Abstract
Mouse mast cell protease (mMCP)-6-null C57BL/6 mice lost less aggrecan proteoglycan from the extracellular matrix of their articular cartilage during inflammatory arthritis than wild-type (WT) C57BL/6 mice, suggesting that this mast cell (MC)-specific mouse tryptase plays prominent roles in articular cartilage catabolism. We used ex vivo mouse femoral head explants to determine how mMCP-6 and its human ortholog hTryptase-β mediate aggrecanolysis. Exposure of the explants to recombinant hTryptase-β, recombinant mMCP-6, or lysates harvested from WT mouse peritoneal MCs (PMCs) significantly increased the levels of enzymatically active matrix metalloproteinases (MMP) in cartilage and significantly induced aggrecan loss into the conditioned media, relative to replicate explants exposed to medium alone or lysates collected from mMCP-6-null PMCs. Treatment of cartilage explants with tetramer-forming tryptases generated aggrecan fragments that contained C-terminal DIPEN and N-terminal FFGVG neoepitopes, consistent with MMP-dependent aggrecanolysis. In support of these data, hTryptase-β was unable to induce aggrecan release from the femoral head explants obtained from Chloe mice that resist MMP cleavage at the DIPEN↓FFGVG site in the interglobular domain of aggrecan. In addition, the abilities of mMCP-6-containing lysates from WT PMCs to induce aggrecanolysis were prevented by inhibitors of MMP-3 and MMP-13. Finally, recombinant hTryptase-β was able to activate latent pro-MMP-3 and pro-MMP-13 in vitro. The accumulated data suggest that human and mouse tetramer-forming tryptases are MMP convertases that mediate cartilage damage and the proteolytic loss of aggrecan proteoglycans in arthritis, in part, by activating the zymogen forms of MMP-3 and MMP-13 which are constitutively present in articular cartilage.
Keywords: mast cells, inflammation, arthritis, cartilage, aggrecan proteoglycan, matrix metalloproteinases, tryptase
Introduction
The most abundant proteins in human synovial mast cells (MCs) are the family of tetramer-forming β-tryptases encoded by the adjacent TPSAB1 and TPSB2 genes on human chromosome 16p13.3 (1–3). Their orthologs are mouse MC protease (mMCP)-6 (4) and mMCP-7 (5) which are encoded by the corresponding Tpsb2/Mcpt6 and Tpsab1/Mcpt7 genes within the tryptase locus on mouse chromosome 17A3.3. While the inheritance of a non-functional allele of the human TPSAB1 and/or TPSB2 genes is frequent (6,7) (also see the GenBank SNP database for both human genes), no one completely deficient in functional hTryptase-β has been identified, thereby suggesting the importance of these MC-restricted enzymes to our survival. In support of this conclusion, transgenic C57BL/6 (B6) mice lacking both mMCP-6 and mMCP-7 cannot combat bacterial and helminth infections effectively (8–10). Moreover, these tryptase-deficient mice cannot efficiently prevent the thrombin-dependent accumulation of life-threatening fibrin deposits and platelet-fibrin clots in their skin 6 h after the induction of a passive cutaneous anaphylaxis reaction (11).
Despite their beneficial roles in innate immunity and blood coagulation, we (12,13) and others (14–17) discovered that hTryptase-β+ MCs have adverse roles in human rheumatoid arthritis (RA) and osteoarthritis (OA). Nevertheless, the definitive contributions of hTryptase-β and other factors exocytosed from activated MCs in the human synovium remain enigmatic due, in part, to the heterogeneous nature of RA and OA. Investigators therefore have focused their attention on the evaluation of MCs and their varied mediators in different animal models. In this regard, van den Broek and coworkers (18) first noted that antigen-induced arthritis was significantly reduced in MC-deficient KitW/KitW-v mice. Lee and coworkers (19) also observed that inflammatory arthritis was significantly reduced 7–10 d after MC-deficient KitW/KitW-v and KitlS/KitlSl-d mice received arthritogenic K/BxN mouse serum.
The fact that MCs release a variety of factors with contrasting bioactivities in vivo makes it difficult to interpret data from studies carried out on MC-deficient mice. Also problematic, the release of these factors from distinct MC compartments uses different secretory mechanisms and is controlled by a precise balance of activating and inhibitory signaling pathways. The functional contribution of these counterbalancing factors and mechanisms is not appreciated when MC-deficient mice are investigated.
To more definitively evaluate the roles of MCs and their granule mediators (e.g., MC proteinases) in the pathogenesis of arthritis, we created novel inbred B6 mouse lines in which a single gene was disrupted. We then employed these mice in various models. To that end, RasGRP4 is a signaling protein expressed in MCs (20) that controls the release of cytokines and preformed mediators (21). Our inability to induce K/BxN arthritis in RasGRP4-null B6 mice (21) supports the conclusion made in earlier KitW/KitW-v mouse studies that synovial MCs release factors that have adverse roles in acute inflammatory arthritis.
We also created a transgenic B6 mouse line that lacks both mMCP-6 and mMCP-7 (8). We then showed that the severities of K/BxN mouse serum- and methylated BSA/IL-1β-induced arthritis were both significantly reduced in these transgenic mice relative to wild-type (WT) B6 mice (22,23). Although evidence for tryptase redundancy was obtained in those studies, mMCP-6 was more important in K/BxN arthritis than mMCP-7. mMCP-6 is packaged in the secretory granules of synovial MCs ionically bound to heparin-containing serglycin proteoglycans. In support of the data obtained using the mMCP-6-null mice, arthritis was significantly reduced in heparin-deficient B6 mice (22,23).
Aggrecan is the major proteoglycan in cartilage and, together with type-II collagen, gives cartilage its intrinsic weight-bearing properties. The protein core of aggrecan has G1 and G2 globular domains that reside at its N terminus, and a G3 domain at its C terminus. The G1 domain immobilizes aggrecan in the cartilage matrix by binding to hyaluronan. The portion of the core protein that separates the G2 and G3 domains contains the glycosaminoglycans (GAGs) that attract water to confer the osmotic swelling pressure that enables cartilage to resist compressive loads.
Aggrecan degradation (termed aggrecanolysis) is a prominent feature of arthritic diseases, yielding fragments of the proteoglycan that are readily detected in the patient’s cartilage and synovial fluids (24). The most detrimental aggrecanolysis occurs by proteolytic cleavage of the core protein within the interglobular domain (IGD) that separates the G1 and G2 domains. Aggrecan is susceptible to proteolysis by numerous neutral proteinases, including matrix metalloproteinase (MMP)-3, MMP-13, aggrecanase-1/ADAMTS-4, and aggrecanase-2/ADAMTS-5 (25–28). The MMPs and aggrecanases preferentially cleave aggrecan within its IGD at the respective DIPEN↓FFGVG (25,26,29) and TEGE↓ALG (30,31) sites. Aggrecan fragments, detected with neoepitope-specific antibodies that recognize the newly formed C- or N-terminal sequences at the cleavage site, readily distinguish the destructive actions of the MMPs and aggrecanases in arthritic cartilage (32). Although it is well known that both families of neutral proteinases are initially translated as latent zymogens, the mechanisms by which they are activated in arthritic cartilage remains to be determined.
Using a proteomics approach, we identified a prominent soluble fragment of aggrecan lacking its hyaluronan-binding domain in the joints of arthritic WT B6 mice (23). Employing histochemical and immunohistochemical approaches, we discovered that the proteolytic loss of aggrecan in two arthritis models was significantly attenuated in our mMCP-6-null B6 mice (22,23). While these data could be a consequence of fewer proteinase-rich neutrophils in the arthritic joints as a result of the tryptase deficiency, we previously noted that an undefined serine proteinase present in rat peritoneal MCs (PMCs) can initiate cleavage of the N terminus of rat and bovine aggrecan in vitro in an unknown manner, thereby resulting in fragments that cannot recognize hyaluronan (33). It is now known that rat PMCs express the ortholog of mMCP-6 and hTryptase-β.
The unique structural features of tetrameric tryptases (34) predict that they are unable to directly cleave aggrecan because the proteoglycan’s bulky N-terminal G1 and C-terminal G3 domains cannot fit inside the small pore in the tryptase’s donut-shaped tetramer unit where the catalytic site of each monomer resides. We therefore hypothesized that the most likely mechanism by which mMCP-6 and hTryptase-β participate in aggrecanolysis is by activation of an MMP and/or aggrecanase zymogen constitutively present in the joint (23). To evaluate that possibility, we now describe an ex vivo system in which the effects of recombinant hTryptase-β, recombinant mMCP-6, and lysates of WT and mMCP-6-null MCs on cartilage explants can be studied in the absence of neutrophils and other proteinase-expressing inflammatory cells present in the arthritic joint. Using this in vitro system, we now show that mouse and human tetramer-forming tryptases initiate aggrecanolysis in an indirect manner by proteolytically activating the latent zymogen forms of MMP-3 and MMP-13 but not ADAMTS-4 and ADAMTS-5. Thus, there is specificity as to which neutral proteinase zymogen in the joint is susceptible to tryptase-dependent activation.
