Abstract
Cardiac hypertrophy induced by pathological stimuli is regulated by a complex formed by the repressor element 1-silencing transcription factor (REST) and its corepressor mSin3A. We previously reported that hypertrophic signaling is blunted by O-linked attachment of β-N-acetylglucosamine (O-GlcNAc) to proteins. Regular exercise induces a physiological hypertrophic phenotype in the heart that is associated with decreased O-GlcNAc levels, but a link between O-GlcNAc, the REST complex, and initiation of exercise-induced cardiac hypertrophy is not known. Therefore, mice underwent a single 15- or 30-min bout of moderate- to high-intensity treadmill running, and hearts were harvested immediately and compared with sedentary controls. Cytosolic O-GlcNAc was lower (P < 0.05) following 15 min exercise with no differences in nuclear levels (P > 0.05). There were no differences in cytosolic or nuclear O-GlcNAc levels in hearts after 30 min exercise (P > 0.05). Cellular compartment levels of O-GlcNAc transferase (OGT, the enzyme that removes the O-GlcNAc moiety from proteins), REST, mSin3A, and histone deacetylases (HDACs) 1, 2, 3, 4, and 5 were not changed with exercise. Immunoprecipitation revealed O-GlcNAcylation of OGT and HDACs 1, 2, 4, and 5 that was not changed with acute exercise; however, exercised hearts did exhibit lower interactions between OGT and REST (P < 0.05) but not between OGT and mSin3A. These data suggest that hypertrophic signaling in the heart may be initiated by as little as 15 min of exercise via intracellular changes in protein O-GlcNAcylation distribution and reduced interactions between OGT and the REST chromatin repressor.
Keywords: exercise, cardiac hypertrophy, chromatin, β-N-acetylglucosamine, histone deacetylase
cellular regulation of cardiac hypertrophy has primarily been examined in the context of pathological remodeling resulting from pressure overload and hypertension (4). While the focus has traditionally been on activation of the fetal gene program by pathological stimuli and subsequent downstream mediators of hypertrophic remodeling, it is now evident that these processes are regulated by chromatin remodeling induced by reduced activation of the repressor element 1-silencing transcription factor (REST) complex. REST is a key transcription repressor found in numerous tissues, including the heart, and interacts with corepressors such as mSin3A to form a complex that recruits histone deacetylases (HDACs), thus altering chromatin structure, inducing epigenetic alterations, and subsequently resulting in gene silencing of hypertrophic mediators (15). Class I HDACs (HDAC 1, 2, 3) promote transcription of hypertrophic genes while class IIa HDACs (HDAC 4, 5) repress hypertrophy (14, 20). While it has long been accepted that exercise training induces a healthy, “physiological” hypertrophic phenotype, the mechanisms underlying this process are not well described. Consistent with its role in pathological hypertrophy, cardiac HDAC 2 activity is increased following 3 days of forced swimming (13), and, in skeletal muscle, HDACs 4 and 5 are exported from the nucleus in response to exercise (19). The role of the REST complex in exercise-induced cardiac hypertrophy is not known.
We recently demonstrated that the O-linked attachment of a β-N-acetylglucosamine (O-GlcNAc) sugar on serine and threonine residues of nuclear and cytoplasmic proteins blunted the hypertrophic response in diabetic hearts (18). O-GlcNAcylation is a dynamic, transient, and reversible regulatory mechanism that is analogous to protein phosphorylation (12, 28–30) and can be upregulated by either increases in substrate availability or cellular stressors such as glucose deprivation (27) or heat shock (33). In contrast to phosphorylation, only two enzymes control O-GlcNAcylation with the attachment of O-GlcNAc mediated by O-GlcNAc transferase (OGT) and the removal catalyzed by β-N-acetylhexosaminidase (O-GlcNAcase; OGA). Recent studies by others reported increased cardiac hypertrophy and decreased cardiac O-GlcNAc and OGT protein in both diabetic and nondiabetic adult mouse hearts after 6 wk of swimming (2, 3). Interestingly, OGT forms a corepressor complex with mSin3A, which regulates gene transcription (5, 31), and both OGT and O-GlcNAc are reported as part of the “histone code” in chromatin (11). However, the role of O-GlcNAc and OGT in regulation of the REST-mSin3A complex control of cardiac hypertrophy is not known.
Exercise-induced cardiac hypertrophy becomes evident after 3 wk of training and is the result of repeated bouts of acute exercise and subsequent recovery (22). Therefore, the goals of this study were to examine the effect of a single bout of exercise on protein O-GlcNAcylation and the REST corepressor complex and to explore potential interactions between these two processes. We hypothesized that acute exercise would upregulate O-GlcNAcylation of components of the REST-mSin3A complex and that interactions between OGT, REST, and mSin3A would be enhanced in exercised hearts.
METHODS
Ethical approval.
This protocol was approved by the Washington State University Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication no. 85–23, 1996).
