Abstract
Purpose
The study’s objective was to determine the proportion and patient characteristics of patients in vasopressor-dependent septic shock who presented without lactatemia.
Methods
A retrospective review of patients presenting to an urban tertiary-care emergency department between December 2007 and September 2008 was conducted. Patients with a final diagnosis of septic shock requiring vasopressors were divided, based on initial lactate, to nonlactate expressors (0–2.4 mmol/L), intermediate (2.5–3.9 mmol/L), and high (>4.0 mmol/L) lactate groups.
Results
Among 123 patients with vasopressor-dependent septic shock, 55 (45%) were nonlactate expressors (lactate ≤2.4 mmol/L). Acute liver injury, history of liver disease, and presence of bacteremia were associated with elevated lactate.
Conclusion
Almost one-half of patients with vasopressor-dependent septic shock did not express lactate on presentation, although a high mortality rate remains in this population. We found a significant association between lactate expressors and liver disease and between lactate expressors and positive blood cultures. The use of lactatemia as the sole indicator of need for additional intravenous fluid or an end point of resuscitation in septic shock may be inadequate.
Keywords: Sepsis, Septic shock, Lactic acid, Critical care, Emergency medicine
1. Introduction
Severe sepsis and septic shock cause significant morbidity and mortality worldwide. In the United States, there are estimated to be 751 000 cases of severe sepsis each year (3.0 per 1000 population), leading to some 215 000 deaths [1]. In addition, sepsis carries a remarkable financial burden averaging $44 600 for the index admission and $78 500 in the first year for each patient [2]. Given this striking morbidity and mortality, the ability to identify and riskstratify septic patients may improve outcomes as well as reduce health care costs.
Lactate has commonly been used by investigators as a marker of severe sepsis and septic shock, and several studies have shown that lactatemia in emergency department (ED) patients with suspected infection is an indicator of poor prognosis [3–5]. However, recent literature has suggested expanding the role of lactate beyond that of a prognostic indicator. Based on this association between lactatemia and mortality, lactate has been proposed as an obligatory criterion for the diagnosis of septic shock [6]. In addition, based on the improved prognosis of patients in septic shock who clear an existing lactatemia, lactate clearance has been proposed as a goal of resuscitation [7[. Although lactatemia and lactate clearance are useful prognostic tools, lactatemia may not develop in all patients in septic shock or in those who eventually die of septic shock. The prevalence of lactatemia and the characteristics of patients who express, or do not express lactate in vasopressor-dependent septic shock, have not been reported in the literature. The identification of patient characteristics that affect the likelihood of developing lactatemia should increase our understanding of the mechanism of lactatemia in septic shock.
We hypothesized that a proportion of patients in septic shock will not express lactate despite refractory hypotension. This group is termed nonlactate expressors because of their failure to mount or “express” a lactate in response to refractory hypotension. To test this hypothesis, we evaluated patients with vasopressor-dependent septic shock to determine the prevalence of nonlactate expressors and to identify patient characteristics associated with the development of lactatemia.
2. Materials and methods
2.1. Study design and setting
We performed a retrospective evaluation of patients with vasopressor-dependent septic shock at an urban, universityaffiliated, tertiary care center with an ED volume of more than 50 000 annual patient visits between December 2007 and September 2008. The ED followed the Multiple Urgent Sepsis Treatments protocol, an aggressive screening and treatment protocol for all patients with suspected bacteremia or sepsis, including initial screening lactic acid level and blood cultures drawn before the initiation of sepsis therapy, followed by rapid fluid resuscitation, empiric antibiotic coverage, and early goal-directed therapy [8]. The institutional review board approved our study protocol and provided a waiver of informed consent.
