Abstract
Mutations in cardiac myosin binding protein C (MyBP-C) are a common cause of familial hypertrophic cardiomyopathy (FHC). The majority of MyBP-C mutations are expected to reduce MyBP-C expression; however, the consequences of MyBP-C deficiency on the regulation of myofilament function, Ca2+ homeostasis, and in vivo cardiac function are unknown. To elucidate the effects of decreased MyBP-C expression on cardiac function, we employed MyBP-C heterozygous null (MyBP-C+/−) mice presenting decreases in MyBP-C expression (32%) similar to those of FHC patients carrying MyBP-C mutations. The levels of MyBP-C phosphorylation were reduced 53% in MyBP-C+/− hearts compared with wild-type hearts. Skinned myocardium isolated from MyBP-C+/− hearts displayed decreased cross-bridge stiffness at half-maximal Ca2+ activations, increased steady-state force generation, and accelerated rates of cross-bridge recruitment at low Ca2+ activations (<15% and <25% of maximum, respectively). Protein kinase A treatment abolished basal differences in rates of cross-bridge recruitment between MyBP-C+/− and wild-type myocardium. Intact ventricular myocytes from MyBP-C+/− hearts displayed abnormal sarcomere shortening but unchanged Ca2+ transient kinetics. Despite a lack of left ventricular hypertrophy, MyBP-C+/− hearts exhibited elevated end-diastolic pressure and decreased peak rate of LV pressure rise, which was normalized following dobutamine infusion. Furthermore, electrocardiogram recordings in conscious MyBP-C+/− mice revealed prolonged QRS and QT intervals, which are known risk factors for cardiac arrhythmia. Collectively, our data show that reduced MyBP-C expression and phosphorylation in the sarcomere result in myofilament dysfunction, contributing to contractile dysfunction that precedes compensatory adaptations in Ca2+ handling, and chamber remodeling. Perturbations in mechanical and electrical activity in MyBP-C+/− mice could increase their susceptibility to cardiac dysfunction and arrhythmia.
Keywords: cardiomyopathy, myosin binding protein C, contractile function, cross-bridge kinetics, myocardium
mutations in cardiac myosin binding protein C (MyBP-C) are among the most common causes of familial hypertrophic cardiomyopathy (FHC), an autosomal dominant disease with variable disease onset, penetrance, and phenotypic presentation (40). Millions of people worldwide carrying MyBP-C mutations are at significantly higher risk for developing cardiac disease and sudden cardiac death (48), and the majority of MyBP-C mutations are expected to produce C-terminal truncated proteins. However, to date these truncated proteins have not been found in myocardial samples isolated from patients with MyBP-C mutations (25, 34, 58), but rather a decrease in the total amount of MyBP-C has been reported, likely due to degradation of unstable mutant MyBP-C by cell surveillance mechanisms (8). Despite an intense research effort in the last few years, the mechanisms by which mutations in MyBP-C cause disease, and specifically mutations that cause a decrease in MyBP-C expression in the heart, are still not understood.
MyBP-C is a thick filament protein localized in regular intervals in the C zone of the A band of the myofilament and regulates myofilament contractile mechanics through its phosphorylation via interactions with actin and myosin (reviewed in Refs. 2, 3, 26, 43). The absence of MyBP-C leads to accelerated cross-bridge kinetics at the myofilament level (29, 50) and to myocyte disarray and fibrosis at the tissue level (7, 22). At the organ level, MyBP-C knockout (MyBP-C−/−) mice exhibit profound left ventricular (LV) hypertrophy (4, 7, 22, 41, 44) and early-onset contractile dysfunction (4, 7, 22). In contrast, heterozygous MyBP-C (MyBP-C+/−) null mice, which express ∼25% less MyBP-C than wild-type (WT) mice, present with very mild or no hypertrophy (7, 22) yet still display early-onset mechanical dysfunction (15). Thus the mechanisms by which altered MyBP-C expression in the sarcomere contribute to altered whole organ contractile dysfunction remain elusive.
The primary effect of MyBP-C mutations is expected to manifest as a defect in the regulation of myofilament function in response to Ca2+ activation, and indeed, mechanical experiments on myocardial preparations isolated from hearts of patients carrying MyBP-C mutations have shown decreased force-generating capacities at maximal Ca2+ activations and increased force-generating capacities at low levels of Ca2+ activation (34, 58). Furthermore, there is also evidence that MyBP-C is dephosphorylated in hearts expressing MyBP-C mutations (13, 58); however, it is not clear how this dephosphorylation, along with decreased MyBP-C expression, contributes to cardiac dysfunction. Also, it is not known if altered myofilament behavior in MyBP-C-deficient hearts causes downstream compensatory changes in signaling cascades that modulate the phosphorylation state of other key regulatory myofilament and Ca2+-handling proteins in the myocyte that could contribute to functional deficits observed at the organ level. Finally, because MyBP-C phosphorylation is crucial for modulating cardiac contractility (2, 3, 26, 43), it is important to investigate whether MyBP-C deficiency impairs the contractile response to β-adrenergic stimulation.
The goal of the present study is to define the functional link between MyBP-C deficiency at the sarcomere and changes in the molecular mechanisms that modulate the function of the myofilament and Ca2+-handling machinery, which together regulate in vivo cardiac contractile function. We employed adult MyBP-C+/− mice, which exhibit levels of MyBP-C deficiency similar to those of hearts of human patients possessing heterozygous MyBP-C mutations, and measured in vitro contractile function in skinned myocardium and intact cardiomyocyte and in vivo cardiac hemodynamic function and electrical activity. The utilization of a comprehensive integrative approach in this study will allow us to gain a deeper insight into the pathogenesis of MyBP-C-related FHC. Our data demonstrate that decreased MyBP-C expression in MyBP-C+/− skinned myocardium causes a significant acceleration of rates of cross-bridge recruitment, resulting in increased force-generating capacity at low Ca2+ activations compared with WT skinned myocardium. Furthermore, the remaining MyBP-C in MyBP-C+/− myocardium is less phosphorylated than WT myocardium, which together with decreased total MyBP-C expression likely contributes to a decrease in cross-bridge stiffness. Treatment of skinned myocardium with protein kinase A (PKA) accelerated cross-bridge kinetics in both WT and MyBP-C+/− skinned myocardium; however, the effect was more pronounced in WT skinned myocardium, thereby abrogating basal differences in cross-bridge behavior between the two groups. Despite a lack of LV hypertrophy, MyBP-C+/− hearts displayed systolic and diastolic hemodynamic dysfunction compared with WT hearts in basal conditions, which was ameliorated following β-agonist infusion. Furthermore, MyBP-C+/− animals displayed altered cardiac electrical activity as evidenced by prolonged ventricular contraction and repolarization. Collectively, these data suggest that altered myofilament function due to MyBP-C deficiency in MyBP-C+/− mice can lead to impaired in vivo cardiac function and an increased risk for sudden cardiac death (SCD) even in the absence of LV hypertrophy. Normalization of contractile function in MyBP-C+/− myofilaments and intact hearts following β-adrenergic stimulation suggests that basal contractile dysfunction in MyBP-C+/− hearts is at least partly mediated by impaired MyBP-C phosphorylation.
MATERIALS AND METHODS
Ethical Approval and Experimental Model
This study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals [DHEW Publication No. (NIH) 85-23, Revised 1996, Office of Science and Health Reports, DRR/NIH, Bethesda, MD 20205], and the procedures of anesthesia, surgery, and general care of the animals were approved by the Institutional Animal Care and Use Committee at Case Western Reserve University. Adult male WT and MyBP-C+/− mice of the SV/129 strain at ∼6 mo of age were used (22).
Echocardiography
Myocardial function was evaluated by echocardiography at ∼6 mo of age (n = 7–10 per group). A Sequoia C256 system (Siemens Medical, Malvern, PA) with a 15-MHz linear array transducer was used as previously described (38) with anesthetized mice (1.5–2.0% isoflurane). Two-dimensional, two-dimensional guided M-mode, and Doppler echocardiographic studies of aortic and transmitral flows were performed via parasternal and foreshortened apical windows. Wall thickness and end-systolic (ESD) and end-diastolic dimensions (EDD) were measured, and ejection fraction (EF) and fractional shortening (FS) were calculated as previously described (38).
Hemodynamic Measurements
In vivo LV contractile properties were assessed at ∼6 mo of age (n = 9 per group). Mice were anesthetized (1.5–2.0% isoflurane), intubated, and ventilated. A Millar 1.4-F microtip pressure-volume transducer catheter (Millar Instruments, Houston, TX) was introduced via the right carotid artery as previously described (45). After baseline (BL) recording, 10 μg/g body wt of dobutamine (Dob) was administered through the jugular vein and the peak β-adrenergic response was analyzed at 5 min postinjection. Endpoints related to heart rate (HR), LV pressure, EF, peak rate of LV pressure rise (dP/dtmax), and the relaxation time constant τ were determined with the aid of PVAN/Chart5 software (Millar Instruments).
Electrocardiogram
The telemetric ambulatory electrocardiogram (ECG) recordings were obtained at ∼6 mo of age (n = 8–11 per group) with implantable transmitters (ETA-F10; Data Sciences International, St. Paul, MN). Mice were anesthetized with isoflurane, and a midline incision was made along the spine. The implantable wireless radiofrequency transmitter was aseptically inserted into a subcutaneous tissue pocket in the back, and the leads were placed at the right shoulder and lower left chest and sutured to the muscle. Following a recovery period from the transmitter implant surgery, the mice were placed in cages overlying a receiver for transmission of ECG signals to a computer for display and analysis. Subsequent to a 1- to 2-h acclimatization period when mice rested in their cages, telemetered ECG tracings were obtained in conscious mice during quiet awake time at rest for a total period of 2 h. Measurements of durations and intervals of waveforms taken from ∼8 min of undisturbed tracings (monitored by activity channel recorded simultaneously) were performed with the aid of Ponemah software (Data Sciences International). Determination of QRS and QT segments was done according to previous studies (10, 46). The corrected QT interval (QTc) was calculated using the Bazett formula: QTc = QT/(RR/100)1/2, which is specifically modified for mouse ECG as previously described (36).
Histological Assessment of Cardiac Morphology
Hearts were harvested (n = 7) for histological evaluation of chamber modifications, potential fibrosis, and myocyte hypertrophy. All hearts were washed in cardioplegic buffer (containing in mM: 110 NaCl, 16 MgCl2, 16 KCl, 10 NaHCO3, 5 dextrose, and 1.2 CaCl2), formalin fixed, and paraffin embedded. The hearts were sliced at mid-LV level using a slicer matrix, and the sections were cut at 5-μm thickness using a microtome. The cross sections were stained with either Masson's trichrome or hematoxylin and eosin (H&E) stain for gross morphology and fibrosis or cellular hypertrophy, respectively.
Myocyte Isolation and In Vitro Shortening and Ca2+ Transients
Ventricular cardiomyocytes were isolated enzymatically from mouse hearts (n = 4–5 per group) using an enzymatic dispersion technique described previously (56). Sarcomere shortening was assessed using a video-based sarcomere length detection system (IonOptix, Milton, MA) (16). To measure intracellular Ca2+ transients, myocytes were loaded with 2 μM indo-1 AM (Invitrogen, Carlsbad, CA) and 0.025% (wt/wt) Pluronic F-127 (Invitrogen) for 20 min at room temperature. The intracellular indo-1 was excited at 355 nm, and the fluorescence emitted at 405 and 485 nm was collected by two matched photomultiplier tubes. Data were filtered at 200 Hz and sampled at 1 kHz. The ratio of the intensity of fluorescence emitted at 405 nm over that at 485 nm (F405/485) was calculated after subtraction of background fluorescence and used to compare the time course and amplitude of intracellular Ca2+ transients between groups of myocytes. The emission field was restricted to a single cell with the aid of an adjustable window (61). Sarcomere shortening and Ca2+ transients were initiated by field stimulation at 2 Hz at room temperature with 1.8 mM Ca2+ and were measured simultaneously. All analysis of sarcomere shortening (n = 55–69 per group) and Ca2+ transients (n = 50–68 per group) was done using IonOptix software (IonOptix).
Myofilament Contractile Function
Solutions for skinned myocardium experiments.