Materials and Methods
Reagents and animals
Reagents were purchased as follows: recombinant hTryptase-β from Promega (Madison, WI); mMCP-6, hMMP-3, and hMMP-13 from R&D Systems (Minneapolis, MN); 1,9 dimethylmethylene blue dye from Polysciences (Warrington, PA); Percoll from Pharmacia Biotech (Uppsala, Sweden); heparin and trypsin from Sigma-Aldrich (St Louis, MO); RPMI-1640 culture medium from GIBCO (Carlsbad, CA); FBS from Hyclone (Logan, UT), and 8% paraformaldehyde from ProSciTech Microscopy (Thuringowa, QLD, Australia). The fluorometric Sensolyte 520 Generic MMP assay kit used to quantitate MMP enzymatic activity in cartilage explants was obtained from Anaspec (San Jose, CA). WT B6 mice3 were purchased from the Animal Resources Centre (Canning Vale, WA, Australia) or from Australian BioResources (Mossvale, NSW, Australia). The mMCP-6-null B6 mouse line used in this study has been previously described (8). Because the mMCP-6 gene was inactivated in mMCP-7-null B6 embryonic stem cells using a homologous recombination approach, the MCs in this transgenic mouse line cannot express mMCP-6 and mMCP-7. Chloe B6 mice have a knock-in mutation in the aggrecan gene that changes the sequence in the IGD from DIPEN↓FFG to DIPEN↓GTR for the purpose of blocking MMP-dependent cleavage of aggrecan (35). All animal studies were reviewed and approved by the Animal Care and Ethics Committee of the University of New South Wales.
Evaluation of the susceptibility of purified aggrecan to hTryptase-β
Purified porcine aggrecan (200 μg/ml) was incubated for 16 h at 37°C in RPMI-1640 medium alone, or medium containing either 12 μg/ml hTryptase-β, 50 ng hMMP-3, or 50 ng hMMP-3 activated with 1 mM 4-aminophenyl-mercuric acetate (APMA; Sigma-Aldrich). Following incubation, samples were deglycosylated with 0.01 U chondroitinase ABC and 60 U keratanase (Seikagaku Corp, Tokyo, Japan). The treated samples were analyzed by SDS-PAGE immunoblotting with the mouse monoclonal 2B6 antibody (36) which recognizes the chondroitinase ABC-generated stubs of chondroitin sulfate remaining attached to the peptide core.
Isolation of mouse PMCs
PMCs were obtained by peritoneal lavage of WT and mMCP-6-null B6 mice with modified Tyrode’s buffer (140 mM NaCl, 2.7 mM KCl, 12 mM NaHCO3, 4.2 mM NaH2PO4, 5.6 mM D-glucose, and 0.1% w/v gelatin). The two groups of mice (aged > 3 months) were sacrificed, and 10 ml of modified Tyrode’s buffer was injected intraperitoneally into each animal. The abdomen was gently massaged for ~30 sec, and then the lavage fluid was aspirated. The cellular exudates collected from twenty WT and mMCP-6-null B6 mice were purified using a Percoll gradient as described (37). The resulting cell pellets of purified PMCs were washed in RPMI-1640 medium supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin, and then stored at −80°C.
Isolation and culture of femoral head cartilage explants
The dissection of femoral heads from 3-wk old WT or Chloe (35) B6 mice was done as previously described (32). The explants were cultured immediately after their removal from the mice, or were freeze-thawed to kill their endogenous chondrocytes prior to culture. The explants were placed in serum-free RPMI-1640 medium supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C with 5% CO2. After 48 h, the conditioned medium was discarded to remove aggrecan fragments released by non-enzymatic, passive loss. Thereafter, the explants were cultured for an additional 24 h in fresh FBS-free RPMI-1640 medium without an additive or with trypsin (5 μg/ml), hTryptase-β (15 μg/ml), mMCP-6 (15 μg/ml), or lysates from 105 lysed PMCs isolated from WT or mMCP-6-null B6 mice. Where indicated, cartilage explants were cultured with the above additives in the presence of 50 μM MMP-3 Inhibitor I (Ac-RCGVPD-NH2; CalBiochem, Darmstadt, Germany) or 80 nM MMP-13 Inhibitor [pyrimidine-4, 6-dicarboxylic acid, bis-(4-fluoro-3-methyl-benzylamide); CalBiochem].
Measurement of MMP activity in femoral head cartilage explants
To measure MMP activity in the femoral head explants, proteins were extracted by placing the tissue in 50 mM Tris-HCl, pH 7.5, containing 10 mM CaCl2, and 2 M guanidine hydrochloride at 4°C for 24 h with gentle rotation. The resulting extracts were dialyzed at 4°C for 48 h against 50 mM Tris-HCl, pH 7.5 supplemented with 0.25 M NaCl, 10 mM CaCl2, and 0.2% Brij-35 (Sigma-Aldrich). The dialysates were concentrated using an Amicon Ultra-0.5 centrifugal filter unit (Millipore, Billerica, MA). The total protein content of the samples was measured employing a bicinchoninic acid protein assay (Bio-Rad, Hercules, CA). The amount of enzymatically active MMP in each sample was then determined using the Sensolyte 520 Generic MMP assay kit. Briefly, 1 μg of total extracted protein was incubated with the FAM-QXL 520 FRET substrate for 1 h in a 96-well plate at room temperature. A standard was obtained using 5-FAM-Pro-Leu-OH. The measurements were read using a Perkin Elmer Victor 3 1420 (Turku, Finland) multi-labeled counter with excitation at 485 nm and emission at 535 nm. Values were normalized to the concentration of the product of enzymatic reaction per microgram of protein.
Histochemistry and immunohistochemistry of femoral head cartilage explants
To assess the degree of cartilage degradation histologically after 24 h of culture, femoral head explants were fixed in 4% paraformaldehyde for 48 h, decalcified in 30% formic acid, and paraffin embedded. Tissue sections were cut at 4 μm and stained with 0.05% toluidine blue (pH 2.5). Immunohistochemistry was done on replicate tissue sections deglycosylated with 0.05 U/ml chondroitinase ABC for 30 min at room temperature. Alternately, they were incubated with a 1:1000 dilution of proteinase K (Roche, Mannheim, Germany) at 37°C for 10 min for detection of the DIPEN neoepitope. Endogenous peroxidase activity was blocked by incubating sections with 3% hydrogen peroxide, while non-specific protein binding was blocked using Dako’s serum-free protein block (Glostrup, Denmark). Sections were incubated overnight at 4°C with 5 μg/ml rabbit anti-mouse aggrecan antibody (AB1031; Millipore), with 0.6 μg/ml rabbit anti-DIPEN antibody, or with a control rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA). In each instance, this was followed by incubation with a 1:600 dilution of goat anti-rabbit horseradish peroxidase-conjugated antibody (Bio-Rad). Immunoreactivity was detected by reaction with the colorimetric DAB substrate reagent (Dako), and counterstaining with Meyer’s haematoxylin (Sigma-Aldrich).
Measurement of aggrecanolysis
To quantify aggrecan released from the cartilage explants, the conditioned medium was collected and the remaining cartilage was digested overnight at 60°C in 0.1 M sodium acetate pH 5.5 buffer containing 5 mM EDTA, 5 mM L-cysteine, and 125 μg/ml papain (Sigma-Aldrich). The liberated GAGs in the culture medium and in the papain digests were assayed as a surrogate measure of aggrecan content using the 1,9-dimethylmethylene blue assay (38) with chondroitin sulfate from shark cartilage (Sigma-Aldrich) employed as a standard. Together, the two samples represent the total aggrecan content of each femoral head explant. The percentage of aggrecan released into the medium in each instance was calculated relative to the total content.
Immunoblotting of aggrecan and its fragments
Pooled samples of the collected conditioned media were dialyzed against 10 mM EDTA, concentrated, deglycosylated with 0.01 U chondroitinase ABC, and electrophoresed on 10% SDS-PAGE reducing gels. Immunoblots of the separated proteins were evaluated for the presence of the DIPEN and FFGVG neoepitopes (39) which are specific for the MMP-mediated cleavage of aggrecan at the DIPEN↓FFGVG sequence. The blots were then evaluated for the presence of the ALG neoepitope (39) which is specific for the ADAMTS-4/ADAMTS-5-mediated cleavage of the extracellular matrix proteoglycan at its TEGE↓ALG sequence.
