Abstract
Background
Higher white blood cell (WBC) count is associated with impaired endothelium-dependent vasodilation. However, the influence of higher WBC count on endothelin (ET)-1 vasoconstrictor activity is currently unknown. We tested the hypothesis that adults with elevated WBC count demonstrate enhanced ET-1 system activity.
Methods
Thirty-four healthy adults were studied: 17 with WBC count < 5.0 × 109 cells/L (lower WBC; 9M/8F; age: 53 ± 2 yr) and 17 with WBC count > 5.0 × 109 cells/L (higher WBC; 10M/7F; 54 ± 3 yr). Forearm blood flow (FBF) responses to intra-brachial infusion of ET-1 (5 pmol/min for 20 min) and selective ETA receptor blockade (BQ-123; 100 nmol/min for 60 min) were measured by venous occlusion plethysmography.
Results
The vasoconstrictor response to ET-1 was significantly blunted (∼60%) in the higher WBC group versus the lower WBC group. The FBF responses to selective ETA receptor blockade were also significantly different (P < 0.05) between the groups. In the lower WBC group, resting FBF increased marginally (∼5%) to BQ-123, whereas the increase in FBF to BQ-123 was significantly greater (∼15%) in higher WBC group. Furthermore, there was a significant relation between WBC count and FBF response to ET-1 (r = −0.43) and BQ-123 (r = 0.41).
Conclusions
Relative elevations in WBC count in middle-aged and older adults, independent of adiposity and other cardiometabolic risk factors, are associated with enhanced ET-1-mediated vasoconstrictor tone. Elevated ET-1 system activity may be a mechanism linking higher WBC count with increased cardiovascular risk.
Keywords: Endothelin, Vasoconstriction, White blood cell
Introduction
White blood cells (WBC) play a critically important role in immunological responses to inflammatory and infectious processes.1 The recognition that inflammation is a key component of atherosclerotic vascular disease and its clinical consequences sparked, and rekindled, interest in the potential link between WBC count and risk of cardiovascular disease.2,3 Indeed, several epidemiological and prospective studies have shown that elevations in WBC count, even within suggested normal ranges, are independently associated with coronary heart disease and stroke incidence and mortality.3–6 A number of biochemical, biomechanical, electrical and rheological mechanisms have been proposed to underlie the pathologic link between WBCs and atherosclerotic vascular disease.3,7 Given the constant interaction between WBCs and the vascular endothelium, endothelial cell damage and dysfunction may be a primary causative factor.
Impaired endothelial cell function, particularly vasomotor dysfunction, is an early component in the development of atherosclerosis. Diminished nitric oxide-mediated endothelium dependent vasodilation and enhanced endothelin (ET)-1 vasoconstrictor tone is recognized as an atherogenic endothelial phenotype associated with increased cardiovascular and cerebrovascular risk.8 Elevations in WBC count have been shown to be inversely related to endothelial vasodilation in both healthy and diseased populations.9,10 It is currently unknown whether WBC count is also related to other endothelial vasomotor abnormalities. Accordingly, we tested the hypothesis that elevations in WBC count, within clinically normal range, are associated with increased ET-1 vasoconstrictor tone in middle-aged and older adults.
Methods
Subjects
Thirty-four healthy sedentary adults (age range: 41–73 years) were studied: 17 (9 males/8 females) with WBC count < 5.0 × 109 cells/L (lower WBC) and 17 (10 males/7 females) with WBC count >5.0 × 109 cells/L (higher WBC).9 The stratification used for WBC was based on a recent study demonstrating associated impairment in endothelial vasodilator function with WBC count >5.0 × 109 cells/L.9 All subjects were normotensive (arterial blood pressure < 140/90 mmHg) and free of overt cardiovascular disease and metabolic abnormalities as assessed by medical history, physical examination, fasting blood chemistries and blood pressure at rest and during incremental exercise performed to exhaustion. None of the subjects smoked or were taking medications (including vitamins). No subjects had WBC count >10.0 × 109 cells/L, and all subjects were free of recent inflammation/infection (<2 weeks) as determined by questionnaire.11 All of the women were at least 1 year postmenopausal and had never taken or had discontinued use of hormone replacement therapy at least 1 year before the start of the study. Prior to participation, all of the subjects had the research study and its potential risks and benefits explained fully before providing written informed consent according to the guidelines of the University of Colorado at Boulder.
