Abstract
Exposure to chronic stress is a reliable predictor of depressive disorders, and social stress is a common ethologically relevant stressor in both animals and humans. However, many animal models of depression were developed in males and are not applicable or effective in studies of postpartum females. Recent studies have reported significant effects of chronic social stress during lactation, an ethologically relevant and effective stressor, on maternal behavior, growth, and behavioral neuroendocrinology. This manuscript will describe this chronic social stress paradigm using repeated exposure of a lactating dam to a novel male intruder, and the assessment of the behavioral, physiological, and neuroendocrine effects of this model. Chronic social stress (CSS) is a valuable model for studying the effects of stress on the behavior and physiology of the dam as well as her offspring and future generations. The exposure of pups to CSS can also be used as an early life stress that has long term effects on behavior, physiology, and neuroendocrinology.
Keywords: Behavior, Issue 76, Neuroscience, Neurobiology, Physiology, Anatomy, Medicine, Biomedical Engineering, Neurobehavioral Manifestations, Mental Health, Mood Disorders, Depressive Disorder, Anxiety Disorders, behavioral sciences, Behavior and Behavior Mechanisms, Mental Disorders, Stress, Depression, Anxiety, Postpartum, Maternal Behavior, Nursing, Growth, Transgenerational, animal model
Introduction
The etiology of mood disorders, including postpartum depression, often involves exposure to chronic stress1,2. Although depression in mothers has negative effects on both parenting 3 and psychological development of the offspring 4 ,the vast majority of studies on depression and anxiety focus on male animals and humans. Although there are a few animal models of altered maternal behavior using chronic stress, most have substantial physiological components which may not pertain to chronic psychosocial stressors commonly experienced by humans or focus mostly on the prepartum period. Chronic restraint stress during pregnancy in rats increases maternal care and anxiety and decreases maternal aggression in some studies 5, but decreases maternal care in others 6. Restraint stress is a potent physical stressor 7, and some reports are inconsistent with the attenuated maternal care observed in human mothers with postpartum depression 4. Prenatal ultramild stress does not affect pup care, but does reduce maternal aggression 8. Ultramild stress paradigms use a variety of randomized stressors (such as 30 degree cage tilt, reversed light cycle, confinement, overnight illumination, and soiled cage) over several days, and the effect of individual components is often unclear. It is likely that some stressors used in ultramild stress paradigms, such as reversed light cycle and overnight illumination directly affect maternal care independent of any stress effects, as maternal care in rats has a circadian rhythm. Exposure to wet bedding is a significant thermal stress to the pups, and can result in pup mortality in less than 24 hr in young litters (personal observations).
Recent rodent studies have been focusing on the use of ethologically relevant stressors, such as chronic social stress (CSS), in the study of the effects of stress on offspring behavior and mood disorders9-13. In an effort to develop an ethological model for postpartum depression, reduce the physiological component of the stressor used, and generate consistent behavioral and physiological results, our lab has focused on the use of social stress. While chronic social defeat is effectively used as a model for social stress-induced depression in male rodents 14, this is not an ecologically relevant behavioral model for many maternal rodents if male intruders are used, as a single social defeat of a maternal rodent usually results in cannibalism of the litter by the male intruder. However, acute exposure to a novel male intruder, without social defeat of the dam, elicits robust behavioral and endocrine stress responses in lactating females15-18. There is also evidence that chronic stress during lactation, including exposure to a male intruder, directly impairs milk production 19,20, which may impact pup growth. These results in maternal rats are supported by studies in virgin females, where chronic social instability (varying isolation and crowding) increases hypothalamic–pituitary–adrenal axis activity 11,21, alters body temperature regulation, reduces sucrose and food intake, and has been proposed as an animal model for female depression11. Self grooming, a measure of anxiety 22,23 is also sensitive to social stress manipulations 24,25.