2.2. Selection of participants
Potential eligible subjects were identified through a query of the ED patient registry. Subjects were included if all of the following inclusion criteria were met: (1) age 18 years or older; (2) presentation to the ED with suspected or confirmed infection and 2 or more systemic inflammatory response criteria [9]; (3) discharge diagnosis of sepsis recorded in the hospital discharge summary; (4) hypotension and requirement for vasopressor (epinephrine, norepinephrine, phenylephrine, vasopressin, or dopamine) support in the ED; and (5) serum lactate level obtained in the ED. For patients with more than 1 hospitalization meeting inclusion criteria, only the first visit within the study time frame was included.
2.3. Data collection
Patient records of all ED patients requiring vasopressors while in the ED were screened by one author to exclude those younger than 18 years or without suspected infection, 2 or more systemic inflammatory response criteria, or an initial lactate sent from the ED. These patients were then confirmed by a second author who additionally verified a recorded discharge diagnosis of sepsis from the inpatient medical record, thereby meeting all inclusion criteria. Two of the authors extracted subject data from our hospital’s electronic medical record system (webOMR), which included both ED and inpatient documentation. We established the data points and abstraction process before study initiation and entered the data directly into a standardized, closed entry, Microsoft Access (Microsoft Corp, Redmond, Washington) database form. Both abstractors met weekly to confirm compliance. Any discrepancies in subject inclusion or chart abstraction were resolved with reevaluation of the patient chart and author consensus. We recorded subject demographics, initial ED vital signs, laboratory and culture data, as well as medical history and current medication use. In addition, we collected information on the hospital course including discharge diagnoses and 28-day mortality. Acute renal injury was defined as an increase over baseline creatinine by 0.5 mg/dL or a 50% increase. Acute liver injury was defined as a new increase in alanine aminotransferase (ALT) or aspartate aminotransferase (AST) to greater than 3 times the normal value, or 120 IU/L. Severity of illness scores including Acute Physiology and Chronic Health Examination (APACHE II) and Sequential Organ Failure Assessment (SOFA) scores were calculated using vital signs and laboratory data as previously described [10,11].
2.4. Primary outcome and data analysis
The primary variable of interest for this investigation was initial lactic acid level in the ED. Lactate categories were defined a priori based on ranges previously used in the literature: (1) Nonlactate expressors (0–2.4 mmol/L), (2) intermediate lactate expressors (2.5–3.9 mmol/L), and (3) high-lactate expressors (>4.0 mmol/L) [5]. The secondary outcome measures for this investigation were the patient characteristics associated with each given lactate category.
We used descriptive statistics to compare baseline characteristics between the 3 lactate groups including counts and percentages for categorical variables, and median and interquartile ranges (IQR) for continuous data. Significant differences between the 3 lactate categories were assessed using Fisher exact test for categorical variables and the Kruskal-Wallis test for continuous variables because of nonparametric data. The Mantel-Haenszel χ2 test was used to assess significant trends across the lactate categories. Statistical significance for all statistical tests was set at P = .05. Statistical analyses were performed in SAS v9.1 (SAS Analytics, Cary, NC).
Univariable and multivariable linear regression analyses were performed with the response variable of log-lactate. Lactate values had a positive skew, but after log transformation, log-lactate had a close to normal distribution. Using log lactate, we performed individual univariable linear regressions with acute liver injury, liver disease, and positive blood cultures followed by the same set of regressions controlled for 28-day mortality. Subsequently, we performed a multivariable regression between log-lactate and acute liver injury, liver disease, positive blood cultures, and 28-day mortality to control for potential confounding among these variables. Residual analysis was performed, and this final model met assumptions of normality per the Shapiro-Wilk test. Sensitivity analysis showed no change with removal of influential points. Regression analysis was performed with Stata Statistical Software: Release 11 (StataCorp LP, 2009, College Station, Texas).
