Abstract
Background
Heart failure with preserved ejection fraction (HFpEF) is a disease of the elderly with cardiovascular stiffening and reduced exercise capacity. Exercise training appears to improve exercise capacity and cardiovascular function in heart failure with reduced ejection fraction. However, it is unclear whether exercise training could improve cardiovascular stiffness, exercise capacity, and ventricular-arterial coupling in HFpEF.
Methods
Eleven HFpEF patients and 13 healthy controls underwent invasive measurements with right heart catheterization to define Starling and left ventricular (LV) pressure-volume curves; secondary functional outcomes included Doppler echocardiography, arterial stiffness, cardiopulmonary exercise testing with cardiac output measurement, and ventricular-arterial coupling assessed by the dynamic Starling mechanism. Seven of 11 HFpEF patients (74.9 ± 6 years; 3 men/4 women) completed 1 year of endurance training followed by repeat measurements. Pulmonary capillary wedge pressures and LV end-diastolic volumes were measured at baseline during decreased and increased cardiac filling. LV compliance was assessed by the slope of the pressure-volume curve. Beat-to-beat LV end-diastolic pressure (estimated from pulmonary arterial diastolic pressure) and stroke volume index were obtained, and spectral transfer function analysis was used to assess the dynamic Starling mechanism.
Results
Before training, HFpEF patients had reduced exercise capacity, distensibility and dynamic Starling mechanism but similar LV compliance and end-diastolic volumes compared to controls albeit with elevated filling pressure and increased wall stress. One year of training had little effect on LV compliance and volumes, arterial stiffness, exercise capacity or ventricular-arterial coupling.
Conclusion
Contrary to our hypothesis, 1 year of endurance training failed to impart favorable effects on cardiovascular stiffness or function in HFpEF.
About half of patients >65 years old admitted with congestive heart failure have a “preserved” ejection fraction (HFpEF).1 HFpEF patients are characterized by a reduced exercise capacity and increased morbidity and mortality similar to those with systolic HF.1,2 Impaired LV diastolic function has been thought to be the primary cause of HF in HFpEF patients,3 who are more likely to be elderly, with hypertension and diabetes mellitus.1 So far, no drug therapy has improved LV lusitropic properties or exercise capacity in HFpEF patients.4
Life-long endurance exercise training prevents age-associated declines of exercise capacity and cardiac compliance in healthy subjects.5 Moreover, several months to a year of exercise training increases exercise capacity in healthy subjects6 and heart failure patients with reduced ejection fraction (HFrEF).7 An improved exercise capacity in HFrEF appears to be related to improvements in peripheral arterial function.7 In HFpEF patients, there are some studies in which exercise capacity was apparently improved after several months of exercise training with8 or without improved LV diastolic function.9,10 However, it is unclear whether and how exercise training could improve LV and arterial function, exercise capacity, or peripheral oxygen extraction in HFpEF patients.
We recently reported characterization of static and dynamic LV diastolic function11 and LV-arterial coupling12 by use of invasive measurements in HFpEF patients. In the present study, we prescribed one year of progressive exercise training for these same patients and repeated a comprehensive and detailed measurement of hemodynamics and LV structure and function. Therefore, these data would be of importance to develop strategies that may improve LV and arterial function, and exercise capacity and eventually reduce adverse outcomes in HFpEF patients.
Methods
Patient population
Eleven HFpEF patients >65 years old (4 men/7 women; mean age 74.9 years), who had been a part of 23 patients diagnosed as HFpEF from a total of 2054 hospitalized HF patients, were enrolled in the present study as previously reported.11 HFpEF patients were defined as having a clear history of HF by Framingham criteria plus confirmatory evidence of pulmonary congestion with an ejection fraction >50% by echocardiography at the time of their index hospitalization. Exclusion criteria for this study included atrial fibrillation, myocardial ischemia, recent myocardial infarction, prior coronary artery bypass graft, moderate to severe valvular heart disease, renal failure, chronic pulmonary disease, previous habitual exercise training, HF admission within 5 months, and warfarin use. Thirteen healthy, sedentary controls used previously in our studies were also enrolled.11 Baseline data from HFpEF patients and healthy controls have been published,11–13 and this study now reports the effects of one year of endurance exercise training on the cardiovascular system and exercise capacity in HFpEF patients. All participants signed an informed consent form approved by the institutional review boards of the University of Texas Southwestern Medical Center at Dallas and Texas Health Presbyterian Hospital Dallas.
Exercise testing
A modified Astrand-Saltin incremental treadmill protocol was used to determine peak oxygen uptake (VO2peak).14 The heart rate at the work rate that elicited the ventilatory/gas exchange threshold was defined as the heart rate at maximal steady state (MSS), which was generally equivalent to approximately 80% to 85 % of the maximal heart rate. Cardiac output was measured by the acetylene rebreathing method.6,11 Arterial-venous oxygen difference (a-vDo2) was calculated by the Fick equation. All the subjects underwent maximal exercise testing to evaluate provocable ischemia by baseline and post-exercise echocardiogram and to become familiar with the maximal exercise testing (day 1). Maximal exercise testing was repeated at least 72 hours after the day1 to measure VO2peak and peak cardiac output (day 2), which were used as the baseline values. All HFpEF patients achieved gas exchange threshold based on an increase in VCO2/VO2 slope. All patients stopped exercise because of exertional fatigue despite vigorous encouragement. No patients had noncardiovascular limitations such as joint pain or difficulty following the protocol. During the year of training, maximal exercise testing was performed quarterly.
Exercise training
The HFpEF patients participated in one year of an exercise training program. Initially, the HFpEF patients walked and/or cycled, 3 times a week for 25 min per session, at the “base pace” in which target heart rates were equivalent to approximately 70% to 80 % of the maximal heart rates at just below the ventilatory threshold. Two patients chose to undergo cycle-based training because of slight balance problems. From the third month, “base pace” was gradually prolonged to 35 to 40 minutes per session. A 30-minute “MSS” session was added monthly, and the frequency of “MSS” was increased to twice a month from the fifth month. At the seventh month, 30 seconds per session of “intervals”, with target heart rates within 5 to 10 beat/min of the maximal heart rate, were added weekly.15 The duration of each “interval” session was gradually prolonged. All the training sessions were supervised closely by exercise physiologists. Training mode, intensity, and duration were documented strictly for every session. HFpEF patients visited our laboratory at least once a month and discussed the intensity and frequency of their training programs. If subjects felt that exercise training was burdensome, training mode and intensity were adjusted to maintain adequate exercise compliance. By the end of the year of training, the average exercise time was about 200 min/wk.