Chronic social stress (CSS) during lactation can disrupt maternal behavior and attenuate growth of the dam and offspring. The daily presentation of a novel male intruder during lactation decreases maternal care, increases maternal aggression towards a novel male intruder and self grooming, and inhibits growth in both the dam and her offspring 10. These effects indicate that CSS is an ethologically relevant and effective model for mood disorders that involve impairments in maternal care, anhedonia, elevated anxiety, and attenuated offspring growth, such as postpartum depression. Both the maternal care and offspring data indicate that CSS may have enduring effects on the future generations, and research on the effects of chronic stress on maternal behavior support the hypothesis that the effects of chronic stress on maternal behavior are hormonally mediated (Murgatroyd and Nephew 2012). Furthermore, recent data from the adult female offspring of stressed dams supports the hypothesis that CSS as a form of early life stress causes changes in central oxytocin (OXT) and prolactin (PRL) which are associated with inefficient nursing behavior 12. The CSS model complements other effective models of stress induced disruptions in maternal behavior, such as limiting nesting material26,27 and maternal separation28,29. Unique aspects of the CSS model are that it is ethologically relevant in both rodents and humans, has a relatively minor physiological component, and can be combined with similar stressors at other life stages (social defeat, chronic social instability).
The primary objectives in developing the CSS paradigm were to use an ethologically and clinically relevant stressor, induce anhedonia (as measured by maternal care and saccharin preference), increase behavioral measures of anxiety, and impair pup physiological, behavioral, and or endocrine development. The purpose of the model is to test current and novel treatments and practices for postpartum depression and anxiety.
Protocol
Adult Sprague Dawley females are bred in our animal facility, litters are culled to 8-10 pups with an even sex ratio, and maternal care and aggression testing is done in the rats home cage. The experimental subjects remain in the same room through the study. One inch high cage separators made of clear Plexiglas are placed in the cage on day 20 or 21 of gestation. These divide the cage into 4 quadrants to prevent pups from crawling back to the dam when placed in the cage. Typical maternal behavior is confirmed for all dams on the day of birth (day 1 of lactation) through direct observation of pup retrieval, grooming, nursing, and self grooming. Intruder males are smaller or similarly sized Sprague Dawley males, and females are never presented with the same male twice. Maternal care and maternal aggression are video recorded on the morning of days 2, 9, and 16 of lactation as described below:
Remove the litter from the dam, weigh males and females separately, and place entire litter in a clean holding cage for 60 min.
At the end of the 60 min separation, weigh males and female pups separately prior to replacing litter in the home cage of the dam, dividing the pups as evenly as possible between the 3 non-nest quadrants of the cage. Start video recording prior to the reintroduction to ensure all pup retrieval is recorded.
Video record maternal care for 30 min. Allow dams to interact with their litters for another 90 min. 120 min (30 min recording + additional 90 min) after placing pups in the dam's cage, weigh them again to determine milk intake over the two hour period. One pup is euthanized from each litter on behavioral testing days following the 120 min nursing period to extract a milk sample from the stomach. The purpose of collecting the milk from the pups is to assess the levels of hormones and other factors that may mediate the behavioral and/or physiology effects of chronic social stress on offspring.
Return pups to dam after weighing, and introduce a smaller or similarly sized novel male intruder to the cage for 30 min to test for maternal aggression. Start video recording during the introduction to ensure that all aggression is recorded. For control subjects, the male is removed after the 30 min recording. For Chronic Social Stress subjects, the males are left in for another 30 min. On non-behavioral testing days during the day 2-16 period, novel intruder males are placed in the cages of CSS subjects for one hour without video recording. The social stress exposures are stopped if injury occurs, and injured animals are treated and removed from the study.
A bottle with 0.02% saccharin is added to the dam home cage at 3 PM on days 2, 9, and 16 alternating between the right and left side. Both plain water and saccharin bottles are weighed at 3 PM, then again at 8 AM the next morning to determine saccharin preference.
Pups are weaned on day 23, and dams are euthanized at this point to obtain basal plasma samples and extract the brain for gene expression analysis of physiologically and behaviorally relevant target nuclei.
