Abstract
Objective
To characterize in vivo the high-affinity CB1 cannabinoid receptor (CB1R) selective anandamide analog AM1346 [alkoxyacid amide of N-eicosa-tetraenylamine] using drug discrimination. Substitution tests involved Δ9-tetrahydrocannabinol (Δ9-THC) and R-(+)-methanandamide (mAEA), a metabolically stable analog of anandamide (AEA), as well as the CB1R antagonist/inverse agonist rimonabant; D-amphetamine and morphine were also examined to assess pharmacological specificity.
Materials and methods
Rats were initially trained to discriminate between i.p.-injected vehicle and 3 mg/kg AM1346 (group 3 mg/kg; t′=20 min); subsequently, the rats were retrained with 5.6 mg/kg AM1346 (group 5.6 mg/kg; t′= 20 min).
Results
Dose-generalization curves of AM1346, Δ9-THC, and mAEA suggested the following order of potency: Δ9-THC > AM1346 > mAEA both for rats discriminating between 3 and 5.6 mg/kg AM1346 from vehicle. In group 3 mg/kg, challenge by 1 mg/kg rimonabant resulted in parallel shifts to the right of the dose-generalization curves for Δ9-THC and AM1346, suggesting surmountable antagonism. Surmountable antagonism was not demonstrated with rimonabant–mAEA combinations. A long duration of effect was indicated when 3 mg/kg AM1346 was examined after different time intervals following i.p. administration (group 3 mg/kg). The in vivo half-life was close to 5 h. Neither D-amphetamine nor morphine generalized in either of groups 3 mg/kg and 5.6 mg/kg, suggesting pharmacological specificity.
Conclusion
Unlike mAEA, the surmountable antagonism between rimonabant and AM1346 showed that the structural features of AEA can be modified to produce novel ligands that reduce the dissociation between the discriminative stimulus and rate decreasing effects of CB1R agonists derived from an AEA template.
Keywords: Δ9-THC, Anandamide analogs [AM-1346 and mAEA · i.e. · R-(+)-methanandamide], Rimonabant, Drug discrimination, Rats
Anandamide (AEA) is an endogenous ligand for the cannabinoid 1 receptor (CB1R). CB1R activation also likely is an essential mechanism for the psychoactive effects following ingestion of Δ9-tetrahydrocannabinol (Δ9-THC), the main psychoactive constituent in Cannabis sativa preparations. AEA is rapidly deactivated by the enzyme fatty acid amide hydrolase (FAAH). Being a good substrate for FAAH presumably is a major reason for the short duration of action of AEA. AEA analogs with better resistance to deactivation by FAAH include O-1812 [(R)-(20-cyano-16,16-dimethyl docosa-cis-5,8,11,14-tetraenoyl)-1′-hydroxy-2′-propylamine], methylated fluoroAEA (2-methylarachidonyl-2′-fluoroethylamide), R-(+)-methanandamide (mAEA; (R)-(+)-arachidonyl-1′-hydroxy-2′-propylamide), and AM1346 [alkoxyacid amide of N-eicosa-(5Z, 8Z, 11Z, 14Z)-tetraenylamide]. Such longer acting analogs are better suited than AEA for pharmacological analysis of the (patho)physiological consequences of CB1R activation.
Notwithstanding such advantage for pharmacological intervention of CB1R function, it has become increasingly clear that CB1R agonists may produce subtle but distinctly different biological effects. The currently known two cannabinoid receptors are G-coupled proteins. Data by Howlett and colleagues (e.g., Houston and Howlett 1998; Mukhopadhyay and Howlett 2005; Shim and Howlett 2006), as well as by others (e.g., Bonhaus et al. 1998; Georgieva et al. 2008; Picone et al. 2005), suggest that different CB1R agonists can activate different signal transduction pathways down stream. Alternatively, CB1R agonists, especially those that are structurally related to AEA, may also interact with other G-coupled-protein receptors such as the vanilloid type-1 receptor (TRPV1) or the recently deorphanized GPR55 (Brown 2007; Howlett 2004; Pacher et al. 2006; Oz 2006 for reviews). Identifying these differences in receptor activation might provide clues for more rational drug design through novel ligands with enhanced pharmacological selectivity.
We previously reported (Järbe et al. 2006) that the AEA analog AM1346 substituted fully for the discriminative stimulus effects of Δ9-THC irrespective of the Δ9-THC training dose (low/high) being used to maintain the drug discriminations. The AM1346 substitution occurred without marked changes in response rate. In contrast, mAEA failed to substitute fully in rats trained with a high dose (5.6 mg/kg) of Δ9-THC (Järbe et al. 1998a, 2000); the highest test dose of mAEA (30 mg/kg) resulted in severe response suppression. Furthermore, surmountable antagonism occurred with AM1346/rimonabant combinations (Järbe et al. 2006) but not convincingly with mAEA/rimonabant combinations (Järbe et al. 2001). Time-course tests indicated that the duration of the psychotropic/cannabimetic effects of AM1346 exceeded those of mAEA (Järbe et al. 2001, 2006).
Thus, although mAEA and AM1346 encompass similar shared features related to the endogenous ligand AEA, the pharmacological profiles seem discernibly different (Järbe et al. 1998b, 2003a, 2006, 2007). To further characterize AM1346 pharmacologically in vivo, the current study employed AM1346 as a discriminative stimulus for rats using an operant two-lever drug discrimination procedure. Cannabinoid drug discrimination appears pharmacologically selective and highly predictive of psychotropic (“cannabimetic”) activity in humans (Balster and Prescott 1992; Järbe and Mathis 1992; Wiley 1999 for overviews). Rats were initially trained with 3 mg/kg AM1346 and then tested for response generalization with (a) AM1346 itself, Δ9-THC, mAEA, D-amphetamine, and morphine, the latter two drugs assessing pharmacological specificity of the discrimination; (b) AM1346, Δ9-THC, and mAEA in combination with the CB1R selective antagonist/inverse agonist rimonabant to assess surmountable antagonism; and (c) 3 mg/kg AM1346 at different intervals after administration to assess the time-course of the discriminative stimulus effects. Once the above tests were completed, the animals were retrained with 5.6 mg/kg AM1346 and retested with the compounds listed above under subheading (a) (systematic replication; Sidman 1960). Thus, the general hypothesis for these studies is that different CB1R agonists activate the receptor through different signaling pathways (e.g., different G proteins).