Solution compositions were calculated using the computer program of Fabiato (17) and stability constants listed by Godt and Lindley (19) corrected to pH 7.0 and 22°C. All solutions contained (in mM) 100 N,N-bis(2-hydroxy-ethyl)-2-aminoethanesulfonic acid (BES), 15 creatine phosphate, 5 dithiothreitol, 1 free Mg2+, and 4 MgATP. pCa 9.0 solution contained (in mM) 7 EGTA and 0.02 CaCl2; pCa 4.5 contained 7 EGTA and 7.01 CaCl2; and preactivating solution contained 0.07 EGTA. Ionic strength of all solutions was adjusted to 180 mM with potassium propionate. Solutions containing different amounts of free Ca2+ were prepared by mixing appropriate volumes of solutions of pCa 9.0 and pCa 4.5.
Apparatus and experimental protocols.
Skinned multicellular ventricular myocardium for mechanical experiments was prepared and attached to the arms of a position motor and force transducer as previously described (9). Motor position and force signals were sampled using SLControl software (6) and saved to computer files for later analysis. All mechanical measurements were performed at 22°C, and sarcomere length was set to 2.1 μm. Data presented for all of the baseline mechanical experiments were the average of 20 fibers from 5 mice per group, and 12 fibers from 4 mice per group for PKA experiments.
Force-pCa Relationships
Methods for obtaining and analysis of force-pCa relationships are described in detail elsewhere (9). Briefly, each myocardial preparation was allowed to develop steady force in solutions of varying free Ca2+ concentration ([Ca2+]). The difference between steady-state force and the force baseline obtained after the 20% slack step was measured as the total force at that free [Ca2+]. Active force was then calculated by subtracting Ca2+-independent force in solution of pCa 9.0 from the total force and was normalized to the cross-sectional area of the preparation, which was calculated from the width of the preparations assuming a cylindrical cross section. Force-pCa relationships were constructed by expressing submaximal force (P) at each pCa as a fraction of maximal force (Po) determined at pCa 4.5, i.e., P/Po. The apparent cooperativity in the activation of force development was inferred from the steepness of the force-pCa relationship and was quantified using a Hill plot transformation of the force-pCa data. The force-pCa data were fit using the equation P/Po = [Ca2+]n/(kn + [Ca2+]n), where n is the Hill coefficient and k is the [Ca2+] required for half-maximal activation (i.e., pCa50).
Rate of Force Development
The rate constant of force development (ktr) was measured in skinned myocardium as a measure of the rate of transitions of cross bridges between weak-binding, non-force-generating states and strong-binding, force-generating states using a release-restretch protocol (51). Each skinned preparation was transferred from relaxing to an activating solution containing Ca2+ (sufficient for activation of ∼10–25% of maximal force) and allowed to generate steady-state force. The myocardial preparation was rapidly (<2 ms) slackened by 20% of its original length, resulting in a rapid reduction of force that was followed by a brief period of unloaded shortening (10 ms), after which the preparation was rapidly restretched to its original length. ktr was estimated by linear transformation of the half-time of force redevelopment, i.e., ktr = 0.693/t1/2, as described previously (51).
Stretch Activation Experiments
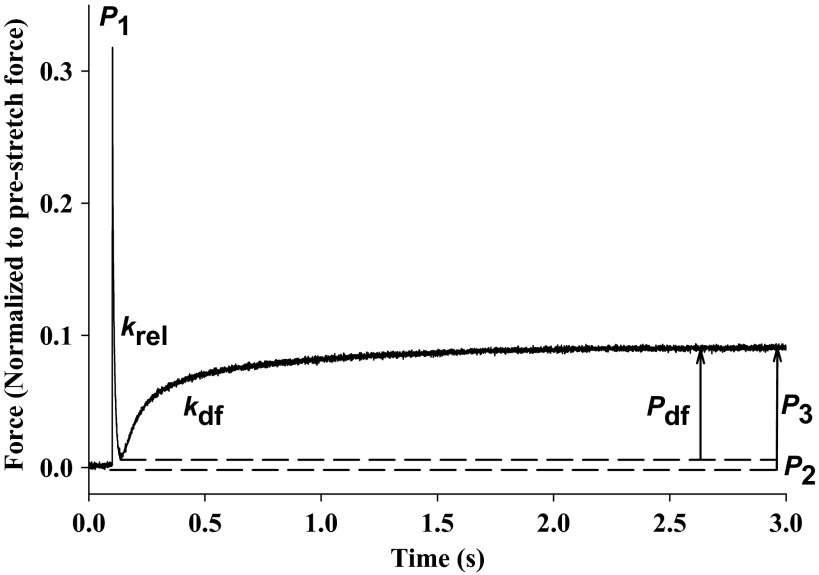
For stretch activation experiments, fiber length in relaxing solution was adjusted to achieve a sarcomere length of ∼2.1 μm for measurement of initial isometric force and for subsequent imposition of stretch. To evoke stretch activation, a rapid stretch (∼10 muscle lengths per second) of 1% of muscle length was imposed on fibers that were activated to develop submaximal forces equivalent to ∼10–25% maximal force (Po). Submaximal Ca2+-activated force (P) was expressed as a fraction of Po generated at pCa 4.5, i.e., P/Po. The method used for measuring the stretch activation variables has been described in detail previously (52). The amplitudes of the phases of the stretch activation responses were measured as follows: P1, measured from prestretch steady-state force to the peak of phase 1; P2, measured from prestretch steady-state force to the minimum force value at the end of phase 2; P3, measured from prestretch steady-state force to the peak value of delayed force; and Pdf, difference between P3 and P2. All amplitudes were normalized to the prestretch Ca2+-activated force to allow comparisons between preparations. Apparent rate constants were calculated for phase 2 (krel, s−1) between the peak of phase 1 and the minimum of phase 2 and for phase 3 (kdf, s−1) from the beginning of force reuptake following phase 2 to the completion of delayed force development. krel was obtained by fitting the time course trace with a single exponential, and kdf was estimated by linear transformation of the half-time of force redevelopment as previously described (52).
A representative stretch activation response in WT skinned myocardium is illustrated in Fig. 1, in which a stretch of 1% of initial length was imposed on a muscle generating Ca2+-activated force that was ∼25% maximal. The characteristic features of the stretch activation response have been elucidated in detail elsewhere (5, 20, 52). Briefly, following achievement of steady-state force generation, acute stretch elicits an immediate increase in force (P1), due to distortion of attached cross bridges. The force transient then begins to rapidly decay due to the detachment of strained cross bridges and reaches a minimum amplitude (P2), which can be positive or negative (i.e., below initial prestretch force). Next, force begins to increase due to a recruitment of new cross bridges into strongly bound states mediated by an increase in muscle length and eventually reaches a new steady-state level (P3), which is sustained over a period of several seconds before decaying back to the original isometric force.
Fig. 1.
Stretch activation responses in murine myocardium. Shown is a representative force transient following a stretch of 1% of muscle length recorded from wild-type (WT) skinned myocardium activated with Ca2+ to a prestretch isometric force of ∼25% maximal. The transient is normalized to prestretch force, and the recorded variables are labeled on the trace and described in detail in the text. P1, P2, P3, and Pdf are phases of stretch activation responses; krel represents the rate of cross-bridge detachment; kdf represents the rate of delayed cross-bridge recruitment.
In experiments assessing the effects of PKA (catalytic subunit of bovine PKA; Sigma) on mechanical properties, skinned preparations were first incubated for 1 h (22°C) in pCa 9.0 solution containing PKA (0.25 U/μl). Mechanical properties were then measured as described above. Because PKA treatment decreased the Ca2+ sensitivity of force in both WT and MyBP-C+/− myocardium, it was necessary to use a pCa solution with a slightly higher [Ca2+] to match the PKA untreated baseline isometric force.
Muscle Fiber Stiffness
Skinned myocardium was incubated in a pCa solution that yielded forces approximately half of maximal, and when the muscle fiber reached a steady-state isometric force, the muscle length was increased in a steplike fashion by 0.25, 0.5, 1.0, 1.5, 2.0, and 2.5% of initial muscle length (ΔL). Cross-bridge stiffness associated with the number of strongly bound cross bridges before stretch was estimated from the relationship between length change in ΔL and the peak force response following stretch (P1). Stiffness is represented as the slope of the linear regression of the relationship between P1 and ΔL.
Quantification of Protein Expression and Phosphorylation
Ventricular tissue (n = 6–10 per group) was homogenized in buffer containing 20 mM Tris-base, 137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2-6H2O, 1 mM (K2)EDTA, 10% glycerol, and 1% Triton X-100, pH 7.8, with protease and phosphatase inhibitor cocktails (Sigma). Protein concentration was quantified by bicinchoninic acid (BCA) protein assay (Thermo Scientific, Lake Barrington, IL). Laemmli buffer (62.5 mM Tris·HCl, 10% glycerol, 2% SDS, 0.01% bromophenol blue, 5% β-mercaptoethanol, pH 6.8) was added to the samples. Ten micrograms of nonboiled protein were loaded onto 7.5% Tris·HCl gels (Bio-Rad, Hercules, CA) for analysis of sarco(endo)plasmic reticulum (SR) Ca2+-ATPase 2 (SERCA2) and Na+-Ca2+ exchanger 1 (NCX1) levels, and 14 μg of nonboiled protein were loaded onto 3–8% Tris-acetate gels (Bio-Rad) for ryanodine receptor 2 (RyR2). Samples were boiled for 10 min, and 10 μg of protein were loaded onto 10% Tris·HCl gels (Bio-Rad) for phosphorylated (p-)Ser273/Ser282/Ser302 MyBP-C (pMyBP-C273, pMyBP-C282, and pMyBP-C302) as well as MyBP-C (14), and 10 μg of boiled samples were loaded onto 15% Tris·HCl gels (Bio-Rad) for phosphorylated (p-)Ser23/24 troponin I (pTnI) as well as TnI, and phosphorylated (p-)Ser16/Thr17 phospholamban (pPLB16 and pPLB17) as well as PLB. Heat shock chaperone 70 (HSC70) was chosen to serve as a loading control. For RyR2, gels were run at 150 V for 1 h 45 min and then transferred to polyvinylidene difluoride (PVDF) membranes at 30 V at 4°C for 30 h and 70 V for 1 h. For the rest of the target proteins, gels were run at 200 V for 1 h and then transferred to PVDF membranes at 100 V for 45 min. Membranes were incubated at 4°C overnight with 1:1,000 anti-MyBP-C (sc-67353; Santa Cruz Biotechnology), 1:1,000 anti-pMyBP-C273, 1:1,000 anti-pMyBP-C282, 1:1,000 anti-pMyBP-C302 (custom-made from 21st Century Biochemicals, Marlborough, MA), 1:1,000 anti-TnI (4002S; Cell Signaling Technology, Danvers, MA), 1:1,000 anti-pTnI (4004S; Cell Signaling Technology), 1:1,000 anti-SERCA2 (MA3-919; Thermo Scientific), 1:1,500 anti-NCX1 (MA1-4672; Thermo Scientific), 1:1,000 anti-PLB (05-205; Millipore, Billerica, MA), 1:1,000 anti-pPLB16 (07-052; Millipore), 1:5,000 anti-pPLB17 (A010-13; Badrilla, Leeds, UK), at room temperature for 30 min with 1:2,000 anti-HSC70 (sc-7298; Santa Cruz Biotechnology), and at room temperature overnight with 1:300 anti-RyR2 (MA3-916; Thermo Scientific), followed by incubations with the appropriate secondary antibodies. Membranes were incubated with chemiluminescence reagents (Thermo Scientific) and exposed to films. Densitometry of bands was determined using ImageJ software (NIH).
To assess regional MyBP-C expression in the hearts of MyBP-C+/− mice, LV tissues were isolated from the apex, mid-LV, and base (5–6 hearts per region). Tissues were then prepared and loaded onto gels for quantification of MyBP-C expression by Western blot as described above.
The protein content of cardiac myosin heavy chain (MHC)-α and -β were determined via gel electrophoresis as previously described (62). Ventricular tissue (n = 5) was homogenized in radioimmunoprecipitation assay buffer. The protein concentration was quantified by BCA protein assay (Thermo Scientific). Ten micrograms of protein were loaded onto a 6% acrylamide gel cross-linked with N-N′ diallyltartardiamide as previously described (62). The gel was run at 10-mA constant current for 19 h at 4°C. Bands were detected using Silver Stain Plus (Bio-Rad) per manufacturer's protocol. The densitometry was analyzed using the GelBandFitter analysis program as previously described (37).