Incubation of latent pro-MMP-3 and pro-MMP-13 with hTryptase-β
Recombinant human pro-MMP-3 (3 μg/ml) and pro-MMP-13 (3 μg/ml) were incubated with recombinant hTryptase-β (15 μg/ml) alone or in the presence of 50 μM MMP-3 inhibitor, 80 nM MMP-13 inhibitor, or 1 mM APMA. The reactions were carried out in a 50-μl final reaction volume of 50 mM Tris-HCl, pH 7.5, 0.15 M NaCl, 10 mM CaCl2, and 0.05% Brij-35 (w/v) at 37°C for 24 h to activate pro-MMP-3, and 2 h to activate pro-MMP-13. The samples were electrophoresed on 10% SDS-PAGE gels under reducing conditions, and visualized by silver staining to identify active enzyme. A Sensolyte-520 generic MMP activity assay was carried out as described above by analyzing samples containing 100 ng of pro-MMP-13 to measure the extent of activation.
Results
hTryptase-β induces aggrecanolysis in cultured femoral head explants
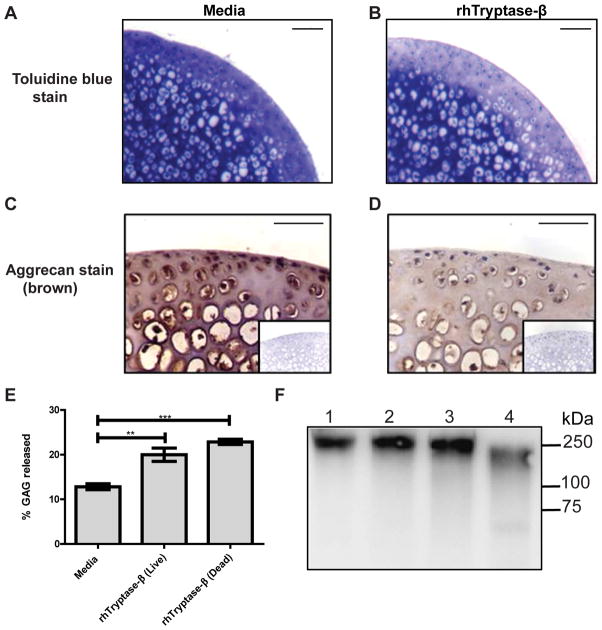
To determine whether or not MC-restricted tetramer-forming tryptases could induce aggrecanolysis in cartilage, femoral head explants were isolated from 3-wk old WT B6 mice and then cultured ex vivo in the presence or absence of recombinant hTryptase-β. Toluidine blue staining (Fig. 1, A and B) and immunohistochemistry (Fig. 1, C and D) of the treated tissue showed appreciably less aggrecan in the explants that had been exposed to hTryptase-β for 24 h (Fig. 1, B and D) relative to replicate explants that had been exposed to culture media alone (Fig. 1, A and C). As expected, toluidine blue loss preferentially occurred in the articular cartilage region of the explant because it is physically difficult for the ~150-kDa, tetramer-forming tryptase to diffuse deep into the tissue biopsy. These data indicated that the MC-restricted tetramer-forming tryptases can induce loss of aggrecan from the cartilage in the absence of neutrophils and the other inflammatory cells that accumulate in arthritic joints.
FIGURE 1.
Induction of aggrecanolysis in cartilage by hTryptase-β. A-D, Measurement of aggrecan in sections of femoral head explants using toluidine blue staining (A, B) and immunohistochemistry with anti-aggrecan antibodies (C, D) showed appreciably greater aggrecan loss from cartilage when explants were incubated for 24 h with 15 μg/ml rhTryptase-β (B, D) compared to culture media alone (A, C). Isotype controls for immunohistochemistry are inset in the panels; scale bars = 50 μm. E, rhTryptase-β (15 μg/ml) induced significant release of aggrecan-derived GAGs from both live and dead femoral head explants compared to media alone. ** p < 0.01; ***, p < 0.001; error bars represent S.E.M.; n = 3. F, rhTryptase-β (12 μg/ml, lane 2) and pro-MMP-3 (50 ng, lane 3) were unable to directly degrade purified pig aggrecan (200 μg/ml) when incubated for 16 h, compared to incubation of aggrecan with media alone (lane 1), or with 50 ng pro-MMP-3 activated with 1 mM APMA (lane 4). Samples were analyzed by immunoblotting using the 2B6 antibody that recognizes the chondroitinase ABC-generated chondroitin sulfate stubs.
Potentially, hTryptase-β could promote aggrecanolysis in cartilage explants by inducing chondrocytes to increase their transcription and translation of an aggrecan-degrading proteinase such as an MMP or ADAMTS. To address that possibility, we compared hTryptase-β-induced aggrecan loss from freshly-isolated live cartilage explants and explants in which chondrocytes were killed by freeze-thawing. Untreated explants that encountered just culture medium released baseline levels of aggrecan. Explants incubated with hTryptase-β released significantly more aggrecan into the conditioned medium whether the explants contained live or dead chondrocytes (Fig. 1E). These data indicate that neither living chondrocytes nor changes in their gene expression are required for hTryptase-β-mediated aggrecanolysis.
To evaluate whether hTryptase-β induced aggrecanolysis via direct cleavage of aggrecan, the purified proteoglycan was incubated with hTryptase-β for 16 h. SDS-PAGE immunoblotting using the 2B6 antibody, which binds to the GAG-attachment region that resides between the G2 and G3 domains, showed that hTryptase-β was unable to cleave the 250-kDa core protein of aggrecan efficiently (Fig. 1F). The accumulated data therefore suggested an indirect mechanism for tryptase-mediated aggrecanolysis, namely by activating an undefined proteinase zymogen constitutively present in the explants.
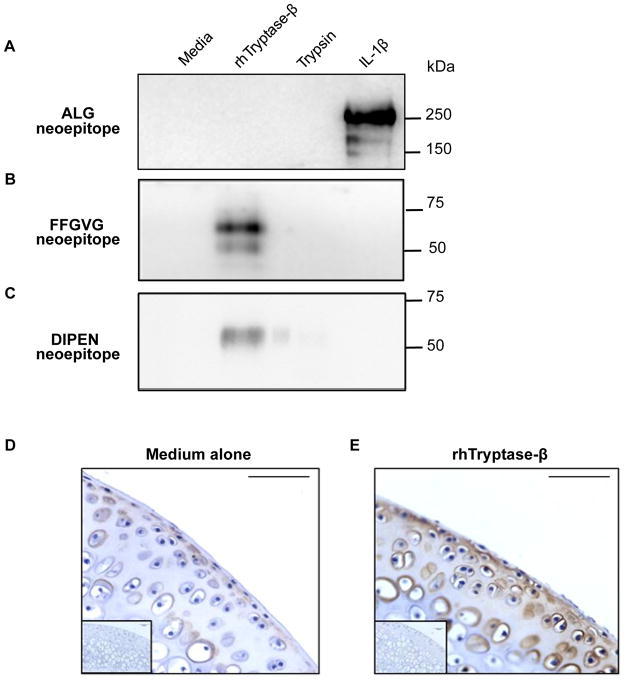
The aggrecan fragments released from the explants into the culture media were next analyzed by immunoblotting. Stimulation of freshly-isolated live explants with IL-1β was used as a positive control experiment because this cytokine induces chondrocytes to increase their expression of enzymatically active aggrecanases which cleave the proteoglycan’s core protein to generate a ~250-kDa fragment that possesses the N-terminal ALG neoepitope, as noted in lane 4 of Figure 2A. Incubation of living (not shown) or devitalized explants with recombinant hTryptase-β did not result in generation of aggrecan fragments that possessed the ALG neoepitope (Fig. 2A, lane 2). In contrast, when the devitalized explants were exposed to the MC tryptase, a prominent fragment containing the N-terminal FFGVG neoepitope was released into the culture media (Fig. 2B, lane 2). Immunostaining of hTryptase-β-exposed cartilage explants demonstrated the presence of the aggrecan fragment that possessed the C-terminal DIPEN neoepitope (Fig. 2E). As expected, this aggrecan fragment was not detected in the explants that had been cultured in medium alone (Fig. 2D). Aggrecan fragments that contained the C-terminal DIPEN neoepitope also were released into the media after exposure to hTryptase-β (Fig. 2C, lane 2). The obtained data were consistent with cleavage of aggrecan by an hTryptase-β-activatable MMP that was constitutively present in the cartilage explants.
FIGURE 2.
