Abstract
Recent data suggests that a history of childhood maltreatment is associated with reductions in hippocampal volume in healthy adults. Because this association is also evident in adults with psychiatric illness, it has been suggested that reductions in hippocampal volume associated with childhood maltreatment may be a risk factor for psychiatric illness. Such an interpretation suggests that healthy adults with a history of childhood maltreatment are more resilient to the effects of maltreatment. Current models of resilience suggest, however, that resiliency should be measured across multiple domains of functioning. The present study sought to investigate childhood maltreatment in relationship to hippocampal volumes in healthy adults and to address the question of whether the putative resiliency extends to other domains of functioning. Sixty-seven healthy Caucasian adults were assessed for a history of childhood emotional abuse, emotional neglect and physical abuse and received high resolution structural MR imaging scans. Participants with and without histories of abuse or neglect were compared on measures of total hippocampal volume, general cognitive ability and subclinical psychopathology. Our results suggest that childhood emotional abuse is associated with reduced hippocampus volume in males, but not in females. However, emotional abuse was associated with higher levels of subclinical psychopathology in both males and females. These data suggest that while females may be more resilient to the neurological effects of childhood maltreatment, they are not more resilient to the psychiatric symptoms associated with childhood maltreatment. Further research is needed to elucidate the mechanisms involved in these different levels of resilience.
Stress has been shown to have a substantial impact on the structure and function of limbic structures, particularly the hippocampus. In rodents, early work found that elevating stress-related hormone levels for an extended period reduced the number of neurons in the hippocampus (Sapolsky et al. 1987) and subsequent work demonstrated that this effect is particularly pronounced during early postnatal development (Brunson et al. 2001; Chen et al. 2004). In humans, evidence for the relation between early-life stress and hippocampal volume derives from studies demonstrating reduced hippocampus volume in adults with a history of childhood maltreatment compared to controls. In most of these studies, however, the study samples were comprised of adults with a psychiatric disorder.
Recently, Teicher and colleagues (2012) examined the relation between childhood maltreatment and hippocampal volumes in a large, general community sample and found a strong negative correlation between severity of childhood maltreatment and volume of several hippocampal subfields. However, participants in this study were selected without regard to psychiatric history and a large percentage of the sample was found to have a diagnosable psychiatric illness. Specifically, approximately 25% of the sample met criteria for major depressive disorder (MDD), 7% met criteria for post-traumatic stress disorder (PTSD) and approximately 25% met criteria for another mood or anxiety disorder. Due to the high percentage of the sample having a diagnosis of a mood or anxiety disorder, it was unclear if the findings indicated a direct relation between childhood maltreatment and reduced hippocampal volumes or between childhood maltreatment and psychiatric illness. The authors attempted to address this issue by conducting a follow-up analysis using structural equation modeling to evaluate whether a diagnosis of MDD or PTSD mediated the association between childhood maltreatment and subfield volumes. Although the authors found no evidence to suggest that a diagnosis of MDD or PTSD mediated the association between childhood maltreatment and reduced hippocampal volumes, this interpretation could not be entirely ruled out because approximately 25% of the sample that had a diagnosis other than MDD or PTSD were excluded from the mediation analysis. Despite this limitation, however, the authors concluded that the reductions in hippocampal volumes they observed were a consequence of maltreatment and a risk factor for the development of psychiatric illness.
To date only a single study has assessed the relation between a history of childhood maltreatment and hippocampal volume in adult subjects with no history of psychiatric illness. Dannlowski and colleagues (2012) used voxel-based morphometry to study morphological alterations of the hippocampus in 148 adults with no history of psychiatric illness. These authors found significant negative correlations between right hippocampal gray matter volume and total score, as well as all subscale scores, on the Childhood Trauma Questionnaire (CTQ). These results suggest a direct relation between childhood maltreatment and reduced hippocampal volumes even in individuals with no history of a psychiatric illness. Similar to the conclusions drawn by Teicher et al. (2012), Dannlowski et al. (2012) concluded that reductions in hippocampal volumes in response to childhood maltreatment were likely risk factors for, rather than a feature of psychiatric illness. Such an interpretation suggests that some individuals may be resilient to the long-term consequences of reductions in hippocampal volume resulting from childhood maltreatment.