For the rest of the myofibrillar proteins, the ventricular tissue (n = 4) was harvested and purified for myofibrillar proteins using a protocol previously described (30). Ten micrograms of protein were loaded onto 10% Tris-glycine mini gels, and the gels were run at 150 V for 1.5 h, followed by Pro-Q Diamond phosphoprotein gel staining (Invitrogen) per manufacturer's protocol. The same gel was then stained with Silver Stain Plus (Bio-Rad) per manufacturer's protocol for total protein expression. Densitometry of bands was determined using ImageJ (NIH). The degree of phosphorylation of a given protein was determined by the slope of a first-order linear regression generated on basis of the Pro-Q optical density and protein content loaded as previously described (53).
To determine the effect of PKA treatment on the level of phosphorylation of MyBP-C and other myofilament proteins, skinned myocardium was isolated from WT and MyBP-C+/− hearts and incubated with PKA (1 U/μl) for 1 h at 30°C as previously described (57). The samples were then denatured with Laemmli buffer, and the protein content was quantified using BCA protein assay. Ten micrograms of boiled samples were loaded onto 4–20% Tris-glycine mini gels, and the gels were run at 150 V for 1.5 h, followed by Pro-Q Diamond phosphoprotein gel staining per manufacturer's protocol. The same gel was then stained with Coomassie blue for total protein expression.
Statistical Analysis
All statistical analyses were performed using SigmaStat software. Regional differences in MyBP-C expression between the apex, mid-LV, and base of MyBP-C+/− myocardium were determined using one-way ANOVA. All the other analyses were done via Student's t-test. Significance was established at P < 0.05. Data are means ± SE.
RESULTS
Myofilament Protein Content and Phosphorylation
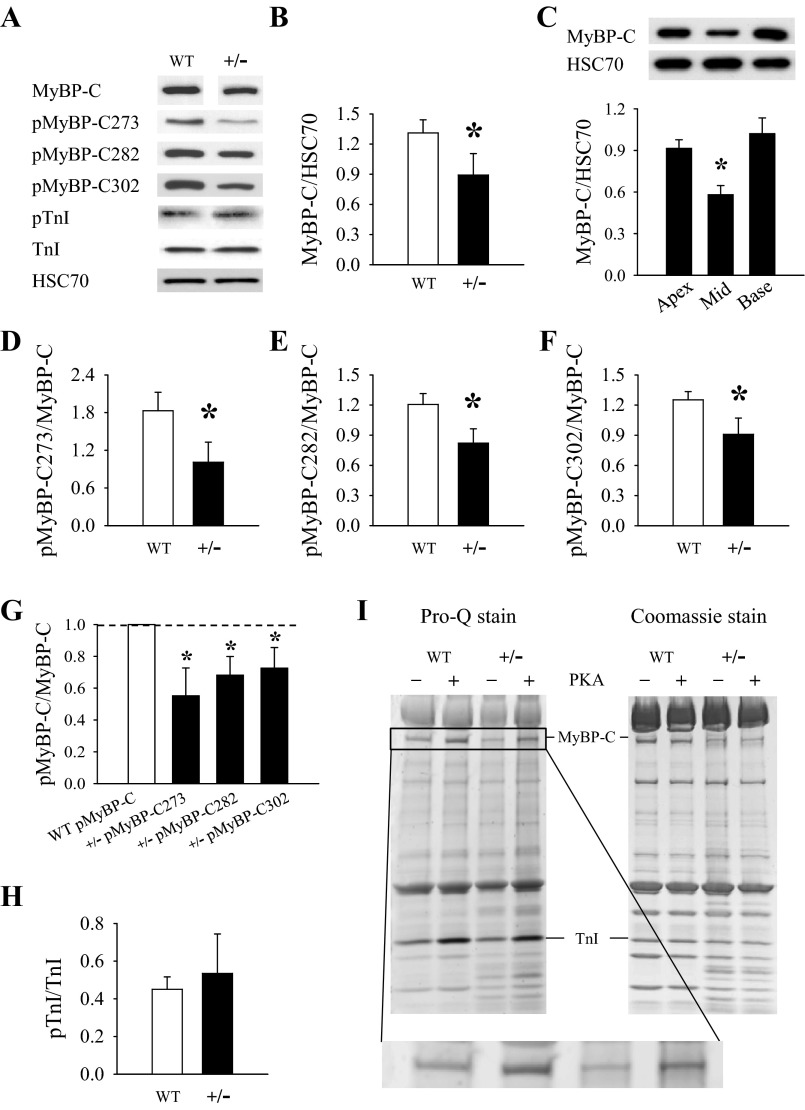
The expression and phosphorylation levels of MyBP-C and other regulatory myofibrillar proteins were quantified in adult animals at age ∼6 mo. The overall expression of MyBP-C in MyBP-C+/− hearts was 32% lower than in WT hearts (Fig. 2B), which was greater than the 25% decline we previously observed in younger MyBP-C+/− mice 2–3 mo of age (15). Further analysis of the pattern of MyBP-C expression in the LV of MyBP-C+/− hearts revealed that MyBP-C expression (normalized to HSC70) was significantly lower in samples isolated from the mid-LV region compared with samples isolated from the apex and base regions (Fig. 2C). Although it is difficult to directly compare MyBP-C expression values derived from a regional LV analysis and a whole LV analysis, the results suggest that MyBP-C haploinsufficiency causes a greater decrease in MyBP-C expression in the mid-LV region compared with the apex and base in MyBP-C+/− hearts.
Fig. 2.
Expression and phosphorylation of myofilament proteins. A: representative Western blots of cardiac myosin binding protein C (MyBP-C), MyBP-C phosphorylated at Ser273 (MyBP-C273), Ser282 (pMyBP-C282), and Ser302 (pMyBP-C302), phosphorylated troponin I (pTnI), troponin I (TnI), and the loading control heat shock chaperone 70 (HSC70). Representative bands depicting MyBPC expression in WT and MyBP-C+/− samples were obtained from the same gel; however, the images have been separated to convey that the gel lanes were not contiguous. B: overall expression of MyBP-C (normalized to HSC70) in MyBP-C+/− whole left ventricular (LV) homogenates (6–10 hearts/group). C: regional differences in MyBP-C expression in LV samples (5–6 hearts/region) isolated from the apex, mid-LV, and base of MyBP-C+/− hearts. Representative Western blots are shown at top and quantified protein expression via densitometry at bottom. D–F: quantification of MyBP-C and TnI phosphorylation in WT and MyBP-C+/− myocardium: pMyBP-C273 (D), pMyBP-C282 (E), and pMyBP-C302 (F), all normalized to MyBP-C. G: data for MyBP-C phosphorylation at specific phosphorylation residues in the MyBP-C+/− group shown in D–F are expressed as fold changes to their WT counterparts (set to 1). MyBP-C phosphorylation levels in MyBP-C+/− samples are decreased at each of the phosphorylation residues compared with WT. H: levels of pTnI (normalized to TnI). I: representative gel images of myofilament proteins before and after PKA treatment. Pro-Q staining of phosphorylated proteins is shown at left and Coomassie-stained total protein expression at right. Magnified Pro-Q-stained MyBP-C bands are shown in inset. *P < 0.05 compared with apex and base LV samples via 1-way ANOVA (C) or compared with WT via Student's t-test (B, D–G).
The phosphorylation levels of the remaining MyBP-C (normalized to MyBP-C expression) at residues Ser273 (Fig. 2D), Ser282 (Fig. 2E), and Ser302 (Fig. 2F) were all decreased in MyBP-C+/− myocardium compared with WT myocardium, by 45, 32, and 27%, respectively. Figure 2G shows the relative ratio of MyBP-C phosphorylation at each phosphorylation residue in MyBP-C+/− hearts compared with its WT counterpart (which is set to 1). TnI expression (normalized to HSC70) and phosphorylation (normalized to TnI expression) (Fig. 2H) were not different between groups. Consistent with previous studies (15), we observed no differences between groups in the composition of MHC isoforms or the expression and phosphorylation of troponin T or myosin regulatory light chain (data not shown), which were quantified by silver staining and Pro-Q phosphostaining of SDS-PAGE gels.
To assess the effects of PKA treatment on MyBP-C and TnI phosphorylation, skinned myocardium preparations isolated from WT and MyBPC+/− hearts were incubated with PKA before electrophoresis, and the representative Pro-Q phosphostained and Coomassie-stained gel images are shown in Fig. 2I. PKA treatment resulted in significant and comparable increases in the phosphorylation of MyBP-C and TnI in both WT and MyBP-C+/− myocardium, suggesting that MyBP-C haploinsufficiency does not impair the phosphorylation of the main myofilament targets of PKA.
Steady-State Force and Dynamic Cross-Bridge Kinetics Before and After PKA Treatment
Skinned myocardium was isolated from WT and MyBP-C+/− hearts to determine steady-state force-pCa relationships and for measurements of cross-bridge kinetics using a slack-restretch maneuver and the dynamic response to acute stretch. The minimum and maximum force generation in pCa 9.0 and 4.5 solutions, respectively, were not different between groups. The Ca2+ sensitivity of force generation (pCa50) and Hill coefficient (nH) were also not different between groups (Table 1). However, incubation of skinned myocardium isolated from MyBP-C+/− hearts in pCa solutions that produced <15% of maximal force (pCa 6.1 and pCa 6.0) resulted in greater force production compared with WT skinned myocardium (Table 1). After PKA treatment, both WT and MyBP-C+/− skinned myocardium displayed a similar decrease in pCa50 (Table 1), without a change in maximal and minimal steady-state force or nH (Table 1).
Table 1.
Steady-state mechanical properties of skinned fibers isolated from WT and MyBP-C+/− myocardium
| Fmin, mN/mm2 | Fsub (P/Po) | Fmax, mN/mm2 | pCa50 | nH | |
|---|---|---|---|---|---|
| WT (−PKA) | 0.54 ± 0.38 | 21.89 ± 1.51 | 5.81 ± 0.02 | 4.41 ± 0.59 | |
| pCa 6.1 | 0.04 ± 0.01 | ||||
| pCa 6.0 | 0.08 ± 0.01 | ||||
| WT (+PKA) | 0.45 ± 0.39 | 20.57 ± 1.42 | 5.70 ± 0.02† | 4.24 ± 0.62 | |
| MyBP-C+/− (−PKA) | 0.73 ± 0.29 | 20.67 ± 1.94 | 5.83 ± 0.03 | 4.08 ± 0.64 | |
| pCa 6.1 | 0.08 ± 0.01* | ||||
| pCa 6.0 | 0.13 ± 0.01* | ||||
| MyBP-C+/− (+PKA) | 0.57 ± 0.26 | 19.83 ± 1.79 | 5.73 ± 0.03† | 3.97 ± 0.53 |
Data are means ± SE of properties in wild-type (WT) and myosin binding protein C heterozygous (MyBP-C+/−) mice with (+PKA) or without protein kinase A (−PKA) treatment. Fmin, Ca2+-independent force at pCa 9.0; Fsub, submaximal Ca2+-activated force at pCa 6.1 and 6.0 (expressed as P/Po, where P is submaximal Ca2+-activated force and Po is maximal force); Fmax, maximal Ca2+-activated force at pCa 4.5; pCa50, pCa required for half-maximal force generation; nH, Hill coefficient for total force-pCa relationship. Data are averages from 20 (−PKA) and 12 fibers/group (+PKA).
P < 0.05 compared with WT.
P< 0.05 compared with untreated (−PKA).
Cross-bridge kinetics measurements in skinned WT and MyBP-C+/− myocardium were performed in pCa solutions that yielded similar isometric forces (P/Po = ∼0.10 and 0.25) to account for slightly increased Ca2+ sensitivity of force in MyBP-C+/− fibers. Skinned myocardium isolated from MyBP-C+/− hearts exhibited accelerated rates of force development (ktr) compared with WT (Table 2). A representative ktr trace recorded from WT and MyBP-C+/− skinned myocardium is presented in Fig. 3A. After PKA treatment, both WT and MyBP-C+/− skinned myocardium displayed accelerated ktr; however, the relative increase was greater in WT myocardium such that basal differences in ktr were no longer apparent following PKA treatment (Table 2).
