Abstract
We collected mosquito immatures from artificial containers during 2010–2011 from 26 communities, ranging in size from small rural communities to large urban centers, located in different parts of Yucatán State in southeastern México. The arbovirus vector Aedes (Stegomyia) aegypti was collected from all 26 examined communities, and nine of the communities also yielded another container-inhabiting Aedes mosquito: Aedes (Howardina) cozumelensis. The communities from which Ae. cozumelensis were collected were all small, rural communities (<6,000 inhabitants) in the north-central part of Yucatán State. These new collection records for Ae. cozumelensis demonstrate that this mosquito has a far broader geographic range in the Yucatán Peninsula than previously known. Ae. cozumelensis immatures were collected from both residential premises and cemeteries, with specimens recovered from rock holes as well as various artificial containers including metal cans, flower vases, buckets, tires and a water storage tank. The co-occurrence with Ae. aegypti in small rural communities poses intriguing questions regarding linkages between these mosquitoes, including the potential for direct competition for larval development sites. Additional studies are needed to determine how commonly Ae. cozumelensis feeds on human blood and whether it is naturally infected with arboviruses or other pathogens of medical or veterinary importance. We also summarize the published records for Ae. cozumelensis, which are restricted to collections from México’s Yucatán Peninsula and Belize, and uniformly represent geographic locations where Ae. aegypti can be expected to occur.
Keyword Index: Aedes aegypti, Aedes cozumelensis, Yucatán State, Méxic
INTRODUCTION
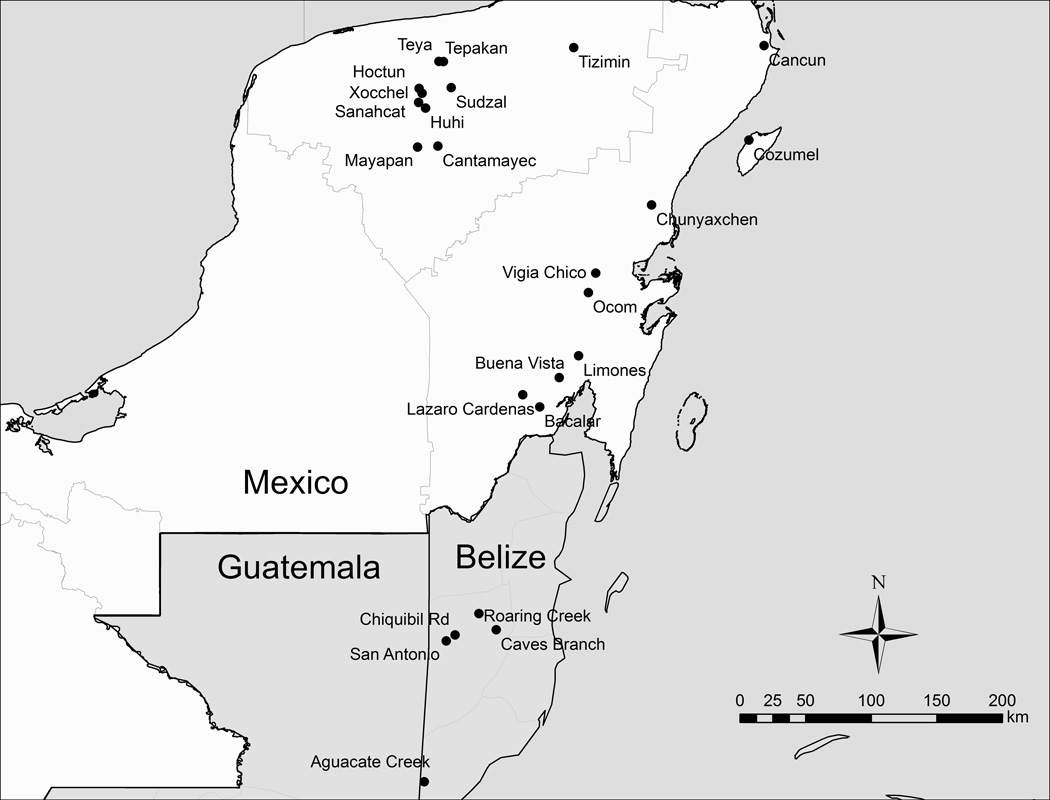
The mosquito Aedes (Howardina) cozumelensis Diaz Najera was first described in 1965 from the island of Cozumel, which is located to the east of the Yucatán Peninsula in southeastern México (Diaz Najera 1966). The original collection consisted of larvae recovered from plastic flower pots and reared to adults. Additional collections of Ae. cozumelensis in the Yucatán Peninsula include: (i) a single larva recovered from the city of Tizimin in northeastern Yucatán State (Berlin 1969), (ii) adults collected from human bait in Cancún in northeastern Quintana Roo State (Pletsch 1977), and (iii) immatures collected from standing water in various artificial containers, leaf axils or leaves in multiple locations (Buena Vista, Bacalar, Ejido Lázaro Cárdenas, Limones, Vigía Chico, Chunyaxchen, and Ocom) in Quintana Roo State (Ortega-Morales et al. 2010a). Records of Ae. cozumelensis outside of the Yucatán Peninsula are restricted to Belize, which is located just to the south of the Yucatán Peninsula (Figure 1). The mosquito was first recorded from Belize in 1967,, through collection of adults coming to human bait, and then again in 1990–1992 through larval collections from artificial containers (1 l plastic bottles) placed at ground level (Bertram 1971,, Heinemann and Belkin 1977a, Pecor et al. 2002). The above-mentioned collections are summarized, together with new records presented herein, in Table 1 and the approximate collection locations are shown in Figure 1.