3. Results
A total of 349 ED visits from 319 distinct patients were reviewed. Of these, 141 patients received vasopressors for an infection-related diagnosis in the ED. Three of these patients did not have an initial lactate sent from the ED, 11 did not have a confirmed hospital discharge diagnosis of sepsis, and 4 had less than 2 systemic inflammatory response criteria. The remaining 123 patients who met all study inclusion criteria during the study period were included in this analysis. The median age of the cohort was 70 (IQR, 58–82) years, and 70 (57%) subjects were male. Additional baseline characteristics for the study population are in Table 1. Nonlactate expressors were the largest group, composed of 55 (45%) patients with an initial lactate below 2.5 mmol/L. There were 31 (25%) patients in the intermediate lactate group (lactate between 2.5 and 3.9 mmol/L) and 37 (30%) in the high lactate group (lactate of 4 mmol/L or higher). A histogram depicting the presenting lactate levels shows a positive skew with a mode of 1.5 to 2.0 mmol/L (Fig. 1).
Table 1.
Baseline characteristics of the study population
| Characteristic | Total population, N = 123 |
|---|---|
| Age (y) | 70 (58–82) |
| Male sex (%) | 70 (57) |
| Comorbid disease | |
| Coronary artery disease (%) | 46 (37) |
| Congestive heart failure (%) | 36 (29) |
| Chronic obstructive pulmonary disease (%) | 27 (21) |
| Diabetes (%) | 43 (35) |
| Hypertension (%) | 63 (51) |
| Kidney disease (%) | 33 (27) |
| Liver disease (%) | 9 (7.3) |
| Initial vitals | |
| Temperature (F) | 98.7 (97.4–100.3) |
| Heart rate (beats per minute) | 100 (82–118) |
| Respiratory rate (beats per minute) | 20 (16–28) |
| Mean arterial pressure (mm Hg) | 70 (61–87) |
| Laboratory data | |
| White blood cell (×103/μL) | 12.6 (7.9–19.8) |
| Hematocrit (%) | 34.3 (30–39) |
| Platelets (×103/μL) | 248 (167–338) |
| Glucose (mg/dL) | 131 (100–171) |
| INR | 1.4 (1.2–2.3) |
| ALT (U/L) | 28 (17–63) |
| AST (U/L) | 35 (24–94) |
| Creatinine | 1.6 (1.1–2.7) |
| Lactic acid (mmol/L) | 2.7 (1.9–4.1) |
| Infection source | |
| Lung (%) | 52 (42) |
| Urine (%) | 27 (22) |
| Intraabdominal (%) | 15 (12) |
| Other/unknown (%) | 29 (24) |
| Blood culture positive | 32 (26) |
| Acute liver injury (%) | 22 (18) |
| Acute renal injury (%) | 59 (48) |
| Intubated | 58 (46) |
| Vasopressor time (h) | 61 (26–101) |
| APACHE II | 20 (13–26) |
| SOFA | 7 (5–10) |
| 28-day mortality (%) | 36 (29) |
Medians and IQR are reported for continuous variables.
Categorical variables are reported as frequencies with percentages.
Fig. 1.
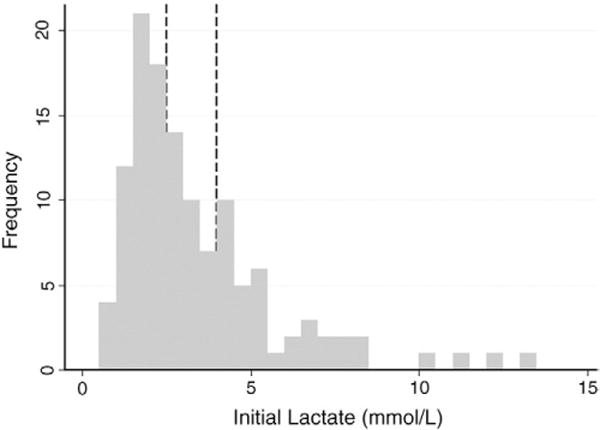
Histogram of lactate values for study subjects. Lines show cutoff values for nonlactate expressors, intermediate, and high-lactate categories.