Aggrecan degradation products induced by hTryptase-β possess MMP- but not ADAMTS-dependent neoepitopes. A-C, Immunoblots of conditioned media from devitalized femoral head explants cultured in media alone, rhTryptase-β (15 μg/ml), trypsin (5 μg/ml), or live cartilage explants stimulated to induce aggrecanase activity using rhIL-1β (10 ng/ml), probed with antibodies that detect the aggrecanase-specific ALG neoepitope (panel A), the MMP-specific FFGVG neoepitope (panel B), or the MMP-specific DIPEN neoepitope (panel C). D and E, Immunohistochemistry with anti-DIPEN antibody in sections of femoral head explants cultured in the absence (D) or presence of 15 μg/ml rhTryptase-β (E). Isotype controls for immunohistochemistry are inset in the panels; scale bars = 50 μm.
Human and mouse MC-restricted tryptases induce MMP activity in femoral head explants
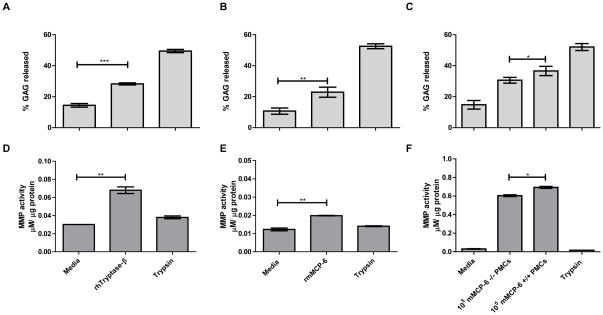
Many allelic isoforms of hTryptase-β have been identified by the Human Genome Consortium due to numerous point mutations in the human TPSAB1 and TPSB2 genes (see the SNP database for GenBank Gene IDs 7177 and 64499). Other isoforms of hTryptase-β have been identified due to differential splicing of the precursor transcripts. To ensure that there was nothing peculiar about the recombinant hTryptase-β isoform we used in our study, we next evaluated the effects of recombinant mMCP-6 in our ex vivo system. Since incubation of explants with hTryptase-β resulted in the release into the conditioned medium of aggrecan fragments that were consistent with MMP-dependent proteolysis (Fig. 2, B and C, lane 2), we measured the MMP enzymatic activity in protein extracts from the explants after a 24-h incubation with hTryptase-β or mMCP-6. As noted in Figures 3D and 3E, both MC-restricted tetramer-forming tryptases induced significantly more MMP activity in the devitalized cartilage explants than replicate explants cultured in media alone. The increase in MMP enzymatic activity occurred in parallel with a significantly greater release of aggrecan fragments into the culture media (Fig. 3, A and B).
FIGURE 3.
Human and mouse MC tryptases induce MMP activity in femoral head explants. A-F, Aggrecan-derived GAG released into the culture media from femoral head explants (panels A, B, and C) and MMP enzymatic activity in extracts from femoral head explants (panels D, E, and F) following incubation with 15 μg/ml rhTryptase-β (panels A and D), 15 μg/ml rmMCP-6 (panels B and E), or lysates isolated from 105 mMCP-6+/+ or mMCP-6−/− PMCs (panels C and F). Culture of femoral heads with media alone or 5 μg/ml trypsin represent negative and positive controls for optimal GAG release respectively. *, p < 0.05; **, p < 0.01; ***, p < 0.001; error bars represent S.E.M.; n = 3.
To determine whether the results obtained using recombinant mMCP-6 and hTryptase-β were representative of the native tetramer-forming tryptases in MCs, devitalized explants were incubated for 24 h in the presence of lysates of mMCP-6+ PMCs isolated from WT B6 mice. To control for the possible effects of other granule neutral proteinases in these lysates (e.g., endogenous mMCP-4, mMCP-5, cathepsin G, and carboxypeptidase A3), the results were compared to lysates of PMCs isolated from mMCP-6-null B6 mice (Fig. 3F). The results showed that lysates of WT mouse PMCs induced the explants to express significantly more enzymatically active MMP than the lysates from mMCP-6-null PMCs. This was associated with a concomitant increase in aggrecan loss from the explants (Fig. 3C).
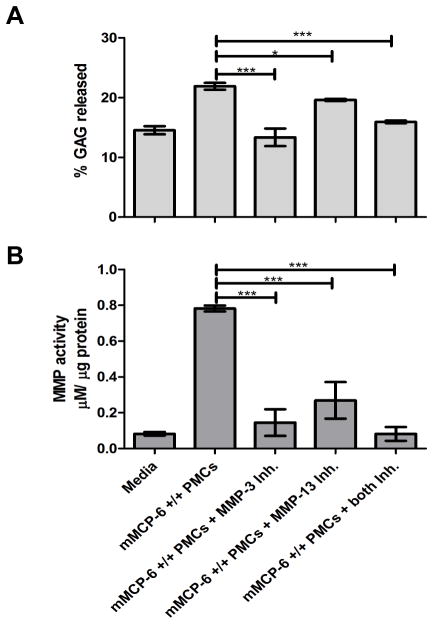
MC tryptase-induced aggrecanolysis is abrogated by specific MMP inhibitors
To assess the in vitro consequences of blocking resident MMPs in the cartilage matrix, devitalized femoral head explants were cultured in media containing lysates of the PMCs isolated from WT and mMCP-6-null B6 mice in the presence or absence of inhibitors of MMP-3 or MMP-13. Figure 4 shows the effects of the two MMP inhibitors on the ability of mMCP-6 from WT PMCs to generate enzymatically active MMPs in the cartilage explants after deduction from each relevant condition of the effects that the mMCP-6-null PMC granules had on aggrecanolysis and MMP activity. The MMP-3 inhibitor significantly reduced the mMCP-6-induced MMP enzymatic activity in the explants (Fig. 4B). Moreover, the inhibitor dampened the mMCP-6-mediated loss of aggrecan (Fig. 4A). The MMP-13 inhibitor also significantly abrogated, but did not completely inhibit, mMCP-6-induced MMP activity in the explants (Fig. 4B) and the subsequent aggrecan loss (Fig. 4A). In the presence of both inhibitors, mMCP-6-mediated MMP activity was further reduced compared with the effects of each inhibitor alone. In support of this finding, aggrecan release also was significantly reduced.
FIGURE 4.
MC tryptase-mediated aggrecanolysis is abrogated by MMP-specific inhibitors. A-B, Aggrecan-derived GAGs released into culture media from femoral head explants (A) and MMP enzymatic activity in extracts from femoral head cartilage explants (B) following incubation with granule proteins isolated from 105 mMCP-6+/+ PMCs alone, in the presence of a selective MMP-3 inhibitor (Inh; 50 μM), a selective MMP-13 Inh (80 nM), or in the presence of both MMP-3 and MMP-13 Inh. *, p < 0.05; ***, p < 0.001; error bars represent S.E.M.; n = 3.
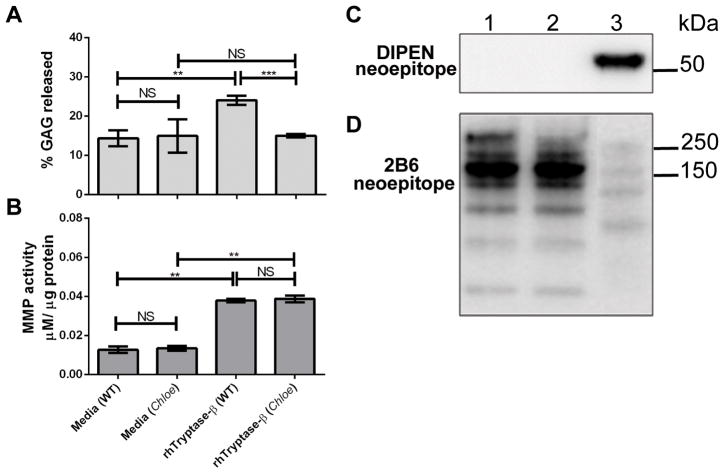
Tryptase is unable to induce aggrecanolysis in femoral head explants from mice resistant to MMP-mediated aggrecan cleavage
Femoral head explants were isolated from Chloe B6 mice. This mouse line carries a mutation in the aggrecan gene that renders the expressed cartilage proteoglycan resistant to MMP cleavage at its DIPEN↓FFGVG site. Similar to the effect on WT cartilage explants, hTryptase-β induced comparable levels of MMP activity when incubated with devitalized explants from Chloe B6 mice (Fig. 5B). Despite this activation of MMPs in Chloe cartilage, hTryptase-β did not induce significant GAG release from the explants of Chloe mice compared to media alone (Fig. 5A). Aggrecan within Chloe explants exposed to hTryptase-β was more resistant to degradation (Fig. 5D, lane 2) than WT explants exposed to hTryptase-β (Fig. 5D, lane 3). Moreover, aggrecan fragments released from Chloe explants exposed to hTryptase-β lacked the MMP-mediated DIPEN neoepitope (Fig. 5C, lane 2), which was evident in fragments released from WT explants (Fig. 5C, lane 3).