Experimental design.
Ten-week-old, male CD-1 mice (Harlan Laboratories, Kent, WA) were housed up to four per cage, maintained on a 12:12-h light-dark cycle, and provided with food and tap water ad libitum. Mice were randomly assigned to sedentary or exercise groups. Irrespective of activity group, all animals were accommodated to a small animal treadmill before acute exercise for 1 h/day for three consecutive days; the first 30 min were spent with the treadmill turned off after which the treadmill was turned on, and the belt was set to move at a very low speed (5 m/min) for the second 30 min. Following accommodation, animals were rested for 48 h, and those that were randomized to the exercise group then underwent either 15 or 30 min (n = 18/group) of treadmill running at 17 m/min for a single bout. Sedentary, control animals (n = 18/group) were placed on the stationary belt for the same duration as their respective exercise-trained cohort. To examine the acute effect of exercise, mice were quickly anesthetized immediately after cessation of exercise using 2–4% isoflurane in 100% oxygen and killed by decapitation. Hearts were rapidly removed, cleaned in ice-cold phosphate-buffered saline (PBS), snap-frozen (n = 16/group) or placed in 4% paraformaldehyde (n = 2/group), and stored for later use. Nonfasting blood glucose levels were measured in whole trunk blood using the Accu-Chek Advantage analyzer (Roche Diagnostics, Basel, Switzerland).
Western blot analysis.
Frozen heart tissue was ground into a fine powder under liquid nitrogen and fractionated into nuclear and cytosolic components using a commercially available kit (Thermo Fisher Scientific, Rockford, IL) in the presence of O-(2-acetamido-2-deoxy-d-glucopyranosylidene)amino-N-phenylcarbamate (PUGNAc; Sigma-Aldrich, St. Louis, MO) to inhibit OGA and prevent the removal of O-GlcNAc as previously described (16) and a protease inhibitor cocktail (Sigma-Aldrich). Protein concentrations were determined using the Bio-Rad Protein Assay Kit (Bio-Rad, Hercules, CA). Solubilized nuclear and cytosolic proteins were suspended in Laemmli buffer (Bio-Rad), and 25 μg protein/sample were separated using SDS-PAGE and transferred to either nitrocellulose (for O-GlcNAc only) or polyvinylidene difluoride membranes at a constant voltage of 100 volts for 75 min. Equal protein loading was initially confirmed by Ponceau-S staining, and immunoblots were then probed for O-GlcNAc (CTD110.6, 1:5,000; Epitope Recognition and Immunodetection Facility, University of Alabama at Birmingham, Birmingham, AL; or RL-2, 1:2,000; Abcam, ab2739, Cambridge, MA), OGT (1:1,500; Sigma-Aldrich, O6139), actin (1:2,000; Abcam, ab28052), atrial natriuretic peptide (1:500; Abcam, ab91250), HDACs 1–5 [1:1,000; Cell Signaling stock nos. 5356, 5113, 3949, 5392, and 2082 (HDAC1–5 respectively), Danvers, MA], mSin3A (1:2,000; Abcam, ab3479), or REST (1:1,000; Millipore, 07–579, Billerica, MA). Blots were then stripped using 1 M NaOH and reprobed with glyceraldehyde-3-phosphate dehydrogenase (1:1,500; Abcam, ab9484) and TATA-binding protein antibodies (1:1,000; Abcam, ab51841) as purity and loading controls for the cytoplasmic and nuclear fractions, respectively, or calsequestrin (1:4,000; Abcam, ab3516) as the protein-loading control for whole cell lysates. After incubation with appropriate secondary antibodies, blots were washed with SuperSignal West Pico or Femto Chemiluminescent Substrate (Thermo Fisher Scientific) and either exposed to film or visualized using the Chemidoc XRS imager (Bio-Rad). Densitometric analysis was performed using ImageJ software (National Institutes of Health, Bethesda, MD).
Coimmunoprecipitation.
Heart tissue was homogenized in 10× wt/vol T-PER buffer (Thermo Fisher Scientific) containing 1% protease inhibitor cocktail (Sigma-Aldrich), 1% each phosphatase inhibitor cocktail 2 and 3 (Sigma-Aldrich), and 0.1% PUGNAc (20 mM stock) and incubated 45 min on ice. Tissue debris was pelleted at 12,000 g for 10 min, and the supernatants were stored at −80°C. Lysates were made to 3 mg/ml with Dulbecco's PBS and precleared over protein A/G resin beads (Thermo Fisher Scientific) for 4 h at 4°C. Beads were pelleted at 10,000 g, and the supernatants were collected. Fresh beads were incubated with antibody (anti-OGT, Abcam, ab50271) or anti-O-GlcNAc (RL-2, Abcam, ab2739) for 4 h at 4°C. Precleared lysate was directly immunoprecipitated onto the antibody-conjugated beads overnight with slow rotation at 4°C. Beads were then pelleted by centrifugation, washed with wash buffer, eluted by boiling for 5 min in Laemmli buffer, and subjected to Western blotting as described above. Heart tissue lysate diluted to the immunoprecipitation (IP) concentration was used for input; precleared lysates immunoprecipitated without antibody were used as IgG controls. Analysis for co-IP was performed by normalizing the densitometry of the coimmunoprecipitated protein to that of the IP target protein.