The baseline demographics of the lactate groups were similar because age and sex did not differ significantly between lactate groups (both P > .05). Table 2 shows additional baseline characteristics across lactate categories. The only element of medical history associated with an elevated lactate level was a history of liver disease, which was more common in patients with a higher initial lactic acid level (P = .03). Laboratory markers of acute liver disease such as ALT (P < .001), AST (P < .001), and acute liver injury (P < .001) were also associated with initial lactate level. Markers of renal disease such as acute renal injury (P = .19) and creatinine (P = .16) did not reach a significant association with lactate. Other laboratory values such as white blood cell count (P = .02) and international normalized ratio of prothrombin time (INR) (P = .03) were also associated with lactate level.
Table 2.
Baseline characteristics across lactate categories
| Characteristic | Low lactate, n = 55 | Intermediate lactate, n = 31 | High lactate, n = 37 | P |
|---|---|---|---|---|
| Age (y) | 68 (58–80) | 78 (55–86) | 70 (58–80) | .41 |
| Male sex (%) | 30 (55) | 20 (65) | 20 (54) | .61 |
| Comorbid disease | ||||
| Coronary artery disease (%) | 24 (44) | 11 (35) | 11 (30) | .39 |
| Congestive heart failure (%) | 20 (36) | 6 (19) | 10 (27) | .24 |
| Chronic obstructive pulmonary disease (%) | 16 (29) | 2 (6.5) | 9 (24) | .05 |
| Diabetes (%) | 19 (35) | 10 (32) | 14 (38) | .89 |
| Hypertension (%) | 30 (55) | 14 (45) | 19 (51) | .71 |
| Kidney disease (%) | 16 (29) | 10 (32) | 7 (19) | .41 |
| Liver disease (%) | 1 (1.8) | 2 (6.5) | 6 (16) | .03 |
| Initial vitals | ||||
| Temperature (°F) | 98.8 (97.6–99.8) | 99.3 (97.8–101.0) | 98.0 (96.2–100.2) | .03 |
| Heart rate (beats per minute) | 96 (83–112) | 108 (91–121) | 94 (71–119) | .17 |
| Respiratory rate (beats per minute) | 20 (16–22) | 20 (16–28) | 27 (18–32) | .01 |
| Mean arterial pressure (mm Hg) | 70 (62–80) | 66 (59–87) | 78 (61–89) | .64 |
| Laboratory data | ||||
| White blood cell (×103/μL) | 11.4 (7.8–5.7) | 11.1 (6.4–19.9) | 16.1 (10.3–23.4) | .02 |
| Hematocrit (%) | 33.4 (29.6–37.8) | 34.6 (28.9–40.8) | 34.9 (31.5–41.3) | .35 |
| Platelets (×103/μ L) | 247 (175–370) | 211 (137–294) | 265 (171–338) | .24 |
| Glucose (mg/dL) | 131 (106–172) | 133 (99–164) | 124 (86–188) | .63 |
| INR | 1.2 (1.1–1.7) | 1.4 (1.2–2.5) | 1.6 (1.3–3.4) | .03 |
| ALT (U/L) | 22 (15–48) | 24 (13–59) | 38 (27–123) | <.001 |
| AST (U/L) | 27 (18–52) | 26 (24–75) | 55 (38–179) | <.001 |
| Creatinine | 1.4 (1.0–2.6) | 1.6 (1.2–2.6) | 2.2 (1.2–3.4) | .16 |
| Lactic acid (mmol/L) | 1.8 (1.3–2.1) | 3.1 (2.7–3.4) | 5.2 (4.4–7.1) | |
| Infection source | ||||
| Lung (%) | 27 (49) | 12 (39) | 13 (35) | .81 |
| Urine (%) | 11 (20) | 8 (26) | 8 (22) | |
| Intraabdominal (%) | 4 (7.3) | 5 (16) | 6 (16) | |
| Other/unknown (%) | 13 (24) | 6 (19) | 10 (27) | |
| Acute liver injury | 5 (9.1) | 3 (10) | 14 (38) | <.001 |
| Acute renal injury | 22 (40) | 15 (48) | 22 (59) | .19 |
| Blood culture positive | 6 (11) | 10 (32) | 16 (43) | .02 |
| Intubated | 22 (37) | 18 (58) | 18 (49) | .16 |
| Vasopressor time (h) | 57 (27–101) | 65 (31–101) | 68 (25–110) | .77 |
| APACHE II | 17 (12–24) | 20 (13–27) | 23 (18–30) | .02 |
| SOFA | 6 (5–9) | 7 (6–12) | 8 (6–10) | .03 |
| 28-day mortality | 11 (20) | 9 (29) | 16 (43) | .06 |
Medians and IQR are reported for continuous variables.
