Abstract
The photothrombotic stroke model aims to induce an ischemic damage within a given cortical area by means of photo-activation of a previously injected light-sensitive dye. Following illumination, the dye is activated and produces singlet oxygen that damages components of endothelial cell membranes, with subsequent platelet aggregation and thrombi formation, which eventually determines the interruption of local blood flow. This approach, initially proposed by Rosenblum and El-Sabban in 1977, was later improved by Watson in 1985 in rat brain and set the basis of the current model. Also, the increased availability of transgenic mouse lines further contributed to raise the interest on the photothrombosis model. Briefly, a photosensitive dye (Rose Bengal) is injected intraperitoneally and enters the blood stream. When illuminated by a cold light source, the dye becomes activated and induces endothelial damage with platelet activation and thrombosis, resulting in local blood flow interruption. The light source can be applied on the intact skull with no need of craniotomy, which allows targeting of any cortical area of interest in a reproducible and non-invasive way. The mouse is then sutured and allowed to wake up. The evaluation of ischemic damage can be quickly accomplished by triphenyl-tetrazolium chloride or cresyl violet staining. This technique produces infarction of small size and well-delimited boundaries, which is highly advantageous for precise cell characterization or functional studies. Furthermore, it is particularly suitable for studying cellular and molecular responses underlying brain plasticity in transgenic mice.
Keywords: Medicine, Issue 76, Biomedical Engineering, Immunology, Anatomy, Physiology, Neuroscience, Neurobiology, Surgery, Cerebral Cortex, Brain Ischemia, Stroke, Brain Injuries, Brain Ischemia, Thrombosis, Photothrombosis, Rose Bengal, experimental stroke, animal models, cortex, injury, protocol, method, technique, video, ischemia, animal model
Introduction
At the beginning of the 21th century, ischemic stroke is a devastating disorder that represents the second cause of long term disability1 and the second cause of mortality worldwide, in which stroke accounted for approximately 5,7 million deaths in 20042. In spite of the many efforts that were put in, there is still no effective treatment available to improve functional recovery after stroke. Animal models of stroke are widely used in the field of stroke research as they allow modeling of the pathophysiology of ischemic damage and test the efficacy of different neuroprotective strategies in vivo. Most of these models aim to induce extensive infarctions by interrupting (temporarily or permanently) the blood flow within the middle cerebral artery, whereas other models were developed to study lesions of small size in specific areas, typically the motor and somatosensory cortices. However, several factors may contribute to generate a certain degree of variability in experimental stroke studies, including the mouse strain used, the age and sex of animals included in the study and, above all, the technique adopted to induce the ischemic damage. With regard to the latter point, the duration and invasiveness of the surgery (i.e. the need for a craniotomy) as well as the surgical skill required to the operator to reliably induce an ischemic lesion are critical determinants for a successful and unbiased in vivo stroke study.
The concept of photothrombosis was initially proposed by Rosenblum and El-Sabban in 19773 and became renowned by its application in rat brain by Watson et al. in 19854 in which the technique was largely improved and set the basis of the current model3-6. The photothrombotic approach aims to induce a cortical infarction through the photo-activation of a light-sensitive dye previously delivered into blood system, which results in local vessel thrombosis in the areas exposed to the light. When the circulating dye is illuminated at the appropriate wavelength by a cold light source, it releases energy to oxygen molecules, which in turn generate a large amount of highly reactive singlet oxygen products. These oxygen intermediates induce endothelial cell membrane peroxidation, leading to platelet adhesion and aggregation, and eventually to the formation of thrombi which determine local cerebral flow interruption7.