Immunohistochemistry.
To visualize the effect of acute exercise on intracellular distribution of O-GlcNAc, horizontal short-axis sections through the mid-left ventricle were fixed in 4% paraformaldehyde, transferred to 70% ethanol until being paraffin embedded, sectioned at 5 μm, and mounted on slides. Slides were then deparaffinized in xylene, rehydrated in ethanol, and blocked with 5% goat serum in 1% bovine serum for 1 h at room temperature. Sections were incubated with primary antibodies against O-GlcNAc (CTD110.6, 1:50; Epitope Recognition and Immunodetection Facility, University of Alabama at Birmingham) and desmin (1:400; Abcam, ab15200) diluted in 5% goat serum in 1% bovine serum overnight at 4°C; appropriate secondary antibodies conjugated to either Alexa Fluor 488 (green) or 594 (red) (Invitrogen, Carlsbad, CA) were used to visualize the specific proteins with 4′,6-diamidino-2-phenylindole (DAPI; blue) to identify nuclei. Image acquisition was performed on a Zeiss Axioplan 2 epifluorescence microscope with an AxioCam MRm cooled CCD camera and AxioVision software (Carl Zeiss Microimaging, Thornwood, NY). Line scans to detect intracellular patterns of O-GlcNAc distribution were generated using ImageJ software.
Statistical analysis.
Data were analyzed using a two-tailed unpaired Student's t-test or by two-way ANOVA or ANOVA on ranks, where appropriate. Interactions for ANOVA were investigated using Bonferroni post hoc analysis. Values are presented as means ± SE, and significance between groups was established at P < 0.05.
RESULTS
Acute exercise differentially alters cytosolic and nuclear O-GlcNAc and OGT protein.
Physical characteristics for sedentary and exercised animals are presented in Table 1. Body weight and heart weight were not different between groups. It is important to note that postexercise blood glucose levels were not different compared with sedentary controls, irrespective of the duration of exercise (P > 0.05), since protein O-GlcNAcylation can be upregulated by increased production of UDP-GlcNAc, the substrate for O-GlcNAc. A lack of difference between sedentary and exercise blood glucose values indicates that any differences observed in O-GlcNAc and OGT protein are not due to changes in glucose-mediated substrate availability.
Table 1.
Animal characteristics
| 15 Min |
30 Min |
|||
|---|---|---|---|---|
| Sedentary | Exercise | Sedentary | Exercise | |
| Body wt, g | 39.3 ± 1.2 | 40.3 ± 1.1 | 39.9 ± 1.2 | 39.1 ± 0.7 |
| Heart wt, mg | 193 ± 8 | 205 ± 11 | 211 ± 8 | 193 ± 8 |
| HW/BW, mg/g | 4.9 ± 0.2 | 5.1 ± 0.2 | 5.3 ± 0.2 | 4.9 ± 0.2 |
| Blood glucose, mmol/l | 11.1 ± 0.6 | 11.6 ± 0.6 | 12.7 ± 1.0 | 11.3 ± 0.4 |
Values are means ± SE. HW, heart weight; BW, body weight.
The effects of a single exercise bout on nuclear and cytosolic levels of O-GlcNAcylation and OGT protein were initially analyzed using Western blotting. Densitometry was first performed on whole lanes to determine the effect of exercise on overall O-GlcNAc protein levels and revealed that cytosolic O-GlcNAc was significantly lower following 15 min of exercise (P < 0.05) with no difference in nuclear levels compared with sedentary hearts (Fig. 1, A and B). There were no differences in cytosolic or nuclear O-GlcNAc levels in hearts from animals that exercised for 30 min (Fig. 2, A and B). Because densitometry of the entire sample lane is dominated by the intense immunoreactive bands at 40 and 90 kDa, the analysis was also performed over the high-, mid-, and low-molecular-weight ranges as indicated in Figs. 1A and 2A. This analysis revealed that the decrease in overall cytosolic O-GlcNAc following 15 min exercise was largely the result of lower O-GlcNAc levels in the mid- and low-molecular-weight protein ranges (Fig. 1C). Despite no effect of exercise on overall nuclear O-GlcNAc levels, lower-molecular-weight proteins exhibited significantly higher protein O-GlcNAcylation following both 15 and 30 min exercise (Figs. 1D and 2D). Acute changes in O-GlcNAc levels in response to cellular stress are likely due to changes in enzyme activity or substrate availability rather than protein levels of the regulatory enzymes (27, 33). Therefore, it was not surprising that a single bout of exercise did not alter OGT protein in either cytosolic (Figs. 1E and 2E) or nuclear (Figs. 1F and 2F) fractions. OGT reportedly exists in three isoforms: 110- to 116-kDa nucleocytoplasmic (ncOGT), 103-kDa mitochondrial (mOGT), and 78-kDa short cytosolic (17); the immunoblots shown in Figs. 1A and 2A are consistent with this, since all three isoforms were present in cytosolic fractions, but we were only able to detect the 110-kDa band in nuclear fractions.