Maternal Behavior Scoring
The maternal care aggression behaviors described in Tables 1 and 2 are scored from the videos using Odlog behavioral analysis software. Keys are assigned to each behavior, and the key is held down while each behavior is being performed to record the duration of that behavior. Frequencies are the sum of the key strokes for each behavior. This program creates both a complete data file of time stamped five second bins and a summary table with the total frequencies and durations for the defined behaviors over the 30 min recording. Both maternal aggression and maternal care are scored for the maternal aggression videos, as the dams do respond maternally to the pups while the male intruder is in the cage.
Soon after placing the pups in the cage, the dam will retrieve each pup individually back to the nest. Full retrieval is scored once when all pups are in the nest. While full retrieval is typically only scored once, it may sometimes be scored twice if the dam relocates the entire litter to a new site after an initial full retrieval. Nesting behavior is scored whenever the nesting material is manipulated by the dam with her mouth or paws. Pup grooming includes licking or manipulation of the pup's fur by the dam with her paws. Nursing scoring is initiated after the dam has been in a stationary crouching or prone nursing posture for 10 seconds. This is to allow time for pups to start suckling. Total maternal care is the combined duration of nesting, pup grooming, and nursing, but may be restricted to grooming and nursing to focus on pup-directed behavior. Self grooming is defined as manipulation of the dam's own fur with her mouth or paws. Activity is scored during any locomotor activity not associated with the previously defined behaviors.
Maternal aggression consists of attacking, biting, kicking, and pinning. Attacking is defined as the boxing or tackling of the intruder male. Biting is scored when the dam uses her teeth on the intruder, and pinning is recorded when the dam holds the male down with her limbs or body. Total aggression is the sum of attacking, biting, kicking, and pinning.
Statistics
Maternal behaviors, milk intake, and saccharin preference of the control and CSS dams were compared on each lactation day with one-tailed t-tests to assess the effects of CSS on individual days. Growth data on days 2, 9, and 16 of lactation, were compared with one-tailed t-tests on weight (in grams) on day 2 or the percentage growth relative to day 2 (day 9 or 16 weight/day 2 weight x 100). Expression levels for OXT mRNA were normalized against three combined housekeeping genes, b-actin, hypoxanthine phosphoribosyltransferase (HPRT) and glyceraldehyde-3- phosphate dehydrogenase (GAPDH). Relative OXT mRNA expression levels were compared with individual t-tests on each peptide or receptor for each brain region. All graphical results are presented as group means + SEM, and the level of statistical significance was p ≤ 0.05.
Representative Results
Exposing a lactating dam to CSS depresses the duration of pup grooming and total maternal care during a 30 min video recording on day 9 of lactation (Nephew and Bridges 2011, Figure 2). On day 16 of lactation, aggression and self grooming, a measure of anxiety, are increased (Nephew and Bridges 2011, Figure 3). Milk intake by the pups of CSS dams is 40% less than control dams on days 9 and 16 of lactation (Figure 4), and pup growth is attenuated on both of these days (Nephew and Bridges 2011, Figure 5). Saccharin preference is lower on day 16 in CSS treated dams (Figure 6).
In the adult F1 female offspring of CSS treated dams, milk intake (Figure 7) and saccharin preference (Figure 8) are depressed on days 2 and 16. Oxytocin gene expression in the supraoptic nucleus is lower in these dams (Figure 9), and similar results were reported in the paraventricular nucleus, central amygdala, and medial amygdala (Murgatroyd and Nephew, 2012).
| BEHAVIOR | DESCRIPTION |
| Retrieval | Carrying of pups back to the nest |
| Full Retrieval | Time required to retrieve all 8 pups |
| Nesting | Manipulation of bedding in nest area with mouth or paws |
| Pup Grooming | Licking or manipulation of the pup’s fur with paws |
| Nursing | When dam is in a crouching or prone nursing posture |
| Total Maternal Care | Total duration of Nesting, Pup Grooming, and Nursing |
| Self Grooming | Manipulation of dams own fur with mouth or paws |
| Activity | Any locomotor activity not associated with above behaviors |
Table 1. Maternal Care.
| BEHAVIOR | DESCRIPTION |
| Attacking | Boxing or tackling of intruder male |
| Biting | Use of teeth to attack intruder male |
| Kicking | Use of feet to strike intruder male, similar to a rabbit kicking |
| Pinning | Maternal female holding male down with limbs or body |
| Total Aggression | Sum of the durations of Attacking, Biting, Kicking, and Pinning |
Table 2. Maternal Aggression.
