Abstract
Mechanistic target of rapamycin (mTOR) is essential for cardiac development, growth, and function, but the role of mTOR in the regulation of cardiac metabolism and mitochondrial respiration is not well established. This study sought to determine cardiac metabolism and mitochondrial bioenergetics in mice with inducible deletion of mTOR in the adult heart. Doxycycline-inducible and cardiac-specific mTOR-deficient mice were generated by crossing cardiac-specific doxycycline-inducible tetO-Cre mice with mice harboring mTOR floxed alleles. Deletion of mTOR reduced mTORC1 and mTORC2 signaling after in vivo insulin stimulation. Maximum and minimum dP/dt measured by cardiac catheterization in vivo under anesthesia and cardiac output, cardiac power, and aortic pressure in ex vivo working hearts were unchanged, suggesting preserved cardiac function 4 wk after doxycycline treatment. However, myocardial palmitate oxidation was impaired, whereas glucose oxidation was increased. Consistent with reduced palmitate oxidation, expression of fatty acid metabolism genes fatty acid-binding protein 3, medium-chain acyl-CoA dehydrogenase, and hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase/enoyl-CoA hydratase (trifunctional protein)-α and -β was reduced, and carnitine palmitoyl transferase-1 and -2 enzymatic activity was decreased. Mitochondrial palmitoyl carnitine respiration was diminished. However, mRNA for peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α and -1β, protein levels of PGC-1α, and electron transport chain subunits, mitochondrial DNA, and morphology were unchanged. Also, pyruvate-supported and FCCP-stimulated respirations were unchanged, suggesting that mTOR deletion induces a specific defect in fatty acid utilization. In conclusion, mTOR regulates mitochondrial fatty acid utilization but not glucose utilization in the heart via mechanisms that are independent of changes in PGC expression.
Keywords: mechanistic target of rapamycin, cardiac substrate metabolism, mitochondrial respiration, peroxisome proliferator-activated receptor-γ coactivator-1α
mechanistic target of rapamycin (mTOR) is an essential gene in mice (11, 20). mTOR engages in two distinct complexes, mTORC1 and mTORC2, distinguished by their specific binding partners, raptor and rictor, respectively (18). Disruption of mTOR affects both complexes; however, disruption of mTORC2 by rictor deletion in metabolic tissues generally has mild effects (1, 6, 7).
mTOR is also required for murine heart development, growth, and function (35, 37). An elegant study using the α-myosin heavy chain (MHC)-mutated estrogen receptor-Cre-mutated estrogen receptor [mer-cre-mer (MCM)] transgene (30) to temporally control mTOR gene deletion after tamoxifen administration revealed that lack of mTOR in the adult heart leads to heart failure and death of the mice 7 wk after initial tamoxifen administration that was characterized by uncontrolled apoptosis, excess autophagy, and altered mitochondrial structure (35). It was proposed that, mechanistically, elevated eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1), particularly nonphosphorylated 4E-BP1, repressed protein translation in mTOR-deficient hearts, leading to heart failure. Crossing MCM-mTOR mice to whole body 4E-BP1-deficient mice doubled median survival duration from 7 to 14 wk; however, full rescue was not achieved, raising the possibility of additional mechanisms.
Disruption of mTORC1 by raptor deletion phenocopies mTOR deletion in the heart (29), supporting the concept that mTORC2 may play a less significant role. This study showed that mTORC1 is required for the adaptive hypertrophy after transverse aortic constriction through regulation of 4E-BP1 and ribosomal protein S6 kinase 1 phosphorylation without changing total protein content. A switch of cardiac substrate oxidation from fatty acid to glucose in the hearts of raptor-deficient mice was observed. These measurements were performed 4 wk after gene deletion, but cardiac function was noted to be maintained for up to 3 wk after gene deletion. The switch of substrate metabolism in the failing heart from fatty acid to glucose is well described (8, 9, 25, 33); thus, the possibility remains that substrate switching in mTORC1-deficient hearts is secondary to cardiac dysfunction.
Differences in nonphosphorylated 4E-BP1 protein content in the aforementioned studies might indicate mTORC2-specific regulation of total 4E-BP1 protein content in the heart. An interesting phenomenon is that an increase of nonphosphorylated 4E-BP1 has also been seen during transverse aortic constriction-induced heart failure (31, 35), raising the possibility that inhibition of protein synthesis by increased 4E-BP1 content could be a secondary effect occurring during late-stage heart failure, rather than being the sole or specific basis for heart failure after mTOR deletion in the heart.
mTORC1 has been shown to regulate mitochondrial biogenesis and oxidation in part via the regulation of peroxisome proliferator-activated receptor (PPAR)-γ coactivator (PGC)-1α expression and activation in skeletal muscle and C2C12 cells (a skeletal muscle-derived cell line) via a complex involving mTOR and yin and yang 1 (YY1) (5). In cardiac muscle, a role for this signaling mechanism has not been rigorously examined, nor has the role of mTOR in the regulation of mitochondrial bioenergetics. We hypothesized that deletion of mTOR would impair mitochondrial biogenesis and ATP generation and lead to cardiac contractile dysfunction.
To test this hypothesis, we used doxycycline-inducible tetO-Cre governed by cardiac-restricted expression of reverse tetracycline-controlled transactivator protein to achieve temporally controlled mTOR deletion in the adult heart. Doxycycline, used for tetO induction, is less toxic than tamoxifen, which has been shown to induce cardiotoxicity and severe transient dilated cardiomyopathy in mice (17). Mitochondrial dysfunction is a commonly observed characteristic of failing hearts. For this reason, we studied mice with cardiomyocyte deficiency of mTOR before the onset of any detectable left ventricular (LV) dysfunction. Our analysis of myocardial substrate utilization, mitochondrial function, and levels of proteins and genes that regulate fatty acid metabolism indicates that mTOR specifically regulates mitochondrial fatty acid metabolism in the absence of changes in PGC-1α and that deletion of mTOR in cardiomyocytes impairs myocardial fatty acid utilization before the onset of cardiac dysfunction.
MATERIALS AND METHODS
Animals.
Inducible and cardiac-specific mTOR-deficient [mTOR knockout (KO)] mice were generated by developing compound transgenic mice harboring a tetO-Cre construct (strain no. 6234, Jackson Laboratories), a reverse tetracycline transactivator under the control of the α-MHC promoter and floxed mTOR alleles (tetO-cretg/+/α-MHC-rtTAtg/+/mTORfl/fl). All mice were on a pure C57BL/6 background. To induce tetO-Cre expression, mTOR KO mice were administered doxycycline hyclate (Sigma, St. Louis, MO) at a dose of 4 mg/kg body wt by intraperitoneal injection at 8 wk of age and then were kept on doxycycline chow (1 g/kg) for 3 wk, after which they were switched back to normal rodent chow for 1 more week to allow doxycycline washout before they were euthanized for experiments. Expression of tetO-Cre results in recombination of the floxed mTOR alleles and deletion of the mTOR gene. mTORfl/fl mice were used as controls for mTOR KO mice.
Chloral hydrate was used as general anesthesia (400 mg/kg body wt, by a single intraperitoneal injection) unless specified. The depth of anesthesia was assessed by pinching the toe; any reaction from the mouse 4 min after the administration of the anesthetic agent indicated that the anesthesia was too light and additional anesthetic agent would be given.
Animals were handled in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996). All animal procedures in this study were approved by the Institutional Animal Care and Use Committee of the University of Utah.
Tissue harvesting.
Random fed mice were anesthetized by chloral hydrate, and hearts were immediately removed and rinsed in cold PBS before being snap frozen in liquid nitrogen. Heart tissues were used for immunoprecipitation and Western blot analysis, quantitative real-time PCR, mitochondrial DNA measurements, citrate synthase (CS) activity, pyruvate dehydrogenase (PDH) activity, glycogen content determination, and metabolomic analysis.