FIGURE 5.
hTryptase-β is unable to induce aggrecanolysis in femoral heads from mice resistant to MMP-mediated proteolysis of aggrecan. A-B, Aggrecan-derived GAG released into culture media from femoral head explants (A) and MMP enzymatic activity in extracts from femoral head explants of Chloe and WT mice (B) following incubation with media or 15 μg/ml rhTryptase-β. **, p < 0.01; ***, p < 0.001; NS, not significant; error bars represent S.E.M.; n = 3. C-D, Immunoblots of conditioned media from devitalized femoral head explants of Chloe mice (lanes 1 and 2), or WT mice (lane 3) cultured in media alone (lane 1), or rhTryptase-β (15 μg/ml; lanes 2 and 3), probed with antibodies that detect the MMP-specific DIPEN neoepitope (panel C) or the aggrecan core protein using the 2B6 antibody (panel D).
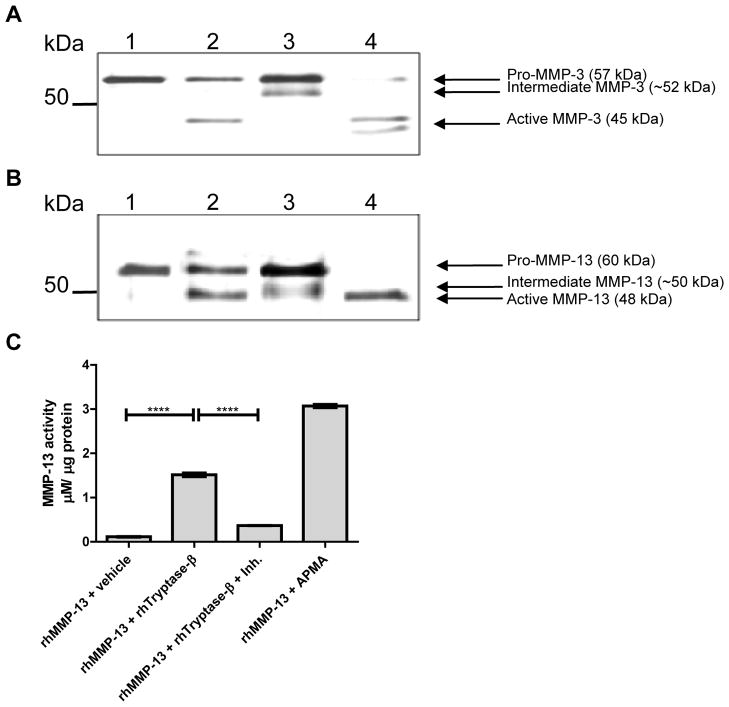
Tryptase activates latent pro-MMP-3 and pro-MMP-13 which are abrogated by specific inhibitors of these neutral proteinases
Finally, to confirm that tetramer-forming tryptases can activate pro-MMP-3 and pro-MMP-13 in vitro, the recombinant human zymogens were incubated with recombinant hTryptase-β. As shown in Figure 6A, hTryptase-β converted latent 57-kDa pro-MMP-3 into its enzymatically active 45-kDa form (lane 2). Similarly, hTryptase-β converted the latent 60-kDa form of pro-MMP-13 into its active 48-kDa form (Fig. 6B, lane 2). However, the pro-forms of MMP-3 and MMP-13 were not as completely activated, as detected following incubation with APMA (Fig. 6, A and B, lanes 4). In the presence of a selective MMP-3 or MMP-13 inhibitor, an intermediate band was generated at ~52 kDa and ~50 kDa, respectively. In addition, the active forms of the respective MMPs at 45 and 48 kDa were not detected (Fig. 6, A and B, lanes 3). The presence of enzymatically active MMP-13 in the hTryptase-β treated sample was confirmed using an enzymatic assay (Fig. 6C). The accumulated data suggest that MMP-3 and MMP-13 are the primary neutral proteinases responsible for the tryptase-induced aggrecanolysis observed in this ex vivo system.
FIGURE 6.
hTryptase-β activates pro-MMP-3 and pro-MMP-13 in vitro. A, rhpro-MMP-3 (3 μg/ml) was incubated alone (lane 1), with 15 μg/ml rhTryptase-β (lane 2), with 15 μg/ml rhTryptase-β and 50 μM MMP-3 inhibitor (lane 3), or with 1 mM APMA (lane 4) for 24 h at 37 °C. B, rhpro-MMP-13 (3 μg/ml) was incubated alone (lane 1), with 15 μg/ml rhTryptase-β (lane 2), with 15 μg/ml rhTryptase-β and 80 nM MMP-13 inhibitor (lane 3), or with 1 mM APMA (lane 4) for 2 h at 37 °C. C, rhMMP-13 enzymatic activity was measured following incubation with rhTryptase-β. ****, p < 0.0001; error bars represent S.E.M.; n = 3.
Discussion
The number of hTryptase-β+ MCs increase up to 25-fold within the synovium of patients with RA or OA (12,13,17). mMCP-6 is the ortholog of hTryptase-β, and the MCs in the joints of arthritic B6 mice express this tryptase, which has been implicated in joint inflammation and aggrecanolysis. It is a tetramer-forming tryptase that mediates leukocyte recruitment, in part, by inducing fibroblast-like synoviocytes (23) and endothelial cells (40) to increase their expression of neutrophil-responsive chemokines. mMCP-6-null B6 mice have impaired recruitment of neutrophils into their peritoneal cavity during a bacterial infection (8) and into synovial joints when two models of inflammatory arthritis were evaluated (22,23). In the latter disease models, aggrecan depletion was markedly attenuated in the arthritic joints of mMCP-6-null mice. It remained to be determined if these data were due to a primary effect of mMCP-6 on cartilage, or was a consequence of decreased extravasation into the joint of neutrophils which are destructive granulocytes that express MMP-8/neutrophil collagenase, elastase, and cathepsin G.
There is some evidence that MC β tryptases have roles in extracellular matrix proteolysis. hTryptase-β has been reported to possess potent gelatin degrading properties, similar to that seen by the gelatinases MMP-2 and MMP-9 (41). The MC enzyme also has been reported to degrade partially denatured type-I collagen, both prominent features in the degradation of the extracellular matrix in normal and pathological conditions such as RA (41). However, there have been no previous reports that a MC-tryptase has aggrecanolysis properties. A model of ex vivo aggrecanolysis was therefore used to determine how mouse and human tetramer-forming tryptases induce aggrecan depletion in the absence of neutrophils and other inflammatory cells. Our results revealed that these MC tryptases mediate rapid (within 24 h) loss of aggrecan from normal cartilage explants, with quantitatively significant aggrecan-derived GAG release into the culture medium.
Native aggrecan has a molecular weight that exceeds 1 million Da. The unique donut-shaped hole in the tetramer unit of mMCP-6 and hTryptase-β where the active site of each monomer resides (34) makes it highly unlikely that these serine proteinases can proteolytically cleave aggrecan even if the proteoglycan’s GAG chains are removed by chondroitinases. As expected, these MC tetramer-forming tryptases were unable to directly cleave the core protein of the large sized aggrecan proteoglycan.
Because aggrecanolysis initiated by hTryptase-β and mMCP-6 did not require living chondrocytes, these tetramer-forming proteinases initiate aggrecanolysis indirectly via activation of one or more proteinase zymogens which are constitutively present in cartilage. It is important to note however that the secretory granule proteins from PMCs isolated from both WT and mMCP-6-null mice induced significantly greater aggrecan proteolysis and MMP activity than media alone. These findings indicate that PMCs contain at least two factors that mediate aggrecanolysis, one of which is mMCP-6. Of the other possible factors, MCs themselves are known to express MMPs (42) as well as other proteinases [e.g., chymases (43)] that can potentially activate MMPs.
