling in cardiomyocytes from failing hearts (12). In the present study, TAC was used to learn how Ryr2D/D mice respond to conditions of stress. We found that pressure overload increased heart weight-to-body weight ratio in Ryr2D/D mice, with only small changes in cardiac parameters of wild-type and homozygous mice subjected to TAC compared with sham-operated mice. RT-PCR indicated a modest increase in ANP mRNA levels in mice subjected to TAC. The results suggest that inhibition of RyR2 by CaM and S100A1 at diastolic Ca2+ may preserve heart weight-to-body weight ratio and ANP expression during stress such as exercise. However, it should be noted that even under pressure overload of Ryr2D/D mice, changes in heart growth and function were smaller than those in 10-day old Ryr2ADA/ADA mice not subjected to TAC.
S100A1 is an EF hand Ca2+ binding protein expressed at high levels in the heart (13). Evidence indicates that S100A1 regulates intracellular Ca2+ homeostasis and cardiac contraction. S100A1, like CaM, regulates cardiac Ca2+ handling proteins such as RyR2, voltage-dependent L-type Ca2+ channel, and the SERCA2a-PLN complex (10, 21, 24, 29). [3H]ryanodine binding and Ca2+ spark measurements indicated that S100A1 has multiple effects on RyR2 channel activity. In [3H]ryanodine binding studies, low concentrations of S100A1 increased RyR2 activity at diastolic 0.1 μM Ca2+ but high concentrations decreased activity. At systolic Ca2+, S100A1 activated RyR2 (14, 20). In permeabilized cardiomyocytes, Ca2+ spark frequency decreased in response to exogenously added S100A1 (28). Similar to previous reports, we observed S100A1 effects on RyR2 function. However, this is the first study that describes a genetically modified mouse with a loss of S100A1 binding to RyR2, thus avoiding the multiple effects of gene delivery such as altering the regulation of SERCA and L-type Ca2+ channel. Single-channel recordings showed that 1 μM S100A1 inhibited wild-type RyR2 at 0.2 and 2 μM Ca2+. The RyR2-L3591D mutation eliminated the inhibitory effect of S100A1 on RyR2 at both Ca2+ concentrations. Nevertheless, Ryr2D/D mice did not demonstrate major changes in cardiac function and growth. Together, the results suggest that S100A1 has a limited role in regulating RyR2 in vivo under normal conditions.
In conclusion, our studies with Ryr2D/D mice show that severe cardiac hypertrophy and early death in Ryr2ADA/ADA mice (33, 35) can be ascribed to impaired RyR2 inhibition by CaM at systolic Ca2+. Single-channel measurements showed that S100A1 and CaM share a single regulatory domain in RyR2. Loss of inhibition of RyR2 by CaM at diastolic Ca2+ and by S100A1 at diastolic and systolic Ca2+ in mice had only modest effects on cardiac morphology and function.
GRANTS
The work was supported by American Heart Association Grant 10SDG3500001 and National Science Foundation Grant EPS-0903795 to N. Yamaguchi and National Heart, Lung, and Blood Institute Grant HL-073051 to G. Meissner.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.Y., A.C., T.H., L.X., and G.M. conception and design of research; N.Y., A.C., T.H., L.X., A.C.G., and D.A.P. performed experiments; N.Y., A.C., T.H., L.X., A.C.G., D.A.P., and G.M. analyzed data; N.Y., A.C., T.H., L.X., D.A.P., and G.M. interpreted results of experiments; N.Y., L.X., and G.M. prepared figures; N.Y. and G.M. drafted manuscript; N.Y., A.C., T.H., L.X., A.C.G., D.A.P., and G.M. edited and revised manuscript; N.Y., A.C., T.H., L.X., A.C.G., D.A.P., and G.M. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of A. Chakraborty: Centre for Cellular and Molecular Biology, Council for Scientific and Industrial Research, Hyderabad 500 007, India.