Photothrombosis is a non-canonical ischemic model that does not occlude or break only one artery as it usually happens in human stroke, but induces lesions in more superficial vessels, which results in selective interruption of blood flow in the areas exposed to light. For this reason, this approach may be suitable for cellular and molecular studies of cortical plasticity. The principal advantage of this technique resides in its simplicity of execution. Moreover, photothrombosis can be easily performed in approximately forty minutes per animal, including twenty-minute wait (3 min for anesthesia; 1 min to shave the scalp; 3 to 5 min to place the animal on the stereotaxic apparatus; 2 min to scrub the scalp with antiseptic solution, make an incision and clean the skull; 2 to 4 min to place the cold light fiber; 1 min to inject the rose Bengal solution; 5 min-wait for intraperitoneal diffusion; 15 min of illumination; and 5 min to clean the wound and suture the animal). Furthermore, no surgical expertise is needed to perform this technique as the lesion is induced through simple illumination of the intact skull. Unlike classical arterial occlusion, this method determines selective occlusions of pial and intraparenchymal microvessels within the irradiated zone and reduces the variability among lesions as no collateral vessel is left to supply oxygen in the targeted area.
In spite of its particular nature, the photothrombotic damage shares essential mechanisms occurring in brain stroke. Similarly to artery occlusion in human stroke, platelet aggregation and clot formation determine interruption of blood flow in the irradiated area7. Likewise, this model also shares essential inflammatory responses as in middle cerebral artery occlusion8. However, because of the well-delimitated boundaries, the penumbral zone, which corresponds to an area of partially preserved metabolism, is very reduced or inexistent after a photothrombotic lesion. This clear border can facilitate the study of cellular responses within ischemic or intact cortical area. Photothrombosis mouse model is particularly suitable for stroke studies in a variety of transgenic animals. Indeed classical models cannot fit to all strains and long period studies in C57BL/6 mouse strain reported a high mortality ratio that can cause bias9.
Protocol
1. Pre-surgery
Weigh Rose Bengal in an 1.5 ml tube and dissolve in sterile saline solution until reaching a final concentration of 15 mg/ml. Filter sterilize through a 0.2 μm filter and store it in the dark at room temperature up to two months.
Sterilize all surgical instruments by autoclaving. The surgical area should be sanitized less than one hour before initiating the surgery.
Record the mouse body weight to adjust the dose of Rose Bengal to be injected. We injected 10 μl/g animal weight in female CD1 mice 12 weeks old in the present protocol. The amount of Rose Bengal needed to produce the desired cortical lesion size can be easily determined in a separate set of preliminary experiments by testing different dosages (typically 2 μl/g, 5 μl/g or 10 μl/g body weight). Note that the amount of Rose Bengal to be injected is highly dependent on the experiment conditions, particularly the type of light source used and the duration of light exposure. In the present study, 10 μl/g (150 μg/g) dose was found to be necessary to induce photothrombosis upon exposure to light for 15 min, whereas others groups reported that either 50 μg/g 6,8 and 100 μg/g10 intraperitoneal injection was sufficient for inducing a photothrombotic lesion.
2. Anesthesia Procedure
Anesthetize mice with isoflurane in a transparent induction chamber (3.5-4% for induction, 1.5-2% for maintaining) in 50% (v/v) oxygen/50% (v/v) dinitrogen monoxide gas mixture. Gaseous anesthesia allows a quick wake up of the animals and the level of anesthetic gas can be adjusted easily. Alternatively, mice can also be anesthetized by a ketamine-xylazine mixture.
When deep anaesthesia is reached, remove the anesthetized animal from the induction chamber, place it in the stereotaxic frame and maintain anesthesia using a face mask. Adjust the isoflurane dose to achieve an adequate anesthesia level. Monitor the breath rate throughout the procedure and make sure it is constant (40- 60 breaths per minute).
Use toe pinches in order to ensure the animal is deeply anaesthetized.
Apply eye ointment, in order to prevent the eyes from drying out.
Gently insert the rectal probe to monitor the temperature throughout the surgical procedures. Set the associated feedback-controlled heating pad for maintaining the mouse body temperature at 37 ± 0.5 °C.
3. Surgery for Illumination of the Target Area
Shave the mouse scalp with an electric razor.
Firmly secure the head and insert the ear bars into external meatus. Be careful not to damage eardrums.
Disinfect the skin on the surface with alternating swipes of 70% ethanol and betadine using cotton swabs.