Figure 1.
Locations in México’s Yucatán Península (in Yucatán State to the north and Quintana Roo State to the east) or Belize where Ae. cozumelensis was collected in the present or previous studies (black circles ●). See Table 1 for more detailed information about the collections.
Table 1.
Summary of collections of Ae. cozumelensis from the present and previous studies.
| Collection location | Approximate coordinates |
Year of collection |
Life stage collected |
Source of collection | Environment | Referencea |
|---|---|---|---|---|---|---|
| México – Yucatán State | ||||||
| Cantamayec | 20°28.23', −89°04.89' | 2011 | Larva | Rock hole | Rural; Homes | 8 |
| Hoctun | 20°51.92′, −89°12.59′ | 2011 | Larva | Bucket, metal can, tire | Rural; Cemetery, Homes | 8 |
| Huhi | 20°43.87', −89°09.94' | 2011 | Larva | Flower vase | Rural; Cemetery | 8 |
| Mayapan | 20°27.83′, −89°13.23′ | 2011 | Larva | Water storage tank | Rural; Homes | 8 |
| Sanahcat | 20°46.17′, −89°12.83′ | 2011 | Larva | Rock hole; Bucket, tire | Rural; Homes | 8 |
| Sudzal | 20°52.24', −88°59.37' | 2011 | Larva | Metal can | Rural; Cemetery | 8 |
| Tepakan | 21°02.94′, −89°02.62′ | 2011 | Larva | Tire | Rural; Homes | 8 |
| Teya | 21°03.05', −89°04.43' | 2011 | Larva | Metal can | Rural; Cemetery | 8 |
| Tiziminb | 21°09', −88°09' | 1960 | Larva | Unknown | Not specified | 2 |
| Xocchel | 20°49.94′, −89°11.39′ | 2011 | Larva | Bucket, metal can | Rural; Cemetery | 8 |
| México – Quintana Roo State | ||||||
| Bacalar | 18°41′, −88°23′ | 2006 | Larva/Pupac | Artificial containers and/or leaf axild | Not specified | 7 |
| Buena Vista | 18°53′, −88°15′ | 2006 | Larva/Pupac | Artificial containers and/or leaf axild | Not specified | 7 |
| Cancúnb | 21°09′, −86°51′ | 1972–75 | Adult | Human bait | Urban, Forest edge | 5 |
| Chunyaxchen | 20°04′, −87°37′ | 2006 | Larva/Pupac | Artificial containers and/or leaf axild | Not specified | 7 |
| Ejido Lázaro Cárdenas | 18°46′, −88°30′ | 2006 | Larva/Pupac | Artificial containers and/or leaf axild | Not specified | 7 |
| Limones | 19°02′, −88°07′ | 2006 | Larva/Pupac | Artificial containers and/or leaf axild | Not specified | 7 |
| Ocom | 19°28′, −88°03′ | 2006 | Larva/Pupac | Artificial containers and/or leaf axild | Not specified | 7 |
| San Miguel de Cozumelb | 20°30′, −86°57′ | 1965 | Larva | Flower pot | Cemetery, Homes | 1 |
| Vigía Chico | 19°36′, −88°00′ | 2006 | Larva/Pupac | Artificial containers and/or leaf axild | Not specified | 7 |
| Belize – Cayo District | ||||||
| Caves Branchb | 17°08′, −88°42′ | 1967 | Adult | Human bait | Forest | 3−4 |
| Chiquibil Rd | 17°07′, −88°58′ | 1990, 1991 | Larva/Pupac | Plastic bottle | Forest | 6 |
| Roaring Creekb | 17°16′, −88°48′ | 1967 | Adult | Human bait | Forest | 3−4 |
| San Antoniob | 17°05′, −89°01′ | 1967 | Adult | Human bait | Forest | 3−4 |
| Belize – Toledo District | ||||||
| Aguacate Creek | 16°09′, −89°05′ | 1992 | Larva/Pupac | Plastic bottle | Forest | 6 |
1, Diaz Najera 1966; 2, Berlin 1969; 3, Bertram 1971; 4, Heinemann and Belkin 1977a; 5, Pletsch 1977; 6, Pecor et al. 2002; 7, Ortega-Morales et al. 2010a; 8, Present study;
Approximate coordinates were determined, based on the description of the collection locations, using Google Earth (Google, Mountain View, CA);
Stage of immatures (larva or pupa) collected not given;
Collection source for Ae. cozumelensis was not specified by artificial container versus natural habitat (rock hole, leaf axils, fallen leaves).
Our knowledge of the biology of Ae. cozumelensis is very restricted. The mosquito has been recovered from both urban areas (Pletsch 1977) and rural settings (Bertram 1971). It exploits artificial containers as development sites for the immature stages (Diaz Najera 1966,, Pecor et al. 2002,, Ortega-Morales et al. 2010a) but also can be found in natural water-holding substrates such as leaf axils (Ortega-Morales et al. 2010a). Adults can be collected using human bait (Pletsch 1977,, Bertram 1971,, Heinemann and Belkin 1977a), which suggests that the females are potential human-biters. The primary activity period, as determined by landing catches on humans, appears to be diurnal (Bertram 1971).
