Abstract
The purpose of this study was to clarify the clinical features most closely associated with gangrenous appendicitis. From among 314 patients who had undergone open appendectomy in our collected database, 222 for whom sufficient data were evaluable were enrolled. The results of univariate analysis revealed that age (≤40/>40 years), sex (female/male), fever (≤37°/>37°C), the serum levels of C-reactive protein and albumin, the Glasgow prognostic score (0, 1/2), and the neutrophil-to-lymphocyte ratio (NLR) (≤8/>8) were associated with gangrenous appendicitis. Among these 7 clinical features, multivariate analysis disclosed that age (≤40/>40 years) (odds ratio, 3.435; 95% confidence interval 1.744–6.766; P < 0.001) and NLR (≤8/>8) (odds ratio, 3.016; 95% confidence interval 1.535–5.926; P = 0.001) were associated with gangrenous appendicitis. The sensitivity and specificity of these two clinical features were 65% and 27%, and 73% and 39%, respectively. NLR (>8) shows a significant association with gangrenous appendicitis in patients undergoing appendectomy.
Keywords: Acute appendicitis, Catarrhal appendicitis, Gangrenous appendicitis, Phlegmonous appendicitis, Neutrophil-to-lymphocyte ratio
Emergency surgery is imperative for patients diagnosed as having acute appendicitis,1,2 because severe phlegmonous or gangrenous appendiceal inflammation can easily lead to peritonitis if perforation occurs. Therefore, correct evaluation of the severity of acute appendicitis provides useful information for surgeons, allowing them to downgrade the risk from life-threatening peritonitis to sepsis. Although recent advances in antibiotic therapy have broadened the range of optional treatments for acute appendicitis other than surgery,3–5 surgery is still the first choice for severe acute appendicitis,1,2 especially gangrenous appendicitis.
Although there are a number of useful diagnostic modalities for acute appendicitis, including evaluation of clinical symptoms, scoring systems,6–8 and imaging methods,9–11 there are few methods for evaluating the severity of appendicitis itself before surgery. In western countries, acute appendicitis is commonly classified into three grades (i.e., focal, suppurative, or gangrenous [phlegmonous]), whereas in Japan it is classified as catarrhal, phlegmonous, or gangrenous. Because the latter classification reflects the severity of acute appendicitis based on inflammation, it may be better correlated with data for inflammatory markers such as white blood cell (WBC) count,12,13 neutrophil count,14 and platelet count15 along with the neutrophil-to-lymphocyte ratio (NLR).16,17 Similarly, C-reactive protein (CRP) and albumin are well known to be acute phase proteins whose serum levels reflect the activity of inflammatory cytokines. CRP in particular is commonly used as an inflammatory marker not only in acute appendicitis,12,18,19 but also in several chronic inflammatory diseases,20,21 including cancer.22
In the present study, therefore, we tried to clarify the clinical features, including inflammatory markers, that are most closely associated with gangrenous appendicitis.
Materials and Methods
We retrospectively reviewed a database of 314 patients who had undergone open appendectomy for acute appendicitis, performed by the same trained surgical team at the Department of Gastroenterological Surgery, Dokkyo Medical University Hospital, between January 2000 and December 2009. Among these patients, 222 (119 men, 103 women), for whom sufficient data were evaluable, were enrolled in the study.
All underwent routine laboratory tests, including assessment of inflammation-related markers such as the serum levels of CRP and albumin, and the Glasgow prognostic score (GPS).
The GPS was estimated as described previously.23,24 Briefly, patients with an elevated CRP level (>1.0 mg/dL) and hypoalbuminemia (<3.5 g/dL) were allocated a score of 2, patients with only 1 of these biochemical abnormalities were allocated a score of 1, and patients with neither were allocated a score of 0.
Similarly, the recommended cutoff value of the preoperative NLR was decided using receiver operating characteristic curve analyses. The recommended cutoff value of the NLR was based on the most prominent point on the receiver operating characteristic curve for sensitivity (0.725) and specificity (0.620). Because these 2 parameters indicated a cutoff value of 8.055, the recommended NLR cutoff value was defined as 8.0. The area under the receiver operating characteristic curve was 0.679.