Use a scalpel to make an incision along the midline from the eye level down to the neck. Apply skin retractors to keep the skull exposed.
Gently retract the periostium to the edges of the skull with a scalpel and let the skull surface dry using sterile cotton swabs. Identify the bregma and lambda. Place a glass micropipette on the bregma as reference point, then move it to the coordinates of your region of interest. The region of interest chosen for this article is roughly roundly shaped, centered approximately 2 mm lateral to the bregma, and covering an area of about 30 mm2, which includes a large part of the sensorimotor cortex according to the mouse brain atlas by Franklin and Paxinos11.
Mark the position of interest with reference points and put an optic fiber in close contact with the skull surface to avoid light scattering but pay attention not to exert pressure on it. The illuminated area can be restricted by applying on the skull a mask with small aperture or a cap at the tip of the optic fiber guide.
4. Rose Bengal Injection and Activation
Load the Rose Bengal solution in a 1 ml syringe and calculate the amount to be injected according to a dose of 10 μl/g of body weight.
Proceed to a slow intraperitoneal injection.
Let the dye diffuse and enter the blood stream. After 5 min, switch on the cold light illuminator. Avoid illumination of the animal by any other source of light. We used fiber optic illuminator of 150 W intensity.
After 15 min of illumination, stop light exposure and suture the wound. Five minutes of illumination already produce infarction and 10 min is likely sufficient to obtain a maximal effect12. In order to minimize variability, we choose to illuminate for 15 min.
5. Suture
Remove the skin retractors and apply sterile saline solution to avoid dehydration.
Close up the wound using reverse cutting needle and silk or nylon suture thread.
Interrupt anesthesia delivery, carefully remove the mouse from the stereotaxic apparatus and put it on a pre-warmed heating pad until it is fully awake, then return it to its cage. Body temperature should be monitored carefully throughout the procedures to limit the variability in the infarct extension. According to the concentration and the route of administration, Rose Bengal can still be detected in the blood stream for several hours after injection13. Prefer the heating blanket to warming lamp to avoid potential secondary damages (Rose Bengal absorption wavelength is in the green spectrum).
Representative Results
This protocol will produce a cortical lesion that is already visible upon dissection of the cortex to the unaided eye (Figures 1A-1C). The photothrombotic lesion develops in superficial and deep cortical layers in which the tissue is sufficiently translucent to allow photo-activation of the Rose Bengal. Measurement of the extent of cerebral infarction can be performed quickly by histological staining with triphenyl-tetrazolium chloride (TTC) on fresh tissue or by cresyl violet after fixation in 4% paraformaldehyde (PFA). Before TTC incubation, the freshly dissected brain is positioned in a brain slicer and sectioned into 1 mm thick slices (Figure 1C). TTC labels intact tissue in red whereas the infarcted region appears pale, which allows a precise measurement of the infarct area (Figures 1D-1E). The zone of incomplete ischemia defined as penumbra is very reduced or inexistent according to the size of the lesion.
Similarly, the cresyl violet staining allows the identification of unstained infarcted areas as compared to the purple-stained surrounding tissue. The reactive proliferation and cell infiltration (which occurs during the first week following the lesion) can also be assessed (Figures 2A1-2A2), as well as the appearance of a glial scar (Figures 2B1-2B2). The negative control is carried out by performing the same surgery and dye injection, but without the exposure to the cold light source. The lesion is reproducible in size and location at different time points after the lesion (Figures 3A-3B). The infarct area generally reaches its maximum size by 4 to 6 hr after Rose Bengal injection14 and subsequently reduces quickly from 2 days to 4 days post photothrombosis. The irradiation occurs in the more superficial layers, but large vessels in the deep cortical layers might also be occluded because of the compression provoked by the lesion7. It is important to investigate the reproducibility of the lesion before treatment because the distribution of brain pial microvasculature can vary between animals of different age or strain.
