Abstract
Transthyretin (TTR) cardiac amyloidosis is an important, often under-recognized and potentially modifiable cause of heart failure with a preserved ejection fraction. The only proven treatment is liver or combined heart/liver transplantation, which, although effective, is not suitable for the vast majority of older adults with this condition. Diflunisal, a nonsteroidal anti-inflammatory drug, can stabilize the TTR tetramer in vitro and may prevent misfolding monomers and dimers from forming amyloid deposits in the heart. It is one of two small molecules assessed in animal safety studies and human clinical trials of TTR polyneuropathy. The authors conducted a single-arm, open-label investigation with a mean follow-up of 0.9±0.3 years to determine the safety and efficacy of diflunisal administration in a cohort of 13 patients with confirmed wild-type or mutant TTR cardiac amyloidosis. Diflunisal was well tolerated from a hematologic standpoint, although a 6% decline in estimated glomerular filtration rate was noted. Therapy was discontinued in one patient who rapidly developed volume overload. There was no significant mean change in cardiac structure (left ventricular mass: −53 g/m2 change, P=.36), function (ejection fraction: −2% change, P=.61), or biomarkers (Troponin I: +0.03 ng/mL, P=.08; BNP: +93 pg/mL change, P=.52) during the course of therapy. These data suggest that at low dosages and with careful monitoring, diflunisal can be safely administered to compensated patients with cardiac TTR amyloidosis. Further study in a randomized placebo-controlled trial is warranted.
Transthyretin amyloidosis (ATTR), the most frequent cause of autosomal-dominant hereditary systemic amyloidosis, is an often under-recognized cause of heart failure with a preserved ejection fraction (HFpEF) that is progressive in nature.2,3 In its normal state, transthyretin (TTR) is a 127-amino acid, 55-kDa homotetramer primarily synthesized by the liver that functions as a plasma transport protein for thyroid hormone and retinol-binding hormone/vitamin A.4 However, in some older adults or adults with any of more than 100 delineated mutations with varying ethnic and geographic specificity,5 the TTR tetramer can destabilize and dissociate into misfolded amyloidogenic monomers that aggregate into insoluble amyloid fibrils. Deposition of these fibrils into the myocardium leading to diastolic dysfunction, restrictive cardiomyopathy, and heart failure is a common presentation.
TTR amyloid cardiomyopathy arises from either genetically wild-type (ATTRwt) TTR deposition, known also as senile systemic amyloidosis (SSA), or from genetically mutant (ATTRm) deposition, known also as familial amyloid cardiomyopathy. ATTRwt cardiac amyloidosis occurs primarily in elderly Caucasian men and in up to one third of patients with HFpEF older than 75 years.6–9 The most common ATTRm allele, the valine to isoleucine substitution at position 122 (V122I),5 is found in approximately 3.5% of the African American population.10,11 Liver or combined heart/liver transplantation has been employed to eradicate the mutant form of TTR12,13 and while potentially effective, it is limited by expense, lifelong immunosuppression, surgical risk in already cardiac compromised patients, and disease progression after liver transplantation.6,14 Additionally, for a majority of patients with ATTR cardiac amyloidosis who are older adults, transplantation is not feasible given the shortage of donor organs. As a strategy to avoid these shortcomings, small molecule agents designed to prevent TTR misfolding and subsequent amyloid deposition are currently in various stages of development, clinical trial, and regulatory approval processes in Europe and the United States.15,16
Diflunisal, a nonsteroidal anti-inflammatory drug (NSAID), has been shown to bind and stabilize common familial TTR variants against acid-mediated fibril formation in vitro and has been tested in animal safety studies and human clinical trials.17–22 Based on this, diflunisal is hypothesized to inhibit tetrameric TTR dissociation and suppress amyloidogenesis. However, chronic NSAID use, because of inhibition of cyclooxygenase (COX) enzymes, can lead to gastrointestinal (GI) bleeding (COX-1), renal dysfunction (COX-2), fluid retention, and hypertension that may precipitate heart failure (COX-2) in vulnerable individuals.23–28 We aimed to investigate in a short-term, single-arm, open-label fashion whether diflunisal could be safely administered to patients with ATTR cardiac amyloid and to gather preliminary data on efficacy.