Categorical variables are reported as frequencies with percentages.
Pneumonia was the most common source of infection (52/123, or 42%) in the study population, but overall infection source was not associated with lactate category (P = .81). Subjects in the high lactate group were significantly more likely to have a positive blood culture (P = .02).
Both mortality and markers of illness severity increased with increasing lactate group. APACHE II and SOFA scores increased with rising lactate (P = .02 and P = .03, respectively). Overall, the 28-day mortality in the population was 29% (36/123). Although there was no significant difference in 28-day mortality across lactate categories (P = .06), there was a significant trend toward increasing mortality rate with increasing lactate category (Mantel-Haenszel χ2, P = .03).
To evaluate for potential confounding because of the association between lactate and increased mortality, we performed individual regressions for acute liver injury, liver disease, and positive blood cultures. Controlling for 28-day mortality did not substantially change the association between each of these factors and lactate because all 3 variables continued to have a significant positive association with lactate before and after controlling for 28-day mortality as shown in Table 3. To evaluate for potential confounding between acute liver injury, liver disease, and positive blood cultures, we performed a multiple linear regression. This regression, shown in Table 4, demonstrates a decrease in the significance of acute liver injury but a continued positive correlation between log lactate and a history of liver disease and positive blood cultures.
Table 3.
Results of individual linear regression analysis with outcome variable of log-lactate with and without adjustment for 28-day mortality
| Unadjusted
|
Adjusted for 28-day mortality
|
|||||
|---|---|---|---|---|---|---|
| β coefficient | SE | P | β coefficient | SE | P | |
| Acute liver injury | .619 | 0.135 | <.001 | .577 | 0.133 | <.001 |
| Liver disease | .697 | 0.205 | .001 | .644 | 0.202 | .002 |
| Positive blood cultures | .372 | 0.132 | .006 | .337 | 0.132 | .013 |
Table 4.
Results of multiple linear regression analysis with outcome variable of log-lactate
| β coefficient | SE | P | |
|---|---|---|---|
| Acute liver injury | .085 | 0.180 | .639 |
| Liver disease | .760 | 0.209 | .001 |
| Positive blood cultures | .332 | 0.121 | .008 |
| 28-day mortality | .219 | 0.132 | .103 |
3.1. Limitations
To ensure enrollment of true septic patients, this study used patient selection criteria of shock requiring vasopressor use combined with 2 or more systemic inflammatory response syndrome criteria and suspected infection in the ED, as well as a confirmed diagnosis of sepsis upon discharge from the hospital. The observational nature of this study allows for inclusion of only patients with infectious etiologies as verified by hospital discharge diagnosis. So, although this study is limited to some degree by the retrospective design, this design allowed for verification of sepsis because inclusion criteria consider future hospital course (eg, a patient with presumed sepsis initially but later diagnosed with massive PE would not be included).
Although all patients included in the study had 2 or more systematic inflammatory response syndrome criteria, a suspicion of infection in the ED, and confirmed sepsis recorded at hospital discharge, the use of vasopressor agents was at the discretion of each ED physician. It is possible that there was variation in clinical practice in terms of threshold for starting vasopressors and that some patients should have been started on vasopressors in the ED but were not or that some patients may have improved simply with additional volume resuscitation and not truly required vasoactive agents. However, the ED in this study has a protocol that encourages immediate volume resuscitation before initiation of vasopressors. This protocol also requires evaluation of initial lactate. Hence, only 3 patients, less than 3%, were eliminated because of lack of initial lactate drawn in the ED, which should have minimal impact on patient selection.