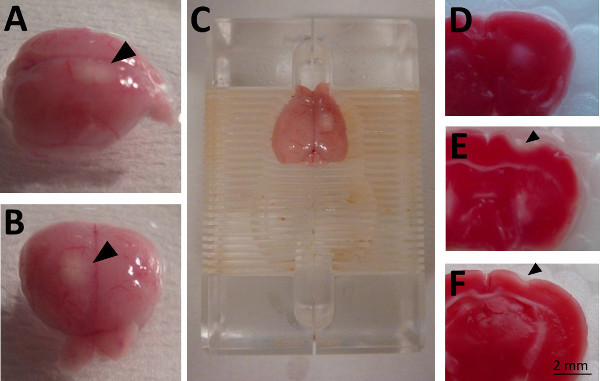 Figure 1. Macroscopical appearance of photothrombotical lesion in CD1 mouse brain 4 days after photothrombosis.A-B. Lateral and frontal view. The superficial infarction (arrowheads) appears as a white region visible to the unaided eye. C. The brain is positioned in brain slicer and sectioned quickly into 1 mm thick sections. D-F. Measurement of the extent of cerebral infarction can be accomplished by TTC staining. Necrotic tissue within the thrombotic lesion is characterized by absence of staining. Coronal slices of the brain illustrated in A-C are represented in rostral to caudal extension from D to F.
Figure 1. Macroscopical appearance of photothrombotical lesion in CD1 mouse brain 4 days after photothrombosis.A-B. Lateral and frontal view. The superficial infarction (arrowheads) appears as a white region visible to the unaided eye. C. The brain is positioned in brain slicer and sectioned quickly into 1 mm thick sections. D-F. Measurement of the extent of cerebral infarction can be accomplished by TTC staining. Necrotic tissue within the thrombotic lesion is characterized by absence of staining. Coronal slices of the brain illustrated in A-C are represented in rostral to caudal extension from D to F.
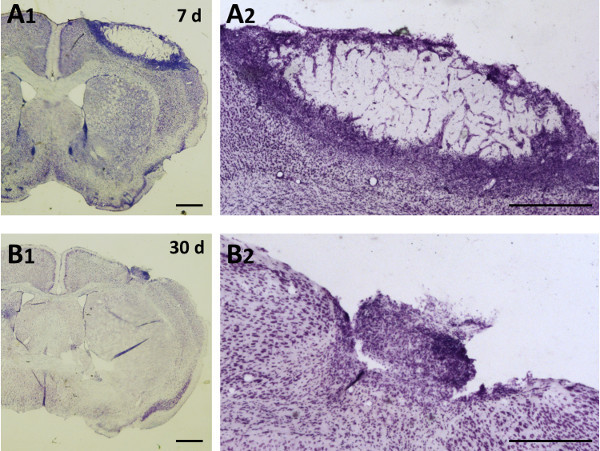 Figure 2. Representative temporal evolution of photothrombotic damage. Coronal sections of adult C57BL/6 mouse brain were stained by cresyl violet. A1, A2. 7 days after injury a high number of cells gathers at the infarct boundary. B1, B2. At 30, the lesion volume highly reduced and glial scar is forming. A2 and B2 are high magnification of A1 and B1 respectively. Scale bars: A1, B1: 1 mm; A2, B2: 0.5 mm.
Figure 2. Representative temporal evolution of photothrombotic damage. Coronal sections of adult C57BL/6 mouse brain were stained by cresyl violet. A1, A2. 7 days after injury a high number of cells gathers at the infarct boundary. B1, B2. At 30, the lesion volume highly reduced and glial scar is forming. A2 and B2 are high magnification of A1 and B1 respectively. Scale bars: A1, B1: 1 mm; A2, B2: 0.5 mm.
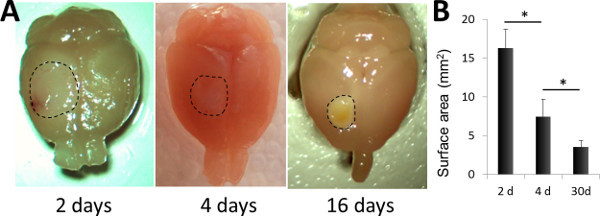 Figure 3. Macroscopic appearance of the brain at 2, 4 and 16 days after photothrombosis.A. Microphotographs of PFA-perfused brains, prepared for further immunohistological analysis. The infarcted area is highlighted (dashed line). B. Comparison of the surface area at 2, 4 and 16 days after the lesion. n=4 in each group. *p <0.05 (Student's t-test).
