Abstract
Intraspecific competition is a key factor shaping space-use strategies and movement decisions in many species, yet how and when neighbors utilize shared areas while exhibiting active avoidance of one another is largely unknown. Here we investigated temporal landscape partitioning in a population of wild baboons (Papio cynocephalus). We used global positioning system (GPS) collars to synchronously record the hourly locations of 5 baboon social groups for ~900 days, and we used behavioral, demographic, and life history data to measure factors affecting use of overlap areas. Annual home ranges of neighboring groups overlapped substantially, as predicted (baboons are considered non-territorial), but home ranges overlapped less when space use was assessed over shorter time scales. Moreover, neighboring groups were in close spatial proximity to one another on fewer days than predicted by a null model, suggesting an avoidance-based spacing pattern. At all time scales examined (monthly, biweekly, and weekly), time spent in overlap areas was greater during time periods when groups fed on evenly dispersed, low-quality foods. The percent of fertile females in social groups was negatively correlated with time spent in overlap areas only during weekly time intervals. This suggests that broad temporal changes in ecological resources are a major predictor of how intensively overlap areas are used, and groups modify these ecologically driven spacing patterns at short time scales based on female reproductive status. Together these findings offer insight into the economics of territoriality by highlighting the dynamics of spacing patterns at differing time scales.
Keywords: biologically informed gas model, home range overlap, landscape partitioning, Papio cynocephalus, territoriality, wild baboons
INTRODUCTION
The home range of an animal is defined as the area it uses in obtaining food and mates, and in caring for young over some specified time period (Burt 1943). By contrast, an animal’s territory is defined as the portion of the home range from which conspecifics are excluded as a consequence of intraspecific competition (Begon et al. 2005). The degree of territoriality exhibited by individuals may thus be measured as the extent to which home ranges do not overlap (e.g., Nemtzov 1997). These definitions of home ranges and territories can apply to both solitary and group-living species, but in the latter case they are determined by the cohesive movements of a social group rather than the movements of an individual.
Across and within populations, the extent of home range overlap between neighboring conspecifics can vary considerably. Theoretical and empirical evidence suggest that the distribution of ecological resources is a fundamental factor influencing this variability. Territorial behavior is adaptive only if the costs of excluding competitors are offset by the benefits of monopolized resource use, i.e. if the defense of a territory is less expensive in terms of fitness than the potential loss of ecological resources to competitors in the absence of defense (Brown 1964). Because spatially and/or temporally clumped ecological resources are more economically defensible than those with an even distribution, territoriality is most commonly observed when ecological resources are clustered in space and time (e.g., Rubenstein 1981; Whitten 1983; Harcourt 1987; Ims 1987; Schoener 1987; Jensen et al. 2005).
It follows conceptually that temporal changes in the availability and distribution of ecological resources will affect the relative costs and benefits of maintaining exclusivity. However, differences in home range overlap across multiple time scales has received relatively little attention except for research focused on comparisons between seasons (e.g., Lambin and Krebs 1991; Gehrt and Fritzell 1998; McLoughlin et al. 2007; Hoset et al. 2008). Thus, we know little about the dynamics of landscape partitioning at finer time intervals. This topic is important because the availability of ecological resources will usually vary considerably within as well as between seasons.
Temporal changes in the availability of sexually receptive partners also affects spacing patterns by modifying the extent to which individual males can defend receptive mates from other males (Emlen and Oring 1977). Yet, to the best of our knowledge, no previous research has evaluated whether variation in the temporal availability of fertile females within multimale-multifemale social groups influences spatial interactions among neighboring groups. Although males may cooperate to exclude extra-group males from access to females in their own group (reviewed in Davies 2000), the effects of temporal changes in the availability of fertile females on landscape partitioning among social groups is poorly understood.
Here we investigate temporal landscape partitioning at the group level in a population of wild baboons (Papio cynocephalus) living in the Amboseli basin of East Africa. Baboons are large, ground-dwelling cercopithecine monkeys. Like other cercopithecines, they live in stable multimale-multifemale social groups of 20–100 individuals (Estes 1991). Males are year-round group residents, though dispersal between groups by individual adult males in search of mating opportunities is common and occurs throughout the year (Alberts and Altmann 1995). Multiple social groups comprise a single population, and the annual home ranges of neighboring groups overlap extensively (e.g., Altmann and Altmann 1970; Shopland 1982).
Baboons do not exhibit seasonality in mating or birth (Altmann 1980; Melnick and Pearl 1987; Bercovitch and Harding 1993; Bentley-Condit and Smith 1997). However, recent environmental conditions can affect reproduction (Alberts et al. 2005). For example, Beehner and colleagues (2006) found that female baboons were less likely to cycle immediately after periods of drought, and Gesquiere and colleagues demonstrated that the reproductive physiology of both females (Gesquiere et al. 2008) and males (Gesquiere et al. 2011) was negatively affected by high ambient temperatures and during dry seasons.
The ensuing peaks in conception and birth likely result from temporal variability in energy availability. Baboons are eclectic omnivores, and both interseasonal and intraseasonal changes in diet have been documented (Norton et al. 1987; S. Altmann 1998; Alberts et al. 2005). In Amboseli, baboons respond to food scarcity by shifting to “fallback foods”, defined as foods that are abundant but require extensive harvesting time relative to the nutrients they provide (Alberts et al. 2005; S. Altmann 2009). Grass corms, the underground stem bases used as storage organs for nutrients and moisture (Esau 1965; Fritsch and Salisbury 1965), are the most notable fallback food for Amboseli baboons. Although evenly dispersed across the landscape and available year-round, grass corms are only consumed in large quantities in the absence of preferred food-types (e.g., flowers and fruits), which are characterized by intraseasonal peaks in their availability (Alberts et al. 2005; S. Altmann 2009). The dietary shift to grass corms results in increased time spent foraging during the period without rainfall (Jun–Oct) relative to “wetter” periods (Nov–May) (Alberts et al. 2005).