Cardiac catheterization and experimental protocol
Right heart catheterization was performed at baseline and after a year of training. Only baseline data in healthy controls are presented in the present study.6,11 A 6F Swan-Ganz catheter was placed to measure pulmonary capillary wedge (PCWP) and right atrial pressures. Baseline measurements including heart rate, blood pressures, LV end-diastolic volume (EDV) by echocardiography, PCWP, and cardiac output and stroke volume by acetylene rebreathing were performed. After the baseline measurements, subjects breathed at 0.2 Hz for 8 minutes with continuous measurements of pulmonary arterial diastolic pressure (PAD) and finger blood pressure by photoplethysmography. The last 6 minutes of data were used for the data analysis.12 Lower-body negative pressure (LBNP) was then used to decrease cardiac filling and measurements were repeated after 5 minutes each of −15 and −30 mm Hg LBNP. Head-up tilt was used in one subject instead of LBNP because of a large body habitus. After repeat measurements confirmed a return to a steady state, cardiac filling was increased by rapid infusion (100–200 mL/min) of saline. Measurements were repeated after 10 to 15 and 20 to 30 mL/kg of saline infusion. Total arterial compliance was determined by the ratio of stroke volume and pulse pressure.16 Effective arterial elastance was defined as brachial systolic blood pressure ×0.9 divided by stroke volume.6,17
Assessment of cardiac catheterization data
To evaluate chamber stiffness properties, LV pressure-volume curves were constructed by relating LVEDV and PCWP. We define 2 different but related mechanical properties of the heart during diastole. (1) Overall chamber stiffness refers to the stiffness constant “a” of the exponential equation describing the pressure-volume curve (see below); and (2) distensibility is used to mean the absolute LVEDV at a given PCWP, independent of stiffness constant “a”.11
To characterize LV pressure-volume curves, we modeled the data according to an exponential equation18: P = P ∞ (expa(v-vo) −1), where P is PCWP, P ∞ is the pressure asymptote of the curve, V is LVEDV index, V0 is equilibrium volume, and “a” is overall LV chamber stiffness. As external constraints affect LV hemodynamics, LV transmural filling pressure (TMP) was calculated as PCWP – right atrial pressure19 and used to construct transmural pressure-volume curves.
The PCWP and stroke volume data were used to construct Starling (stroke volume index/PCWP) curves. The LVEDV, stroke volume, and mean arterial pressure were used to construct preload-recruitable stroke work relationships. LV contractility was assessed by the slopes of the stroke work-LVEDV relationship.20
Echocardiography
LV images were obtained at all loading conditions. LV volumes were determined from the apical 4-chamber view.6,11 LV early (E) and late (A) filling peak velocities were recorded, and the E/A ratio was used to assess global LV diastolic function. The peak early diastolic mitral annular velocity was measured in both septal and lateral sides of the mitral annulus and values were averaged to obtain tissue Doppler imaging (TDI) E mean. Color M-mode Doppler was obtained and the mitral inflow propagation velocity (Vp) was measured.6,11 Isovolumic relaxation time (IVRT) was also determined.
Dynamic Starling mechanism
Beat-to-beat PAD was used as an index for LV preload and beatto-beat changes in stroke volume were derived from the Modelflow method.12,21 Spectral transfer functional analysis between PAD and stroke volume index during the controlled breathing (0.2 Hz) were applied to obtain gain, phase, and coherence as previously reported.12,22 The gain between PAD and stroke volume index was used as an index of the dynamic Starling mechanism which reflects the beat-to-beat modulation of stroke volume from changes in LV preload as previously reported.12,22
Assessment of overall cardiovascular function
The primary outcome variables in the present study included a) LV stiffness assessed from the pressure-volume curves, which reflects LV static diastolic function; and b) global LV performance assessed from Starling curves and preload-recruitable stroke work. Secondary outcomes included (a) VO2peak; (b) LV dynamic diastolic function by echo Doppler variables; (c) arterial stiffness by arterial elastance; (d) arterial function by total aortic compliance; and (e) ventricular-arterial coupling by dynamic Starling mechanism.
Statistical analysis
Continuous data were expressed as mean ± SD except for graphics, in which SEM was used. Baseline data in HFpEF patients and controls were compared by an unpaired t test or nonparametric Mann-Whitney rank sum test. For data obtained during cardiac catheterization, 2-way repeated analysis of variance (ANOVA) with post hoc testing was applied to evaluate the differences between the groups. To evaluate the effects of training, paired t test and 2-way repeated ANOVA with post hoc testing were used. For pressure-volume curves, a multivariate regression analysis was conducted on the repeated measures data, modeling pressure by use of the covariates volume and subject group. P <.05 was considered significant.
This study was supported by the National Institutes of Health grant AG17479-02.
Results
Subject characteristics
Seven of 11 HFpEF patients completed a year of exercise training. Four patients, who were unable to exercise consistently as prescribed or felt that training was too burdensome, dropped out from the study at the third, fourth, sixth, and eighth months. Hemodynamic data were analyzed in the remaining 7 HFpEF patients before and after training and were compared with baseline data in 13 healthy controls, Table I. Baseline data in these subjects have been previously published.6,11 After a year of training, no effects of exercise training on blood pressures or heart rate (P = .37) were observed. None of these HFpEF patients experienced exacerbation of HF related to training; although occasionally medications had to be adjusted by the patients’ physicians during the year of training, all patients were studied after being on exactly the same type and dose of medications for at least one week on the post training study, that they had been on for the pre-training study.
Table I.
