Abstract
The nature and origin of human diversity has been a source of intellectual curiosity since the beginning of human history. Contemporary advances in cultural and biological sciences provide unique opportunities for the emerging field of cultural neuroscience. Research in cultural neuroscience examines how cultural and genetic diversity shape the human mind, brain and behavior across multiple time scales: situation, ontogeny and phylogeny. Recent progress in cultural neuroscience provides novel theoretical frameworks for understanding the complex interaction of environmental, cultural and genetic factors in the production of adaptive human behavior. Here, we provide a brief history of cultural neuroscience, theoretical and methodological advances, as well as empirical evidence of the promise of and progress in the field. Implications of this research for population health disparities and public policy are discussed.
I. A brief history of cultural neuroscience
Understanding human diversity has been a source of intellectual curiosity since the beginning of human history. Isidori of Seville, in his 7th century encyclopedia Etymologiae, wrote that human diversity exists not only in physical appearance, such as color or body size, but also in mental content (Jahoda, 2002). The study of human diversity gained further prominence in the late 19th century with the emergence of the field of anthropology, which examines cultural variation in customs, practices, values and beliefs of different cultural groups and the genetic origins of such variation. More recently, advances in the study of human behavior have led to the emergence of cultural psychology, which examines human behavioral diversity, and human neuroscience, which has been largely influenced by conventional biological notions of natural selection; for instance, a majority of cognitive neuroscience studies have examined what aspects of the mind and brain serve as adaptive information processing mechanisms (Barkow et al., 1992).
Developmental and lifespan psychological approaches have emphasized the importance of timescale in explaining the interaction of cultural and biological factors. For instance, work on probabilistic epigenesis, by prominent developmental psychologists including D’Arcy Thompson and C. Waddington, emphasized the importance of the biological trajectories unfolding throughout development as a result of interactions with environmental input (Johnson, 1997). Biocultural constructivism furthered these concepts by discussing the significance of plasticity across development and in particular the role of plasticity in altering both the path and end state (Li, 2003).
Building on these developmental and lifespan approaches to an understanding of the mind and brain, early investigations in cultural neuroscience were motivated by a neuroscientific investigation of aging and culture (Park & Gutchess, 2002). Culture serves an important role later in life as a compensatory mechanism for the decline in cognitive abilities due to neural changes in cellular and structural organization of the brain. While much evidence within cultural psychology indicates cultural variation in how people think as early as young adulthood (Nisbett et al., 2001), brain changes in structural and functional organization due to aging may result in even greater cultural variation in how people think in older age (Park & Gutchess, 2002). The goal of a cultural neuroscience of aging is to facilitate an understanding of both environmental influences as well as biological constraints to cognitive functioning in late adulthood (Park & Gutchess, 2002).
A cultural neuroscience framework was then introduced to explain how theoretical and empirical approaches across distinct fields within the social and natural sciences may further an understanding of how cultural and genetic factors influence the human mind, brain and behavior not only across the lifespan, but also within the situation and across evolutionary timescales (Chiao & Ambady, 2007). Advances in our understanding of cultural influences on cognitive brain function led to the development of transcultural imaging which includes empirical work on cognitive variation in brain function across cultures, with event-related potentials and neuroimaging techniques (Han & Northoff, 2008). Discovery of cultural influences on neural representations of self and identity led to further conceptual development within cultural neuroscience, and in particular, the introduction of the concept of “looping effects,” or the notion that culture is a dynamical system of bidirectional influences with the individual, including psychological and biological processes that facilitate social interaction (Vogeley & Roepstorff, 2009). Given that culture mutually influences individual processes, such as mind, brain and behavior, important questions in cultural neuroscience include culture mapping, or the kinds of cognitive processes that vary across cultures at the neural level, and source analysis, where cultural universals and differences emerge from (Ambady & Bharucha, 2009).
Our understanding of culture-biology interactions not only across the lifespan, but also across evolutionary timescales has advanced with the discovery of culture-gene coevolutionary models of human behavior, including the cultural and genetic selection of specific traits in the production of adaptive behavior (Chiao & Blizinsky, 2010; Way & Lieberman, 2010; Nikolaidis & Gray, 2010). A neuro-culture interaction model was then developed to suggest a causal trajectory such that cultural practices reinforce values and tasks that become “culturally patterned neural activities” due to neuroplasticity or neuronal change, which then facilitates social survival via biological adaptation and reproductive success (Kitayama & Uskul, 2011). Culture-gene coevolutionary processes may also produce cultural variation in core cognitive and neural architecture (e.g., structure and function) across phylogeny and generations, due to geographical variation in environmental pressures (Chiao & Immordino-Yang, in press). For instance, environmental factors, such as pathogen prevalence, are known to lead to cultural selection of individualism-collectivism, due at least in part to genetic selection of the short (S) allele of the serotonin transporter gene (Chiao & Blizinsky, 2010). Additionally, ecological pressures, such as food deprivation and national vulnerability to natural disasters, are known to lead to cultural selection of tightness-looseness, due at least in part to genetic selection of the short (S) allele of the serotonin transporter gene as well (Mrazek et al., under review). Co-selection of cultural and genetic factors likely serves an adaptive function, such as reduced pathogen prevalence and psychopathology as well as reduced anxiety and mood disorders. Importantly, once cultural selection becomes adaptive, genetic selection causes further refinement of core cognitive and neural architecture necessary for the storage and transmission of adaptive cultural capacities (Boyd & Richerson, 1985; Chiao & Blizinsky, 2010; Mrazek et al, under review). Within this unprecedented decade of research in cultural neuroscience, researchers have made significant progress in theoretical and empirical knowledge of how culture and biology interact across multiple timescales. Here we review the conceptual framework and model of cultural neuroscience, as well as empirical progress and future promise of the field.
II. A framework of cultural neuroscience
Theory
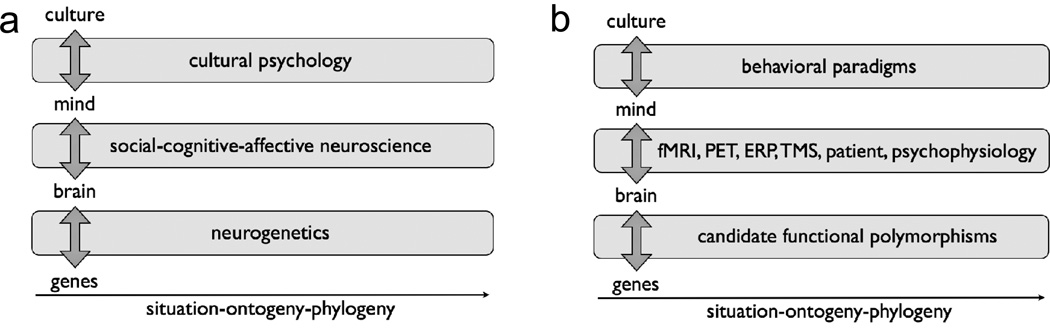
Research in cultural neuroscience bridges theories from distinct fields, including anthropology, cultural psychology, neuroscience/neurogenetics and population genetics (Chiao & Ambady, 2007; Chiao, 2009; Chiao et al., 2010; Chiao, 2011; Figure 1). In the past four decades, researchers in cultural science have developed novel theories to explain the key dimensions along which nations and cultures may vary, such as individualism-collectivism (IND-COL), power distance (PD), short-term-long-term orientation (SLO), uncertainty avoidance (UA) and masculinity-femininity (MF) (Hofstede, 2001). Cultural constructs such as analytic-holistic cognition (Nisbett, Peng, Choi, Norenzayan, 2001), socioeconomic class (SES; Snibbe & Markus, 2005; Savani et al., 2008) and tightness-looseness (Gelfand et al., 2011) further describe how people may think, feel and behave differently across geography and culture.
Figure 1. Framework of cultural neuroscience (Chiao & Ambady, 2007; Chiao, 2009/2011).
(a-b) Research in cultural neuroscience integrates theory and methods from cultural psychology, social-cognitive-affective neuroscience and neurogenetics across multiple time scales, specifically situation, ontogeny and phylogeny.
People from Western and Eastern cultures vary in how they reason about themselves and the world around them (Nisbett et al., 2001). East Asians are more likely to engage in holistic cognition, specifically attending to the entire field of a scene and relying on dialectical reasoning; by contrast, Westerners are more likely to show analytic cognition, attending to objects rather than their context and using rules to reason (Nisbett et al., 2001). People from high and low socioeconomic (SES) backgrounds may process the world differently (Stephens, Markus, Fryberg, 2012); for instance, people of high SES are more likely to value choice, whereas people of low SES may find choice daunting. Tightness-looseness refers to cultural dimensions that affect how sensitive people are to social norm compliance and violations. For instance, people who live in tight cultures are more likely to be socially sensitive to social norms, whereas people who live in loose cultures are more likely to be tolerant of social deviance (Gelfand et al., 2011).