Objectives
The main objective of this study was to evaluate how spatial interactions between neighboring baboon groups varied across different time scales. To do so, we first calculated home range overlap and the percent of time pairs of groups spent in overlap areas at 3 time scales: monthly, biweekly (every 2 weeks), and weekly. These “static” measures (sensu Macdonald et al. 1980; Doncaster 1990) are a fundamental expression of the extent of shared space, but do not directly characterize the nature of group-level spacing behavior (neutral, avoidance-based, or attractive). Thus, to gain more insight into the interrelated movements of neighboring groups, we next compared observed encounter rates in overlap regions with those predicted by the ideal gas model, which assumes that groups move independently of one another (Hutchinson and Waser 2007). If groups are attracted to each other, the observed frequency should be higher than the predicted frequency calculated by the ideal gas model; conversely, if groups avoid each other, the observed frequency should be lower than the predicted frequency. The most straightforward version of the ideal gas model also assumes that groups move at constant speeds and that their turning angles are randomly distributed, whereas in reality, travel speed may vary and turning angles may be directionally biased. Despite these simplifications, it has proven to be a valuable null model (Hutchinson and Waser 2007). Lastly, we evaluated how the availability of ecological resources and mates affected the percent of time spent in overlap areas. Extending theories on the relationship between territoriality and relative peaks in ecological resource availability, we hypothesized that the time spent in overlap areas would be greater during periods characterized by low rather than high resource availability. We used the percent time spent feeding as a behavioral assay of food quality; we assumed that increased time spent feeding indicated that the baboons were relying upon evenly distributed but lower quality, non-defensible foods (i.e. grass corms). For temporal availability of mates, we hypothesized that “herding” behavior by males when groups came in spatial proximity would intensify as the percent of females in the high fertility portion of their cycle increased. “Herding” occurs when males closely follow fertile females and drive them away from other groups (Cheney and Seyfarth 1977; Kitchen et al. 2004). This is the group-level analogue of mate-guarding, in which a male keeps his female consort away from other males within his group, a behavior that particularly occurs during the female’s high fertility 5-day periovulatory period (Gesquiere et al. 2007). Because of this hypothesized increase in herding, we predicted that social groups would spend less time in overlap areas as the percent of fertile females relative to the total number of females increases.
METHODS
Since 1971, the study population of wild baboons living in the Amboseli basin of East Africa has been the focus of consistent, year-round behavioral and ecological monitoring as part of the Amboseli Baboon Research Project (ABRP). Alberts and colleagues (2005) provide a thorough description of the ecology in the Amboseli region, with particular emphasis on seasonality (dry vs. “wetter” seasons) within the hydrological year (Nov–Oct). Complete details on ABRP monitoring effort and data collection protocols can be accessed online (http://amboselibaboons.nd.edu/).
We used global positioning system (GPS) collars (model G2110B, Advanced Telemetry Systems Inc. Isanti, MN) to simultaneously monitor the movements of the 5 ABRP study groups. Collars were deployed in 3 sequential rounds (Mar 2008–Jan 2009, Jan 2009–Nov 2009, and Nov 2009–Sep 2010). Each collar deployment lasted approximately 300 days, a time duration based upon manufacturer calculations for expected battery life and ABRP field testing of equipment (Markham and Altmann 2008). For logistical reasons, collar deployment and subsequent retrieval date varied slightly between groups. To avoid analytical complications of asynchronous monitoring, data included in all analyses herein were limited to the time window during which all groups were collared (1 Apr 2008–31 Aug 2010).
Within this time window, each of the 5 study groups was monitored an average of 878 days (± 11.0 SE, range 834–890 days). There was minor intergroup asynchrony in collar monitoring effort such that synchronous data for all pairs of groups ranged from 811–869 days. During the time of field deployment (excluding dates for collar attachment and drop-off), the collars were extremely successful at capturing scheduled GPS locations; average success rate was 98.6% across groups (range: 98.2–98.8%).
In each deployment round, we collared a single individual at a time within each study group and programmed that individual’s collar to record hourly locations during the portion of the day that baboons are active (0600–1900). The time period between 1900 and 0600 the following morning are hours when baboons are usually asleep, and spatial displacement is minimal: in an analysis of GPS collar data from 5,649 paired 1900 and 0600 readings, we found that baboons moved 16.8 ± 0.61 m (mean ± SE) between 1900 and 0600 the following morning. On average, GPS locations used in analyses (N=59462 locations) were captured 47.2 sec (± 0.07 SE) after the targeted time. Spatial precision of the GPS collar locations was high: mean position dilution of precision (PDOP) was 2.73 (± 0.004 SE). Because coordinate data of a single collared individual was used to represent the entire group’s location, we used sex and age-class criteria to select collaring candidates who had a minimal risk of dispersal. We preferentially collared adult females (N=14) because females remain in their natal groups throughout their lives. When no females were suitable candidates at the time of collar deployment, we collared a young subadult male (N=1; average age during collar deployment=6.12 y) who had not yet reached the median age of natal dispersal (8.45 y: Alberts and Altmann 1995). Finally, when neither females nor young subadult males were suitable candidates, we collared an adult male (N=1); duration and stability of his tenure in the group suggested a low probability of imminent dispersal. Observers routinely monitored each study group 2–3 days per week and observed no instances of collared individuals leaving their group during the months of collar deployment. One collared female died 52 d post-deployment (cause of death was not attributable to the collar); we immediately collared another individual in the group to resume the original study design.
Collar deployment required immobilization for collar attachment. We darted each baboon from within 10 m using a blowpipe injected syringe containing Telazol™ (Fort Dodge Animal Health in Fort Dodge, IA, USA) (tiletamine hydrochloride and zolazepam); for details, see Altmann et al. (1993) and Sapolsky and Altmann (1991). Because each collar was equipped with an automated release mechanism pre-programmed to disengage, we did not need to recapture the baboons to retrieve the collar/data except for 1 instance when the collar failed to discharge. Subsequent manufacturer assessment of this collar revealed that the release mechanism fired as programmed, but corrosion of metal parts prevented drop-off. One plausible cause of the corrosion was an infected abrasion on the animal’s neck following an unusually heavy tick-bite infestation.