Patient characteristics
| Control | HFpEF |
P (pre-post) | |||
|---|---|---|---|---|---|
| Before training | After training | ||||
| Number of subjects | 13 | 7 | − | ||
| Female gender, n (%) | 6 (38 %) | 4 (57 %) | − | ||
| Age, yrs | 70.2 ± 4 | 74.9 ± 6 | − | ||
| Height, cm | 168 ± 10 | 165 ± 11 | − | ||
| Weight, kg | 73 ± 10 | 87 ± 26 | 84 ± 27 | .07 | |
| Body surface area, m2 | 1.85 ±0.17 | 1.98 ± 0.34 | 1.94 ± 0.35 | .07 | |
| Body mass index, kg/m2 | 25.7 ± 2.3* | 31.5 ± 7.1 | 30.3 ± 7.3 | .06 | |
| Heart rate, beat/min | 69 ± 10 | 77 ± 26 | 70 ± 15 | .37 | |
| Systolic blood pressure, mm Hg | 123 ± 10 | 130 ± 17 | 128 ± 17 | .80 | |
| Diastolic blood pressure, mm Hg | 73 ± 6 | 64 ± 9 | 65 ± 13 | .89 | |
| Hematocrit, % | 40.6 ± 2.7 * | 36.7 ± 2.9 | 35.7 ± 3.8 | .23 | |
| Norepinephrine, pg/mL | 279 ±127 * | 466 ± 233 | 405 ±188 | .44 | |
| Co-morbid conditions | |||||
| Hypertension, n (%) | − | 7 (100 %) | |||
| Diabetes | − | 4 (57 %) | |||
| Hyperlipidemia | − | 6 (86 %) | |||
| Renal insufficiency | − | 1 (14 %) | |||
| Coronary artery disease | − | 0 (0 %) | |||
| Medications, n (%) | |||||
| Diuretic | − | 6 (86 %) | |||
| Beta blocker | − | 4 (57 %) | |||
| ACE-I/ARB | − | 5 (71 %) | |||
| Calcium channel blocker | − | 5 (71 %) | |||
| HMG-CoA reductase inhibitor | − | 6 (86 %) | |||
Values are mean ± SD. HFpEF indicates Heart failure with preserved ejection fraction; ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; HMG-CoA, 3-hydroxy-3-methyl-glutaryl-coenzyme A. Systolic and diastolic blood pressures were obtained from 24-hour blood pressure monitoring.
P < .05 for healthy controls versus HFpEF patients before training, and P values for HFpEF after training compared to before training.
LV structure and Doppler measures of diastolic function
Baseline data were shown in Table II. A year of training had no effects on LV volumes, ejection fraction or Doppler variables such as TDI E mean (6.8 ± 1.6 vs. 7.1 ± 2.0 cm/s, P = .50), mitral inflow propagation velocity (Vp) or IVRT in HFpEF patients.
Table II.
Doppler echocardiography
| Controls | HFpEF |
P (pre-post) | ||
|---|---|---|---|---|
| Before training | After training | |||
| LVEDV, mL | 87.1 ± 25.0 | 81.4 ± 14.1 | 82.2 ± 10.7 | .80 |
| LVEDV index, mL/m2 | 46.8 ±11.1 | 41.6 ± 7.1 | 42.1 ± 6.3 | .77 |
| LVESV, mL | 29.8 ± 12.1 | 19.6 ± 6.8 | 20.8 ± 4.1 | .55 |
| LV ejection fraction, % | 65 ± 6* | 76 ± 8 | 75 ± 5 | .55 |
| E-wave velocity, cm/s | 58.1 ± 10.3 | 82.1 ± 29.9 | 88.7 ± 25.8 | .42 |
| A-wave velocity, cm/s | 73.4 ± 19.6 * | 108.1 ± 34.1 | 99.5 ± 23.3 | .33 |
| E/A ratio | 0.83 ± 0.20 | 0.75 ± 0.11 | 0.89 ± 0.14 | .03 |
| TDI Emean, cm/s | 9.5 ± 1.5* | 6.8 ± 1.6 | 7.1 ± 2.0 | .50 |
| TDI Amean, cm/s | 11.1 ±2.1 | 9.3 ± 3.1 | 9.1 ± 3.0 | .94 |
| IVRT, ms | 147 ± 19 | 122 ± 39 | 113 ± 35 | .15 |
| Vp, cm/s | 34 ± 7 | 50 ± 28 | 40 ± 14 | .22 |
Values are mean ± SD. Left ventricular volumes obtained by two-dimensional echocardiography. ESV, End-systolic volume; TDI Emean, average of the peak early velocities of septal and lateral mitral annulus; TDI Amean, average of the peak late velocities of septal and lateral mitral annulus.
P < .05 for healthy controls versus HFpEF before training, and P values for HFpEF after training compared to before training.
Resting vascular function and hemodynamics
Baseline data were previously reported, Table III.11 A year of training imparted no favorable effects on the vascular characteristics such as total arterial compliance (P = .55) or arterial elastance (P = .47). No increases in resting cardiac output or stroke volume were observed after training in HFpEF patients.
Table III.
Ventricular-vascular function
| Controls | HFpEF |
P (pre-post) | |||
|---|---|---|---|---|---|
| Before training | After training | ||||
| LV function | PCWP,mm Hg | 11.4 ± 2.0 | 16.1 ± 5.6* | 15.2 ± 3.6 | .65 |
| LV TMP, mm Hg | 3.4 ± 1.4 | 6.8 ± 2.6 | 5.0 ± 1.7 | .21 | |
| CO (reb), L/min | 5.03 ± 0.63 | 6.51 ± 3.47 | 6.33 ± 2.35 | .78 | |
| CI (reb), L/min/m2 | 2.73 ± 0.27 | 3.27 ± 1.57 | 3.20 ± 1.09 | .83 | |
| SV (reb), mL | 75.5 ± 19.8 | 81.4 ± 20.1 | 88.9 ± 18.9 | .36 | |
| SV index (reb), mL/m2 | 40.7 ± 8.9 | 41.4 ± 9.1 | 45.1 ± 7.6 | .38 | |
| LV stiffness constant | 0.060 ± 0.029 | 0.057 ± 0.046 | 0.057 ± 0.019 | .99 | |
| LV equilibrium volume, mL | 19.3 ± 9.2 | 8.9 ± 7.9* | 13.7 ± 6.8 | .37 | |
| Pressure asymptote, mm Hg | 4.7 ± 3.5 | 8.3 ± 10.4 | 4.6 ± 1.7 | .58 | |
| LV stiffness constant (TMP) | 0.060 ± 0.045 | 0.044 ± 0.023 | 0.049 ± 0.032 | .74 | |
| LV equilibrium volume (TMP), mL | 19.6 ± 10.3 | 10.4 ± 12.5 | 9.8 ± 12.3 | .93 | |
| Pressure asymptote (TMP), mm Hg | 1.7 ± 0.8 | 3.4 ± 0.9* | 2.8 ± 1.4 | .43 | |
| Vascular Function | Pulse pressure, mm Hg | 59.3 ± 8.6 | 76.1 ± 12.1* | 78.4 ± 20.8 | .74 |
| TAC, mL/mm Hg | 1.31 ± 0.45 | 1.09 ± 0.30 | 1.19 ± 0.32 | .55 | |
| Arterial elastance, mm Hg/mL | 1.75 ± 0.50 | 1.74 ± 0.60 | 1.55 ± 0.41 | .47 | |
Values are mean ± SD. CO (reb), Cardiac output by acetylene rebreathing (reb) technique; CI, cardiac index; SV, stroke volume; stiffness constant (TMP), stiffness constant by use of LVTMP (TMP); TAC, total arterial compliance.
