Abstract
In 2010, World Health Organization classified gastric neuroendocrine tumor (NET) as follows: NET grade (G) 1, NET G2, neuroendocrine carcinoma (NEC). We reviewed 22 gastric NETs that were encountered in our institutions. Nine, 6, and 4 were NET G1, G2, and NEC, respectively. We also encountered 3 NET G3. NET G1 was treated with observation in 2 patients, endoscopic mucosal resection (EMR) in 3, and gastrectomy in 4 patients. No recurrence was experienced during a median of 53 months of follow-up. All NET G2 was treated with gastrectomy. No patient experienced recurrence during a median of 25 months of follow-up. NET G3 was treated with gastrectomy. One patient died of liver metastasis 52 months after gastrectomy. For NEC, gastrectomy was performed in 3 cases and no patients died of tumor-related death. We conclude that the prognoses of NET G1 and G2 were good. We also experienced long-term survivors of NEC. An accumulation of more patients is needed for further investigation.
Keywords: Stomach neoplasms, Neuroendocrine tumors, Carcinoid tumor, Neuroendocrine carcinoma, Small cell carcinoma, Gastrin, Mitosis, Ki-67 antigen, Gastrectomy, World Health Organization
Gastric (neuro)endocrine tumor (NET) is a rare neoplasm that includes carcinoid, neuroendocrine carcinoma, and small cell carcinoma. The classification and terms have been often confused. In 2010, the World Health Organization (WHO) revised these classifications.1 As such, most NETs are classified into NET grade (G)1, NET G2, and neuroendocrine carcinoma (NEC). G stands for grading according to mitotic count and Ki-67 index. NET G1 is usually benign, whereas NET G2 and NEC are malignant. However, their clinical behaviors have not been fully characterized. We reviewed cases of gastric NETs that were encountered in our institutions and analyzed their clinical behaviors.
Materials and Methods
We encountered 1303 gastric cancers between 1998 and 2011 at Higashiosaka City General Hospital and 1447 gastric cancers between 2000 and 2009 at National Hospital Organization Kure Medical Center/Chugoku Cancer Center. Among these 2750 cases, 22 (0.8%) were histopathologically diagnosed as NETs.
The WHO 2010 classification was used to classify NETs. According to the classification, NETs are classified into 5 categories: NET G1 (carcinoid), NET G2, NEC (large cell or small cell type), mixed adenoneuroendocrine carcinoma, and hyperplastic and preneoplastic lesions. Most cases belong to the former three tiers.
NET G1 and G2 are well-differentiated neuroendocrine neoplasms that are composed of cells with features similar to those of normal gut endocrine cells expressing general markers of neuroendocrine differentiation with mild-to-moderate nuclear atypia and a low number of mitoses. This definition encompasses neoplasms termed carcinoid tumor in the WHO 2000 classification.2 G stands for grading based on proliferation with the following definitions of mitotic count and Ki-67 index: G1, mitotic count <2 per 10 high power fields (HPF) and Ki-67 index ≤2%; G2, mitotic count 2 to 20 per 10 HPF or 3% to 20% Ki-67 index; and G3, mitotic count >20 per 10 HPF or >20% Ki-67 index.1 HPF is 2 mm2 and is evaluated in at least 50 fields in areas of the highest mitotic density. The Ki-67 index is expressed with the use of the MIB1 antibody as a percentage of 500 to 2000 tumors cells in areas with the strongest nuclear labeling. If grade differs for mitotic count compared with the Ki-67 index, it is suggested that the higher grade be assumed.
NEC is a poorly differentiated, high-grade malignant neoplasm that is composed of small cells or large-to-intermediate cells with marked nuclear atypia, multifocal necrosis, and a high number of mitoses (>20 per 10 HPF). This definition refers to neoplasms that were previously classified as small cell carcinoma, large cell (neuro)endocrine carcinoma, or poorly differentiated (neuro)endocrine carcinoma.
The 22 patients with NET were staged according to the WHO 2010 classification. Their clinical characteristics, treatment, and prognoses were retrospectively reviewed. The TNM classification by the International Union Against Cancer (UICC)3 was used to describe tumor features. In addition, Rindi's type was used to classify gastric carcinoid tumors.4 Type I was the most common and was associated with chronic atrophic gastritis, known as type A gastritis. Type II was associated with multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome. Type III was a biologically more aggressive, sporadic lesion. Cause-specific survival was shown on Kaplan-Meier survival curve.