The studied patients were divided into three groups based on the pathologic grades of acute appendicitis used in Japan: group A, those who had catarrhal appendicitis; group B, those who had phlegmonous appendicitis; and group C, those who had gangrenous appendicitis.
Univariate analysis was performed to examine the relationships between gangrenous appendicitis and clinical features such as age (≤40/>40 years), sex (female/male), body mass index (BMI) (kg/m2), fever (≤37°/>37°C), time until surgery (≤1/>1 day), WBC count (×103/mm3), neutrophil count (×103/mm3), platelet count (×104/mm3), CRP (mg/dL), albumin (g/dL), GPS (0, 1/2), and NLR (≤8/>8).
Multivariate analysis was then performed using the clinical features found to have a significance level of P < 0.05 in the univariate analysis to clarify those most closely associated with gangrenous appendicitis.
The sensitivity and specificity of the features selected by multivariate analysis were also determined to estimate their diagnostic value.
Statistical analysis
Data are presented as mean ± SD. Differences among the groups were analyzed using the χ2 test and Kruskal-Wallis test. Odds ratios (OR) with 95% confidence interval (CI) were calculated using univariate or multivariate analysis, performed using logistic regression. Statistical analyses were performed using the SPSS statistical software package, version 16.0 (SPSS Inc, Chicago, Illinois) at a significance level of P < 0.05.