Table 2.
Cross-bridge kinetics of skinned fibers isolated from WT and MyBP-C+/− myocardium
| Force (P/Po) | ktr, s−1 | kdf, s−1 | krel, s−1 | P2 | P3 | ||
|---|---|---|---|---|---|---|---|
| WT (−PKA) | 0.09 ± 0.02 | 1.99 ± 0.16 | 2.48 ± 0.24 | 204 ± 22 | 0.03 ± 0.01 | 0.16 ± 0.01 | 0.13 ± 0.01 |
| 0.22 ± 0.03 | 3.59 ± 0.20 | 4.96 ± 0.29 | 193 ± 21 | 0.03 ± 0.01 | 0.12 ± 0.01 | 0.09 ± 0.01 | |
| WT (+PKA) | 0.13 ± 0.04 | 3.68 ± 0.33† | 4.97 ± 0.51† | 253 ± 17† | −0.03 ± 0.02† | 0.18 ± 0.02 | 0.21 ± 0.02† |
| 0.25 ± 0.04 | 5.90 ± 0.52† | 9.02 ± 0.67† | 247 ± 15† | −0.03 ± 0.02† | 0.14 ± 0.02 | 0.17 ± 0.02† | |
| MyBP-C+/− (−PKA) | 0.11 ± 0.02 | 3.05 ± 0.18* | 4.01 ± 0.29* | 241 ± 26 | 0.00 ± 0.01* | 0.16 ± 0.01 | 0.16 ± 0.01* |
| 0.24 ± 0.03 | 4.88 ± 0.23* | 7.37 ± 0.38* | 230 ± 24 | 0.01 ± 0.01* | 0.12 ± 0.01 | 0.12 ± 0.01* | |
| MyBP-C+/− (+PKA) | 0.15 ± 0.04 | 4.13 ± 0.35† | 5.69 ± 0.54† | 287 ± 18† | −0.04 ± 0.02† | 0.18 ± 0.02 | 0.22 ± 0.01† |
| 0.28 ± 0.04 | 6.55 ± 0.56† | 10.11 ± 0.79† | 269 ± 16† | −0.04 ± 0.02† | 0.14 ± 0.02 | 0.18 ± 0.01† |
Data are means ± SE of measurements performed in pCa solutions yielding similar isometric forces (P/Po = ∼0.10 and 0.25). ktr, rate constant of force development after slack-restretch maneuver; kdf, rate constant of force development during stretch activation; krel, rate constant of force decay during stretch activation; P2, the change between prestretch steady-state force and the minimum force value at the end of phase 2 of stretch-activation protocol; P3, the change between prestretch steady-state force and the peak value of delayed force of stretch-activation protocol; Pdf: the difference between P3 and P2. Comparisons were made at equivalent levels of activation. Data are averages from 20 (−PKA) and 12 fibers/group (+PKA).
P < 0.05 compared with WT.
P < 0.05 compared with untreated (−PKA).
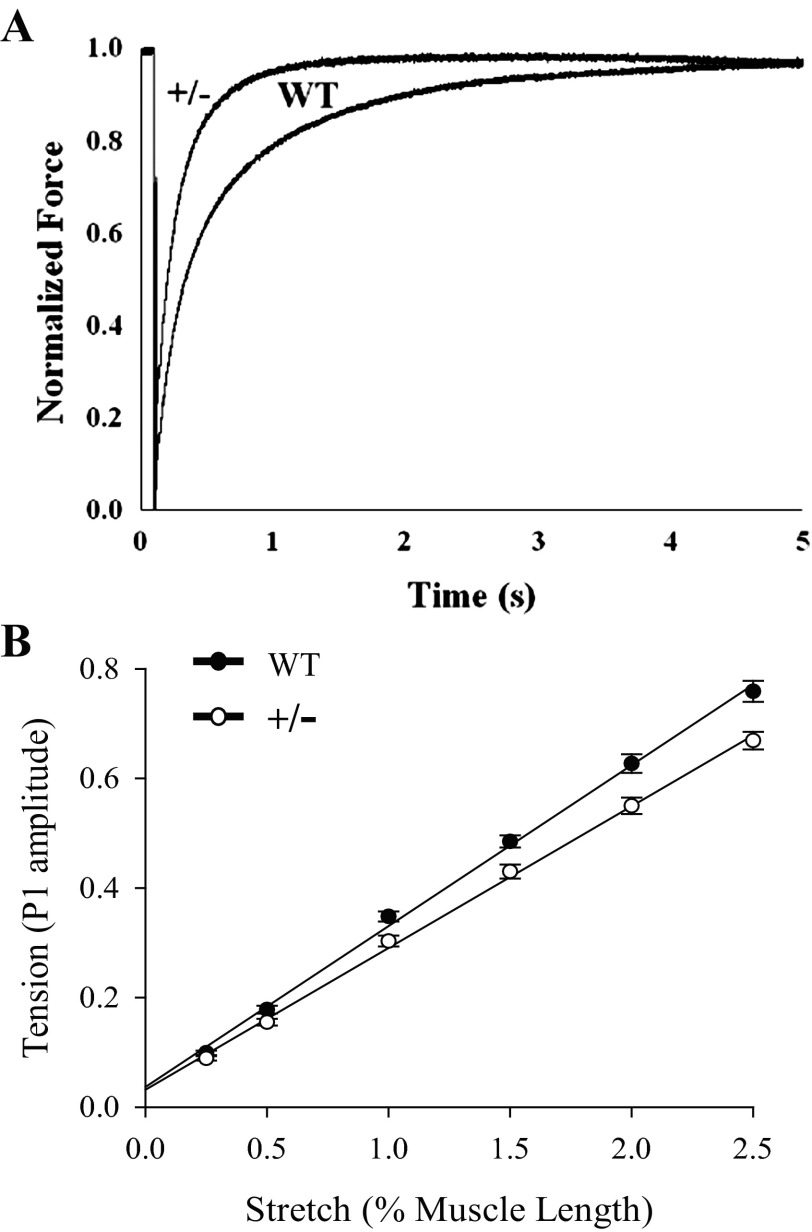
Fig. 3.
Rate of force development and fiber stiffness. A: representative force traces following a slack and release-restretch protocol showing accelerated rates of force development in MyBP-C+/− skinned myocardium. In this example fibers were activated in solutions containing sufficient Ca2+ to yield ∼25% of maximal force. B: stiffness, as the slope of stretch-tension relationship. The tension was measured following achievement of steady-state force at approximately half-maximal activation level by application of a series of stretch increases in muscle length (0.25–2.5% of initial muscle length), completed in 0.2 ms. The amplitude of the force transient following stretch (P1) was recorded, and peak force was plotted against the step length. The stiffness was significantly (P < 0.05) decreased in MyBP-C+/− fibers compared with WT. Data are averages of 20 fibers/group.
Stretch activation experiments revealed that the rate of delayed cross-bridge recruitment (kdf) was accelerated in MyBP-C+/− skinned myocardium compared with WT, but the rate of cross-bridge detachment (krel) was not different between groups (Table 2). The net delayed recruitment of cross bridges following stretch (P3) was similar between groups at both activation levels; however, the greater decline in the amplitudes of P2 following stretch in MyBP-C+/− skinned myocardium increased the overall amplitude of the phase 3 force transient compared with that in WT, as indicated by a larger increase in Pdf for MyBP-C+/− (Table 2). Similar to ktr measurements, PKA treatment of WT and MyBP-C+/− skinned myocardium resulted in accelerated cross-bridge kinetics, indicated by increases in kdf and krel, and there were no differences in kdf between groups following PKA treatment (Table 2). Consistent with previous studies (53, 57), PKA treatment significantly decreased the P2 amplitude, thereby resulting in an increase in Pdf for both groups (Table 2).
Stepwise rapid stretches of increasing ΔL (0.25–2.5%) were imposed on skinned myocardium isolated from WT and MyBP-C+/− hearts at steady-state activations of ∼50% of maximal activation. The peak amplitude of the force transient following stretch normalized to prestretch isometric force (P1 amplitude) was plotted against stretch length (ΔL) as an estimate of muscle fiber stiffness. The amplitude of P1 was significantly smaller for MyBP-C+/− skinned myocardium at stretch lengths >0.25% of muscle length, and the slope of the relationship between stretch (ΔL) and tension (P1 amplitude) was shallower, indicating a decrease in cross-bridge stiffness (Fig. 3B).
Isolated Myocyte Contractile Properties
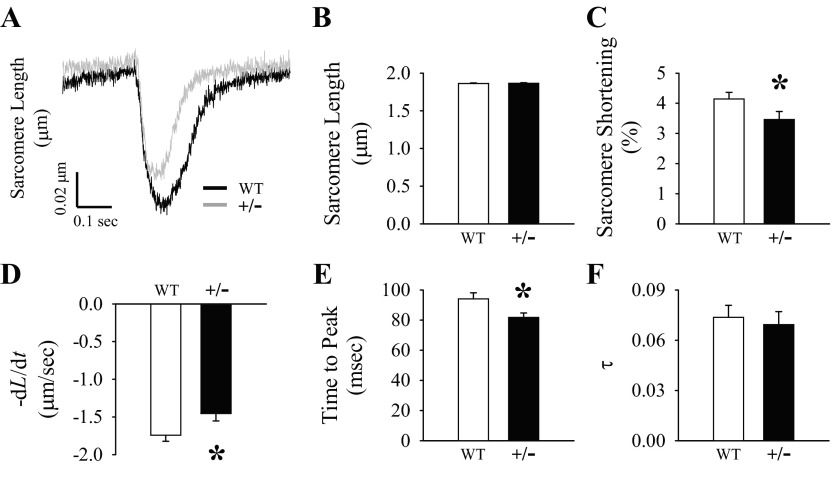
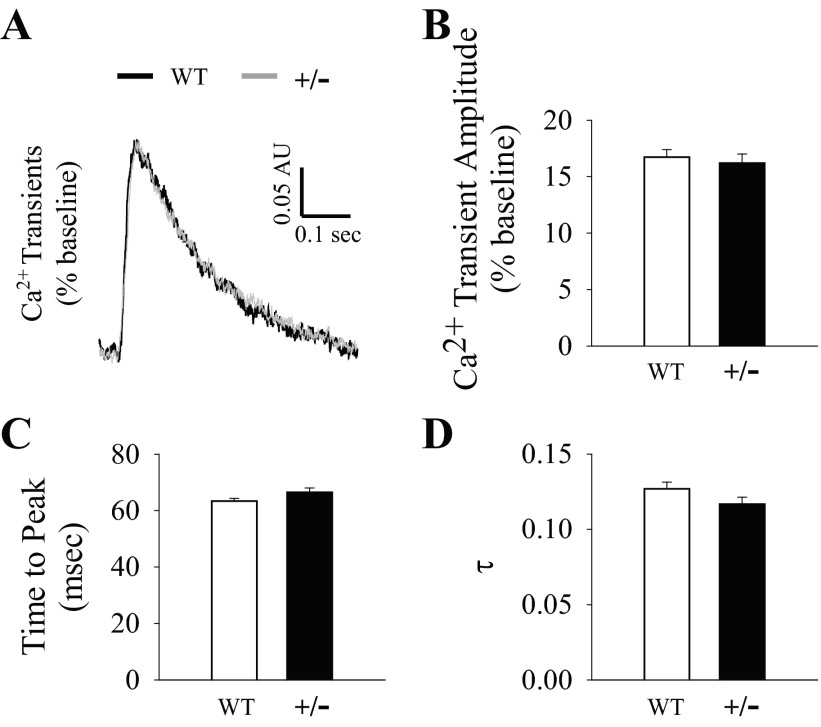
To evaluate the in vitro contractile properties, we assessed sarcomere shortening in intact ventricular cardiomyocytes isolated from WT and MyBP-C+/− hearts. Representative tracings of sarcomere shortening are shown in Fig. 4A. Diastolic sarcomere length was not different between groups (Fig. 4B); however, the percentage of sarcomere shortening (normalized to diastolic sarcomere length) was depressed in MyBP-C+/− myocytes compared with WT myocytes (Fig. 4C). The rate of sarcomere shortening (−dL/dt) was also depressed in MyBP-C+/− myocytes compared with WT myocytes (Fig. 4D), but the rate of sarcomere relengthening (+dL/dt) was not different between groups (WT: 1.21 ± 0.08 μm/s, MyBP-C+/−: 1.16 ± 0.11 μm/s). The time to peak shortening was shorter in MyBP-C+/− myocytes compared with WT myocytes (Fig. 4E); however, the time to 50% relaxation (WT: 0.16 ± 0.01 s, MyBP-C+/−: 0.14 ± 0.01 s), time to 90% relaxation (WT: 0.23 ± 0.01 s, MyBP-C+/−: 0.22 ± 0.01 s), and the relaxation time constant τ (Fig. 4F) were not different between groups.