The past two decades of human neuroscience research have focused primarily on discovering mappings between mind, brain and behavior. Specifically, what kinds of brain structure and function predict behavior? How can we understand the mind as a product of neural systems? Theories such as modularity of mind indicate that the mind and brain are comprised of modules that are information-encapsulated mechanisms, that from birth rapidly and automatically process specialized kinds of information and are found across species (Fodor, 1983). Major advances within human neuroscience research have led to the discovery of several modules within the human brain for processing distinct kinds of information, such as faces, objects, houses, scenes and people (Kanwisher, 2010). Important theoretical directions within cultural neuroscience are to understand the role that culture and genetic diversity may play shaping neural representations within domain-specific modules or adaptive neural machinery (Chiao & Immordino-Yang, in press).
Methods
Methods in cultural neuroscience vary across levels of analysis; however, all studies in cultural neuroscience rely on quantitative methods or a mixture of qualitative and quantitative methods (Chiao et al., 2010, Figure 1). Cultural scientists, including anthropologists and cultural psychologists, typically include behavioral surveys in order to examine cultural values, practices and beliefs in one’s self and others (Chiao et al., 2010). Open-ended interviews or ethnography comprise another important way that cultural scientists study the behavior of others. During open-ended interviews and ethnography, researchers attempt to better understand what others value, what kinds of cultural customs they practice and what they believe and why from their perspective (Gelfand et al., 2012).
Neuroscientists measure neural response and correlate this with mental and behavioral responses via indirect methods that vary in spatial and temporal resolution (Gazzaniga et al., 2002; Heeger and Rees, 2002; Handy, 2005). For instance, noninvasive neuroimaging methods, such as functional magnetic resonance imaging (fMRI) and positron emission tomography (PET), that measure neural response indirectly and noninvasively, have a relatively poor temporal resolution (seconds), but good spatial resolution (millimeters). Electrophysiological methods, that measure neural response directly from the scalp, have very good temporal resolution (milliseconds), but poor spatial resolution (recordings directly from the scalp). Neuropsychological studies of people with brain damage provide another important window into understanding how the brain works. When psychological ability is impaired due to brain damage, researchers are able to determine what brain regions are necessary to perform a given psychological function.
Studies within cross-cultural neuropsychology have shown that racial/ethnic variation in performance on cognitive neuropsychological tests are sometimes due to cultural factors, such as language ability, acculturation and level of education (Manly et al., 2004). Finally, behavioral genetic and neurogenetic studies rely on integrating genetic information with behavioral and neuroscientific data. The majority of these behavioral and neurogenetic studies examine gene-behavior and gene-neural-behavior associations within a given culture or population. Notably, recent advances in population genetics indicate significant variation in allele frequencies across the globe as a function of population structure due to multiple evolutionary factors, including natural selection, genetic drift, mutation and gene flow (Tishkoff & Kidd, 2004). Current empirical directions in cultural neuroscience research examine behavioral genetics and neurogenetic associations across cultures as a means of better understanding the mutual influence of cultural and genetic factors on mind, brain and behavior.
III. Model of cultural neuroscience
Culture-gene coevolutionary theory
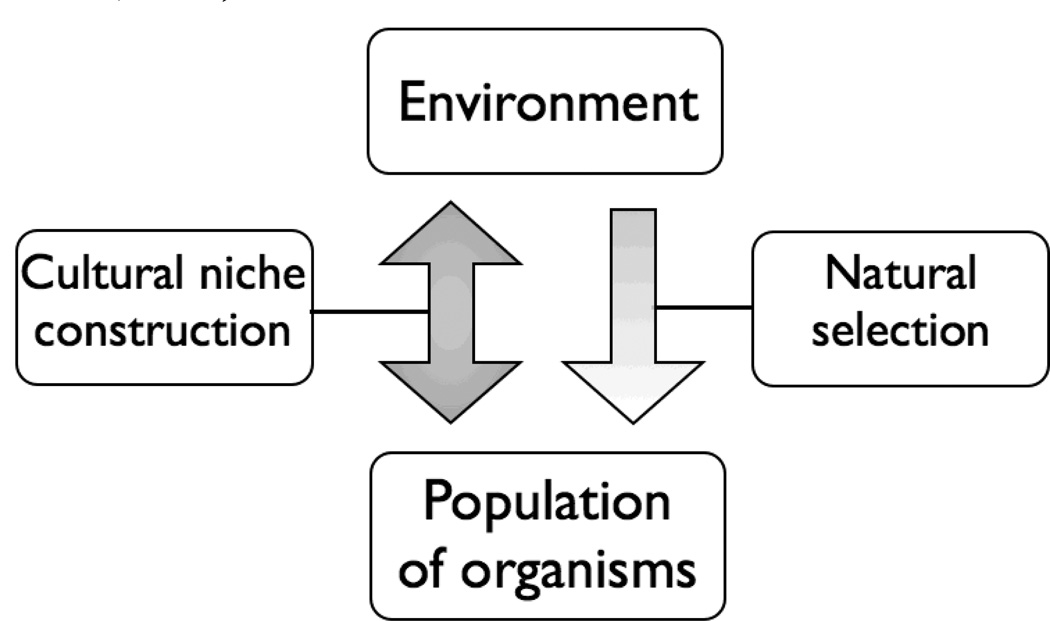
Culture-gene coevolutionary theory asserts that adaptive behavior is the product of two interacting and complementary evolutionary processes, specifically cultural and genetic selection (Cavalli-Sforza & Feldman, 1981; Lumsden & Wilson, 1981; Boyd & Richerson, 1985). This dual inheritance theory of human behavior posits that cultural traits are adaptive and emerge due to environmental and ecological pressures that vary across geography under which genetic selection occurs (Boyd & Richerson, 1985). Additionally, niche construction provides a complementary evolutionary process by which not only are environmental pressures affecting favorable traits or characteristics via the process of natural selection, but in creating or altering their environments or niches, humans can shift the kinds of traits that are being selected for (Olding-Smee, Laland, Feldman, 2003) (Figure 2). A prominent example of culture-gene coevolution is lactose tolerance. Regions within northern Europe that have an increased frequency of people who digest milk also have an increased frequency of cattle who produce milk, indicating a culture-gene coevolution between cattle milk protein genes and human lactase genes (Beja-Pereira et al., 2003). Despite evidence of culture-gene coevolution in dietary practices, such as lactose tolerance, less well understood is culture-gene coevolution of human behavior (Chiao & Blizinsky, 2010).
Figure 2. Model of dual inheritance theory (adapted from Stone, Lurquin & Cavalli-Sforza, 2006).
Cultural neuroscience model of human behavior
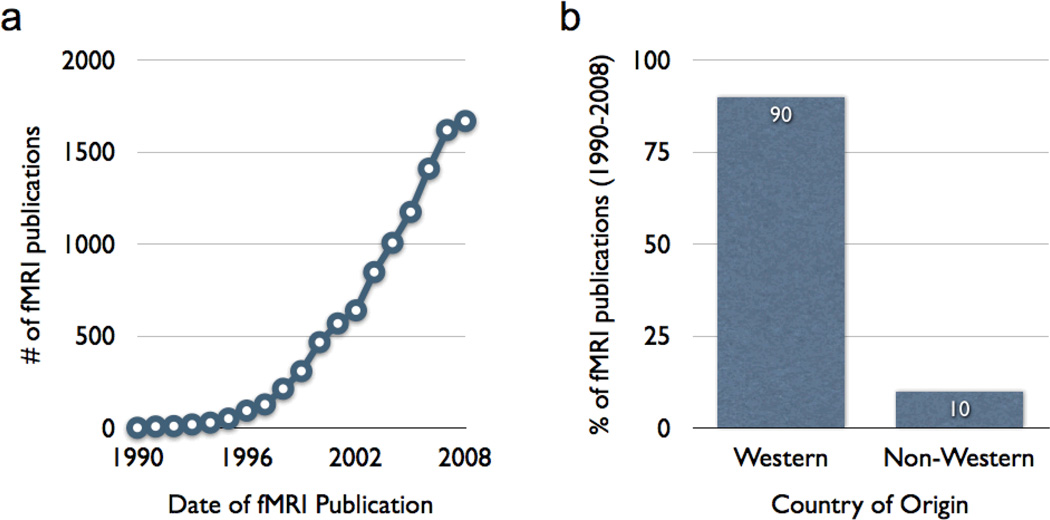
A majority of the research in behavioral and brain sciences has focused on understanding how natural selection produces adaptive human behavior by shaping psychological and neural architecture (Chiao & Cheon, 2010; Henrich, Heine & Norenzayan, 2010). However, approximately 95% of psychological studies come from countries with only 12% of the world’s population (Arnett, 2008); similarly, 90% of the neuroimaging studies examine mind-brain-behavior mappings in people from Western, industrialized, rich, educated, democratic (WEIRD) populations (Chiao, 2009; Henrich, Heine & Norenzayan, 2010, Figure 3). Culture-gene coevolutionary theory of human behavior indicates that a key goal in cultural neuroscience research is to understand how both cultural and genetic selection further shape neural and psychological architecture in the maintenance and production of adaptive human behavior (Figure 4). Identifying the specific cultural and genetic traits that serve adaptive functions and shape psychological and neural architecture is a chief goal of cultural neuroscience research.
Figure 3. Publication bias in peer-reviewed human neuroimaging literature (adapted from Chiao, 2009).