Immunoprecipitation and Western blot analysis.
Cytosolic proteins were extracted from whole heart lysates using homogenization buffer containing 0.1% Triton X-100 and protease/phosphatase inhibitors (Pierce, Rockford, IL). The protein concentration was determined by a Micro BCA Protein Assay kit (Pierce).
Immunoprecipitation was performed with Millipore (Billerica, MA)-provided protocols: 0.5 mg protein lysates in 1 ml lysis buffer containing both protease and phosphatase inhibitors were incubated with 10 μg carnitine palmitoyl transferase (CPT)-1b antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C on a rocker for 3 h, and the antibody-protein complexes were later captured by protein A agarose beads (Millipore) by 1-h incubation on a rocker at 4°C. The immunocomplexes were then dissociated from the beads by boiling with 2× Tris-glycine SDS sample buffer (Invitrogen, Carlsbad, CA) containing 5% β-mercaptoethanol at 95°C for 10 min. The supernatant was used for Western blot analysis.
For Western blot analysis, protein samples were mixed with 2× Tris-glycine SDS sample buffer containing 5% β-mercaptoethanol and boiled at 95°C for 10 min. Identical amounts of protein in equivalent volumes were loaded, resolved by SDS-PAGE, and transferred to either polyvinylidene difluoride (low fluorescence) or nitrocellulose membranes for immunoblot detection with specific antibodies. Detection and quantification were performed by measuring the intensity of fluorescence from secondary antibodies using the Odyssey infrared imaging system and accompanying software (LI-COR Biosciences, Lincoln, NE).
The primary antibodies used were as follows: LC3, actin, and tubulin antibodies were purchased from Sigma; pyruvate dehydrogenase kinase (PDK)-1 antibody was purchased from Stressgen; VDAC antibody was purchased from MBL (Woburn, MA); 4-hydroxynonenal (4-HNE), 4E-BP1, PDH, and PDK4 antibodies were purchased from Abcam (Cambridge, MA); PDK2, PDK3, CPT-1b, and phospho-PDH (Ser293) antibodies were purchased from Santa Cruz Biotechnology; glucose transporter (GLUT)1 and GLUT4 antibodies were purchased from Millipore; all mitochondrial oxidative phosphorylated (OXPHOS) antibodies were purchased from Molecular Probes (now part of Invitrogen); and all other antibodies were purchased from Cell Signaling (Danvers, MA). Primary antibodies were used at the recommended dilutions.
The fluorophore-conjugated secondary antibodies used were as follows: goat anti-rabbit Alexa 680, goat anti-mouse Alexa 680, donkey anti-rabbit Alexa 680, and donkey anti-goat Alexa 680 antibodies were purchased from Invitrogen; and goat anti-rabbit IRDye 800 and goat anti-mouse IRDye 800 were purchased from Li-Cor. Secondary antibodies were used at 1:10,000 dilutions.
RNA extraction, cDNA synthesis, and quantitative real-time PCR.
RNA extraction was performed using TRIzol (Life Technologies, Grand Island, NY), and cDNA synthesis was done using the Superscript III cDNA synthesis kit (Life Technologies) using the manufacturer's protocols. Transcript levels of genes were measured by quantitative cyber green real-time PCR and expressed as fold changes compared with controls. Primers of genes were designed using National Center for Biotechnology Information Primer Blast. The ribosomal protein 16S gene was used as an internal reference.
Hyperinsulinemic euglycemic clamp.
Hyperinsulinemic euglycemic clamps were performed in nonsedated mice as previously described with minor changes (14). In summary, the mouse jugular vein was catheterized under avertin anesthesia (250 mg/kg body wt, by a single intraperitoneal injection). Mice were then allowed to recover for 5 days with one heparin flush on day 3 before the clamp procedure. All mice were fasted overnight before the clamp procedure day to synchronize the metabolic state. On the day of the procedure, mice were single housed in a standard housing cage with a tether arm attached to the catheter. A dual infusion pump (Harvard Apparatus) was used to infuse insulin at a constant flow rate (10 mU·kg−1·min−1). Glucose solution was infused at a variable rate to maintain plasma glucose at a target value of 75–110 mg/dl and held at that level for 60 min. The procedure took an average of 90–100 min. A comparable rate of saline infusion was used as the control. Glucose was monitored using tail vein blood at 5-min intervals with a glucometer (Glucometer Elite, Tarrytown, NY). At the end of the procedure, mice were anesthetized with chloral hydrate; serum was collected from the supernatant after spinning of whole blood at 13,000 rpm in a centrifuge at 4°C for 30 min. Heart tissue was collected and snap frozen in liquid nitrogen. Insulin concentration was measured to ensure the success of the clamp using the MAGPIX analysis system (Luminex, Austin, TX). Serum insulin concentrations were 93.15 ng/ml in control mice and 89.27 ng/ml in mTOR KO clamped mice, respectively (P = 0.78). Insulin levels were undetectable in saline-infused mice.
Substrate metabolism in ex vivo working hearts.
Mice were anesthetized with chloral hydrate, and hearts were rapidly excised in ice-cold buffer. The aorta was then cannulated, and the heart was perfused in the working mode at a constant pressure of 60 mmHg with Krebs buffer containing 5 mM glucose and 0.4 mM palmitate prebound to 3% BSA at 37°C saturated with 95% O2-5% CO2, as previously described (4, 19). Palmitate oxidation was determined by measuring the amount of 3H2O released from [9,10-3H]palmitate (Perkin-Elmer, Waltham, MA). Glycolysis and glucose oxidation were determined by measuring the amount of 3H2O released from the metabolism of [5-3H]glucose (Perkin-Elmer) and 14CO2 released by the metabolism of [U-14C]glucose (Perkin-Elmer), respectively. Cardiac output, cardiac efficiency, and myocardial O2 consumption were determined as previously described (19).
Mitochondrial respiration.
Fibers were separated from the LV and permeabilized with saponin (Sigma) as previously described (3). O2 consumption was measured at 25°C as V0 in buffer supplemented with 20 μM palmitoyl-carnitine (palmitoyl-carnitine respiration) or 10 mM pyruvate (pyruvate respiration). ADP and oligomycin were then added in sequence to measure respiration after the addition of ADP and oligomycin. The rate of ATP synthesis was determined by sampling the respiratory buffer six times at 10-s intervals shortly after the addition of ADP. Final respiration rates were normalized by dry heart fiber weight.
Mitochondrial DNA quantification.
DNA was extracted from 5 mg of heart tissue and purified with the DNeasy Tissue kit (Invitrogen). Real-time PCR was performed using an ABI Prism qRT-PCR instrument (Applied Biosystems, Foster City, CA) in a 96-well plate format with SYBR green I for detection. Mitochondrial DNA content (D-loop noncoding region) was expressed relative to genomic NADH-ubiquinone-oxidoreductase flavoprotein (Ndufv)1. Primers for the mitochondrial D-loop noncoding region and genomic Ndufv1 gene were obtained from a previous publication (29).
Electron microscopy.
Cardiac tissue samples were removed and fixed in electron microscopy (EM) fixation buffer containing 2.5% glutaraldehyde and 1% paraformaldehyde. They were processed as previously described at the University of Utah EM Core (3).
Echocardiography.
Echocardiography was done as previously described (22). In detail, mice were anesthetized with 2% isoflurane gas with an inflow rate of 1 ml/min and placed on a heated stage (37°C). Chest hair was then removed with a topical depilatory agent before the echocardiogram. Short- and long-axis two-dimensional guided M-mode images were taken with a 13-MHz linear probe from GE Medical Systems (Milwaukee, WI) for measurements of LV dimensions and wall thickness. Fractional shortening (in %) was calculated as 100 × [(LVDd − LVDs)/LVDd], where LVDd is the LV dimension at diastole and LVDs is the LV dimension at systole.