There are two key areas in which Ae. cozumelensis may be of importance in the context of medical entomology. Firstly, it may compete with container-inhabiting arbovirus vectors, such as Aedes (Stegomyia) aegypti (L.) and Aedes (Stegomyia) albopictus (Skuse), for larval development sites. There is a rich literature on the effects of competition between immatures of Ae. aegypti and Ae. albopictus (e.g., Macdonald 1956,, Black et al. 1989,, Leisnham et al. 2009,, Reiskind and Lounibos 2009). In some settings, Ae. albopictus immatures can, under certain circumstances, outcompete Ae. aegypti leading to reductions in the abundance of the latter species (Juliano 1998,, Braks et al. 2004,, Juliano et al. 2004,, Lounibos et al. 2010). It therefore is interesting to speculate on the potential effects of a third container-inhabiting Aedes species competing for larval development sites with these two important arbovirus vectors. The primary dengue virus vector Ae. aegypti is ubiquitous in urban settings in the Yucatán Peninsula and Belize, and Ae. albopictus, which also can transmit dengue virus, recently was recorded from Cancún in the Yucatán Peninsula as well as from Belize (Ortega-Morales et al. 2010b, Salomón-Grajales et al. 2012). The geographic range of Ae. cozumelensis thus overlaps with those of Ae. aegypti and Ae. albopictus. Moreover, Ae. cozumelensis previously was collected together with Ae. aegypti from artificial containers in Quintana Roo State in the eastern part of the Yucatán Peninsula (Ortega-Morales et al. 2010a).
Secondly, we cannot rule out the possibility that Ae. cozumelensis bites humans as well as domestic or wild animals, and thus may serve as a vector of known or yet to be discovered arboviruses in the Yucatán Peninsula or Belize. Dengue is a major health problem in the Yucatán Peninsula (Loroño-Pino et al. 1993,, 2004;; García-Rejón et al. 2008), and recent studies in this area revealed the presence of other mosquito-borne viruses (e.g., Cache Valley virus, Cholul virus, Kairi virus, South River virus, T’Ho virus, and West Nile virus) with known or potential human health relevance but poorly understood local enzootic transmission cycles (Farfán-Ale et al. 2004,, 2006,, 2009,, 2010;; Soto et al. 2009;; Blitvich et al. 2012a, b). Moreover, the recognition of mosquito species potentially serving as secondary vectors can change our perception of virus transmission cycles. For instance, transmission dynamics of dengue virus may differ between areas where Ae. aegypti is the sole vector compared to areas where other, secondary vectors also are involved. Salomón-Grajales et al. (2012) speculated that the introduction of Ae. albopictus to the Yucatán Peninsula could result in dengue virus transmission being intensified in rural areas because Ae. albopictus is more likely to occur in this setting compared to Ae. aegypti, which more commonly is encountered in densely populated urban areas in the Americas (Braks et al. 2003). The association of Ae. cozumelensis with peridomestic environments (Table 1) enhances its potential to serve as a vector to humans of pathogenic organisms and underscores the importance of future studies to determine the potential importance of this species as a pathogen vector.
Our previous studies on mosquito immatures and adults in Yucatán State, with collection of immatures from artificial containers and indoor/outdoor backpack aspiration of adults, focused on urban settings within the city of Mérida and did not produce any specimens of Ae. cozumelensis (García-Rejón et al. 2008,, 2011a, b). However, during 2010–2011 we visited a number of other communities in different parts of Yucatán State to recover immatures of Ae. aegypti from artificial containers for rearing to adults and subsequent insecticide resistance profiling. We report here on the collection of Ae. aegypti and Ae. cozumelensis from these communities in Yucatán State, and present a summary of published collection records for Ae. cozumelensis.
MATERIALS AND METHODS
This study included 26 communities located in different parts of Yucatán State in southeastern México (Table 2, Figure 2). The population size of the examined communities ranged from <2,000 to >750,000 (Table 2). Collections of mosquito immatures, primarily from artificial containers, were undertaken in these communities from March 2010 – February 2011 to provide material for a state-wide survey of the presence and frequency of a mutation (Ile1,016) in the voltage-gated sodium channel of Ae. aegypti that confers knockdown resistance to synthetic pyrethroid insecticides. Yucatán State has a subtropical climate with seasonal heavy rainfall from June-October (typically >100 mm rainfall per month) and sporadic rainfall during the remaining, drier part of the year (García-Rejón et al. 2011b). With the two notable exceptions of the city of Mérida (where immatures were collected continuously during the March 2010 – February 2011 study period) and Ciudad Caucel (where collections were made during the wet season from August-October 2010), collections in the remaining 24 communities were made during the drier part of the year from November 2010 – February 2011. Monthly rainfall for this 4-mo period ranged from 5–19 mm in the city of Mérida (based on data from a weather station at the Mérida airport operated by Comision Nacional del Agua).
Table 2.
Collections of mosquito immatures from homes and cemeteries in communities inYucatán State, March 2010 – February 2011.