P < .05 for HFpEF before training versus controls, and P values for HFpEF after training compared to before training.
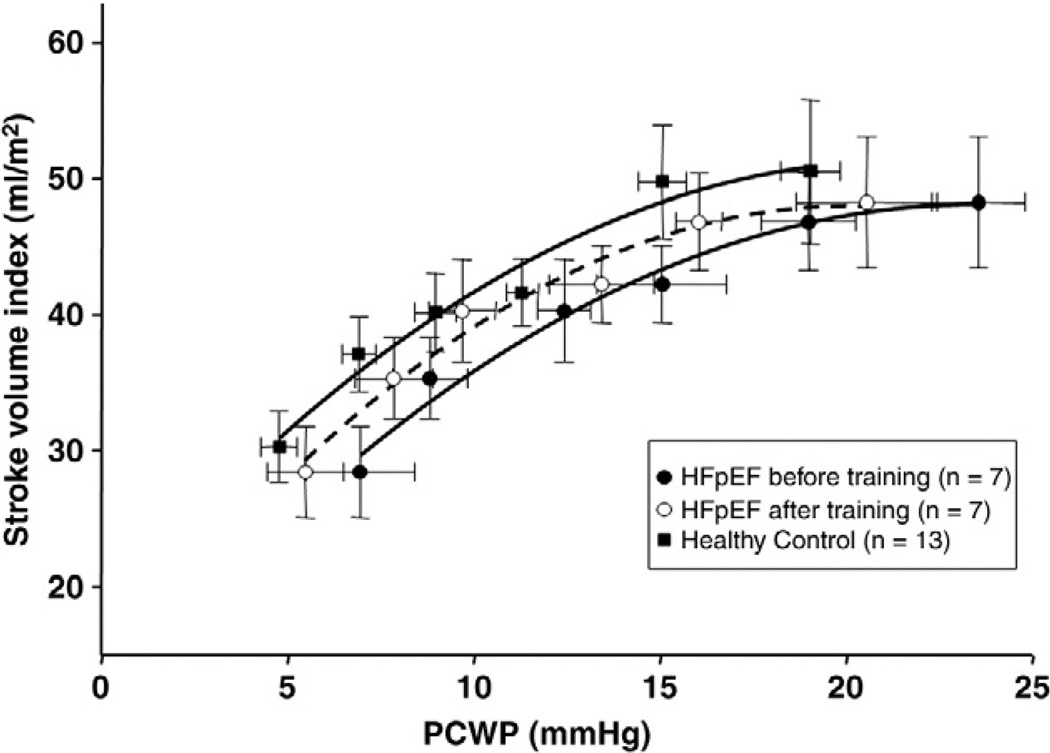
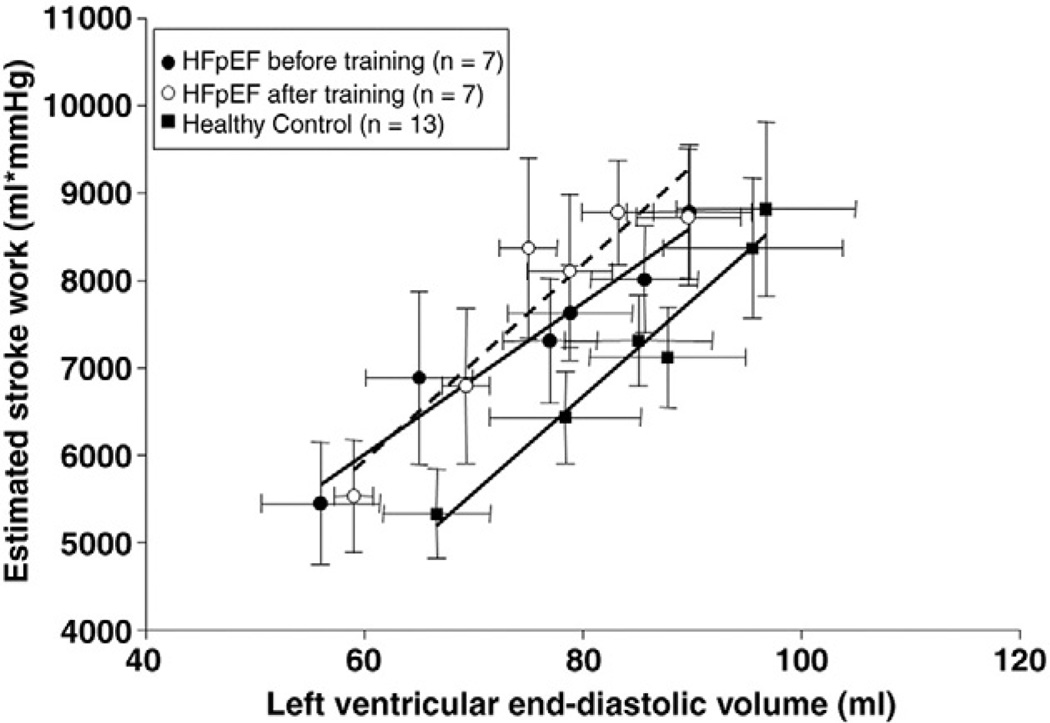
Catheterization data
As reported previously,11 overall contractile function assessed by Frank-Starling curves or preload-stroke work relations was similar between controls and HFpEF (Figures 1 and 2). PCWP was unaffected by exercise training in HFpEF patients (16.1 ± 5.6 vs. 15.2 ± 3.6mm Hg, P = .65). A year of training had no effect on Starling curves or stroke work-LVEDV relations. The slopes of the individual stroke work-LVEDV relation were also unchanged after exercise training (P = .75), suggesting no change in LV contractile function.
Figure 1.
Frank Starling relationship. Lines represent results of second linear regression analyses for HFpEF patients before (r = 0.99) and after (r = 0.99) training and controls (r = 0.99). Note no differences in stroke volume index for any given PCWP in HFpEF patients before and after training.
Figure 2.