(a) Graph illustrating the growth in peer-reviewed human neuroimaging studies from 1990–2008; (b) Graph illustrating the publication bias within the human neuroimaging literature whereby the vast majority (~90%) of publications to date originate from a Western country.
Figure 4. Cultural neuroscience model of human behavior (adapted from Chiao & Immordino-Yang, in press; Chiao & Blizinsky, under review).
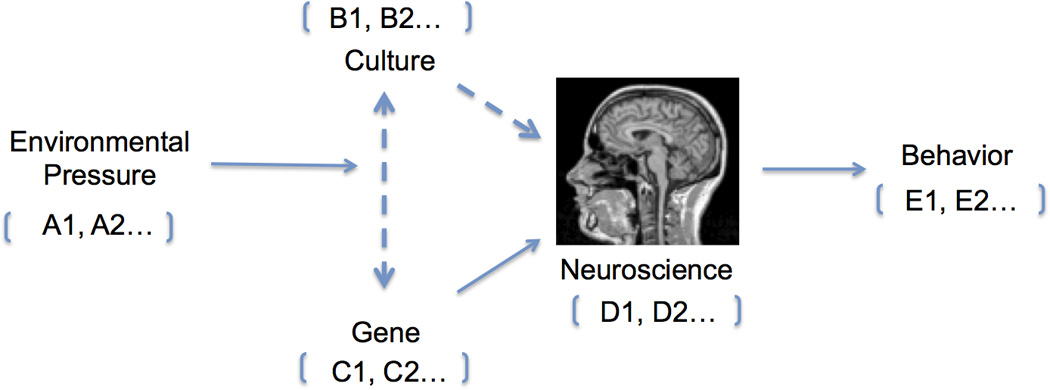
Dotted lines refer to empirical advances within the field of cultural neuroscience, which have emphasized the influence of culture-gene interactions on neural processes and behavior as well as cultural influences on mind-brain-behavior relations.
Individualism-collectivism (IND-COL) and the serotonin transporter gene (5-HTTLPR)
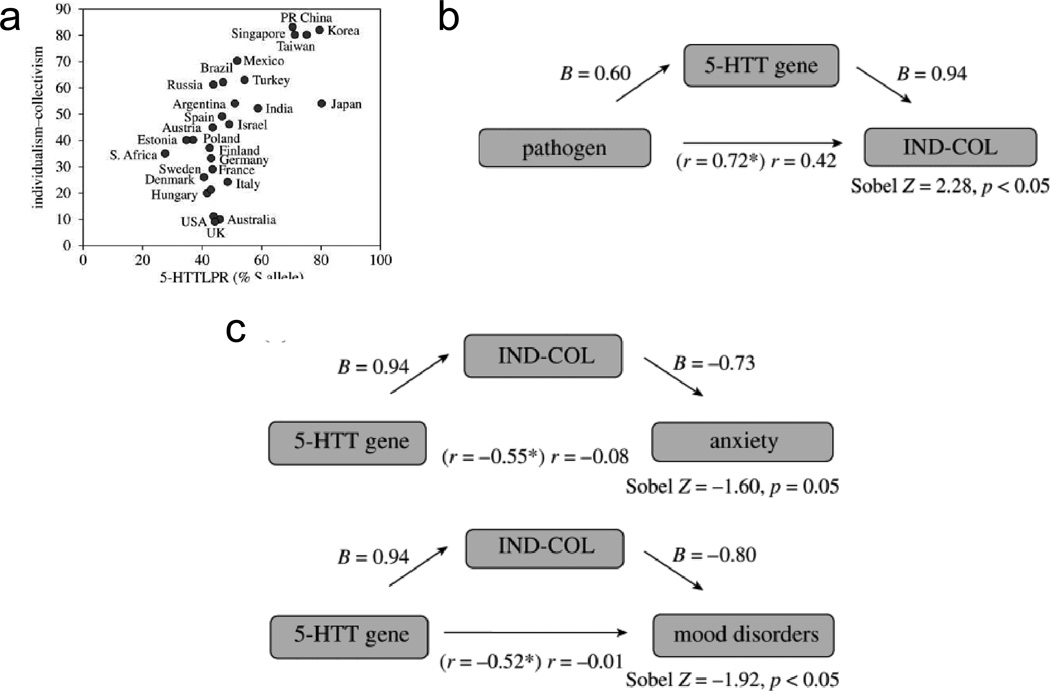
Towards this goal, we recently discovered that cultural values of individualism-collectivism coevolved with the serotonin transporter gene (SLC6A4) in the production of adaptive human behavior (Chiao & Blizinsky, 2010; Figure 5). Specifically, nations with increased prevalence of environmental pressures, such as pathogen prevalence, are more likely to endorse collectivistic values due to anti-pathogen functions of cultural collectivism. In geographical regions with increased prevalence of infectious disease, people from collectivistic nations show increased preference for group members and reduced contact with out-group members as a means of reducing the potential transmission of infectious disease (Fincher et al., 2008). Furthermore, collectivistic nations show increased prevalence of people carrying the short (S) allele of the 5HTTLPR compared to the long (L) allele, due in part to cultural and genetic selection. Hence, collectivism not only serves an anti-pathogen function but also an anti-psychopathological function; specifically, collectivistic nations show reduced prevalence of anxiety and mood disorders, due in part to genetic selection of the serotonin transporter gene (Chiao & Blizinsky, 2010).
Figure 5. Culture-gene coevolution of individualism-collectivism and the serotonin transporter gene.
(a) nations with increased collectivism also show increased prevalence of the S allele; (b) nations with increased contemporary pathogen prevalence are more likely to show collectivistic values due to increased frequency of the S allele of the serotonin transporter gene; (c) nations with increased S allele of the serotonin transporter gene are more likely to show reduced anxiety and mood disorders due to increased prevalence of cultural collectivism (adapted from Chiao & Blizinsky, 2010).
Tightness-looseness (TL) and the serotonin transporter gene (SLC6A4)
Culture-gene coevolution may not only explain adaptive behaviors that prevent psychopathology, such as anxiety and mood disorders, but also psychopathy or antisocial personality disorder linked to impairments in the ability to feel empathy and recognize emotional cues (Mrazek et al., under revision). Recent evidence from our lab demonstrates that cultural values of tightness-looseness likely coevolved with the serotonin transporter gene in the production of moral behavior (Mrazek, Chiao, Blizinsky, Lun, Gelfand, under revision). Tightness and looseness (TL) refer to the degree to which nations are sensitive to social norm compliance and violations, respectively. Tight nations are more likely to endorse strict adherence to social norms, whereas loose nations are more likely to be tolerant of violations of social norms. Gelfand and colleagues (2011) recently showed that nations vary in tightness-looseness due to ecological and historical threats, such as population density, resource scarcity, territorial threats, disease and environmental threats. To determine whether or not culture-gene coevolutionary theory may explain geographical variation in tightness-looseness, we tested a coevolutionary model of cultural-tightness and the serotonin transporter gene in the production of moral justifiability (e.g., are the following behaviors, such as cheating on taxes, justifiable?), due to variation in ecological threat (Mrazek, Chiao, Blizinsky, Lun, Gelfand, under revision). Across 21 nations, we found that ecological threat predicts cultural tightness-looseness due to frequency of S allele variation, and that frequency of S allele variation predicts justifiability of moral behavior, due to cultural tightness-looseness.
Our findings further indicate that adaptive moral behavior results from both cultural and genetic selection of tightness-looseness and the serotonin transporter gene. People living in tight nations may show increased social norm sensitivity as a function of carrying the S allele, a gene previously associated with sensitivity or vigilance to negative affective cues in the environment (Munafo, Clark, Flint, 2005). By contrast, people living in loose nations may show increased tolerance for social norm violations as a function of carrying the L allele, a gene previously associated with sensitivity to positive affective cues in the environment (Fox, Ridgewell, Ashwin, 2009). Finally, cultural and genetic selection of TL and the serotonin transporter gene may lead to adaptive moral behavior. For instance, people living in tight nations who carry the serotonin transporter gene may show increased negative response to behaviors that are considered moral violations (e.g., not paying taxes or receiving government benefits for which one is not entitled to).
In geographic regions with either man-made or historical threats, people carrying the S allele may show enhanced or heightened vigilance as an adaptive response to ecological pressure and thus be more likely to demonstrate behaviors that facilitate the cultural transmission of tight versus loose attitudes towards social norms. Hence, our findings demonstrate that culture-gene coevolutionary theory provides an important window into understanding the role that both cultural and genetic factors play in adaptive behavior.