Results
Nine patients were classified as having NET G1, 6 were NET G2, 3 were NET G3, and the remaining 4 patients were NEC (Table 1). The WHO 2010 classification does not define NET G3; however, the clinical behaviors of the three cases we experienced were obviously different from those of NET G1 and G2. Although they were well-differentiated tumors, their Ki-67 indices were as high as 32% to 58%. In addition, all patients had invasion into the subserosa and had metastases to the lymph nodes or liver.
Table 1.
Gastric neuroendocrine tumors encountered in our institutions
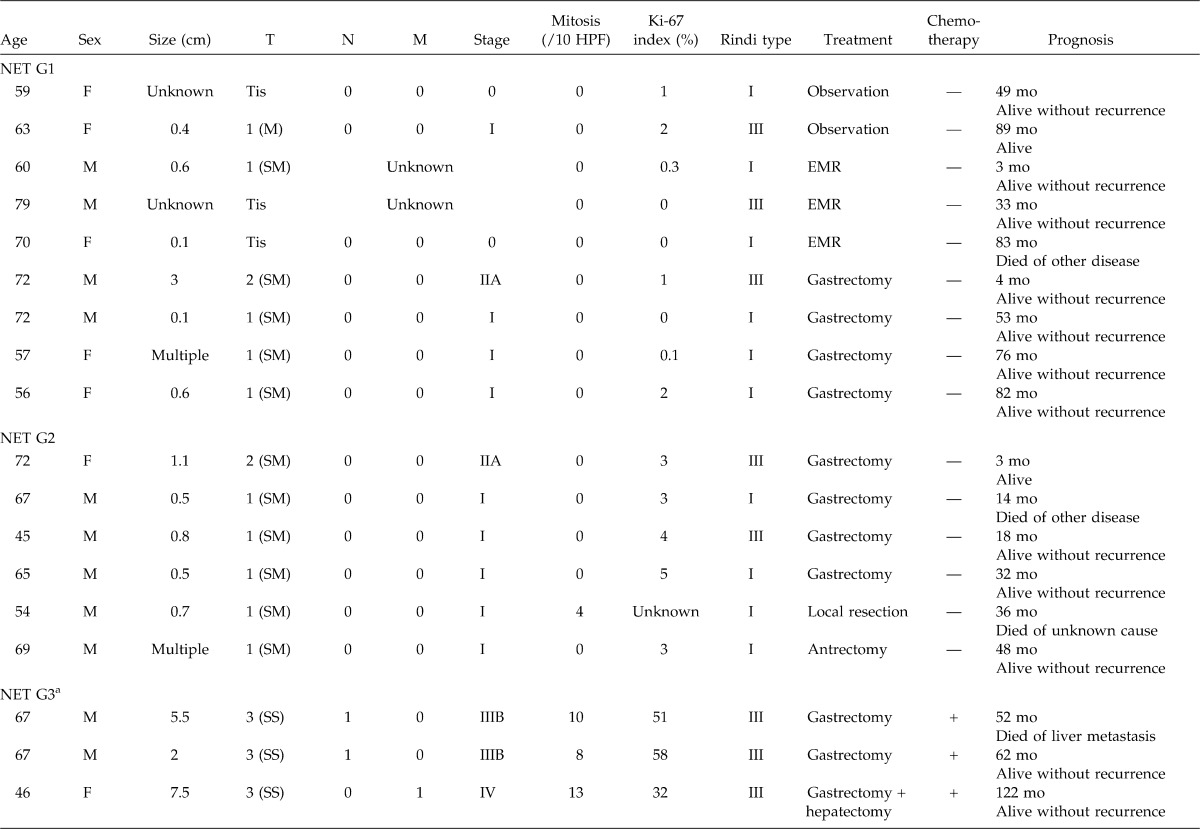
Table 1.