In addition to using remotely acquired data from the GPS collars, we used ABRP observer-recorded data on demography, reproductive status, and behavior of mature females. All baboons within the study population were individually identifiable by ABRP field researchers, and each of the 5 social groups was the focus of detailed observations several days each week. Consequently, demographic and reproductive data were typically accurate to within a few days. Details on assessing reproductive status and maturational milestones relevant to calculating the number of fertile females are provided by Alberts and Altmann (2005). Similarly, the ABRP focal animal sampling protocols relevant to our time budget analysis are described in Altmann and Muruthi (1988). For the purposes of this investigation, we were interested only in the percent of time spent feeding by mature females, which we calculated as the number of sample points when animals were feeding relative to the total sample points for which any activity was recorded. In all statistical analyses, pairs of groups were the unit of analysis. For each pair of social groups, we averaged the two group-specific values to provide a single measure for that pair for both percent of fertile females and percent time spent feeding.
Home range delineation and overlap
We used the digitized polygon method for home range delineation at each time scale. By this method, autocorrelated locational data are converted to daily travel paths which are, in turn, buffered with an observer-defined distance that reflects the area of influence for the individual or, in social species, group (Pulliainen 1984; Ostro et al. 1999). Home ranges are delineated by merging all buffered daily travel paths within the observer’s time scale of interest (e.g. month, year).
This approach is well suited to our analyses for two key reasons. First, it makes use of the temporal sequence of all locational points collected, thereby recognizing autocorrelated observations as an intrinsic and relevant property of movement data (Nathan et al. 2008; Boyce et al. 2010). Second, it is robust to variability in sample size. Because home ranges assessed over longer time intervals incorporated more locational points than home ranges assessed over shorter intervals, use of the digitized polygon method avoids problems of varying sampling effort present in other methods of home range delineation (Reynolds and Laundré 1990) in our comparisons of home range overlap across multiple time scales.
We used ArcView 3.3 and ArcGIS 9.2 (Environmental Systems Resource Institute Inc., Redlands, CA, USA) and the Xtools extension (Data East, Novosibirsk, Russia) to delineate home ranges and overlap areas for pairs of groups. First, we created daily travel paths by linking all temporally-sequenced GPS positions captured for an animal with straight-lines. We then added a buffer width of 125 m on each side of the travel path to represent the group’s area of influence (250 m). This buffer measure is based upon the expert knowledge gained from long-term observations of baboons in the wild and incorporates an estimate of the average spread of the group as well as the distance at which one group influences the behavior (e.g. increased vigilance, vocalizations) though not necessarily movement of another group. At greater distances, neighboring groups may be within visual range, depending upon the openness of the surrounding habitat, but groups are often relatively unresponsive to each other. Finally, we merged the buffered daily travel paths for each of the time scales to create weekly, biweekly, and monthly home ranges. To account for differences in the number of days in each calendar month, we standardized the duration of each time scale as follows: weeks were defined as 7-day time intervals, biweeks were defined as 14-day intervals (1 biweek = 2 weeks), and months were defined as 28-day intervals (1 month = 2 biweeks = 4 weeks). We began all time intervals on the first day of the month; data from the 29–31 day of each month, when applicable, were excluded from the analyses. Annual home ranges were calculated for the single complete hydrological year of observation (1 Nov 2008–31 Oct 2009).
To quantify percent time spent in overlap areas, we used the “simple ratio index” (reviewed in Cairns and Schwager 1987):
where n1 and n2 refer to the number of records that were within the overlap area, and N1 and N2 refer to the total number of locations (inside and outside the overlap area) that were recorded for Group 1 and Group 2, respectively. The “simple ratio index” reflects two important aspects of space use and movement: (1) it indirectly incorporates the absolute size of the overlap region, and (2) it directly assesses the intensity of use of that area.
Group-level spacing behavior: Testing a random gas model and predictors of time spent in overlap areas
For analyzing encounters between two groups within their overlap area, we used hourly coordinate data from GPS collars to determine both observed and predicted intergroup encounter rates. Intergroup encounters were defined when pairs of groups were within a detection distance (D) of 250 m; as noted above, this distance incorporates both the average group spread and the distance at which one group influences the behavior of another group. To confirm robustness of our findings, we additionally considered detection distances of 500 m and 750 m. Encounter days were defined as days on which a pair of groups had at least 1 hourly encounter. Observed encounter rate (total number of encounter days divided by total days of synchronous observation) was determined from the GPS collar data. Because the baboons moved very little during the nighttime hours (1900–0600), we did not include 0600 hourly readings when determining encounter days; doing so would have resulted in a slight overestimate of encounter days. Predicted encounter rates for each pair of groups were given by ideal gas model equation (Hutchinson and Waser 2007):
where v was the average speed of the pair of groups on days and ρ was density of groups. We only used days on which a group was in the overlap area when computing v, and we assumed constant speed following the most straightforward version of the ideal gas model. We note if we instead assumed variable speed with a Maxwell-Boltzman distribution (Hutchinson and Waser 2007), the predicted encounter rates would deviate even further from observed.
Following Hutchinson and Waser (2007), we calculated ρ as:
where overlap use1 and overlap use2 refer to the proportion of days that Group 1 and Group 2 used the area of overlap, respectively, and area of overlap12 refers to the space shared by both groups during the study period. Days of synchronous observation, v, ρ, and area of overlap were calculated separately for each pair of groups (Table 1). This method of computing density ensures that we account for the fact that the area of overlap between home ranges of groups is partial, and that frequency of usage of overlap area could be different for each group.
Table 1.