Fig. 1.
Effect of 15 min of exercise (EX) on β-N-acetylglucosamine (O-GlcNAc) levels and O-GlcNAc transferase (OGT) protein in cytosolic and nuclear compartments of mouse hearts compared with sedentary (SED) controls. Representative Western blots (A) and densitometric analyses of protein levels of O-GlcNAc using analysis of the entire lane (B) or high-, mid-, and low-molecular weights in the cytosolic (C) or nuclear (D) fractions are shown in addition to cytosolic (E) and nuclear (F) OGT. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and TATA-binding protein (BP) antibodies are shown as purity and loading controls for the cytoplasmic and nuclear fractions, respectively; n = 8/group. *P < 0.05 vs. sedentary.
Fig. 2.
Effect of 30 min of exercise on O-GlcNAc levels and OGT protein in cytosolic and nuclear compartments of mouse hearts compared with sedentary controls. Representative Western blots (A) and densitometric analyses of protein levels of O-GlcNAc using analysis of the entire lane (B) or high-, mid-, and low-molecular weights in the cytosolic (C) or nuclear (D) fractions are shown in addition to cytosolic (E) and nuclear (F) OGT. GAPDH and TATA-binding protein antibodies are shown as purity and loading controls for the cytoplasmic and nuclear fractions, respectively; n = 8/group. *P < 0.05 vs. sedentary.
Exercise does not alter intracellular O-GlcNAc distribution.
The effects of exercise on the distribution of cytosolic and nuclear O-GlcNAcylation can be qualitatively compared via the line scans shown in Figs. 3 and 4, respectively. Cytosolic O-GlcNAc exhibited a striated pattern in cardiomyocytes that appears to be closely associated with the z-lines indicated by desmin staining (Fig. 3); to our knowledge, this is the first study to demonstrate this pattern in the mouse heart and is consistent with our previous report showing a similar distribution in the rat heart (16). Interestingly, neither 15 nor 30 min of exercise alters the localization of O-GlcNAc-modified proteins to the z-lines.
Fig. 3.
Cytosolic distribution of O-GlcNAc (green) in sedentary and exercised mouse hearts compared with z-lines indicated by desmin (red). Dashed lines indicate the direction of the corresponding line scans that are shown in the panels on the right. Scale bars, 10 μm.
Fig. 4.
Nuclear distribution of O-GlcNAc (green) in sedentary and exercised mouse hearts compared with 4′,6-diamidino-2-phenylindole (DAPI, blue). Dashed lines indicate the direction of the corresponding line scans that are shown in the panels on the right. Scale bars, 10 μm.
Punctate O-GlcNAc and DAPI staining was present in the nuclei of both sedentary and exercise-trained hearts (Fig. 4). The punctate DAPI staining is characteristic of mouse nuclei, since mouse chromatin contains increased AT-rich regions of DNA to which DAPI preferentially binds (10). The line scans shown in Fig. 4 clearly demonstrate that the pattern of O-GlcNAc immunofluorescence is markedly different from that seen with DAPI, particularly in the AT-rich DAPI-positive regions where O-GlcNAc staining is very low. Moreover, the nuclear distribution of protein O-GlcNAcylation does not appear to be altered by either 15 or 30 min of exercise compared with sedentary controls.
Chromatin proteins are not altered by acute exercise.
Figure 5 demonstrates the presence of class I HDACs 2 and 3, class II HDACs 4 and 5, and mSin3A in both cellular and nuclear compartments, whereas HDAC1 and REST appear to reside exclusively in the nucleus. To our knowledge, this is the first demonstration of compartment localization of HDACs, REST, and mSin3A in the adult heart. Regardless of duration, an acute bout of intense exercise does not alter compartmentalized protein levels of class I or II HDACs, REST, or mSin3A.
Fig. 5.

Immunoblots (A) and densitometric analysis of cytosolic (B) and nuclear (C) distribution of histone deacetylase (HDAC) 1, HDAC2, HDAC3, HDAC4, HDAC5, repressor element 1-silencing transcription factor (REST), and mSin3A in sedentary and exercised mouse hearts compared with GAPDH or TATA-binding protein; n = 6/group. ND, not detected.
O-GlcNAcylation of OGT is decreased with acute exercise.