Cardiac catheterization.
Cardiac catheterizations were performed as previously described (22). Mice were anesthetized with a single intraperitoneal injection of 400 mg chloral hydrate per 1 kg body wt and placed on a heating pad (37°C). A Millar Mikro-Tip catheter (1.0-Fr) was then inserted into the LV via the right carotid artery, and hemodynamic measurements (maximum and minimum dP/dt) were obtained using LabChart7 Pro software (AD Instruments, Colorado Springs, CO).
Measurement of enzymatic activity.
Total CPT and CPT-2 activity were measured in isolated mitochondria from fresh heart tissue as previously described (2). In detail, mitochondria with 100 μg protein content were assayed in 1 ml of reaction buffer containing 20 mM HEPES, 1 mM EGTA, 220 mM sucrose, 40 mM KCl, 0.1 mM 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB), 1.3 mg/ml BSA, and 40 μM palmitoyl-CoA (pH 7.4 at 25°C). The reaction was started by the addition of 1 mM carnitine and was monitored at 412 nm for 4 min with an Ultrospec 3000 spectrophotometer. CPT-2 activity was measured with the same reaction as for total CPT activity but in the presence of 10 μM malonyl-CoA , which inhibits CPT-1 activity. CPT-1 activity was calculated by subtracting CPT-2 activity from total CPT activity. The result was normalized to mitochondrial protein content.
CS activity was assessed in frozen cardiac tissue as previously described (2). In detail, heart tissue was dounce homogenized on ice in homogenization buffer containing 20 mM HEPES and 10 mM EDTA at pH 7.4. Homogenates were subjected to two freeze and thaw cycles to liberate CS from the mitochondrial matrix and then diluted 1:10 to an approximate final protein concentration of 1 μg/μl. The reaction was performed in 1 ml of reaction buffer containing 20 mM HEPES, 1 mM EDTA, 220 mM sucrose, 40 mM KCl, 0.1 mM DTNB, and 0.1 mM acetyl-CoA (pH 7.4 at 25°C). The reaction was started by the addition of 0.05 mM oxaloacetate and monitored at 412 nm for 3 min with an Ultrospec 3000 spectrophotometer. The result was normalized to protein content.
PDH activity was assayed in tissue homogenates using a purchased kit with manufacturer's protocols (Novagen). The assay started with lysates containing identical amounts of protein. PDH activity was expressed as the initial rate of reaction, determined from the slopes of the absorption curve generated. All data were normalized to the control group.
Glycogen measurement.
Glycogen content in frozen cardiac tissues was measured based on the measurement of glucose released from glycogen. The detailed protocol has been described in a previous publication (34).
Metabolomics.
Gas chromatography-mass spectrometry (GC-MS) was used to measure glucose, glucose-6-phosphate, and other metabolic intermediates in snap-frozen heart tissue using previously described protocols at the University of Utah Metabolomics Core (36).
Hematoxylin-eosin and trichrome staining.
Cardiac tissues were fixed in 10% zinc formalin (Fisher), embedded in paraplastic, and sectioned at 8 μm thickness. Slides were then stained with hematoxylin and eosin or with a trichrome staining kit (Sigma) using manufacturer's protocols.
Statistics.
Data are expressed as means ± SE (n ≥ 3). Statistical significance of two different groups was evaluated by a Student's t-test. Statistical significance for multiple groups was estimated by ANOVA followed by a Bonferroni post hoc test performed with Prism Graphpad software. P values of <0.05 were considered significant.
RESULTS
Cardiac-specific deletion of mTOR.
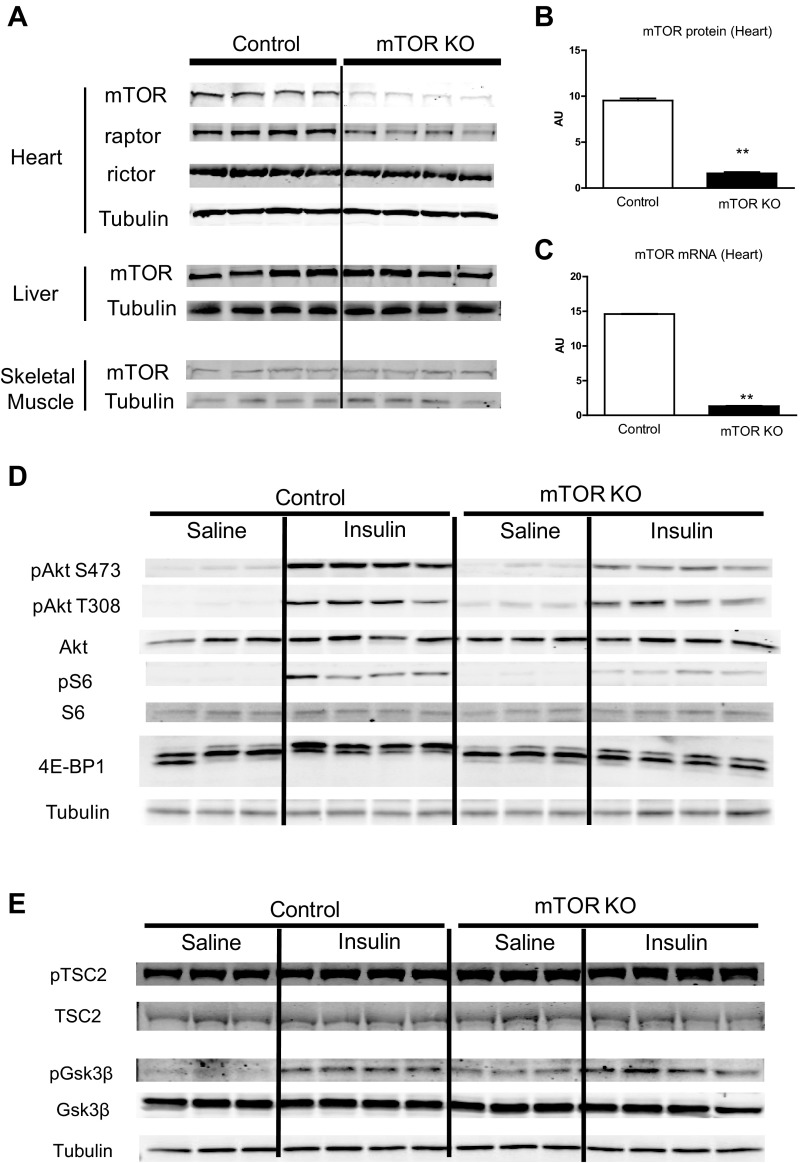
Cardiac mTOR deletion was achieved by doxycycline-induced cardiac-specific overexpression of Cre recombinase. Four weeks after the first doxycycline injection, there was an 83.2% decrease in mTOR protein in whole heart homogenates from mTOR KO mice compared with control mice, whereas in other tissues, such as liver and skeletal muscle, mTOR protein levels were maintained (Fig. 1, A and B). mTOR mRNA was also reduced by 90.9% in mTOR KO hearts compared with control hearts (Fig. 1C).
Fig. 1.
Generation and verification of mechanistic target of rapamycin (mTOR) deletion in mTOR knockout (KO) mice. A: Western blots of mTOR, raptor, and rictor in the heart and Western blots of mTOR in the liver and skeletal muscle from control and mTOR KO mice. B: quantification of mTOR protein levels in control and mTOR KO hearts. n = 4. C: mTOR mRNA measured by quantitative RT-PCR in control and mTOR KO cardiac tissue. n = 6. D: basal and insulin-stimulated Akt, S6 (Ser235/236), and eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) phosphorylation in control and mTOR KO hearts. There were three bands for 4E-BP1. The bottom band is nonphosphorylated 4E-BP1, the middle band is phosphorylated 4E-BP1, and the top band is hyperphosphorylated 4E-BP1. Phosphorylation of 4E-BP1 by insulin leads to a shift from the bottom to top band. E: basal and insulin-stimulated glycogen synthase kinase (GSK)-3β (Ser9) and tuberous sclerosis complex subunit 2 (TSC2) (Thr1462) phosphorylation in control and mTOR KO hearts. **P < 0.01.