Materials and methods
Animals
Male Sprague–Dawley rats (Taconic Farms, Germantown, NY, USA) were individually housed in a colony room with an average temperature of 20°C and a 12-h light/dark cycle (rats were trained and tested during the light phase). Animals (~90-day old at the beginning of the study) were experimentally naïve at the time of shaping the lever pressing response (see below). The average (±SEM) weights of the rats were 302 (±5.14) g at the beginning and 403 (±8.03) g at the end of the study. Post-session supplemental feeding with Harlan Rat Chow® (#2018) was restricted to approximately 12 to 14 g/day. All procedures were approved by the Animal Care and Use Committee of Temple University, Philadelphia, PA, USA. The “Principles of Animal Laboratory Care” (National Institutes of Health, 1996) was followed.
Apparatus
Training and testing occurred in eight chambers (ENV-001, Med. Associates, St Albans, VT, USA) equipped with two non-retractable response levers, house-and lever lights, and a grid floor. Each chamber was enclosed within sound- and light-attenuating boxes with an exhaust fan and interfaced with a DOS/Windows compatible computer. Response contingencies were programmed using Med-PC software (v. 1.16; Med. Associates).
Training
Rats were trained to eat food pellets (45 mg, formula A, Noyes®) from a food receptacle located midway between the two response levers and shaped to lever press for food reinforcement until they responded ten times for each reinforcer (fixed-ratio ten schedule of reinforcement; FR-10). Under our conditions, when the house light was off and the stimulus lights above the response levers lit, completion of ten presses on the state-appropriate lever resulted in the delivery of two 45 mg food pellets, followed by a 10-s time-out period with only the house light on. At the end of the 10-s time-out period, the stimulus lights above the levers were lit, the house light turned off, and the FR-10 schedule of reinforcement reinstated. Termination of a session was indicated by all lights in the box being turned off.
Once daily, beginning 20 min after i.p. injection, the rats were trained in this two-choice task to respond on drug- or vehicle-appropriate levers. The position of drug-appropriate levers was randomly assigned among subjects so that it was to the right of the food cup for half the subjects and left for the other half. Throughout the session, the aforementioned schedule was in effect. Presses on the incorrect lever were recorded but had no programmed consequences. The order of drug or vehicle administrations was nonsystematic, with no more than two consecutive drug or vehicle sessions. Approximately an equal number of drug and vehicle training sessions occurred throughout the study. To avoid the influence of odor cues left in a chamber by a preceding subject, the order in which drug and vehicle training sessions were conducted for animals trained in the same chamber was randomized (Extance and Goudie 1981). Training took place Monday through Friday and lasted for 20 min. Training continued until animals reached the acquisition criterion of selecting the lever appropriate for the training condition on at least eight out of ten consecutive training days. Correct selection was defined as total presses before the first reinforcement (FRF) being equal to or less than 14 (i.e., the incorrect lever not pressed more than four times before completing ten responses on the lever appropriate for the prevailing training condition; FRF≤14).
Testing
After animals reached acquisition criterion, test (T) sessions were conducted on an average of three times every 2 weeks; on interim days, regular drug (D) or vehicle (V) training sessions of 20-min duration took place. Approximately 2 weeks before initial testing, animals began receiving two i.p. injections 20 min before the training sessions (i.e., drug and vehicle or vehicle and vehicle) to accustom the animals to a double injection procedure such as that used for antagonism testing. Typically, the order of sessions was D, V, T, V, D (week 1); V, T, V, D, T (week 2); V, D, T, D, V (week 3); and D, T, D, V, T (week 4), wherein T stands for test. A drug training session preceded half the test sessions; the other half was preceded by a vehicle session. Tests were conducted only if responding during the preceding training sessions had been correct (FRF≤14) during the initial six reinforcement cycles of the session. If incorrect, animals were retrained for at least three sessions where FRF≤14 before additional testing. During test sessions, reinforcers were delivered for ten presses on either lever for six reinforcers or until 20 min had elapsed, whichever occurred first. There was one session per test day. Doses were examined in a mixed order. For each dose tested, the percentage of responding on the drug-appropriate lever was calculated from the ratio of the number of presses on the AM1346 associated lever to the total number of lever presses in a test session (excluding responding during the time-out periods). Only data for animals receiving at least one reinforcer during the test session were considered for this measure, i.e., animals must have made a minimum of ten presses on one of the two levers. Additionally, response rate (responses per second) across all subjects was calculated. This measure was based on the performance of all animals, including non-responders. Responding during time-out periods was not included in the rate data.
Statistics
Response rate was averaged (±SEM) among rats and plotted as a function of dose. The effects of a drug on response rate were considered significant when the mean rate of responding was not within the 95% confidence limits (±95% CL) of the mean control response rate. This was defined in individual rats as the mean response rate pertaining to the initial six reinforcement cycles calculated from vehicle training sessions in which the criteria for testing were met.
Linear regression analyses of dose generalization and antagonism data after log-dose transformation were performed using Prism 5 software (v. 5.0, GraphPad Software, San Diego, CA, USA; www.graphpad.com) to provide ED50 estimates and their ±95% CL. Time-course data were similarly analyzed but without log transformation. For time-course replicates, the mean of those was used for the analysis. Using the F test, the Prism program also estimates if slopes are equal (parallel) and if the elevations or intercepts are equal (a measure of potency). All data shown in the Results were obtained from test sessions.
Drugs
mAEA [(R)-(+)-arachidonyl-1′-hydroxy-2′-propylamide; Ki (CB1)=28 nM; Ki (CB2)=867 nM], synthesized according to Abadji et al. (1994), and AM1346 [alkoxyacid amide of N-eicosa-(5Z, 8Z, 11Z, 14Z)-tetraenylamide; Ki (CB1)=1.5 nM; Ki (CB2)=152 nM], were sent to the site of behavioral evaluation in argon-capped vials on a monthly basis. This shipment schedule was implemented to minimize the likelihood of drug decomposition over time. Upon arrival, mAEA and AM1346 were dissolved in ethanol, appropriate amounts withdrawn, the ethanol evaporated under a stream of nitrogen, the residue then dissolved (w/v) in a solution of propylene glycol (PG) and Tween-80 (T-80), and stored at −20°C. Shortly before being used, the solute was diluted with normal (0.9%) saline after the solute had been sonicated for 20–30 min. This procedure was followed for preparing suspensions of Δ9-THC as well. The levo isomer of Δ9-THC, dissolved in ethanol (200 mg/ml), was kindly provided by the National Institute on Drug Abuse (NIDA; Bethesda, Maryland, USA) and also stored at −20°C until used. Rimonabant, as the base (N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide), was also provided by NIDA, and the compound was stored and refrigerated at 4°C before being dissolved in the PG/T-80 mixture (final suspension 5%/3% for all cannabinoids ligands) before being diluted with saline (92%). The ligands mAEA and AM1346 were synthesized in the Department of Pharmaceutical Sciences, University of Connecticut at Storrs. Cannabinoid ligand doses were administered i.p. in a volume of 2 ml/kg (Δ9-THC, AM1346, and rimonabant) or 3 ml/kg mAEA. Suspensions were prepared fresh daily. Morphine SO4 and D-amphetamine SO4 were purchased from Sigma (St Louis, MO, USA) and dissolved in physiological saline and administered i.p. in a volume of 1 ml/kg. All drugs were administered 20 min prior to testing except when examining the time-course of AM1346 where different injection-to-test intervals were studied (see “Results” section below). Doses are expressed as the forms indicated.