Fig. 4.
Ventricular cardiomyocyte sarcomere shortening. A: representative tracings of sarcomere shortening at 2 Hz with 1.8 mM Ca2+ averaged from 8 peaks from the same cell from each group. Diastolic sarcomere length (B), %sarcomere shortening (C), rate of sarcomere shortening (−dL/dt) (D), time to peak (E), and the relaxation time constant τ (F) were measured on fresh isolated intact ventricular cardiomyocytes at 2 Hz with 1.8 mM Ca2+. Data are averages of 50–69 myocytes/group. *P < 0.05 compared with WT.
Myocyte Ca2+ Handling Properties
In vitro Ca2+ handling properties were evaluated by measuring the amplitude and kinetics of Ca2+ transients recorded from isolated intact ventricular cardiomyocyte loaded with indo-1. The representative superimposed Ca2+ transient tracings are shown in Fig. 5A. There were no differences between groups in baseline Ca2+ levels [(in ratio F405/485) WT: 1.07 ± 0.06, MyBP-C+/−: 1.22 ± 0.08], Ca2+ transient amplitude as %baseline (Fig. 5B), time to peak Ca2+ (Fig. 5C), time to 50% Ca2+ decay (WT: 150 ± 3 ms, MyBP-C+/−: 147 ± 3 ms), time to 90% Ca2+ decay (WT: 349 ± 4 ms, MyBP-C+/−: 349 ± 4 ms), or the relaxation time constant τ (Fig. 5D).
Fig. 5.
Ventricular cardiomyocyte Ca2+ transients. A: representative superimposed Ca2+ transient tracings at 2 Hz with 1.8 mM Ca2+ averaged from 8 peaks from the same cell from each group. Ca2+ transient amplitude (normalized to baseline) (B), time to peak (C), and the relaxation time constant τ (D) were measured on fresh isolated intact ventricular cardiomyocytes loaded with indo-1 at 2 Hz with 1.8 mM Ca2+. Data are averages of 50–68 myocytes/group.
Expression and Phosphorylation of Ca2+ Handling Proteins
To determine if differences in myocyte contractile function were related to Ca2+ handling properties, the expression and phosphorylation of several key Ca2+ handling proteins were quantified by Western blot analysis. The expression of Ca2+ handling proteins (all normalized to HSC70) examined in this study were not different between groups, which is consistent with a lack of difference between groups in Ca2+ transient measurements. This includes RyR2, the Ca2+-releasing channel (WT: 0.68 ± 0.10, MyBP-C+/−: 0.60 ± 0.08); SERCA2, the primary protein responsible for sequestering Ca2+ from the cytosol back to the SR (WT: 1.16 ± 0.60, MyBP-C+/−: 1.07 ± 0.55); PLB, the inhibitor of SERCA2 (WT: 1.86 ± 0.11, MyBP-C+/−: 1.96 ± 0.11); the SERCA2-to-PLB ratio (both normalized to HSC70; WT: 0.66 ± 0.35, MyBP-C+/−: 0.59 ± 0.30); NCX1, which contributes to extrusion of Ca2+ into the extracellular space, (WT: 0.27 ± 0.04, MyBP-C+/−: 0.35 ± 0.05); and pPLB17 [(normalized to PLB) WT: 1.63 ± 0.21, MyBP-C+/−: 1.10 ± 0.31]. pPLB16-to-PLB ratio was decreased in MyBP-C+/− hearts compared with WT hearts (WT: 0.78 ± 0.09, MyBP-C+/−: 0.49 ± 0.05, P < 0.05), which would be expected to reduce the inhibition on the activity of SERCA2, but by itself did not induce a change in Ca2+ transient properties.
In Vivo Hemodynamic Function
In vivo LV hemodynamic function was assessed in WT and MyBP-C+/− hearts by pressure-volume catheterization at BL and following Dob infusion. HR was not different between groups (Table 3). The end-systolic pressure (ESP) was not different between groups; however, end-diastolic pressure (EDP) was elevated in MyBP-C+/− hearts at BL (Table 3), which could be indicative of diastolic dysfunction. EF was not different between groups; however, there was evidence for systolic dysfunction in MyBP-C+/− hearts, indicated by a decrease in dP/dtmax compared with that in WT hearts at BL (Table 3). Additionally, differences in dP/dtmax between groups were no longer apparent after Dob treatment (Table 3). The relaxation time constant τ was not different between groups at BL or after Dob treatment (Table 3).
Table 3.
LV hemodynamic function at baseline and following β-adrenergic stimulation
| WT |
MyBP-C+/− |
|||
|---|---|---|---|---|
| BL | Dob | BL | Dob | |
| HR, beats/min | 410 ± 16 | 441 ± 16 | 404 ± 5 | 442 ± 12 |
| ESP, mmHg | 94.4 ± 4.1 | 80.2 ± 11.0 | 93.9 ± 5.2 | 79.5 ± 8.7 |
| EDP, mmHg | 5.01 ± 0.53 | 6.04 ± 1.83 | 7.58 ± 1.08* | 4.75 ± 0.81* |
| EF, % | 75.0 ± 4.9 | 90.2 ± 3.2 | 65.0 ± 6.9 | 91.0 ± 4.1 |
| dP/dtmax, mmHg/s | 7964 ± 273 | 11072 ± 961 | 6314 ± 643* | 9899 ± 1067 |
| τ, ms | 9.50 ± 0.87 | 8.15 ± 0.56 | 10.6 ± 0.7 | 9.70 ± 0.99 |
Data are means ± SE of responses determined at baseline (BL) or 5 min after dobutamine (Dob) injection. HR, heart rate; ESP, end-systolic pressure; EDP, end-diastolic pressure; EF, ejection fraction; dP/dtmax, peak rate of pressure rise; τ, relaxation time constant. Data are averages from 9 mice/group.
P < 0.05 compared with WT.
Echocardiography
We also investigated the degree of chamber remodeling and in vivo LV function of the hearts using echocardiography. Body weight, LV mass-to-body weight ratio, posterior wall thickness at end diastole, ESD, and EDD were all comparable between groups (Table 4), indicating that MyBP-C+/− hearts did not display evidence of hypertrophy. Consistent with previous reports (7, 22), we did not observe any functional differences between groups, including EF, FS, ejection time, and isovolumic relaxation time (Table 4).
Table 4.
LV morphology and in vivo function measured by echocardiography
| WT | MyBP-C+/− | |
|---|---|---|
| BW, g | 31.1 ± 1.2 | 30.5 ± 0.8 |
| LV mass/BW | 3.95 ± 0.21 | 3.84 ± 0.24 |
| PWTd, mm | 0.86 ± 0.03 | 0.86 ± 0.03 |
| ESD, mm | 2.13 ± 0.20 | 2.26 ± 0.22 |
| EDD, mm | 3.78 ± 0.07 | 3.77 ± 0.13 |
| HR, beats/min | 427 ± 17 | 412 ± 1 |
| EF, % | 80.7 ± 4.4 | 77.2 ± 3.9 |
| FS | 0.44 ± 0.04 | 0.41 ± 0.04 |
| ET, ms | 60.9 ± 2.6 | 65.1 ± 2.0 |
| IVRT, ms | 22.9 ± 2.2 | 20.1 ± 2.4 |
Data are means ± SE. BW, body weight; LV, left ventricular; PWTd, posterior wall thickness in diastole; ESD, end-systolic dimension; EDD, end-diastolic dimension; FS, fractional shortening; ET, ejection time; IVRT, isovolumic relaxation time. Data are averages from 7–10 mice/group.
Histological Assessment of Cardiac Morphology
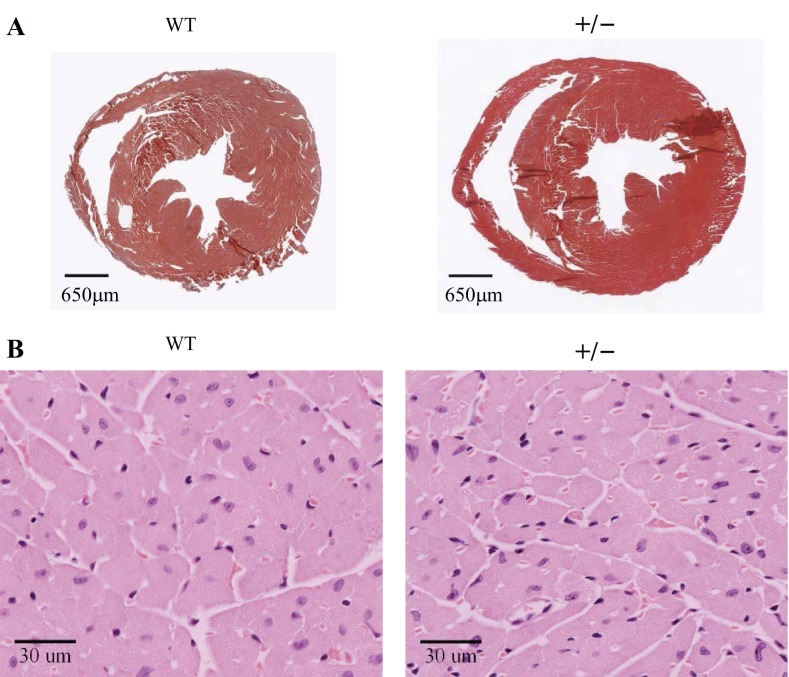
We performed histological staining studies on cross sections isolated from WT and MyBP-C+/− hearts, and representative images are presented in Fig. 6. Masson's trichrome-stained cross sections at mid-LV level revealed similar LV geometry in both groups, with no obvious chamber remodeling or hypertrophy (Fig. 6A). Also, there was no overt fibrosis in either group, because very little blue staining was detectable (Fig. 6A). Additionally, H&E staining showed no appreciable differences in cardiomyocyte morphology (Fig. 6B), because no cellular hypertrophy or disarray was evident in either group.
Fig. 6.
Morphology of the hearts and cardiomyocytes. A: representative cross sections of WT and MyBP-C+/− hearts at the mid-LV level, stained with Masson's trichrome (×10 magnification). The viable myocardium is red and fibrotic area blue (insignificant). B: representative magnified cross sections at mid-LV level of WT and MyBP-C+/− hearts stained with hematoxylin and eosin (×40 magnification). The cardiomyocytes are pink and their nuclei blue.
Electrocardiography
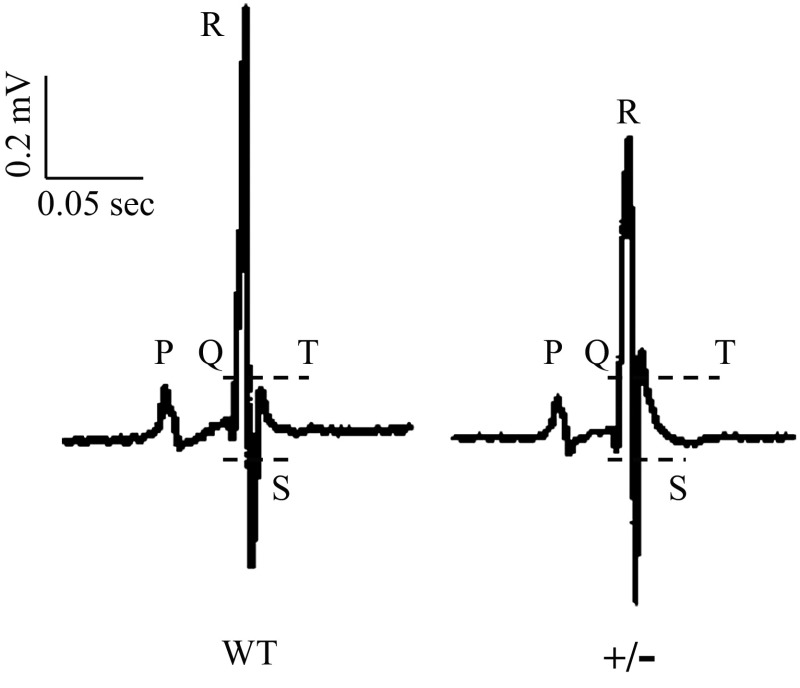
To further explore the in vivo mechanisms of MyBP-C deficiency in the heart, we performed continuous ECG recordings in conscious animals using surgically implanted radio telemetry devices. The representative ECG waveforms of WT and MyBP-C+/− mice are shown in Fig. 7. The RR interval, P-wave duration, and PR interval were not different between groups; however, the QRS duration, QT interval, and QTc interval were all prolonged in MyBP-C+/− mice compared with WT mice (Table 5).