Generally, resilience refers to an individual’s ability to successfully adapt to acute or chronic stress (Feder et al. 2009). A growing literature has recently begun to focus on understanding resilience among people with a history of childhood maltreatment. Among the key developments in this area of research is the recognition that resilience defined by a single outcome measure is inadequate (Afifi and Macmillan 2011). Current definitions of resilience suggest that it is a dynamic process that may be observed in some domains of functioning but not in others (Walsh et al. 2010) and should be measured across many different phenotypic levels (Feder et al. 2009). Thus, examining the effects of childhood maltreatment on measures of hippocampal volume concurrently with other potential measures of resilience could contribute to a more integrative model of resilience to psychiatric illness.
The present study sought to examine the relationship between childhood maltreatment and hippocampal volume in an adult sample with no history of psychiatric illness. Moreover, we also sought to specifically address whether individuals with a history of childhood maltreatment would show evidence of resilience in other domains of functioning. Specifically, in addition to examining the relation between childhood maltreatment and hippocampal volume, we also assessed the relation between childhood maltreatment and other key domains of functioning, including cognition and subclinical levels of positive, negative and depressive symptoms. Moreover, because resilience often shows robust sex-differences, we sought to assess differences between males and females in all of these domains.
Although the relation between childhood maltreatment and cognitive deficits has not been widely studied in healthy populations, data suggest that exposure to trauma during childhood may lead to significant deficits in memory and executive function (Majer et al. 2010; Spann et al. 2012). Similarly, although a limited number of studies have examined the relation between childhood maltreatment and subclinical positive, negative and depressive symptoms in healthy populations, a recent meta-analysis indicated that individuals exposed to childhood stress or trauma were 2 times more likely to report subclinical psychotic experiences (including positive, negative and depressive symptoms) than those without such histories (Linscott and van Os 2010). Combined with data demonstrating significant relations between childhood maltreatment and cognitive deficits in patients with psychiatric illness (Aas et al. 2012) and the plethora of data demonstrating the relation between childhood maltreatment and psychiatric illness, these domains are ideal candidates for the present study. Finally, a considerable body of data suggests that females may be more resilient to the effects of stress than their male counterparts. Several studies conducted in PTSD suggest that maltreated males may show more evidence of adverse brain development than maltreated females (De Bellis et al. 1999; 2002; 2003). Thus, the present study also sought to assess whether sex differences could be observed in any of our primary dependent measures.
Method
Participants
Caucasian healthy volunteers (30 males, 37 females, Mage = 36.94±14.77) were recruited from the general population via word of mouth, newspaper and internet advertisements and posted flyers. All participants provided written informed consent to a protocol approved by the Institutional Review Board of the North Shore-Long Island Jewish Health System. Participants were excluded from the study if they had an Axis I diagnosis, active or recent substance abuse, a first-degree relative with a known or suspected Axis I disorder, based on family history questionnaire, contraindications to magnetic resonance imaging, prior psychosurgery and pregnancy. The sample used in the present study represents a subset of a larger healthy volunteer sample and were selected based on the availability of MRI data.
Clinical Assessments
Diagnostic rule-out
Participants were initially administered the Structured Clinical Interview for DSM-IV, Non-Patient edition (SCID-I/NP) to rule out a past or present Axis I psychiatric disorder (First et al. 2002). Information obtained from the SCID was compiled into a narrative case summary and absence of pathology was determined by two expert diagnosticians from the ZHH faculty.