Preload recruitable stroke work. Lines represent results of linear regression analyses for HFpEF patients before (r = 0.97) and after (r = 0.94) training and controls (r = 0.98). Stroke work was unaffected across all loading conditions in HFpEF patients after training (P = .239).
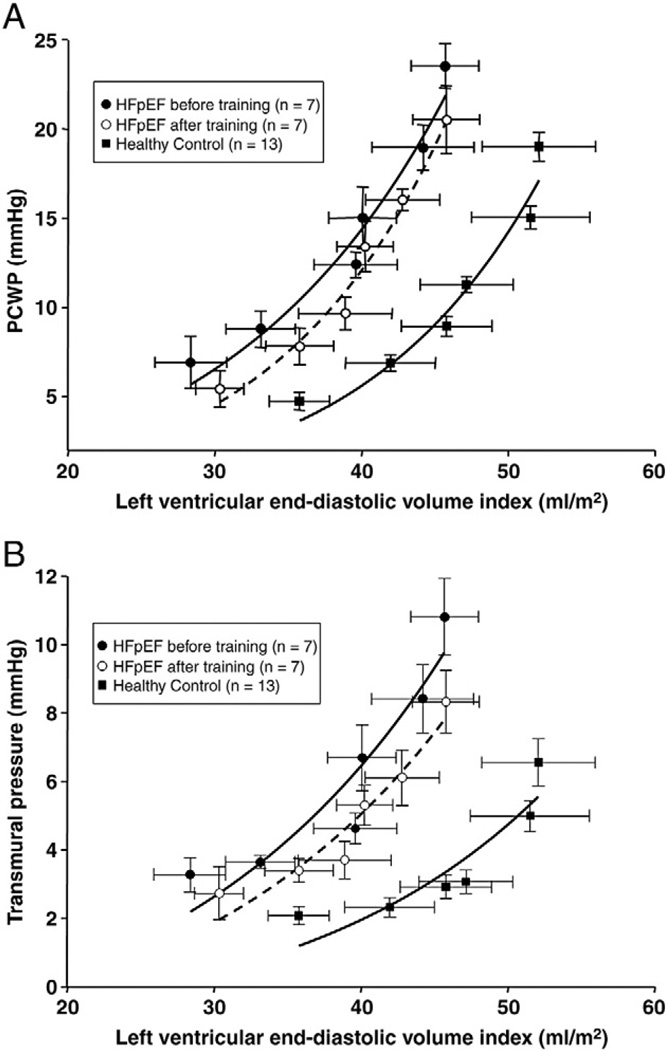
LV pressure-volume curves
As shown in Figure 3A, the pressure-volume curve for HFpEF patients before training was upward and leftward shifted compared to controls, with similar static LV compliance.11 After training, no significant changes in pressure-volume curves were observed in HFpEF patients. When LVTMP was used instead of PCWP, the pressure-volume curves were also unaffected after training, Figure 3B. The stiffness constant “a” using PCWP before and after training was 0.067 and 0.085.
Figure 3.
A, B. Diastolic pressure-volume relationships. A, Pressure-volume curves for HFpEF patients before (r = 0.98) and after (r = 0.99) training and controls (r = 0.97). No significant changes in pressure-volume curves were observed after training. B, Transmural pressures-volume curves for HFpEF patients before (r = 0.94) and after (r = 0.96) training and controls (r = 0.91). No significant changes were observed in pressure-volume curves after training.
When analyzed individually and statistically, a year of training showed no effect on LV stiffness constant “a” by use of PCWP (0.057 ± 0.046 vs. 0.057 ± 0.019, P = .99; difference, 0.0002; SD, 0.048; 95% CI, −0.044 to 0.044) or stiffness constant “a” by use of LVTMP (0.044 ± 0.023 vs. 0.049 ± 0.032, P = .74) (Table III).
Hemodynamics during controlled breathing
As previously reported, the gain between PAD and stroke volume index was smaller in HFpEF patients compared to healthy controls, indicating a lower dynamic Starling mechanism in HFpEF patients (Table IV).12 After training, neither the spectral power of PAD variability (P = .81) nor that of stroke volume index variability (P = .79) was altered, resulting in no improvement of the gain between PAD and stroke volume index (0.26 ± 0.14 vs. 0.29 ± 0.17 mL/m2 per mm Hg, P = .45) in HFpEF, Table IV. In contrast to the controls,22 HFpEF patients exhibited no improvement in the dynamic Starling mechanism after training.
Table IV.
Hemodynamic variables during controlled breathing
| Controls | HFpEF |
P (pre-post) | ||
|---|---|---|---|---|
| Before training | After training | |||
| Heart rate, beat/min | 67.2 ± 9.1 | 74.4 ± 25.7 | 69.4 ± 15.4 | .49 |
| PAD mean, mm Hg | 7.6 ± 2.0* | 13.2 ± 4.0 | 12.8 ± 4.0 | .86 |
| PAD SD, mm Hg | 2.2 ± 0.7* | 4.0 ± 1.1 | 4.0 ± 1.8 | .99 |
| PSD PAD, mmHg2 | 3.8 ± 2.9* | 10.5 ± 9.2 | 13.6 ± 14.3 | .81 |
| SV index mean, mL/m2 | 39.5 ± 8.3 | 41.4 ± 8.9 | 45.1 ± 7.6 | .37 |
| SV index SD, mL/m2 | 1.8 ± 0.7 | 2.2 ± 0.7 | 2.4 ± 0.8 | .52 |
| PSD SV index, (mL/m2)2 | 0.66 ± 0.53 | 0.87 ±1.10 | 0.75 ± 0.55 | .79 |
| Gain PAD-SV index, mL/m2/mm Hg | 0.37 ± 0.11* | 0.26 ± 0.14 | 0.29 ±0.17 | .45 |
| Coherence PAD-SV index | 0.74 ± 0.09 | 0.79 ± 0.20 | 0.68 ±0.16 | .44 |
| Phase PAD-SV index | −0.15 ± 0.77 | −0.10 ± 0.77 | −0.47 ± 0.70 | .17 |
Values are mean ± SD. PAD SD, Time series SD of PAD; PSD PAD, power spectral density of PAD; SV mean, mean of beat-to-beat stroke volume; PSD SV index, power spectral density of SV index; gain PAD-SV index, transfer functional gain between PAD and SV index at respiratory frequency; coherence PAD-SV index, coherence function between PAD and SV index; phase PAD-SV index, transfer function phase between PAD and SV index.