As aggrecan catabolism in arthritic cartilage is collectively orchestrated by the ADAMTS and MMP families of neutral proteinases, we next analyzed the conditioned media for aggrecan fragments known to be generated by these two proteinase families. Our findings revealed that these fragments contained either the C-terminal DIPEN neoepitope or the N-terminal FFGVG neoepitope which are indicative of MMP-dependent proteolysis. Not detected were aggrecan fragments that contained the ALG neoepitope caused by ADAMTS-4 and ADAMTS-5 cleavage. Aggrecan fragments containing the C-terminal DIPEN neoepitope also were detected within the cartilage of tryptase-treated explants. Moreover, the amount of MMP enzymatic activity was higher in the cartilage explants that encountered the MC tryptases. In support of these data, substantial proteolysis of aggrecan did not occur when explants from Chloe mice were incubated with hTryptase-β. These data implicate tetramer-forming tryptases in a relatively early phase of aggrecan degradation in arthritis by activating latent MMPs which then cleave the core protein of aggrecan proteoglycan at its N-terminal DIPEN↓FFGVG site. The importance of identifying the onset of MMP-driven aggrecan catabolism is a necessary step in preventing the irreversible joint destruction evident in RA and OA, as previous studies have shown MMP-mediated degradation of the aggrecan core protein to be associated with the complete loss of an ability to repair damage to type-II collagen fibrils and severe attenuation of aggrecan synthesis by chondrocytes (44).
Within the inflamed joint, immunolocalization studies reveal high levels of extracellular tryptase at sites of cartilage erosion, which correlate with the detection of MMP-3 at sites of MC distribution (45). The discovery of an intermediate form of MMP-3 suggests that hTryptase-β and mMCP-6 use a nibbling approach to remove the inhibitory propeptide of pro-MMP-3 (Fig. 6A). MMP-3 can cleave many of the components of cartilage’s extracellular matrix, and MMP-3 is believed to be a key activator of the collagenase subfamily of MMPs (e.g., MMP-1, MMP-8, and MMP-13) (46–48). The importance of enzymatically active MMP-3 in antigen-induced arthritis was shown by van Meurs and coworkers in terms of its ability to activate procollagenases to expedite collagenolysis, as well as to generate the aggrecan fragment that results in the DIPEN neoepitope (49). Indeed, the findings that the levels of activated MMP-3 in the serum correlate with the radiographic progression of joint damage in patients with RA re-iterates the importance of this neutral proteinase in RA pathogenesis (50).
Like MMP-3, MMP-13 is thought to have a key role in the joint destruction observed in patients with RA. Both of these proteinases are selectively up-regulated by fibroblast-like synoviocytes and chondrocytes within the rheumatoid lesion (51). MMP-13 alone participates in the degradation of aggrecan and type-II collagen; it is often found at sites of cartilage erosion (52). A deficiency in this MMP was recently shown to significantly impair cartilage erosion in a mouse model of OA (53). Inhibition of MMP-13 also results in significantly reduced cartilage erosion in collagen-induced arthritis and in the SCID mouse co-implantation animal model of RA (54). We now show that pro-MMP-13 also is efficiently activated by hTryptase-β. This was an unexpected finding due to substantial negative charge of the propeptides of mouse and human pro-MMP-13.
As observed with MMP-3, the activation cascade of latent pro-MMP-13 by hTryptase-β to its mature 48-kDa form appears to occur in a stepwise fashion since a 50-kDa intermediate was initially detected (Fig. 6B). The likely reason why mature MMP-3 and MMP-13 are not susceptible to hTryptase-β or mMCP-6 is because their large hemopexin domains cannot physically gain access to the tetramer ring. In contrast, the tryptase-susceptible propeptide domains of these MMPs are solvent exposed and accessible to proteolytic attack.
As in RA, there is a growing recognition of the contribution of MCs within the joint of patients with OA. Although there is relatively little evidence for inflammation in OA, the detection of MC mediators in synovial fluid (16) and increases in MC numbers within the OA synovium (15) suggest potential involvement in the pathogenesis of this degenerative joint disease. MCs are one of the primary inflammatory cells represented in the OA synovium, with a selective increase in the number of tryptase-positive, chymase-negative MCs (55). To date, there are no published studies that have evaluated the roles of MC-tryptases in OA. In support of a potential involvement of tryptases in the osteoarthritic joint, MMP-13 is the primary collagenase responsible for the degradation of type-II collagen fibrils in human OA (56). Findings collected from a transgenic mouse model of MMP-13 over-expression detected significant cartilage erosion due to collagen and aggrecan depletion resembling the joint destruction evident in this degenerative disease (57). Thus, the implications of our study presented here may be as significant for OA as for RA.
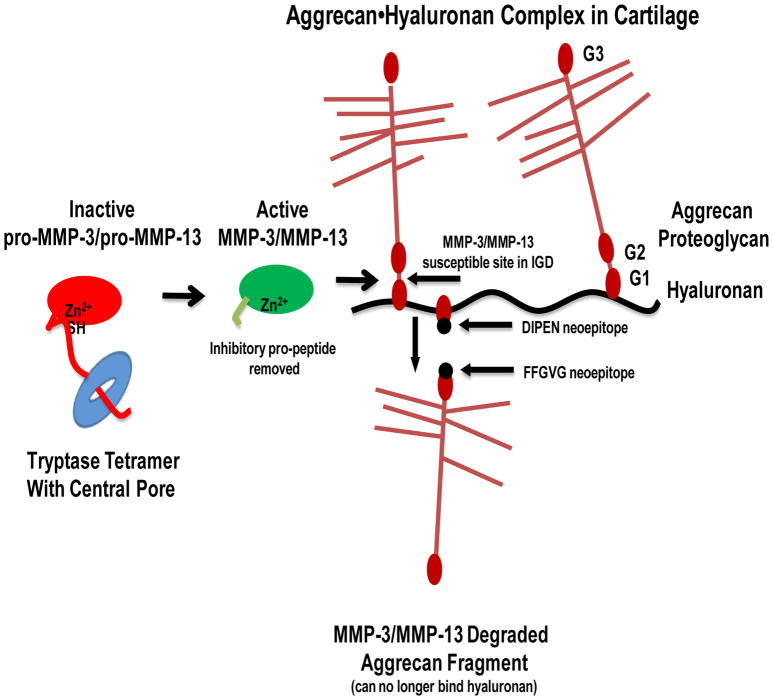
Our data, summarized in Figure 7, highlight a novel immunopathological role for MC-restricted tetramer-forming tryptases in joint pathology and loss of aggrecan by activation of latent MMP-3 and MMP-13 constitutively present in cartilage. With the recognized role of mMCP-6 and hTryptase-β in inducing leukocyte recruitment, and together with recent studies suggesting that synoviocytes in the synovial tissue promote the accumulation of mMCP-6 within the arthritic joints (58), hTryptase-β and mMCP-6 affect inflammatory joint pathology in at least two ways, namely by promoting the extravasation of neutrophils into the joint and secondly by activating some of the MMP zymogens constitutively present in cartilage. No endogenous protein has been identified in humans that is an effective inhibitor of MC tetramer-forming tryptases. Although the beneficial immunological roles of MC-restricted tetramer-forming tryptases in innate immunity (8–10) cannot be over looked, our data raise the possibility that chemical inhibition of the closely related proteinases derived from the TPSAB1 and TPSB2 genes might have therapeutic benefit in patients with arthritis.
FIGURE 7.
Schematic of the mechanisms of aggrecanolysis induced by MC-derived tetramer-forming tryptases in the rheumatic joint. Within the inflamed arthritic joint, degranulation of mouse and human MCs results in respective release of mMCP-6 or hTryptase-β into the synovial tissue and joint space. The exocytosed tetramer-forming tryptases activate latent pro-MMP-3 and pro-MMP-13 that are constitutively produced by fibroblast-like synoviocytes, chondrocytes, and other cell types in the arthritic cartilage. This situation then results in the proteolysis of aggrecan and the release of fragments into the synovial fluid that can no longer bind hyaluronan.
Abbreviations used in this article
- APMA
4-aminophenyl-mercuric acetate
- B6
C57BL/6
- GAG
glycosaminoglycan
- IGD
interglobular domain
- MC
mast cell
- mMCP
mouse MC protease
- MMP
matrix metalloproteinase
- OA
osteoarthritis
- PMC
peritoneal MC
- RA
rheumatoid arthritis
- and WT
wild-type
Footnotes
This work was funded in part by the National Institutes of Health AI059746 (RLS), AI065858 (RLS), a research fellowship from the Harvard Club of Australia Foundation (RLS), Cancer Prevention and Research Institute of Texas (CPRIT) RP110166 (RA), and project grants from Arthritis Australia (HPM).
In contrast to most WT mouse strains, the MCs in WT B6 mice cannot express mMCP-7 due to an exon 2/intron 2 splice-site mutation in its gene.