Figure 3. Macroscopic appearance of the brain at 2, 4 and 16 days after photothrombosis.A. Microphotographs of PFA-perfused brains, prepared for further immunohistological analysis. The infarcted area is highlighted (dashed line). B. Comparison of the surface area at 2, 4 and 16 days after the lesion. n=4 in each group. *p <0.05 (Student's t-test).
Discussion
Modifications and substitutions
Because of its absorption peak at 562 nm, a green light laser from a filtered xenon arc lamp was originally chosen to irradiate the photosensitive Rose Bengal. Although laser-mediated excitation was still used recently5, it can be replaced by cold light lamp that also ensure dye excitation10,15. Cold light optic fibers are easier to manipulate and less expensive than laser sources. However, it should be noticed that lasers are commonly used to target individual surface arterioles after craniotomy for an in vivo vessel-specific clotting10.
Delivery of sensitive dye is usually accomplished by tail vein injection, which requires some training for obtaining homogenous and reproducible results. By contrast, intraperitoneal injection is easier to perform and Rose Bengal is detectable in the blood stream a few minutes after13. Effective photothrombosis can be achieved by exciting the dye 5 min after intraperitoneal injection8, however high intraperitoneal dose of Rose Bengal in rat showed that plasma concentration of the dye continue to increase even 60 min after administration13. Infused intravenous injection of Rose Bengal can increase the reproducibility of the lesion13, however this way of administration can be associated to hypotension in the case the infusion is performed too rapidly (<1.5 min) or at high dye concentration5. For avoiding fluctuations in blood pressure, other photosensitive substances such as Erythrosin B can be used to produce vessels occlusions, however this molecule is considered less efficient than Rose Bengal for the same range of concentration5.
Critical steps
Different factors affect lesion size and depth such as the concentration of the sensitive dye, the latency between the dye injection and illumination, the intensity of the light source, the diameter of the enlightened surface, the exposure duration and the body temperature during and after surgery. The angle of the light source on the skull can also influence the shape of the infarct. A series of preliminary experiments should be carried out to test the reproducibility and to determine the minimum amount of dye and irradiation time which produce a lesion affecting selectively the target area and/or obtain significative behavioral deficit.
Limitations of the technique
This technique was developed in order to mimic human stroke by inducing platelet aggregation as observed during cerebral ischemia4. However photothrombotic damage slightly differs from human stroke. First of all, thrombosis is triggered in large number of vessels in the illuminated area, whereas stroke is typically caused by interruption of blood flow within a single terminal artery. As a consequence, some regions of the brain, whose metabolism is only partially supported by the artery undergoing the blood flow interruption are initially less affected as they may receive blood supply by collateral arteries and do not undergo necrotic cell death. At the contrary the well-defined boundary produced by photothrombosis results in a very limited penumbra, that is the main target of post-ischemia neuroprotective agents. Also, in a subset of stroke patients, reperfusion occurs spontaneously and may elicit secondary damages (including the reperfusion injury). To study this particular aspect of the ischemia, transient occlusion models are more appropriate.
The pattern of infarction itself displays some characteristics that differ from human stroke. MRI shows a simultaneous development of the ischemic infarction and vasogenic edema in large photothrombotic lesion whereas the development of the ischemic infarction prevails over vasogenic edema in human stroke16. Conversely, photothrombosis may not be adequate for the study of anti-thrombotic agent studies due to the fact that photothrombotic infarction occurs also after blocking of platelets or inhibition of intrinsic coagulation pathway17. Indeed it has been suggested that in peculiar conditions the platelet coagulation was not necessary to photothrombotic occlusion in which disruption of the endothelial integrity would induce edema and associated compression of the surrounding vessels. Moreover in the same study MRI analysis did not show modification of the infarct size after blockade of platelet function or depletion of platelets17.