Results
Acquisition of the AM1346 drug discrimination
Rats required an average (±SEM) of 31.5 (±3.2) training sessions with the presence/absence of 3 mg/kg AM1346 to fulfill the 8/10 acquisition criterion (range 13 to 47 sessions). However, testing did not commence until all the animals had been trained for drug discrimination for 71 sessions. When retrained with 5.6 mg/kg AM1346, testing resumed after 16 training sessions equally divided between drug and vehicle.
Substitution tests with CB1R agonists, D-amphetamine, and morphine (SD=3 mg/kg AM1346)
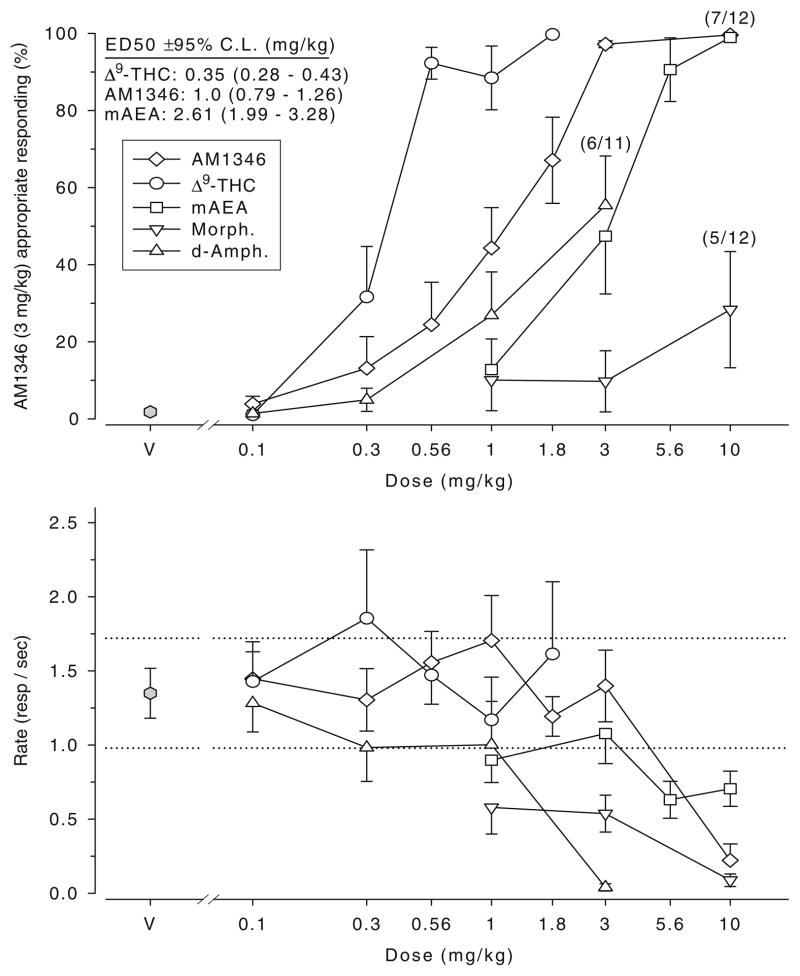
Figure 1 shows the generalization test results of three cannabinoid ligands for animals trained to discriminate between vehicle and 3 mg/kg AM1346. The ED50 estimates (±95% CL) for these generalization curves are also summarized in Fig. 1 (AM1346 doses of 0.1 and 10 mg/kg were not included in the regression analysis). Clearly, the discriminative stimulus associated with AM1346 training generalized to both Δ9-THC and mAEA. The linear regressions suggested no deviations from parallelism (p>0.05). Hence, the following rank order of potency was established: Δ9-THC > AM1346 > mAEA. Thus, Δ9-THC was roughly 2.9 times more potent than AM1346 and 7.5 times more potent than mAEA; AM1346 was 2.6 times more potent than mAEA. D-Amphetamine and morphine did not generalize to AM1346, i.e., did not exceed 80% drug-appropriate responding. The effective dose ranges of the non-cannabinoid drugs were examined as indicated by marked rate suppression (see below). Thus, the 3-mg/kg-AM1346 discrimination exhibited pharmacological selectivity.
Fig. 1.
Generalization test results (top) and corresponding response rate data (bottom) for AM1346 (n=11–12), Δ9-THC (n=12), and mAEA [R-(+)-methanandamide; n=10–12], as well as for D-amphetamine (D-Amph., n=11–12) and morphine (Morph., n=12) in AM1346-(3 mg/kg) versus vehicle-trained rats; sessions began 20 min after i.p. administration. The generalization results represent the mean (±SEM) percentage of lever presses on the drug (AM1346) appropriate lever out of the total number of lever presses emitted during a test session (Y axis); doses examined in milligram per kilogram (X axis). Rate refers to the mean (±SEM) number of lever presses per second emitted during a test session (Y axis); doses in milligram per kilogram (X axis). Dotted lines represent the ±95% confidence limits of vehicle control response rate determined from the initial six reinforcement cycles of the vehicle training sessions preceding these tests; symbols outside the confidence limits are considered significantly different from control. Data points are based on one observation for each rat and were obtained on separate test days. Numbers within brackets indicate the number of rats responding (i.e., obtaining at least one reinforcement) out of the total number used for the test; note that (7/12) refers to 10 mg/kg AM1346. Data are based on test sessions of a maximum of six reinforcers or 20 min, whichever occurred first. V Vehicle
The lower graph in Fig. 1 shows the mean (±SEM) rate of responding during the above generalization tests. The dotted horizontal lines represent the ±95% CL of the vehicle rate. Data points outside these lines are considered significant. Hence, all three test doses of morphine and the highest test dose of D-amphetamine resulted in a reduced rate of responding as did the two higher test doses of mAEA, as well as the highest test dose of AM1346 (10 mg/kg).