METHODS
Seventy-seven patients treated in the Center for Advanced Cardiac Care of Columbia University Medical Center between June 2009 to December 2011 with ATTRwt or ATTRm cardiac amyloidosis were evaluated to participate in an open-label study of the safety and efficacy of diflunisal. Patients were predominantly older adults (Table I) with biopsy-proven ATTR cardiac amyloid and clinical manifestations. Patients who had an NSAID drug allergy or hypersensitivity, active or recent GI bleed, significant renal impairment (estimated glomerular filtration rate [eGFR] <30 mL/min/m2) estimated by Cockroft-Gault, anticipated survival <1 year, or use of other NSAIDs or >2 blood thinners were excluded from participation. One patient was offered access to the drug but did not have biopsy-proven cardiac amyloidosis and, therefore, was not included in this analysis. No patients refused access to the drug.
TABLE I.
Mean Baseline Demographic and Clinical Parameters of the ATTRwt and ATTRm Population Studied (N=13)
| Demographics | No. (%) |
|---|---|
| Age (±SE), y | 69±3 |
| Men | 11 (85) |
| White race | 9 (69) |
| African American race | 2 (15) |
| Hispanic ethnicity | 4 (31) |
| ATTR wild-type | 7 (54) |
| ATTR mutant | 6 (46) |
| Follow-up, mean±SE, d | 321±101 |
| Comorbid conditions | |
| Hypertension | 4 (31) |
| Diabetes | 1 (8) |
| Coronary artery disease | 1 (8) |
| Atrial fibrillation | 3 (23) |
| Cerebral vascular accident | 1 (8) |
| Electrocardiographic | |
| Low QRS voltage | 4 (31) |
| Infarct pattern | 9 (69) |
| Pacemaker/defibrillator | 4 (31) |
Abbreviations: ATTR, transthyretin amyloidosis; SE, standard error.
Thirteen patients were prescribed diflunisal 250 mg twice a day along with either a histamine receptor antagonist or proton pump inhibitor (PPI) in an open-label fashion. Follow-up occurred every 3 months or as indicated by the treating physician (MSM), during which complete blood cell count, basic metabolic panel, Troponin I (Tr I), brain natriuretic peptide (BNP), electrocardiograms (ECGs), and transthoracic echocardiograms were obtained. For safety, changes in hemoglobin (Hgb), creatinine, modified body mass index (BMI), a reflection of serum albumin×BMI,29 mean arterial pressure (MAP), and hospital admission rates were evaluated. For efficacy, changes in Tr I, BNP, left ventricular ejection fraction (EF), and left ventricular (LV) mass were evaluated. Statistical analysis involved calculation of mean±standard errors for interval data and percentages for categoric variables. Changes in safety and efficacy parameters were compared from baseline to latest follow-up using the Kruskal-Wallis test for nonparametric data.
RESULTS
Patients were on average older adults, 69±3 years, and almost exclusively men, 85% (Table I). Participants were patients with ATTRwt (54%) and ATTRm (46%) with a broad range of represented mutations: Val122Ile, Thr60Ala, Ser23Asn, and Glu89Gln. Mean length of follow-up was 0.9±0.3 years (range 0.2– 3.4 years). At baseline, patients had a phenotype consistent with cardiac amyloidosis: fatigue 46%, presyncope 38%, dyspnea on exertion 69%, orthopnea 31%, paroxysmal nocturnal dyspnea 23%, peripheral edema 46%, palpitations 23%, peripheral neuropathy 46%, and macroglossia 8%, consistent with ATTR symptomatology described previously.6 Functionally, these symptoms translated to an average baseline 69% with New York Heart Association (NYHA) class I or II and 31% class III or IV heart failure with a low-normal EF of 50%±3. Electrocardiographically, 31% of patients had baseline evidence of low QRS voltage, 69% had an infarct pattern characteristic of amyloidosis, 30 and 31% had a pacemaker/defibrillator.