Number of synchronous days of observation, mean daily speed (km/day), and home range overlap area (km2) for 10 pairs of baboon social groups from 1 Apr 2008–31 Aug 2010
| Pair of groups | Synchronous days |
Observed encounter days |
Mean speed (km/day) |
Overlap area (km2) |
|---|---|---|---|---|
| Linda-Nyayo | 865 | 64 | 4.48 | 17.6 |
| Linda-Omo | 811 | 18 | 4.85 | 11.3 |
| Linda-Viola | 868 | 43 | 4.74 | 10.7 |
| Linda-Weaver | 862 | 10 | 5.34 | 7.1 |
| Nyayo-Omo | 812 | 86 | 4.88 | 13.6 |
| Nyayo-Viola | 867 | 70 | 4.75 | 9.5 |
| Nyayo-Weaver | 878 | 15 | 5.10 | 8.3 |
| Omo-Viola | 817 | 82 | 5.14 | 18.6 |
| Omo-Weaver | 810 | 75 | 5.52 | 34.2 |
| Viola-Weaver | 866 | 113 | 5.37 | 18.5 |
Statistical analyses
To test whether observed encounter rates differed significantly from rates predicted by the null (gas) model, we used a Wilcoxon signed-ranks test. To test for predictors of the percent time spent in overlap areas, we used linear mixed models. Percent time spent in overlap areas was arcsin-square root transformed, and only time periods during which the home ranges of pairs of groups overlapped were included in this analysis. As fixed effects, we used the average percent time spent feeding and the percent of fertile females. Collinearity between covariates was checked using Pearson’s correlation; following Tabachnick and Fidell (2006), a cutoff criterion of 0.60 was used. We only included time periods in the analyses in which (1) both groups were monitored by the GPS collars for all days within the targeted time period and (2) when at least N min of data on time budgets were available from each group, where N=25, 50, 100 for analyses at the weekly, biweekly, and monthly scales, respectively. This resulted in 188 time periods analyzed at the monthly time scale, 354 time periods analyzed at the biweekly time scale, and 553 time periods analyzed at the weekly time scale. Group pair identity was fitted as a random intercept in all models to account for repeated observations of the same pair of groups (Pinheiro and Bates 2000; Börger et al. 2008), and temporal autocorrelation in sequential indices of the dependent variable was controlled for by a first-order autoregressive structure (Brockwell and Davis 1991; Box et al. 2008). All statistical tests were performed in SPSS 19.0 (SPSS Inc., Chicago, IL, USA). The alpha value for statistical significance was set to 0.05 for all analyses.
RESULTS
The extent of home range overlap varied across time scales, with the extent of overlap declining as time scale became increasingly fine-grained. On a monthly scale, mean (± SE) home range overlap for the 10 pairs of groups was 2.5 km2 (± 0.12); at the biweekly scale, it was 1.4 km2 (± 0.05); and at the weekly scale, it was 0.69 km2 (± 0.02; see also Table 2).
Table 2.
Mean extent of home range overlap (km2) for pairs of groups at monthly, biweekly, and weekly time scales. Error reflects variability within each time scale for a specific pair of groups. Sample sizes indicate number of monthly, biweekly, or weekly time periods included in the analyses for each pair of groups
| Group-pair | Monthly | Biweekly | Weekly | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean ± SE | N | Mean ± SE | N | Mean ± SE | N | |
| Linda-Nyayo | 3.0 ± 0.28 | 27 | 1.7 ± 0.14 | 56 | 0.8 ± 0.06 | 112 |
| Linda-Omo | 1.0 ± 0.21 | 25 | 0.6 ± 0.11 | 52 | 0.3 ± 0.04 | 104 |
| Linda-Viola | 1.5 ± 0.19 | 27 | 0.8 ± 0.10 | 56 | 0.4 ± 0.04 | 112 |
| Linda-Weaver | 0.6 ± 0.17 | 26 | 0.3 ± 0.07 | 55 | 0.2 ± 0.03 | 111 |
| Nyayo-Omo | 2.9 ± 0.17 | 25 | 1.8 ± 0.11 | 52 | 1.0 ± 0.05 | 106 |
| Nyayo-Viola | 1.5 ± 0.12 | 28 | 0.9 ± 0.07 | 57 | 0.4 ± 0.03 | 114 |
| Nyayo-Weaver | 1.1 ± 0.13 | 27 | 0.6 ± 0.07 | 56 | 0.2 ± 0.03 | 114 |
| Omo-Viola | 3.5 ± 0.32 | 25 | 2.0 ± 0.16 | 52 | 1.0 ± 0.07 | 105 |
| Omo-Weaver | 5.5 ± 0.52 | 24 | 3.0 ± 0.20 | 51 | 1.4 ± 0.08 | 105 |
| Viola-Weaver | 4.7 ± 0.31 | 27 | 2.6 ± 0.18 | 56 | 1.3 ± 0.08 | 113 |
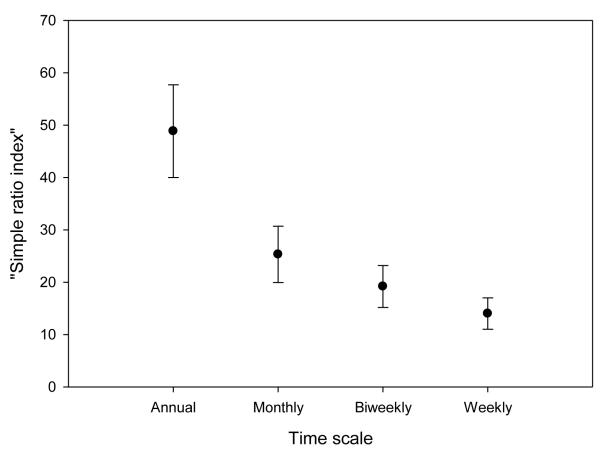
Percent time spent within overlap areas, assessed using the “simple ratio index” (see Methods), also decreased at shorter relative to longer time scales. On a monthly scale (N=261), mean (± SE) percent time spent in overlap areas for the 10 pairs of groups was 28.8% (± 1.30); at the biweekly scale (N=543), it was 22.4% (± 0.81); and at the weekly scale (N=1096) it was 16.3% (± 0.49). Fig. 1 shows the percent time spent in overlap areas for the subset of the data during the single complete hydrological year of our study.
Fig. 1.