Body composition
Body mass was measured to the nearest 0.1 kg using a medical beam balance. Percent body fat was determined by dual energy X-ray absorptiometry (Lunar Corp., Madison, WI). Body mass index (BMI) was calculated as weight (kilograms) divided by height (meters) squared. Minimal waist circumference was measured according to published guidelines.12
Maximal oxygen consumption
To assess aerobic fitness, subjects performed incremental treadmill exercise with a modified Balke protocol. Maximal oxygen consumption (VO2 max) was measured with on-line computer-assisted open circuit spirometry as described previously.13
WBC count and metabolic measurements
WBC count was determined by standard Coulter counter technique (Beckman Coulter Ac T 5diff CP) by the Clinical and Translational Research Center (CTRC) core laboratory. Plasma lipid, lipoprotein, glucose and insulin concentrations were also determined by the CTRC core laboratory using standard methods. Plasma concentrations of C-reactive protein and oxidized LDL were determined by enzyme immuno-assay.
Intra-arterial infusion protocol
All studies were performed between 7 and 10 AM after a 12-hour overnight fast in a temperature-controlled room. Under strict aseptic conditions a 5-cm, 20-gauge catheter was inserted into the brachial artery of the nondominant arm under local anesthesia (1% lidocaine). Heart rate and arterial blood pressure were continuously measured throughout the infusion protocol. Forearm blood flow (FBF) at rest and in response to each pharmacologic agent was measured using strain-gauge venous occlusion plethysmography (D. E. Hokanson, Bellevue, WA), as previously described by our laboratory.14 Baseline FBF was measured for 5 min and for 5 min before each drug infusion thereafter. To rule out the possibility of nonspecific differences to vasoconstrictor agents between WBC count groups, vascular responses to norepinephrine were determined. Norepinephrine was infused at a rate of 260 pmol/min for 5 min, and FBF was measured during the last 3 min. After a 20-minute rest period to allow FBF to return to baseline levels, ET-1 (Clinalfa, AG) was infused at a rate of 5 pmol/min for 20 min, and FBF was measured during the last 3 min. After a 30-minute rest period to allow resting FBF to return to baseline, BQ-123 (Clinalfa, AG), a selective ETA receptor antagonist, was infused at a rate of 100 nmol/min for 60 min, and FBF was measured every 10 min. The selected dose of BQ-123 has been shown to completely inhibit the vasoconstrictor effect of endogenous ET-1 in the human forearm of healthy adults.15
Statistical analysis
Differences in subject baseline characteristics, WBC count and the magnitude of change in FBF to norepinephrine and ET-1 were determined by between-groups analysis of variance (ANOVA). Group differences in FBF responses to BQ-123 were determined by repeated-measures ANOVA. Relations between variables of interest were assessed by linear regression analysis. There were no significant gender interactions in any of the primary variables; therefore the data were pooled and presented together. All data are expressed as means ± SEM. Statistical significance was set a priori at P < 0.05.
Results
Selected subject characteristics are presented in Table 1. By design, WBC count was significantly different (∼30%) between the lower and higher WBC count groups. Aside from WBC count, there were no significant differences between the groups in any anthropometric, hemodynamic or metabolic variables.
Table 1.