Power distance (PD), the serotonin transporter gene (SLC6A4) and the oxytocin receptor polymorphism (OXTR)
Another important cultural dimension that may have coevolved with the genetic selection of the serotonin transporter gene across geography is power distance or preference for social hierarchy (Chiao, 2010). Serotonin is found to play an important role in detecting and maintaining social dominance status across the animal kingdom from reptiles, lobsters, crayfish and fish to apes and humans (Chiao, 2010; Edwards & Kravitz, 1997; Kiser, Steemers, Branchi, & Homberg, 2012). For instance, elevating chronic serotonin levels using selective serotonin reuptake inhibitor in dominant male lizards, Anolis Carolinensis, causes them to become subordinate males (Larson & Summers, 2001). Similarly, in Arctic charr fish, Salvelinus alpines, the level of the major serotonin metabolite, 5-HIAA, is higher in subordinate fish than in dominant ones (Winberg, Nilsson, & Olsén, 1991). Furthermore, altering the level of serotonin using drugs (e.g., tryptophan and fenfluramine) influences social dominance acquisition in both vervet monkeys (Raleigh, McGuire, Brammer, Pollack, & Yuwiler, 1991) and human subjects (Moskowitz, Pinard, Zuroff, Annable, & Young, 2001). More recently, research by Beacher and colleague (2011) suggested a causal link between serotonin and social perception by lowering the serotonin levels of women participants using the tryptophan depletion technique. These participants later showed attenuation in positive ratings toward happy faces and in arousal ratings toward angry faces. This perhaps suggests that people who have a lower concentration of serotonin may be less influenced by social cues, and in turn, that serotonin may also be involved in social dominance perception.
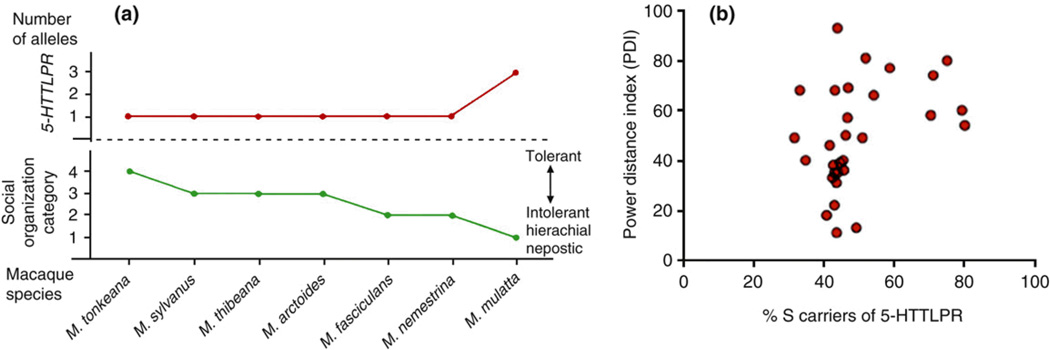
Similar to studies that directly manipuated serotonin levels, genetic studies also show the relationship between and social perception; S-allele carriers, who are often thought to have higher concentration of serotonin at the synaptic cleft (Lesch, et al., 1996), are found to be more sensitive to social cues than L carriers. For example, rhesus macaques with one S-allele (S/L) show larger pupil diameters when looking at photos of high versus low social dominant macaques than those without S-allele (L/L) (Watson, Ghodasra, & Platt, 2009). In addition, when new rhesus female monkeys were introduced in a new group of five varying in terms of serotonin transporter gene and had to form a new social status hierarchy, S-allele carriers expressed highest levels of both submission and aggression among the five (Jarrell, et al., 2008). This is perhaps analogous to social dominance behavior of inhabitants in high PDI cultures in human societies: high-power individuals are high in aggression, and low-power individuals are high in submission. Supporting this notion, rhesus monkey species that have higher tolerant societies with more lenient hierarchy and relaxed dominance usually carry only the L-allele (Chiao, 2010, Figure 6). However, species that are intolerant and have a strict hierarchy, including M. mulatta, carry at least one S-allele. Strikingly similar, countries that are high in PDI are more likely to have greater prevalence of 5-HTTLPR S-allele carriers (Chiao, 2010; Figure 6). This suggests the interplay between socio-cultural values and interacting genes on social dominance processes at the societal level, and provides further evidence for the co-evolution of power distance hypothesis.
Figure 6. Cross-species relation of allelic variation of the serotonin transporter gene (5-HTTLPR) and social hierarchy.
(a) allelic variation in the serotonin transporter gene in non-human primates; (b) allelic variation in the serotonin transporter gene in humans (Chiao, 2010).
IV. Cultural influences on brain function
Evidence for culture-gene coevolutionary theory of human behavior indicates that another key goal in cultural neuroscience is to understand how culture influences brain structure and function. Recent progress in cultural neuroscience has identified at least three distinct cultural dimensions that modulate neural bases of social and emotional behavior: individualism-collectivism, power distance or preference for social hierarchy, and racial identification. In the next section, we review recent empirical progress in our understanding of how these distinct cultural dimensions shape the human brain and behavior.
Individualism-collectivism and neural bases of the self
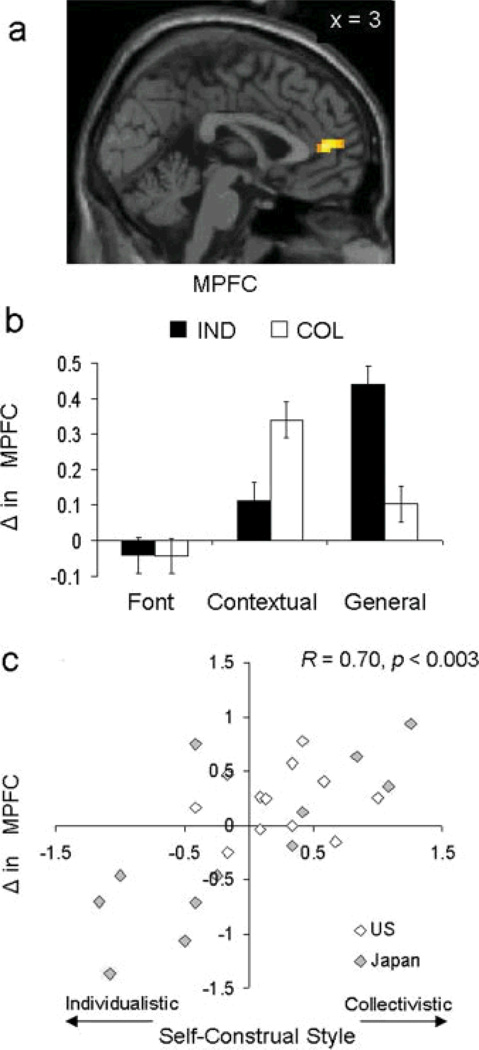
One of the most important aspects of social interaction is the capacity to have knowledge and awareness of one’s self and others. Cross-cultural and cultural psychological research has shown that there exist two primary dimensions for how people define themselves and their relation to others: individualism and collectivism (Hofstede, 1991; Triandis & Gelfand, 1998; Markus & Kitayama, 1991). Individualism refers to the self defined as autonomous from others, whereas collectivism refers to the self as connected to or defined by others or the social situation. Recent evidence from our lab indicates that cultural values of individualism and collectivism modulate neural responses during self processing. People living in the US and Japan who endorse individualistic cultural values are more likely to show increased neural response within medial prefrontal cortex (MPFC) to general (e.g., I am humble) compared to contextual self statements (e.g., When talking to my mother, I am humble); by contrast, people who endorse collectivistic cultural values are more likely to show increased MPFC response to contextual compared to general self statements (Chiao et al., 2009, Figure 7). Convergent evidence from Ray and colleagues (2010) indicates that neural response within MPFC and posterior prefrontal cortex (PCC) correlates with interdependence when even monocultural individuals, such as third generation Americans, make judgments of the self and their mother.
Figure 7. Cultural influences on the social brain (adapted from Chiao et al., 2009).
(a) Cultural values of individualism-collectivism modulate neural response within the medial prefrontal cortex during self judgments; (b) People show increased neural response within MPFC to culturally-congruent self judgments (e.g., individualists-general self; collectivists-contextual self); (c) Degree of individualism-collectivism predicts medial prefrontal response to general compared to contextual self judgments in both the US and Japan.
These results indicate that cultural values, rather than nationality or race, modulate neural response during self processing. Furthermore, cultural influences on neural bases of the self may not only reflect distinct kinds of stable knowledge representation, but also transient or dynamic knowledge representations of self and others. Even temporarily heightening awareness of cultural values of individualism and collectivism in bicultural Asian-Americans modulates neural response within cortical midline structures, specifically MPFC and PCC response, during general and contextual self compared to font judgments (e.g., is the phrase “I am humble” written in italics) (Chiao et al., 2010).
Cultural values of individualism-collectivism may not only modulate neural responses during explicit, but also during implicit self processing. For instance, Harada and colleagues (2011) found that cultural priming modulates neural response within dorsal, but not ventral, portions of the MPFC when people are shown autobiographical facts as well as facts about a close other, compared to an unfamiliar other. Importantly, when people are primed with individualism, dorsal regions of the MPFC show less deactivation during implicit self evaluation of father, but not self-relevant information compared to control information about an unfamiliar other. These findings indicate that culture may dynamically shape neural response during the evaluation, but not detection of self-relevant information. A majority of the cross-cultural neuroimaging studies within the past decade show cultural modulation of neural responses during self processing occurring even in the absence of cultural variation in behavioral response (e.g., the extent to which people agree with self statements), which indicates the importance of understanding the influence of cultural values of individualism and collectivism at not only the behavioral, but also the neurobiological level.