Fig. 7.
Electrocardiograph (ECG) waveforms. Representative ECG waveforms of conscious animals were recorded at baseline using telemetry implants. The intervals of QRS and QT are indicated with dotted lines.
Table 5.
Electrocardiographic data acquired by radio telemetry
| WT | MyBP-C+/− | |
|---|---|---|
| RR interval, ms | 131 ± 4 | 135 ± 4 |
| P wave duration, ms | 17.5 ± 0.6 | 18.3 ± 0.3 |
| PR interval, ms | 39.5 ± 1.0 | 39.0 ± 0.8 |
| QRS duration, ms | 27.3 ± 1.1 | 30.6 ± 0.8* |
| QT interval, ms | 45.1 ± 1.8 | 50.4 ± 1.1* |
| QTc interval, ms | 39.2 ± 1.4 | 44.3 ± 0.9* |
Data are means ± SE. QTc, heart rate-adjusted QT interval determined using the Bazett formula: QTc = QT/(RR/100)1/2. Data are averages from 8–11 mice/group.
P < 0.05 compared with WT.
DISCUSSION
The majority of FHC mutations in MyBP-C are predicted to result in MyBP-C haploinsufficiency; however, the link between decreased MyBP-C expression and the development of cardiac dysfunction is not clear. We have previously shown that hearts of young MyBP-C+/− mice (i.e., 8–10 wk of age) display impairments in in vivo mechanical function, which is related to altered myofilament function (15). However, the functional effects of decreased MyBP-C expression in the sarcomeres of MyBP-C+/− hearts on myofilament behavior and posttranslational modifications and on regulatory mechanisms downstream from the myofilaments, such as Ca2+ homeostasis and the electrical activity in the heart, have not been thoroughly investigated. We found that partial MyBP-C deficiency in the heart produced altered function at the molecular and whole organ levels, and these alterations occurred in the absence of LV chamber remodeling and hypertrophy.
Our results show that reduced expression and phosphorylation of MyBP-C accelerated rates of cross-bridge attachment at low Ca2+ and reduced cross-bridge stiffness in skinned myocardium. Altered myofilament function in turn contributed to reduced sarcomere shortening, −dL/dt, and time to peak shortening in intact myocytes isolated from MyBP-C+/− hearts without any alterations in Ca2+ transient kinetics. In vivo hemodynamic systolic and diastolic function in MyBP-C+/− hearts were also impaired as evidenced by decreased dP/dtmax and elevated EDP, respectively. Furthermore, basal differences in cross-bridge behavior and in vivo contractile function between WT and MyBP-C+/− mice were largely reversed following β-adrenergic stimulation by PKA and Dob, respectively. ECG measurements in conscious animals revealed prolonged QRS, QT, and QTc durations in MyBP-C+/− animals, which may predispose them to an increased risk of arrhythmia and SCD. Collectively, these data demonstrate that in a mouse model that closely mimics the loss of MyBP-C expression in the sarcomere as in human patients carrying MyBP-C mutations, the primary consequence that contributes to impaired in vivo mechanical and electrical function in the heart is abnormal myofilament contractile function.
We observed a significant increase in the rates of force development (ktr) and stretch-induced cross-bridge recruitment (kdf) at low levels of Ca2+ activation in MyBP-C+/− skinned myocardium, the latter being consistent with results from our previous study (15). Assuming that ktr is the sum of the forward (f) and reverse (g) rate constants of the transitions between force-generating and non-force-generating cross-bridge states, the lack of acceleration in krel in MyBP-C+/− skinned myocardium suggests that accelerated ktr was mostly due to accelerated cross-bridge recruitment and transitions to force-generating states (i.e., greater f). It has previously been shown that complete ablation of MyBP-C in the sarcomere of MyBP-C−/− mice radially displaces the heads of myosin cross bridges closer to actin and increases the probability of actomyosin interactions, thereby accelerating rates of cross-bridge recruitment and force generation (11). Force generation at low Ca2+ activation is highly cooperative in that strong binding of cross bridges to actin can promote further cross-bridge recruitment within the thin filament. At low levels of Ca2+ activation, cooperative cross-bridge recruitment slows the rate of force development as strongly bound cross bridges gradually recruit additional cross bridges into strongly bound force-generating states. It is possible that although the decrease in MyBP-C expression in the MyBP-C+/− sarcomere is substantially smaller than in the MyBP-C−/− mouse, it may be sufficient to enhance the probability of actomyosin interactions in regions of the sarcomere where MyBP-C is absent (15, 55) by accelerating rates of cross-bridge binding throughout the sarcomere, thereby reducing the time course of cooperative cross-bridge recruitment and accelerating force development. At higher levels of Ca2+ activation, force generation becomes less dependent on cross bridge-induced cooperative recruitment of cross bridges, because increased binding of Ca2+ to troponin C would recruit the majority of available cross bridges to interact with open actin sites. Cross-bridge kinetics were not different between WT and MyBP-C+/− skinned myocardium at Ca2+ activations yielding forces greater than ∼30% of maximal (data not shown), suggesting that decreased MyBP-C expression accelerates cooperative recruitment of cross bridges.
Accelerated rates of cross-bridge kinetics at low Ca2+ activation in MyBP-C+/− skinned myocardium were also accompanied by an increase in steady-state force generation (Table 1). An increase in steady-state force at low Ca2+ activations was also previously noted in MyBP-C−/− skinned myocardium (51), which can be attributed to increased actomyosin interactions due to the closer juxtaposition of actin and myosin in the absence of MyBP-C (11). Thus it is possible that a decrease in MyBP-C content in MyBP-C+/− skinned myocardium may also contribute to increased force generation by a mechanism similar to that in MyBP-C−/− skinned myocardium. Moreover, because we observed an increase in the rate of stretch-induced cross-bridge recruitment (kdf) but not cross-bridge detachment (krel) in MyBP-C+/− myocardium, it is possible that increased force generation at low Ca2+ activations may reflect an increase in the number of cross bridges bound at a given time without a decrease in the amount of time cross bridges are in strongly bound states (i.e., no change in rates of cross-bridge detachment). Furthermore, because MyBP-C phosphorylation has been proposed to decrease force generation at submaximal Ca2+ activations (9), presumably by decreasing the time that cross bridges are in strongly bound states (12), the overall decrease in MyBP-C phosphorylation in MyBP-C+/− hearts could also have contributed to increased cross-bridge dwell time, and thereby force generation.
Basal differences in cross-bridge kinetics between WT and MyBP-C+/− skinned myocardium were abolished following treatment with PKA (Table 2), because both groups displayed accelerations in ktr and stretch activation kinetics. The overall response to PKA was smaller in MyBP-C+/− compared with WT skinned myocardium due to a reduction in the amount of MyBP-C in the sarcomere available to be phosphorylated. However, the data suggest that reduced MyBP-C expression in MyBP-C+/− myocardium does not impair the ability to accelerate cross-bridge kinetics in response to PKA phosphorylation. The decrease in Ca2+ sensitivity following PKA phosphorylation was also similar in WT and MyBP-C+/− skinned myocardium (Table 1), suggesting that decreased MyBP-C expression also does not impair PKA-dependent TnI phosphorylation.
The presence of MyBP-C in the sarcomere and its phosphorylation have been shown to be important for maintaining mechanical rigidity of the myofilament lattice, which is an important determinant of the properties of cross-bridge binding to actin (39, 42), and a decrease in both MyBP-C content and MyBP-C phosphorylation in the sarcomeres has been shown to significantly reduce radial and longitudinal lattice stiffness (42). Consistent with this notion, we found that skinned myocardium isolated from MyBP-C+/− hearts displayed reduced cross-bridge stiffness compared with WT skinned myocardium when subjected to stepwise acute stretches at half-maximal Ca2+ activations (Fig. 3B). Furthermore, we observed a greater decline in the minimum amplitude of the force transient (P2) following stretch in MyBP-C+/− skinned myocardium at low Ca2+ activations (Table 2), corresponding to greater cross-bridge detachment in response to stretch, which may suggest increased cross-bridge compliance (50, 53). The precise mechanisms for decreased cross-bridge stiffness in MyBP-C+/− myocardium in response to acute stretch are unclear but are unlikely to be due to differences in the number of strongly bound cross bridges prior to stretch because steady-state force at half-maximal activation was not different between WT and MyBP-C+/− skinned myocardium. Rather, it is possible that individual MyBP-C+/− cross bridges are inherently more compliant and detach more easily when subjected to significant strain such as acute stretch. It has been proposed that MyBP-C normally provides a significant elastic and viscous load that is important for storing elastic recoil energy to facilitate subsequent relaxation function (42). Thus a decrease in cross-bridge stiffness resulting in more compliant myofilaments in MyBP-C+/− hearts may impair LV mechanical relaxation in vivo, as we have previously observed (15).
A few studies have examined the mechanical properties of skinned myocardium isolated from human hearts expressing MyBP-C mutations (23, 58, 59). These investigations have consistently shown decreased MyBP-C expression, MyBP-C phosphorylation, and TnI phosphorylation, which together result in large deficits in maximal force generation and increases in submaximal force generation (when expressed relative to maximal force). Although the decrease in MyBP-C expression in the hearts of the MyBP-C+/− mice studied presently (32%) mirrors the loss of MyBP-C in myectomy samples from hearts of human patients expressing MyBP-C truncation mutations (33%) (58, 59), we did not observe any changes in maximal force generation between MyBP-C+/− and WT skinned myocardium. Furthermore, the increases in submaximal force generation in MyBP-C+/− skinned myocardium were small and limited to very low activation levels (<15% of maximal). It is likely that altered steady-state force generation in skinned myocardium isolated from human MyBP-C truncation mutation carriers is related to large decreases in TnI phosphorylation (58, 59). However, the finding that myectomy samples isolated from human FHC hearts that did not express MyBP-C mutations displayed similar force generation properties suggests that these changes are general features related to FHC and not decreased MyBP-C expression per se (59). Thus our results suggest that in the absence of LV remodeling and activation of compensatory cascades, the initial response to MyBP-C insufficiency is an acceleration in the rates of cross-bridge attachment at low Ca2+ activation and increased steady-state force generation, which in conjunction with decreased MyBP-C phosphorylation result in decreased cross-bridge stiffness.
Impairments in cardiac contractility can be mediated by disruptions in Ca2+ cycling at the SR, which modulates the availability of Ca2+ to the myofilaments, and thereby force generation. We investigated whether MyBP-C insufficiency in MyBP-C+/− sarcomeres results in altered Ca2+-handling properties in intact myocytes. Indeed, MyBP-C+/− myocytes displayed abnormal in vitro contractile properties as evidenced by reduced sarcomere shortening, −dL/dt, and a reduced time to peak shortening. However, altered MyBP-C+/− myocyte contractility was not related to changes in baseline Ca2+ levels, Ca2+ release kinetics, peak Ca2+ transient amplitude, and Ca2+ transient clearance/decay. The expression and phosphorylation of Ca2+ handling proteins responsible for the individual components of Ca2+ transient measurements were not different between groups with the exception of decreased pPLB16 in MyBP-C+/− hearts. However, we did not observe differences in the rates of the Ca2+ transient decay; therefore, it is unlikely that in this case decreased pPLB16 significantly affected SERCA2 function. Interestingly, faster Ca2+ transient decay was seen in myocytes isolated from a knockin mouse model expressing an FHC missense mutation in MyBP-C that results in decreased MyBP-C expression and a large increase in Ca2+ sensitivity, and was hypothesized to be a compensatory mechanism to improve diastolic relaxation in the hypercontractile MyBP-C mutant knockin hearts (18). In contrast, the increases in Ca2+ sensitivity of force generation observed in the present study in MyBP-C+/− skinned myocardium may not have been substantial enough to invoke a compensatory response in Ca2+ clearance, suggesting that depressed MyBP-C+/− myocyte contractile behavior was due to impaired myofilament function rather than altered Ca2+-handling properties.