Selected subject characteristics.
| Variable | Lower WBC count (n = 17) | Higher WBC count (n = 17) |
|---|---|---|
| Age, yr | 53 ± 2 | 55 ± 2 |
| Gender, M/F | 9/8 | 10/7 |
| WBC count, 109 cells/L | 4.4 ± 0.2 | 6.3 ± 0.2* |
| Body mass, kg | 78.4 ± 4.2 | 82.4 ± 3.6 |
| BMI, kg/m2 | 26.7 ± 0.9 | 27.9 ± 1.0 |
| Body fat, % | 35.2 ± 2.1 | 33.9 ± 2.2 |
| Waist circumference, cm | 91.4 ± 3.6 | 91.0 ± 3.0 |
| Systolic BP, mmHg | 122 ± 2 | 126 ± 2 |
| Diastolic BP, mmHg | 76 ± 2 | 79 ± 1 |
| VO2 max, mL/kg/min | 28.7 ± 1.9 | 29.0 ± 1.9 |
| Total cholesterol, mmol/L | 5.3 ± 0.2 | 5.1 ± 0.2 |
| LDL-cholesterol, mmol/L | 3.3 ± 0.2 | 3.0 ± 0.2 |
| HDL-cholesterol, mmol/L | 1.3 ± 0.1 | 1.3 ± 0.1 |
| Triglycerides, mmol/L | 1.5 ± 0.1 | 1.6 ± 0.2 |
| Glucose, mmol/L | 5.1 ± 0.1 | 5.1 ± 0.1 |
| Insulin, pmol/L | 47.3 ± 5.4 | 55.7 ± 8.9 |
| C-reactive protein, mg/L | 2.6 ± 0.7 | 3.7 ± 0.7 |
| Oxidized LDL, U/L | 64.1 ± 3.7 | 60.2 ± 4.4 |
Values are mean ± SEM. WBC: white blood cell count; BMI: body mass index; BP: blood pressure; VO2max: maximal oxygen consumption; LDL: low-density lipoprotein; HDL: high-density lipoprotein;
P < 0.05 vs. Lower WBC count.
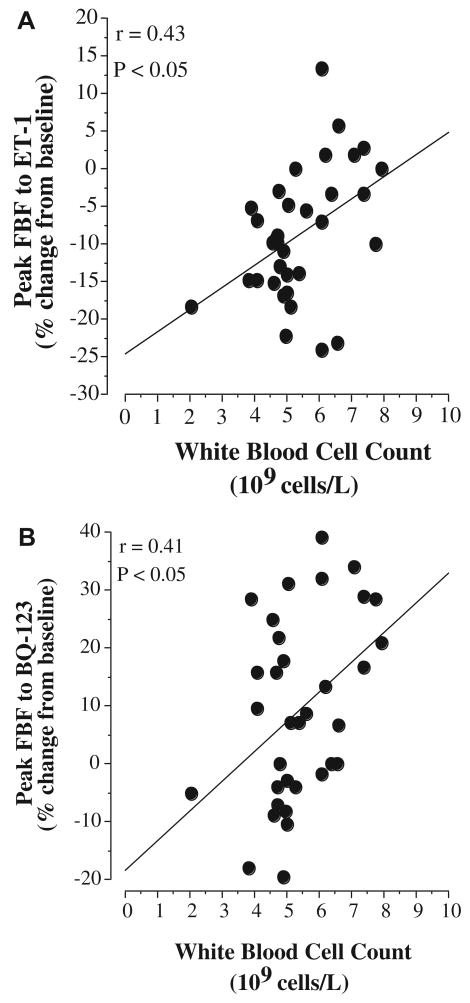
The vasoconstrictor response to norepinephrine was not significantly different between groups, as FBF was reduced by 24% in both groups (data not shown). In contrast, the vasoconstrictor response to ET-1 was markedly different (∼60%; P = 0.01) between the WBC groups. In the higher WBC group, there was a 5.1 ± 2.5% reduction in resting FBF to ET-1 compared with a 12.2 ± 1.2% reduction in the lower WBC group (Fig. 1). The FBF responses to selective ETA receptor blockade were also significantly different between the groups. In the lower WBC group, resting FBF increased marginally (∼5%) to BQ-123, whereas the increase in FBF to BQ-123 was approximately three-fold greater (∼15%) in higher WBC group (Fig. 1). In the overall study population, WBC count was inversely related to the FBF response to ET-1 (r = −0.43, P = 0.01) and positively correlated with the peak FBF response to BQ-123 (r = 0.41, P = 0.02) (Fig. 2).