Comorbid conditions within this study population were common (Table I). Over the study period, there were no hospitalizations for worsening heart failure. One patient underwent an orthotopic heart transplant at 7 months into the study, while another patient underwent a combined heart/liver transplant at 8 months. One patient’s therapy was discontinued after 2 weeks for a >10-lb weight gain, and another patient’s diflunisal dose was halved to 125 mg twice daily due to concern for volume overload. Pharmacologically, there was no necessitation for an increase in diuretic dose between baseline and follow-up (25±7 to 28±8 mg/d of furosemide equivalents).
Diflunisal was well tolerated from a hematologic and hemodynamic standpoint. Comparison between mean baseline and follow-up parameters showed stable Hgb, modified BMI, and MAP levels and a nonsignificant 6% decrease in eGFR (Table II, Figure). Of note, the decline in GFR was strongly related to the duration of therapy (r=−0.82, P<0.002). From the standpoint of efficacy, there was no significant mean change in cardiac structure (stable LV mass), function (stable EF), or biomarkers (Tr I and BNP), although there was a nonsignificant uptrend in Tr I and BNP (Table II, Figure).
TABLE II.
Biochemical and Echocardiographic Characteristicsa (N=13)
| Baseline | Follow-Up | P Value | |
|---|---|---|---|
| Biochemical | |||
| Hemoglobin, g/dL | 13.7±0.3 | 14.0±0.4 | .55 |
| Platelets, 109/L | 179±12 | 168±11 | .81 |
| BUN, mg/dL | 22±2 | 27±3 | .44 |
| Creatinine, mg/dL | 1.2±0.1 | 1.2±0.1 | .74 |
| eGFR, mL/min | 82±8 | 77±8 | .35 |
| Troponin I, ng/mL | 0.04±0.01 | 0.07±0.02 | .08 |
| BNP, pg/mL | 388±92 | 481±102 | .52 |
| Albumin, g/dL | 4.1±0.1 | 4.0±0.1 | .33 |
| Modified BMI, kg g/m2 dL | 100±4 | 95±4 | .21 |
| Echocardiographic | |||
| LV ejection fraction, % | 50±3 | 48±6 | .61 |
| LV end-diastolic diameter, cm | 4.4±0.1 | 4.5±0.1 | .33 |
| Interventricular septal thickness, cm | 1.8±0.1 | 1.6±0.1 | .25 |
| LV posterior wall thickness, cm | 1.6±0.1 | 1.5±0.2 | .50 |
| LA diameter, cm | 4.6±0.2 | 4.5±0.2 | .80 |
| LV mass, g/m2 | 384±37 | 331±65 | .36 |
Abbreviations: BMI, body mass index; BNP, B-type natriuretic peptide; BUN, serum urea nitrogen; eGFR, estimated glomerular filtration rate; LA, left atrial; LV, left ventricular.
Mean±standard error at baseline and follow-up, change, and P value from Kruskal-Wallis test are shown.
FIGURE.
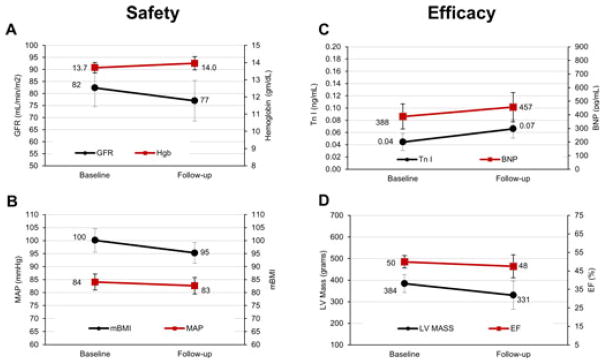
Comparison between mean baseline and follow-up safety parameters show a trend towards decrease in glomerular filtration rate (GFR) but a stable hemoglobin (A), modified body mass index (mBMI), and mean arterial pressure (MAP) (B). With regard to efficacy, trends between mean baseline and follow-up metrics show a mild increase in B-type natriuretic peptide (BNP) and Troponin (Tr) I (C), stable left ventricular (LV) mass, and a stable ejection fraction (EF) (D). Standard error bars are shown.