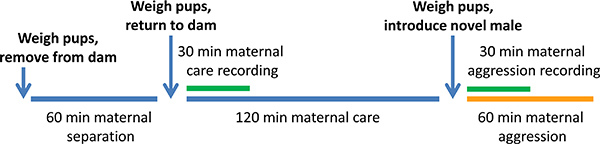 Figure 1. Schedule of the chronic social stress protocol.
Figure 1. Schedule of the chronic social stress protocol.
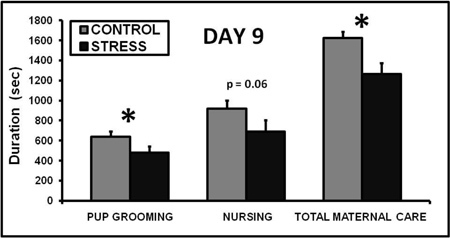 Figure 2. Chronic social stress decreases maternal care on day 9 of lactation. Exposing a lactating dam to a novel male intruder for one hour per day for 7 days decreased the time spent pup grooming and total maternal care, which includes pup grooming, nursing, and nesting behaviors over a 30 min maternal behavior video recording (Nephew and Bridges, 2011). * Indicates a significant difference between control and stress groups, p<0.05, n's = 9-10.
Figure 2. Chronic social stress decreases maternal care on day 9 of lactation. Exposing a lactating dam to a novel male intruder for one hour per day for 7 days decreased the time spent pup grooming and total maternal care, which includes pup grooming, nursing, and nesting behaviors over a 30 min maternal behavior video recording (Nephew and Bridges, 2011). * Indicates a significant difference between control and stress groups, p<0.05, n's = 9-10.
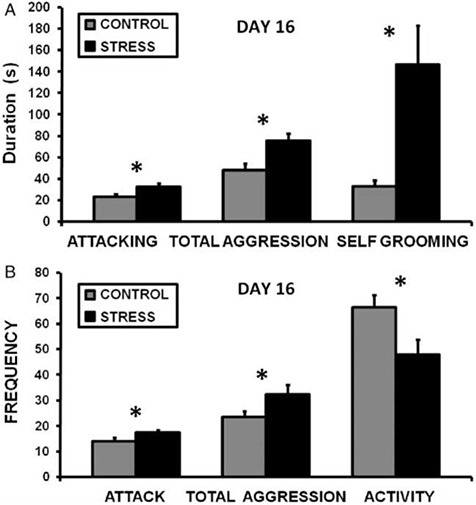 Figure 3. Chronic social stress increases maternal aggression and anxiety on day 16 of lactation. Exposing a lactating dam to a novel male intruder for one hour per day for 14 days increased the duration (A) and frequency (B) of aggression and self grooming and decreased locomotor activity over a 30 min maternal aggression video recording (Nephew and Bridges, 2011). * Indicates a significant difference between control and stress groups, p<0.05, n's =9-10.
Figure 3. Chronic social stress increases maternal aggression and anxiety on day 16 of lactation. Exposing a lactating dam to a novel male intruder for one hour per day for 14 days increased the duration (A) and frequency (B) of aggression and self grooming and decreased locomotor activity over a 30 min maternal aggression video recording (Nephew and Bridges, 2011). * Indicates a significant difference between control and stress groups, p<0.05, n's =9-10.
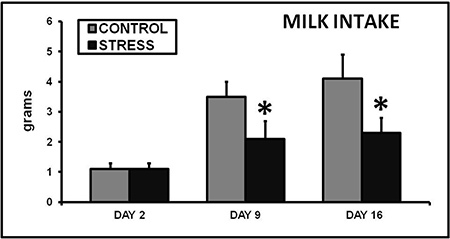 Figure 4. Chronic social stress decreases milk intake by the offspring. Total milk intake by litters of 8-10 pups over a two hour nursing interval was reduced in stressed dams on days 9 and 16 of lactation. * Indicates a significant difference between control and stress groups, p<0.05, n's = 12-14.