Results
Table 1 shows the relationships between the clinical background features and patients with different forms of acute appendicitis. Mean patient age was 40 years (40 ± 19 years, mean ± SD, range, 12–90 years), and the median age was 34 years. There were 43 patients in group A (catarrhal appendicitis), 99 in group B (phlegmonous appendicitis), and 80 in group C (gangrenous appendicitis). There were significant intergroup differences in age (≤40/>40 years) (P < 0.001), sex (P = 0.018), fever (≤37°/>37°C) (P = 0.013), appendix perforation (absence/presence) (P < 0.001), time until surgery (≤1/>1 day) (P = 0.049), operation time (≤60/>60 minutes) (P = 0.004), GPS (0/1/2) (P < 0.001), and NLR (≤8/>8) (P < 0.001).
Table 1.
Relationship between clinical background features and patients with different forms of acute appendicitis
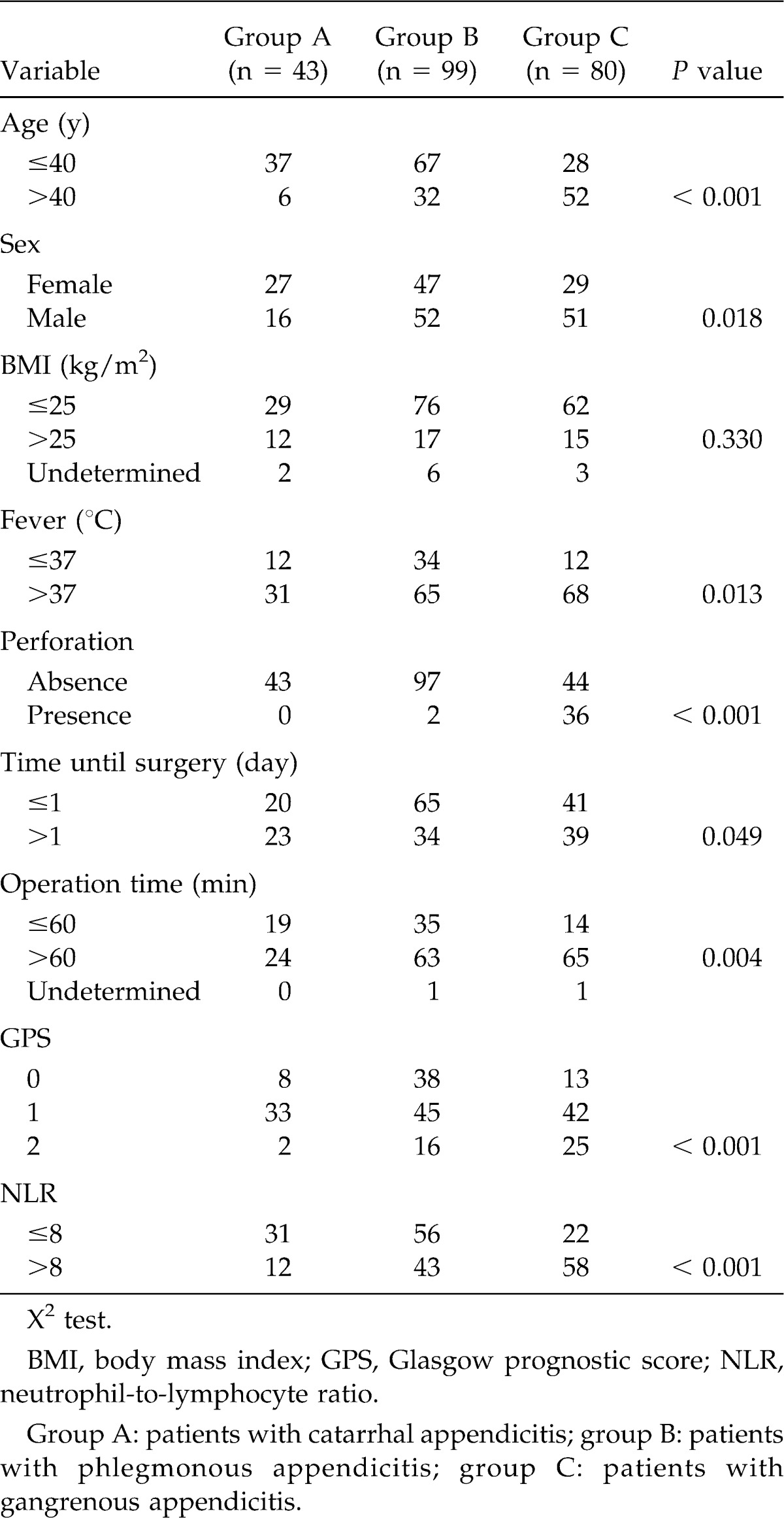
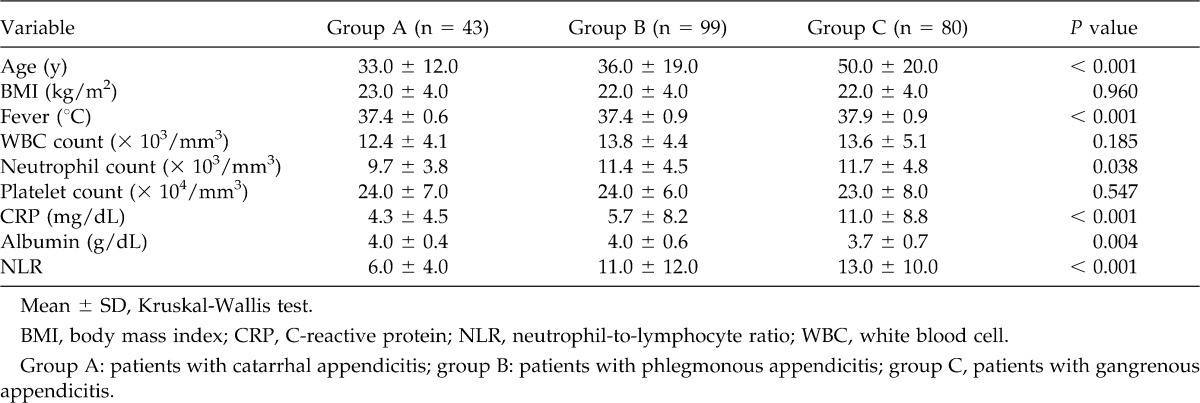
Table 2 shows the relationships between the clinical/laboratory parameters and patients with different forms of acute appendicitis. There were significant intergroup differences in age (in years) (P < 0.001), fever (in °C) (P < 0.001), neutrophil count (P = 0.038), serum CRP level (in milligrams per deciliter) (P < 0.001), serum albumin level (P = 0.004), and NLR (P < 0.001) (by Kruskal-Wallis test).
Table 2.