Because protein O-GlcNAcylation was increased in nuclear extracts following 15 min of exercise (Fig. 1), we subsequently examined O-GlcNAcylation of proteins that modulate chromatin regulation of hypertrophic signaling through IP. As shown in Fig. 6A, HDACs 1, 2, 4, and 5 and OGT are O-GlcNAcylated in the mouse heart, but this is not altered by acute exercise. Furthermore, as shown in Fig. 6A, the 110-kDa ncOGT isoform appears to be more O-GlcNAcylated than are the 103-kDa mOGT and 78-kDa OGT isoforms; however, this may be a result of antibody-antigen affinity. We were unable to identify O-GlcNAcylation of HDAC3 through IP because of its molecular weight corresponding to the signal of the IgG band. We show here, for the first time, that both REST and mSin3A interact with OGT in the mouse heart (Fig. 6, C and D). Interestingly, IP of OGT primarily captured the 110-kDa nucleocytosolic isoform that was associated with decreased levels of total protein O-GlcNAcylation (P < 0.05), and a trend toward lower protein O-GlcNAcylation of the 110-kDa band (P = 0.072) in response to exercise. Acute exercise was associated with a lower interaction between REST and OGT (P < 0.05), with no differences in OGT-mSin3A and no effect of exercise duration when analysis was performed relative to the OGT 110-kDa band.
Fig. 6.
Coimmunoprecipitation (IP) of O-GlcNAc (A and B) and OGT (C and D) and subsequent densitometric analysis with immunoblots (IB) for O-GlcNAc, OGT, HDAC1, HDAC2, HDAC4, and HDAC5. Note that the input and IgG lanes were present on the same membranes as the sedentary and exercise samples; the position from which additional lanes were removed is indicated by the lined box and white spaces; n = 6/group. *P < 0.05, main effect of exercise.
DISCUSSION
The mechanisms regulating cardiac hypertrophy have primarily focused on pathological remodeling, particularly downstream mediators of this process, whereas the processes that mediate exercise-induced physiological hypertrophy are not well understood. The REST corepressor complex alters chromatin architecture via interactions with HDACs and subsequent acetylation and deacetylation of histones, thus regulating access of transcription factors to DNA and gene transcription of hypertrophic mediators (9, 21, 23, 32). We have previously demonstrated that protein O-GlcNAcylation blunts the hypertrophic response in cardiomyocytes (18). When taken together with other reports that mSin3A, a component of the REST complex, interacts with OGT and is subsequently O-GlcNAcylated (31), and that cardiac O-GlcNAc levels are decreased following endurance exercise training (2, 3), we postulated that exercise-induced hypertrophy may be mediated by interactions between the REST complex and OGT. In the current study, we have demonstrated 1) a differential effect of acute exercise on nuclear and cytosolic O-GlcNAc levels, 2) O-GlcNAcylation of HDACs and components of the REST complex that are not altered with exercise, and 3) an established interaction between OGT and REST that decreases in the heart with exercise. It must be emphasized that these changes are independent of metabolic increases in UDP-GlcNAc, the substrate for O-GlcNAc, and also have occurred following a single exercise session and are therefore independent of any recovery signaling that may occur in the hours following exercise training.
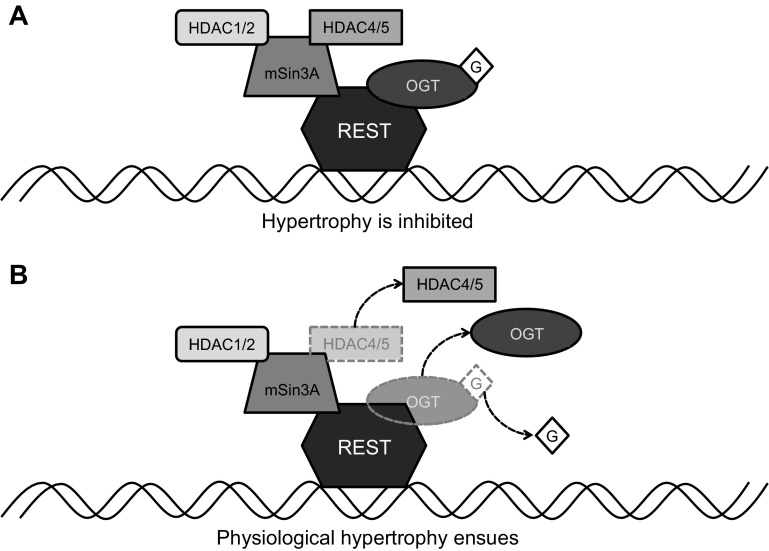
Because cellular O-GlcNAc increases in response to stress, we had hypothesized that acute exercise would upregulate protein O-GlcNAcylation in the heart and that the lower O-GlcNAc reported in chronically exercise-trained hearts (2, 3) was an adaptive response to repeated increases in O-GlcNAc. We actually found that acute exercise differentially alters cytosolic and nuclear protein O-GlcNAcylation in the mouse heart. Indeed, a single 15-min bout of exercise induced a decrease in cytosolic O-GlcNAc in the mouse heart that was not evident after 30 min of exercise. We also found that 15 min of exercise induced a significant elevation in O-GlcNAcylation of low-molecular-weight proteins in the nucleus that remained elevated after 30 min of exercise. Because the previous studies in this area focused solely on analysis of whole tissue lysates, it is not known whether the decreases reported were evident in both the cytosolic and nuclear compartments. The differential response between subcellular compartments in response to 15 min of exercise, particularly in the lower-molecular-weight range, is intriguing and may reflect translocation of O-GlcNAcylated proteins to the nucleus, a