Figure 1.
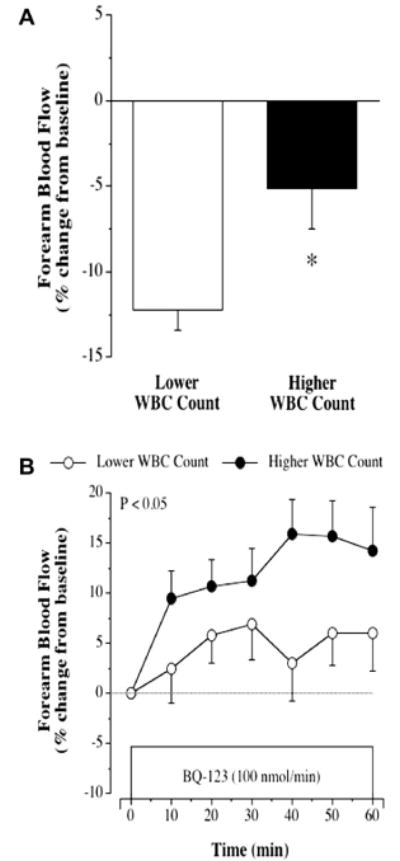
FBF responses to ET-1 (5 pmol/min for 20 min) (panel A) and BQ-123 (panel B) in the lower and higher WBC count groups. Values are mean ± SEM. *P < 0.05 vs lower WBC count.
Figure 2.
Relation between white blood cell count and peak FBF response to ET-1 (panel A) and BQ-123 (panel B) in the study population.
Discussion
The primary new finding of the present study is that elevations in WBC count, within clinically normal range and independent of other cardiovascular risk factors, is associated with increased ET-1-mediated vasoconstrictor tone. To the best of our knowledge this is the first study to determine a potential link between WBC count and ET-1 system activity. Increased ET-1 vasoconstrictor tone may contribute to the heightened risk of cardiovascular disease and stroke reported with elevations in WBC count.
WBC count in both healthy and diseased adults has been shown to be associated with, and predictive of, cardiovascular and cerebrovascular events.4–6,16–19 For example, in the Atherosclerosis Risk in Communities Study4 the incidence of coronary artery disease was almost two-fold higher in Caucasian and African American adults with WBC counts >7.0 × 109 cells/L compared with adults of similar age with WBC counts < 4.8 × 109 cells/L. Elkind and colleagues5 reported that elevations in WBC count were associated with an increased risk of ischemic stroke, independent of other risk factors, in a population cohort of the Northern Manhattan Study. Moreover, the risk of future stroke was estimated to increase by ∼20% per 1.8 × 109 cells/L increment in WBC count. Several studies have also noted the prognostic value of WBC count for morbidity and mortality following percutaneous coronary intervention.16–18 The mechanisms underlying the vascular risk with elevated WBCs are not completely understood.