Figure 4. Chronic social stress decreases milk intake by the offspring. Total milk intake by litters of 8-10 pups over a two hour nursing interval was reduced in stressed dams on days 9 and 16 of lactation. * Indicates a significant difference between control and stress groups, p<0.05, n's = 12-14.
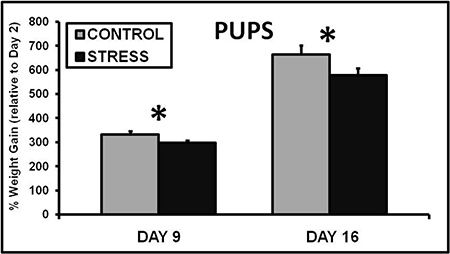 Figure 5. Chronic social stress attenuates pup growth on days 9 and 16 of lactation. Exposing dams to chronic social stress for 15 days during lactation results in attenuated pup growth (Nephew and Bridges 2011). * Indicates a significant difference between control and stress groups, p<0.05, n's =9-10.
Figure 5. Chronic social stress attenuates pup growth on days 9 and 16 of lactation. Exposing dams to chronic social stress for 15 days during lactation results in attenuated pup growth (Nephew and Bridges 2011). * Indicates a significant difference between control and stress groups, p<0.05, n's =9-10.
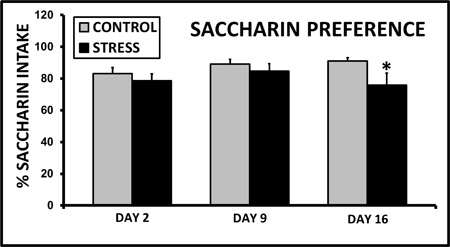 Figure 6. Chronic social stress induces decreased saccharin intake on lactation day 16. Fifteen days of social stress decreases the percentage intake of saccharin relative to water over a 17 hr period. * Indicates a significant difference between control and stress groups, p<0.05, n's =9-10.
Figure 6. Chronic social stress induces decreased saccharin intake on lactation day 16. Fifteen days of social stress decreases the percentage intake of saccharin relative to water over a 17 hr period. * Indicates a significant difference between control and stress groups, p<0.05, n's =9-10.
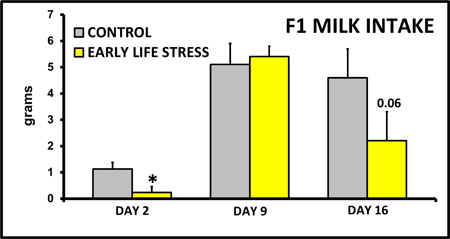 Figure 7. Chronic early life social stress decreases milk intake by the offspring of F1 dams. Total milk intake by the offspring of F1 dams (litters of 8-10 pups) over a two hour nursing interval was reduced on days 2 and 16 of lactation. * Indicates a significant difference between control and stress groups, p<0.05, n's = 12-14.
Figure 7. Chronic early life social stress decreases milk intake by the offspring of F1 dams. Total milk intake by the offspring of F1 dams (litters of 8-10 pups) over a two hour nursing interval was reduced on days 2 and 16 of lactation. * Indicates a significant difference between control and stress groups, p<0.05, n's = 12-14.
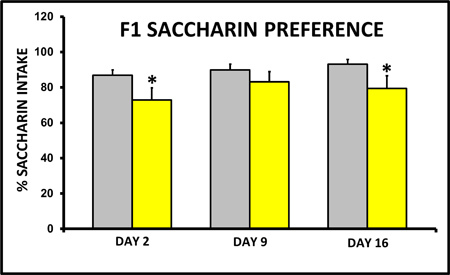 Figure 8. Chronic early life social stress induces decreased saccharin intake on lactation days 2 and 16. Fifteen days of social stress decreases the percentage intake of saccharin relative to water over a 17 hr period. * Indicates a significant difference between control and stress groups, p<0.05, n's =12-14.