Relationship between clinical/laboratory parameters and patients with different forms of acute appendicitis
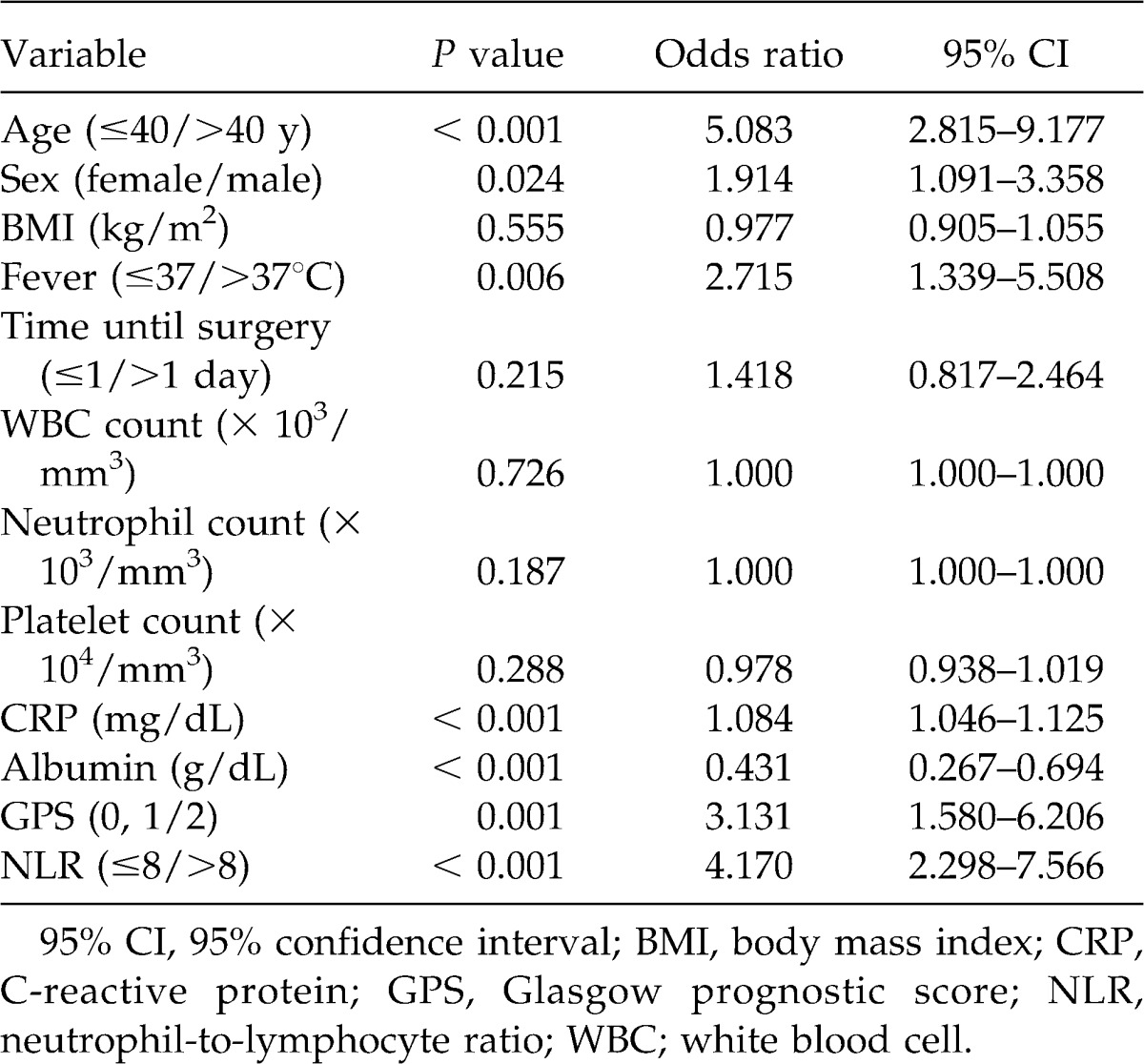
Univariate analysis to evaluate the association between clinical/laboratory parameters and gangrenous appendicitis demonstrated that age (≤40/>40 years) (OR, 5.083; 95% CI 2.815–9.177; P < 0.001), sex (female/male) (OR, 1.914; 95% CI 1.091–3.358; P = 0.024), fever (≤37°/>37°C) (OR, 2.715; 95% CI 1.339–5.508; P = 0.006), serum CRP level (OR, 1.084; 95% CI 1.046–1.125; P < 0.001), serum albumin level (OR, 0.431; 95% CI 0.267–0.694; P < 0.001), GPS (0, 1/2) (OR, 3.131; 95% CI 1.580–6.206; P = 0.001), and NLR (≤8/>8) (OR, 4.170; 95% CI 2.298–7.566; P < 0.001) were associated with gangrenous appendicitis (Table 3).
