Abstract
We encountered a rare case of a well-differentiated endocrine carcinoma originating from the bile duct in association with a congenital choledochal cyst (CCC). The patient is a 28-year-old woman referred to our clinic for pruritus. Laboratory data showed mild elevation of serum hepatobiliary enzymes. Computed tomography and magnetic resonance imaging demonstrated pancreatobiliary maljunction and a Todani type IV-A CCC from the inferior bile duct to the bilateral intrahepatic bile ducts. A solid tumor was detected in the middle portion of the common bile duct. Pancreatoduodenectomy and total extrahepatic bile duct resection was performed. Based on pathologic and immunohistochemical examinations, a diagnosis of well-differentiated endocrine carcinoma was made according to the World Health Organization criteria. To our knowledge, this is the third report of a neuroendocrine tumor originating from the bile duct in association with a CCC.
Keywords: Congenital choledochal cyst, Well-differentiated endocrine carcinoma, Bile duct, Pancreatobiliary maljunction
Congenital choledochal cysts (CCCs) are found in approximately 1 in 100,000 to 150,000 persons.1 According to a nationwide survey in Japan, carcinoma of the biliary tract was identified in 16.2% of patients with CCCs.2 Pathologically, most cases are of epithelial origin.3–7
We present a rare case of a well-differentiated endocrine carcinoma originating from the bile duct in association with a CCC. Extrahepatic bile ducts are the rarest primary sites for neuroendocrine tumors (NETs), accounting for 0.01% of all gastrointestinal NETs.8 To our knowledge, this is the third case of NET of bile duct origin in association with a CCC.
Case Report
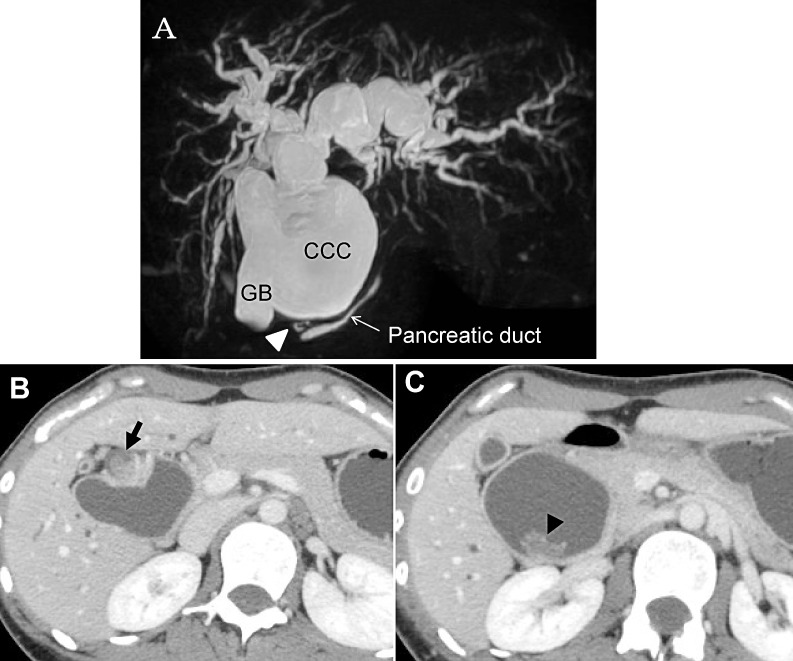
A 28-year-old woman was referred to our clinic for pruritus. Physical examination showed slight jaundice. Biochemical examination showed slightly elevated levels of hepatobiliary enzymes. Magnetic response cholangiopancreatography demonstrated the presence of a CCC from the bilateral intrahepatic bile ducts to the inferior portion of the common bile duct (CBD), which was diagnosed as Todani type IV-A.1 The presence of pancreatobiliary maljunction (PBM), in which the dilated bile duct tapered to enter the pancreatic duct at a right angle with the dilated common channel, was also identified (Fig. 1A). A solid tumor (Fig. 1B) and a polypoid tumor (Fig. 1C) were detected in the middle portion of the CBD. Based on these findings, a provisional diagnosis of cholangiocarcinomas with a CCC was made, and pancreatoduodenectomy combined with total extrahepatic bile duct resection was planned.