In hearts from overnight fasted mice, phosphorylation of Akt on the Ser473 residue was barely detectable in control and mTOR KO hearts, and phosphorylation of mTORC1 downstream targets S6 and 4E-BP1 was also low in both groups. In contrast, mTOR deletion increased basal Akt Thr308 phosphorylation by 8.3-fold (Fig. 1D). Insulin treatment (hyperinsulinemic euglycemic clamp experiment) robustly increased Akt phosphorylation on both Ser473 and Thr308 sites in control hearts, resulting in an increase of S6 and 4E-BP1 phosphorylation. Deletion of mTOR reduced insulin-stimulated Akt Ser473 phosphorylation compared with control hearts stimulated with insulin, whereas insulin-stimulated Akt Thr308 phosphorylation in mTOR KO hearts was comparable to control hearts, supporting the fact that mTORC2 specifically phosphorylates Akt on the Ser473 residue. Insulin-stimulated S6 phosphorylation and 4E-BP1 phosphorylation (top band) were also reduced in mTOR KO hearts, confirming that deletion of mTOR also resulted in decreased mTORC1 downstream signaling in the heart (Fig. 1D). Basal glycogen synthase kinase (GSK)-3β and tuberous sclerosis complex subunit 2 (TSC2) phosphorylation were increased in mTOR KO hearts compared with control hearts. Densitometric analysis showed that phosphorylation of TSC2 and GSK-3β were increased by 31.8% and 269.0%, respectively, in mTOR KO hearts under basal conditions, likely reflecting increased Akt activity as a consequence of the increase in Akt Thr308 phosphorylation (Fig. 1E). Insulin increased both GSK-3β and TSC2 phosphorylation in mTOR KO hearts to a similar level as control hearts.
Myocardial metabolism and substrate oxidation.
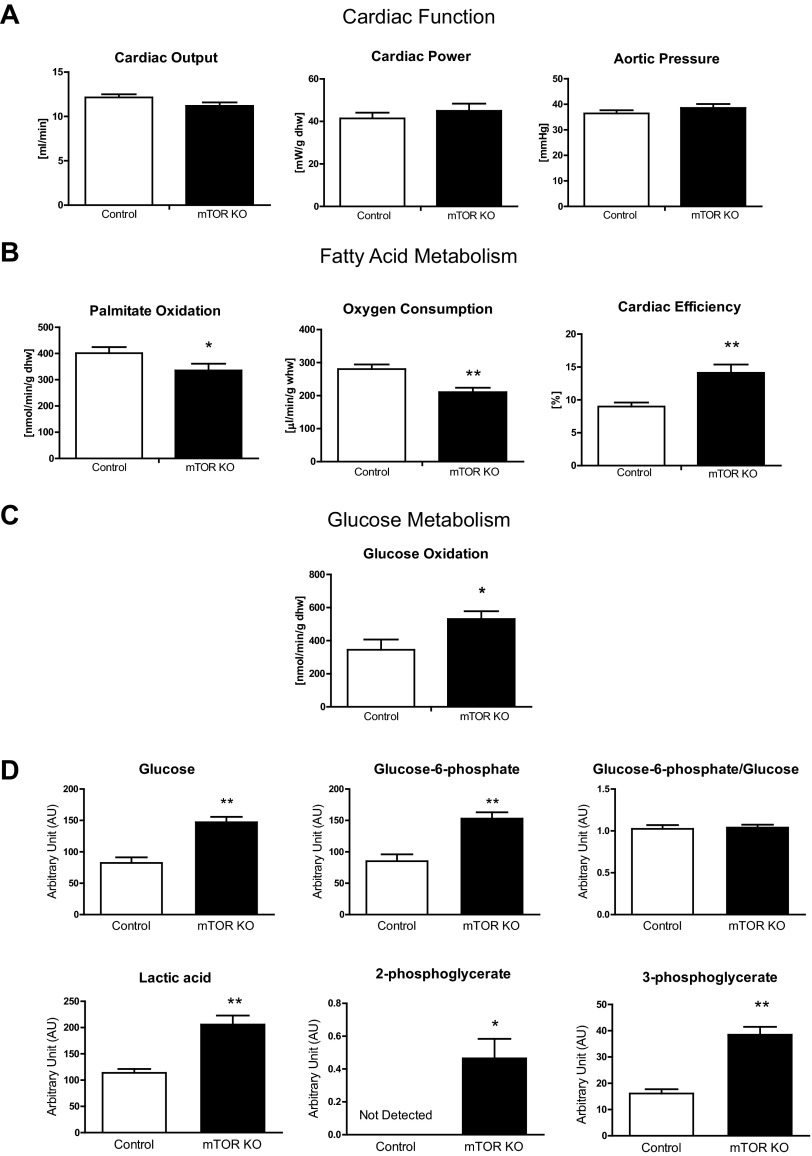
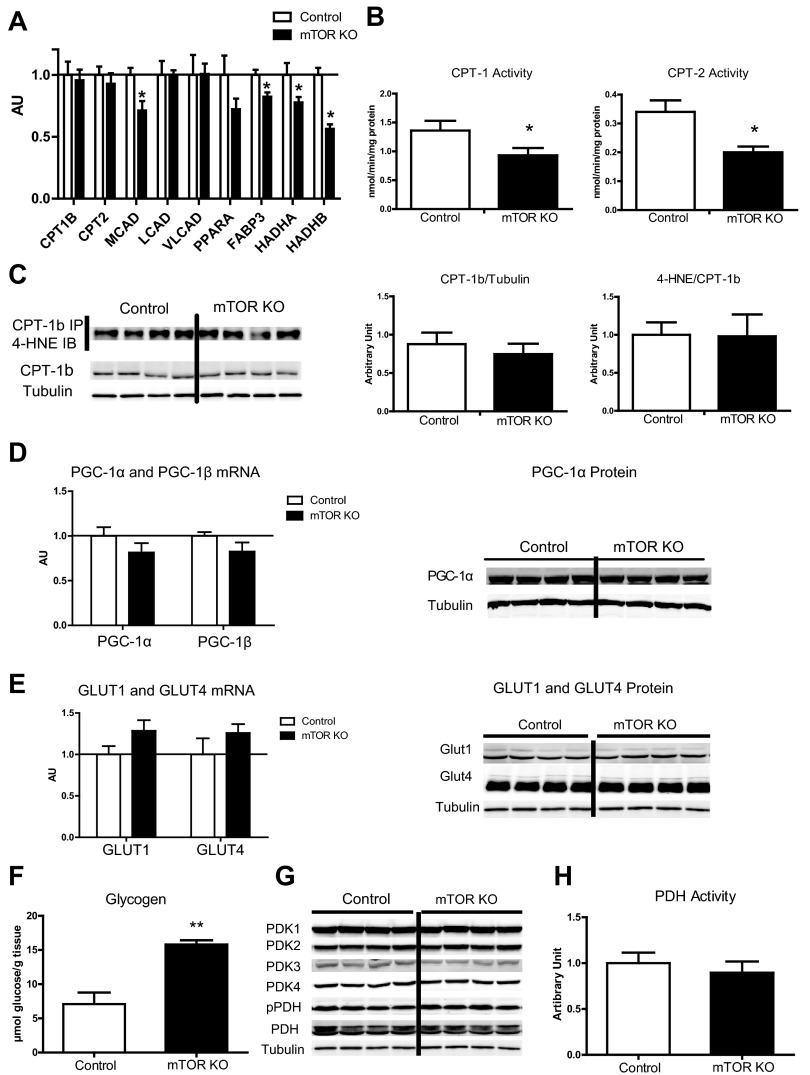
Cardiac output, cardiac power, and aortic pressure measured in ex vivo working hearts were not changed 4 wk after doxycycline treatment, suggesting that there was no cardiac dysfunction at this stage in mTOR KO hearts (Fig. 2A). Myocardial fatty acid oxidation (FAO) and glucose oxidation were also determined in ex vivo working hearts (Fig. 2, B and C), and free glucose and glycolytic intermediates were measured by GC-MS (Fig. 2D). Specifically, myocardial FAO was reduced by 16.4%, and O2 consumption was reduced by 24.9% in mTOR KO hearts compared with control hearts; concurrently, cardiac efficiency was increased by 57.1% (Fig. 2B). Transcription of fatty acid utilization genes such as FABP3, medium-chain acyl-CoA dehydrogenase (MCAD), and hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase/enoyl-CoA hydratase (trifunctional protein)-α and -β (HADHA and HADHB, respectively) were repressed in mTOR KO hearts (Fig. 3A). Although CPT transcripts (Fig. 3A) were not changed, CPT-1 and CPT-2 enzymatic activities measured in vitro were decreased by 41% and 32%, respectively (Fig. 3B). Oxidative modifications have been reported to alter CPT function (28), but we found no increase in HNE adducts (4-HNE) in immunocaptured CPT-1b protein (Fig. 3C). Taken together, these data suggest that impaired FAO in mTOR-deficient hearts occurs at multiple levels, including fatty acid transport into mitochondria as well as β-oxidation within the mitochondria. However, neither PGC-1α nor PGC-1β gene expression was affected (Fig. 3D). The PGC-1α protein level was also not changed (Fig. 3D), suggesting that the decreases of MCAD, FABP3, HADHA, and HADHB were regulated independently of changes in PGC expression.