Table 3.
Univariate analysis of clinical features in relation to gangrenous appendicitis
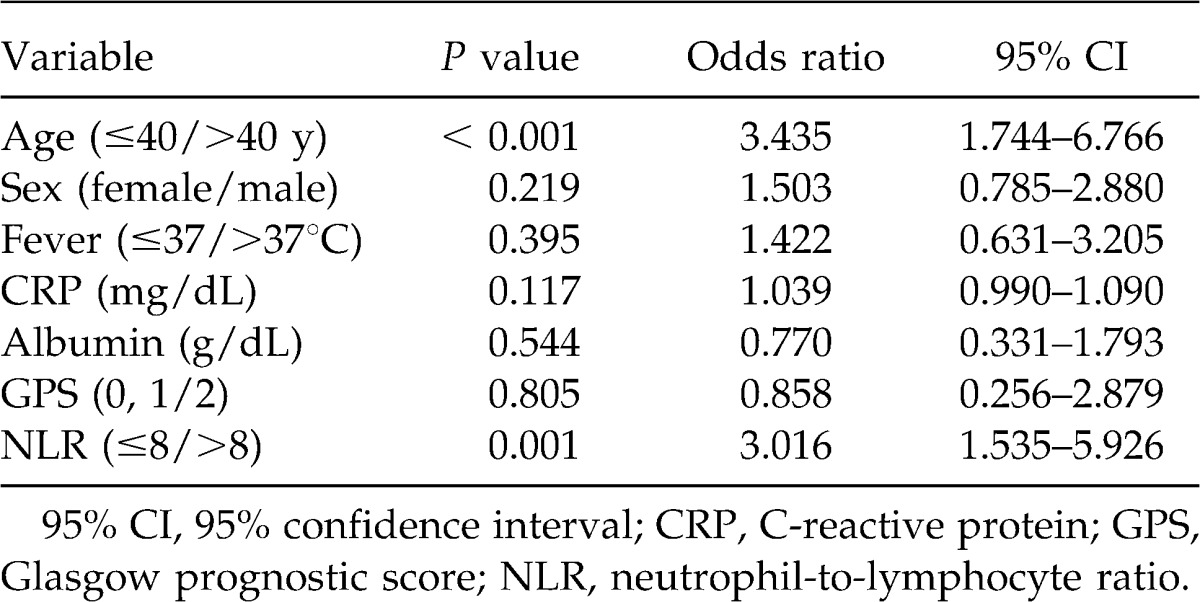
Multivariate analysis of the 7 clinical features to assess those most closely associated with gangrenous appendicitis demonstrated that both age (≤40/>40 years) (OR, 3.435; 95% CI 1.744–6.766; P < 0.001) and NLR (≤8/>8) (OR, 3.016; 95% CI 1.535–5.926; P = 0.001) were closely associated (Table 4), with sensitivities and specificities of 65% and 27%, and 73% and 39%, respectively.
Table 4.
Multivariate analysis of selected clinical features in relation to gangrenous appendicitis
Discussion
Although it is difficult to fully explain why group C had a higher ratio of men to women than groups A and B in the present retrospective study, a recent report25 described a similar phenomenon. In a comparison of laparoscopic and open appendectomy, the latter group had a higher ratio of men than women. Although that study investigated children rather than adults undergoing surgery for acute appendicitis, the results demonstrated that male patients tended to have severe appendicitis, for which open appendectomy, rather than laparoscopic appendectomy, is indicated.
With regard to age, group C in the present study had a higher ratio of older patients (>40 years) than groups A and B. Acute appendicitis tends to occur in relatively young individuals. It is well known that diagnosis of appendicitis in older patients is more difficult than in younger patients. Several previous studies have demonstrated that older patients sometimes lack not only the typical symptoms of appendicitis, but also the characteristic laboratory data abnormalities.26 As a result, a higher proportion of older patients tend have gangrenous appendicitis.