Tests for surmountable antagonism: rimonabant and CB1R agonists (SD=3 mg/kg AM1346)
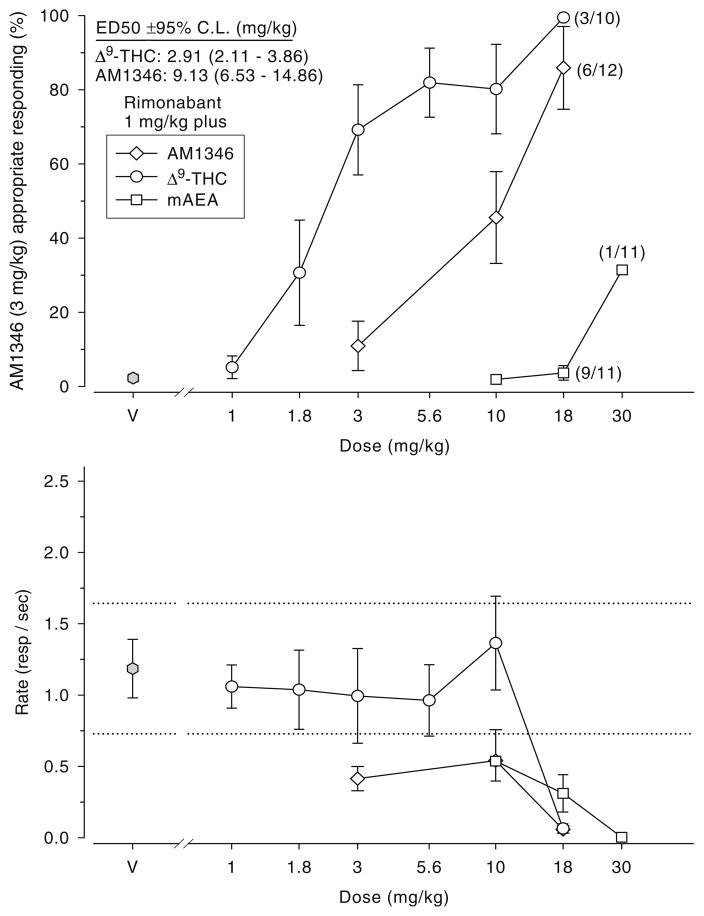
Figure 2 (top graph) shows that the generalization gradients (dose–response curves) for both Δ9-THC and AM1346 were shifted to the right when these two CB1R agonists were tested in the presence of 1 mg/kg rimonabant, suggesting surmountable antagonism. The relative order of potency for the two drugs was similar to that observed when the two cannabinoid agonists were examined singly. Thus, the potency of Δ9-THC was about three times that of AM1346 also when examined in the presence of 1 mg/kg rimonabant. There was no evidence of surmountable antagonism when rimonabant was combined with mAEA. Rate of responding was reduced in all tests with the anandamide analogs, as well as in the test involving 18 mg/kg Δ9-THC and 1 mg/kg rimonabant.
Fig. 2.
Surmountable antagonism test results (top) and corresponding response rate data (bottom) for combinations of 1 mg/kg rimonabant and (1) Δ9-THC (open circles; n=10–11), (b) AM1346 (open diamonds; n=12), and (c) mAEA [R-(+)-methanandamide; open squares; n=10–11] 20 min post i.p. administration in the 3 mg/kg AM1346- versus vehicle-trained rats. Data in condition V (vehicle) shown at top left indicate the percentage of drug responding when the animals were tested with vehicle (two injections of 2 ml/kg vehicle). The corresponding rate data are shown at the bottom graph. Drug lever responding results (top) represent the mean (±SEM) percentage of lever presses on the AM1346 (3 mg/kg) appropriate lever out of the total number of lever presses emitted during a test session (Y axis); doses examined in milligram per kilogram (X axis). Rate (bottom) refers to the mean (±SEM) number of lever presses per second emitted during a test session (Y axis); doses in milligram per kilogram (X axis). Dotted lines represent the ±95% confidence limits of vehicle control response rate determined from the initial six reinforcement cycles of the vehicle training sessions preceding these tests; symbols outside the confidence limits are considered significantly different from control. Data points are based on one observation for each rat and were obtained on separate days. Data are based on test sessions of a maximum of six reinforcers or 20 min, whichever occurred first. V Vehicle
Time-course tests with 3 mg/kg AM1346 (SD=3 mg/kg AM1346)
Figure 3 shows the duration of effect when 3 mg/kg of i.p.-administered AM1346 was examined alone at different post-injection intervals (i.e., 20, 60, 120, 240, 360, and 480 min with replicates at the 240- and 360-min intervals after administration; open diamonds). Also, the AM1346 vehicle was examined singly (gray diamonds); the vehicle tests resulted in a low degree of drug (AM1346) appropriate responding (≤10%). The in vivo half-life post-administration of 3 mg/kg AM1346 was estimated to being close to 5 h (see Fig. 3). There were no major changes in the rate of responding for either drug or vehicle (lower graph, Fig. 3).
Fig. 3.
Time-course of i.p.-administered AM1346 (open diamonds; n=12) and vehicle (gray diamonds; n=12 for the 20-min post-injection interval; n=6 for all the other vehicle post-injection intervals) in animals trained to discriminate between vehicle and 3 mg/kg AM1346. Drug lever responding results (top) represent the mean (±SEM) percentage of lever presses on the AM1346 (3 mg/kg) appropriate lever out of the total number of lever presses emitted during a test session (Y axis); doses examined in milligram per kilogram (X axis). Rate (bottom) refers to the mean (±SEM) number of lever presses per second emitted during a test session (Y axis); doses in milligram per kilogram (X axis). Dotted lines represent the ±95% confidence limits of vehicle control response rate determined from the initial six reinforcement cycles of the vehicle training sessions preceding these tests; symbols outside the confidence limits are considered significantly different from control. Data points are based on one observation for each rat and were obtained on separate days (note the two separately shown replications at 240 and 360 min post-injection for AM1346; for the regression analysis, the mean of each of the two the replicates was used). Data are based on test sessions of a maximum of six reinforcers or 20 min, whichever occurred first
Substitution tests with CB1R agonists, D-amphetamine, and morphine (SD=5.6 mg/kg AM1346)
Figure 4 shows the generalization test results of three CB1R agonists for animals retrained to discriminate between vehicle and 5.6 mg/kg AM1346 (group 5.6 mg/kg). The ED50 estimates (±95% CL) for these generalization curves are also summarized in Fig. 4. The response associated with AM1346 training clearly generalized to Δ9-THC and to a lesser extent, mAEA. The linear regressions suggested no deviations from parallelism (p>0.05). Hence, the following potency rank order: Δ9-THC > AM-1346 > mAEA. Thus, Δ9-THC was roughly 3.5 times more potent than AM1346 and 24 times more potent than mAEA; AM1346 was approximately seven times more potent than mAEA. The relative difference in potency between mAEA on the one hand and Δ9-THC and AM1346 on the other hand suggests that mAEA was less efficacious as a substitute in rats retrained with 5.6 mg/kg AM1346 compared to testing these same animals when they were trained with 3 mg/kg AM1346. In support, pair-wise comparisons of the slopes obtained in tests with the three CB1R agonists in groups 3 mg/kg and 5.6 mg/kg suggested a significant difference for mAEA (p≤0.05) but not for Δ9-THC or AM1346 (p> 0.05). Thus, the slope appeared less steep when mAEA was examined in group 5.6 mg/kg compared to the corresponding slope observed in group 3 mg/kg. D-Amphetamine and morphine did not generalize to 5.6 mg/kg AM1346. That effective dose ranges of the non-cannabinoid drugs were examined is indicated by marked rate suppression (see below). Thus, the 5.6 mg/kg AM1346 discrimination exhibited pharmacological specificity.