Figure 8. Chronic early life social stress induces decreased saccharin intake on lactation days 2 and 16. Fifteen days of social stress decreases the percentage intake of saccharin relative to water over a 17 hr period. * Indicates a significant difference between control and stress groups, p<0.05, n's =12-14.
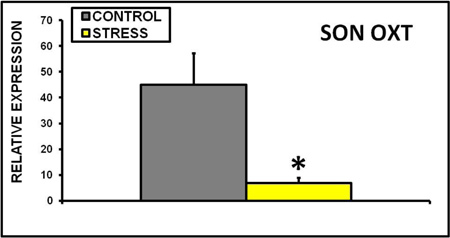 Figure 9. Chronic early life social stress decreases oxytocin mRNA expression in the supraoptic nucleus. Adult female offspring of dams exposed to chronic social stress have attenuated central oxytocin activity, which is associated with decreased nursing efficiency (Murgatroyd and Nephew, 2012). Expression levels for OXT mRNA were normalized against three combined housekeeping genes, b-actin, hypoxanthine phosphoribosyltransferase (HPRT) and glyceraldehyde-3- phosphate dehydrogenase (GAPDH).* Indicates a significant difference between control and stress groups, p<0.05, n's = 16-18.
Figure 9. Chronic early life social stress decreases oxytocin mRNA expression in the supraoptic nucleus. Adult female offspring of dams exposed to chronic social stress have attenuated central oxytocin activity, which is associated with decreased nursing efficiency (Murgatroyd and Nephew, 2012). Expression levels for OXT mRNA were normalized against three combined housekeeping genes, b-actin, hypoxanthine phosphoribosyltransferase (HPRT) and glyceraldehyde-3- phosphate dehydrogenase (GAPDH).* Indicates a significant difference between control and stress groups, p<0.05, n's = 16-18.
Discussion
The data obtained from the CSS paradigm is most likely to be consistent and repeatable in a stable behavioral research environment. It is argued that the housing and husbandry of lab animals is a component that needs to be more carefully considered in any type of study, especially behavioral studies. Housing and husbandry practices which may introduce confounds include: starting with pregnant dams shipped from the supplier, moving dams between rooms daily for behavioral testing, variation in the time of day of behavioral testing, excessive exposure of dams to novel sights, sounds, or odors, not using home cage for behavioral testing, a lack of or inconsistent nesting material, and poor routine animal care (e.g. exposure to wet and soiled bedding). In addition, the inclusion of other behavioral tests during lactation (e.g. forced swim test or elevated plus maze) may introduce confounds. However, the use of a novel cage environment or other acute stressor may be useful to reveal behavioral differences not apparent with home cage testing due to potential interactions between chronic stress, acute stress, and behavior.
The currently described protocol is effective with Sprague Dawley rats, but modifications of the protocol may be needed for other strains and species. For example, pups must be protected with a barrier from highly aggressive Long Evans males to prevent injury. Critical practices for the social stress administration include the use of smaller or similarly sized males and having the first interaction of a male intruder with a highly aggressive day 2 dam to ensure defeat and consistently submissive behavior in the males. If males do attack a litter, they should be removed from the study, as it is likely that they will attack subsequent litters. One option for reducing the likelihood of overly aggressive males is to have them defeated by a larger male prior to the start of the study. For the behavioral scoring, the recording and analysis of both frequency and duration of most behaviors is necessary to provide a complete picture of the behavior, and the use of shorter recording intervals or spot checks may result in the collection of insufficient data to thoroughly test the hypotheses. Thorough and consistent training of scorers can be accomplished with the use previously scored behavioral videos.
The main application of the CSS model is the testing of current and novel drug treatments for postpartum depression and anxiety. An additional use of this model is to investigate the effects of common practices and procedures on maternal care, such as cesarean section births, induced labor, and various lactation schedules. Further investigation of potential effects of stress on lactation will be valuable given the lack of studies on this topic. While milk samples from the dams would be useful, the need for anesthesia and exogenous OXT in the collection of these samples introduces several behavioral and physiological confounds, so studies on the effects of stress on lactation may not be conducive to simultaneous behavioral analysis. The potential for transgenerational studies with the CSS model is great, and these investigations may also include various stress treatments of the adult offspring to investigate potential interactions between early life stress and later stress exposure.