References
- 1.Schwartz LB, Lewis RA, Austen KF. Tryptase from human pulmonary mast cells: purification and characterization. J Biol Chem. 1981;256:11939–11943. [PubMed] [Google Scholar]
- 2.Miller JS, Moxley G, Schwartz LB. Cloning and characterization of a second complementary DNA for human tryptase. J Clin Invest. 1990;86:864–870. doi: 10.1172/JCI114786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanderslice P, Ballinger SM, Tam EK, Goldstein SM, Craik CS, Caughey GH. Human mast cell tryptase: multiple cDNAs and genes reveal a multi-gene serine protease family. Proc Natl Acad Sci USA. 1990;87:3811–3815. doi: 10.1073/pnas.87.10.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reynolds DS, Gurley DS, Austen KF, Serafin WE. Cloning of the cDNA and gene of mouse mast cell protease 6: transcription by progenitor mast cells and mast cells of the connective tissue subclass. J Biol Chem. 1991;266:3847–3853. [PubMed] [Google Scholar]
- 5.McNeil HP, Reynolds DS, Schiller V, Ghildyal N, Gurley DS, Austen KF, Stevens RL. Isolation, characterization, and transcription of the gene encoding mouse mast cell protease 7. Proc Natl Acad Sci USA. 1992;89:11174–11178. doi: 10.1073/pnas.89.23.11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soto D, Malmsten C, Blount JL, Muilenburg DJ, Caughey GH. Genetic deficiency of human mast cell α tryptase. Clin Exp Allergy. 2002;32:1000–1006. doi: 10.1046/j.1365-2222.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 7.Trivedi NN, Tamraz B, Chu C, Kwok PY, Caughey GH. Human subjects are protected from mast cell tryptase deficiency despite frequent inheritance of loss-of-function mutations. J Allergy Clin Immunol. 2009;124:1099–1105. doi: 10.1016/j.jaci.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thakurdas SM, Melicoff E, Sansores-Garcia L, Moreira DC, Petrova Y, Stevens RL, Adachi R. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J Biol Chem. 2007;282:20809–20815. doi: 10.1074/jbc.M611842200. [DOI] [PubMed] [Google Scholar]
- 9.McNeil HP, Adachi R, Stevens RL. Mast cell-restricted tryptases: structure and function in inflammation and pathogen defense. J Biol Chem. 2007;282:20785–20789. doi: 10.1074/jbc.R700017200. [DOI] [PubMed] [Google Scholar]
- 10.Shin K, Watts GF, Oettgen HC, Friend DS, Pemberton AD, Gurish MF, Lee DM. Mouse mast cell tryptase mMCP-6 is a critical link between adaptive and innate immunity in the chronic phase of Trichinella spiralis infection. J Immunol. 2008;180:4885–4891. doi: 10.4049/jimmunol.180.7.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prieto-Garcia A, Zheng D, Adachi R, Xing W, Lane WS, Chung K, Anderson P, Hansbro PM, Castells M, Stevens RL. Mast cell restricted mouse and human tryptase-heparin complexes hinder thrombin-induced coagulation of plasma and the generation of fibrin by proteolytically destroying fibrinogen. J Biol Chem. 2012;287:7834–7844. doi: 10.1074/jbc.M111.325712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gotis-Graham I, McNeil HP. Mast cell responses in rheumatoid synovium: association of the MCTC subset with matrix turnover and clinical progression. Arthritis Rheum. 1997;40:479–489. doi: 10.1002/art.1780400314. [DOI] [PubMed] [Google Scholar]
- 13.Gotis-Graham I, Smith MD, Parker A, McNeil HP. Synovial mast cell responses during clinical improvement in early rheumatoid arthritis. Ann Rheum Dis. 1998;57:664–671. doi: 10.1136/ard.57.11.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gruber B, Poznansky M, Boss E, Partin J, Gorevic P, Kaplan AP. Characterization and functional studies of rheumatoid synovial mast cells: activation by secretagogues, anti-IgE, and a histamine-releasing lymphokine. Arthritis Rheum. 1986;29:944–955. doi: 10.1002/art.1780290802. [DOI] [PubMed] [Google Scholar]
- 15.Malone DG, Irani AM, Schwartz LB, Barrett KE, Metcalfe DD. Mast cell numbers and histamine levels in synovial fluids from patients with diverse arthritides. Arthritis Rheum. 1986;29:956–963. doi: 10.1002/art.1780290803. [DOI] [PubMed] [Google Scholar]
- 16.Buckley MG, Walters C, Wong WM, Cawley MI, Ren S, Schwartz LB, Walls AF. Mast cell activation in arthritis: detection of α- and β-tryptase, histamine and eosinophil cationic protein in synovial fluid. Clin Sci. 1997;93:363–370. doi: 10.1042/cs0930363. [DOI] [PubMed] [Google Scholar]
- 17.Tetlow LC, Woolley DE. Distribution, activation and tryptase/chymase phenotype of mast cells in the rheumatoid lesion. Ann Rheum Dis. 1995;54:549–555. doi: 10.1136/ard.54.7.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van den Broek MF, van den Berg WB, van de Putte LB. The role of mast cells in antigen induced arthritis in mice. J Rheumatol. 1988;15:544–551. [PubMed] [Google Scholar]
- 19.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297:1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, Li L, Wong GW, Krilis SA, Madhusudhan MS, Šali A, Stevens RL. RasGRP4, a new mast cell-restricted Ras guanine nucleotide releasing protein with calcium- and diacylglycerol-binding motifs: identification of defective variants of this signaling protein in asthma, mastocytosis, and mast cell leukemia patients and demonstration of the importance of RasGRP4 in mast cell development and function. J Biol Chem. 2002;277:25756–25774. doi: 10.1074/jbc.M202575200. [DOI] [PubMed] [Google Scholar]
- 21.Adachi R, Krilis SA, Nigrovic PA, Hamilton MJ, Chung K, Thakurdas SM, Boyce JA, Anderson P, Stevens RL. Ras guanine nucleotide-releasing protein-4 involvement in experimental arthritis and colitis. J Biol Chem. 2012;287:20047–20055. doi: 10.1074/jbc.M112.360388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNeil HP, Shin K, Campbell IK, Wicks IP, Adachi R, Lee DM, Stevens RL. The mouse mast cell-restricted tetramer-forming tryptases mouse mast cell protease 6 and mouse mast cell protease 7 are critical mediators in inflammatory arthritis. Arthritis Rheum. 2008;58:2338–2346. doi: 10.1002/art.23639. [DOI] [PubMed] [Google Scholar]
- 23.Shin K, Nigrovic PA, Crish J, Boilard E, McNeil HP, Larabee KS, Adachi R, Gurish MF, Gobezie R, Stevens RL, Lee DM. Mast cells contribute to autoimmune inflammatory arthritis via their tryptase/heparin complexes. J Immunol. 2009;182:647–656. doi: 10.4049/jimmunol.182.1.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Struglics A, Larsson S, Pratta MA, Kumar S, Lark MW, Lohmander LS. Human osteoarthritis synovial fluid and joint cartilage contain both aggrecanase- and matrix metalloproteinase-generated aggrecan fragments. Osteoarthr Cartilage. 2006;14:101–113. doi: 10.1016/j.joca.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Flannery CR, Lark MW, Sandy JD. Identification of a stromelysin cleavage site within the interglobular domain of human aggrecan; evidence for proteolysis at this site in vivo in human articular cartilage. J Biol Chem. 1992;267:1008–1014. [PubMed] [Google Scholar]
- 26.Fosang AJ, Neame PJ, Hardingham TE, Murphy G, Hamilton JA. Cleavage of cartilage proteoglycan between G1 and G2 domains by stromelysins. J Biol Chem. 1991;266:15579–15582. [PubMed] [Google Scholar]
- 27.Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, Little CB, Last K, Farmer PJ, Campbell IK, Fourie AM, Fosang AJ. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648–652. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- 28.Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, Flannery CR, Peluso D, Kanki K, Yang Z, Majumdar MK, Morris EA. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 29.Fosang AJ, Last K, Knauper V, Murphy G, Neame PJ. Degradation of cartilage aggrecan by collagenase-3 (MMP-13) FEBS Lett. 1996;380:17–20. doi: 10.1016/0014-5793(95)01539-6. [DOI] [PubMed] [Google Scholar]
- 30.Sandy JD, Neame PJ, Boynton RE, Flannery CR. Catabolism of aggrecan in cartilage explants: identification of a major cleavage site within the interglobular domain. J Biol Chem. 1991;266:8683–8685. [PubMed] [Google Scholar]