Increased ET-1 system activity has been linked with a number of cardiovascular risk factors associated with elevated WBC count, such as obesity20 and hypertension,21 and is considered to be an important contributor to the etiology of atherosclerotic vascular disease.22 In the present study, WBC count was associated with increase ET-1 system activity. Indeed, there were marked differences in the vascular responses to ET-1 and selective ETA receptor blockade between the higher and lower WBC groups. The forearm vasoconstrictor response to exogenous ET-1 was significantly blunted in the adults with higher WBC count compared with lower WBC count. The reduced vasoconstrictor effect of the peptide in the higher WBC count group suggests greater endogenous ET-1 bioavailability and continuous receptor activation.15 It has been shown that chronic exposure to ET-1 does result in a reduction in ET-1 receptors on vascular smooth cells.23 Of note, it is unlikely that the observed WBC count-related differences in the ET-1 response were due to a nonspecific decline in contractile function of the vascular smooth muscle, because the vasoconstrictor response to norepinephrine was similar between the groups. The vascular response to exogenous ET-1 is often used as a bioassay of endogenous ET-1 production given the inaccuracy and unreliability of circulating ET-1 concentrations as an indicator of vascular ET-1 production.15 Coupled with the blunted response to ET-1, the higher WBC count group demonstrated a significantly greater vasodilator response to the selective ETA receptor antagonist BQ-123 than the lower WBC count group suggesting enhanced ETA receptor-mediated ET-1 vasoconstrictor tone in the higher WBC count group. Additionally, we observed significant correlations between WBC count and the vascular responses to both ET-1 and BQ-123 reflecting an association between WBC count and the ET-1 system. No other factors were associated with these blood flow responses in the present study.
The mechanisms responsible for the apparent WBC-related increase in ET-1 system activity are unclear and outside the scope of this cross-sectional study. However, it is important to note that aside from WBC count, there were no other group differences in anthropometric, cardiometabolic, inflammatory or oxidative factors that have been shown to influence ET-1 system activity between the groups.15,24,25 As a result our findings suggests a primary link between WBCs and vascular function. Indeed, the results presented herein complement and extend the results of previous studies demonstrating that relative elevations in WBC count are associated with a reduction in endothelial vasodilator function.9,10 For example, a recent study by Walker et al.,9 involving a similar population of healthy, sedentary middle-aged and older adults stratified by the same WBC count criteria as the present study, demonstrated impaired nitric oxide-mediated endothelium-dependent vasodilation in adults with higher (6.0 ± 0.2 × 109 cells/L) compared with lower (4.1 ± 0.1 × 109 cells/L) WBC count. These findings and those presented herein indicate that relative elevations in WBC count, independent of other risk factors, are associated with a proatherogenic endothelial phenotype characterized by enhanced ET-1-mediated vasoconstriction and reduced nitric oxide-mediated vasodilation. Future studies are needed determine the underlying causes for this apparent deleterious interaction between WBCs and vascular endothelial function.
Given the inherent limitations of the cross-sectional design employed in the present study we are unable to dismiss that genetic and/or lifestyle factors may have influenced our results. To minimize the influence of lifestyle behaviors, we studied subjects who were nonsmokers, not currently taking any medication that could influence WBC or endothelial vasomotor function, and did not differ in body composition or habitual physical activity. In addition, in an effort to isolate the primary influence of WBC, we studied adults free of other cardiometabolic risk factors that have been shown to influence WBC and endothelin-1 system activity such as hypertension21 and type 2 diabetes.26,27 Although the plasma concentrations of CRP were slightly, but not significantly, higher in the WBC group, we do not believe that these higher CRP concentrations influenced our findings, and in turn, the interpretation the data. Indeed, in the present study we observed no relation between CRP and either WBC or the FBF responses to ET-1 or BQ-123. This finding is consistent with previous studies from our laboratory involving CRP and ET-1 system activity.28 Unfortunately, due to a reduction in drug availability during the course of this study, we were unable to administer the selective ETB receptor antagonist BQ-788 and therefore cannot comment on the influence of WBC on the vascular actions of the ETB receptor and non-selective ET-1-receptor blockade.
In conclusion, the results of this study demonstrate that WBC count is associated with significant differences in FBF responses to exogenous ET-1 administration and ET-1 receptor antagonism in healthy, sedentary, non-smoking middle-aged and older adults. Increased ET-1-mediated vasoconstrictor tone may contribute to the increased risk of cardiovascular and cerebrovascular disease and events associated with relative elevations in WBC count. In addition, the apparent link between WBC count and vascular endothelial function may also underlie the reported prognostic value of WBC count to cardiovascular outcomes.