With regard to perforative appendicitis, although group C had a higher ratio of perforation (45.0%, 36/80) than group B (2.0%, 2/99), there were no patients with perforation in group A (0, 0/43). These results clearly confirm that emergency surgery is mandatory for patients with gangrenous appendicitis to prevent peritonitis due to perforation. Because operation time was significantly longer in group C than in groups A and B27 suggests that patients with gangrenous appendicitis also have problems related to surgery in view of the severe and more advanced nature of the inflammation.
Similarly, it was obvious that the inflammatory state in group C patients was more severe than in groups A and B. Patients in group C not only had higher fever but also more severe inflammation in terms of clinical scores such as the GPS and NLR. However, although these phenomena might reflect the degree of activity of inflammatory cytokines, including interleukin-6,28 resulting from local inflammation, there were some discrepancies in clinical parameters such as the WBC and platelet counts. Although most patients (56.8%, 126/222) developed typical symptoms and underwent surgery within 24 hours, there were no intergroup differences in complete blood count (CBC) components, except for neutrophil count.
Generally, under hypercytokinemic conditions, the increases of CBC components are very rapid in comparison to the dynamics of acute phase proteins, such as the increase in the serum level of CRP and the decrease in the serum level of albumin. This is because protein synthesis in the liver requires a longer time than proliferation of CBC components in the bone marrow. Therefore, it seems rational that gangrenous appendicitis would have a closer association with acute phase proteins than with CBC components, except for the neutrophil count, because of the longer period until surgery than that for other grades of appendicitis.
In addition, the results of univariate analysis revealed that age,26 sex,29 fever,30 CRP,19 albumin, GPS, and NLR16 were associated with gangrenous appendicitis. Among these clinical parameters, CRP and albumin are the components of the GPS, as well as being acute phase proteins. These features might reflect the activity of inflammatory cytokines, along with fever. Among the CBC components, only NLR was associated with gangrenous appendicitis.13
Interestingly, however, multivariate analysis demonstrated that age and NLR were associated with gangrenous appendicitis.
With regard to NLR, a recent study17 has assessed its relationship with acute appendicitis based on an analysis of 1117 patients lrss than 16 years of age.31 Although the investigators used a statistical cutoff value of P < 0.1, they concluded that NLR appears to have greater diagnostic accuracy than either WBC or CRP alone. This conclusion strongly supports the results of our present study, in that NLR, rather than either CRP or albumin alone, showed a close relationship with gangrenous appendicitis. In addition, because the GPS has been established as an inflammation-based prognostic system for several types of cancer associated with chronic inflammation,32,33 there is a degree of difficulty in applying it for assessment of severe inflammation such as that in acute appendicitis.
Finally, comparison of age and NLR demonstrated that the latter was diagnostically superior to the former, the respective sensitivities and specificities being 65% and 27%, and 73% and 39%. Because NLR was a better diagnostic indicator than age for gangrenous appendicitis, patients undergoing surgery for acute appendicitis can be regarded as having a greater risk of gangrenous appendicitis (OR, 4.170) if they show a high NLR level (>8).
Thus, preoperative NLR (>8) appears to be the most valuable predictor of gangrenous appendicitis in patients undergoing surgery for acute appendicitis. In addition, although older patients with acute appendicitis sometimes wait longer to operation because of the lack of typical symptoms,26 higher preoperative NLR level (>8) can predict severe acute appendicitis as well as appropriate imaging studies such as computed tomography9 and magnetic resonance imaging.34
Footnotes
Reprint requests: Mitsuru Ishizuka, MD, Department of Gastroenterological Surgery, Dokkyo Medical University, 880 Kitakobayashi, Mibu, Tochigi 321-0293, Japan.