Variation in the percent time spent in overlap areas (using the “simple ratio index”) plotted as a function of whether space use was evaluated on an annual, monthly, biweekly, or weekly scale. Points and error bars represent mean ± SE; error reflects variability between pairs of groups. Data depicted were collected between 1 Nov 2008 and 31 Oct 2009 (the Amboseli hydrological year)
Group-level spacing behavior: Testing a random gas model and predictors of time spent in overlap areas
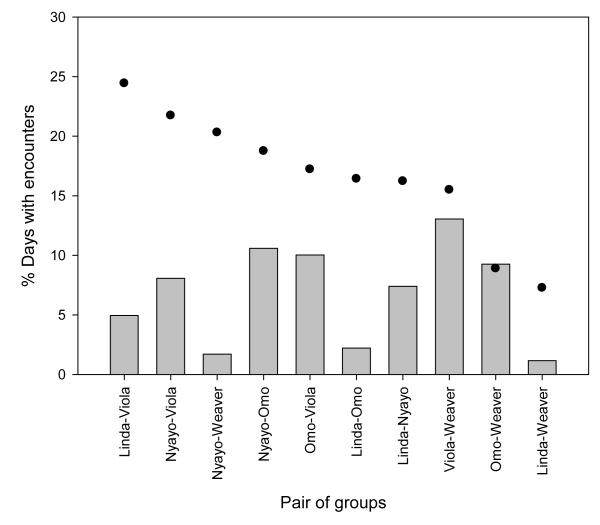
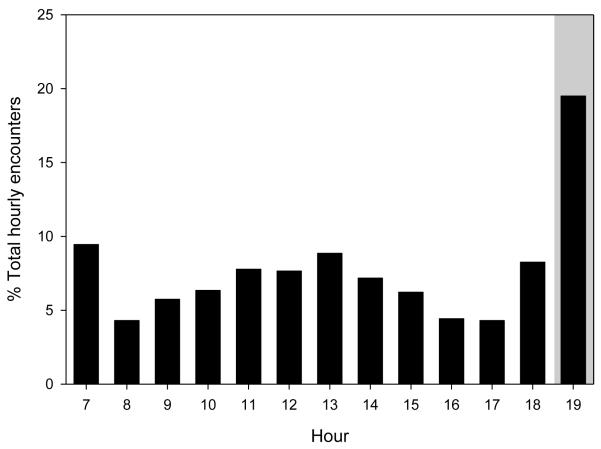
Despite considerable spatial overlap between groups, the mean percent of encounter days (6.8 ± 1.31 SE) was significantly lower than the values predicted by the null model (16.7 ± 1.68; Wilcoxon signed-ranks test: Z=-2.70, N=10, P=0.007; Fig. 2). These findings were consistent when we increased the detection distance to 500 m (Wilcoxon signed-ranks test: Z=-2.60, N=10, P=0.009) and 750 m (Wilcoxon signed-ranks test: Z=-2.60, N=10, P=0.009) to examine the robustness of these results. Most encounter days (67.6%; N=390) had only a single synchronous hourly reading in which one group was within the 250 m detection distance of another group; the number of synchronous readings within the 250 m detection distance of another group on days with multiple encounters ranged from 2–7. Hourly encounters were most common at 1900 (19.5%; N=163), when baboons are typically arboreal in sleeping groves (Fig. 3).
Fig. 2.
Observed (bars) vs. predicted (points) percent of encounter days for each pair of groups. Predicted percent encounter days were calculated using the ideal gas model (Hutchinson and Waser 2007); see text for details
Fig. 3.
Hourly distribution of intergroup encounters; intergroup encounters were defined as occurring when pairs of groups were within of 250 m of each other (measured using GPS coordinate data). Shaded box indicates hours of the day when baboons are usually asleep; non-shaded area indicates hours of the day when baboons are active
Time spent feeding and time spent in overlap areas were significantly positively correlated at the monthly, biweekly and weekly time scales indicating that groups overlapped more when they spent more time feeding. Percent of fertile females was a negative predictor of the time spent in the overlap area, but only at the weekly time scale. Summary statistics of the linear mixed model at the monthly, biweekly, and weekly time scales are given in Table 3.
Table 3.
Effects of ecological (percent time spent feeding) and reproductive (percent adult females in the high-fertility phase) resource availability on the percent time spent in overlap areas at monthly, biweekly, and weekly time scales. Statistically significant results are shown in bold
| Fixed Effects | Estimate | Numerator df |
Denominator df |
F | Sig. |
|---|---|---|---|---|---|
| Monthly | |||||
| Intercept | 0.3715 | 1 | 30.783 | 15.076 | 0.001 |
| Average % fertile females | −0.0008 | 1 | 162.306 | 0.495 | 0.483 |
| Average % feeding time | 0.0050 | 1 | 143.252 | 12.085 | 0.001 |
|
| |||||
| Biweekly | |||||
| Intercept | 0.3876 | 1 | 19.426 | 34.260 | <0.001 |
| Average % fertile females | −0.0011 | 1 | 312.073 | 1.212 | 0.272 |
| Average % feeding time | 0.0027 | 1 | 317.623 | 8.629 | 0.004 |
|
| |||||
| Weekly | |||||
| Intercept | 0.3512 | 1 | 22.659 | 50.116 | <0.001 |
| Average % fertile females | −0.0024 | 1 | 538.352 | 4.267 | 0.039 |
| Average % feeding time | 0.0023 | 1 | 463.120 | 10.560 | 0.001 |
DISCUSSION
Despite considerable home range overlap, the proportion of days during which neighboring groups were in close spatial proximity was much lower than predicted if groups moved independently of each other, suggesting that one or both groups reduced the probability of encounter by active avoidance. How this avoidance was achieved is not yet clear. One possibility is that baboons rely upon visual cues in the relatively open and flat savannah habitat of Amboseli, although we are aware of no quantitative assessment of the visual range for baboons or similar species in comparable habitats. Baboons probably also rely upon auditory signals; indeed, acoustic research on chacma baboons (P. ursinus) has highlighted the potential for long-distance signal transmission of some call-types (Fischer et al. 2002). Olfactory cues may additionally play a role in intergroup communication under some conditions: Hausfater and Meade (1982) hypothesized that odor associated with fecal accumulation beneath sleeping sites may mediate avoidance of sites that have been recently used by other baboon groups (see below).