History of childhood maltreatment
To assess the history of childhood maltreatment we utilized the 28-item Childhood Trauma Questionnaire (CTQ; Bernstein et al. 2003). The CTQ is a 5-point Likert-type self-report questionnaire that measures several dimensions of abuse and neglect during childhood including physical abuse (PA), emotional abuse (EA) and sexual abuse (SA) as well as emotional neglect (EN) and physical neglect (PN). Examination of the CTQ scores in the present sample revealed that none of the dimensional scores were normally distributed and thus, not amenable to investigation using parametric tests. Therefore, participants were dichotomized into maltreatment positive and negative groups on each subscale. Group assignment was based on whether or not they endorsed any history of the specific type of maltreatment. Specifically, those participants who scored a 5 on any one subscale were placed in the maltreatment negative (−) group for that type of trauma, while those who scored above 5 were placed in the maltreatment positive (+) group for that type of trauma. Due to low and high frequency of endorsement, respectively, sexual abuse and physical neglect could not be explored in the present study. Although higher cut-offs for maltreatment positive groupings have been suggested in the literature (Walker et al, 1999), the distribution of scores in our data was limited and the average scores were lower than typically reported (see Table 1).
Table 1.
Participant demographics
| Maltreatment Type | Age (SD) | Sex | WRAT-3 (SD) | Mean CTQ Subscale Score (SD) |
|---|---|---|---|---|
| Emotional Abuse | ||||
| Positive (EA+) | 38.70 (15.39) | 14M/16F | 49.15 (2.71) | 8.53 (3.14) |
| Negative (EA−) | 35.51 (14.29) | 16M/21F | 47.72 (4.04) | *5 (0) |
| Emotional Neglect | ||||
| Positive (EN+) | 42.28 (13.39) | 17M/18F | 48.70 (4.00) | 9.86 (2.85) |
| Negative (EN−) | 31.10 (14.15) | 13M/19F | 48.00 (3.03) | *5 (0) |
| Physical Abuse | ||||
| Positive (PA+) | 48.04 (13.91) | 10M/7F | 49.20 (3.30) | 7.35 (1.46) |
| Negative (PA−) | 33.17 (13.16) | 20M/30F | 48.07 (3.63) | *5 (0) |
Note: Scores for the Wide Range Achievement Test-Third Edition-Reading Subtest (WRAT-3) are presented in their raw form.
Groups were dichotomized such that the groups indicated as (−) did not endorse any history of that type of maltreatment. A score of 5 indicates that a participant did not endorse any items on that subscale.
History of subclinical psychopathology
Lifetime history of subclinical psychopathology was assessed using the Community Assessment of Psychic Experiences (CAPE; Stefanis et al. 2002). The CAPE is a 42-item, self-report questionnaire that measures three dimensions of subclinical psychopathology including positive, negative and depressive symptoms. The CAPE provides scores on each of the three dimensions of as well as an overall score. The present study utilized the overall score as the primary dependent measure as we were only interested in assessing the relation between childhood maltreatment and general psychopathology.
Magnetic Resonance Imaging
MRI exams were conducted at North Shore University Hospital and acquired in the coronal plane using a 3D spoiled gradient (SPGR) sequence (TR = 7.5 ms, TE = 3 ms, matrix = 256x256, FOV = 240 mm) on a single 3T scanner with (GE Signa HDx; General Electric, Milwaukee, Wisconsin), producing 216 contiguous images (slice thickness = 1mm) through the whole head. All scans were reviewed for quality assurance by a neuroradiologist and a member of the research team. Scans from two subjects contained significant artifacts and were excluded from the current sample.
Cortical reconstruction and volumetric segmentation was performed with the Freesurfer image analysis suite, which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/). The technical details of these procedures are described in prior publications (e.g. Dale et al. 1999, Reuter et al. 2012). Briefly, this processing includes motion correction, removal of non-brain tissue using a hybrid watershed/surface deformation procedure (Segonne et al. 2004), automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures (Fischl et al. 2004) intensity normalization (Sled et al. 1998), tessellation of the gray matter white matter boundary, automated topology correction (Fischl et al. 2001; Segonne et al. 2007), and surface deformation following intensity gradients to optimally place the gray/white and gray/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class (Fischl and Dale 2000). Given our a priori hypotheses, volumes of the right and left hippocampi were generated for analysis.