Continued
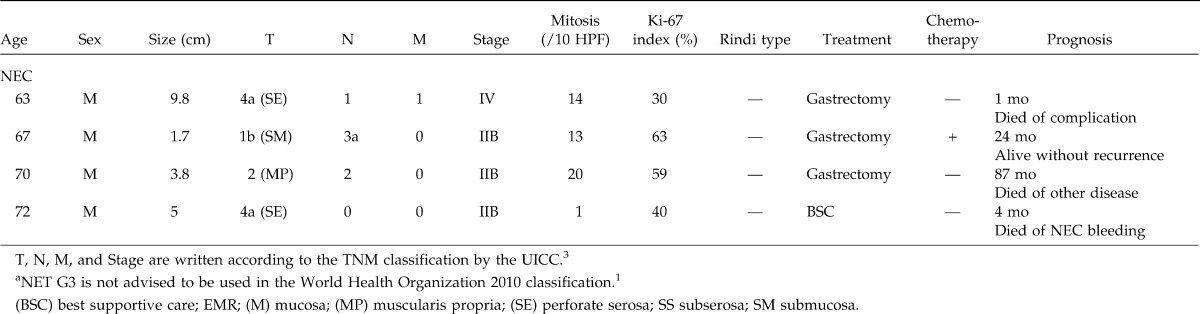
As for patients staged at NET G1, the tumor invaded within the submucosa in all patients. No case showed metastases. Two patients were observed without treatment. Endoscopic mucosal resection (EMR) was performed in 3 cases and gastrectomy in 4 cases. Regardless of type or treatment, no patient showed recurrence or tumor-related death during the median 53-month follow-up (range, 3–89 months) (Fig. 1).
Fig. 1.
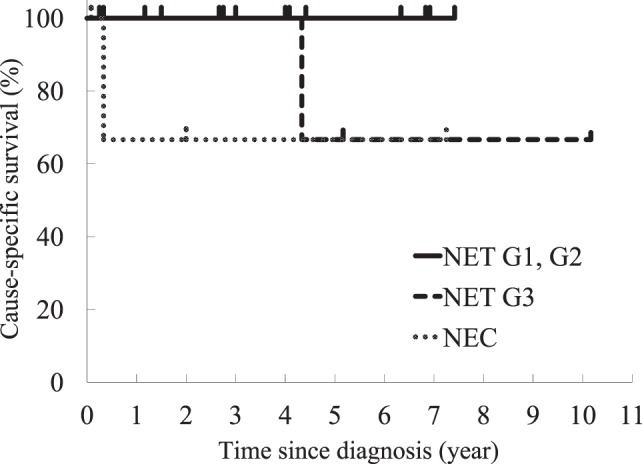
Cause-specific survivals of gastric neuroendocrine tumor grade 1 (NET G1), NET G2, NET G3, and neuroendocrine carcinoma (NEC) were shown on Kaplan-Meier curve. For NET G1 and G2, the 3-year and 5-year survival rates were 100% and 100%, respectively. For NET G3, the 3-year and 5-year survival rates were 100% and 67%, respectively. For NEC, the 3-year and 5-year survival rates were 67% and 67%, respectively, containing a best supportive care case.
As for patients staged at NET G2, all tumors invaded the submucosa. No patient showed metastases. All patients were treated with gastrectomy. Antrectomy was also effective for a patient with multiple tumors of the fundus, hypergastrinemia, and type A atrophic gastritis. Including this case, we experienced no recurrence for the median 25-month follow-up (range, 3–48 months) (Fig. 1).
As for patients staged at NET G3, tumor sizes were more than those of G1 and G2. The tumor invaded the subserosa in all patients. Two patients had lymph node metastases and 1 had liver metastasis. Mitotic counts were 8 to 13 per 10 HPF and Ki-67 indices were as high as 32% to 58%. These were thought to be Rindi's type III. All patients underwent radical surgical resection and adjuvant chemotherapy. One patient with stage IIIB died of liver metastasis 52 months after a total gastrectomy; however, the other 2 patients lived without recurrence for 62 and 122 months, respectively (Fig. 1).
As for NEC, three patients showed lymph node metastases and 1 showed peritoneal metastases and liver metastases. Treatment included gastrectomy in 3 patients, and best supportive care in 1 patient. Two gastectomized patients died from other diseases, and 1 lived for 24 months without recurrence (Fig. 1).
Discussion
There have been many classifications and terms to define the clinical aspects of NET. In 2000, WHO classified endocrine tumors of the gastrointestinal tract as follows: well-differentiated endocrine tumor, carcinoid; well-differentiated endocrine carcinoid, malignant carcinoid; and poorly differentiated endocrine carcinoma, small cell carcinoma. The WHO 2010 classification, the latest classification, is based on the grading of neuroendocrine neoplasms of the digestive system. Mitotic count and the Ki-67 index are thought to be an indicator of malignancy. In this classification, “NET G3” is not advised to be used because G3 cases were supposed to be NEC. However, we experienced 3 cases of NET G3 that were not poorly differentiated.