decrease or increase in cytosolic and nuclear protein O-GlcNAcylation, respectively, or a combination of these processes. Another possibility is the utilization of cellular glucose as fuel for exercise within 15 min, which results in decreased O-GlcNAc. Protein O-GlcNAcylation is then increased through a cellular stress response in exercise lasting 30 min, which may occur independent of substrate availability (27, 33). Because increased levels of O-GlcNAc are cardioprotective in ischemia-reperfusion (16), and we observed decreased O-GlcNAcylation of OGT and lower association of REST with OGT in exercised hearts, we postulate that this may be an early initiator of physiological, or exercise-induced, cardiac hypertrophy. More specifically, we propose that decreased O-GlcNAcylation of OGT allows initiation of exercise-induced cardiac hypertrophy via reduced recruitment of the chromatin repressor REST. In human skeletal muscle, it was previously established that exercising for 60 min induces ejection of class II HDACs from the nucleus to cytoplasm, a mechanism known to initiate hypertrophic signaling (19). However, to our knowledge, our study is the first to report a link between REST and OGT in regulation of cardiac hypertrophy. As such, we propose the mechanism illustrated in Fig. 7 with inhibition of REST/mSin3A signaling and cardiac hypertrophy when OGT is present in this complex (Fig. 7A). O-GlcNAcylation of OGT during acute exercise causes dissociation of OGT and the anti-hypertrophic class II HDACs from the REST/mSin3A complex, thus triggering physiological hypertrophy signaling (Fig. 7B).
Fig. 7.
Proposed roles of O-GlcNAc and OGT in REST-mediated regulation of exercise-induced physiological cardiac hypertrophy. A: at rest, O-GlcNAcylation of OGT promotes the interaction of OGT with components of the REST complex, inhibiting hypertrophy. B: acute exercise causes the removal of the O-GlcNAc moiety from OGT, which triggers the dissociation of both OGT and HDACs 4 and 5 from the REST complex, initiating physiological hypertrophic signaling. G, O-GlcNAc.
The O-GlcNAc moiety is a very small, uncharged molecule, and its attachment does not prevent proteins crossing membranes, for example, O-GlcNAc modification of NeuroD1 and cyclic adenosine monophosphate response element-binding protein 2 induces translocation of the modified protein to the nucleus (1, 7). The fact that lower-molecular-weight molecular proteins were lower in the cytosol and higher in the nucleus following 15 min of exercise suggests that translocation of O-GlcNAcylated proteins to the nucleus may have been induced by exercise; however, the lack of discernible exercise-induced changes in patterns of cytosolic and nuclear distributions of O-GlcNAc visualized using immunohistochemistry do not fully support this notion. Changes in metabolic demand may contribute to the differential responses between intracellular compartments, since it was recently reported that nicotinamide adenine dinucleotide (NAD+) decreases O-GlcNAc in cardiomyocytes in a time- and dose-dependent manner that is independent of both OGT and OGA (8); however, because increased cardiac work induced by intense exercise is driven by a transient reduction in cytosolic and mitochondrial NAD+ (34), it appears unlikely that this mechanism can explain the decreased cytosolic O-GlcNAc following 15 min of exercise.
A limitation of this study is the use of forced treadmill exercise, since this elicits a clear psychological and physiological stress response that may confound any changes observed in the heart (6). Ideally, a voluntary exercise protocol would be used to limit the stress response; however, this is not practical for the purposes of examining the response to a single bout of exercise because of the inability to control the duration or intensity of exercise in the rodent model. Thus, to limit the stress associated with the treadmill and/or forced exercise, all animals were accommodated to the treadmill in the days preceding the experiment, and mice in the sedentary group were placed on the treadmill for the same duration as the exercise-trained animals before death. Finally, because our observations suggest transient modifications in the state of protein O-GlcNAcylation, caution should be used if extrapolating these data to any longer-duration or chronic exercise applications. However, training-induced adaptations occur through repeated exposures to acute stress; therefore, we suggest that such adaptations occur because of cumulative effects of the transient findings observed in our study.