Fig. 4.
Generalization test results (top) and corresponding response rate data (bottom) for AM1346 (n=8–11), Δ9-THC (n=7–10), and mAEA [R-(+)-methanandamide; n=8], as well as for D-amphetamine (D-Amph., n=10) and morphine (Morph., n=10) in AM1346- (5.6 mg/kg) versus vehicle-trained rats; sessions began 20 min after i.p. administration. The generalization results represent the mean (±SEM) percentage of lever presses on the drug (5.6 mg/kg AM1346) appropriate lever out of the total number of lever presses emitted during a test session (Y axis); doses examined in milligram per kilogram (X axis). Rate refers to the mean (±SEM) number of lever presses per second emitted during a test session (Y axis); doses in milligram per kilogram (X axis). Dotted lines represent the ±95% confidence limits of vehicle control response rate determined from the initial six reinforcement cycles of the vehicle training sessions preceding these tests; symbols outside the confidence limits are considered significantly different from control. Data points are based on one observation for each rat and were obtained on separate test days. Numbers within brackets indicate the number of rats responding (i.e., obtaining at least one reinforcement) out of the total number used for the test; note that (4/10) refers to Δ9-THC (rather than AM1346). Data are based on test sessions of a maximum of six reinforcers or 20 min, whichever occurred first. V Vehicle
The lower graph of Fig. 4 shows the mean (±SEM) rate of responding pertaining to the above generalization tests. The dotted horizontal lines represent the ±95% CL of the vehicle rate. Data points outside these lines are considered significant. Hence, the highest test doses of Δ9-THC, morphine, and D-amphetamine resulted in a reduced rate of responding, as did the two higher test doses of mAEA. An increased rate of responding occurred in testing with 3 mg/kg AM1346.
Discussion
This study was undertaken to further characterize the discriminative stimulus functions of the CB1R selective high-affinity anandamide analog AM1346 in vivo. To that end, rats were trained to discriminate between vehicle and AM1346, initially 3 mg/kg and subsequently also 5.6 mg/kg. Under both training conditions, the rank order of potencies was Δ9-THC > AM1346 > mAEA; the non-cannabinoid drugs D-amphetamine and morphine did not generalize. Antagonism by 1 mg/kg rimonabant of the discriminative stimulus effects of AM1346 was surmountable with increasing doses of AM1346. Surmountable antagonism was also evident for the response generalization by Δ9-THC but not for the response generalization by mAEA. In both cases of surmountable antagonism, there was approximately an eight- to nine-fold parallel shift to the right of the two CB1R agonist dose-generalization curves in the presence of rimonabant compared to the curves when the agonists were evaluated singly. The duration of effect for AM1346 appeared quite long compared to those of, e.g., Δ9-THC and mAEA (see below).
The finding that the three cannabinoids agonists substituted fully in the animals trained to discriminate between vehicle and 3 mg/kg AM1346 (group 3 mg/kg) was expected. Δ9-THC was more potent than AM1346, which in turn was more potent than mAEA. Thus, Δ9-THC was close to three times more potent than AM1346 and 7.5 times more potent than mAEA, and the potency difference between the two AEA analogs was approximately 2.5. These estimates correspond quite well with those previously reported for rats discriminating between vehicle and Δ9-THC or mAEA (Järbe et al. 2006). Thus, rats trained with a lower Δ9-THC dose (1.8 mg/kg) showed a two-fold difference, and rats trained with a higher Δ9-THC dose (5.6 mg/kg) showed a four-fold difference in potency. For rats discriminating between vehicle and 10 mg/kg mAEA, the potency difference was 2.5 when evaluated 30 min after AM1346 injections. The relatively large potency difference between Δ9-THC and mAEA is consistent with the majority of previous drug discrimination studies in rats and monkeys (Järbe et al. 1998a, 2000, 2001; McMahon 2006; Solinas et al. 2007a), exceptions being studies by Burkey and Nation (1997) and Alici and Appel (2004) where the potency differences for rats were considerably less. It is noteworthy that mAEA did not substitute in mice trained to discriminate between 10 mg/kg Δ9-THC and vehicle, nor did mAEA significantly alter the discriminative stimulus effects of Δ9-THC when the two compounds were tested in combination (McMahon et al. 2008), signifying potential species differences.