Fig. 1.
(A) Magnetic resonance cholangiopancreatography showed a congenital choledochal cyst from the bilateral intrahepatic bile ducts to the inferior portion of the common bile duct, diagnosed as Todani type IV-A. The dilated bile duct tapered to enter the pancreatic duct at a right angle with the dilated common channel (arrowhead), which reveals presence of pancreatobiliary maljunction, classified as Komi type Ib. CCC, congenital choledochal cyst; GB, gallbladder. (B) A solid tumor, measuring 26 × 15 mm, located in the middle portion of the common bile duct in the axial view (arrow). (C) A polypoid tumor, 20 × 15 mm in size, was located in the middle portion of the common bile duct (arrowhead).
The operation was successfully performed. There were finally five orifices (i.e., B1l, B2-4, B5+6, B7, and B8). Reconstruction was done according to the modified Child method. She has been recurrence-free for 3 years without adjuvant chemotherapy.
Pathologic findings
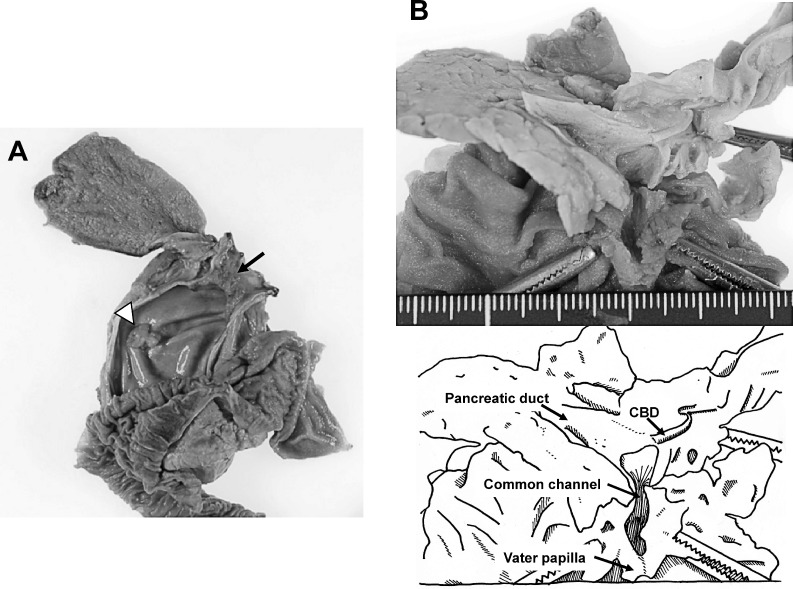
Macroscopically, the surgical specimens showed dilation of the bile duct from the distal stump of the bilateral intrahepatic bile duct to the inferior portion of the CBD that tapered to join with the pancreatic duct (Fig. 2A). The confluence was outside of the pancreas, 23 mm from the Vater papilla, with cystic dilation of the common channel (Fig. 2B). A nodular tumor measuring 30 × 18 × 15 mm and a polypoid tumor measuring 20 × 15 × 8 mm were located in the middle portion of the CBD (Fig. 2A).
Fig. 2.
(A) The narrowed common bile duct (CBD) connected to the pancreatic duct outside of the pancreas, with cystic dilation of the common channel. A nodular expanding tumor measuring 30 × 18 × 15 mm (arrow) and a polypoid tumor 20 × 15 × 8 mm in size (white arrowhead) were located in the middle portion of the CBD. (B) Dilation of the bile duct involved the distal stump of the bilateral intrahepatic bile ducts to the inferior portion of the CBD.