Fig. 2.
Cardiac substrate oxidation in mTOR KO hearts measured in ex vivo working hearts. A: cardiac output, cardiac power, and aortic pressure measured in working hearts. n = 5–8. B: palmitate oxidation, O2 consumption, and cardiac efficiency measured in working hearts. n = 5–8. C: glucose oxidation measured in working hearts. n = 5–7. D: free glucose, glucose-6-phosphate, lactic acid, 2-phosphoglycerate, and 3-phosphoglycerate in control and mTOR KO hearts measured by gas chromatography-mass spectrometry. n = 6–8. AU, arbitrary units. *P < 0.05; **P < 0.01.
Fig. 3.
Genes and proteins involved in fatty acid utilization and glucose utilization. A: expression of genes in fatty acid transport and fatty acid metabolism measured by quantitative RT-PCR in control and mTOR KO hearts. n = 6. B: carnitine palmitoyl transferase (CPT)-1 and CPT-2 enzymatic activity in isolated mitochondria. n = 4. C: 4-hydroxynonenal (4-HNE) Western blots of immunocaptured CPT-1b protein. Quantification is shown on the right of the blots; n = 4 for quantification. D: peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α and PGC-1β gene expression measured by quantitative RT-PCR in control and mTOR KO hearts. n = 6. PGC-1α protein levels in control and mTOR KO hearts were detected by Western blot analysis. The western blot was done with the same set of samples used in Fig. 1A. The same tubulin blot was used as a loading control. E: expression of glucose transporter (GLUT)1 and GLUT4 measured by quantitative RT-PCR. n = 6. GLUT1 and GLUT4 protein levels were detected by Western blot analysis. F: glycogen content in control and mTOR KO hearts. n = 5. G: four cardiac pyruvate dehydrogenase kinase (PDK) isoforms, phosphorylated pyruvated dehydrogenase (PDH), and total PDH protein levels detected by Western blot analysis. The Western blot was done with the same set of samples used in Fig. 3E. The same tubulin blot was used as a loading control. H: PDH activity of control and mTOR KO hearts. n = 7–8. *P < 0.05; **P < 0.01.
Glucose oxidation is a multiple-step, multiple enzyme-catalyzed process, occurring after the initial transport of glucose into cells. The unchanged GLUT1 and GLUT4 gene expression and protein content (Fig. 3E) support preserved glucose uptake capacity in mTOR KO hearts. Free glucose and glucose-6-phosphate were increased in mTOR KO hearts relative to control hearts (Fig. 2D), suggesting increased glucose uptake. This could be due to an increase in basal Akt activity in mTOR KO hearts, as suggested by increased phosphorylation of Akt downstream kinases (Fig. 1E). Lactic acid and the glycolytic pathway intermediates 2-phosphoglycerate and 3-phosphoglycerate were also increased (Fig. 2D), suggesting an increase in glycolysis. Glycogen content was also increased in mTOR KO hearts by 122.5% (Fig. 3F).
Glucose oxidation in mTOR KO hearts was increased by 53.7% (Fig. 2C). Glucose oxidation can be regulated by the activity of PDH. PDH can be inactivated by PDK through inhibitory phosphorylation. However, the four known isoforms of PDK in mTOR hearts were unchanged relative to control hearts, as was phosphorylation of PDH on the Ser293 residue (Fig. 3G). Consistent with the phosphorylation state, PDH activity was not changed in mTOR KO hearts relative to control hearts (Fig. 3H). However, the lack of difference in in vitro PDH activity does not exclude the possibility of allosteric regulation in vivo, resulting from reduced FAO in mTOR KO hearts (15, 21).
Myocardial mitochondrial respiration and morphology.
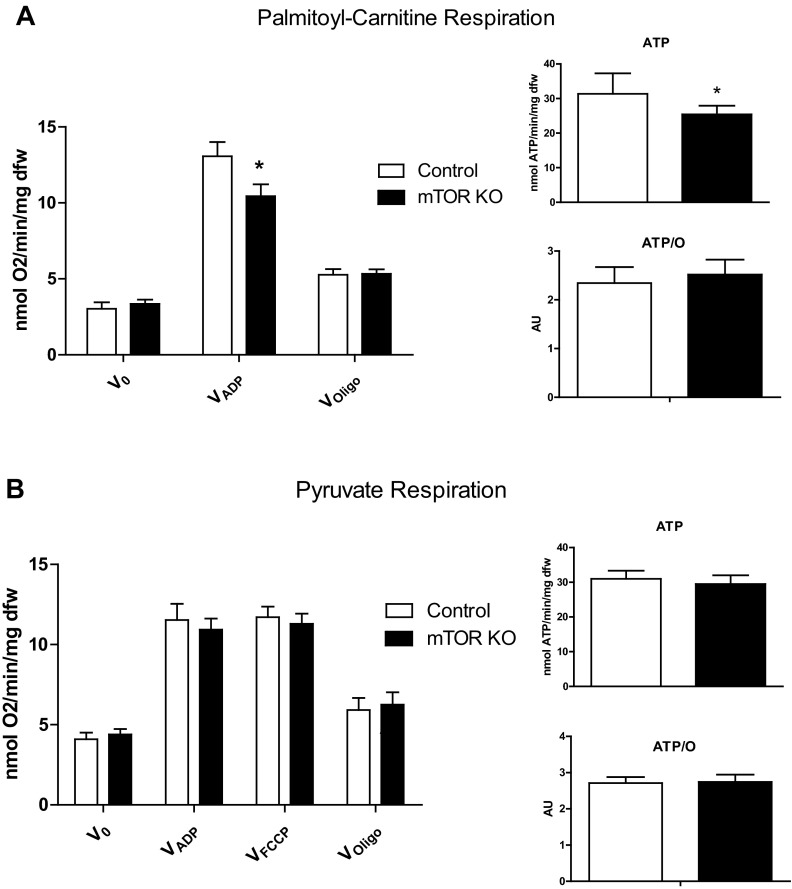
To investigate if reduced FAO was secondary to impaired mitochondrial substrate oxidation, O2 consumption rates were measured in saponin-permeabilized cardiac fibers supplemented with different substrates. Consistent with a reduction in FAO, mitochondrial respiration with palmitoyl-carnitine was reduced by 20.0% in mTOR KO heart fibers. As a result of reduced respiration, ATP production was reduced by 18.9%. However, the ATP-to-O ratio was not changed, indicating that the decrease in ATP production is independent of mitochondrial uncoupling in mTOR KO hearts (Fig. 4A). When isolated mTOR KO cardiac fibers were supplemented with pyruvate, respiration rates were similar to fibers from control hearts. ATP production and the ATP-to-O ratio were also unchanged (Fig. 4B). FCCP is a mitochondrial uncoupler that maximizes mitochondrial respiration. Thus, FCCP respiration rates can be used as an indicator of maximal mitochondrial oxidation capacity. FCCP (1 μM), in the presence of ADP and pyruvate, stimulated O2 consumption equivalently in control fibers and mTOR KO fibers, indicating preserved electron transport chain capacity in mTOR KO hearts (Fig. 4B).