Antagonism of the discriminative stimulus effects of Δ9-THC and AM1346 was surmountable, i.e., increasing doses of the two CB1R agonists overcame the blockade induced by 1 mg/kg rimonabant. Similarly, when Δ9-THC (1.8 mg/kg)-trained rats were challenged with 0.3 mg/kg rimonabant, increasing doses of Δ9-THC, as well as of AM1346, overcame the antagonism. There was approximately a five-fold difference in the ED50 values for the response curves in the absence and presence of 0.3 mg/kg rimonabant. Except for the highest test dose of AM1346 (10 mg/kg), response rates were not significantly affected (Järbe et al. 2006). However, in the current study, where a higher dose of rimonabant was employed, response rates were significantly lower for all AM1346/rimonabant combinations. As before (Järbe et al. 2001), surmountable antagonism for mAEA/rimonabant combinations did not occur. Such tests were accompanied by reduced rates of responding (see also Järbe et al. 2003b, 2008). It is possible that route of administration may play a role as Solinas et al. (2007a) observed a limited shift to the right of the mAEA generalization curve when increasing doses of the drug were administered intravenously following pre-treatment with i.p.-administered rimonabant (3 mg/kg). This rightward shift of the generalization curve occurred without marked changes in the response rates. Yet, similar tests involving AEA and rimonabant were accompanied by significantly reduced response rates (Solinas et al. 2007a). An emerging pattern appears to be that response rate following administration of CB1R agonists in rats is less amenable to antagonism by rimonabant, particularly rate changes associated with AEA and its analogs (Järbe et al. 2003b; Wiley et al. 2004). In addition, mAEA (but not Δ9-THC) decreased rates of responding that were not antagonized by rimonabant in CB1R-deficient mice (Baskfield et al. 2004), indicating that these behavioral depressant effects of mAEA are not mediated by CB1R. Wiley et al. (2005) also reported that the degree of cross-tolerance in mice between Δ9-THC on the one hand and AEA, 2-methylAEA and O-1812 on the other hand, varied with the test (locomotor activity, antinociception, temperature, and catalepsy) employed to examine cross-tolerance. Although the neural mechanisms responsible for these varied effects are unclear, it is worth noting that vanilloid mechanism(s) do not seem important for the discriminative stimulus effects of CB1R agonists because (a) the TRPV1 antagonist capsazepine did not block the Δ9-THC-like effects of AEA (Solinas et al. 2007a) and (b) the TRPV1 agonist O-1839 did not substitute for either Δ9-THC or O-1812 in rats discriminating between vehicle and either of these two CB1R agonists (Wiley et al. 2004).
The current half-life estimate of AM1346 being close to 5 h suggests that the relative duration of action of this CB1R ligand is longer than the time-course noted for Δ9-THC (Järbe et al. 1981; 1986; see also Gold et al. 1992) and much longer lasting than that of mAEA (Järbe et al. 2001) in rats. Our previously reported estimate suggested that the duration of action was similar for Δ9-THC and AM1346 (Järbe et al. 2006). However, the previous assessment of the duration of action for AM1346 was less than ideal because of limited drug availability at the time of conducting that study. Metabolically stable AEA analogs are expected to be more resistant to degradation by the enzyme FAAH compared to AEA (Cravatt et al. 1996; Lin et al. 1998). Thus, the long duration of action of AM1346 can be attributed, at least partly, to the ligand being a poor substrate for FAAH (Khanolkar and Makriyannis 1999). Whether AM1346 represents the longest acting “cannabimetic” AEA analog developed to date is unclear as, to the best of our knowledge, no time-course data related to the discriminative stimulus effects have been reported for 2-methylAEA, O-1812, or other AEA analogs examined using drug discrimination as the end-point (Wiley et al. 1998).
After being retrained with 5.6 mg/kg AM1346, the generalization curves for Δ9-THC and AM1346 shifted slightly to the right as reflected by somewhat higher ED50 values. This was expected and commonly observed in drug discrimination studies. The potency ratio between Δ9-THC and AM1346 also rose slightly (from 2.9 to 3.5), perhaps reflecting on our previous observation that the potency ratio was 2 for the low-dose compared to 4 for the high-dose Δ9-THC training conditions (Järbe et al. 2006). However, of more significance was the marked increase in the ED50 value derived from the mAEA substitution tests. A comparison of the mAEA slope functions in groups 3 mg/kg and 5.6 mg/kg suggested that the slope of the generalization curve was significantly shallower in the animals trained with 5.6 mg/kg compared to the same animals trained with 3 mg/kg AM1346. In other words, the potency ratios (mAEA/Δ9-THC and mAEA/AM1346) increased approximately three times as a function of the AM1346 training dose. This is reminiscent of previous findings employing different training doses of Δ9-THC for evaluating substitution by mAEA (Järbe et al. 1998a, 2000). Thus, in this respect, there appears to be more overlap in the discriminative stimulus effects between AM1346 and Δ9-THC compared to mAEA and these two other CB1R agonists.
The non-cannabinoid compounds D-amphetamine and morphine produced limited CB1R agonist-like responding. This is congruent with previous reports evaluating these agents in rats discriminating between 3.0 to 3.2 mg/kg Δ9-THC and vehicle (Barrett et al. 1995; for overviews, see Balster and Prescott 1992; Browne and Weissman 1981; Järbe and Mathis 1992; Weissman 1978; Wiley 1999). Limited drug-like responding in tests with D-amphetamine and morphine also occurred in rats trained with lower (1.8 mg/kg) and higher (5.6 mg/kg) doses of Δ9-THC, as well as in rats trained with 10 mg/kg mAEA (Järbe et al. 1998a, 2006). Yet, mAEA has been reported to augment methamphetamine self-administration in rodents (Vinklerová et al. 2002), and in common with other drugs of abuse, CB1R agonists have been shown to result in increased dopamine levels in the nucleus accumbens (discussed in Solinas et al. 2007b). Although speculative, such commonality in end result might have been a contributing factor for the relatively high degree of drug-like responding observed with D-amphetamine in the rats discriminating between 3 mg/kg AM1346 and vehicle. Clearly, the degree of AM1346-like responding after D-amphetamine treatment was much attenuated when the rats discriminated between 5.6 mg/kg AM1346 and vehicle. This would be congruent with an enhancement of pharmacological specificity as a function of training dose characteristically observed in drug discrimination assays, although the current training history may also have contributed to the outcome.
In conclusion, the surmountable antagonism between rimonabant and AM1346 (or Δ9-THC) but not mAEA (and presumably also AEA) and differential substitution patterns as demonstrated here and elsewhere (Järbe et al. 1998a, 2000, 2001, 2006) supports the conclusion that CB1R activation may engage different signaling pathways. This may constitute a basis for the design of pharmacologically new, more selective ligands, which ultimately may lead to novel CB1R-based medications with reduced side effects.
Acknowledgments
United States Public Health Service Grants DA 09064, 00253, and 13429 (Philadelphia) and DA 03801, 9158, 7215, and 00152 (Boston) from the National Institute on Drug Abuse (NIDA) supported this work. We thank Ms. M. Harris for technical assistance and Mr. B. LeMay and Ms. S. Tai, as well as three anonymous reviewers, for comments on earlier drafts of the manuscript. We also thank NIDA for supplies of (−)-Δ9-THC and rimonabant (as the base).
Contributor Information
Torbjörn U. C. Järbe, Email: t.jarbe@neu.edu, Department of Psychology, Temple University, 265-67 Weiss Hall, 1701 North 13th Street, Philadelphia, PA 19122, USA. Department of Pharmaceutical Sciences, Center for Drug Discovery (CDD), Northeastern University, 116 Mugar, 360 Huntington Ave, Boston, MA 02115, USA
Chen Li, Department of Pharmaceutical Sciences, University of Connecticut, U-92, Storrs, CT 06269, USA.