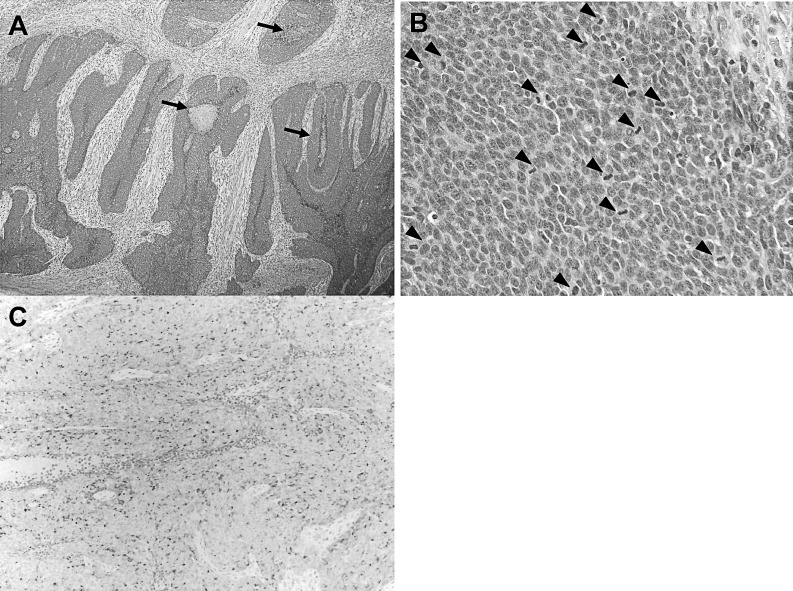
Microscopically, the nodular tumor showed massive growth in the mucosa of the bile duct without infiltration of the adjacent connective tissue and pancreas. The tumor cells formed solid structures with areas of central necrosis (Fig. 3A). The cells were round or oval, hyperchromatic, and had an increased nucleus-to-cytoplasm ratio. Mitotic figures were quite frequent, seen in 70 to 80 cells per 10 high-power fields (Fig. 3B). Immunohistochemically, tumor cells were diffusely positive for chromogranin-A and synaptophysin, membrane proteins usually present in neuroendocrine cells (Fig. 3C). Tumor cells were negative for Pan-Keratin and CD56, which are typically expressed on the surface of neurons or endothelial cells. The Ki-67 labeling index was 89.8%. These findings are consistent with well-differentiated neuroendocrine carcinoma according to the World Health Organization criteria for the clinicopathologic classification of gastroenteropancreatic NETs.9 Vertical invasion was limited to within the fibromuscular layer. There was no microscopic tumor invasion in the resected bile duct stumps. The polypoid tumor consisted of columnar cells with metaplasia, proliferating in an irregular papillotubular pattern. The basal epithelium in the dilated portion of the CBD showed hyperplasia.
Fig. 3.
(A) Tumor cells formed focal solid structures. Arrows indicate areas of central necrosis. H&E (×40). (B) Tumor cells were round to oval in shape with scanty cytoplasm. They were hyperchromatic and showed a high frequency of aberrant mitosis. Arrowheads indicate mitotic figures. H&E (×400). (C) Tumor cells were diffusely positive for chromogranin-A (×40).
No lymph nodes metastasis was observed, and surgical margins were clear. According to the Union for International Cancer Control guidelines, the final classification was stage 1A (pT1N0M0).
Discussion
We report a rare case of a well-differentiated neuroendocrine carcinoma originating from the bile duct in association with a CCC. Biliary NETs are so rare that there are no biological classification criteria. Because of the poor prognosis of biliary neuroendocrine carcinomas and prominent differences in treatment strategies,10–12 standard classification criteria, including biological criteria, are needed to provide reliable prediction of tumor behavior and delineation of treatment strategies for biliary NETs.
NETs are considered to originate from enterochromaffin or Kulchitsky cells in the crypts of Lieberkühn, which are now believed to be of endodermal origin.13 The biliary tract has a very small number of Kulchitsky cells, which explains the rarity of NETs at this site. In the biliary tract, Kulchitsky cells usually appear as a part of a metaplastic process commonly associated with chronic inflammation. The most common types of metaplasia found in the biliary tract and gallbladder are gastric and intestinal metaplasia.13 PBM correlates with dysplasia and carcinogenesis in CCCs. The mixture of pancreatic juice and bile increases the cytotoxic potential for damage to the epithelium of the biliary tract under conditions of infection, inflammation, biliary stasis, decreased trypsin inhibitor concentrations, and presence of enterokinase.14 Multicentric development of biliary carcinoma may occur in patients with PBM, and mutations of oncogenes, such as K-ras,15,16 and tumor-suppressing genes, such as p53,17 are detected in noncancerous biliary epithelium with hyperplasia, metaplasia, and even inflammation before neoplastic changes were histologically detected. In the present case, chronic inflammation of the bile duct due to PBM could first lead to metaplasia, resulting in an increase in the number of Kulchitsky cells that acted as a predisposing factor for the development of neuroendocrine carcinoma. Actually, there were hyperplastic changes in the basal epithelium of the dilated bile duct and a metaplastic tumor in the CBD. Subsequent continuous stimulation by PBM contributed to carcinogenesis.