Neuropsychological Assessments
All subjects were administered a brief battery of standardized neurocognitive tests. Because a full scale IQ measure was not available, scores on the Wide Range Achievement Test-Third Edition-Reading Subtest (WRAT-3) were used to provide a general estimate of IQ (Keefe et al. 2005). Additionally, participants were administered the Wechsler Adult Intelligence Test-Revised (WAIS-R)-Digit Span, Animal Naming and Trail Making Tests A&B (Spreen and Strauss, 1998). Our primary-dependent measure was general cognitive ability (g), the first factor score derived from an unrotated principal components analysis, as previously described (Carroll, 1997).
Data reduction was achieved through factor analysis using principal components as the extraction method. Raw scores on Digit Span, Animal Naming and Trail Making Tests A and B were initially transformed to standardized scores and then entered into the model. A total of 10 participants did not have complete score data and were excluded from this analysis. The first unrotated factor explained 49% of the variance and represented our general cognitive ability factor. Each of the individual measures loaded onto the first factor with covariance of >0.59. Factor scores representing g are standardized using a Z-score scale with a mean of 0 and standard deviation of 1.
Statistical Analysis
Preliminary analyses compared the emotional abuse (EA), physical abuse (PA) and emotional neglect (EN) + and − groups on basic demographic characteristics including age, sex and estimated IQ scores using a multivariate analysis of variance (MANOVA). To rule out sex differences in overall CTQ scores, males and females were compared on each subscale using t-tests for raw scores and chi square for dichotomized groups. Spearman’s rho was then used to assess the overall relationship between total CTQ score and hippocampus volume. This analysis was followed up with a series of univariate analyses of covariance (ANCOVA) to compare the EA+ and EA− groups, the PA+ and PA− groups and the EN+ and EN− groups on total hippocampal volume, which were corrected for total intracranial volume. In each of these models, sex was entered into the model as a fixed factor and age was entered as a covariate. Any significant group differences we observed were followed-up with additional analyses comparing those groups on general cognitive ability and subclinical psychopathology using the same model structure as used in the primary analysis.
Results
Preliminary analyses revealed no differences between the EA+ and EA− group on sex, age or estimated IQ. Comparison of the PA+ and PA− groups revealed a significant difference in age (MPA+ = 33.17; MPA− = 48.04; t(65) = 3.97; p < .001) but no differences in sex or estimated IQ. Comparison of the EN+ and EN− groups also revealed a significant difference in age (MEN+ = 31.10; MEN− = 42.28; t(65) = 3.33; p = .001) but no differences in sex or estimated IQ. No differences were observed between males and females on any of the raw subscale scores. Moreover, no difference in sex distribution was observed in any of the the dichotomized groups. Demographic characteristics of each group are shown in Table 1.
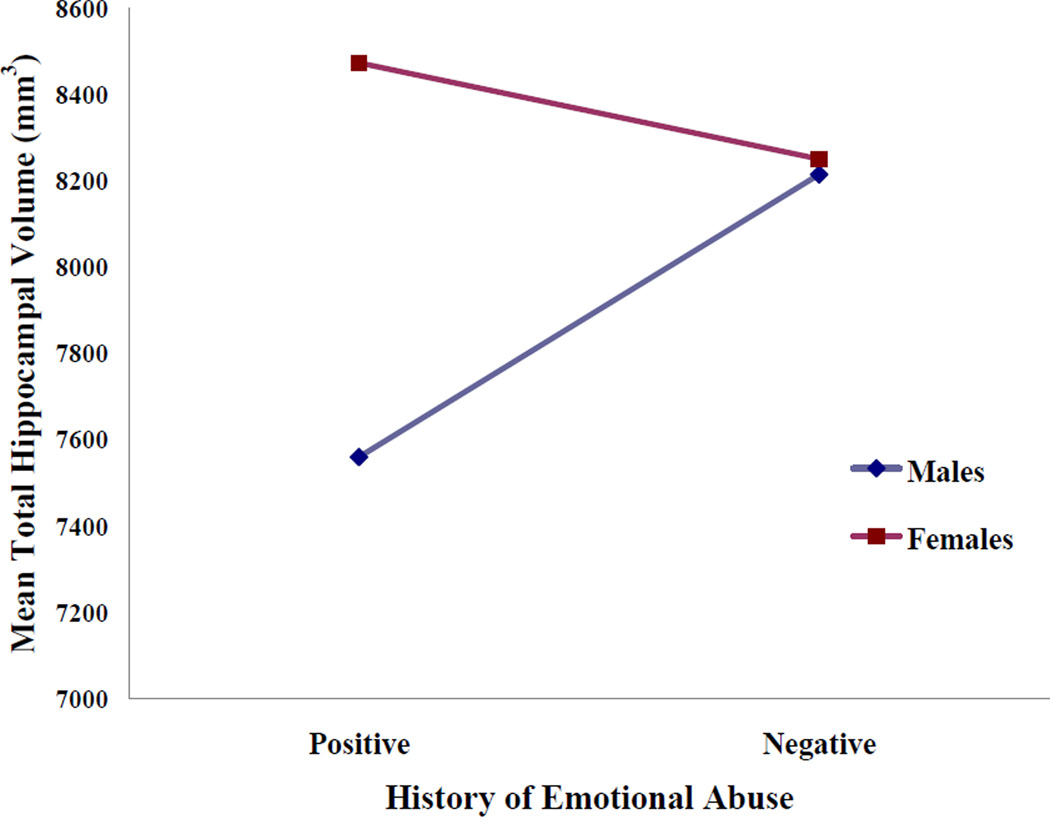