| Community | Approximate coordinates |
Population sizea |
Collection environmentb |
Collection period |
Source of collection | Number collected | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ae. aegypti | Ae. cozumelensis | Other speciesc | |||||||||
| Larvae | Pupae | Larvae | Pupae | Larvae | Pupae | ||||||
| Cantamayec | 20°28.23', −89°04.89' | 1,695 | Rural | Jan 2011 | Artificial containers | 260 | 15 | 0 | 0 | 160 | 0 |
| Rock holes | 20 | 0 | 15 | 0 | 0 | 0 | |||||
| Chemax | 20°39.56′, −87°56.38′ | 14,885 | Rural | Dec 2010 | Artificial containers | 210 | 20 | 0 | 0 | 150 | 20 |
| Ciudad Caucel | Multiple sites within Ciudad Cauceld | 6,988 | Urban | Aug–Oct 2010 | Artificial containers | 2,525 | 81 | 0 | 0 | 200 | 15 |
| Hoctun | 20°51.92′, −89°12.59′ | 4,686 | Rural | Jan 2011 | Artificial containers | 310 | 16 | 14 | 0 | 200 | 0 |
| Huhi | 20°43.87', −89°09.94' | 4,745 | Rural | Jan 2011 | Artificial containers | 220 | 15 | 10 | 0 | 150 | 0 |
| Hunucma | 21°01.27′, −89°53.01′ | 24,910 | Rural | Nov 2010 | Artificial containers | 250 | 30 | 0 | 0 | 200 | 30 |
| Rock holes | 30 | 5 | 0 | 0 | 0 | 0 | |||||
| Kanasin | 20°56.24′, −89°33.74′ | 7,724 | Rural | Nov 2010 | Artificial containers | 200 | 30 | 0 | 0 | 150 | 20 |
| Maxcanu | 20°35.15', −90°00.15' | 12,621 | Rural | Nov 2010 | Artificial containers | 330 | 25 | 0 | 0 | 200 | 30 |
| Mayapan | 20°27.83′, −89°13.23′ | 3,263 | Rural | Jan 2011 | Artificial containers | 330 | 20 | 10 | 0 | 400 | 0 |
| Mérida | Multiple sites within Méridad | 777,615 | Urbane | Mar 2010–Feb 2011 | Artificial containers | 6,990 | 555 | 0 | 0 | 1,063 | 286 |
| Rock or tree holes | 80 | 15 | 0 | 0 | 0 | 0 | |||||
| Motul | 21°05.29′, −89°17.43′ | 23,240 | Rural | Dec 2010 | Artificial containers | 200 | 30 | 0 | 0 | 200 | 35 |
| Oxkutzcab | 20°18.46′, −89°25.81′ | 23,096 | Mixed | Nov 2010 | Artificial containers | 205 | 15 | 0 | 0 | 150 | 10 |
| Peto | 20°07.21′, −88°55.72′ | 19,821 | Rural | Dec 2010 | Artificial containers | 200 | 20 | 0 | 0 | 150 | 10 |
| Progresso | 21°16.84′, −89°40.67′ | 37,369 | Mixed | Nov 2010 | Artificial containers | 280 | 21 | 0 | 0 | 400 | 100 |
| Sanahcat | 20°46.17′, −89°12.83′ | 1,619 | Rural | Jan 2011 | Artificial containers | 260 | 13 | 20 | 0 | 400 | 20 |
| Rock holes | 30 | 1 | 15 | 0 | 0 | 0 | |||||
| Sudzal | 20°52.24', −88°59.37' | 1,261 | Rural | Feb 2011 | Artificial containers | 280 | 21 | 10 | 0 | 200 | 20 |
| Tekax | 20°11.80′, −89°16.65′ | 25,751 | Rural | Dec 2010 | Artificial containers | 300 | 35 | 0 | 0 | 100 | 20 |
| Telchac Puerto | 21°20.36′, −89°15.56′ | 1,722 | Rural | Jan 2011 | Artificial containers | 320 | 15 | 0 | 0 | 195 | 15 |
| Tepakan | 21°02.94′, −89°02.62′ | 2,064 | Rural | Jan 2011 | Artificial containers | 240 | 25 | 10 | 0 | 0 | 0 |
| Teya | 21°03.05', −89°04.43' | 1,975 | Rural | Jan 2011 | Artificial containers | 250 | 16 | 10 | 0 | 200 | 0 |
| Ticul | 20°23.84′, −89°32.71′ | 32,796 | Mixed | Nov 2010 | Artificial containers | 250 | 25 | 0 | 0 | 180 | 30 |
| Tinum (Piste) | 20°45.99', −88°23.52' | 2,111 | Rural | Dec 2010 | Artificial containers | 200 | 20 | 0 | 0 | 150 | 20 |
| Tizimin | 21°08.75', −88°08.99' | 46,971 | Mixed | Nov 2010 | Artificial containers | 330 | 33 | 0 | 0 | 200 | 35 |
| Tree holes | 40 | 5 | 0 | 0 | 0 | 0 | |||||
| Valladolid | 20°41.53′, −88°13.34′ | 48,973 | Mixed | Nov 2010 | Artificial containers | 230 | 27 | 0 | 0 | 150 | 30 |
| Uman | Multiple sites within Umand | 39,611 | Mixed | Nov 2010 – Feb 2011 | Artificial containers | 990 | 91 | 0 | 0 | 280 | 80 |
| Xocchel | 20°49.94′, −89°11.39′ | 3,229 | Rural | Jan 2011 | Artificial containers | 195 | 16 | 15 | 0 | 100 | 0 |
Based on data for 2010 obtained from Mexico’s Instituto Nacional de Estadística Geografía e Informática;
Rural, Urban or Mixed rural and urban;
Predominantly Culex spp. with sporadic collections of Aedes (Ochlerotatus) taeniorhynchus and Toxorhynchites spp.;
General coordinates for the cities: 20°59.75', −89°41.82' (Ciudad Caucel), 20°58.20', −89°37.20' (Mérida), and 20°52.91', −89°44.81' (Uman);
Predominantly urban environment with a few rural collection sites at the edges of the city.