References
- 1.Fitzmaurice GJ, McWilliams B, Hurreiz H, Epanomeritakis E. Antibiotics versus appendectomy in the management of acute appendicitis: a review of the current evidence. Can J Surg. 54:6610. doi: 10.1503/cjs.006610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varadhan KK, Humes DJ, Neal KR, Lobo DN. Antibiotic therapy versus appendectomy for acute appendicitis: a meta-analysis. World J Surg. 34:199–209. doi: 10.1007/s00268-009-0343-5. [DOI] [PubMed] [Google Scholar]
- 3.Park HC, Kim BS, Lee BH. Efficacy of short-term antibiotic therapy for consecutive patients with mild appendicitis. Am Surg. 77:752–755. [PubMed] [Google Scholar]
- 4.Styrud J, Eriksson S, Nilsson I, Ahlberg G, Haapaniemi S, Neovius G, et al. Appendectomy versus antibiotic treatment in acute appendicitis: a prospective multicenter randomized controlled trial. World J Surg. 2006;30:1033–1037. doi: 10.1007/s00268-005-0304-6. [DOI] [PubMed] [Google Scholar]
- 5.Eriksson S, Granstrom L. Randomized controlled trial of appendicectomy versus antibiotic therapy for acute appendicitis. Br J Surg. 1995;82:166–169. doi: 10.1002/bjs.1800820207. [DOI] [PubMed] [Google Scholar]
- 6.Chong CF, Adi MI, Thien A, Suyoi A, Mackie AJ, Tin AS, et al. Development of the RIPASA score: a new appendicitis scoring system for the diagnosis of acute appendicitis. Singapore Med J. 51:220–225. [PubMed] [Google Scholar]
- 7.Jang SO, Kim BS, Moon DJ. Application of alvarado score in patients with suspected appendicitis [in Korean] Kor J Gastroenterol. 2008;52:27–31. [PubMed] [Google Scholar]
- 8.Brigand C, Steinmetz JP, Rohr S. The usefulness of scores in the diagnosis of appendicitis [in French] J Chir (Paris) 2009;146:2–7. doi: 10.1016/j.jchir.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Al-Khayal KA, Al-Omran MA. Computed tomography and ultrasonography in the diagnosis of equivocal acute appendicitis. A meta-analysis. Saudi Med J. 2007;28:173–180. [PubMed] [Google Scholar]
- 10.Terasawa T, Blackmore CC, Bent S, Kohlwes RJ. Systematic review: computed tomography and ultrasonography to detect acute appendicitis in adults and adolescents. Ann Intern Med. 2004;141:537–546. doi: 10.7326/0003-4819-141-7-200410050-00011. [DOI] [PubMed] [Google Scholar]
- 11.Parks NA, Schroeppel TJ. Update on imaging for acute appendicitis. Surg Clin North Am. 91:141–154. doi: 10.1016/j.suc.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Sanjuan JC, Martin-Parra JI, Seco I, Garcia-Castrillo L, Naranjo A. C-reactive protein and leukocyte count in the diagnosis of acute appendicitis in children. Dis Colon Rectum. 1999;42:1325–1329. doi: 10.1007/BF02234223. [DOI] [PubMed] [Google Scholar]
- 13.Keskek M, Tez M, Yoldas O, Acar A, Akgul O, Gocmen E, et al. Receiver operating characteristic analysis of leukocyte counts in operations for suspected appendicitis. Am J Emerg Med. 2008;26:769–772. doi: 10.1016/j.ajem.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 14.Shafi SM, Afsheen M, Reshi FA. Total leucocyte count, C-reactive protein and neutrophil count: diagnostic aid in acute appendicitis. Saudi J Gastroenterol. 2009;15:117–120. doi: 10.4103/1319-3767.48969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albayrak Y, Albayrak A, Albayrak F, Yildirim R, Aylu B, Uyanik A, et al. Mean platelet volume: a new predictor in confirming acute appendicitis diagnosis. Clin Appl Thromb Hemost. 2011;17:362–366. doi: 10.1177/1076029610364520. [DOI] [PubMed] [Google Scholar]
- 16.Goodman DA, Goodman CB, Monk JS. Use of the neutrophil:lymphocyte ratio in the diagnosis of appendicitis. Am Surg. 1995;61:257–259. [PubMed] [Google Scholar]
- 17.Markar SR, Karthikesalingam A, Falzon A, Kan Y. The diagnostic value of neutrophil: lymphocyte ratio in adults with suspected acute appendicitis. Acta Chir Belg. 110:543–547. [PubMed] [Google Scholar]
- 18.Gurleyik E, Gurleyik G, Unalmiser S. Accuracy of serum C-reactive protein measurements in diagnosis of acute appendicitis compared with surgeon's clinical impression. Dis Colon Rectum. 1995;38:1270–1274. doi: 10.1007/BF02049151. [DOI] [PubMed] [Google Scholar]