Acknowledgments
We would like to thank all of the subjects who participated in the study. This study was supported by National Institute of Health Awards HL077450, HL076434, MO1 RR00051 and 1 UL1 RR025780.
References
- 1.Shaykhiev R, Bals R. Interactions between epithelial cells and leukocytes in immunity and tissue homeostasis. J Leukoc Biol. 2007;82:1–15. doi: 10.1189/jlb.0207096. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman M, Blum A, Baruch R, Kaplan E, Benjamin M. Leukocytes and coronary heart disease. Atherosclerosis. 2004;172:1–6. doi: 10.1016/s0021-9150(03)00164-3. [DOI] [PubMed] [Google Scholar]
- 4.Lee CD, Folsom AR, Nieto FJ, Chambless LE, Shahar E, Wolfe DA. White blood cell count and incidence of coronary heart disease and ischemic stroke and mortality from cardiovascular disease in African-American and White men and women: atherosclerosis risk in communities study. Am J Epidemiol. 2001;154:758–64. doi: 10.1093/aje/154.8.758. [DOI] [PubMed] [Google Scholar]
- 5.Elkind MS, Sciacca RR, Boden-Albala B, Rundek T, Paik MC, Sacco RL. Relative elevation in baseline leukocyte count predicts first cerebral infarction. Neurology. 2005;64:2121–5. doi: 10.1212/01.WNL.0000165989.12122.49. [DOI] [PubMed] [Google Scholar]
- 6.Kannel WB, Anderson K, Wilson PW. White blood cell count and cardiovascular disease. Insights from the Framingham Study JAMA. 1992;267:1253–6. [PubMed] [Google Scholar]
- 7.Madjid M, Awan I, Willerson JT, Casscells SW. Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol. 2004;44:1945–56. doi: 10.1016/j.jacc.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen A, Thorin-Trescases N, Thorin E. Working under pressure: coronary arteries and the endothelin system. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1188–94. doi: 10.1152/ajpregu.00653.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker AE, Seibert SM, Donato AJ, Pierce GL, Seals DR. Vascular endothelial function is related to white blood cell count and myeloperoxidase among healthy middle-aged and older adults. Hypertension. 2010;55:363–9. doi: 10.1161/HYPERTENSIONAHA.109.145870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elkind MS, Sciacca RR, Boden-Albala B, Tondella ML, Feikin DR, Fields BS, et al. Leukocyte count is associated with reduced endothelial reactivity. Atherosclerosis. 2005;181:329–38. doi: 10.1016/j.atherosclerosis.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Macko R, Ameriso S, Gruber A, Griffin J, Fernandez J, Brandt R, et al. Impairments of the protein C system and fibrinolysis in infection-associated stroke. Stroke. 1996;27:2005–11. doi: 10.1161/01.str.27.11.2005. [DOI] [PubMed] [Google Scholar]
- 12.Lohman T, Roche A, Mortorell R. Athropometric standardization reference manual. Champaign, IL: Human Kinetics; 1988. [Google Scholar]
- 13.DeSouza C, Shapiro L, Clevenger C, Dinenno F, Monahan KD, Tanaka H, et al. Regular aerobic exercise prevents and restores the age-related decline in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–7. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 14.Van Guilder GP, Westby CM, Greiner JJ, Stauffer BL, DeSouza CA. Endothelin-1 vasoconstrictor tone increases with age in healthy men but can be reduced by regular aerobic exercise. Hypertension. 2007;50:403–9. doi: 10.1161/HYPERTENSIONAHA.107.088294. [DOI] [PubMed] [Google Scholar]
- 15.Cardillo C, Kilcoyne C, Waclawiw M, Cannon R, Panza J. Role of endothelin in the increased vascular tone of patients with essential hypertension. Hypertension. 1999;33:753–8. doi: 10.1161/01.hyp.33.2.753. [DOI] [PubMed] [Google Scholar]