4. Discussion
We observed that almost half of all patients do not express lactate even in the setting of septic shock. In our study, 45% of the patients in vasopressor-dependent septic shock did not express lactate, yet this group of patients had a mortality of 20%. Although there is a basic understanding of the pathophysiologic mechanisms involved in the formation and clearance of lactic acid, the reason why some patients with septic shock express an elevated lactate while others do not remains unclear. Although a useful adjunct for identification of hypoperfusion, our data demonstrate that lactate is not expressed by a large proportion of critically ill septic patients and thus may not be adequate as the only marker of severity when evaluating patients in septic shock. Recent suggestions to include elevated lactate as an obligatory criteria for the diagnosis of septic shock would therefore have missed 45% of the patients during their initial evaluation and management. Previous studies supporting this criteria have used a lactate greater than 2.5 mmol/L during the first 24 hours, which is not useful in the setting of the ED [6]. With an increased emphasis on early aggressive sepsis therapy, using this criteria could lead to decreased identification of early septic shock and the subsequent resuscitation. Furthermore, lactate clearance has been used as an end point of resuscitation; this variable becomes potentially misleading if the patient is starting from a low lactate level despite being in septic shock [7].
The secondary objective of this study was to characterize elements of comorbidities, laboratory values, or physical examination that could contribute to the understanding of why some patients in septic shock are lactate expressors, whereas others are not. Despite many years of research and multiple mechanistic proposals, the cause of elevated lactate in septic shock remains unclear. The general cellular mechanism begins with glycolysis, resulting in the formation of pyruvate, which is transported into the mitochondria and oxidized to adenosine triphosphate. Without sufficient cellular oxygen or in cases of mitochondrial dysfunction, pyruvate is converted to lactate, which is in turn released into the bloodstream. Clearance of lactate is performed mostly by the liver through the Cori Cycle. [12].
The etiology of lactatemia in septic shock remains controversial and may be related to increased production from pyruvate conversion to lactate, decreased clearance, or a combination of both. Increased production of lactate may originate from hypoperfusion secondary to macrocirculatory dysfunction, hypermetabolic state (including increased catecholamine response), microcirculatory dysfunction leading to hypoperfusion, or mitochondrial dysfunction [13–16]. Previous investigations into the etiology of lactatemia are conflicting and probably reflect the multifactorial reasons for lactate elevation, individual patient variability, and the stage of disease. For example, a patient in the ED setting who has not received any form of resuscitation may be more likely to mount lactatemia from macrocirculatory dysfunction. In contrast, a well-resuscitated patient in their third hospital day in the intensive care unit may be more likely to have persistent lactatemia from mitochondrial dysfunction. The current investigation focused on hypotensive patients in the ED who may have been more prone to macrocirculatory dysfunction, although microcirculatory defects have been described in this setting as well [17]. Our principal finding is that a large percentage of patients did not express significant lactatemia and the reasons for this remain unknown. The timing of the lactate measurement preceded most resuscitative efforts (ie, before volume expansion with intravenous fluids), and nonlactate expressors were still judged clinically to require volume resuscitation per standard septic shock management (ie, most patients with normal lactates still received fluid resuscitation). Withholding fluid resuscitation to patients in septic shock because of a “normal” lactate would not seem to be clinically warranted, and our data support the concept that many patients in septic shock will present with normal lactate levels. Clinicians should be cautioned against underresuscitating septic patients based on perceived adequate perfusion as judged by a normal lactate level before provision of intravenous fluids.