Significance with respect to existing methods
Numerous well-standardized models of permanent, transient, focal and global ischemia, mechanically or chemically induced, were developed in rodents in order to closely mimic the human stroke pathophysiology and to develop new neuroprotective strategies. The photothrombosis as presented here is a model of permanent focal ischemia that consistently induces a stroke injury of the desired size in any cortical or subcortical site in a very reproducible and minimally invasive manner. This model shows a high survival rate that that reached 100% in our experiments (25 animals) while more extensive studies obtained 98.4%18. The photothrombotic lesion can be customized in order to affect a precise function according to the targeted area and behavioral recuperation can be assessed by multiple tests6,19. Moreover this approach is not time-consuming, few expensive and can be quickly learnt as it is not technically demanding as opposed to other models, including the filament model. Additionally the vessel occlusion is achieved by inducing the local formation of thrombi that are structurally similar to those observed in human stroke. However, the photothrombotic approach was also adapted for producing occlusions of the middle cerebral artery20. The ring lesion model was later developed in order to obtain a larger penumbra. It consists of an illumination under the shape of a circle, in the center of which cortical region of viable tissue is surrounded by a circular damaged tissue and share the biochemical and molecular characteristics of the ischemic penumbra21,22. Variants of technique are also available for performing stroke in the developing brain23 or in subcortical tissue24.
In conclusion, photothrombosis is an easy to perform ischemic model with high reproducibility. Furthermore, it is suitable for a number of experimental stroke studies, particularly for the evaluation of cortical plasticity in response to brain injury, at both cellular and molecular level.
Disclosures
No conflicts of interest declared.
Acknowledgments
We thank Annalisa Buffo for insightful suggestions and comments, and Maurizio Grassano, Marina Boido and Ermira Pajaj for the shooting. This work was funded by FP7-MC-214003-2 (Marie Curie Initial Training Network AXREGEN) and the Compagnia di San Paolo, gliarep project.
References
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors. Lancet. 2001;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- Mathers CD, Boerma T, Ma Fat D. Global and regional causes of death. Br. Med. Bull. 2009;92:7–32. doi: 10.1093/bmb/ldp028. [DOI] [PubMed] [Google Scholar]
- Rosenblum WI, El-Sabban F. Platelet aggregation in the cerebral microcirculation: effect of aspirin and other agents. Circ. Res. 1977;40:320–328. doi: 10.1161/01.res.40.3.320. [DOI] [PubMed] [Google Scholar]
- Watson BD, Dietrich WD, Busto R, Wachtel MS, Ginsberg MD. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann. Neurol. 1985;17:497–504. doi: 10.1002/ana.410170513. [DOI] [PubMed] [Google Scholar]
- Bergeron M. Inducing photochemical cortical lesions in rat brain. Curr. Protoc. Neurosci. 2003;Chapter 9:Unit 9 16. doi: 10.1002/0471142301.ns0916s23. [DOI] [PubMed] [Google Scholar]
- Lee JK, et al. Photochemically induced cerebral ischemia in a mouse model. Surg. Neurol. 2007;67:620–625. doi: 10.1016/j.surneu.2006.08.077. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Watson BD, Busto R, Ginsberg MD, Bethea JR. Photochemically induced cerebral infarction. I. Early microvascular alterations. Acta Neuropathol. 1987;72:315–325. doi: 10.1007/BF00687262. [DOI] [PubMed] [Google Scholar]