REFERENCES
- 1.Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol 51: 468–473, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balshaw DM, Xu L, Yamaguchi N, Pasek DA, Meissner G. Calmodulin binding and inhibition of cardiac muscle calcium release channel (ryanodine receptor). J Biol Chem 276: 20144–20153, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Barrick CJ, Rojas M, Schoonhoven R, Smyth SS, Threadgill DW. Cardiac response to pressure overload in 129S1/SvImJ and C57BL/6J mice: temporal- and background-dependent development of concentric left ventricular hypertrophy. Am J Physiol Heart Circ Physiol 292: H2119–H2130, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Brinks H, Rohde D, Voelkers M, Qiu G, Pleger ST, Herzog N, Rabinowitz J, Ruhparwar A, Silvestry S, Lerchenmuller C, Mather PJ, Eckhart AD, Katus HA, Carrel T, Koch WJ, Most P. S100A1 genetically targeted therapy reverses dysfunction of human failing cardiomyocytes. J Am Coll Cardiol 58: 966–973, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunting M, Bernstein KE, Greer JM, Capecchi MR, Thomas KR. Targeting genes for self-excision in the germ line. Genes Dev 15: 1524–1528, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capes EM, Loaiza R, Valdivia HH. Ryanodine receptors. Skelet Muscle 1: 18, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du XJ, Cole TJ, Tenis N, Gao XM, Kontgen F, Kemp BE, Heierhorst J. Impaired cardiac contractility response to hemodynamic stress in S100A1-deficient mice. Mol Cell Biol 22: 2821–2829, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fruen BR, Bardy JM, Byrem TM, Strasburg GM, Louis CF. Differential Ca2+ sensitivity of skeletal and cardiac muscle ryanodine receptors in the presence of calmodulin. Am J Physiol Cell Physiol 279: C724–C733, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Gangopadhyay JP, Ikemoto N. Aberrant interaction of calmodulin with the ryanodine receptor develops hypertrophy in the neonatal cardiomyocyte. Biochem J 438: 379–387, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gusev K, Ackermann GE, Heizmann CW, Niggli E. Ca2+ signaling in mouse cardiomyocytes with ablated S100A1 protein. Gen Physiol Biophys 28: 371–383, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Hamilton SL, Serysheva II. Ryanodine receptor structure: progress and challenges. J Biol Chem 284: 4047–4051, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hino A, Yano M, Kato T, Fukuda M, Suetomi T, Ono M, Murakami W, Susa T, Okuda S, Doi M, Kobayashi S, Yamamoto T, Koseki N, Kyushiki H, Ikemoto N, Matsuzaki M. Enhanced binding of calmodulin to the ryanodine receptor corrects contractile dysfunction in failing hearts. Cardiovasc Res 96: 433–443, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato K, Kimura S, Haimoto H, Suzuki F. S100a0 (alpha alpha) protein: distribution in muscle tissues of various animals and purification from human pectoral muscle. J Neurochem 46: 1555–1560, 1986 [DOI] [PubMed] [Google Scholar]
- 14.Kettlewell S, Most P, Currie S, Koch WJ, Smith GL. S100A1 increases the gain of excitation-contraction coupling in isolated rabbit ventricular cardiomyocytes. J Mol Cell Cardiol 39: 900–910, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Kim HS, Lee G, John SW, Maeda N, Smithies O. Molecular phenotyping for analyzing subtle genetic effects in mice: application to an angiotensinogen gene titration. Proc Natl Acad Sci USA 99: 4602–4607, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kishimoto I, Tokudome T, Horio T, Garbers DL, Nakao K, Kangawa K. Natriuretic peptide signaling via guanylyl cyclase (GC)-A: an endogenous protective mechanism of the heart. Curr Cardiol Rev 5: 45–51, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meissner G. Molecular regulation of cardiac ryanodine receptor ion channel. Cell Calcium 35: 621–628, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Meissner G. Regulation of ryanodine receptor ion channels through posttranslational modifications. Curr Top Membr 66: 91–113, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meissner G, Henderson JS. Rapid calcium release from cardiac sarcoplasmic reticulum vesicles is dependent on Ca2+ and is modulated by Mg2+, adenine nucleotide, and calmodulin. J Biol Chem 262: 3065–3073, 1987 [PubMed] [Google Scholar]