Qian Liu, Department of Pharmaceutical Sciences, University of Connecticut, U-92, Storrs, CT 06269, USA.
Alexandros Makriyannis, Department of Pharmaceutical Sciences, Center for Drug Discovery (CDD), Northeastern University, 116 Mugar, 360 Huntington Ave, Boston, MA 02115, USA.
References
- Abadji V, Lin S, Taha G, Griffin G, Stevenson LA, Pertwee RG, Makriyannis A. (R)-Methanandamide: a chiral novel anandamide possessing higher potency and metabolic stability. J Med Chem. 1994;37:1889–1893. doi: 10.1021/jm00038a020. [DOI] [PubMed] [Google Scholar]
- Alici T, Appel JB. Increasing the selectivity of the discriminative stimulus effects of Δ9-tetrahydrocannabinol: complete generalization with methanandamide. Pharmacol Biochem Behav. 2004;79:431–437. doi: 10.1016/j.pbb.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Balster RL, Prescott WR. Δ9-Tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neurosci Biobehav Rev. 1992;16:55–62. doi: 10.1016/s0149-7634(05)80051-x. [DOI] [PubMed] [Google Scholar]
- Barrett RL, Wiley JL, Balster RL, Martin BR. Pharmacological specificity of delta-9-tetrahydrocannabinol discrimination in rats. Psychopharmacology (Berl) 1995;118:419–424. doi: 10.1007/BF02245942. [DOI] [PubMed] [Google Scholar]
- Baskfield CY, Martin BR, Wiley JL. Differential effects of Δ9-tetrahydrocannabinol and methanandamide in CB1 knockout and wild-type mice. J Pharmacol Exp Ther. 2004;309:86–91. doi: 10.1124/jpet.103.055376. [DOI] [PubMed] [Google Scholar]
- Bonhaus DW, Chang LK, Kwan J, Martin GR. Dual activation and inhibition of adenylyl cyclase by cannabinoid receptor agonists: evidence for agonist-specific trafficking of intracellular responses. J Pharmacol Exp Ther. 1998;287:884–888. [PubMed] [Google Scholar]
- Brown AJ. Novel cannabinoid receptors. Br J Pharmacol. 2007;152:567–575. doi: 10.1038/sj.bjp.0707481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne RG, Weissman A. Discriminative stimulus properties of delta-9-tetrahydrocannabinol: mechanistic studies. J Clin Pharmacol. 1981;21(Suppl):227S–234S. doi: 10.1002/j.1552-4604.1981.tb02599.x. [DOI] [PubMed] [Google Scholar]
- Burkey RT, Nation JR. (R)-Methanandamide, but not anandamide, generalizes to Δ9-THC in a drug-discrimination procedure. Exp Clin Psychopharm. 1997;5:195–202. doi: 10.1037//1064-1297.5.3.195. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Extance K, Goudie AJ. Inter-animal olfactory cues in operant drug discrimination procedures in rats. Psychopharmacology (Berl) 1981;73:363–371. doi: 10.1007/BF00426467. [DOI] [PubMed] [Google Scholar]
- Georgieva T, Devanathan S, Stropova D, Park CK, Salamon Z, Tollin G, Hruby VJ, Roeske WR, Yamamura HI, Varga E. Unique agonist-bound cannabinoid CB(1) receptor conformations indicate agonist specificity in signaling. Eur J Pharmacol. 2008;581:19–29. doi: 10.1016/j.ejphar.2007.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold LH, Balster RL, Barrett RL, Britt DT, Martin BR. A comparison of the discriminative stimulus properties of delta9-tetrahydrocannabinol and CP 55,940 in rats and rhesus monkeys. J Pharmacol Exp Ther. 1992;262:479–486. [PubMed] [Google Scholar]
- Houston DB, Howlett AC. Differential receptor-G-protein coupling evoked by dissimilar cannabinoid receptor agonists. Cell Signal. 1998;9:667–674. doi: 10.1016/s0898-6568(98)00013-8. [DOI] [PubMed] [Google Scholar]
- Howlett AC. Efficacy in CB1 receptor-mediated signal transduction. Br J Pharmacol. 2004;142:1209–1218. doi: 10.1038/sj.bjp.0705881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe TUC, Mathis DA. Dissociative and discriminative stimulus functions of cannabinoids and cannabinergics. In: Bartke A, Murphy L, editors. Marijuana/cannabinoids: neurobiology and neurophysiology. CRC; Boca Raton FL: 1992. pp. 425–459. [Google Scholar]
- Järbe TUC, Swedberg MDB, Mechoulam R. A repeated tests procedure to assess onset and duration of the cue properties of (−)-delta-9-THC, (−)-delta-8-THC-DMH and (+)-delta-8-THC. Psychopharmacology (Berl) 1981;75:152–157. doi: 10.1007/BF00432178. [DOI] [PubMed] [Google Scholar]
- Järbe TUC, Hiltunen AJ, Lander N, Mechoulam R. Cannabinergic activity (delta-1-THC cue) of cannabidiol monomethyl ether and two stereoisomeric hexahydrocannabinols in rats and pigeons. Pharmacol Biochem Behav. 1986;25:393–399. doi: 10.1016/0091-3057(86)90015-8. [DOI] [PubMed] [Google Scholar]
- Järbe TUC, Lamb RJ, Makriyannis A, Lin S, Goutopoulos A. Δ9-THC training dose as a determinant for (R)-methanandamide generalization in rats. Psychopharmacology (Berl) 1998a;140:519–522. doi: 10.1007/s002130050797. [DOI] [PubMed] [Google Scholar]
- Järbe TUC, Sheppard R, Lamb RJ, Makriyannis A, Lin S, Goutopoulos A. Effects of delta-9-tetrahydrocannabinol and (R)-methanandamide on open-field behavior in rats. Behav Pharmacol. 1998b;9:169–174. [PubMed] [Google Scholar]
- Järbe TUC, Lamb RJ, Lin S, Makriyannis A. Δ9-THC training dose as a determinant for (R)-methanandamide generalization in rats: a systematic replication. Behav Pharmacol. 2000;11:81–86. doi: 10.1097/00008877-200002000-00009. [DOI] [PubMed] [Google Scholar]
- Järbe TUC, Lamb RJ, Lin S, Makriyannis A. (R)-Methanandamide and Δ9-THC as discriminative stimuli in rats: tests with the cannabinoid antagonist SR-141716 and the endogenous ligand anandamide. Psychopharmacology (Berl) 2001;156:369–380. doi: 10.1007/s002130100730. [DOI] [PubMed] [Google Scholar]
- Järbe TUC, DiPatrizio NV, Li C, Makriyannis A. The cannabinoid receptor antagonist SR-141716 does not readily antagonize open-field effects induced by the cannabinoid receptor agonist (R)-methanandamide in rats. Pharmacol Biochem Behav. 2003a;75:809–821. doi: 10.1016/s0091-3057(03)00168-0. [DOI] [PubMed] [Google Scholar]