Consistent with previous investigations employing echocardiography to study contractile function in heterozygous null MyBP-C hearts (7, 22), we did not find differences in LV chamber wall dimensions or indexes of cardiac function compared with WT hearts (Table 4). However, invasive pressure-volume catheterization studies revealed that despite relatively preserved EF, MyBP-C+/− hearts displayed significantly depressed dP/dtmax compared with WT hearts, indicative of systolic dysfunction (Table 3). This finding differs from a previous study that found no significant changes in contractile function between WT and MyBP-C+/− hearts at 9 mo of age (7). The causes for the differences between studies are unclear but may reflect differences in the genetic backgrounds of the MyBP-C+/− animals employed and, perhaps, may be due to the greater decrease in cardiac MyBP-C expression in the mice used here (∼32%) compared with those used by Carrier et al. (7) (∼25%), and/or MyBP-C phosphorylation, which was not reported by Carrier et al. (7), all of which can contribute to the more pronounced contractile impairments observed in the present study. Our finding that dP/dtmax was not different between WT and MyBP-C+/− hearts following Dob treatment (Table 3) suggests that decreased MyBP-C phosphorylation may have contributed to basal systolic impairment in MyBP-C+/− hearts and underscores the importance of MyBP-C phosphorylation in maintaining normal cardiac function.
Impaired systolic function in MyBP-C+/− hearts is consistent with measurements in intact myocytes showing reduced sarcomere shortening and rates of sarcomere shortening (−dL/dt). In addition to systolic dysfunction, MyBP-C+/− hearts also display evidence for diastolic dysfunction, as evidenced by increased EDP, compared with WT hearts. Although the increase in EDP in MyBP-C+/− hearts is modest, it could be indicative of the early phases of diastolic dysfunction that could further impair systolic function. Elevated EDP can be associated with decreased LV compliance (21), which in this case could be due to enhanced cross-bridge binding and accelerated rates of force generation in skinned myocardium and to reduced time to peak shortening in intact myocytes at low cytosolic Ca2+ concentrations. Ultimately, this hypercontractile state prevents the myofilaments from relaxing completely, thereby impairing ventricular filling during diastole (18, 44).
Our finding here that in vivo systolic function is impaired in MyBP-C+/− hearts is consistent with previous data showing depressed systolic torsion and circumferential strain in MyBP-C+/− hearts compared with WT hearts (15). Although accelerated rates of cooperative cross-bridge activation and force generation at low Ca2+ activations in MyBP-C+/− skinned myocardium would be expected to enhance systolic function in vivo, MyBP-C+/− hearts displayed depressed dP/dtmax. The precise cause for systolic dysfunction in MyBP-C+/− hearts is unclear but could be related to a disruption in the timing of fiber shortening in early- and late-activated regions of the heart during the early isovolumic contraction phase due to accelerated cross-bridge kinetics at submaximal workloads. Depressed mechanical function could also be due to regional inhomogeneity in rates of force development due to variable MyBP-C expression within sarcomeres such that regions with less MyBP-C content may exhibit accelerated cross-bridge kinetics compared with regions with higher MyBP-C content, which ultimately results in uncoordinated sarcomere shortening and stretching. We found that MyBP-C expression in MyBP-C+/− hearts was more depressed in the mid-LV region compared with the apex and base (Fig. 2C). The cause of the differences in regional MyBP-C expression in MyBP-C+/− hearts is unclear, but a greater decrease in MyBP-C expression in the mid-LV is consistent with our previous observations in younger MyBP-C+/− mice, which shows that the mid-LV region displays greater mechanical dysfunction than the apex and base as evidenced by greater abnormalities in measurements of radial and circumferential strains (15). Furthermore, LV rotational mechanics during systole were particularly depressed in the regions between the mid-LV and base in MyBP-C+/− mice, resulting in a decrease in overall LV twist, which compromises systolic function and efficiency (15).
Arrhythmia-induced SCD is the most common cause of death in FHC patients (33) and can affect young and asymptomatic individuals. LV hypertrophy is considered an independent risk factor for SCD (49); however, in many cases arrhythmia and SCD occur in the absence of overt LV hypertrophy (1, 31, 32, 60, 63). In this study we recorded electrical activity in unanesthetized ambulatory MyBP-C+/− and WT mice, and despite a lack of LV hypertrophy, MyBP-C+/− hearts displayed abnormal ECG patterns associated with increased risk of arrhythmia and cardiac dysfunction. Specifically, MyBP-C+/− mice had prolonged QT intervals compared with WT mice, perhaps indicative of prolonged action potential duration and ventricular repolarization (46, 47), a feature that has also been documented in patients carrying MyBP-C mutations (27). Furthermore, we also observed a prolongation of ventricular depolarization, as evidenced by an increase in QRS duration in MyBP-C+/− mice compared with WT mice, which is associated with impaired ventricular conductance (46, 47). The precise causes that underlie the abnormal ECG patterns in MyBP-C+/− animals are unclear; however, enhanced myofilament Ca2+-sensitivity and dyssynchronous mechanical function have been implicated as potential triggers for abnormal cardiac electrical activity (24, 28, 54), and both of these characteristics have been observed in MyBP-C+/− hearts (present study and Ref. 15). Regardless of the mechanisms, our ECG data suggest that MyBP-C+/− animals are at an increased risk for developing arrhythmias, especially in conditions of increased cardiac stress.
Collectively, our data show that decreased expression of MyBP-C in MyBP-C+/− hearts and concomitant downregulation of MyBP-C phosphorylation in the sarcomere results in altered cross-bridge function at the myofilament level and contributes to in vivo contractile dysfunction and ECG abnormalities. Myofilament dysfunction in MyBP-C+/− hearts was not accompanied by compensatory adaptations in Ca2+ transient kinetics and was independent of changes in LV chamber remodeling and hypertrophy. Because decreased MyBP-C expression is a common feature in MyBP-C-mediated FHC, increasing MyBP-C expression in the sarcomeres in MyBP-C-deficient hearts may normalize cross-bridge function, thereby providing a therapeutic application to delay the emergence of FHC or reverse the pathological course of the disease (35).
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01 HL-114770-01 (to J. E. Stelzer) and American Heart Association Grant 09SDG2050195 (to J. E. Stelzer).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.C., D.S.R., M.P.C., and J.E.S. conception and design of research; Y.C., X.W., T.A.M., X.C., K.S.G., and J.E.S. performed experiments; Y.C., X.W., T.A.M., X.C., K.S.G., M.P.C., and J.E.S. analyzed data; Y.C., X.W., T.A.M., X.C., K.S.G., D.S.R., M.P.C., and J.E.S. interpreted results of experiments; Y.C., X.W., K.S.G., and J.E.S. prepared figures; Y.C., M.P.C., and J.E.S. drafted manuscript; Y.C., X.W., D.S.R., M.P.C., and J.E.S. edited and revised manuscript; Y.C., X.W., T.A.M., X.C., K.S.G., D.S.R., M.P.C., and J.E.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The experiments were performed at the Department of Physiology and Biophysics and The Heart and Vascular Research Center at the MetroHealth Campus, Case Western Reserve University, Cleveland, OH. We thank the Tissue Resources Core Facility at Case Comprehensive Cancer Center for tissue processing and histological staining, and Scott Howell, Ph.D., Department of Ophthalmology and The Visual Sciences Research Center at Case Western Reserve University, for expert technical assistance with the histological assessments.
REFERENCES
- 1. Adabag AS, Casey SA, Kuskowski MA, Zenovich AG, Maron BJ. Spectrum and prognostic significance of arrhythmias on ambulatory Holter electrocardiogram in hypertrophic cardiomyopathy. J Am Coll Cardiol 45: 697– 704, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Bardswell S, Cuello F, Kentish J, Avkiran M. cMyBP-C as a promiscuous substrate: phosphorylation by non-PKA kinases and its potential significance. J Muscle Res Cell Motil 33: 53– 60, 2012 [DOI] [PubMed] [Google Scholar]
- 3. Barefield D, Sadayappan S. Phosphorylation and function of cardiac myosin binding protein-C in health and disease. J Mol Cell Cardiol 48: 866– 875, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brickson S, Fitzsimons DP, Pereira L, Hacker T, Valdivia H, Moss RL. In vivo left ventricular functional capacity is compromised in cMyBP-C null mice. Am J Physiol Heart Circ Physiol 292: H1747– H1754, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Campbell KB, Chandra M. Functions of stretch activation in heart muscle. J Gen Physiol 127: 89– 94, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Campbell KS, Moss RL. SLControl: PC-based data acquisition and analysis for muscle mechanics. Am J Physiol Heart Circ Physiol 285: H2857– H2864, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Carrier L, Knöll R, Vignier N, Keller DI, Bausero P, Prudhon B, Isnard R, Ambroisine ML, Fiszman M, Ross J, Schwartz K, Chien KR. Asymmetric septal hypertrophy in heterozygous cMyBP-C null mice. Cardiovasc Res 63: 293– 304, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Carrier L, Schlossarek S, Willis MS, Eschenhagen T. The ubiquitin-proteasome system and nonsense-mediated mRNA decay in hypertrophic cardiomyopathy. Cardiovasc Res 85: 330– 338, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen Y, Somji A, Yu X, Stelzer JE. Altered in vivo left ventricular torsion and principal strains in hypothyroid rats. Am J Physiol Heart Circ Physiol 299: H1577– H1587, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chu V, Otero JM, Lopez O, Morgan JP, Amende I, Hampton TG. Method for non-invasively recording electrocardiograms in conscious mice. BMC Physiol 1: 1– 6, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Colson BA, Bekyarova T, Locher MR, Fitzsimons DP, Irving TC, Moss RL. Protein kinase A-mediated phosphorylation of cMyBP-C increases proximity of myosin heads to actin in resting myocardium. Circ Res 103: 244– 251, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Colson BA, Patel JR, Chen PP, Bekyarova T, Abdalla MI, Tong CW, Fitzsimons DP, Irving TC, Moss RL. Myosin binding protein-C phosphorylation is the principal mediator of protein kinase A effects on thick filament structure in myocardium. J Mol Cell Cardiol 53: 609– 616, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Copeland ON, Sadayappan S, Messer AE, Steinen GJM, van der Velden J, Marston SB. Analysis of cardiac myosin binding protein-C phosphorylation in human heart muscle. J Mol Cell Cardiol 49: 1003– 1011, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Coulton AT, Stelzer JE. Cardiac myosin binding protein C and its phosphorylation regulate multiple steps in the cross-bridge cycle of muscle contraction. Biochemistry 51: 3292– 3301, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Desjardins CL, Chen Y, Coulton AT, Hoit BD, Yu X, Stelzer JE. Cardiac myosin binding protein C insufficiency leads to early onset of mechanical dysfunction. Circ Cardiovasc Imaging 5: 127– 136, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. El-Armouche A, Wahab A, Wittköpper K, Schulze T, Böttcher F, Pohlmann L, King SB, DuMond JF, Gerloff C, Böger RH, Eschenhagen T, Carrier L, Donzelli S. The new HNO donor, 1-nitrosocyclohexyl acetate, increases contractile force in normal and [beta]-adrenergically desensitized ventricular myocytes. Biochem Biophys Res Commun 402: 340– 344, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol 157: 378– 417, 1988 [DOI] [PubMed] [Google Scholar]