In the past 3 decades, knowledge of the development and biological behavior of NETs have increased dramatically due to advances in clinical and morphologic diagnosis. Carcinoid, a conventional term, is no longer adequate to cover the entire morphologic and biological spectrum of neoplasms of the disseminated neuroendocrine cell system. The World Health Organization revised the classification criteria of gastroenteropancreatic NETs in 2010.9 Under these new criteria, gastroenteropancreatic NETs are classified according to tumor location (i.e., stomach, duodenum, proximal jejunum, ileum including the distal jejunum, appendix, colon-rectum, and pancreas). Furthermore, gastroenteropancreatic NETs are distinguished according to malignant potential and morphologic differentiation (i.e., well-differentiated endocrine tumor, well-differentiated endocrine carcinoma, and poorly differentiated endocrine carcinoma). Biological criteria reflect malignant potential and prognosis (i.e., tumor size, angioinvasion, histologic differentiation, and Ki-67 labeling index). Because of the paucity of cases, there is still no biological classification criteria established for biliary NETs.
Well-differentiated biliary neuroendocrine carcinoma shows slow progression even when it exhibits gross local invasion or distant metastasis.18 Complete resection including metastases is the most important prognostic factor.19 Despite a few successful cases with cisplatin-based combination chemotherapy,20 well-differentiated biliary endocrine carcinoma is basically resistant to chemotherapy. Poorly differentiated biliary endocrine carcinoma shows rapid progression with high proliferative activity.10 It is usually treated by multidisciplinary management including chemotherapy, surgery, and radiation therapy.10 Due to the poor prognosis and prominent differences in treatment strategies for these types of biliary endocrine carcinomas,10–12 pathologic classification including biological criteria is essential to provide proper treatment. In the present case, the nodular tumor was morphologically a well-differentiated carcinoma. However, considering that the tumor had a high mitotic index and a high Ki-67 labeling index, it was expected that the tumor was biologically aggressive, implying a poor prognosis. Despite discrepancies between the morphologic diagnosis and biological features, the patient was recurrence-free for 3 years without adjuvant chemotherapy. This case highlights the importance of a complete resection for the treatment of biologically aggressive well-differentiated endocrine carcinoma. Accumulation of cases and surveillance are needed to establish biological classification criteria for biliary NETs.
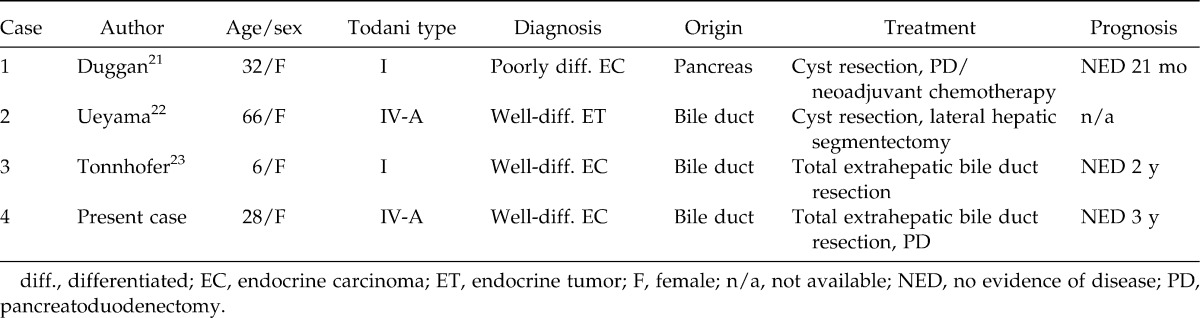
A MEDLINE literature search of English-language articles revealed only 3 cases of NETs in association with CCCs (Table 1).21–23 The patients were all women, and the origin was the bile duct in 2 cases and the pancreas in the remaining case. The CCCs were classified as Todani type I or IV-A, implying the existence of PBM.24 Regarding pathologic diagnosis, there was 1 well-differentiated neuroendocrine tumor, 1 well-differentiated endocrine carcinoma, and 1 poorly differentiated neuroendocrine carcinoma. Our patient is the fourth case of NET associated with a CCC. With regard to the bile duct origin, our case is the third in the world.