Individualism-collectivism and neural bases of emotion
Understanding the feelings or phenomenological experience of others is central to an understanding of self and others (Barrett, 2009; Hechtman, Pornpattanangkul, Chiao, 2012; Lindquist et al., 2012a/b). Expressing one’s feelings to others either with facial or bodily expressions and vocal tones provides communicative signals that allow people to understand as well as to experience the phenomenology of others. Perceiving or recognizing the feelings or phenomenological experiences of others serves an adaptive function, allowing one to know about whether or not environmental or ecological pressures, such as danger or reward, are present as well as what kinds of behaviors may be adaptive in a given situation.
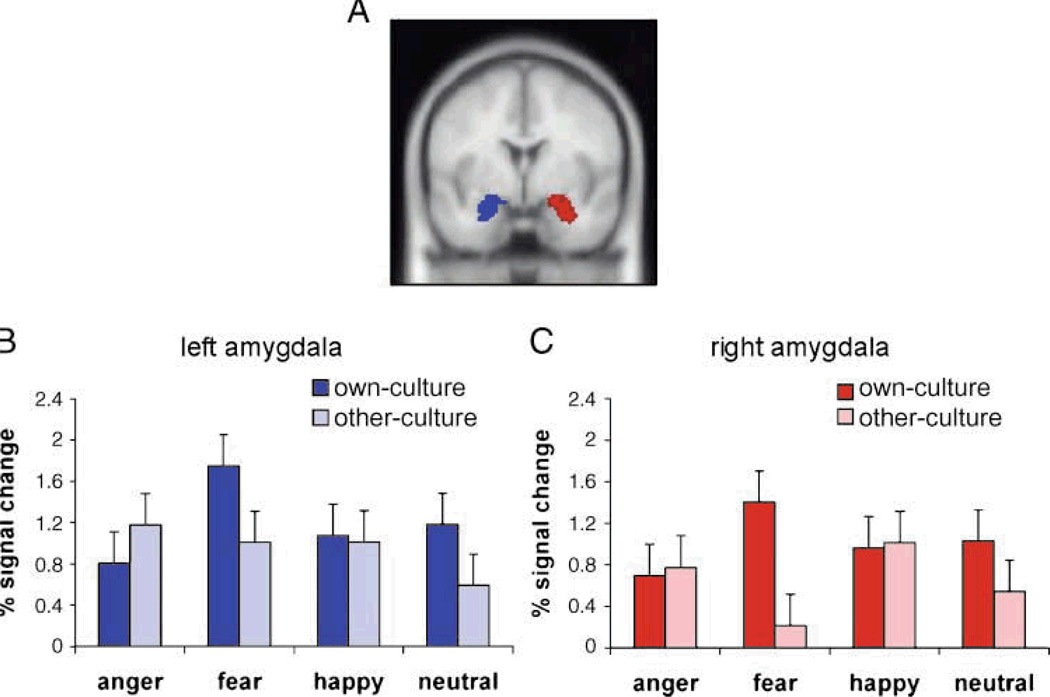
Culture not only affects neural processes associated with self processing, but also affective processing, such as perceiving emotional expressions in the environment. People living in the US and Japan show increased amygdala response to own-culture compared to other-culture fear faces, even in the absence of behavioral differences in the ability to recognize emotion (Chiao et al., 2008; Figure 8). Heightened amygdala response to emotional faces of one’s own cultural group is likely to reflect differences associated with acculturation. For instance, for Asians living in Europe, amygdala response to emotions expressed by Caucasian-Europeans depends on length of stay in Europe (Derntl et al., 2012). Increased experience or exposure to emotional expressions of members from distinct cultural groups likely modulates amygdala response in a manner similar to effects of experience in other brain regions important for social communication, such as language (Kuhl, Williams, Lacerda, Stevens & Lindblom, 1992; Perrachione, Chiao, Wong, 2010) and face recognition (Golby, Gabrieli, Chiao, Eberhardt, 2001).
Figure 8. Cultural influences on the emotional brain (adapted from Chiao et al., 2008).
(a-c) People living in the Japan and United States show increased bilateral amygdala response to fear expressed by members of their own cultural group.
Cultural influences on amygdala response likely not only reflect cultural variation in perceptual, but also conceptual representations of emotion. For instance, recent evidence indicates that cultural values of individualism-collectivism modulate amygdala response during emotion processing. People living in a collectivistic culture, such as Japan, show increased amygdala response compared to people living in an individualistic culture, such as the United States, independent of other known modulators of amygdala response, such as genotype, gender, personality and urbanicity (Chiao et al, in revision). Even temporarily heightening awareness of individualistic and collectivistic values with cultural priming modulates amygdala response when people perceive negative compared to neutral scenarios (Chiao et al, in preparation). These results indicate that culture affects evolutionary ancient neural circuitry, such as brain regions within the limbic system. One possible explanation for this cultural variation in evolutionary ancient limbic circuitry is that geographic variation in environmental and ecological pressures may lead to cultural selection of values, such as individualism and collectivism, which encourage distinct psychological and neural repertoires of emotional and social communication, as a means of producing adaptive behavior.
Notably, neural response within evolutionarily ancient circuitry, such as the limbic system, may not only vary as a function of culture, but also due to population genetic variation. For instance, amygdala response to affective cues is known to be modulated by allelic variation of the serotonin transporter gene (5-HTTLPR), a functional polymorphism known to be associated with socioemotional processes (Canli & Lesch, 2007; Munafo, Brown, Hariri, 2008). Within Western nations, people who carry the S compared to the L allele of the serotonin transporter gene are more likely to show increased amygdala response to negative compared to neutral emotional cues (Hariri et al., 2002; Munafo, Brown, Hariri, 2008). Within Western nations, amygdala response to affective cues may also vary due to interaction of both genetic and environmental factors, such as acute (Drabant et al., 2012) and chronic life stress (Canli et al., 2006). Consistent with a culture-gene coevolutionary theory of emotion, it is likely that both cultural selection of individualism-collectivism and genetic selection of the serotonin transporter gene produce heightened amygdala reactivity to affective cues in response environmental or ecological pressures.
Preference for social hierarchy and neural bases of empathy
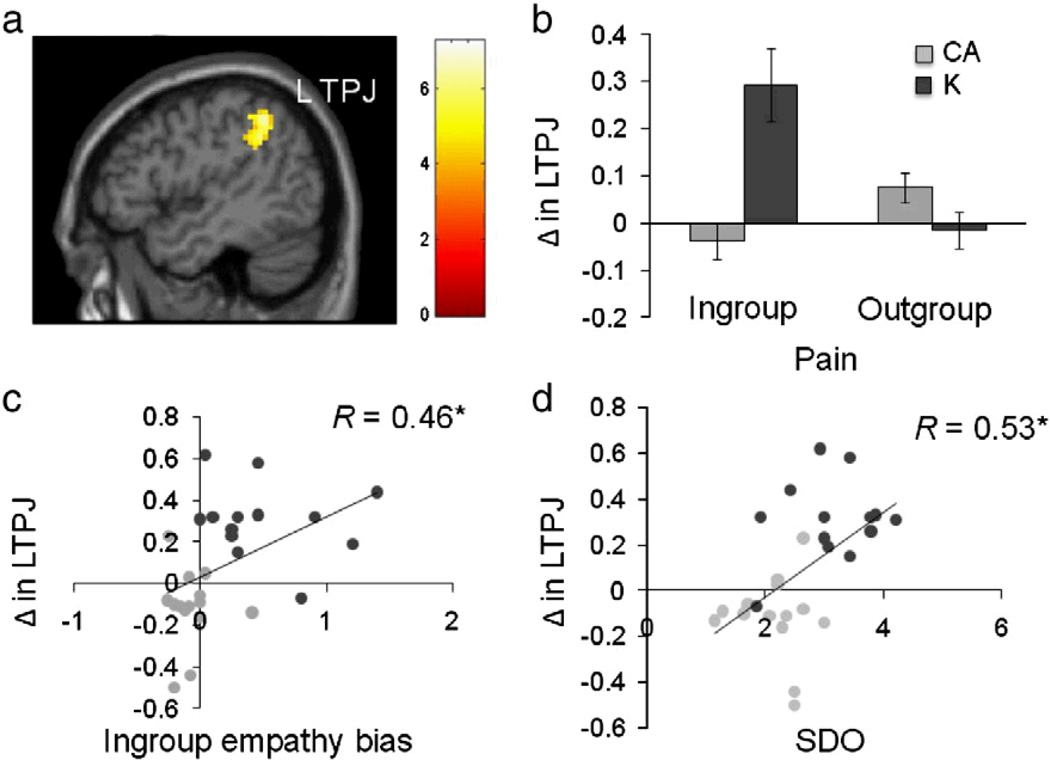
Another key cultural dimension that shapes neural architecture is power distance or preference for social hierarchy. People and cultures vary in the extent to which they prefer social hierarchy, a phenomenon known as social dominance orientation (Pratto et al., 1994; Sidanius & Pratto, 1999). Heightened preference for social hierarchy typically results in reduced empathy for others (Pratto et al., 1994). Recently we found that people living in nations that prefer social hierarchy, such as Korea, show increased empathic neural response within the left temporoparietal junction (L-TPJ) when viewing people from their own culture in painful compared to neutral scenarios and when compared to people living in nations that prefer egalitarianism, such as the United States (Cheon et al., 2011, Figure 9). Furthermore, degree of preference for social hierarchy predicts degree of parochial empathy, due to increased neural response within the L-TPJ, indicating the importance of recruiting brain regions typically associated with theory of mind when responding to the pain of others in a hierarchical culture. People living in cultures that prefer hierarchy, such as Korea, may not display their emotions in social situations due to strong display rules that emphasize low emotional expressivity (Matsumoto et al., 2008), possibly as a means of increasing adherence to status-based social norms. Theory of mind, or a rule-based understanding of status-based social norms or rules that guide social interaction, may be necessary in order to detect and respond to the suffering of others in hierarchical cultures (Saxe, Schulz, Jiang, 2006).