A non-parametric correlational analysis revealed a significant relation between overall CTQ score and hippocampus volumes (rho = −.26; p = .03). The ANCOVA analyses comparing maltreatment positive and negative groups on total hippocampal volume revealed no significant main effects of physical abuse, emotional neglect or sex were observed. Analysis of the effect of emotional abuse and sex on hippocampal volume also revealed no significant findings. However, a significant interaction between EA and sex was observed (F(67) = 5.89; p = .01). Specifically, a positive history of emotional abuse was significantly associated with total hippocampal volume in males (F(30) = 6.61; p = .01) but not in females. This interaction is shown in Figure 1. When left and right hippocampus was assessed separately, the interaction between EA and sex was only significant for the left hippocampus (F(67) = 5.51; p = .02). Finally, follow-up analyses assessing the main effects of history of emotional abuse and sex on general cognitive ability revealed no significant effects. However, history of emotional abuse was associated with overall levels of subclinical positive, negative and depressive symptoms (MEA+ = 55.43; MEA− = 49.31; F(63) = 12.05; p = .001) but no main effect of sex and no interaction was observed.
Figure 1.
Mean total hippocampal volumes for males and females with positive or negative histories of childhood emotional abuse. Although no significant main effects of sex or emotional abuse history were observed, the interaction was significant.
Discussion
The present findings suggest that childhood emotional abuse directly influences hippocampal volume even in individuals with no history of psychiatric illness. This finding is consistent with two recent reports in the literature (Teicher et al. 2012; Dannlowski et al. 2012). Interestingly, however, in the present study the association between history of emotional abuse and reduced hippocampal volume was only observed for males. Although earlier studies seeking to assess the relation between childhood maltreatment and hippocampal volumes did not look specifically at sex-based differences, our findings our comparable to findings reported for global brain volume measures. Specifically, in samples comprised of maltreated children and adolescents, males with PTSD had significantly greater reductions in corpus callosum volume (De Bellis et al. 1999), greater reductions in overall cerebral volume, and greater lateral ventricular volume (De Bellis et al. 2003) than maltreated females with PTSD. Combined with our data, these findings may suggest that the brains of healthy males are more vulnerable to the effects of childhood maltreatment than healthy females. Alternatively, the observed sex difference in the present study might suggest that maltreated females who evidence decreases in hippocampal volumes are more likely than males to develop a psychiatric illness. Such an interpretation would be consistent with findings suggesting that the incidence of post-traumatic stress disorder is greater in females despite males being exposed to more traumatic events (Breslau et al. 2001; Breslau et al. 2002). However, although the overall EA+ group differed significantly from those with no history of emotional abuse on our measure of subclinical psychopathology, the difference was not sex-dependent. The comparable levels of subclinical positive, negative and depressive symptoms observed in males and females in the present study despite the sex differences observed in hippocampal volume suggest that females are equally susceptible to some, but not all, of the effects of childhood maltreatment than males.