- 31.Lohmander LS, Neame PJ, Sandy JD. The structure of aggrecan fragments in human synovial fluid: evidence that aggrecanase mediates cartilage degradation in inflammatory joint disease, joint injury, and osteoarthritis. Arthritis Rheum. 1993;36:1214–1. doi: 10.1002/art.1780360906. [DOI] [PubMed] [Google Scholar]
- 32.Stanton H, Golub SB, Rogerson FM, Last K, Little CB, Fosang AJ. Investigating ADAMTS-mediated aggrecanolysis in mouse cartilage. Nat Protoc. 2011;6:388–404. doi: 10.1038/nprot.2010.179. [DOI] [PubMed] [Google Scholar]
- 33.Stevens RL, Somerville LL, Sewell D, Swafford JR, Caulfield JP, Levi-Schaffer F, Hubbard JR, Dayton ET. Serosal mast cells maintain their viability and promote the metabolism of cartilage proteoglycans when cocultured with chondrocytes. Arthritis Rheum. 1992;35:325–335. doi: 10.1002/art.1780350312. [DOI] [PubMed] [Google Scholar]
- 34.Pereira PJ, Bergner A, Macedo-Ribeiro S, Huber R, Matschiner G, Fritz H, Sommerhoff CP, Bode W. Human β-tryptase is a ring-like tetramer with active sites facing a central pore. Nature. 1998;392:306–311. doi: 10.1038/32703. [DOI] [PubMed] [Google Scholar]
- 35.Little CB, Meeker CT, Hembry RM, Sims NA, Lawlor KE, Golub SB, Last K, Fosang AJ. Matrix metalloproteinases are not essential for aggrecan turnover during normal skeletal growth and development. Mol Cell Biol. 2005;25:3388–3399. doi: 10.1128/MCB.25.8.3388-3399.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caterson B, Christner JE, Baker JR, Couchman JR. Production and characterization of monoclonal antibodies directed against connective tissue proteoglycans. Fed Proc. 1985;44:386–393. [PubMed] [Google Scholar]
- 37.Hachisuka H, Kusuhara M, Higuchi M, Okubo K, Sasai Y. Purification of rat cutaneous mast cells with Percoll density centrifugation. Arch Dermatol Res. 1988;280:358–362. doi: 10.1007/BF00426614. [DOI] [PubMed] [Google Scholar]
- 38.Sabiston P, Adams ME, Ho YA. Automation of 1,9-dimethylmethylene blue dye-binding assay for sulfated glycosaminoglycans with application to cartilage microcultures. Anal Biochem. 1985;149:543–548. doi: 10.1016/0003-2697(85)90611-6. [DOI] [PubMed] [Google Scholar]
- 39.Fosang AJ, Last K, Stanton H, Golub SB, Little CB, Brown L, Jackson DC. Neoepitope antibodies against MMP-cleaved and aggrecanase-cleaved aggrecan. Methods Mol Biol. 2010;622:312–347. doi: 10.1007/978-1-60327-299-5_19. [DOI] [PubMed] [Google Scholar]
- 40.Huang C, Friend DS, Qiu WT, Wong GW, Morales G, Hunt J, Stevens RL. Induction of a selective and persistent extravasation of neutrophils into the peritoneal cavity by tryptase mouse mast cell protease 6. J Immunol. 1998;160:1910–1919. [PubMed] [Google Scholar]
- 41.Fajardo I, Pejler G. Human mast cell β-tryptase is a gelatinase. J Immunol. 2003;171:1493–1499. doi: 10.4049/jimmunol.171.3.1493. [DOI] [PubMed] [Google Scholar]
- 42.Di GN, Indoh I, Jackson N, Wakefield D, McNeil HP, Yan W, Geczy C, Arm JP, Tedla N. Human mast cell-derived gelatinase B (matrix metalloproteinase-9) is regulated by inflammatory cytokines: role in cell migration. J Immunol. 2006;177:2638–2650. doi: 10.4049/jimmunol.177.4.2638. [DOI] [PubMed] [Google Scholar]
- 43.Tchougounova E, Lundequist A, Fajardo I, Winberg JO, Abrink M, Pejler G. A key role for mast cell chymase in the activation of pro-matrix metalloprotease-9 and pro-matrix metalloprotease-2. J Biol Chem. 2005;280:9291–9296. doi: 10.1074/jbc.M410396200. [DOI] [PubMed] [Google Scholar]
- 44.Karsdal MA, Madsen SH, Christiansen C, Henriksen K, Fosang AJ, Sondergaard BC. Cartilage degradation is fully reversible in the presence of aggrecanase but not matrix metalloproteinase activity. Arthritis Res Ther. 2008;10:R63. doi: 10.1186/ar2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tetlow LC, Woolley DE. Mast cells, cytokines, and metalloproteinases at the rheumatoid lesion: dual immunolocalisation studies. Ann Rheum Dis. 1995;54:896–903. doi: 10.1136/ard.54.11.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu JJ, Lark MW, Chun LE, Eyre DR. Sites of stromelysin cleavage in collagen types II, IX, X, and XI of cartilage. J Biol Chem. 1991;266:5625–5628. [PubMed] [Google Scholar]
- 47.Hasty KA, Reife RA, Kang AH, Stuart JM. The role of stromelysin in the cartilage destruction that accompanies inflammatory arthritis. Arthritis Rheum. 1990;33:388–397. doi: 10.1002/art.1780330312. [DOI] [PubMed] [Google Scholar]
- 48.Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997;378:151–160. [PubMed] [Google Scholar]
- 49.van Meurs J, van Lent P, Stoop R, Holthuysen A, Singer I, Bayne E, Mudgett J, Poole R, Billinghurst C, van der Kraan P, Buma P, van den Berg W. Cleavage of aggrecan at the Asn341-Phe342 site coincides with the initiation of collagen damage in murine antigen-induced arthritis: a pivotal role for stromelysin-1 in matrix metalloproteinase activity. Arthritis Rheum. 1999;42:2074–2084. doi: 10.1002/1529-0131(199910)42:10<2074::AID-ANR7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 50.Houseman M, Potter C, Marshall N, Lakey R, Cawston T, Griffiths I, Young-Min S, Isaacs JD. Baseline serum MMP-3 levels in patients with rheumatoid arthritis are still independently predictive of radiographic progression in a longitudinal observational cohort at 8 years follow up. Arthritis Res Ther. 2012;14:R30. doi: 10.1186/ar3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cunnane G, Fitzgerald O, Hummel KM, Youssef PP, Gay RE, Gay S, Bresnihan B. Synovial tissue protease gene expression and joint erosions in early rheumatoid arthritis. Arthritis Rheum. 2001;44:1744–1753. doi: 10.1002/1529-0131(200108)44:8<1744::AID-ART309>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 52.Konttinen YT, Ainola M, Valleala H, Ma J, Ida H, Mandelin J, Kinne RW, Santavirta S, Sorsa T, Lopez-Otin C, Takagi M. Analysis of 16 different matrix metalloproteinases (MMP-1 to MMP-20) in the synovial membrane: different profiles in trauma and rheumatoid arthritis. Ann Rheum Dis. 1999;58:691–697. doi: 10.1136/ard.58.11.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Little CB, Barai A, Burkhardt D, Smith SM, Fosang AJ, Werb Z, Shah M, Thompson EW. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60:3723–3733. doi: 10.1002/art.25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jungel A, Ospelt C, Lesch M, Thiel M, Sunyer T, Schorr O, Michel BA, Gay RE, Kolling C, Flory C, Gay S, Neidhart M. Effect of the oral application of a highly selective MMP-13 inhibitor in three different animal models of rheumatoid arthritis. Ann Rheum Dis. 2010;69:898–902. doi: 10.1136/ard.2008.106021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buckley MG, Gallagher PJ, Walls AF. Mast cell subpopulations in the synovial tissue of patients with osteoarthritis: selective increase in numbers of tryptase-positive, chymase-negative mast cells. J Pathol. 1998;186:67–74. doi: 10.1002/(SICI)1096-9896(199809)186:1<67::AID-PATH132>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 56.Minond D, Lauer-Fields JL, Cudic M, Overall CM, Pei D, Brew K, Visse R, Nagase H, Fields GB. The roles of substrate thermal stability and P2 and P1′ subsite identity on matrix metalloproteinase triple-helical peptidase activity and collagen specificity. J Biol Chem. 2006;281:38302–38313. doi: 10.1074/jbc.M606004200. [DOI] [PubMed] [Google Scholar]
- 57.Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, Kulik J, Turner J, Wu W, Billinghurst C, Meijers T, Poole AR, Babij P, DeGennaro LJ. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001;107:35–44. doi: 10.1172/JCI10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaieda S, Shin K, Nigrovic PA, Seki K, Lee RT, Stevens RL, Lee DM. Synovial fibroblasts promote the expression and granule accumulation of tryptase via interleukin-33 and its receptor ST-2 (IL1RL1) J Biol Chem. 2010;285:21478–21486. doi: 10.1074/jbc.M110.114991. [DOI] [PMC free article] [PubMed] [Google Scholar]