- 18. Fraysse B, Weinberger F, Bardswell SC, Cuello F, Vignier N, Geertz B, Starbatty J, Krämer E, Coirault C, Eschenhagen T, Kentish JC, Avkiran M, Carrier L. Increased myofilament Ca2+ sensitivity and diastolic dysfunction as early consequences of Mybpc3 mutation in heterozygous knock-in mice. J Mol Cell Cardiol 52: 1299– 1307, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Godt RE, Lindley BD. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J Gen Physiol 80: 279– 297, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gollapudi SK, Mamidi R, Mallampalli SL, Chandra M. The N-terminal extension of cardiac troponin T stabilizes the blocked state of cardiac thin filament. Biophys J 103: 940– 948, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gutierrez C, Blanchard DG. Diastolic heart failure: challenges of diagnosis and treatment. Am Fam Physician 69: 2609– 2616, 2004 [PubMed] [Google Scholar]
- 22. Harris SP, Bartley CR, Hacker TA, McDonald KS, Douglas PS, Greaser ML, Powers PA, Moss RL. Hypertrophic cardiomyopathy in cardiac myosin binding protein-C knockout mice. Circ Res 90: 594– 601, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Hoskins AC, Jacques A, Bardswell SC, McKenna WJ, Tsang V, dos Remedios CG, Ehler E, Adams K, Jalilzadeh S, Avkiran M, Watkins H, Redwood C, Marston SB, Kentish JC. Normal passive viscoelasticity but abnormal myofibrillar force generation in human hypertrophic cardiomyopathy. J Mol Cell Cardiol 49: 737– 745, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huke S, Knollmann BC. Increased myofilament Ca2+-sensitivity and arrhythmia susceptibility. J Mol Cell Cardiol 48: 824– 833, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jacques A, Hoskins A, Kentish J, Marston S. From genotype to phenotype: a longitudinal study of a patient with hypertrophic cardiomyopathy due to a mutation in the MYBPC3 gene. J Muscle Res Cell Motil 29: 239– 246, 2008 [DOI] [PubMed] [Google Scholar]
- 26. James J, Robbins J. Signaling and myosin-binding protein C. J Biol Chem 286: 9913– 9919, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jouven X, Hagege A, Charron P, Carrier L, Dubourg O, Langlard JM, Aliaga S, Bouhour JB, Schwartz K, Desnos M, Komajda M. Relation between QT duration and maximal wall thickness in familial hypertrophic cardiomyopathy. Heart 88: 153– 157, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kohl P, Sachs F. Mechanoelectric feedback in cardiac cells. Philos Trans R Soc Lond A Math Phys Sci 359: 1173– 1185, 2001 [Google Scholar]
- 29. Korte FS, McDonald KS, Harris SP, Moss RL. Loaded shortening, power output, and rate of force redevelopment are increased with knockout of cardiac myosin binding protein-C. Circ Res 93: 752– 758, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Layland J, Cave AC, Warren C, Grieve DJ, Sparks E, Kentish JC, Solaro RJ, Shah AM. Protection against endotoxemia-induced contractile dysfunction in mice with cardiac-specific expression of slow skeletal troponin I. FASEB J 19: 1137– 1139, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Maron BJ, Kragel AH, Roberts WC. Sudden death in hypertrophic cardiomyopathy with normal left ventricular mass. Br Heart J 63: 308– 310, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maron BJ, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ, Seidman CE, Shah PM, Spencer Iii WH, Spirito P, Ten Cate FJ, Wigle ED, Vogel RA, Abrams J, Bates ER, Brodie BR, Danias PG, Gregoratos G, Hlatky MA, Hochman JS, Kaul S, Lichtenberg RC, Lindner JR, O'rourke RA, Pohost GM, Schofield RS, Tracy CM, Winters WL, Jr, Klein WW, Priori SG, Alonso-Garcia A, Blomström-Lundqvist C, De Backer G, Deckers J, Flather M, Hradec J, Oto A, Parkhomenko A, Silber S, Torbicki A. American College of Cardiology/European Society of Cardiology Clinical Expert Consensus Document on Hypertrophic Cardiomyopathy: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol 42: 1687– 1713, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Maron BJ, Olivotto I, Spirito P, Casey SA, Bellone P, Gohman TE, Graham KJ, Burton DA, Cecchi F. Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation 102: 858– 864, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Marston S, Copeland O, Jacques A, Livesey K, Tsang V, McKenna WJ, Jalilzadeh S, Carballo S, Redwood C, Watkins H. Evidence from human myectomy samples that MYBPC3 mutations cause hypertrophic cardiomyopathy through haploinsufficiency. Circ Res 105: 219– 222, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Merkulov S, Chen X, Chandler MP, Stelzer JE. In vivo cardiac myosin binding protein C gene transfer rescues myofilament contractile dysfunction in cardiac myosin binding protein C null mice. Circ Heart Fail 5: 635– 644, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mitchell GF, Jeron A, Koren G. Measurement of heart rate and Q-T interval in the conscious mouse. Am J Physiol Heart Circ Physiol 274: H747– H751, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Mitov MI, Holbrook AM, Campbell KS. Myocardial short-range force responses increase with age in F344 rats. J Mol Cell Cardiol 46: 39– 46, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Morgan EE, Faulx MD, McElfresh TA, Kung TA, Zawaneh MS, Stanley WC, Chandler MP, Hoit BD. Validation of echocardiographic methods for assessing left ventricular dysfunction in rats with myocardial infarction. Am J Physiol Heart Circ Physiol 287: H2049– H2053, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Nyland LR, Palmer BM, Chen Z, Maughan DW, Seidman CE, Seidman JG, Kreplak L, Vigoreaux JO. Cardiac myosin binding protein-C is essential for thick-filament stability and flexural rigidity. Biophys J 96: 3273– 3280, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Page SP, Kounas S, Syrris P, Christiansen M, Frank-Hansen R, Andersen PS, Elliott PM, McKenna WJ. Cardiac myosin binding protein-C mutations in families with hypertrophic cardiomyopathy: disease expression in relation to age, gender, and long term outcome. Circ Cardiovasc Genet 5: 156– 166, 2012 [DOI] [PubMed] [Google Scholar]
- 41. Palmer BM, Georgakopoulos D, Janssen PM, Wang Y, Alpert NR, Belardi DF, Harris SP, Moss RL, Burgon PG, Seidman CE, Seidman JG, Maughan DW, Kass DA. Role of cardiac myosin binding protein C in sustaining left ventricular systolic stiffening. Circ Res 94: 1249– 1255, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Palmer BM, Sadayappan S, Wang Y, Weith AE, Previs MJ, Bekyarova T, Irving TC, Robbins J, Maughan DW. Roles for cardiac MyBP-C in maintaining myofilament lattice rigidity and prolonging myosin cross-bridge lifetime. Biophys J 101: 1661– 1669, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pfuhl M, Gautel M. Structure, interactions and function of the N-terminus of cardiac myosin binding protein C (MyBP-C): who does what, with what, and to whom? J Muscle Res Cell Motil 33: 83– 94, 2012 [DOI] [PubMed] [Google Scholar]
- 44. Pohlmann L, Kröger I, Vignier N, Schlossarek S, Krämer E, Coirault C, Sultan KR, El-Armouche A, Winegrad S, Eschenhagen T, Carrier L. Cardiac myosin-binding protein C is required for complete relaxation in intact myocytes. Circ Res 101: 928– 938, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Rennison JH, McElfresh TA, Okere IC, Patel HV, Foster AB, Patel KK, Stoll MS, Minkler PE, Fujioka H, Hoit BD, Young ME, Hoppel CL, Chandler MP. Enhanced acyl-CoA dehydrogenase activity is associated with improved mitochondrial and contractile function in heart failure. Cardiovasc Res 79: 331– 340, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Salama G, London B. Mouse models of long QT syndrome. J Physiol 578: 43– 53, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sarkozy A, Brugada P. Sudden cardiac death and inherited arrhythmia syndromes. J Cardiovasc Electrophysiol 16: S8– S20, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Seidman CE, Seidman JG. Identifying sarcomere gene mutations in hypertrophic cardiomyopathy: a personal history. Circ Res 108: 743– 750, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Spirito P, Bellone P, Harris KM, Bernabò P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med 342: 1778– 1785, 2000 [DOI] [PubMed] [Google Scholar]
- 50. Stelzer JE, Dunning SB, Moss RL. Ablation of cardiac myosin-binding protein-C accelerates stretch activation in murine skinned myocardium. Circ Res 98: 1212– 1218, 2006 [DOI] [PubMed] [Google Scholar]
- 51. Stelzer JE, Fitzsimons DP, Moss RL. Ablation of myosin-binding protein-C accelerates force development in mouse myocardium. Biophys J 90: 4119– 4127, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stelzer JE, Larsson L, Fitzsimons DP, Moss RL. Activation dependence of stretch activation in mouse skinned myocardium: implications for ventricular function. J Gen Physiol 127: 95– 107, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stelzer JE, Patel JR, Walker JW, Moss RL. Differential roles of cardiac myosin-binding protein C and cardiac troponin I in the myofibrillar force responses to protein kinase A phosphorylation. Circ Res 101: 503– 511, 2007 [DOI] [PubMed] [Google Scholar]
- 54. ter Keurs H. Electromechanical coupling in the cardiac myocyte; stretch-arrhythmia feedback. Pflügers Arch 462: 165– 175, 2011 [DOI] [PubMed] [Google Scholar]
- 55. Theis JL, Bos JM, Theis JD, Miller DV, Dearani JA, Schaff HV, Gersh BJ, Ommen SR, Moss RL, Ackerman MJ. Expression patterns of cardiac myofilament proteins: genomic and protein analysis of surgical myectomy tissue from patients with obstructive hypertrophic cardiomyopathy. Circ Heart Fail 2: 325– 333, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tian XL, Yong SL, Wan X, Wu L, Chung MK, Tchou PJ, Rosenbaum DS, Van Wagoner DR, Kirsch GE, Wang Q. Mechanisms by which SCN5A mutation N1325S causes cardiac arrhythmias and sudden death in vivo. Cardiovasc Res 61: 256– 267, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tong CW, Stelzer JE, Greaser ML, Powers PA, Moss RL. Acceleration of crossbridge kinetics by protein kinase A phosphorylation of cardiac myosin binding protein C modulates cardiac function. Circ Res 103: 974– 982, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. van Dijk SJ, Dooijes D, dos Remedios C, Michels M, Lamers JMJ, Winegrad S, Schlossarek S, Carrier L, ten Cate FJ, Stienen GJM, van der Velden J. Cardiac myosin-binding protein C mutations and hypertrophic cardiomyopathy. Circulation 119: 1473– 1483, 2009 [DOI] [PubMed] [Google Scholar]
- 59. van Dijk SJ, Paalberends ER, Najafi A, Michels M, Sadayappan S, Carrier L, Boontje NM, Kuster DW, van Slegtenhorst M, Dooijes D, dos Remedios C, ten Cate FJ, Stienen GJ, van der Velden J. Contractile dysfunction irrespective of the mutant protein in human hypertrophic cardiomyopathy with normal systolic function. Circ Heart Fail 5: 36– 46, 2012 [DOI] [PubMed] [Google Scholar]
- 60. Varnava AM, Elliott PM, Baboonian C, Davison F, Davies MJ, McKenna WJ. Hypertrophic cardiomyopathy: histopathological features of sudden death in cardiac troponin T disease. Circulation 104: 1380– 1384, 2001 [DOI] [PubMed] [Google Scholar]
- 61. Wan X, Laurita KR, Pruvot EJ, Rosenbaum DS. Molecular correlates of repolarization alternans in cardiac myocytes. J Mol Cell Cardiol 39: 419– 428, 2005 [DOI] [PubMed] [Google Scholar]
- 62. Warren CM, Greaser ML. Method for cardiac myosin heavy chain separation by sodium dodecyl sulfate gel electrophoresis. Anal Biochem 320: 149– 151, 2003 [DOI] [PubMed] [Google Scholar]
- 63. Watkins H, McKenna WJ, Thierfelder L, Suk HJ, Anan R, O'Donoghue A, Spirito P, Matsumori A, Moravec CS, Seidman JG, Seidman CE. Mutations in the genes for cardiac troponin T and α-tropomyosin in hypertrophic cardiomyopathy. N Engl J Med 332: 1058– 1065, 1995 [DOI] [PubMed] [Google Scholar]