Several researchers have suggested that females may be more resilient to the effects of stress than their male counterparts (McGloin and Widom 2001; Haatainen et al. 2003; Teicher et al. 2004; Dumont et al. 2007; Hager et al. 2012). While the present results suggest that females may be more resilient to childhood maltreatment at the level of neurodevelopment, they do not appear to be resilient to the subclinical psychiatric symptoms associated with childhood maltreatment. Further research is needed to elucidate the mechanisms involved in these different levels of resiliency.
In the present study we did not find evidence suggesting an association between history of childhood emotional abuse and general cognitive ability. Only one prior study has assessed the relation between childhood emotional abuse and cognition in healthy adults. Majer and colleagues (2010) investigated the relationship between childhood trauma exposure and cognitive function in 47 healthy adults and found that childhood emotional abuse was significantly associated with impaired spatial working memory performance. In the present study, however, we did not assess specific domains of cognitive function. Because the cognitive data available in the present sample was limited, and unfortunately lacking hippocampus-dependent cognitive skill, we chose to assess a more general measure of cognitive ability. At the level of general cognitive ability our results are consistent with data showing that trauma exposure during childhood was not associated with lower estimates of IQ (Saigh et al. 2006).
Some limitations of the present study should be addressed. Contrary to the original report linking childhood maltreatment to reductions in hippocampal volume in healthy adults (Dannlowski et al. 2012), our findings did not indicate a pervasive relationship between hippocampal volumes and all of the domains of maltreatment. Although we found a significant correlation between total hippocampus volume and CTQ total score, the only specific relation we found when we examined the sub-scale scores was an association to emotional abuse. Although it is unclear why we did not find a more generalized association, it should be noted that Dannlowski and colleagues (2012) found that the history of emotional abuse was one of the strongest predictors of hippocampal volume reductions. Given the significant correlation between the subscale scores (all rho’s > .40; all p’s < .001) it is likely that that the smaller sample size, combined with the low scores overall and the narrow distribution of scores in the present study reduced our power to detect a significant association to emotional neglect and physical abuse.
Additionally, the present study and all studies reporting on the effects of childhood maltreatment on the adult hippocampus relied on retrospective assessment of childhood experience. Although it could be argued that healthy adults over-report childhood maltreatment, several studies have demonstrated that adults actually underreport such experiences (Williams 1994; Shaffer et al. 2008). Moreover, the retrospective assessment used in the current study did not take into consideration the timing of the maltreatment being reported. Recently, Enlow and colleagues (2012) reported that children exposed to maltreatment and/or violence against the mother had lower scores on a broad range of cognitive tests at several time points across an 8 year follow-up period. These effects, however, were most noticeable for children who had experienced this type of trauma during the first two years of their lives. Thus, it is possible that the specific timing of the trauma may play a significant role in determining the effects of childhood maltreatment on cognitive function.
Despite these limitations, the present results add to the growing literature suggesting that childhood maltreatment has significant health consequences at many levels of development, even in adults with no history of psychiatric illness. Our findings suggesting that females may be more resilient to the effects of childhood maltreatment at the level of hippocampal volume but not at the level of subclinical psychopathology are of particular interest. Given prior findings of substantial molecular and cellular sex differences in response to stress, particularly in relation to gonadal hormones (Valentino et al. 2012; Lupien et al. 2009), these findings may provide the basis for future studies seeking to elucidate how environmental adversity during childhood may interact with hormonal status.
Acknowledgments
Funding/Support This work was supported in part by grants from the National Institute of Mental Health to Dr. DeRosse (MH086756), Dr. Szeszko (R01 MH076995), Dr. Malhotra (MH079800), the NSLIJ Research Institute General Clinical Research Center (M01 RR018535), an Advanced Center for Intervention and Services Research (P30 MH090590) and a Center for Intervention Development and Applied Research (P50 MH080173).