Daily patterns in the frequency of encounters revealed a higher rate of encounters at 1900, when baboons are typically arboreal in sleeping groves. Sleeping sites are an important ecological resource for baboons because they reduce the risk of attack from nocturnal predators (Altmann and Altmann 1970; Hamilton 1982). In Amboseli, each social group uses multiple groves over time, i.e. groups are “multiple central place foragers” (sensu Chapman et al. 1989; McLaughlin and Montgomerie 1989). However, baboon groups choose among potential sleeping groves selectively – not all available woodlands are used as sleeping sites and frequency of use varies among those that are used (Hamilton 1982). Our finding that groups in Amboseli exhibited avoidance-based spacing overall, with a bias towards proximity when sleeping, suggests that the distribution of sleeping sites constrains spacing behaviors. It follows conceptually that removal of encounters occurring in the context of sleeping sites would result in lower observed encounter rates, thereby strengthening the finding that groups exhibit avoidance-based spacing. Groups may exhibit a forced “tolerance” during evening hours that reflects the limited availability and clustered distribution of desirable sleeping sites. Although mutual occupation of the same or adjacent sleeping sites sometimes occurs, much more commonly a single group monopolizes a sleeping site on a given night and other groups are excluded (ABRP unpubl. data). However, under some conditions such as high predation pressure, nearness to other groups at night may be advantageous. Close group proximity may augment the within-group dilution effect (reviewed in Pulliam and Caraco 1984) by further reducing the probability that any one individual becomes the victim of a successful predator attack. Thus proximity to neighboring groups – in addition to grove-specific attributes (e.g., grove size, canopy height) – may influence sleeping site selection. Whether and when encounters between groups at sleeping sites result in displacement of one group to an alternative and potentially less preferred sleeping site is currently under investigation.
S. Altmann (1974) suggested that choice of a time scale for space use evaluation in baboons is important because the value of an area changes with variations in the ecological and social environment. At all time scales we examined (monthly, biweekly, and weekly), overlap areas were used more intensively during periods characterized by low availability of food. By contrast, as the percent of fertile females in a group increased, overlap with neighboring groups decreased, as predicted, but only at the weekly time scale. These results support the idea that broad temporal changes in ecological resources are strong and consistent predictors of overlap across a considerable range of time scales, but that groups may modify spacing patterns based on reproductive opportunities at short time scales. Our findings thus underscore the importance of discerning biologically appropriate time scales for behavioral analyses: while analyses of movement patterns and occupancy over longer time scales can reveal the effects of resources with relatively constant and stable availability (i.e. some food resources), finer-grained analyses are necessary to capture the effects of resources that can change rapidly in availability (in this case, the number of high fertility females).
Further, our findings have intriguing implications for how home range overlap affects male dispersal patterns. Among primates, evidence suggests that dispersal decisions are influenced both by potential reproductive opportunities (e.g., Cheney and Seyfarth 1983; Olupot and Waser 2001) and extent of home range overlap (e.g., Janmaat et al. 2009). For baboons specifically, Packer (1979) found that most males in his study groups of anubis baboons (P. anubis) in Tanzania dispersed into neighboring groups, and Cheney and Seyfarth (1977) and Kitchen and colleagues (2004) found that resident chacma baboon males in Botswana “herd” cycling females when groups are in close contact, presumably to mobilize fertile females away from nearby groups. In addition, Altmann (2000) and Alberts and Altmann (1995) found that males tended to immigrate into groups with more available females relative to males. Thus, in the short term, intergroup encounters may provide potential dispersing males with the opportunity to identify the group that would provide the most post-dispersal mating opportunities. However, exclusivity may be one tactic used by resident males to reduce competition from immigrants and thus increase fitness.
ACKNOWLEDGMENTS
We are grateful to the government of the Republic of Kenya, to the Kenya Wildlife Services, the staff and wardens of Amboseli National Park, and the local community of the Amboseli region. Tremendous thanks go to ABRP researchers for their contributions to data collection and outstanding dedication in the field: R. Mututua, S. Sayialel, J.K. Warutere, G. Marinka, B. Oyath, and I. Longida. We also thank M. Akinyi, L. Gesquiere, N. Learn, and L. Maryott for their invaluable assistance. T. Garin and Advanced Telemetry Systems, Inc., provided exceptional product support. I. Couzin, A. Dobson, D. Rubenstein, P. Waser, and M. Wikelski commented on an earlier draft of this manuscript. Financial support was provided by American Society of Primatologists (to A.C.M.), Animal Behavior Society (to A.C.M.), International Primatological Society (to A.C.M.), Max Planck Institute of Ornithology (to M. Wikelski), National Institute on Aging (R01AG034513-01 to J.A. and S.C.A.), National Science Foundation (IBN-0322613 to J.A. and S.C.A.; IOS-0919200 to S.C.A.; BCS-0851750 to J.A. and A.C.M.), and Sigma Xi (to A.C.M.).
ETHICAL STANDARDS All project protocols complied with regulations in Kenya (Republic of Kenya Research Permits NCST/5/002/R/776 to J.A. and NCST/5/002/R/777 to S.C.A.) and in the United States (Princeton University IACUC 1649).