Fig. 4.
Mitochondrial respiration supplemented with palmitoyl-carnitine (A) or pyruvate (B). V0 is basal respiration, VADP is respiration after the addition of ADP, VFCCP is respiration after the addition of FCCP, and VOligo is respiration after the addition of oligomycin. ATP is ATP generation from fibers after the addition of ADP when supplemented with the indicated substrate, and the ATP-to-O ratio (ATP/O) is ATP generation normalized by VADP, which is an indicator of mitochondrial coupling. *P < 0.05.
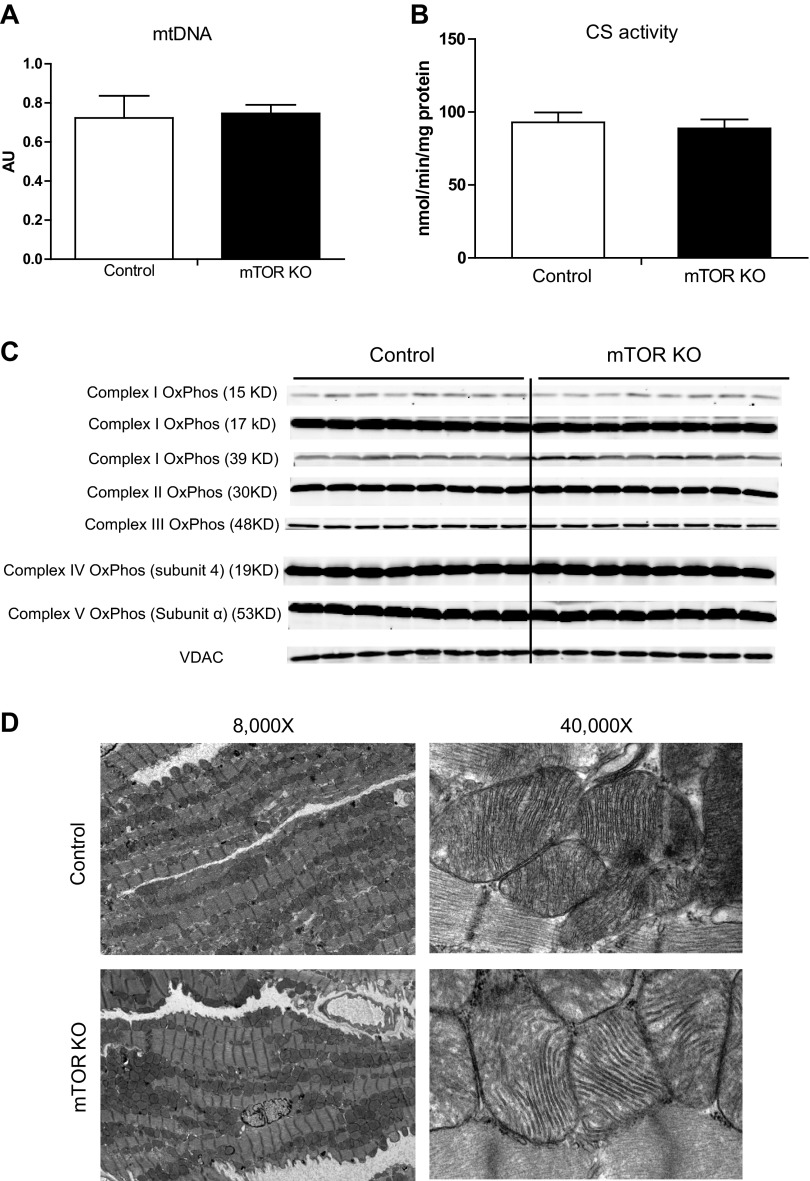
Mitochondrial DNA content was not reduced in mTOR KO hearts (Fig. 5A), mitochondrial CS activity was also maintained (Fig. 5B), and protein levels of representative mitochondrial complex proteins were not reduced (Fig. 5C), relative to controls. Ultrastructure revealed by EM showed a normal alignment of mitochondria in mTOR-deficient hearts and normal mitochondrial cristae structure similar to controls (Fig. 5D). Taken together, mTOR deletion does not result in global mitochondrial dysfunction but specifically precipitates a defect in FAO.
Fig. 5.
Mitochondrial (mt)DNA content, citrate synthase (CS) activity, mitochondrial electron transport complex subunit proteins, and mitochondrial morphology. A: mtDNA content measured by quantitative RT-PCR in control and mTOR KO hearts. n = 8. B: mitochondrial CS activity measured in control and mTOR KO hearts. n = 5–6. C: representative mitochondrial complex subunit proteins detected by Western blot analysis. OxPhos, oxidative phosphorylation. D: mitochondrial ultrastructure revealed by electron microscopy.
Development of cardiac dysfunction and demise of mTOR KO mice.
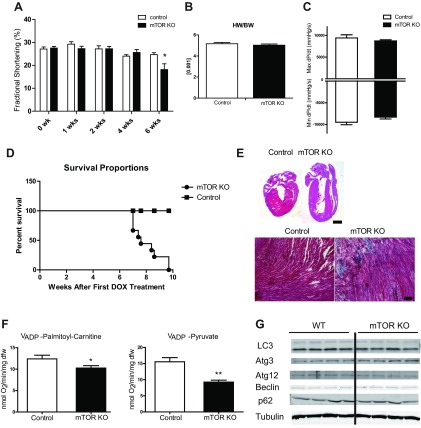
Consistent with ex vivo cardiac function measurements in working hearts, cardiac function evaluated by echocardiography showed that fractional shortening in mTOR KO hearts was maintained up to 4 wk after doxycycline treatment (Fig. 6A). Heart weight normalized to body weight was not changed 4 wk after doxycycline treatment (Fig. 6B). Maximum and minimum dP/dt of mTOR KO hearts measured by cardiac catheterization also showed no change 4 wk after doxycycline treatment (Fig. 6C). However, 6 wk after doxycycline treatment, mTOR KO mice had a 26.5% decrease in fractional shortening, suggesting impaired cardiac function (Fig. 6A). Shortly thereafter, mTOR KO mice started to die; by 10 wk after doxycycline treatment, all mTOR KO mice were dead (Fig. 6D), supporting an indispensable role of mTOR in the adult heart. Additionally, 6 wk after doxycycline treatment, mTOR KO hearts were dilated and showed increased fibrosis (Fig. 6E). Saponin-permeabilized mTOR KO cardiac fibers also showed 17.2% and 40.4% reductions, respectively, in ADP-stimulated O2 consumption when supplemented with palmitoyl-carnitine or pyruvate, indicating a reduction in mitochondrial function. However, the onset of heart failure was not associated with increased myocardial autophagy, as measured by Western blot analysis of autophagy-related proteins from mTOR KO hearts 4 wk after doxycycline treatment (Fig. 6G).