- 16.Kruk M, Karcz M, Przyluski J, Bekta P, Kepka C, Kalinczuk L, et al. White blood cell count adds prognostic information to the thrombolysis in myocardial infarction risk index in patients following primary percutaneous coronary intervention (ANIN Myocardial Infarction Registry) Int J Cardiol. 2007;116:376–82. doi: 10.1016/j.ijcard.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 17.Ndrepepa G, Braun S, Iijima R, Keta D, Byrne RA, Schulz S, et al. Total leucocyte count, but not C-reactive protein, predicts 1-year mortality in patients with acute coronary syndromes treated with percutaneous coronary intervention. Clin Sci (Lond) 2009;116:651–8. doi: 10.1042/CS20080298. [DOI] [PubMed] [Google Scholar]
- 18.Kojima S, Sakamoto T, Ishihara M, Kimura K, Miyazaki S, Tei C, et al. The white blood cell count is an independent predictor of no-reflow and mortality following acute myocardial infarction in the coronary interventional era. Ann Med. 2004;36:153–60. doi: 10.1080/07853890310021553. [DOI] [PubMed] [Google Scholar]
- 19.Rasouli M, Kiasari AM, Bagheri B. Total and differential leukocytes counts, but not hsCRP, ESR, and five fractioned serum proteins have significant potency to predict stable coronary artery disease. Clin Chim Acta. 2007;377:127–32. doi: 10.1016/j.cca.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Pratley RE, Wilson C, Bogardus C. Relation of the white blood cell count to obesity and insulin resistance: effect of race and gender. Obes Res. 1995;3:563–71. doi: 10.1002/j.1550-8528.1995.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 21.Shankar A, Klein BE, Klein R. Relationship between white blood cell count and incident hypertension. Am J Hypertens. 2004;17:233–9. doi: 10.1016/j.amjhyper.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Kelly J, Whitworth J. Endothelin-1 as a mediator in cardiovascular disease. Clin Exp Pharmacol Physiol. 1999;26:158–61. doi: 10.1046/j.1440-1681.1999.03011.x. [DOI] [PubMed] [Google Scholar]
- 23.Kuc RE, Davenport AP. Endothelin-A-receptors in human aorta and pulmonary arteries are downregulated in patients with cardiovascular disease: an adaptive response to increased levels of endothelin-1? J Cardiovasc Pharmacol. 2000;36:S377–9. doi: 10.1097/00005344-200036051-00109. [DOI] [PubMed] [Google Scholar]
- 24.Mather K, Mirzamohammadi B, Lteif A, Steinberg H, Baron A. Endothelin contributes to basal vascular tone and endothelial dysfunction in human obesity and type 2 diabetes. Diabetes. 2002;51:3517–23. doi: 10.2337/diabetes.51.12.3517. [DOI] [PubMed] [Google Scholar]
- 25.Cardillo C, Kilcoyne C, Cannon R, Panza J. Increased activity of endougenous endothelin in patients with hypercholesterolemia. J Am Coll Cardiol. 2000;36:1483–8. doi: 10.1016/s0735-1097(00)00910-4. [DOI] [PubMed] [Google Scholar]
- 26.Moradi S, Kerman SR, Rohani F, Salari F. Association between diabetes complications and leukocyte counts in Iranian patients. J Inflamm Res. 2012;5:7–11. doi: 10.2147/JIR.S26917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Targher G, Seidell JC, Tonoli M, Muggeo M, De Sandre G, Cigolini M. The white blood cell count: its relationship to plasma insulin and other cardiovascular risk factors in healthy male individuals. J Intern Med. 1996;239:435–41. doi: 10.1046/j.1365-2796.1996.815000.x. [DOI] [PubMed] [Google Scholar]
- 28.Weil BR, Mestek ML, Westby CM, Van Guilder GP, Greiner JJ, Stauffer BL, et al. Short sleep duration is associated with enhanced endothelin-1 vasoconstrictor tone. Can J Physiol Pharmacol. 2010;88:777–81. doi: 10.1139/Y10-046. [DOI] [PubMed] [Google Scholar]