To our knowledge, this study is the first to demonstrate an effect of a single bout of exercise on cardiac O-GlcNAcylation in the heart and an effect of exercise on interactions between O-GlcNAc, OGT, and chromatin corepressors of hypertrophic signaling. Specifically, O-GlcNAc differentially modifies nuclear and cytosolic cardiac proteins in response to a single bout of exercise, and this occurs independently of changes in compartment levels of OGT and OGA. Although others have clearly demonstrated a role of O-GlcNAc and OGT in regulation of gene transcription (24–26), our study is the first to demonstrate O-GlcNAcylation of HDACs 1, 2, 4, and 5, mSin3A, and REST in cardiac tissue. Finally, the finding that acute exercise decreases the interaction between OGT and REST suggests that this could be the mechanism by which the O-GlcNAc pathway regulates cardiac hypertrophy and may be the initial step in stimulation of exercise-induced physiological hypertrophy. This finding is important, since HDACs are involved in both pathological and physiological hypertrophic signaling, and changes in O-GlcNAc have been demonstrated in both processes in the heart. We believe this is the first step toward increasing understanding of the paradoxical nature of protein O-GlcNAcylation and may provide a new and novel target for therapies specifically designed to mimic the beneficial effect of exercise on the heart.
GRANTS
This work was supported by the National Institute of Health Grant HL-104549, the Washington State University (WSU) Spokane Office of Research, and the WSU College of Pharmacy. H. M. Medford is supported by a William Townsend Porter Predoctoral Fellowship from the American Physiological Society.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: H.M.M., K.P., and S.A.M. conception and design of research; H.M.M., K.P., and S.A.M. performed experiments; H.M.M., K.P., and S.A.M. analyzed data; H.M.M., K.P., and S.A.M. interpreted results of experiments; H.M.M., K.P., and S.A.M. prepared figures; H.M.M. and S.A.M. drafted manuscript; H.M.M., K.P., and S.A.M. edited and revised manuscript; H.M.M., K.P., and S.A.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful for the technical assistance provided by Kyla E. Hall, Emily J. Cox, and Dr. Lindsey E. Miller.
REFERENCES
- 1.Andrali SS, Qian Q, Ozcan S. Glucose mediates the translocation of NeuroD1 by O-linked glycosylation. J Biol Chem 282: 15589–15596, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belke DD. Swim-exercised mice show a decreased level of protein O-GlcNAcylation and expression of O-GlcNAc transferase in heart. J Appl Physiol 111: 157–162, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Bennett CE, Johnsen VL, Shearer J, Belke DD. Exercise training mitigates aberrant cardiac protein O-GlcNAcylation in streptozotocin-induced diabetic mice. Life Sci 92: 657–663, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther 128: 191–227, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Bowe DB, Sadlonova A, Toleman CA, Novak Z, Hu Y, Huang P, Mukherjee S, Whitsett T, Frost AR, Paterson AJ, Kudlow JE. O-GlcNAc integrates the proteasome and transcriptome to regulate nuclear hormone receptors. Mol Cell Biol 26: 8539–8550, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown DA, Johnson MS, Armstrong CJ, Lynch JM, Caruso NM, Ehlers LB, Fleshner M, Spencer RL, Moore RL. Short-term treadmill running in the rat: what kind of stressor is it? J Appl Physiol 103: 1979–1985, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Dentin R, Hedrick S, Xie J, Yates J, 3rd, Montminy M. Hepatic glucose sensing via the CREB coactivator CRTC2. Science 319: 1402–1405, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Durgan DJ, Pat BM, Laczy B, Bradley JA, Tsai JY, Grenett MH, Ratcliffe WF, Brewer RA, Nagendran J, Villegas-Montoya C, Zou C, Zou L, Johnson RL, Jr, Dyck JR, Bray MS, Gamble KL, Chatham JC, Young ME. O-GlcNAcylation, novel post-translational modification linking myocardial metabolism and cardiomyocyte circadian clock. J Biol Chem 286: 44606–44619, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu H, Liang Y, Mandel G, Roizman B. Components of the REST/CoREST/histone deacetylase repressor complex are disrupted, modified, and translocated in HSV-1-infected cells. Proc Natl Acad Sci USA 102: 7571–7576, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guenatri M, Bailly D, Maison C, Almouzni G. Mouse centric and pericentric satellite repeats form distinct functional heterochromatin. J Cell Biol 166: 493–505, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanover JA. Epigenetics gets sweeter: O-GlcNAc joins the “histone code.” Chem Biol 17: 1272–1274, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanover JA. Glycan-dependent signaling: O-linked N-acetylglucosamine. FASEB J 15: 1865–1876, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Kee HJ, Eom GH, Joung H, Shin S, Kim JR, Cho YK, Choe N, Sim BW, Jo D, Jeong MH, Kim KK, Seo JS, Kook H. Activation of histone deacetylase 2 by inducible heat shock protein 70 in cardiac hypertrophy. Circ Res 103: 1259–1269, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Kee HJ, Sohn IS, Nam KI, Park