Fig. 6.
Development of cardiac dysfunction and lethality in mTOR KO mice. A: serial echocardiography of control and mTOR KO mice. n = 5–8. B: heart weight of control and mTOR KO mice normalized to body weight 4 wk after doxycycline (DOX) treatment. n = 7–10. C: cardiac hemodynamic parameters measured by catheterization 4 wk after DOX treatment. n = 6–7. D: survival curve of mTOR KO mice after DOX treatment. n = 9–10. E, top: four-champer view of a control heart and an mTOR KO heart 6 wk after DOX treatment stained with hematoxylin and eosin. Scale bar = 1 mm. Bottom, trichrome staining of histological sections from control and mTOR KO hearts. Scale bar = 50 μm. F: mitochondrial respiration of cardiac fibers from control and mTOR hearts 6 wk after DOX treatment supplemented with palmitoyl-carnitine or pyruvate. G: Western blots of autophagy-related proteins in control and mTOR KO hearts 4 wk after DOX treatment. The Western blot was done with the same set of samples used in Fig. 1A. The same tubulin blot was used as a loading control. *P < 0.05; **P < 0.01.
DISCUSSION
mTOR regulates many aspects of cell physiology in vivo, including but not limited to protein synthesis, proliferation, autophagy, and mitochondrial biogenesis (18). So it is not surprising that deletion of mTOR in mice resulted in lethality of the embryo shortly after implantation (11, 20). The role of mTOR is distinct in different tissues, and it is indispensable for the heart. However, the mechanism by which deletion of mTOR in the heart leads to cardiac failure is incompletely understood (35).
In this study, temporal deletion of mTOR in the adult heart led to cardiac failure and death of the mice, which supports the results of a previously published study (35) demonstrating that mTOR is indispensable in maintaining cardiac function and survival. However, similar to raptor deletion in the heart (29), we did not observe an increase of total 4E-BP1, suggesting that an uncontrolled increase of 4E-BP1, particularly the nonphosphorylated form, might not be the initial or inciting cause of heart failure in mTOR-deficient hearts.
We initially hypothesized that deletion of mTOR would result in a reduction of PGC-1α or impair its activity to decrease total mitochondrial respiratory capacity. However, we did not observe decreased PGC-1α mRNA or protein or reduced OXPHOS subunit proteins, which supports findings in raptor-deficient hearts in which PGC-1α expression was preserved 2 wk after raptor deletion (29). A general role for mTOR in the transcriptional regulation of PGC-1α is controversial. A mechanism by which mTORC1-PGC-1α-YY1 regulates the expression of mitochondrial biogenesis genes, including PGC-1α itself, in skeletal muscle and C2C12 cells has been proposed (5). Conversely, a direct interaction of endogenous mTORC1, PGC-1α, and YY1 has not been supported by fractionation and imaging studies (24, 26, 27), which revealed relatively low or absent endogenous mTORC1 in the nucleus. In our study, we did not observe any change in PGC-1α at both RNA and protein levels. The preserved mitochondrial FCCP respiration, normal levels of OXPHOS mitochondrial proteins, and normal mitochondrial morphology also argue against a primary role for mTOR in the regulation of global mitochondrial biogenesis. Thus, the possibility exists that previously reported mitochondrial damage in MCM-mTOR or raptor-deficient hearts (29, 35) could be secondary to the effect of cardiac dysfunction or failure or a combination of adverse effects of mTOR or raptor deletion acting in concert with tamoxifen treatment.
Attempts to increase PGC-1α expression or activity did not rescue the myopathic phenotypes of mice with skeletal muscle deletion of raptor (23). Bezafibrate, a PPAR pan agonist that activates the PPAR/PGC-1α pathway, was used on skeletal muscle raptor-deficient mice. Although bezafibrate increased mitochondrial function, such as oxidative capacity and the expression of mitochondrial genes, it did not prevent the progression of myopathy (23). Moreover, genetic overexpression of PGC-1α also did not reverse myopathy (23). Taken together, these observations suggest that the skeletal muscle myopathy induced by the disruption of mTORC1 is not regulated through PGC-1α. Our results suggest that this could also be true in the heart.
Another possible mechanism by which mTOR deletion could induce heart failure is unrestrained autophagy, given the role that mTOR plays in the suppression of autophagy (12, 16). However, there was no change in autophagy, as measured by LC3-II levels and other autophagy-related proteins in mTOR KO hearts (Fig. 6G), and transmission EM also revealed no increase in autophagosome formation in mTOR KO hearts relative to control hearts (Fig. 5D).
In the present study, the earliest change observed in mTOR KO hearts was reduced FAO, reduced mitochondrial palmitoyl-carnitine respiration, and decreased ATP generation. Although the results of the present study do not definitively prove that altered substrate metabolism represents the basis for the ultimate reduction in cardiac function in mTOR-deficient hearts, it revealed a potential mechanism that may contribute in part to impaired cardiac contractility. The heart has an extraordinarily high level of metabolism and heavily relies on fatty acid metabolism as a major source of ATP. Thus, changes in fatty acid metabolism and ATP production have the potential to impact cellular energetics (32), a normal level of which is required to maintain contractile function as well as protein synthesis, damage repair pathways, and other physiological functions in cardiomyocytes. Transportation of long-chain fatty acyl-CoAs by CPT into mitochondria is a rate-limiting step for FAO. Despite preserved CPT transcript and protein levels, CPT activities were reduced. Concentrations of malonyl-CoA, a known metabolic intermediate that allosterically inhibits CPT1 activity, were not changed (control vs. mTOR KO = 1 vs. 1.197, measured by GC-MS, P = 0.47), suggesting that regulation of CPT-1 activity could result from posttranscriptional modifications. It has been shown that oxidation of CPT-1 can reduce CPT-1 activity (28); however, no increase in HNE adducts of CPT-1 were observed, suggesting no increase in CPT-1 oxidation in mTOR KO hearts. CPT-1 can also be phosphorylated, acetylated, or nitrated (10). These posttranslational modifications were not evaluated in the present study. As such, the effect of those modifications on CPT-1 enzymatic activity in mTOR-deficient hearts remains to be elucidated and will be the subject of future studies.
Our study adds to the body of evidence underscoring that mTOR is essential for cardiac survival and function. Many hypotheses have been proposed to explain how deletion of mTOR leads to heart failure in mice. However, no single unifying mechanism has been identified. Given that mTOR controls a wide range of cellular functions by activating diverse downstream targets, it is likely that the heart failure that develops as a consequence of mTOR deletion is multifactorial. In contrast to mTOR KO hearts, chronic rapamycin treatment does not lead to heart failure (13), and the impact of rapamycin treatment on cardiac fatty acid utilization is not known. Thus, a comparative analysis of gene expression and phosphoproteomics in mTOR-deficient versus rapamycin-treated hearts may yield unique insights, not only into the regulation of myocardial metabolism by mTOR but also mechanisms by which mTOR deficiency ultimately leads to heart failure.
GRANTS
This work was supported by National Institutes of Health Grants R01-DK-092065 and R01-HL-108379 (to E. D. Abel). A. R. Wende was supported by an advanced postdoctoral fellowship from the Juvenile Diabetes Research Foundation. C. Riehle was supported by a postdoctoral fellowship from the German Research Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.Z. and E.D.A. conception and design of research; Y.Z., J.S., B.A., C.R., Y.C.Z., A.R.W., and D.J. performed experiments; Y.Z., J.S., B.A., C.R., Y.C.Z., A.R.W., D.J., and E.D.A. analyzed data; Y.Z., J.S., C.R., Y.C.Z., A.R.W., and E.D.A. interpreted results of experiments; Y.Z. prepared figures; Y.Z. drafted manuscript; Y.Z. and E.D.A. edited and revised manuscript; Y.Z., J.S., B.A., C.R., Y.C.Z., A.R.W., D.J., D.A.M., and E.D.A. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. George Thomas and Dr. Sara C. Kozma (University of Cincinnati, Cincinnati, OH), who provided the mTOR floxed mice used in this study.