Figure 9. Cultural influences on the empathic brain (adapted from Cheon et al., 2011).
(a-b) Compared to Caucasian-Americans, Koreans show increased neural response within left temporoparietal junction (L-TPJ) to ingroup members in painful compared to neutral situations; (c) Neural response within the left TPJ positively correlates with ingroup empathy bias; (d) Cultural preference for social hierarchy predicts increased neural response within L-TPJ for painful compared to neutral situations of ingroup compared to outgroup members.
For people living in cultures that prefer egalitarianism, such as the United States, reliance on brain regions associated with theory of mind may not be as necessary given that social norms allow for greater emotional expressivity, thus increasing the degree of social cues in the environment that may help facilitate the likelihood of empathic understanding for and altruistic response to others’ feelings when in painful or neutral situations. For instance, in a recent study of African-Americans (AA) and Caucasian-Americans (CA) living in the United States, we found that neural response within one brain region, the medial prefrontal cortex (MPFC), predicted greater parochial empathy for AA compared to CA individuals in pain compared to no pain conditions (Mathur, Harada, Lipke, Chiao, 2010). MPFC is typically associated with mentalizing, or simulating others’ thoughts, feelings and intentions (Amodio & Frith, 2006), rather than rule-based social cognition (Saxe, Schulz, Jiang, 2006). We theorize that there may be distinct neural systems for social cognition due to geographical variation in environmental pressures; specifically, MPFC is likely a primary neural substrate for mentalizing or simulating the thoughts and feelings of others in egalitarian cultures, whereas TPJ is likely a primary neural substrate for understanding the thoughts and feelings of others in hierarchical cultures.
Racial identification and neural bases of pain perception and experience
Racial identification refers to the degree to which people identify with one’s cultural or ethnic group, a sociocultural factor known to serve as a buffer against environmental stressors, such as racial discrimination. Racial identification occurs due to self-claimed group membership, which reflects the value or significance of belonging to a social group (e.g., amount of commitment to a particular group). The importance of racial identification to psychological well-being, educational outcomes and physical health is well-understood (Phinney, 1992; Sellers & Shelton, 2003; Sellers, Rowley, Chavous, Shelton, Smith, 1997) and is particularly adaptive for minorities living in multicultural communities. For instance, African-Americans demonstrate increased racial identification often in response to increased perceived racial discrimination, global psychological distress and self-esteem. Heightened identification with other racial minority group members can increase academic performance (Smith et al., 2009) and decrease risk for cardiovascular disease (Chae et al., 2010).
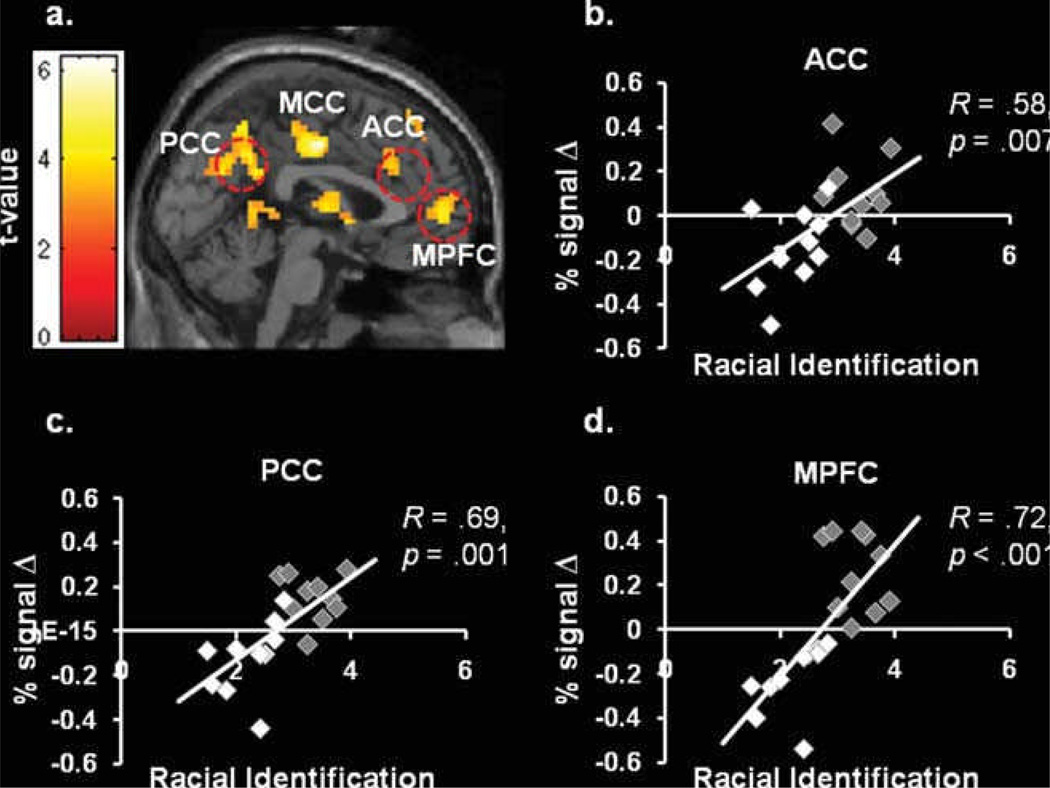
Recently, we discovered that racial identification modulates neural response within cortical midline structures, including the medial prefrontal cortex (MPFC), anterior cingulate cortex (ACC) and the posterior cingulate cortex (PCC), as well as medial temporal lobe regions, including bilateral parahippocampal gyrus when people empathize with others (Mathur, Harada, Chiao, 2012, Figure 10). Specifically, African-Americans who show greater racial identification compared to Caucasian-Americans also demonstrate increased neural response within cortical midline structures; by contrast, African-Americans who show decreased racial identification compared to Caucasian-Americans show decreased neural response within medial temporal lobe structures, specifically parahippocampal gyrus, when empathizing with the pain of other members of their racial group (Mathur, Harada, Chiao, 2012). Our findings demonstrate for the first time the importance of racial identification on neural response when empathizing with people of one’s own racial group.
Figure 10. Racial identification and the empathic pain (Mathur, Harada, Chiao, 2011).
(a-d) African-Americans and Caucasian-Americans who show increased racial identification also show enhanced response within cortical midline structures, specifically MPFC, ACC and PCC, in response to people viewing painful compared to neutral scenarios.
Socioeconomic status and neural bases of social cognition
People from low and high socioeconomic status (SES) are thought to display differences in neural bases of sociocognitive processes. For instance, people with high SES are more likely to show increased neural response within ventral striatum to high status information about others, whereas people with low SES are more likely to show increased ventral striatum response to low status information, indicating that processing social information about others in one’s SES is rewarding (Ly et al., 2011; Figure 11). After perceiving others in pain, people with high SES are more likely to show a positive association between neural response within empathic pain regions and charitable donation, whereas people with low SES show a negative association (Ma, Wang, Han, 2011). Finally, people of lower social status are more likely to recruit neural response within neural regions associated with mentalizing, specifically dorsolateral prefrontal cortex (DLPFC), medial prefrontal cortex (MPFC), precuneus and posterior cingulate cortex (PCC) (Muscatell et al., 2012). Hence, perceived and actual SES play a key role in modulating neural response within social and affective brain regions.
Figure 11. Socioeconomic status (SES) influences on the social brain (adapted from Ly et al., 2011).
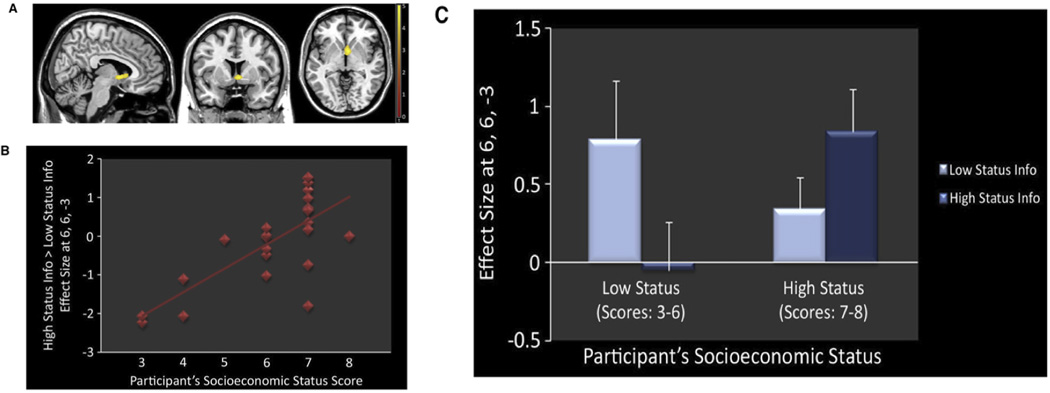
(a) Increased ventral striatum (VS) response when people evaluate social information of others within their own SES; (b) people from higher SES are more likely to show increased VS response to social information of higher SES; (c) Within the ventral striatum, people of low and high SES show increased response to social information of their own social status.