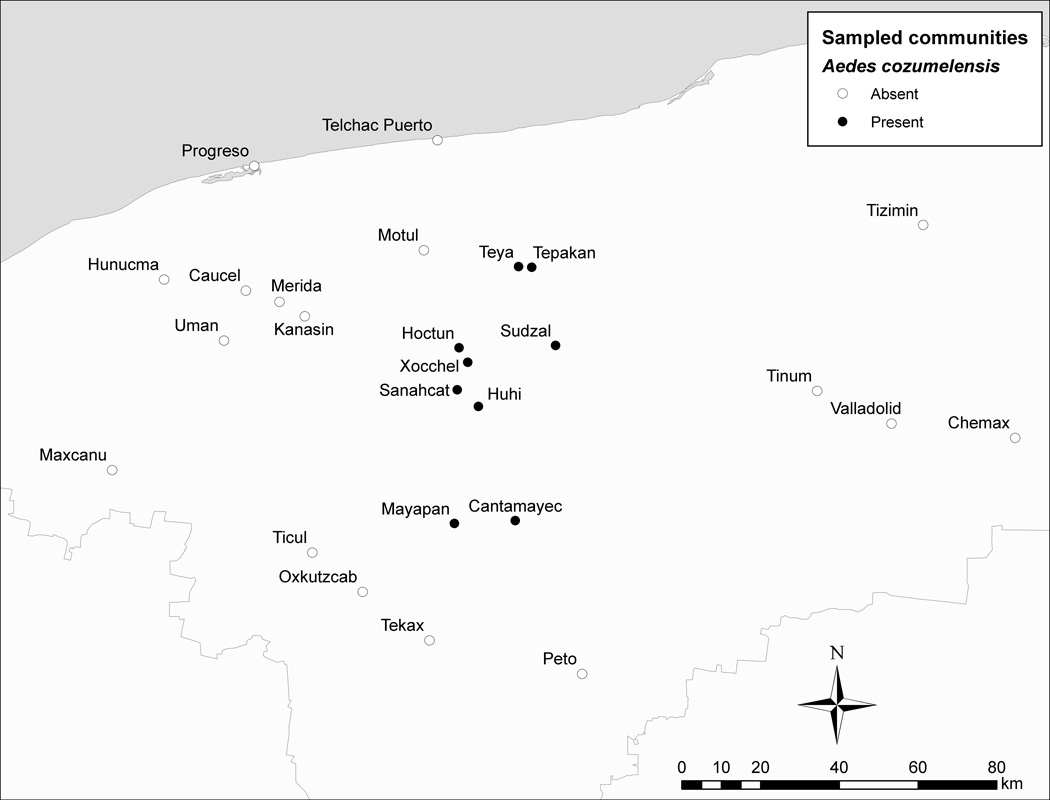
Figure 2.
Mosquito collection locations in Yucatán State from March 2010 – February 2011. All 26 collection locations produced Ae. aegypti. Locations from which both Ae. aegypti and Ae. cozumelensis were collected are denoted by black circles (●); locations producing Ae. aegypti but not Ae. cozumelensis are shown as hollow circles (○). Tizimin yielded Ae. cozumelensis in a previous study (Berlin 1969) but not in this one. See Table 2 for more detailed information about the collections.
Specific collection locations within the communities were subjectively classified as urban (homes with small yards or cemeteries located within densely populated areas) versus rural (homes in rural settings with larger yards and more vegetation, or homes or cemeteries located on the edge of a populated area). The mosquito collection locations were georeferenced using a Global Positioning System receiver (Garmin, Olathe, KS) and mapped in a Geographic Information System (ArcGIS 9.2; ESRI, Redlands, CA).
Mosquito immatures were collected from a wide range of artificial containers encountered on residential premises (including small containers such as bottles, cans and flower pots, medium-sized containers such as buckets and tires, and larger containers such as cement water troughs and water tanks) or in cemeteries (including flower vases, bottles, cans, buckets, and water storage tanks). Immatures also were occasionally recovered from “natural containers” including rock holes and tree holes. Because the purpose of the mosquito collection was to gather sufficient numbers of specimens of Ae. aegypti for insecticide resistance profiling, no specific efforts were made to collect all immatures present in the collection locations or to determine the overall abundance of immatures.
The collected immatures were transported to a laboratory at Universidad Autónoma de Yucatán in Mérida, and all Aedes immatures were reared to the adult stage. During the subsequent species identification, we discovered that some of the adult Aedes specimens belonged to a species not previously encountered by us in Yucatán State. Selected specimens were shipped to the Walter Reed Biosystematics Unit (WRBU) of the Walter Reed Army Institute of Research, United States Army Medical Research and Materiel Command, Silver Spring, MD, for identification. This confirmed their identity as Aedes (Howardina) cozumelensis (courtesy of James Pecor of WRBU) based on the identification key for the neotropical subgenus Howardina of Aedes published by Berlin (1969). Subsequent species identification was done at Universidad Autónoma de Yucatán using the identification key of Berlin (1969) together with the identification key of Darsie and Ward (2005).
RESULTS
All 26 examined communities in Yucatán State, ranging from very small rural communities to large urban centers, yielded at least 200 Ae. aegypti immatures (Table 2, Figure 2). From nine of these communities (Cantamayec, Hoctun, Huhi, Mayapan, Sanahcat, Sudzal, Tepakan, Teya, and Xocchel), we also collected immatures of Ae. cozumelensis (Figures 1–2; Table 2). The communities from which Ae. cozumelensis were collected were all small, rural communities (<6,000 inhabitants) located in the north-central part of Yucatán State (Figure 2, Table 2). Collections of Ae. cozumelensis were made during January–February 2011 and came from residential premises (Cantamayec, Hoctun, Mayapan, Sanahcat, and Tepakan) as well as cemeteries (Hoctun, Huhi, Sudzal, Teya, and Xocchel). Ae. cozumelensis was recovered from rock holes as well as various artificial containers including metal cans, flower vases, buckets, tires and a water storage tank.