JE, Qian YR, Yin Z, Ahn Y, Jeong MH, Bang YJ, Kim N, Kim JK, Kim KK, Epstein JA, Kook H. Inhibition of histone deacetylation blocks cardiac hypertrophy induced by angiotensin II infusion and aortic banding. Circulation 113: 51–59, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Kuwahara K, Saito Y, Ogawa E, Takahashi N, Nakagawa Y, Naruse Y, Harada M, Hamanaka I, Izumi T, Miyamoto Y, Kishimoto I, Kawakami R, Nakanishi M, Mori N, Nakao K. The neuron-restrictive silencer element-neuron-restrictive silencer factor system regulates basal and endothelin 1-inducible atrial natriuretic peptide gene expression in ventricular myocytes. Mol Cell Biol 21: 2085–2097, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laczy B, Marsh SA, Brocks CA, Wittmann I, Chatham JC. Inhibition of O-GlcNAcase in perfused rat hearts by NAG-thiazolines at the time of reperfusion is cardioprotective in an O-GlcNAc-dependent manner. Am J Physiol Heart Circ Physiol 299: H1715–H1727, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazarus BD, Love DC, Hanover JA. Recombinant O-GlcNAc transferase isoforms: identification of O-GlcNAcase, yes tyrosine kinase, and tau as isoform-specific substrates. Glycobiology 16: 415–421, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Marsh SA, Dell'Italia LJ, Chatham JC. Activation of the hexosamine biosynthesis pathway and protein O-GlcNAcylation modulate hypertrophic and cell signaling pathways in cardiomyocytes from diabetic mice. Amino Acids 40: 819–828, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGee SL, Fairlie E, Garnham AP, Hargreaves M. Exercise-induced histone modifications in human skeletal muscle. J Physiol 587: 5951–5958, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakagawa Y, Kuwahara K, Harada M, Takahashi N, Yasuno S, Adachi Y, Kawakami R, Nakanishi M, Tanimoto K, Usami S, Kinoshita H, Saito Y, Nakao K. Class II HDACs mediate CaMK-dependent signaling to NRSF in ventricular myocytes. J Mol Cell Cardiol 41: 1010–1022, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Naruse Y, Aoki T, Kojima T, Mori N. Neural restrictive silencer factor recruits mSin3 and histone deacetylase complex to repress neuron-specific target genes. Proc Natl Acad Sci USA 96: 13691–13696, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Neill BT, Kim J, Wende AR, Theobald HA, Tuinei J, Buchanan J, Guo A, Zaha VG, Davis DK, Schell JC, Boudina S, Wayment B, Litwin SE, Shioi T, Izumo S, Birnbaum MJ, Abel ED. A conserved role for phosphatidylinositol 3-kinase but not Akt signaling in mitochondrial adaptations that accompany physiological cardiac hypertrophy. Cell Metab 6: 294–306, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ooi L, Wood IC. Chromatin crosstalk in development and disease: lessons from REST. Nat Rev Genet 8: 544–554, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Sakabe K, Hart GW. O-GlcNAc transferase regulates mitotic chromatin dynamics. J Biol Chem 285: 34460–34468, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakabe K, Wang Z, Hart GW. Beta-N-acetylglucosamine (O-GlcNAc) is part of the histone code. Proc Natl Acad Sci USA 107: 19915–19920, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinclair DA, Syrzycka M, Macauley MS, Rastgardani T, Komljenovic I, Vocadlo DJ, Brock HW, Honda BM. Drosophila O-GlcNAc transferase (OGT) is encoded by the Polycomb group (PcG) gene, super sex combs (sxc). Proc Natl Acad Sci USA 106: 13427–13432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor RP, Parker GJ, Hazel MW, Soesanto Y, Fuller W, Yazzie MJ, McClain DA. Glucose deprivation stimulates O-GlcNAc modification of proteins through up-regulation of O-linked N-acetylglucosaminyltransferase. J Biol Chem 283: 6050–6057, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Vosseller K, Wells L, Lane MD, Hart GW. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3–L1 adipocytes. Proc Natl Acad Sci USA 99: 5313–5318, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wells L, Vosseller K, Hart GW. Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science 291: 2376–2378, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Whelan SA, Hart GW. Proteomic approaches to analyze the dynamic relationships between nucleocytoplasmic protein glycosylation and phosphorylation. Circ Res 93: 1047–1058, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Yang X, Zhang F, Kudlow JE. Recruitment of O-GlcNAc transferase to promoters by corepressor mSin3A: coupling protein O-GlcNAcylation to transcriptional repression. Cell 110: 69–80, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Yang XJ, Seto E. Collaborative spirit of histone deacetylases in regulating chromatin structure and gene expression. Curr Opin Genet Dev 13: 143–153, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Zachara NE, O'Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem 279: 30133–30142, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Zhou L, Cabrera ME, Okere IC, Sharma N, Stanley WC. Regulation of myocardial substrate metabolism during increased energy expenditure: insights from computational studies. Am J Physiol Heart Circ Physiol 291: H1036–H1046, 2006 [DOI] [PubMed] [Google Scholar]