Dr. Malhotra has served as consultant or speaker for Bristol-Myers Squibb, Astra Zeneca, Vanda Pharmaceuticals and Clinical Data, Inc, and has received research support from Pfizer, Janssen Pharmaceuticals, Bristol-Myers Squibb, and Eli Lilly.
We thank Christopher Morell for the recruitment and assessment of the participants used in the present study. We also thank Jamie Wagner for coordination of the imaging in these participants. Finally, we would like to thank the participants who contributed their time and effort to help us better understand the basis of psychiatric illness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Drs. DeRosse, Ikuta, Szeszko and Ms. Samplin report no competing interests.
References
- Aas M, Navari S, Gibbs A, Mondelli V, Fisher HL, Morgan C, Morgan K, MacCabe J, Reichenberg A, Zanelli J, Fearon P, Jones PB, Murray RM, Pariante CM, Dazzan P. Is there a link between childhood trauma, cognition, and amygdala and hippocampus volume in first-episode psychosis? Schizophrenia Research. 2012;137:73–79. doi: 10.1016/j.schres.2012.01.035. [DOI] [PubMed] [Google Scholar]
- Afifi TO, Macmillan HL. Resilience following child maltreatment: a review of protective factors. Canadian Journal of Psychiatry. 2011;56:266–272. doi: 10.1177/070674371105600505. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse and Neglect. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Breslau N. The epidemiology of posttraumatic stress disorder: what is the extent of the problem? Journal of Clinical Psychiatry. 2001;62:16–22. [PubMed] [Google Scholar]
- Breslau N. Gender differences in trauma and posttraumatic stress disorder. The Journal of Gender-Specific Medicine. 2002;5:34–40. [PubMed] [Google Scholar]
- Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proceedings of the National Academy of Sciences. 2001;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JB. Psychometrics, intelligence, and public policy. Intelligence. 1997;24:25–52. [Google Scholar]
- Chen Y, Bender RA, Brunson KL, Pomper JK, Grigoriadis DE, Wurst W, Baram TZ. Modulation of dendritic differentiation by corticotropin-releasing factor in the developing hippocampus. Proceedings of the National Academy of Sciences. 2004;101:15782–15787. doi: 10.1073/pnas.0403975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, Lindner C, Postert C, Konrad C, Arolt V, Heindel W, Suslow T, Kugel H. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry. 2012;71:286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, Frustaci K, Ryan ND. Developmental traumatology. Part II: Brain Development. Biological Psychiatry. 1999;15:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan M, Shifflett H, Iyengar S, Beers SR, Hall J, Moritz G. Brain structures in pediatric maltreatment-related PTSD: a sociodemographically matched study. Biological Psychiatry. 2002;52:1066–1078. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS. Sex differences in brain maturation in maltreatment-related pediatric posttraumatic stress disorder. Neuroscience and Biobehavioral Reviews. 2003;27:103–117. doi: 10.1016/s0149-7634(03)00013-7. [DOI] [PubMed] [Google Scholar]
- DuMont KA, Widom CS, Czaja SJ. Predictors of resilience in abused and neglected children grown-up: the role of individual and neighborhood characteristics. Child Abuse and Neglect. 2007;31:255–274. doi: 10.1016/j.chiabu.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Enlow MB, Egeland B, Blood EA, Wright RO, Wright RJ. Interpersonal trauma exposure and cognitive development in children to age 8 years: a longitudinal study. Journal of Epidemiology and Community Health. 2012;66:1005–1010. doi: 10.1136/jech-2011-200727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nature Reviews. Neuroscience. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York: New York State Psychiatric Institute. Biometrics Research; 2002. [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Transactions on Medical Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 2004;23:S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Haatainen KM, Tanskanen A, Kylmä J, Honkalampi K, Koivumaa-Honkanen H, Hintikka J, Antikainen R, Viinamäki H. Gender differences in the association of adult hopelessness with adverse childhood experiences. Social Psychiatry and Psychiatric Epidemiology. 2003;38:12–17. doi: 10.1007/s00127-003-0598-3. [DOI] [PubMed] [Google Scholar]