In addition to the multiple causes of lactatemia mentioned above, the liver (and adequate metabolism) plays a particular role in both the creation and clearance of lactate, especially in hypoperfused states [18]. Preexisting liver disease leads to decreased lactate clearance even in a simple exercise model without hypoperfusion [19]. Whether from increased formation or decreased clearance, multiple cohort studies have shown that septic patients with either cirrhosis or acute liver injury have elevated lactate expression and increased mortality [20–22]. In our study, lactate expressors have an increased incidence of previous liver disease and acute liver injury. In addition, the INR, which can reflect the synthetic function of the liver, also increased with increasing lactate, although this could also reflect underlying coagulation derangements such as disseminated intravascular coagulation. Other data support the association between acute liver injury and lactate expression, including Mikkelsen et al [4], who showed a correlation between liver injury and elevated lactate in septic shock, although they defined liver injury in terms of elevated bilirubin. Our study suggests that future investigations with lactatemia might consider controlling for underlying liver disease or acute liver injury.
Presence of sufficient cofactors to allow for adequate metabolism may have an essential role in terms of lactate production as well. A recent investigation found a statistically significant association between high lactate levels and low thiamine levels when accounting for acute liver injury [23]. Thiamine is a key cofactor for pyruvate dehydrogenase, and absolute deficiency will lead to impaired metabolic breakdown of pyruvate into acetyl–coenzyme A and entry into the Krebs cycle. This study raises the potential concept of a relative thiamine deficiency as a contributor to lactatemia, and a clinical trial is currently underway to test this hypothesis (ClinicalTrials.gov ID: NCT01070810).
Our data showed an association between bacteremia and lactate expression. The overall rate of positive blood cultures was similar to comparable studies [6,11]. This association is potentially confounded by severity of disease and liver disease, as previous studies have shown that liver disease is an independent predictor of bacteremia [24]. However, the association between bacteremia and elevated lactate remained even when using multivariable regression to control for potential confounders including mortality and liver disease, confirming the direct association between lactatemia and bacteremia. Lactate expression was associated with several markers of increased severity such as SOFA, APACHE II, and mortality. However, besides liver disease and acute liver injury, neither a medical history of kidney disease nor other markers of potential organ failure such as creatinine, platelets, oxygen saturation, or need for intubation, showed a statistically significant correlation with lactate expression.
The lactate expressor group had a higher severity of illness and increased mortality, consistent with previous investigations in both overall mortality rates and the association between lactate elevation and mortality [3–5]. Although mortality does increase with elevated lactate, there is still a 20% mortality rate in the patients who do not express lactatemia. Of the 36 patients in this study who died in less than 28 days, 11 (31%) of them did not have lactatemia at presentation. Thus, nonlactate expressors represent an important group in terms of the overall mortality from septic shock and resuscitation strategies. Not accounting for this subset of patients could theoretically lead to increased mortality for these patients.
5. Conclusion
Almost one-half of a cohort of patients with vasopressordependent septic shock did not express lactatemia on presentation, despite an overall high mortality in this population. The use of lactatemia as the only indication of perfusion status or a sole end point of resuscitation in septic shock may be inadequate. Lactate expression is associated with acute liver injury, a history of liver disease, and positive blood cultures.