P < .05 for healthy controls versus HFpEF before training, and P values for HFpEF after training compared to before training.
Effects of exercise training on exercise capacity
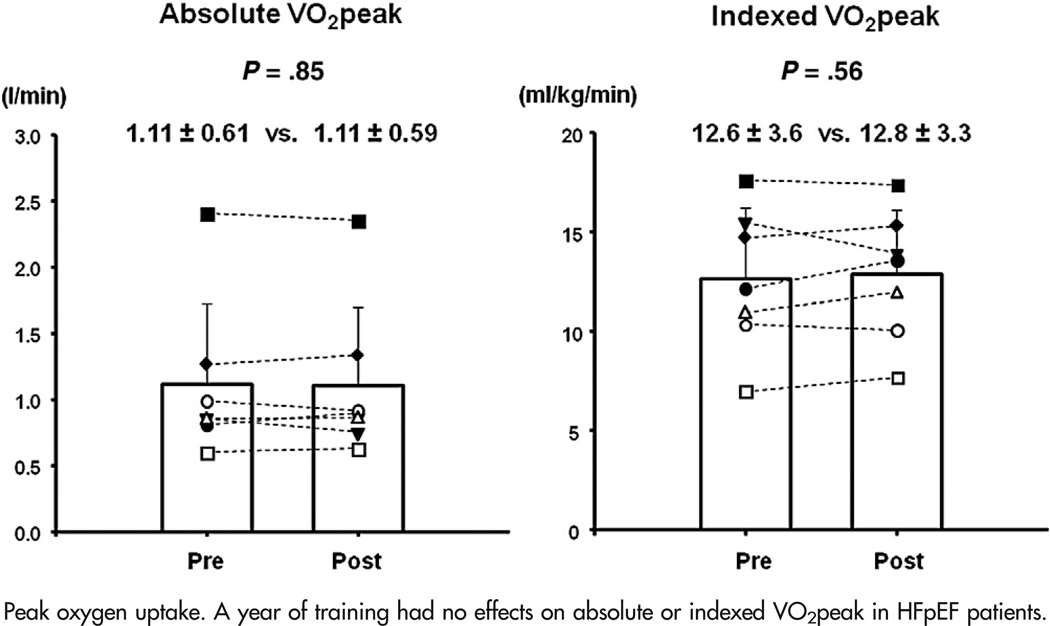
HFpEF patients exhibited a slightly larger VO2peak (11.8 ± 3.4 vs. 12.6 ± 3.6 mL/kg per minute) on day 2 than day 1 before training, probably because of a small increase in maximal heart rates (118 ± 24 vs. 128 ± 30 beat/min) (Table V). After training, HFpEF patients exhibited no improvements in absolute VO2peak (P = .85) or indexed VO2peak (P = .56) when compared to that on day 2 before training, Figure 4. No changes in VO2peak were observed in any quarterly studies (P = .45). Neither a-vDO2 (7.5 ± 2.1 vs. 7.8 ± 2.3 vol%, P = .33) nor peak cardiac index was improved by exercise training in HFpEF patients. When VO2peak after training was compared to that on day 1 before training, there was a modest increase in VO2peak after training (9%, 11.8 ± 3.4 vs. 12.8 ± 3.3 mL/kg per minute, P = .023).
Table V.
Subject characteristics during maximal exercise test
| HFpEF (n = 7) |
||||
|---|---|---|---|---|
| Before training |
After training |
ANOVA |
||
| Day 1 | Day 2 | P | ||
| At peak exercise | ||||
| VO2peak, L/min | 1.05 ± 0.57 | 1.11 ± 0.61 | 1.11 ± 0.59 | .10 |
| Indexed VO2peak, mL/kg/min | 11.8 ± 3.4 | 12.6 ± 3.6* | 12.8 ± 3.3* | .03 |
| Heart rate, beat/min | 118 ± 24 | 128 ± 30 | 127 ± 21 | .33 |
| Systolic blood pressure, mm Hg | 190 ± 13 | 186 ± 37 | 166 ± 34 | .45 |
| Diastolic blood pressure, mm Hg | 91 ± 17 | 84 ± 20 | 81 ± 18 | .23 |
| a-vDo2peak, vol% | 7.4 ± 2.8 | 7.5 ± 2.1 | 7.8 ± 2.3 | .59 |
| VCO2peak, L/min | 1.03 ± 0.60 | 1.06 ± 0.61 | 1.08 ± 0.67 | .39 |
| VE, L/min | 42 ± 14 | 45 ± 17 | 49 ± 22 | .16 |
| Respiratory exchange ratio | 0.97 ± 0.11 | 0.95 ±0.10 | 0.97 ± 0.09 | .49 |
| VE/VCO2 slope | 44 ± 8 | 45 ± 8 | 48 ± 8 | .17 |
| Cardiac index (reb), L·min−1·m−2 | 7.36 ± 2.20 | 7.52 ± 1.69 | 7.24 ± 1.89 | .86 |
| Stroke volume index (reb), mL·m−2 | 61.7 ± 11.2 | 58.2 ± 10.0 | 56.8 ± 17.5 | .62 |
| Arterial elastance, mm Hg/mL | 1.5 ± 0.4 | 1.5 ± 0.4 | 1.5 ± 0.8 | .97 |
Values are mean ± SD. VCO2peak, Carbon dioxide production at maximal exercise; VE, minute ventilation; (reb), by acetylene rebreathing (reb) technique.
P < .05 compared to day 1 before training.
Figure 4.
Peak oxygen uptake. A year of training had no effects on absolute or indexed VO2peak in HFpEF patients.
Discussion
In the present study, we demonstrated that 1 year of progressive and vigorous exercise training, encompassing 200 min/wk, in HFpEF patients failed to have a major impact on cardiac compliance, exercise capacity, LV and arterial function, or its coupling from a comprehensive and detailed measurement of hemodynamics and cardiovascular function.
Effects of exercise training on LV stiffness
In the present study, a year of exercise training showed no changes in static LV stiffness assessed by the pressure-volume curves using PCWP or TMP in HFpEF patients. These results suggest that exercise training had no favorable effects on overall LV chamber stiffness or intrinsic myocardial stiffness in these patients. To our knowledge, this is the first study evaluating the effects of a very long exercise training program on LV stiffness in HFpEF.