The significance of the CSS model and associated behavioral testing centers on ethological and translational relevance. It is postulated that the lack of effectiveness of current treatments for depression and anxiety is due to a lack of ethological overlap between animal and human studies. Animal models of depression and anxiety often rely on robust physical stressors or highly selected strains which may explain the finding that current antidepressant treatments are most effective with severe depression. Physical stressors such as restraint and exposure to a wide variety of randomized stressors do not simulate common human experiences. The rationale for the randomized exposure used on chronic mild stress paradigms is to prevent acclimation. This is a significant concern, but the ideal situation may be to use a protocol which animals do not acclimate to that more closely resembles the etiology of depression and anxiety in humans, such as social stress. In addition to promoting the use of more ethological stressors, another advantage of the CSS model is the use of a relevant measure of anhedonia, maternal behavior. This is a robust reward mediated behavior which directly mediates the adverse effects of depression in mothers on offspring. The combined incorporation of ethological stressors and measures of anhedonia in depression and anxiety research will be especially beneficial in the identification of safe and effective preventative measures and treatments.
Social stress has been used successfully in males and nulliparous females for several years, and the expansion of this work into maternal females is likely to produce a wealth of novel data for postpartum depression as well as early life stress associated disorders in both males and females. It is postulated that the increased use of ethological animal models in depression and anxiety research will lead to more effective treatments due to the collection of behavioral data that is ethologically and translationally relevant, less variable, and more repeatable.
Disclosures
The authors have no competing financial interests.
Acknowledgments
We would like to thank Chantal Lau for advice on assessing milk intake, Klaus Miczek for advice on the use of social stress, and Sheryl Goodman for feedback on the clinical relevance of chronic social stress. The development of this protocol was supported by NICHD K99/R00 HDO59943 to BCN.
References
- Hammen C. Interpersonal stress and depression in women. Journal of Affective Disorders. 2003;74:49–57. doi: 10.1016/s0165-0327(02)00430-5. [DOI] [PubMed] [Google Scholar]
- Tennant C. Life events, stress and depression: a review of recent findings. Australian and New Zealand Journal of Psychiatry. 2002;36:173–182. doi: 10.1046/j.1440-1614.2002.01007.x. [DOI] [PubMed] [Google Scholar]
- Lovejoy MC, Graczyk PA, et al. Maternal depression and parenting behavior: A meta-analytic review. Clinical Psychology Review. 2000;20:561–592. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- Goodman SH. Depression in Mothers. Annu. Rev. Clin. Psychol. 2007;3:107–135. doi: 10.1146/annurev.clinpsy.3.022806.091401. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Badiani A, et al. Prepartal chronic stress increases anxiety and decreases aggression in lactating female mice. Behav. Neurosci. 1991;105:663–668. doi: 10.1037//0735-7044.105.5.663. [DOI] [PubMed] [Google Scholar]
- Smith JW, Seckl JR, et al. Gestational stress induces post-partum depression-like behavior and alters maternal care in rats. Psychoneuroendocrinology. 2004;29:227–244. doi: 10.1016/s0306-4530(03)00025-8. [DOI] [PubMed] [Google Scholar]
- King JA, Garelick TS, et al. Procedure for minimizing stress for fMRI studies in conscious rats. Journal of Neuroscience Methods. 2005;148:154–160. doi: 10.1016/j.jneumeth.2005.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardon MC, Gérardin P, et al. Influence of prepartum chronic ultramild stress on maternal pup care behavior in mice. Biological Psychiatry. 2000;47:858–863. doi: 10.1016/s0006-3223(99)00253-x. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA. Prenatal Social Stress in the Rat Programmes Neuroendocrine and Behavioural Responses to Stress in the Adult Offspring: Sex-Specific Effects. Journal of Neuroendocrinology. 2010;22:258–271. doi: 10.1111/j.1365-2826.2010.01969.x. [DOI] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Effects of chronic social stress during lactation on maternal behavior and growth in rats. Stress. 2011;14:677–684. doi: 10.3109/10253890.2011.605487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog CJ, Czéh B, et al. Chronic social instability stress in female rats: A potential animal model for female depression. Neuroscience. 2009;159:982–992. doi: 10.1016/j.neuroscience.2009.01.059. [DOI] [PubMed] [Google Scholar]