- 20.Most P, Pleger ST, Völkers M, Heidt B, Boerries M, Weichenhan D, Löffler E, Janssen PM, Eckhart AD, Martini J, Williams ML, Katus HA, Remppis A, Koch WJ. Cardiac adenoviral S100A1 gene delivery rescues failing myocardium. J Clin Invest 114: 1550–1563, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Most P, Remppis A, Pleger ST, Loffler E, Ehlermann P, Bernotat J, Kleuss C, Heierhorst J, Ruiz P, Witt H, Karczewski P, Mao L, Rockman HA, Duncan SJ, Katus HA, Koch WJ. Transgenic overexpression of the Ca2+ binding protein S100A1 in the heart leads to increased in vivo myocardial contractile performance. J Biol Chem 278: 33809–33817, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Nitu FR, Fruen BR, Rohde D, Most P, Bers DM, Thomas DD, Cornea RL. FRET detection of CaM-RyR2 binding modulation by S100A1. Biophys J 104: 446a, 2013 [Google Scholar]
- 23.Pleger ST, Shan C, Ksienzyk J, Bekeredjian R, Boekstegers P, Hinkel R, Schinkel S, Leuchs B, Ludwig J, Qiu G, Weber C, Raake P, Koch WJ, Katus HA, Muller OJ, Most P. Cardiac AAV9-S100A1 gene therapy rescues post-ischemic heart failure in a preclinical large animal model. Sci Transl Med 3: 92ra64, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prosser BL, Hernandez-Ochoa EO, Schneider MF. S100A1 and calmodulin regulation of ryanodine receptor in striated muscle. Cell Calcium 50: 323–331, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prosser BL, Wright NT, Hernandez-Ochoa EO, Varney KM, Liu Y, Olojo RO, Zimmer DB, Weber DJ, Schneider MF. S100A1 binds to the calmodulin-binding site of ryanodine receptor and modulates skeletal muscle excitation-contraction coupling. J Biol Chem 283: 5046–5057, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sutko JL, Airey JA, Welch W, Ruest L. The pharmacology of ryanodine and related compounds. Pharmacol Rev 49: 53–98, 1997 [PubMed] [Google Scholar]
- 27.Treves S, Scutari E, Robert M, Groh S, Ottolia M, Prestipino G, Ronjat M, Zorzato F. Interaction of S100A1 with the Ca2+ release channel (ryanodine receptor) of skeletal muscle. Biochemistry 36: 11496–11503, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Volkers M, Loughrey CM, Macquaide N, Remppis A, DeGeorge BR, Jr, Wegner FV, Friedrich O, Fink RH, Koch WJ, Smith GL, Most P. S100A1 decreases calcium spark frequency and alters their spatial characteristics in permeabilized adult ventricular cardiomyocytes. Cell Calcium 41: 135–143, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Volkers M, Rohde D, Goodman C, Most P. S100A1: a regulator of striated muscle sarcoplasmic reticulum Ca2+ handling, sarcomeric, and mitochondrial function. J Biomed Biotech 2010: 1–10, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wehrens XH, Lehnart SE, Marks AR. Intracellular calcium release and cardiac disease. Annu Rev Physiol 67: 69–98, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Xu L, Meissner G. Mechanism of calmodulin inhibition of cardiac sarcoplasmic reticulum Ca2+ release channel (ryanodine receptor). Biophys J 86: 797–804, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu L, Tripathy A, Pasek DA, Meissner G. Ruthenium red modifies the cardiac and skeletal muscle Ca2+ release channels (ryanodine receptors) by multiple mechanisms. J Biol Chem 274: 32680–32691, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi N, Chakraborty A, Pasek DA, Molkentin JD, Meissner G. Dysfunctional ryanodine receptor and cardiac hypertrophy: role of signaling molecules. Am J Physiol Heart Circ Physiol 300: H2187–H2195, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaguchi N, Prosser BL, Ghassemi F, Xu L, Pasek DA, Eu JP, Hernandez-Ochoa EO, Cannon BR, Wilder PT, Lovering RM, Weber D, Melzer W, Schneider MF, Meissner G. Modulation of sarcoplasmic reticulum Ca2+ release in skeletal muscle expressing ryanodine receptor impaired in regulation by calmodulin and S100A1. Am J Physiol Cell Physiol 300: C998–C1012, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamaguchi N, Takahashi N, Xu L, Smithies O, Meissner G. Early cardiac hypertrophy in mice with impaired calmodulin regulation of cardiac muscle Ca2+ release channel. J Clin Invest 117: 1344–1353, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamaguchi N, Xu L, Pasek DA, Evans KE, Meissner G. Molecular basis of calmodulin binding to cardiac muscle Ca2+ release channel (ryanodine receptor). J Biol Chem 278: 23480–23486, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Yano M, Yamamoto T, Ikeda Y, Matsuzaki M. Mechanisms of disease: ryanodine receptor defects in heart failure and fatal arrhythmia. Nat Clin Pract Cardiovasc Med 3: 43–52, 2006 [DOI] [PubMed] [Google Scholar]