DISCUSSION
We have demonstrated in a population of 13 patients with ATTR cardiac amyloidosis that diflunisal can be safely administered with regular renal and hematologic monitoring. Additionally, the absence of significant changes in cardiac structure and function as evidenced by stable LV mass index and EF over the study period suggest that diflunisal may have therapeutic benefit in slowing the progression of cardiac amyloidosis. The slight uptrend observed in BNP and Tr I may relate to the natural progression of the disease and/or the effect of diflunisal on renal function, which highlight the need for ongoing clinical and biochemical monitoring.
Clinical features and survival in patients with ATTR cardiac amyloid have been described by Connors and colleagues31 in a study of 82 ATTRwt patients studied at a single institution between 1994 and 2009. Patients were found to be predominantly men (96%) who presented with dominant cardiac involvement evidenced by abnormal echocardiogram (increased median interventricular septal thickness of 1.6 cm and reduced median LVEF 50%), ECG (low-voltage QRS 33% and supraventricular arrhythmias 57%), and/or elevated cardiac biomarkers (mean Tr I 0.15±0.107 [n=19] and BNP 422±279 [n=41]). Heart failure was found more frequently than arrhythmias and compared with patients with ATTRm, ATTRwt patients were diagnosed at an older age. Median survival in ATTRwt patients was <4 years, which was similar to ATTR(V122I), but significantly lower than ATTR(T60A) and ATTR(S77Y), in which median survivals were ≥5 years. At Columbia, our experience in a cohort of 68 ATTRwt and ATTRm patients has revealed a 5.6-year median survival.
In the largest, prospective, longitudinal investigation to date, the Transthyretin Amyloidosis Cardiac Study (TRACS), the progression of ATTR CA was monitored in 29 ATTRwt and ATTR (V122I) patients.32 The TRACS population and our diflunisal-treated group had a similar baseline age, percentage of men, NYHA classification, and serum creatinine. The TRACS population had more African American patients, more baseline hypertension, higher B-type natriuretic peptide (BNP) levels, and a higher percentage of low QRS voltage and pacemaker/defibrillators than the present cohort, suggesting a more severe phenotype. During a mean of 15.5 months, paired TRACS data revealed that the clinical progression of heart failure as demonstrated by increasing N-terminal pro-BNP and worsening NYHA classification with a concurrent decrease in LVEF was significant. In our diflunisal population, the progression of disease over a similar mean follow-up of approximately 1 year was notably lower, with the exception of a decline in renal function reflected by a 6% reduction in eGFR, a known complication of chronic NSAID use.24 The absence of significant hematologic side effects in terms of hospitalizations for GI bleeding in our study likely reflects the protective benefit of prophylaxis with a histamine antagonist or PPI. Alternatively, a reasonable assumption is that the event rate of GI bleeding in this population may be <1/13.
While there is a suggestion of improved survival in our diflunisal population compared with the TRACS population, differences between the two populations in regard to duration of disease and phenotype severity preclude conclusions that might be drawn about drug efficacy. For instance, the African American population in the TRACS study was 38% while in ours was 15%, a notable difference considering prior reports of worse outcomes in African American patients with the V122I mutation. At Columbia, our group studied 58 ATTR patients, in which the clinical features and outcomes in 36 ATTRwt patients were compared against 22 individuals with the V122I mutation, all of whom were African American.33 We found that despite the ability to test for the V122I allele, these patients presented later to our institution and at a more advanced stage of disease than ATTRwt patients in whom serologic testing for early diagnosis is not available. On average, outcome metrics were also worse in V122I patients compared with those with ATTRwt disease as defined by a trend towards higher Tr I (0.31 vs 0.19, P=.4), higher BNP (1191 vs 822 pg/mL, P=.05), lower EF (26% vs 47%, P<0.0001), a trend towards lower cardiac output (3.1 vs 3.5 L/min, P=.09), and higher peripheral vascular resistance (398 vs 240 dynes, P=.03).