REFERENCES
- Alberts SC, Altmann J. Balancing costs and opportunities: Dispersal in male baboons. Am Nat. 1995;145:279–306. [Google Scholar]
- Alberts SC, Hollister-Smith J, Mututua RS, Sayialel SN, Muruthi PM, Warutere JK, Altmann J. Seasonality and long-term change in a savanna environment. In: Brockman DK, van Schaik CP, editors. Seasonality in primates: Studies of living and extinct human and non-human primates. Cambridge University Press; Cambridge: 2005. pp. 157–195. [Google Scholar]
- Altmann J. Baboon mothers and infants. Harvard University Press; Cambridge: 1980. [Google Scholar]
- Altmann J. Predicting male distribution among primate groups. In: Kappeler P, editor. Primate males: Causes and consequences of variation in group composition. Cambridge University Press; NY: 2000. pp. 236–247. [Google Scholar]
- Altmann J, Muruthi P. Differences in daily life between semiprovisioned and wild-feeding baboons Am J Primatol. 1988;15:213–221. doi: 10.1002/ajp.1350150304. [DOI] [PubMed] [Google Scholar]
- Altmann J, Schoeller D, Altmann SA, Muruthi P, Sapolsky RM. Body size and fatness of free-living baboons reflect food availability and activity levels. Am J Primatol. 1993;30:149–161. doi: 10.1002/ajp.1350300207. [DOI] [PubMed] [Google Scholar]
- Altmann SA. Baboons, space, time, and energy. Am Zool. 1974;14:221–248. [Google Scholar]
- Altmann SA. Foraging for survival: Yearling baboons in Africa. University of Chicago Press; Chicago: 1998. [Google Scholar]
- Altmann SA. Fallback foods, eclectic omnivores, and the packaging problem. Am J Phys Anthropol. 2009;140:615–629. doi: 10.1002/ajpa.21097. [DOI] [PubMed] [Google Scholar]
- Altmann SA, Altmann J. Baboon ecology. University of Chicago Press; Chicago: 1970. [Google Scholar]
- Beehner JC, Onderdonk DA, Alberts SC, Altmann J. The ecology of conception and pregnancy failure in wild baboons. Behav Ecol. 2006;17:741–750. [Google Scholar]
- Begon M, Townsend CR, Harper JL. Ecology: From individuals to ecosystems. 2nd edn Blackwell; Oxford: 2005. [Google Scholar]
- Bentley-Condit V, Smith EO. Female reproductive parameters of the Tana River yellow baboons. Inter J Primatol. 1997;18:581–596. [Google Scholar]
- Bercovitch FB, Harding RSO. Annual birth patterns of savanna baboons (Papio cynocephalus anubis) over a ten-year period at Gilgil, Kenya. Folia Primatol. 1993;61:115–122. doi: 10.1159/000156738. [DOI] [PubMed] [Google Scholar]
- Börger L, Dalziel BD, Fryxell JM. Are there general mechanisms of animal home range behaviour? A review and prospects for future research. Ecol Lett. 2008;11:637–650. doi: 10.1111/j.1461-0248.2008.01182.x. [DOI] [PubMed] [Google Scholar]
- Box GEP, Jenkins GM, Reinsel GC. Time series analysis: Forecasting and control. John Wiley and Sons; New York: 2008. [Google Scholar]
- Boyce MS, Pitt J, Northrup JM, Morehouse AT, Knopff KH, Cristescu B, Stenhouse GB. Temporal autocorrelation functions for movement rates from global positioning system radiotelemetry data. Philos T R Soc B. 2010;365:2213–2219. doi: 10.1098/rstb.2010.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockwell PG, Davis R. Time series: Theory and methods. 2nd edn Springer-Verlag; New York: 1991. [Google Scholar]
- Brown JL. The evolution of diversity in avian territorial systems. Wilson Bull. 1964;76:160–169. [Google Scholar]
- Burt WH. Territoriality and home range concepts as applied to mammals. J Mammal. 1943;24:346–352. [Google Scholar]
- Cairns SJ, Schwager SJ. A comparison of association indices. Anim Behav. 1987;35:1454–1469. [Google Scholar]
- Chapman CA, Chapman LG, McLaughlin RL. Multiple central place foraging by spider monkeys: Travel consequences of using many sleeping sites. Oecologia. 1989;79:506–511. doi: 10.1007/BF00378668. [DOI] [PubMed] [Google Scholar]
- Cheney DL, Seyfarth RM. Behaviour of adult and immature male baboons during inter-group encounters. Nature. 1977;269:404–406. [Google Scholar]
- Cheney DL, Seyfarth RM. Non-random dispersal in free-ranging vervet monkeys: Social and genetic consequences. Am Nat. 1983;122:392–412. [Google Scholar]
- Cowlishaw G. Behavioural patterns in baboon group encounters: The role of resource competition and male reproductive strategies. Behaviour. 1995;132:75–86. [Google Scholar]
- Davies NB. Multi-male breeding groups in birds: Ecological causes and social conflict. In: Kappeler PM, editor. Primate males: Causes and consequences of variation in group composition. Cambridge University Press; Cambridge: 2000. pp. 11–20. [Google Scholar]
- Doncaster CP. Non-parametric estimates of interaction from radio-tracking data. J Theor Biol. 1990;143:431–443. [Google Scholar]
- Dunbar RIM. Primate social systems. Croom Helm; London: 1988. [Google Scholar]
- Emlen ST, Oring LW. Ecology, sexual selection, and the evolution of mating systems. Science. 1977;197:215–223. doi: 10.1126/science.327542. [DOI] [PubMed] [Google Scholar]
- Esau K. Plant anatomy. John Wiley and Sons; New York: 1965. [Google Scholar]
- Estes RD. The behavior guide to African mammals. University of California Press; Berkeley: 1991. [Google Scholar]
- Fischer J, Hammerschmidt K, Cheney DL, Seyfarth RM. Acoustic features of male baboon loud calls: Influences of context, age, and individuality. J Acoust Soc Am. 2002;111:1465–1474. doi: 10.1121/1.1433807. [DOI] [PubMed] [Google Scholar]
- Fritsch FE, Salisbury E. Plant form and function. G Bell and Sons; London: 1965. [Google Scholar]
- Gehrt SD, Fritzell EK. Resource distribution, female home range dispersion and male spatial interactions: Group structure in a solitary carnivore. Anim Behav. 1998;55:1211–1227. doi: 10.1006/anbe.1997.0657. [DOI] [PubMed] [Google Scholar]