Table 1.
Case reports of neuroendocrine tumors in association with congenital choledochal cysts
Footnotes
Reprint requests: Kazuhiro Takahashi, MD, 1-1-1 Tennodai, Tsukuba, Japan, 305-8575.
References
- 1.Todani T, Tabuchi K, Watanabe Y, Kobayashi T. Carcinoma arising in the wall of congenital bile cysts. Cancer. 1979;44(3):1134–1141. doi: 10.1002/1097-0142(197909)44:3<1134::aid-cncr2820440350>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe Y, Toki A, Todani T. Bile duct cancer developed after cyst excision for choledochal cyst. J Hepatobiliary Pancreat Surg. 1999;6(3):207–212. doi: 10.1007/s005340050108. [DOI] [PubMed] [Google Scholar]
- 3.Rossi RL, Silverman ML, Braasch JW, Munson JL, Remine SG. Carcinoma arising in cystic condition of the bile ducts: a clinical and pathologic study. Ann Surg. 1987;205(4):377–384. doi: 10.1097/00000658-198704000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okamura K, Hayakawa H, Kuze M, Takahashi H, Kosaka A, Mizumoto R, et al. Triple carcinomas of the biliary tract associated with congenital choledochal dilation and pancreaticobiliary maljunction. J Gastroenterol. 2000;35(6):465–471. doi: 10.1007/s005350070093. [DOI] [PubMed] [Google Scholar]
- 5.Terada T. Adenosquamouscarcinoma in a congenital choledochal cyst associated with pancreatico-biliary maljunction. Pathol Int. 2009;59(7):482–485. doi: 10.1111/j.1440-1827.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 6.Price L, Kozarek R, Agoff N. Squamous cell carcinoma arising within a choledochal cyst. Dig Dis Sci. 2008;53(10):2822–2825. doi: 10.1007/s10620-007-0182-x. [DOI] [PubMed] [Google Scholar]
- 7.Harris M, Angus P, Davis ID. Choledochal cyst and squamous-cell carcinoma of the biliary tract. Intern Med J. 2002;32(9-10):491. doi: 10.1046/j.1445-5994.2002.t01-1-00247.x. [DOI] [PubMed] [Google Scholar]
- 8.Sethi H, Madanur M, Srinivasan P, Portmann B, Heaton N, Rela M. Non-functioning well-differentiated neuroendocrine tumor of the extrahepatic bile duct: an unusual suspect. Hepatobiliary Pancreat Dis Int. 2007;6(5):549–552. [PubMed] [Google Scholar]
- 9.Albores-Saavedra J, Kloppel G, Adsay NV, Sripa B, Crawford JM, Tsui WMS, et al. Carcinoma of the gallbladder and extrahepatic bile ducts. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System; World Health Organization of Tumours. 4th ed. Lyon: IARC; 2010. pp. 266–273. [Google Scholar]
- 10.Okamura Y, Maeda A, Matsunaga K, Kanemoto H, Boku N, Furukawa H, et al. Small-cell carcinoma in the common bile duct treated with multidisciplinary management. J Hepatobiliary Pancreat Surg. 2009;16(10):575–578. doi: 10.1007/s00534-009-0051-4. [DOI] [PubMed] [Google Scholar]
- 11.Shinchi H, Takao S, Maemura K, Takigawa J, Ohi Y, Aikou T. A case of andenoendocrine cell carcinoma of the extrahepatic bile duct. Nippon Shokakigeka Gakkai Zasshi (Jpn Gastroenterol Surg) 2005;38(2):179–184. [Google Scholar]
- 12.Iwasa S, Morizane C, Okusaka T, Ueno H, Ikeda M, Kondo S, et al. Cisplatin and etoposide as first-line chemotherapy for poorly differentiated neuroendocrine carcinoma of the hepatobiliary tract and pancreas. Jpn J Clin Oncol. 2010;40(4):313–318. doi: 10.1093/jjco/hyp173. [DOI] [PubMed] [Google Scholar]