In healthy elderly individuals, we recently reported no improvement in LV stiffness after a year of training.6 Todaka et al reported an improvement of LV stiffness after exercise training in dogs with HFrEF,23 whereas no study has evaluated the effects of exercise training on LV stiffness by use of invasive pressure-volume curves in HFrEF patients. A large meta-analysis in HFrEF patients reported that training does not always induce favorable LV remodeling,24 which might support our finding of lack of LV remodeling in HFpEF.
Greater myocardial collagen content and collagen cross-linking have been observed in HFpEF patients, which could be associated with myocardial fibrosis and increased LV stiffness.25 Healthy aging is also associated with an increased collagen cross-linking.26 The mean age of the present study cohort was 75 ± 6 years and all the patients suffered from comorbidities such as hypertension or diabetes. Therefore, it may be possible that our HFpEF patients had substantial myocardial collagen and irreversible collagen cross-linking, thus limiting their ability to adapt to training.
Effects of exercise training on Doppler measures of LV diastolic function
Exercise training increases total blood volume.27 Although TDI E has been reported to be a relatively load-independent measure of LV relaxation, a significant positive correlation of TDI E with preload has been observed.28 Therefore, careful attention should be paid to preload when the effects of exercise training on LV relaxation are evaluated by TDI E. In the present study, no increase in PCWP, LVEDV, or total blood volume (unpublished data) were observed after training, due in part to the use of diuretics. One study demonstrated an improved TDI E after training in HFpEF.8 However, other previous studies observed no changes in TDI E after several months of training.9,10 These results suggest that exercise training may have no influence on LV early diastolic function in HFpEF.
Effects of exercise training on arterial stiffness and ventricular-arterial coupling
Neither arterial stiffness nor ventricular-arterial coupling was improved after training in our HFpEF patients. Contrary to the present results, improvements in arterial stiffness6 and ventricular-arterial coupling22 were observed in healthy elderly individuals after a year of training, resulting in an increased VO2peak by 19% in healthy elderly individuals.6 Six months of training was also reported to improve arterial stiffness in middle-age patients with HFrEF.29 Arterial stiffness was comparable in HFrEF patients and healthy controls,30 whereas it was increased in HFpEF patients compared to healthy controls,31 probably because of collagen cross-linking in arterial walls. In the present study, pulse pressure was larger and total arterial compliance tended to be lower in HFpEF patients compared to controls before training. Therefore, it is possible that our HFpEF patients had such stiff arteries at baseline that exercise training failed to improve arterial stiffness or function.
Effects of a year of exercise training on exercise performance
Previous studies demonstrated that a relatively short period of training (~months) improved VO2peak in HFpEF patients.8,10 A recent multicenter study by Edelmann et al demonstrated that combined aerobic and strength training improved LV diastolic function, and increased VO2peak by 16%8 in mild to moderate HFpEF patients. On the contrary, no improvements in VO2peak or peak a-vDo2 were observed in our HFpEF patients who had impaired skeletal muscle oxidative metabolism before training.11,13 Results may have differed if we had prescribed strength training alone or combined aerobic and strength exercise training in our HFpEF patients.
There are few data regarding the effects of long-term exercise training on LV hemodynamics and mechanics in HFpEF. The length of our training program was twice as long as any previous studies in HFpEF,8–10 and we observed no improvements in cardiovascular stiffness or function after one year of training. It may be possible that short-term training effects previously observed in other studies may not have been durable over time. In HFrEF, the HF-ACTION trial, which was the largest exercise intervention trial ever reported in this population and extended for 2 years of training, showed only a modest improvement in VO2peak,32 which was similar in magnitude to the change in VO2peak from day 1 baseline in the present study. Together, these findings might also suggest that long-term training does not yield large improvements in VO2peak in HF patients.
Although a single test appeared sufficient to measure VO2peak in HF patients as well as healthy subjects,33 there has been no report evaluating the reproducibility of VO2peak in HFpEF patients. In our laboratory, 2 maximal exercise tests were performed to enroll subjects in our studies; the main purpose of the first maximal exercise test was to assess myocardial ischemia, and the second test was to assess VO2peak. In the present study, VO2peak on day 2 was closely correlated with that on day 1 (r2 = 0.96). However, VO2peak was larger on day 2 than day 1 by 7% (P = .038), with a small increase in peak heart rate, Table V. This observation raises the possibility that at least some of the changes observed in our study from day 1 to day 2, and perhaps some of the changes in exercise capacity observed in other studies may be partly influenced by a learning effect, that is enhanced by regular exercise training in the intervention groups.
Study limitations
There are several limitations. First, the number of HFpEF patients who completed a year of training was very small. However, power analysis for LV stiffness showed that the sample size (n = 7, difference 0.0002; SD, 0.048; 95% confidence interval, −0.044 to 0.044) was sufficient to detect a true difference in the mean change of LV stiffness of −0.061 or 0.061 with a probability of type II error at <20%. Therefore, it is possible but unlikely that a physiologically meaningful difference was missed due to a type II error. Second, we enrolled no HFpEF patients who served as controls without exercise training because of the challenge in recruiting patients who met our strict inclusion criteria.11 If we had studied HFpEF patients after a year with no exercise training, it is possible that LV stiffness could have deteriorated and the exercise training actually preserved LV diastolic function. Third, LV pressure-volume curves were evaluated using mean PCWP as a surrogate for LV filling pressure. However, none of our patients had clinically significant valvular abnormalities or pulmonary disease which might alter this relationship. Lastly, Ea was not assessed by use of directly measured central aortic pressure. As there may be significant variability in the reflected wave contribution to end-systolic pressure,34 the failure to identify improvements in Ea in our patients might be related to the lack of measurements of central aortic pressure in the derivation of Ea.
Conclusions
One year of endurance training failed to impart favorable effects on cardiovascular stiffness or function in HFpEF. However, this “dose” of exercise training had at least no deleterious effects on such clinical predictors of mortality and morbidity in HFpEF.