- Hager AD, Runtz MG. Physical and psychological maltreatment in childhood and later health problems in women: An exploratory investigation of the roles of perceived stress and coping strategies. Child Abuse and Neglect. 2012;36:393–403. doi: 10.1016/j.chiabu.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Eesley CE, Poe MP. Defining a cognitive function decrement in schizophrenia. Biological Psychiatry. 2005;57:688–691. doi: 10.1016/j.biopsych.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Linscott RJ, van Os J. Systematic reviews of categorical versus continuum models of psychosis: evidence for discontinuous subpopulations underlying a psychometric continuum. Implications for DSM-V, DSM-VI, and DSM-VII. Annual Review of Clinical Psychology. 2010;6:391–419. doi: 10.1146/annurev.clinpsy.032408.153506. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Majer M, Nater UM, Lin JMS, Capuron L, Reeves WC. Association of childhood trauma with cognitive function in healthy adults: a pilot study. BioMedCentral Neurology. 2010;10:1–10. doi: 10.1186/1471-2377-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGloin JM, Widom CS. Resilience among abused and neglected children grown up. Development and Psychopathology. 2001;13:1021–1038. doi: 10.1017/s095457940100414x. [DOI] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-Subject Template Estimation for Unbiased Longitudinal Image Analysis. NeuroImage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigh PA, Yasik AE, Oberfield RA, Halamandaris PV, Bremner JD. The intellectual performance of traumatized children and adolescents with or without posttraumatic stress disorder. Journal of Abnormal Psychology. 2006;115:332–340. doi: 10.1037/0021-843X.115.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal damage. Trends in Neurosciences. 1987;10:346–349. [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Transactions on Medical Imaging. 2007;26:518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- Shaffer A, Huston L, Egeland B. Identification of child maltreatment using prospective and self-report methodologies: a comparison of maltreatment incidence and relation to later psychopathology. Child Abuse and Neglect. 2008;32:682–692. doi: 10.1016/j.chiabu.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Spann MN, Mayes LC, Kalmar JH, Guiney J, Womer FY, Pittman B, Mazure CM, Sinha R, Blumberg HP. Childhood abuse and neglect and cognitive flexibility in adolescents. Child Neuropsychology. 2012;18:182–189. doi: 10.1080/09297049.2011.595400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 2nd edn. New York, NY: Oxford University Press; 1998. [Google Scholar]
- Stefanis NC, Hanssen M, Smirnis NK, Avramopoulos DA, Evdokimidis IK, Stefanis CN, Verdoux H, van Os J. Evidence that three dimensions of psychosis have a distribution in the general population. Psychological Medicine. 2002;32:347–358. doi: 10.1017/s0033291701005141. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, Andersen SL. Childhood neglect is associated with reduced corpus callosum area. Biological Psychiatry. 2004;56:80–85. doi: 10.1016/j.biopsych.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proceedings of the National Academy of Sciences. 2012;109:563–572. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Reyes B, Van Bockstaele E, Bangasser D. Molecular and cellular sex differences at the intersection of stress and arousal. Neuropharmacology. 2012;62:13–20. doi: 10.1016/j.neuropharm.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EA, Gelfand A, Katon WJ, Koss MP, Von Korff M, Bernstein D, Russo J. Adult health status of women with histories of childhood abuse and neglect. The American Journal of Medicine. 1999;107:332–339. doi: 10.1016/s0002-9343(99)00235-1. [DOI] [PubMed] [Google Scholar]
- Walsh WA, Dawson J, Mattingly MJ. How are we measuring resilience following childhood maltreatment? Is the research adequate and consistent? What is the impact on research, practice, and policy? Trauma, Violence and Abuse. 2010;11:27–41. doi: 10.1177/1524838009358892. [DOI] [PubMed] [Google Scholar]
- Williams LM. Recall of childhood trauma. A prospective study of women’s memories of child sexual abuse. Journal of Consulting and Clinical Psychology. 1994;62:1167–1176. doi: 10.1037//0022-006x.62.6.1167. [DOI] [PubMed] [Google Scholar]