Cultural transmission and neural bases of imitation
Cultural dimensions that coevolve with specific genes in response to environmental or ecological demands affect brain processes not only by modulating neural representations of specific psychological processes, but also by orienting attentional and cognitive mechanisms towards the imitation or transmission of specific social and affective signals between group members. For instance, in individualistic or collectivistic cultures, orientation or attention to positive and negative affective cues, respectively, may be advantageous to the imitation and subsequent transmission of positive and negative social signals, such as facial expressions of joy or fear. Brain regions associated with imitation, specifically within the left inferior frontal cortex and right superior parietal lobule, are associated with both observation and imitation of motor action (Iacoboni et al., 1999), which may provide the basic neural building blocks for imitation of more complex social signals, such as facial and bodily expressions.
Notably, cultural transmission within members of one’s own social group, such as people of the same gender, is associated with increased neural response within reward regions, such as ventral and dorsal striatum, orbitofrontal cortex and amygdala, during motor imitation (Losin, Iacoboni, Martin, Dapretto, 2012). By contrast, for Caucasian-Americans, neural response within frontal, parietal and occipital brain regions show increased response when imitating motor actions of African-Americans, compared to Caucasian-Americans and Chinese-Americans, possibly due to between-group variation in similarity or status (Losin, Iacoboni, Martin, Cross, Dapretto, 2012). These findings indicate that the capacity to learn cultural practices from others is affected by group membership, specifically; and psychological and neural architecture that facilitates cultural transmission varies depending on whether or not the communication of social knowledge occurs within or between members of distinct groups.
V. Culture-gene-environment interaction and behavior
Most of the recent advances in our understanding of cultural, genetic and environment interactions are in the behavioral, not neural, levels of analysis. In particular, cross-cultural behavioral genetics studies are demonstrating the importance of culture-gene-environment interactions in the production and maintenance of adaptive behaviors, such as psychological well-being.
Culture, oxytocin receptor (OXTR) polymorphism and support seeking
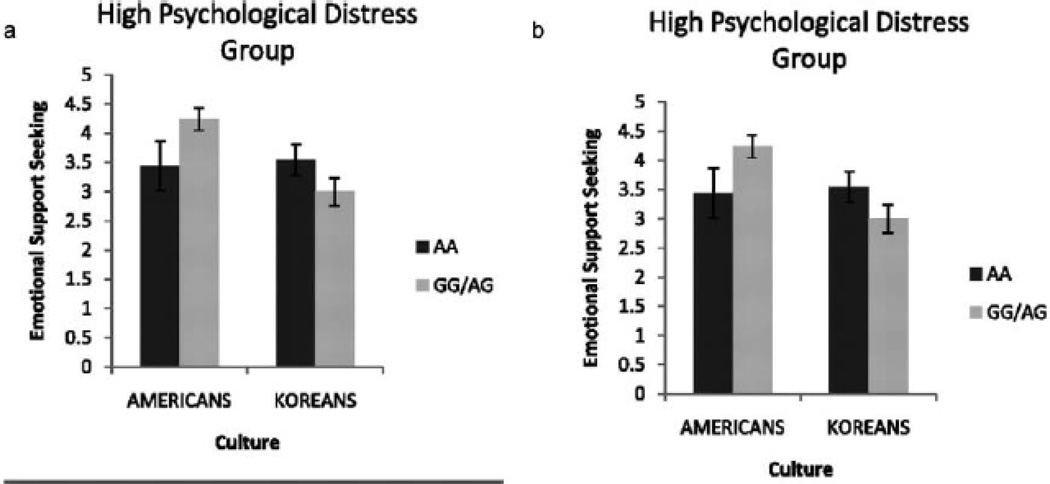
Cultures vary in how people seek social support. When faced with different kinds of environmental stressors in the educational, social or health domain, Asians are less like to seek emotional support from others compared to Caucasian-Americans (Kim, Sherman, Taylor, 2008; Taylor et al., 2004; Kim, Sherman, Ko, Taylor, 2006). Recent behavioral evidence indicates that Asians and Asian-Americans benefit psychologically and biologically from implicit rather than explicit social support (Taylor, Welch, Kim, Sherman, 2007). For instance, when performing the Trier Social Stress Task (TSST), psychological stress response is reduced for European-Americans, but heightened for Asians and Asian-Americans when they receive explicit social support; similarly, European-Americans show heightened cortisol response during implicit social support, whereas Asians and Asian-Americans show heightened cortisol response during explicit social support. Genes also affect the extent to which social support seeking is adaptive across cultures. Specifically, compared to Koreans, Americans who carry one or two copies of the G allele of the oxytocin receptor polymorphism (OXTR) rs53576 when experiencing psychological distress are more likely to seek social support (Kim et al., 2010; Figure 12). These findings indicate that culture interacts with the oxytocin receptor polymorphism (OXTR) in the production of psychological well-being.
Figure 12. Culture-gene interaction in emotional support seeking and psychological distress (adapted from Kim et al., 2010).
Culture, oxytocin receptor (OXTR) polymorphism and emotion regulation
Culture also plays an important role in how people regulate emotions in self and others (Butler, Lee, Gross, 2007). For instance, people from collectivistic cultures are more likely to prefer expressive suppression, whereas people from individualistic culture are more likely to prefer cognitive reappraisal (Butler, Lee, Gross, 2007). Recent cross-cultural behavioral genetics evidence indicates a culture-gene interaction such that Koreans who carry the A are more likely to prefer emotional suppression compared to Koreans who carry the G allele, whereas Americans showed the opposite pattern (Kim et al., 2011). These findings demonstrate that culture interacts with the oxytocin receptor polymorphism in preference for emotion regulation strategy.
Religion, oxytocin receptor gene (OXTR) and psychological well-being
Cross-cultural behavioral genetics studies indicate that nations vary in gene-behavior associations; however, the specific cultural traits that have been selected with genetic traits remains unknown. One possible candidate for cultural selection is the cultural value of religiosity, or the system of beliefs and practices for those who have faith in divinity. Recent cross-cultural behavioral genetics indicates that Koreans who showed greater religiosity and are homozygous for the G allele of the OXTR receptor polymorphism r253576 were more likely to report greater psychological well-being; by contrast, Caucasian-Americans who showed greater religiosity and carried two copies of the G allele (G/G) of the OXTR polymorphism showed reduced psychological well-being, indicating that commitment to religiosity is associated with increased psychological well-being in Koreans, but not Caucasian-Americans, who carry a genetic sensitivity towards social sensitivity (Sasaki, Kim, Xu, 2011). These results further demonstrate the importance of the interaction of cultural and genetic factors in mental health.
Religion, dopamine receptor (DRD4) polymorphism and prosociality
Religion is not only a system of cultural values and practices that people may endorse consistently throughout their lives, but also environmental or situational cultural information that can be temporarily heightened or transmitted to others. Behavioral genetics evidence from Sasaki and colleagues (in press) further shows that priming or temporarily heightening religious beliefs modulates prosocial behavior, or altruism, depending on the presence of specific genes, such as the dopamine receptor polymorphism (DRD4 2-/7-repeat allele). Specifically, for both Caucasian-Americans and Asian-Americans living in the United States, people who carry the 2-/7 repeat allele and are primed with religious beliefs are more likely to show increased prosocial behavior, compared to people who do not carry the 2-/7 repeat allele. Interestingly, when religious beliefs are not primed, prosocial behavior does not vary across people without the 2-/7-repeat allele of the dopamine genotype (DRD4). Hence, environmental exposure to religiosity may increase prosociality, such as donating time to a prosocial organization (e.g., environmental conservation), by lowering dopaminergic signaling and hence increasing novelty seeking as well as sensitivity to environmental cues (e.g., religious priming) (Sasaki et al., 2011).
Serotonin transporter gene (SLC6A4) and intergroup bias
Cultural selection may occur not only for traits, such as religiosity, but also for social attitudes, such as feelings towards people of different groups, which are typically acquired or transmitted through social interactions with others or with the environment. Prior social psychological evidence indicates that negative feelings towards people outside of one’s social group, or intergroup bias, results from fear conditioning or the cultural acquisition or learning of fear towards members outside of one’s group (Olsson, Ebert, Banaji, Phelps, 2005), whereas nonprejudice or positive feelings towards people outside of one’s group results from cultural acquisition or learning of happiness towards members outside of one’s group (Livingston & Drwecki, 2007). Importantly, Cheon and colleagues (under review) recently discovered that the psychological ability to acquire negative or positive feelings towards other people varies as a function of genes, specifically the serotonin transporter polymorphism (5-HTTLPR). Specifically, Cheon and colleagues (under review) show that Caucasian-Americans living in the United States who carry one or two copies of the S allele of the serotonin transporter polymorphism (5-HTTLPR) are more likely to demonstrate implicit intergroup bias, particularly when faced with numerous experiences with potential threat, such as negative prior contact with members of different social groups, specifically Blacks and Arabs, and a perception of a dangerous social environment, such as beliefs in a dangerous world.