Despite the potential differences between the current population and the TRACS study, 11 patients in the TRACS study died from either heart failure (n=3), sudden death (n=3), sepsis (n=3), or unclassified causes (n=2), and 1 patient received a heart transplant, altogether representing 41% of the entire cohort. There were no deaths in our population, but 2 patients progressed to either heart or combined heart/liver transplantation, representing 15% of our population. In addition, 12 patients (41%) of those in the TRACS study required hospitalization for cardiovascular causes whereas none of our diflunisal patients required hospitalization within the study period.
A placebo-controlled, multicenter, international clinical trial to test the efficacy of diflunisal for the treatment of familial amyloid polyneuropathy (FAP) is currently fully enrolled.34 A cohort of 130 patients with ATTR (V30M) and non-V30M mutations that caused a peripheral neuropathy were randomized to receive diflunisal or placebo. Results on the primary efficacy end point utilizing a diabetic polyneuropathy metric and neuropathic impairment score are expected within the next year, but preliminary data show that diflunisal was well tolerated. Congestive heart failure in 2 patients and GI bleeding in a third resulted in drug discontinuation. Five disease-related deaths have been reported, all off the study drug. While the effect of diflunisal on ATTR cardiac amyloid was not studied in this group, a significant percentage of these patients had evidence of cardiac involvement. These results coupled with our own findings suggest that clinical trials of diflunisal could include patients with cardiac amyloid in order to more fully evaluate safety and efficacy.
STUDY LIMITATIONS
Several limitations to our investigation are worth noting. First, the lack of a placebo-controlled arm in our open-label design limits our ability to determine whether observed changes within the study population resulted from diflunisal administration or as a consequence of the natural history of the disease itself. However, such studies are required to evaluate safety of novel therapies prior to embarking on controlled trials. Second, this study represents data from a single amyloid center with a small cohort, limiting statistical power to detect differences in measured parameters over time. Third, the low proportion of African American patients with the V122I allele (14%) may narrow the generalizability of our findings across racial and ethnic groups to the predominant Caucasian population in our cohort. Fourth, our data may not be applicable to high-risk patients whose burden of atherosclerotic disease or severe renal dysfunction may be prohibitive to NSAID administration. Fifth, our cohort presented relatively early in the disease process with relative high NYHA functional status and low diuretic requirements, which limits the applicability of our results to patients with more advanced cardiac amyloidosis. Finally, echocardiogram interpretation was limited by interobserver reader variability in addition to the fact that changes in EF or LV mass usually take years to detect; a longer follow-up or use of a more sensitive measure (eg, LV strain analysis) would increase sensitivity of detecting changes in cardiac structure in future studies. A prospective, multicenter, randomized, double-blinded, placebo-controlled study with sufficient power to detect differences among mutant and wild-type forms of ATTR cardiac amyloid appears warranted to determine whether diflunisal can be safely and effectively administered to patients with ATTR cardiac amyloidosis.
CONCLUSIONS
Diflunisal, at a dose of 250 mg orally twice a day, was reasonably well tolerated in ATTR cardiac amyloid patients. We observed an association with adverse changes in renal function when administered chronically, suggesting that careful monitoring is essential to safe administration. The absence of significant changes in cardiac structure and function as evidenced by stable LV mass index and EF in our ATTR cardiac amyloid population suggest that larger, multicenter, randomized, double-blinded, and placebo-controlled studies would be valuable to assess for long-term efficacy and safety.
Footnotes
Disclosure: The authors report no conflicts of interest.