- Gesquiere LR, Khan M, Shek L, Wango TL, Wango EO, Alberts SC, Altmann J. Coping with a challenging environment: Effects of seasonal variability and reproductive status on glucocorticoid concentrations of female baboons (Papio cynocephalus) Horm Behav. 2008;54:410–416. doi: 10.1016/j.yhbeh.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesquiere LR, Onyango PO, Alberts SC, Altmann J. Endocrinology of year-round reproduction in a highly seasonal habitat: Environmental variability in testosterone and glucocorticoids in baboon males. Am J Phys Anthropol. 2011;144:169–176. doi: 10.1002/ajpa.21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesquiere LR, Wango EO, Alberts SC, Altmann J. Mechanisms of sexual selection: Sexual swellings and estrogen concentrations as fertility indicators and cues for male consort decisions in wild baboons. Horm Behav. 2007;51:114–125. doi: 10.1016/j.yhbeh.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Hamilton WJ., III Baboon sleeping site preferences and relationships to grouping patterns. Am J Primatol. 1982;3:41–53. doi: 10.1002/ajp.1350030104. [DOI] [PubMed] [Google Scholar]
- Harcourt AH. Dominance and fertility among female primates. J Zool. 1987;213:471–487. [Google Scholar]
- Hausfater G, Meade BJ. Alternation of sleeping groves by yellow baboons (Papio cynocephalus) as a strategy for parasite avoidance. Primates. 1982;23:287–297. [Google Scholar]
- Hoset KS, Le Galliard J-F, Gundarsen G, Steen H. Home range size and overlap in female root voles: Effects of season and density. Behav Ecol. 2008;19:139–145. [Google Scholar]
- Hutchinson JMC, Waser PM. Use, misuse and extensions of “ideal gas” models of animal encounter. Biol Rev. 2007;82:335–359. doi: 10.1111/j.1469-185X.2007.00014.x. [DOI] [PubMed] [Google Scholar]
- Ims RA. Male spacing systems in microtine rodents. Am Nat. 1987;130:475–484. [Google Scholar]
- Janmaat KRL, Olupot W, Chancellor RL, Arlet ME, Waser PM. Long-term site fidelity and individual home range shifts in Lophocebus albigena. Int J Primatol. 2009;30:443–466. doi: 10.1007/s10764-009-9352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen SP, Gray SJ, Hurst JL. Excluding neighbours from territories: Effects of habitat structure and resource distribution. Anim Behav. 2005;69:785–795. [Google Scholar]
- Kitchen DM, Cheney DL, Seyfarth RM. Factors mediating inter-group encounters in savannah baboons (Papio cynocephalus ursinus) Behaviour. 2004;141:197–218. [Google Scholar]
- Lambin X, Krebs CJ. Spatial organization and mating system of Microtus townsendii. Behav Ecol Sociobiol. 1991;28:353–363. [Google Scholar]
- Macdonald DW, Ball EG, Hough NG. The evaluation of home range size and configuration using radiotracking data. In: Amlaner CJ, Macdonald DW, editors. A handbook on biotelemetry and radio tracking. Pergamon Press; Oxford: 1980. pp. 405–425. [Google Scholar]
- Markham AC, Altmann J. Remote monitoring of primates using automated GPS technology in open habitats. Am J Primatol. 2008;70:495–499. doi: 10.1002/ajp.20515. [DOI] [PubMed] [Google Scholar]
- McLaughlin RL, Montgomerie RD. Brood dispersal and multiple central place foraging in Lapland longspur parents. Behav Ecol Sociobiol. 1989;25:207–215. [Google Scholar]
- McLoughlin PD, Gaillard J-M, Boyce MS, Bonenfant C, Messier F, Duncan P, Delorme D, Van Moorter B, Saïd S, Klein F. Lifetime reproductive success and composition of the home range in a large herbivore. Ecology. 2007;88:3192–3201. doi: 10.1890/06-1974.1. [DOI] [PubMed] [Google Scholar]
- Melnick DJ, Pearl MC. Cercopithecines in multi-male groups: Genetic diversity and population structure. In: Smuts BB, Cheney DL, Seyfarth R, Wrangham RW, Struhsaker TT, editors. Primate societies. University of Chicago Press; Chicago: 1987. pp. 121–134. [Google Scholar]
- Nathan R, Getz WM, Revilla E, Holyoak M, Kadmon R, Saltz D, Smouse PE. A movement ecology paradigm for unifying organismal movement research. P Natl Acad Sci USA. 2008;105:19052–19059. doi: 10.1073/pnas.0800375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemtzov SC. Intraspecific variation in home range exclusivity by female green razorfish, Xyrichtys splendens (family Labridae), in different habitats. Environ Biol Fishes. 1997;50:371–381. [Google Scholar]
- Norton GW, Rhine RJ, Wynn GW, Wynn RD. Baboon diet: A five-year study of stability and variability in the plant feeding and habitat of the yellow baboons (Papio cynocephalus) of Mikumi National Park, Tanzania. Folia Primatol. 1987;48:78–120. doi: 10.1159/000156287. [DOI] [PubMed] [Google Scholar]
- Olupot W, Waser PM. Correlates of intergroup transfer in male grey-cheeked mangabeys. Int J Primatol. 2001;22:169–187. [Google Scholar]
- Ostro LET, Young TP, Silver SC, Koontz FW. A geographic information system method for estimating home range size. J Wildl Manage. 1999;63:748–755. [Google Scholar]
- Packer C. Inter-troop transfer and inbreeding avoidance in Papio anubis. Anim Behav. 1979;27:1–36. [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS. Springer Verlag; New York: 2000. [Google Scholar]
- Pulliainen E. Use of the home range by pine martens (Martes martes L.) Acta Zool Fennica. 1984;171:271–274. [Google Scholar]
- Pulliam HR, Caraco T. Living in groups: Is there an optimal group size? In: Krebs HR, Davies N, editors. Behavioural ecology. Blackwell Scientific; Oxford: 1984. pp. 122–147. [Google Scholar]
- Reynolds TD, Laundré JW. Time intervals for estimating pronghorn and coyote home ranges and daily movements. J Wildl Manage. 1990;54:316–322. [Google Scholar]
- Rubenstein DI. Behavioural ecology of island feral horses. Equine Vet J. 1981;13:27–34. [Google Scholar]
- Sapolsky RM, Altmann J. Incidence of hypercortisolism and dexamethasone resistance increases with age among wild baboons. Biol Psychiat. 1991;30:1008–1016. doi: 10.1016/0006-3223(91)90121-2. [DOI] [PubMed] [Google Scholar]
- van Schaik CP, Assink PR, Salafsky N. Territorial behavior in southeast Asian langurs: Resource defense or mate defense? Am J Primatol. 1992;26:233–242. doi: 10.1002/ajp.1350260402. [DOI] [PubMed] [Google Scholar]
- Schoener TW. Time budgets and territory size: Some simultaneous optimization models for energy maximizers. Am Zool. 1987;27:259–291. [Google Scholar]
- Shopland JM. An intergroup encounter with fatal consequences in yellow baboons (Papio cynocephalus) Am J Primatol. 1982;3:263–266. doi: 10.1002/ajp.1350030123. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 5th edn Allyn and Bacon; New York: 2006. [Google Scholar]
- Whitten PL. Diet and dominance among female vervet monkeys (Cercopithecus aethiops) Am J Primatol. 1983;5:139–159. doi: 10.1002/ajp.1350050205. [DOI] [PubMed] [Google Scholar]