The published records for Ae. cozumelensis, which currently is known to occur in México’s Yucatán Peninsula and in Belize, are summarized in Table 1 and the approximate collection locations are shown in Figure 1. The known range of Ae. cozumelensis falls entirely within the larger geographic range of Ae. aegypti in the Americas, and the geographic locations where Ae. cozumelensis has been collected uniformly represents settings where Ae. aegypti can be expected to occur.
DISCUSSION
Our study confirmed that, as expected, the arbovirus vector Ae. aegypti is present throughout Yucatán State. This mosquito was readily collected in large urban centers (Mérida) and medium-sized cities (e.g., Kanasín, Progreso, Tizimín, Umán, and Valladolid) as well as in small towns and rural communities. Another important container-inhabiting arbovirus vector, Aedes (Stegomyia) albopictus, was recently recorded from Cancún, which is located in Quintana Roo State in the eastern part of the Yucatán Peninsula (Salomón-Grajales et al. 2012). Ae. albopictus was not encountered by us in Yucatán State but, should the mosquito become established in Cancún, it likely will spread across the Yucatán Peninsula, similar to its previous rapid spread across the southeastern U.S. (Moore 1999). The potential implications of the introduction of Ae. albopictus into the Yucatán Peninsula for Ae. aegypti bionomics and local dengue virus transmission cycles were reviewed previously by Salomón-Grajales et al. (2012).
A third container-inhabiting Aedes species, Ae. cozumelensis, had previously been recorded from several locations in Quintana Roo State (Diaz Najera 1966,, Pletsch 1977,, Ortega-Morales et al. 2010a) but only from a single location (Tizimín) in Yucatán State (Berlin 1969). We collected Ae. cozumelensis from an additional nine locations in Yucatán State (Cantamayec, Sudzal, Hoctun, Huhi, Mayapan, Sanahcat, Tepakan, Teya, and Xocchel; Figures 1–2), which demonstrates that this mosquito has a far broader geographic range in the Yucatán Peninsula than previously known. Our collections of Ae. cozumelensis all came from small rural communities (<6,000 inhabitants) in the north-central part of Yucatán State, located between the urban centers of Mérida to the west and Tizimín and Valladolid to the east. Because collections were made during the drier part of the year, when the availability of water-holding containers is most limited and abundance of container-inhabiting mosquitoes presumably is reduced, we speculate that further sampling during the wet season may reveal the presence of Ae. cozumelensis in additional locations in Yucatán State. The exception in this regard is the city of Mérida, where extensive collections of immatures during the rainy season have failed to produce Ae. cozumelensis (Table 2; García-Rejón et al. 2008,, 2011b). The collections of Ae. cozumelensis produced larvae but no pupae (Table 2). This could have resulted from the low overall number of Ae. cozumelensis specimens collected (~130 larvae) or from pupae of this species behaving in such a way that they evade capture during cursory surveys where no special efforts are made to ensure that all specimens are removed from the containers.
The new records for Ae. cozumelensis in Yucatán State, and the co-occurrence with Ae. aegypti in small rural communities, poses intriguing questions regarding linkages between these mosquitoes, including the potential for direct competition for larval development sites, feeding by both species on human blood, and transmisson of arboviruses. Indeed, the geographic locations where Ae. cozumelensis has been collected (Yucatán State and Quintana Roo State in México’s Yucatán Peninsula, and the neighboring Belize to the south; Figure 1, Table 1) uniformly represents areas where Ae. aegypti can be expected to occur (Table 2; Heinemann and Belkin 1977a, b; Pletsch 1986;; Ortega-Morales et al. 2010a). The manner in which immatures were collected in our study unfortunately precludes a quantitative analysis of the respective abundance of Ae. aegypti and Ae. cozumelensis in containers where they occurred separately versus together, but this should be addressed in future studies.
Aedes cozumelensis has been collected from human bait and thus may take blood from humans (Pletsch 1977,, Bertram 1971,, Heinemann and Belkin 1977a). We speculate that Ae. cozumelensis potentially could serve as a vector for arboviruses to humans in rural areas of the Yucatán Peninsula. As a case in point, another member of the Aedes subgenus Howardina, Ae. bahamensis, is capable of transmitting St. Louis encephalitis virus (Shroyer 1991). Future studies are needed to determine how commonly Ae. cozumelensis feeds on human blood and whether it is naturally infected with arboviruses or other pathogens of medical or veterinary importance.
ACKNOWLEDGEMENTS
We thank Carlos Estrella-Macias of Universidad Autónoma de Yucatán for technical assistance, and James Pecor of the Walter Reed Biosystematics Unit of the Walter Reed Army Institute of Research, United States Army Medical Research and Materiel Command, Silver Sping, MD, for assistance with mosquito identification. The study was supported by the Innovative Vector Control Consortium and by the National Institutes of Health/National Institute of Allergy and Infectious Diseases (International Collaborations in Infectious Disease Research Program U01-AI-088647). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAID or NIH.