- Murgatroyd CA, Nephew BC. Effects of early life social stress on maternal behavior and neuroendocrinology. Psychoneuroendcrinology. 2013;38:219–228. doi: 10.1016/j.psyneuen.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillerer KM, Reber SO, et al. Exposure to Chronic Pregnancy Stress Reverses Peripartum-Associated Adaptations: Implications for Postpartum Anxiety and Mood Disorders. Endocrinology. 2011;152:3930–3940. doi: 10.1210/en.2011-1091. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva NN, Avgustinovich DF. Behavioral and physiological markers of experimental depression induced by social conflicts (DISC) Aggressive Behavior. 1998;24(1998):271–286. [Google Scholar]
- Nephew BC, Caffrey MK, et al. Blood oxygen level-dependent signal responses in corticolimbic 'emotions' circuitry of lactating rats facing intruder threat to pups. European Journal of Neuroscience. 2009;30:934–945. doi: 10.1111/j.1460-9568.2009.06875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann I, Toschi N, et al. Maternal defense as an emotional stressor in female rats: correlation of neuroendocrine and behavioural parameters and involvement of brain oxytocin. Eur. J. Neurosci. 2001;13:1016–1024. doi: 10.1046/j.0953-816x.2001.01460.x. [DOI] [PubMed] [Google Scholar]
- Douglas AJ, Meddle SL, et al. Social stress induces hypothalamo-pituitary-adrenal axis responses in lactating rats bred for high trait anxiety. European Journal of Neuroscience. 2007;25:1599–1603. doi: 10.1111/j.1460-9568.2007.05380.x. [DOI] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Central actions of arginine vasopressin and a V1a receptor antagonist on maternal aggression, maternal behavior, and grooming in lactating rats. Pharmacology Biochemistry and Behavior. 2008;91:77–83. doi: 10.1016/j.pbb.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C. Effects of Stress on Lactation. Pediatric Clinics of North America. 2001;48(05):221–234. doi: 10.1016/s0031-3955(05)70296-0. [DOI] [PubMed] [Google Scholar]
- Lau C, Simpson C. Animal models for the study of the effect of prolonged stress on lactation in rats. Physiology & Behavior. 2004;82:193–197. doi: 10.1016/j.physbeh.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Baranyi J, Bakos N, et al. Social instability in female rats: The relationship between stress-related and anxiety-like consequences. Physiology & Behavior. 2005;84:511–518. doi: 10.1016/j.physbeh.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Lazosky A, Britton D. Effects of 5-HT-1A receptor agonists on CRF-induced behavior. Psychopharmacology. 1991;104:132–136. doi: 10.1007/BF02244567. [DOI] [PubMed] [Google Scholar]
- Spruijt BM, Van Hooff JA, et al. Ethology and neurobiology of grooming behavior. Physiological Reviews. 1992;72:825–852. doi: 10.1152/physrev.1992.72.3.825. [DOI] [PubMed] [Google Scholar]
- Denmark A, Tien D, et al. The effects of chronic social defeat stress on mouse self-grooming behavior and its patterning. Behavioural Brain Research. 2010;208:553–559. doi: 10.1016/j.bbr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Wheaton M, et al. What's wrong with my mouse model?: Advances and strategies in animal modeling of anxiety and depression. Behavioural Brain Research. 2007;179:1–18. doi: 10.1016/j.bbr.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Ivy AS, Brunson KL, et al. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: A clinically relevant model for early-life stress. Neuroscience. 2008;154:1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Kramár E, et al. Mechanisms of Late-Onset Cognitive Decline after Early-Life Stress. The Journal of Neuroscience. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, et al. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat. Neurosci. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, et al. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]