References
- 1.Benson MD. Amyloidosis. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 7. Vol. 7 New York, NY: McGraw-Hill; 1995. [Google Scholar]
- 2.Lachmann HJ, Booth DR, Booth SE, et al. Misdiagnosis of hereditary amyloidosis as AL (primary) amyloidosis. N Engl J Med. 2002;346:1786–1791. doi: 10.1056/NEJMoa013354. [DOI] [PubMed] [Google Scholar]
- 3.Bhuiyan T, Helmke S, Patel AR, et al. Pressure-volume relationships in patients with transthyretin (ATTR) cardiac amyloidosis secondary to V122I mutations and wild-type transthyretin: Transthyretin Cardiac Amyloid Study (TRACS) Circ Heart Fail. 2011;4:121–128. doi: 10.1161/CIRCHEARTFAILURE.109.910455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robbins J. Thyroxine-binding proteins. Prog Clin Biol Res. 1976;5:331–355. [PubMed] [Google Scholar]
- 5.Connors LH, Lim A, Prokaeva T, et al. Tabulation of human transthyretin (TTR) variants, 2003. Amyloid. 2003;10:160–184. doi: 10.3109/13506120308998998. [DOI] [PubMed] [Google Scholar]
- 6.Rapezzi C, Quarta CC, Riva L, et al. Transthyretin-related amyloidoses and the heart: a clinical overview. Nat Rev Cardiol. 2010;7:398–408. doi: 10.1038/nrcardio.2010.67. [DOI] [PubMed] [Google Scholar]
- 7.Tanskanen M, Peuralinna T, Polvikoski T, et al. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: a population-based autopsy study. Ann Med. 2008;40:232–239. doi: 10.1080/07853890701842988. [DOI] [PubMed] [Google Scholar]
- 8.Cornwell GG, III, Murdoch WL, Kyle RA, et al. Frequency and distribution of senile cardiovascular amyloid. A clinicopathologic correlation. Am J Med. 1983;75:618–623. doi: 10.1016/0002-9343(83)90443-6. [DOI] [PubMed] [Google Scholar]
- 9.Mirzoyev SA, Edwards WD, Mohammed SF, et al., editors. Cardiac Amyloid Deposition is Common in Elderly Patients with Heart Failure and Preserved Ejection Fraction. Chicago, IL: AHA; 2010. [Google Scholar]
- 10.Jacobson DR, Gorevic PD, Buxbaum JN. A homozygous transthyretin variant associated with senile systemic amyloidosis: evidence for a late-onset disease of genetic etiology. Am J Hum Genet. 1990 Jul;47:127–136. [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson DR, Pastore RD, Yaghoubian R, et al. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med. 1997;336:466–473. doi: 10.1056/NEJM199702133360703. [DOI] [PubMed] [Google Scholar]
- 12.Suhr OB, Herlenius G, Friman S, Ericzon BG. Liver transplantation for hereditary transthyretin amyloidosis. Liver Transpl. 2000;6:263–276. doi: 10.1053/lv.2000.6145. [DOI] [PubMed] [Google Scholar]
- 13.Pilato E, Dell’Amore A, Botta L, Arpesella G. Combined heart and liver transplantation for familial amyloidotic neuropathy. Eur J Cardiothorac Surg. 2007;32:180–182. doi: 10.1016/j.ejcts.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 14.Liepnieks JJ, Zhang LQ, Benson MD. Progression of transthyretin amyloid neuropathy after liver transplantation. Neurology. 2010;75:324–327. doi: 10.1212/WNL.0b013e3181ea15d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammarstrom P, Wiseman RL, Powers ET, Kelly JW. Prevention of transthyretin amyloid disease by changing protein misfolding energetics. Science. 2003;299:713–716. doi: 10.1126/science.1079589. [DOI] [PubMed] [Google Scholar]
- 16.Alhamadsheh MM, Connelly S, Cho A, et al. Potent kinetic stabilizers that prevent transthyretin-mediated cardiomyocyte proteotoxicity. Sci Transl Med. 2011;3:97ra81. doi: 10.1126/scitranslmed.3002473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekijima Y, Dendle MA, Kelly JW. Orally administered diflunisal stabilizes transthyretin against dissociation required for amyloidogenesis. Amyloid. 2006;13:236–249. doi: 10.1080/13506120600960882. [DOI] [PubMed] [Google Scholar]