- Järbe TUC, Lamb RJ, Liu Q, Makriyannis A. (R)-Methanandamide and Δ9-THC induced operant rate decreases in rats are not readily antagonized by SR-141716A. Eur J Pharmacol. 2003b;466:121–127. doi: 10.1016/s0014-2999(03)01491-2. [DOI] [PubMed] [Google Scholar]
- Järbe TUC, Lamb RJ, Liu Q, Makriyannis A. Discriminative stimulus functions of AM-1346, a CB1R selective anandamide analog in rats trained with Δ9-THC or (R)-methanandamide (AM-356) Psychopharmacology (Berl) 2006;188:315–323. doi: 10.1007/s00213-006-0517-x. [DOI] [PubMed] [Google Scholar]
- Järbe TUC, DiPatrizio NV, Li C, Makriyannis A. Effects of AM1346, a high-affinity CB1 receptor selective anandamide analog, on open-field behavior in rats. Behav Pharmacol. 2007;18:673–680. doi: 10.1097/FBP.0b013e3282f00bbf. [DOI] [PubMed] [Google Scholar]
- Järbe TUC, Li C, Vadivel SK, Makriyannis A. Discriminative stimulus effects of the cannabinoid CB(1) receptor antagonist rimonabant in rats. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-008-1076-0. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanolkar AD, Makriyannis A. Structure–activity relationships of anandamide, an endogenous cannabinoid ligand. Life Sci. 1999;65:607–616. doi: 10.1016/s0024-3205(99)00283-0. [DOI] [PubMed] [Google Scholar]
- Lin S, Khanolkar AD, Fan P, Goutopoulos A, Qin C, Papahadjis D, Makriyannis A. Novel analogues of arachidonylethanolamide (anandamide): affinities for the CB1 and CB2 cannabinoid receptors and metabolic stability. J Med Chem. 1998;41:5353–5361. doi: 10.1021/jm970257g. [DOI] [PubMed] [Google Scholar]
- McMahon LR. Characterization of cannabinoid agonists and apparent pA2 analysis of cannabinoid antagonists in rhesus monkeys discriminating Δ9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2006;319:1211–1218. doi: 10.1124/jpet.106.107110. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Ginsburg BC, Lamb RJ. Cannabinoid agonists differentially substitute for the discriminative stimulus effects of Δ9-tetrahydrocannabinol in C57BL/6J mice. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-007-0900-2. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Howlett AC. Chemically distinct ligands promote differential CB1 cannabinoid receptor–Gi protein interactions. Mol Pharmacol. 2005;67:2016–2024. doi: 10.1124/mol.104.003558. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Principles of animal laboratory care. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Oz M. Receptor-independent actions of cannabinoids on cell membranes: focus on endocannabinoids. Pharmacol Ther. 2006;111:114–144. doi: 10.1016/j.pharmthera.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picone RP, Khanolkar AD, Xu W, Ayotte LA, Thakur GA, Hurst DP, Abood ME, Reggio PH, Fournier DJ, Makriyannis A. (−)-7′-Isothiocyanato-11-hydroxy-1′,1′-dimethylheptylhexahydro-cannabinol (AM841), a high-affinity electrophilic ligand, interacts covalently with a cysteine in helix six and activates the CB1 cannabinoid receptor. Mol Pharmacol. 2005;68:1623–1635. doi: 10.1124/mol.105.014407. [DOI] [PubMed] [Google Scholar]
- Shim JY, Howlett AC. WIN55212-2 docking to the CB1 cannabinoid receptor and multiple pathways for conformational induction. J Chem Inf Model. 2006;46:1286–1300. doi: 10.1021/ci0504824. [DOI] [PubMed] [Google Scholar]
- Sidman M. Tactics of scientific research—evaluating experimental data in psychology. Basic Books; NY, New York: 1960. [Google Scholar]
- Solinas M, Tanda G, Justinova Z, Wertheim CE, Yasar S, Piomelli D, Vadivel SK, Makriyannis A, Goldberg SR. The endogenous cannabinoid anandamide produces Δ9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. J Pharmacol Exp Ther. 2007a;321:370–380. doi: 10.1124/jpet.106.114124. [DOI] [PubMed] [Google Scholar]
- Solinas M, Yasar S, Goldberg SR. Endocannabinoid system involvement in brain reward processes related to drug abuse. Pharmacol Res. 2007b;56:393–405. doi: 10.1016/j.phrs.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinklerová J, Nováková J, Sulcová A. Inhibition of methamphetamine self-administration in rats by cannabinoid receptor antagonist AM 251. J Psychopharmacol. 2002;16:139–143. doi: 10.1177/026988110201600204. [DOI] [PubMed] [Google Scholar]
- Weissman A. Generalization of the discriminative stimulus properties of delta-9-tetrahydrocannabinol to cannabinoids with therapeutic potential. In: Colpaert FC, Rosecrans JA, editors. Stimulus properties of drugs: ten years of progress. Elsevier/North Holland Biomed; Amsterdam: 1978. pp. 99–122. [Google Scholar]
- Wiley JL. Cannabis: discrimination of “internal bliss”? Pharmacol Biochem Behav. 1999;64:257–260. doi: 10.1016/s0091-3057(99)00059-3. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Ryan WJ, Razdan RK, Martin BR. Evaluation of cannabimimetic effects of structural analogs of anandamide in rats. Eur J Pharmacol. 1998;355:113–118. doi: 10.1016/s0014-2999(98)00502-0. [DOI] [PubMed] [Google Scholar]
- Wiley JL, LaVecchia KL, Karp NE, Kulasegram S, Mahadevan A, Razdan RK, Martin BR. A comparison of the discriminative stimulus effects of Δ9-tetrahydrocannabinol and O-1812, a potent and metabolically stable anandamide analog, in rats. Exp Clin Psychopharmacol. 2004;12:173–179. doi: 10.1037/1064-1297.12.3.173. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Smith FL, Razdan RK, Dewey WL. Task specificity of cross-tolerance between Δ9-tetrahydrocannabinol and anandamide analogs in mice. Eur J Pharmacol. 2005;510:59–68. doi: 10.1016/j.ejphar.2005.01.006. [DOI] [PubMed] [Google Scholar]