REFERENCES CITED
- Berlin OGW. Mosquito studies (Diptera, Culicidae). XII. A revision of the neotropical subgenus Howardina of Aedes. Contr. Am. Entomol. Inst. 1969;4(2):1–190. [Google Scholar]
- Bertram DS. Mosquitoes of British Honduras, with some comments on malaria, and on arbovirus antibodies in man and equines. Trans. R. Soc. Trop. Med. Hyg. 1971;65:742–762. doi: 10.1016/0035-9203(71)90089-7. [DOI] [PubMed] [Google Scholar]
- Black IV WC, Rai KS, Turco BJ, Arroyo DC. Laboratory study of competition between United States strains of Aedes albopictus and Aedes aegypti (Diptera: Culicidae) J. Med. Entomol. 1989;26:260–271. doi: 10.1093/jmedent/26.4.260. [DOI] [PubMed] [Google Scholar]
- Blitvich BJ, Staley M, Loroño-Pino MA, García-Rejón JE, Farfán-Ale JA, Dorman KS. Identification of a novel subtype of South River virus (family Bunyaviridae) Arch. Virol. 2012a doi: 10.1007/s00705-012-1280-4. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitvich BJ, Saiyasombat R, Dorman KS, García-Rejón JE, Farfán-Ale JA, Loroño-Pino MA. Sequence and phylogenetic data indicate that an orthobunyavirus recently detected in the Yucatan Peninsula of Mexico is a novel reassortant of Potosi and Cache Valley viruses. Arch. Virol. 2012b doi: 10.1007/s00705-012-1279-x. In Press. [DOI] [PubMed] [Google Scholar]
- Braks MAH, Honorio NA, Lourenco-De-Oliveira R, Juliano SA, Lounibos LP. Convergent habitat segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in southeastern Brazil and Florida. J. Med. Entomol. 2003;40:785–794. doi: 10.1603/0022-2585-40.6.785. [DOI] [PubMed] [Google Scholar]
- Braks MAH, Honorio NA, Lounibos LP, Lourenco-De-Oliveira R, Juliano SA. Interspecific competition between two invasive species of container mosquitoes, Aedes aegypti and Aedes albopictus (Diptera: Culicidae), in Brazil. Ann. Entomol. Soc. Am. 2004;97:130–139. [Google Scholar]
- Darsie RF, Jr, Ward RA. Identification and geographical distribution of the mosquitoes of North America, North of Mexico. Gainesville, FL: University Press of Florida; 2005. [Google Scholar]
- Diaz Najera A. Aedes de México. Descripción de una nueva especie del subgénero Howardina (Diptera: Culicidae) Rev. Inst. Salud Publ. (Méx.) 1966;26:331–344. [PubMed] [Google Scholar]
- Farfán-Ale JA, Blitvich BJ, Loroño-Pino MA, Marlenee NL, Rosado-Paredes EP, García-Rejón JE, Flores-Flores LF, Chulim-Perera L, Lopez-Uribe M, Perez-Mendoza G, Sanchez-Herrera I, Santamaria W, Moo-Huchim J, Gubler DJ, Cropp BC, Calisher CH, Beaty BJ. Longitudinal studies of West Nile virus infection in avians, Yucatan State, Mexico. Vector-Borne and Zoonotic Dis. 2004;4:3–14. doi: 10.1089/153036604773082942. [DOI] [PubMed] [Google Scholar]
- Farfán-Ale JA, Blitvich BJ, Marlenee NL, Loroño-Pino MA, Puerto-Manzano F, García-Rejón JE, Rosado-Paredes EP, Flores-Flores LF, Ortega-Salazar A, Chavez-Medina J, Cremieux-Grimaldi JC, Correa-Morales F, Hernandez-Gaona G, Mendez-Galvan JF, Beaty BJ. Antibodies to West Nile virus in asymptomatic mammals, birds, and reptiles in the Yucatan Peninsula of Mexico. Am. J. Trop. Med. Hyg. 2006;74:908–914. [PubMed] [Google Scholar]
- Farfán-Ale JA, Loroño-Pino MA, García-Rejón JE, Hovav E, Powers AM, Lin M, Dorman KS, Platt KB, Bartholomay LC, Soto V, Beaty BJ, Lanciotti RS, Blitvich BJ. Detection of RNA from a novel West Nile-like virus and high prevalence of an insect-specific flavivirus in mosquitoes in the Yucatan Peninsula of Mexico. Am. J. Trop. Med. Hyg. 2009;80:85–95. [PMC free article] [PubMed] [Google Scholar]
- Farfán-Ale JA, Loroño-Pino MA, García-Rejón JE, Soto V, Lin M, Staley M, Dorman KS, Bartholomay LC, Hovav E, Blitvich BJ. Detection of flaviviruses and orthobunyaviruses in mosquitoes in the Yucatan Peninsula of Mexico in 2008. Vector-Borne Zoonotic Dis. 2010;10:777–783. doi: 10.1089/vbz.2009.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Rejón J, Loroño-Pino MA, Farfán-Ale JA, Flores-Flores L, Rosado-Paredes ED, Rivero-Cardenas N, Najera-Vazquez R, Gomez-Carro S, Lira-Zumbardo V, Gonzalez-Martinez P, Lozano-Fuentes S, Elizondo-Quiroga D, Beaty BJ, Eisen L. Dengue virus-infected Aedes aegypti in the home environment. Am. J. Trop. Med. Hyg. 2008;79:940–950. [PubMed] [Google Scholar]
- García-Rejón JE, Loroño-Pino MA, Farfán-Ale JA, Flores-Flores LF, Lopez-Uribe MP, Najera-Vazquez MD, Nuñez-Ayala G, Beaty BJ, Eisen L. Mosquito infestation and dengue virus infection in Aedes aegypti females in schools in Merida, Mexico. Am. J. Trop. Med. Hyg. 2011a;84:489–496. doi: 10.4269/ajtmh.2011.10-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Rejón JE, López-Uribe MP, Loroño-Pino MA, Farfán-Ale JA, Najera-Vazquez MR, Lozano-Fuentes S, Beaty BJ, Eisen L. Productive container types for Aedes aegypti immatures in Mérida, México. J. Med. Entomol. 2011b;48:644–650. doi: 10.1603/me10253. [DOI] [PubMed] [Google Scholar]