- 19.Yokoyama S, Takifuji K, Hotta T, Matsuda K, Nasu T, Nakamori M, et al. C-Reactive protein is an independent surgical indication marker for appendicitis: a retrospective study. World J Emerg Surg. 2009;4:36. doi: 10.1186/1749-7922-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karoui S, Laz S, Serghini M, Bibani N, Boubaker J, Filali A. Correlation of C-reactive protein with clinical and endoscopic activity in patients with ulcerative colitis. Dig Dis Sci. 2011;56:1801–1805. doi: 10.1007/s10620-010-1496-7. [DOI] [PubMed] [Google Scholar]
- 21.Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:250–255. doi: 10.1164/rccm.200605-713OC. [DOI] [PubMed] [Google Scholar]
- 22.Ishizuka M, Kita J, Shimoda M, Rokkaku K, Kato M, Sawada T, et al. Systemic inflammatory response predicts postoperative outcome in patients with liver metastases from colorectal cancer. J Surg Oncol. 2009;100:38–42. doi: 10.1002/jso.21294. [DOI] [PubMed] [Google Scholar]
- 23.Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. Br J Cancer. 2004;90:1704–1706. doi: 10.1038/sj.bjc.6601789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dagg K, Scott HR. A prospective longitudinal study of performance status, an inflammation-based score (GPS) and survival in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2005;92:1834–1836. doi: 10.1038/sj.bjc.6602591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SL, Yaghoubian A, Kaji A. Laparoscopic vs open appendectomy in children: outcomes comparison based on age, sex, and perforation status. Arch Surg 2011, Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.Kraemer M, Franke C, Ohmann C, Yang Q. Acute appendicitis in late adulthood: incidence, presentation, and outcome. Results of a prospective multicenter acute abdominal pain study and a review of the literature. Langenbeck's Arch Surg. 2000;385:470–481. doi: 10.1007/s004230000165. [DOI] [PubMed] [Google Scholar]
- 27.Korner H, Sondenaa K, Soreide JA, Andersen E, Nysted A, Lende TH, et al. Incidence of acute nonperforated and perforated appendicitis: age-specific and sex-specific analysis. World J Surg. 1997;21:313–317. doi: 10.1007/s002689900235. [DOI] [PubMed] [Google Scholar]
- 28.Sack U, Biereder B, Elouahidi T, Bauer K, Keller T, Trobs RB. Diagnostic value of blood inflammatory markers for detection of acute appendicitis in children. BMC Surg. 2006;6:15. doi: 10.1186/1471-2482-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luckmann R, Davis P. The epidemiology of acute appendicitis in California: racial, gender, and seasonal variation. Epidemiology. 1991;2:323–330. doi: 10.1097/00001648-199109000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Cardall T, Glasser J, Guss DA. Clinical value of the total white blood cell count and temperature in the evaluation of patients with suspected appendicitis. Acad Emerg Med. 2004;11:1021–1027. doi: 10.1197/j.aem.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Lee SL, Ho HS. Acute appendicitis: is there a difference between children and adults. Am Surg. 2006;72:409–413. doi: 10.1177/000313480607200509. [DOI] [PubMed] [Google Scholar]
- 32.Ishizuka M, Nagata H, Takagi K, Horie T, Kubota K. Inflammation-based prognostic score is a novel predictor of postoperative outcome in patients with colorectal cancer. Ann Surg. 2007;246:1047–1051. doi: 10.1097/SLA.0b013e3181454171. [DOI] [PubMed] [Google Scholar]
- 33.Ramsey S, Lamb GW, Aitchison M, Graham J, McMillan DC. Evaluation of an inflammation-based prognostic score in patients with metastatic renal cancer. Cancer. 2007;109:205–212. doi: 10.1002/cncr.22400. [DOI] [PubMed] [Google Scholar]
- 34.Heverhagen JT, Pfestroff K, Heverhagen AE, Klose KJ, Kessler K, Sitter H. Diagnostic accuracy of magnetic resonance imaging: a retrospective evaluation of patients with suspected appendicitis (diamond) J Magn Reason Imaging. 2012;35:617–623. doi: 10.1002/jmri.22854. [DOI] [PubMed] [Google Scholar]