References
- 1.Owan T, Hodge D, Herges R, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 3.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part II: Causal mechanisms and treatment. Circulation. 2002;105:1503–1508. doi: 10.1161/hc1202.105290. [DOI] [PubMed] [Google Scholar]
- 4.Kitzman DW, Daniel K. Diastolic heart failure in the elderly. Heart Fail Clin. 2007;3:437–453. doi: 10.1016/j.hfc.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Arbab-Zadeh A, Dijk E, Prasad A, et al. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110:1799–1805. doi: 10.1161/01.CIR.0000142863.71285.74. [DOI] [PubMed] [Google Scholar]
- 6.Fujimoto N, Prasad A, Hastings JL, et al. Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation. 2010;122:1797–1805. doi: 10.1161/CIRCULATIONAHA.110.973784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan MJ, Higginbotham MB, Cobb FR. Exercise training in patients with severe left ventricular dysfunction. Hemodynamic and metabolic effects. Circulation. 1988;78:506–515. doi: 10.1161/01.cir.78.3.506. [DOI] [PubMed] [Google Scholar]
- 8.Edelmann F, Gelbrich G, Düngen HD, et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol. 2011;58:1780–1791. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 9.Smart N, Haluska B, Jeffriess L, et al. Exercise training in systolic and diastolic dysfunction: effects on cardiac function, functional capacity, and quality of life. Am Heart J. 2007;153:530–156. doi: 10.1016/j.ahj.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Kitzman DW, Brubaker PH, Morgan TM, et al. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail. 2010;3:659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prasad A, Hastings JL, Shibata S, et al. Characterization of static and dynamic left ventricular diastolic function in patients with heart failure and a preserved ejection fraction. Circ Heart Fail. 2010;3:617–626. doi: 10.1161/CIRCHEARTFAILURE.109.867044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shibata S, Hastings JL, Prasad A, et al. Congestive heart failure with preserved ejection fraction is associated with severely impaired dynamic Starling mechanism. J Appl Physiol. 2011;110:964–971. doi: 10.1152/japplphysiol.00826.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhella PS, Prasad A, Heinicke K, et al. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:1296–1304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balke B, Nagle FJ, Daniels J. Altitude and maximum performance in work and sports activity. JAMA. 1965;194:646–649. [PubMed] [Google Scholar]
- 15.Gibala MJ, Little JP, Macdonald MJ, et al. Physiological adaptations to low- volume, high-intensity interval training in health and disease. J Phsyiol. 2012;590:1077–1084. doi: 10.1113/jphysiol.2011.224725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chemla D, Hébert JL, Coirault C, et al. Total arterial compliance estimated by stroke volume-to-aortic pulse pressure ratio in humans. Am J Physiol. 1998;274:H500–H505. doi: 10.1152/ajpheart.1998.274.2.H500. [DOI] [PubMed] [Google Scholar]
- 17.Kelly RP, Ting CT, Yang TM, et al. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86:513–521. doi: 10.1161/01.cir.86.2.513. [DOI] [PubMed] [Google Scholar]
- 18.Mirsky I. Assessment of diastolic function: suggested methods and future considerations. Circulation. 1984;69:836–841. doi: 10.1161/01.cir.69.4.836. [DOI] [PubMed] [Google Scholar]
- 19.Belenkie I, Kieser TM, Sas R, et al. Evidence for left ventricular constraint during open heart surgery. Can J Cardiol. 2002;18:951–959. [PubMed] [Google Scholar]
- 20.Glower DD, Spratt JA, Snow ND, et al. Linearity of the Frank-Starling relationship in the intact heart: the concept of preload recruitable stroke work. Circulation. 1985;71:994–1009. doi: 10.1161/01.cir.71.5.994. [DOI] [PubMed] [Google Scholar]
- 21.Fisher ML, De Felice CE, Parisi AF. Assessing left ventricular filling pressure with flow-directed (Swan- Ganz) catheters. Detection of sudden changes in patients with left ventricular dysfunction. Chest. 1975;68:542–547. doi: 10.1378/chest.68.4.542. [DOI] [PubMed] [Google Scholar]
- 22.Shibata S, Hastings JL, Prasad A, et al. “Dynamic” Starling mechanism: effects of ageing and physical fitness on ventricular-arterial coupling. J Physiol. 2008;586:1951–1962. doi: 10.1113/jphysiol.2007.143651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Todaka K, Wang J, Yi GH, et al. Impact of exercise training on ventricular properties in a canine model of congestive heart failure. Am J Physiol. 1997;272:H1382–H1390. doi: 10.1152/ajpheart.1997.272.3.H1382. [DOI] [PubMed] [Google Scholar]
- 24.Haykowsky MJ, Liang Y, Pechter D, et al. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: the benefit depends on the type of training performed. J Am Coll Cardiol. 2007;49:2329–2336. doi: 10.1016/j.jacc.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 25.Kasner M, Westermann D, Lopez B, et al. Diastolic tissue Doppler indexes correlate with the degree of collagen expression and cross-linking in heart failure and normal ejection fraction. J Am Coll Cardiol. 2011;57:977–985. doi: 10.1016/j.jacc.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 26.Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens. 2003;21:3–12. doi: 10.1097/00004872-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Convertino VA. Blood volume response to physical activity and inactivity. Am J Med Sci. 2007;334:72–79. doi: 10.1097/MAJ.0b013e318063c6e4. [DOI] [PubMed] [Google Scholar]
- 28.Nagueh SF, Sun H, Kopelen HA, et al. Hemodynamic determinants of the mitral annulus diastolic velocities by tissue Doppler. J Am Coll Cardiol. 2001;37:278–285. doi: 10.1016/s0735-1097(00)01056-1. [DOI] [PubMed] [Google Scholar]
- 29.Hambrecht R, Gielen S, Linke A, et al. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: a randomized trial. JAMA. 2000;283:3095–3101. doi: 10.1001/jama.283.23.3095. [DOI] [PubMed] [Google Scholar]
- 30.Shah A, Gkaliagkousi E, Ritter JM, et al. Endothelial function and arterial compliance are not impaired in subjects with heart failure of non-ischemic origin. J Card Fail. 2010;16:114–120. doi: 10.1016/j.cardfail.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 31.Desai A, Mitchell GF, Fang JC, et al. Central aortic stiffness is increased in patients with heart failure and preserved ejection fraction. J Card Fail. 2009;15:658–664. doi: 10.1016/j.cardfail.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 32.O'Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bensimhon DR, Leifer ES, Ellis SJ, et al. Reproducibility of peak oxygen uptake and other cardiopulmonary exercise testing parameters in patients with heart failure (from the Heart Failure and A Controlled Trial Investigating Outcomes of exercise traiNing) Am J Cardiol. 2008;102:712–717. doi: 10.1016/j.amjcard.2008.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schofield RS, Schuler BT, Edwards DG, et al. Amplitude and timing of central aortic pressure wave reflections in heart transplant recipients. Am J Hypertens. 2002;15:809–815. doi: 10.1016/s0895-7061(02)02968-0. [DOI] [PubMed] [Google Scholar]