Acknowledgments
Funding
The project described was supported in part by Grant Number UL1 RR025758-Harvard Clinical and Translational Science Center, from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Michael W. Donnino is support by the NIH-R21AT005119.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Weycker D, Akhras KS, Edelsberg J, et al. Long-term mortality and medical care charges in patients with severe sepsis. Crit Care Med. 2003;31:2316–23. doi: 10.1097/01.CCM.0000085178.80226.0B. [DOI] [PubMed] [Google Scholar]
- 3.Howell MD, Donnino M, Clardy P, et al. Occult hypoperfusion and mortality in patients with suspected infection. Intensive Care Med. 2007;33:1892–9. doi: 10.1007/s00134-007-0680-5. [DOI] [PubMed] [Google Scholar]
- 4.Mikkelsen ME, Miltiades AN, Gaieski DF, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37:1670–7. doi: 10.1097/CCM.0b013e31819fcf68. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro NI, Howell MD, Talmor D, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45:524–8. doi: 10.1016/j.annemergmed.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez G, Castro R, Romero C, et al. Persistent sepsis-induced hypotension without hyperlactatemia: is it really septic shock? J Crit Care. 2011;26:435.e9–435.e14. doi: 10.1016/j.jcrc.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Jones AE, Shapiro NI, Trzeciak S, et al. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303:739–46. doi: 10.1001/jama.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro NI, Howell M, Talmor D. A blueprint for a sepsis protocol. Acad Emerg Med. 2005;12:352–9. doi: 10.1197/j.aem.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 9.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–74. [PubMed] [Google Scholar]
- 10.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 11.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine Intensive. Care Med. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 12.Fall PJ, Szerlip HM. Lactic acidosis: from sour milk to septic shock. J Intensive Care Med. 2005;20:255–71. doi: 10.1177/0885066605278644. [DOI] [PubMed] [Google Scholar]
- 13.Gore DC, Jahoor F, Hibbert JM, DeMaria EJ. Lactic acidosis during sepsis is related to increased pyruvate production, not deficits in tissue oxygen availability. Ann Surg. 1996;224:97–102. doi: 10.1097/00000658-199607000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James JH, Luchette FA, McCarter FD, Fischer JE. Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis. Lancet. 1999;354:505–8. doi: 10.1016/S0140-6736(98)91132-1. [DOI] [PubMed] [Google Scholar]
- 15.Jones AE, Puskarich MA. Sepsis-induced tissue hypoperfusion. Crit Care Clin. 2009;25:769–79. doi: 10.1016/j.ccc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Rady MY. The role of central venous oximetry, lactic acid concentration and shock index in the evaluation of clinical shock: a review. Resuscitation. 1992;24:55–60. doi: 10.1016/0300-9572(92)90173-a. [DOI] [PubMed] [Google Scholar]
- 17.Trzeciak S, Dellinger RP, Parrillo JE, et al. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med. 2007;49:88–98. doi: 10.1016/j.annemergmed.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Schroder R, Gumpert JR, Pluth JR, et al. The role of the liver in the development of lactic acidosis in low flow states. Postgrad Med J. 1969;45:566–70. doi: 10.1136/pgmj.45.526.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almenoff PL, Leavy J, Weil MH, et al. Prolongation of the half-life of lactate after maximal exercise in patients with hepatic dysfunction. Crit Care Med. 1989;17:870–3. doi: 10.1097/00003246-198909000-00004. [DOI] [PubMed] [Google Scholar]
- 20.De Jonghe B, Cheval C, Misset B, et al. Relationship between blood lactate and early hepatic dysfunction in acute circulatory failure. J Crit Care. 1999;14:7–11. doi: 10.1016/s0883-9441(99)90002-3. [DOI] [PubMed] [Google Scholar]
- 21.Moreau R, Hadengue A, Soupison T, et al. Septic shock in patients with cirrhosis: hemodynamic and metabolic characteristics and intensive care unit outcome. Crit Care Med. 1992;20:746–50. doi: 10.1097/00003246-199206000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Tsai MH, Chen YC, Lien JM, et al. Hemodynamics and metabolic studies on septic shock in patients with acute liver failure. J Crit Care. 2008;23:468–72. doi: 10.1016/j.jcrc.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Donnino MW, Carney E, Cocchi MN, et al. Thiamine deficiency in critically ill patients with sepsis. J Crit Care. 2010;25:576–81. doi: 10.1016/j.jcrc.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Bates DW, Sands K, Miller E, et al. Predicting bacteremia in patients with sepsis syndrome. Academic Medical Center Consortium Sepsis Project Working Group. J Infect Dis. 1997:176. 1538–51. doi: 10.1086/514153. [DOI] [PubMed] [Google Scholar]