Few reports in the literature are based on the WHO 2010 classification or grading. Rindi et al5 investigated 102 cases of gastric NET. In that article, 81 cases were NET G1, 5 were NET G2, and 16 were NEC. All NET G2 and NEC cases had metastases and 3-year survival rates were 20% and 7%, respectively, whereas NET G1 showed a 3-year 100% survival. However, in our series, the prognoses of NET G2 were rather good. Our NET G3 cases were thought to be highly malignant considering their high mitotic counts and Ki-67 indices; however, we actually experienced a 10-year survival case. Although the number of NEC cases was limited to 4, their prognoses seemed to be better than reported.
The North American Neuroendocrine Tumor Society published guidelines in 2010,6,7 and the National Comprehensive Cancer Network (NCCN) did so in 2011.8 These guidelines were based on Rindi's type and tumor size; however, they were not based on the WHO 2010 classification. According to the latest NCCN guidelines, for carcinoid tumors, which seemed to correspond to NET G1 and G2, management is described as follows. For hypergastrinemic patients, which indicates that the tumors are Rindi's type I or II, if the tumors are ≦2 cm, options include endoscopic resection, observation, or octreotide. For hypergastrinemic patients, with tumors >2 cm, endoscopic resection or surgical resection is indicated. Patients with locoregional Rindi's type III tumor, they are usually treated with radical resection of the tumor and removal of the perigastric lymph nodes. For resectable poorly differentiated neuroendocrine tumors, which seemed to correspond to NEC, surgical resection and chemotherapy with a small cell lung cancer regimen is advised.
In conclusion, we reviewed 22 cases of gastric NETs that were encountered in our institutions. In the current study, we experienced 3 NET G3 cases. The prognoses of NET G2 and NEC were found to be better than those in previous reports; therefore, we could not clarify whether the WHO 2010 classifications were useful in predicting their clinical behaviors nor in deciding treatment strategy. An accumulation of more cases from several institutions is needed to establish a common classification of malignancy and effective guidelines.
Acknowledgments
The authors are grateful for the prognostic investigations of Health Information Managers Chiga Iitani at Higashiosaka City General Hospital and Fumiko Matsufuru at National Hospital Organization Kure Medical Center/Chugoku Cancer Center.
Footnotes
Reprint requests: Tsutomu Dousei, MD, Department of Surgery, Higashiosaka City General Hospital, 3-4-5 Nishiiwata, Higashiosakashi, Osaka, 578-8588 Japan.
References
- 1.Rindi G, Arnold R, Bosman FT, Capella C, Klimstra DS, Klöppel G, et al. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumors of the Digestive System. 4th ed. Lyon: International Agency for Research on Cancer; 2010. pp. 13–14. [Google Scholar]
- 2.Solcia E, Capella C, Klöppel G, Heitz PU, Sobin LH, Rosai J. Endocrine tumors of the gastrointestinal tract. In: Solcia E, Klöppel G, Sobin LH, editors. Histological Typing of Endocrine Tumors. 2nd ed. Heidelberg, New York: Springer Verlag; 2000. pp. 61–64. [Google Scholar]
- 3.Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumors. 7th ed. Oxford: Wiley-Blackwell; 2009. [Google Scholar]
- 4.Rindi G, Luinetti O, Cornaggia M, Capella C, Solcia E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology. 1993;104:994–1006. doi: 10.1016/0016-5085(93)90266-f. [DOI] [PubMed] [Google Scholar]
- 5.Rindi G, Azzoni C, La Rosa S, Klersy C, Paolotti D, Rappel S, et al. ECL cell tumor and poorly differentiated endocrine carcinoma of the stomach: prognostic evaluation by pathological analysis. Gastroenterology. 1999;116:532–542. doi: 10.1016/s0016-5085(99)70174-5. [DOI] [PubMed] [Google Scholar]
- 6.Kulke MH, Anthony LB, Bushnell DL, de Herder WW, Goldsmith SJ, Klimstra DS, et al. North American Neuroendocrine Tumor Society (NANETS) treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas. 2010;39:735–752. doi: 10.1097/MPA.0b013e3181ebb168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strosberg JR, Coppola D, Klimstra DS, Phan AT, Kulke MH, Wiseman GA, et al. The North American Neuroendocrine Tumor Society (NANETS) consensus guidelines for the diagnosis and management of poorly differentiated (high-grade) extrapulmonary neuroendocrine carcinomas. Pancreas. 2010;39:799–800. doi: 10.1097/MPA.0b013e3181ebb56f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network. NCCN Guidelines Version 1.2011 Neuroendocrine Tumors. http://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf. Accessed November 30, 2011. [Google Scholar]