- Heinemann SJ, Belkin JN. Collection records of the project “Mosquitoes of Middle America”. 8. Central America: Belize (BH), Guatemala (GUA), El Salvador (SAL), Honduras (HON), Nicaragua (NI, NIC) Mosq. Syst. 1977a;9:403–454. [Google Scholar]
- Heinemann SJ, Belkin JN. Collection records of the project “Mosquitoes of Middle America”. 9. Mexico (MEX, MF, MT, MX) Mosq. Syst. 1977b;9:483–535. [Google Scholar]
- Juliano SA. Species introduction and replacement among mosquitoes: Interspecific resource competition or apparent competition? Ecology. 1998;79:255–268. [Google Scholar]
- Juliano SA, Lounibos LP, O'Meara GF. A field test for competitive effects of Aedes albopictus on A. aegypti in South Florida: Differences between sites of coexistence and exclusion? Oecologia. 2004;139:583–593. doi: 10.1007/s00442-004-1532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisnham PT, Lounibos LP, O'Meara GF, Juliano SA. Interpopulation divergence in competitive interactions of the mosquito Aedes albopictus. Ecology. 2009;90:2405–2413. doi: 10.1890/08-1569.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loroño-Pino MA, Farfán-Ale JA, Rosado-Paredes EP, Kuno G, Gubler DJ. Epidemic dengue 4 in the Yucatan, Mexico, 1984. Rev. Inst. Med. Trop. Sao Paulo. 1993;35:449–455. doi: 10.1590/s0036-46651993000500011. [DOI] [PubMed] [Google Scholar]
- Loroño-Pino MA, Farfán-Ale JA, Zapata-Peraza AL, Rosado-Paredes EP, Flores-Flores LF, García-Rejón JE, Diaz FJ, Blitvich BJ, Andrade-Narvaez M, Jimenez-Rios E, Blair CD, Olson KE, Black IV WC, Beaty BJ. Introduction of the American/Asian genotype of dengue 2 virus into the Yucatan State of Mexico. Am. J. Trop. Med. Hyg. 2004;71:485–492. [PubMed] [Google Scholar]
- Lounibos LP, O'Meara GF, Juliano SA, Nishimura N, Escher RL, Reiskind MH, Cutwa M, Greene K. Differential survivorship of invasive mosquito species in South Florida cemeteries: Do site-specific microclimates explain patterns of coexistence and exclusion? Ann. Entomol. Soc. Am. 2010;103:757–770. doi: 10.1603/AN09142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald WW. Aedes aegypti in Malaya. II. Larval and adult biology. Ann. Trop. Med. Parasit. 1956;50:399–414. [PubMed] [Google Scholar]
- Moore CG. Aedes albopictus in the United States: Current status and prospects for further spread. J. Am. Mosq. Contr. Assoc. 1999;15:221–227. [PubMed] [Google Scholar]
- Ortega-Morales AI, Mis-Avila P, Elizondo-Quiroga A, Harbach RE, Siller-Rodríguez QK, Fernández-Salas I. The mosquitoes of Quintana Roo State, Mexico (Diptera: Culicidae) Acta Zool. Mex. 2010a;26:36–46. [Google Scholar]
- Ortega-Morales AI, Mis-Avila P, Dominguez-Galera M, Canul-Amaro G, Esparza-Aguilar J, Carlos-Azueta J, Badillo-Perry S, Marin P, Polanco J, Fernández-Salas I. First record of Stegomyia albopicta (Aedes albopictus) in Belize. Southw Entomol. 2010b;35:197–198. [Google Scholar]
- Pecor JE, Harbach RE, Peyton EL, Roberts DR, Rejmankova E, Manguin S, Palanko J. Mosquito studies in Belize, Central America: Records, taxonomic studies, and a checklist of species. J. Am. Mosq. Contr. Assoc. 2002;18:241–276. [PubMed] [Google Scholar]
- Pletsch DJ. Mosquito investigation and control in tourist development areas in Mexico: III. Species, densities and associations of mosquitoes found in Cancun, Quintana Roo from December, 1972 through December, 1975. Mosq. News. 1977;37:222–226. [Google Scholar]
- Pletsch DJ. A comparison of faunal lists of mosquito species in Florida, in Cuba and in the State of Quintana Roo, Yucatan Peninsula, Mexico. J Florida Anti-Mosquito Assoc. 1986;57:29–32. [Google Scholar]
- Reiskind MH, Lounibos LP. Effects of intraspecific larval competition on adult longevity in the mosquitoes Aedes aegypti and Aedes albopictus. Med. Vet. Entomol. 2009;23:62–68. doi: 10.1111/j.1365-2915.2008.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomón-Grajales J, Lugo-Moguel GV, Tinal-Gordillo VR, de La Cruz-Velázquez J, Beaty BJ, Eisen L, Lozano-Fuentes S, Moore CG, García-Rejón JE. Aedes albopictus mosquitoes, Yucatan Peninsula, Mexico. Emerg. Infect. Dis. 2012;18:525–527. doi: 10.3201/eid1803.111626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroyer DA. Preliminary studies of Aedes bahamensis as a host and potential vector of St. Louis encephalitis virus. J. Am. Mosq. Contr. Assoc. 1991;7:63–65. [PubMed] [Google Scholar]
- Soto V, Dorman KS, Miller WA, Farfán-Ale JA, Loroño-Pino MA, García-Rejón JE, Blitvich BJ. Complete nucleotide sequences of the small and medium RNA genome segments of Kairi virus (family Bunyaviridae) Arch. Virol. 2009;154:1555–1558. doi: 10.1007/s00705-009-0465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]