- Schroeter M, Jander S, Stoll G. Non-invasive induction of focal cerebral ischemia in mice by photothrombosis of cortical microvessels: characterization of inflammatory responses. J. Neurosci. Methods. 2002;117:43–49. doi: 10.1016/s0165-0270(02)00072-9. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, et al. Cerebral ischemia after bilateral carotid artery occlusion and intraluminal suture occlusion in mice: evaluation of the patency of the posterior communicating artery. J. Cereb. Blood Flow Metab. 1998;18:570–579. doi: 10.1097/00004647-199805000-00012. [DOI] [PubMed] [Google Scholar]
- Sigler A, Goroshkov A, Murphy TH. Hardware and methodology for targeting single brain arterioles for photothrombotic stroke on an upright microscope. J. Neurosci. Methods. 2008;170:35–44. doi: 10.1016/j.jneumeth.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 1st. New York: Academic Press; 1997. [Google Scholar]
- Piao MS, Lee JK, Jang JW, Kim SH, Kim HS. A mouse model of photochemically induced spinal cord injury. J. Korean Neurosurg. Soc. 2009;46:479–483. doi: 10.3340/jkns.2009.46.5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva VM, Corson N, Elder A, Oberdorster G. The rat ear vein model for investigating in vivo thrombogenicity of ultrafine particles (UFP) Toxicol. Sci. 2005;85:983–989. doi: 10.1093/toxsci/kfi142. [DOI] [PubMed] [Google Scholar]
- Watson BD, Prado R, Dietrich WD, Ginsberg MD, Green BA. Photochemically induced spinal cord injury in the rat. Brain Res. 1986;367:296–300. doi: 10.1016/0006-8993(86)91606-9. [DOI] [PubMed] [Google Scholar]
- Van Reempts J, Van Deuren B, Van de Ven M, Cornelissen F, Borgers M. Flunarizine reduces cerebral infarct size after photochemically induced thrombosis in spontaneously hypertensive rats. Stroke. 1987;18:1113–1119. doi: 10.1161/01.str.18.6.1113. [DOI] [PubMed] [Google Scholar]
- Carmichael ST. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx. 2005;2:396–409. doi: 10.1602/neurorx.2.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschnitz C, et al. Blocking of platelets or intrinsic coagulation pathway-driven thrombosis does not prevent cerebral infarctions induced by photothrombosis. Stroke. 2008;39:1262–1268. doi: 10.1161/STROKEAHA.107.496448. [DOI] [PubMed] [Google Scholar]
- Porritt MJ, et al. Photothrombosis-induced infarction of the mouse cerebral cortex is not affected by the Nrf2-activator sulforaphane. PLoS One. 2012;7:e41090. doi: 10.1371/journal.pone.0041090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin YK, Dietrich WD, Green EJ. Two effective behavioral tasks for evaluating sensorimotor dysfunction following traumatic brain injury in mice. J. Neurosci Methods. 2003;129:87–93. doi: 10.1016/s0165-0270(03)00212-7. [DOI] [PubMed] [Google Scholar]
- Markgraf CG, et al. Comparative histopathologic consequences of photothrombotic occlusion of the distal middle cerebral artery in Sprague-Dawley and Wistar rats. Stroke. 1993;24:286–292. doi: 10.1161/01.str.24.2.286. [DOI] [PubMed] [Google Scholar]
- Wester P, Watson BD, Prado R, Dietrich WD. A photothrombotic 'ring' model of rat stroke-in-evolution displaying putative penumbral inversion. Stroke. 1995;26:444–450. doi: 10.1161/01.str.26.3.444. [DOI] [PubMed] [Google Scholar]
- Hu X, Wester P, Brannstrom T, Watson BD, Gu W. Progressive and reproducible focal cortical ischemia with or without late spontaneous reperfusion generated by a ring-shaped, laser-driven photothrombotic lesion in rats. Brain Res. Brain Res. Protoc. 2001;7:76–85. doi: 10.1016/s1385-299x(01)00046-0. [DOI] [PubMed] [Google Scholar]
- Maxwell KA, Dyck RH. Induction of reproducible focal ischemic lesions in neonatal mice by photothrombosis. Dev. Neurosci. 2005;27:121–126. doi: 10.1159/000085983. [DOI] [PubMed] [Google Scholar]
- Kuroiwa T, et al. Development of a rat model of photothrombotic ischemia and infarction within the caudoputamen. Stroke. 2009;40:248–253. doi: 10.1161/STROKEAHA.108.527853. [DOI] [PMC free article] [PubMed] [Google Scholar]