- 13.Barrón-Rodríguez LP, Manivel JC, Méndez-Sánchez N, Jessurun J. Carcinoid tumor of the common bile duct: evidence for its origin in metaplastic endocrine cells. Am J Gastroenterol. 1991;86(8):1073–1076. [PubMed] [Google Scholar]
- 14.Todani T, Watanabe Y, Urushihara N, Morotomi Y, Maeba T. Choledochal cyst, pancreatobiliary malunion, and cancer. J Hepatobiliary Pancreat Surg. 1994;1(3):247–251. [Google Scholar]
- 15.Matsubara T, Sakurai Y, Sasayama Y, Hori H, Ochiai M, Funabiki T, et al. K-ras point mutation in cancerous and noncancerous biliary epithelium in patients with pancreotico-biliary maljunction. Cancer. 1996;77(8 suppl):1752–1757. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1752::AID-CNCR51>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 16.Tomono H, Nimura Y, Aono K, Nakashima I, Iwamoto T, Nakashima N. Point mutations of the c-Ki-ras gene in carcinoma and atypical epithelium associated with congenital biliary dilation. Am J Gastroenterol. 1996;91(6):1211–1214. [PubMed] [Google Scholar]
- 17.Matsubara T, Funabiki T, Jinno O, Sakurai Y, Hasegawa S, Imazu H, et al. p53 gene mutations and overexpression of p53 product in cancerous and noncancerous biliary epithelium in patients with pancreaticobiliary maljunction. J Hepatobiliary Pancreat Surg. 1999;6(3):286–293. doi: 10.1007/s005340050120. [DOI] [PubMed] [Google Scholar]
- 18.Noronha YS, Raza AS. Well-differentiated neuroendocrine (carcinoid) tumors of the extrahepatic biliary ducts. Arch Pathol Lab Med. 2010;134(7):1075–1079. doi: 10.5858/2008-0764-RS.1. [DOI] [PubMed] [Google Scholar]
- 19.Squillaci S, Marchione R, Piccolomini M, Colombo F, Bucci F, Bruno M, et al. Well-differentiated neuroendocrine carcinoma (malignant carcinoid) of the extrahepatic biliary tract: report of two cases and literature review. APMIS. 2010;118(8):543–556. doi: 10.1111/j.1600-0463.2010.02633.x. [DOI] [PubMed] [Google Scholar]
- 20.Moertel CG, Kovals LK, O'Connell MJ, Rubin J. Treatment of neuroendocrine carcinoma with combined etoposide and cisplatin: evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer. 1991;68(2):227–232. doi: 10.1002/1097-0142(19910715)68:2<227::aid-cncr2820680202>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 21.Duggan DB, Anderson B, Gordon LP. Small cell carcinoma of the pancreas in association with a choledochal cyst: immunohistochemical characterization and complete response to combination chemotherapy. Med and Pediatr Oncol. 1989;17(6):506–509. doi: 10.1002/mpo.2950170531. [DOI] [PubMed] [Google Scholar]
- 22.Ueyama T, Ding J, Hashimoto H, Tsuneyoshi M, Enjoji M. Carcinoid tumor arising in the wall of a congenital bile duct cyst. Arch Pathol Lab Med. 1992;116(3):291–293. [PubMed] [Google Scholar]
- 23.Tonnhofer U, Balassy C, Reck CA, Koller A, Horcher E. Neuroendocrine tumor of the common bile duct, mimicking a choledochal cyst in a 6-year-old child. J Pediatr Surg. 2009;44(6):E23–25. doi: 10.1016/j.jpedsurg.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 24.Söreide K, Körner H, Havnen J, Söreide JA. Bile duct cysts in adults. Br J Surg. 2004;91(12):1538–1548. doi: 10.1002/bjs.4815. [DOI] [PubMed] [Google Scholar]