- 18.Tojo K, Sekijima Y, Kelly JW, Ikeda S. Diflunisal stabilizes familial amyloid polyneuropathy-associated transthyretin variant tetramers in serum against dissociation required for amyloidogenesis. Neurosci Res. 2006;56:441–449. doi: 10.1016/j.neures.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Johnson SM, Connelly S, Fearns C, et al. The transthyretin amyloidoses: from delineating the molecular mechanism of aggregation linked to pathology to a regulatory-agency-approved drug. J Mol Biol. 2012 Jan 5; doi: 10.1016/j.jmb.2011.12.060. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller SR, Sekijima Y, Kelly JW. Native state stabilization by NSA-IDs inhibits transthyretin amyloidogenesis from the most common familial disease variants. Lab Invest. 2004;84:545–552. doi: 10.1038/labinvest.3700059. [DOI] [PubMed] [Google Scholar]
- 21.Adamski-Werner SL, Palaninathan SK, Sacchettini JC, Kelly JW. Diflunisal analogues stabilize the native state of transthyretin. Potent inhibition of amyloidogenesis. J Med Chem. 2004;47:355–374. doi: 10.1021/jm030347n. [DOI] [PubMed] [Google Scholar]
- 22.Berk JL, Dyck PJ, Obici L, et al. The diflunisal trial: update on study drug tolerance and disease progression. Amyloid. 2011;18(suppl 1):191–192. doi: 10.3109/13506129.2011.574354073. [DOI] [PubMed] [Google Scholar]
- 23.Kearney PM, Baigent C, Godwin J, et al. Do selective cyclo-oxygen-ase- 2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006;332:1302–1308. doi: 10.1136/bmj.332.7553.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epstein M. Non-steroidal anti-inflammatory drugs and the continuum of renal dysfunction. J Hypertens Suppl. 2002;20:S17–S23. [PubMed] [Google Scholar]
- 25.Marnett LJ, Kalgutkar AS. Cyclooxygenase 2 inhibitors: discovery, selectivity and the future. Trends Pharmacol Sci. 1999;20:465–469. doi: 10.1016/s0165-6147(99)01385-1. [DOI] [PubMed] [Google Scholar]
- 26.Wallace JL. Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol. 2001;15:691–703. doi: 10.1053/bega.2001.0229. [DOI] [PubMed] [Google Scholar]
- 27.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286:954–959. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- 28.Page J, Henry D. Consumption of NSAIDs and the development of congestive heart failure in elderly patients: an underrecognized public health problem. Arch Intern Med. 2000;160:777–784. doi: 10.1001/archinte.160.6.777. [DOI] [PubMed] [Google Scholar]
- 29.Suhr O, Danielsson A, Holmgren G, Steen L. Malnutrition and gastrointestinal dysfunction as prognostic factors for survival in familial amyloidotic polyneuropathy. J Intern Med. 1994;235:479–485. doi: 10.1111/j.1365-2796.1994.tb01106.x. [DOI] [PubMed] [Google Scholar]
- 30.Murtagh B, Hammill SC, Gertz MA, et al. Electrocardiographic findings in primary systemic amyloidosis and biopsy-proven cardiac involvement. Am J Cardiol. 2005;95:535–537. doi: 10.1016/j.amjcard.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 31.Connors LH, Doros G, Sam F, et al. Clinical features and survival in senile systemic amyloidosis: comparison to familial transthyretin cardiomyopathy. Amyloid. 2011;18(suppl 1):152–154. doi: 10.3109/13506129.2011.574354059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruberg FL, Maurer MS, Judge DP, et al. Prospective evaluation of the morbidity and mortality of wild-type and V122I mutant transthyretin amyloid cardiomyopathy: the transthyretin amyloidosis cardiac study (TRACS) Am Heart J. 2012 doi: 10.1016/j.ahj.2012.04.015. (in press) [DOI] [PubMed] [Google Scholar]
- 33.Russo C, Green P, Maurer MS, editors. Comparison of V12I and senile cardiac amyloidosis: differences in clinical features and outcomes. 13th International Symposium on Amyloid; 2012; Groningen, Netherlands. [Google Scholar]
- 34.Berk JL, Suhr OB, Sekijima YS, et al., editors. The diflunisal trial: study accrual and drug tolerance. VIIIth International Symposium on Familial Amyloidotic Polyneuropathy; 2011 November 20–23; Kumamoto, Japan. [Google Scholar]