REFERENCES
- 1. Bentzinger CF, Romanino K, Cloëtta D, Lin S, Mascarenhas JB, Oliveri F, Xia J, Casanova E, Costa CF, Brink M, Zorzato F, Hall MN, Rüegg MA. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab 8: 411– 424, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Boudina S, Sena S, O'Neill BT, Tathireddy P, Young ME, Abel ED. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation 112: 2686– 2695, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, Abel ED. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes 56: 2457– 2466, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, Cooksey RC, Litwin SE, Abel ED. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology 146: 5341– 5349, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1α transcriptional complex. Nature 450: 736– 740, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Cybulski N, Hall MN. TOR complex 2: a signaling pathway of its own. Trends Biochem Sci 34: 620– 627, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Cybulski N, Polak P, Auwerx J, Ruegg MA, Hall MN. mTOR complex 2 in adipose tissue negatively controls whole-body growth. Proc Natl Acad Sci USA 106: 9902– 9907, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davila-Roman VG, Vedala G, Herrero P, de las Fuentes L, Rogers JG, Kelly DP, Gropler RJ. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol 40: 271– 277, 2002 [DOI] [PubMed] [Google Scholar]
- 9. de las Fuentes L, Herrero P, Peterson LR, Kelly DP, Gropler RJ, Davila-Roman VG. Myocardial fatty acid metabolism: independent predictor of left ventricular mass in hypertensive heart disease. Hypertension 41: 83– 87, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Distler AM, Kerner J, Hoppel CL. Post-translational modifications of rat liver mitochondrial outer membrane proteins identified by mass spectrometry. Biochim Biophys Acta 1774: 628– 636, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gangloff YG, Mueller M, Dann SG, Svoboda P, Sticker M, Spetz JF, Um SH, Brown EJ, Cereghini S, Thomas G, Kozma SC. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol Cell Biol 24: 9508– 9516, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ganley IG, Lam du H, Wang J, Ding X, Chen S, Jiang X. ULK1, ATG13. FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem 284: 12297– 12305, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460: 392– 395, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hebert LF, Jr, Daniels MC, Zhou J, Crook ED, Turner RL, Simmons ST, Neidigh JL, Zhu JS, Baron AD, McClain DA. Overexpression of glutamine:fructose-6-phosphate amidotransferase in transgenic mice leads to insulin resistance. J Clin Invest 98: 930– 936, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hue L, Taegtmeyer H. The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab 297: E578– E591, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, Kundu M, Kim DH. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell 20: 1992– 2003, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koitabashi N, Bedja D, Zaiman AL, Pinto YM, Zhang M, Gabrielson KL, Takimoto E, Kass DA. Avoidance of transient cardiomyopathy in cardiomyocyte-targeted tamoxifen-induced MerCreMer gene deletion models. Circ Res 105: 12– 15, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci 122: 3589– 3594, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mazumder PK, O'Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, Boudina S, Abel ED. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes 53: 2366– 2374, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Murakami M, Ichisaka T, Maeda M, Oshiro N, Hara K, Edenhofer F, Kiyama H, Yonezawa K, Yamanaka S. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol Cell Biol 24: 6710– 6718, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Randle PJ. Metabolic fuel selection: general integration at the whole-body level. Proc Nutr Soc 54: 317– 327, 1995 [DOI] [PubMed] [Google Scholar]
- 22. Riehle C, Wende AR, Zaha VG, Pires KM, Wayment B, Olsen C, Bugger H, Buchanan J, Wang X, Moreira AB, Doenst T, Medina-Gomez G, Litwin SE, Lelliott CJ, Vidal-Puig A, Abel ED. PGC-1β deficiency accelerates the transition to heart failure in pressure overload hypertrophy. Circ Res 109: 783– 793, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Romanino K, Mazelin L, Albert V, Conjard-Duplany A, Lin S, Bentzinger CF, Handschin C, Puigserver P, Zorzato F, Schaeffer L, Gangloff YG, Ruegg MA. Myopathy caused by mammalian target of rapamycin complex 1 (mTORC1) inactivation is not reversed by restoring mitochondrial function. Proc Natl Acad Sci USA 108: 20808– 20813, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosner M, Hengstschlager M. Cytoplasmic and nuclear distribution of the protein complexes mTORC1 and mTORC2: rapamycin triggers dephosphorylation and delocalization of the mTORC2 components rictor and sin1. Hum Mol Genet 17: 2934– 2948, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation 94: 2837– 2842, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320: 1496– 1501, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 40: 310– 322, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Serviddio G, Giudetti AM, Bellanti F, Priore P, Rollo T, Tamborra R, Siculella L, Vendemiale G, Altomare E, Gnoni GV. Oxidation of hepatic carnitine palmitoyl transferase-I (CPT-I) impairs fatty acid β-oxidation in rats fed a methionine-choline deficient diet. PLos One 6: e24084, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shende P, Plaisance I, Morandi C, Pellieux C, Berthonneche C, Zorzato F, Krishnan J, Lerch R, Hall MN, Ruegg MA, Pedrazzini T, Brink M. Cardiac raptor ablation impairs adaptive hypertrophy, alters metabolic gene expression, and causes heart failure in mice. Circulation 123: 1073– 1082, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, Penninger JM, Molkentin JD. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res 89: 20– 25, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Song X, Kusakari Y, Xiao CY, Kinsella SD, Rosenberg MA, Scherrer-Crosbie M, Hara K, Rosenzweig A, Matsui T. mTOR attenuates the inflammatory response in cardiomyocytes and prevents cardiac dysfunction in pathological hypertrophy. Am J Physiol Cell Physiol 299: C1256– C1266, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Taegtmeyer H. Cardiac metabolism as a target for the treatment of heart failure. Circulation 110: 894– 896, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Taegtmeyer H, Overturf ML. Effects of moderate hypertension on cardiac function and metabolism in the rabbit. Hypertension 11: 416– 426, 1988 [DOI] [PubMed] [Google Scholar]
- 34. Wende AR, Schaeffer PJ, Parker GJ, Zechner C, Han DH, Chen MM, Hancock CR, Lehman JJ, Huss JM, McClain DA, Holloszy JO, Kelly DP. A role for the transcriptional coactivator PGC-1α in muscle refueling. J Biol Chem 282: 36642– 36651, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Zhang D, Contu R, Latronico MV, Zhang JL, Rizzi R, Catalucci D, Miyamoto S, Huang K, Ceci M, Gu Y, Dalton ND, Peterson KL, Guan KL, Brown JH, Chen J, Sonenberg N, Condorelli G. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J Clin Invest 120: 2805– 2816, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhu Y, Pereira RO, O'Neill BT, Riehle C, Ilkun O, Wende AR, Rawlings TA, Zhang YC, Zhang Q, Klip A, Shiojima I, Walsh K, Abel ED. Cardiac PI3K-Akt impairs insulin-stimulated glucose uptake independent of mTORC1 and GLUT4 translocation. Mol Endocrinol 27: 172– 184, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhu Y, Pires KM, Whitehead KJ, Olsen CD, Wayment B, Zhang YC, Bugger H, Ilkun O, Litwin SE, Thomas G, Kozma SC, Abel ED. Mechanistic target of rapamycin (mtor) is essential for murine embryonic heart development and growth. PLoS One 8: e54221, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]