These results provide novel evidence of a gene-environment model of intergroup bias and demonstrate an important role of a specific gene, the serotonin transporter polymorphism, in the acquisition of unconscious prejudice, particularly when encountering exposure to environmental threat (Cheon, Livingston, Hong, Chiao, under review). Importantly, future behavioral genetics research may examine the notable role that the serotonin transporter gene may play in the acquisition of nonprejudice or positive feelings towards members of other social groups, such as when encountering exposure to environmental safety or security. Additionally, future cross-cultural behavioral genetics research may be able to further demonstrate the extent to which the interaction of genetic and environmental factors in the production of intergroup relations varies as a function of cultural values, practices and beliefs.
VI. Promise of cultural neuroscience
Understanding population health disparities
One of the most important contributions of cultural neuroscience to science and public policy is the capacity to enhance our understanding of the etiology of population health disparities in health. Population health disparities arise due to cultural and genetic variation in psychological and neural processes that emerge due to environmental and ecological pressures (Collins et al., 2011; Shields et al., 2005; Wang & Sue, 2005). For instance, Ashkenazi Jews are more likely to develop Tay-Sachs disease, while people from Northern Europe are more likely to develop cystic fibrosis (Exner, Dries, Domanski, Cohen, 2001; Wang & Sue, 2005). Additionally, prevalence of substance abuse, such as nicotine addiction, varies across racial and ethnic groups, due at least in part to allelic frequency of the CYP2A6 gene. Protective forms of the CYP2A6 gene are very rare in Europeans and Africans (~3%), but more prevalent in Japanese and Koreans (~24%) (Shields et al., 2005). Cross-national epidemiological studies, such as the 2008 World Health Organization, have shown that global variation of mental health disorders exist for affective disorders, such as anxiety and major depression (Kessler & Ustun, 2008; Weissman, 1996). Other mental health disorders related to atypical social behavior, such as autism, William’s syndrome and psychopathy, have not yet been studied systematically at an epidemiological level, possibly due to theoretical or methodological difficulties with comprehensively studying these disorders across cultural contexts. Additionally, increasing the sophistication of our understanding of how social psychological factors, such as the cultural attitudes, beliefs and practices that may produce inequities in access or quality of health care is essential to better understanding how and why disparities in health exist across racial groups (Dovidio & Fiske, 2012; Williams et al., 2012).
Research in cultural neuroscience shows that key environmental features, including cultural, geographic and socioeconomic factors modulate genetic, neural and behavioral mechanisms underlying mental health (Chiao, 2011; Chiao & Blizinsky, under review). Sociocultural variation in biological mechanisms underlying mental health disorders may explain, in part, population disparities in mental health across ethnic groups within and across nations. However, given that our current basis of knowledge of biological mechanisms underlying mental health disorders is restricted to predominantly Western industrialized nations (Chiao & Cheon, 2010), increased awareness of researcher biases as well as enhancing research capacity in non-Western industrialized nations or low-to-middle income countries (LMIC) is necessary in order to fully develop understanding of comprehensive etiology of and treatment for mental health disorders around the world (Chiao & Blizinsky, under review). Promoting the scientific study of cultural neuroscience, including development of interdisciplinary educational infrastructure and research capacity for studying human diversity across multiple levels of analysis, is key to closing gaps in population health disparities in the near and far future.
Challenges
There are several challenges facing progress in the field of cultural neuroscience. One of the main challenges is in improving research infrastructure in LMIC countries in order to enhance research capacity and to improve ownership of theory and methods in cultural neuroscience. Due to the limitations in access to and maintenance of necessary laboratory settings and equipment within LMIC countries, researchers who aim to understand cultural and biological factors that contribute to health in the developing world remain constrained. For instance, the appropriateness of the kinds of theories or empirical paradigms developed in LMIC compared to HIC countries may vary; however, without adequate resources to develop novel scientific knowledge, there will continue to exist a significant need for research that effectively addresses health problems within the developing world.
Another main challenge in cultural neuroscience research is in establishing appropriate ethical standards for the creation and collection of cultural, psychological and biological measurements in both developing and non-developing worlds. In recent history, there have been numerous instances of exploitation of underrepresented minorities for the purposes of scientific advancement (e.g., African-Americans during the Tuskegee syphilis experiments between 1932–1972 in Tuskegee, Alabama within the United States). People living in geographic regions with economic and ecological (e.g., food deprivation, territorial disputes, natural disasters) challenges are more likely to be vulnerable to financial benefits that may be offered for participating in scientific research that may or may not be conducted with adequate protection of human participants. Furthermore, there may be cultural differences in what ethical treatment of human participants in scientific research is, and these differences may require cooperation and coordination of scientific agencies across nations in order to ensure that cross-national neuroscientific and genetic studies are conducted with equivalent ethical standards.
Developing culturally-appropriate research-training infrastructure in cultural neuroscience is another key challenge for the field. Western, developed nations typically require students to train in specific fields within the social and natural sciences (e.g., anthropology, psychology, neuroscience, genetics). However, less emphasis is placed on large-scale team science, or developing an emphasis on interdisciplinary training across the social and natural sciences. Encouraging students to enroll in interdisciplinary training programs to conduct research with scientists who are not in their traditional discipline is key to promoting creative and influential cultural neuroscience research. For nations in the developing world, creating training infrastructures within high schools, colleges and universities in both cultural and biological sciences that emphasize interdisciplinary training may provide an advantage for enhancing quality of education, but also may require additional financial resources in order to ensure that students and researchers receive the necessary education to meet their nation’s health agenda priorities. For instance, simply providing teachers and students with accurately translated research and educational materials is a necessary, but sometimes costly, component to ensuring that developed and developing nations have the resources they need to generate novel knowledge. Providing effective guidelines for promoting interdisciplinary training infrastructures in cultural neuroscience across educational institutions in both developing and developed worlds is necessary in order for students and researchers to achieve quality scientific and educational advancement.
Future Promise
There exists an increasing awareness of the importance of team science (Wuchty, Jones, Uzzi, 2007), such that novel, influential scientific advances are more likely to arise from research conducted in increasingly large research teams. For instance, large-scale team science is more frequently cited (Valderas, 2007); a team-authored paper is 6.3 times more likely to be cited at least 1,000 times compared to a solo-authored paper (Wuchty, Jones, Uzzi, 2007). Encouraging junior and senior scientists to engage in large-scale global team science to solve research questions related to the origins of human diversity is one of the most promising directions for future research progress. Hong & Page (2004) have recently argued with computational modeling that a team of randomly selected agents will outperform a team of best-performing agents, primarily because best-performing agents tend to think and behave similarly, leading to a reduced solution space during problem solving. Teams of diverse problem solvers that encourage cultural sensitivity and equality in conversational turn-taking may further enhance their collective intelligence, or the general ability of the group to perform a range of tasks (Woolley et al., 2010).
When large-scale team science encourages problem solving with researchers from distinct nations and cultures, it is likely that more novel and creative solutions may be achieved for solving some of the oldest questions regarding the origin of human nature and human diversity. Although students and researchers from the developing world may require additional research capacity and infrastructure to discover novel scientific knowledge, collaborating together with students and researchers from both the developed and developing world in large research teams may provide effective solutions to expanding the breadth and sophistication of theory and evidence of cultural neuroscience research in the future.
References
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Arnett JJ. The neglected 95%: why American psychology needs to become less American. American Psychologist. 2008;63((7)):602–614. doi: 10.1037/0003-066X.63.7.602. [DOI] [PubMed] [Google Scholar]
- Barkow JH, Cosmides L, Tooby J, editors. The adapted mind: evolutionary psychology and the generation of culture. New York: Oxford University Press; 1992. [Google Scholar]
- Barrett LF. Variety is the spice of life: psychological constructionist approach to understanding variability in emotion. Cognition and Emotion. 2009;23:1284–1306. doi: 10.1080/02699930902985894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beacher FD, Gray MA, Minati L, Whale R, Harrison NA, Critchley HD. Acute tryptophan depletion attenuates conscious appraisal of social emotional signals in healthy female volunteers. Psychopharmacology (Berl) 2011;213((2–3)):603–613. doi: 10.1007/s00213-010-1897-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beja-Pereira A, Luikart G, England PR, Bradley DG, Jann OC, Bertorelle G, Chamberlain AT, Nunes TP, Metodiev S, Ferrand N, Erhardt G. Gene-culture coevolution between cattle milk protein genes and human lactase genes. Nature Genetics. 2003;35:311–313. doi: 10.1038/ng1263. [DOI] [PubMed] [Google Scholar]
- Boyd R, Richerson PJ. Culture and the evolutionary process. Chicago: The University of Chicago Press; 1985. [Google Scholar]
- Butler EA, Lee TL, Gross JJ. Emotion regulation and culture: Are the social consequences of emotion suppression culture-specific? Emotion. 2007;7:30–48. doi: 10.1037/1528-3542.7.1.30. [DOI] [PubMed] [Google Scholar]
- Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nature Neuroscience. 2007;10:1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Canli T, Qiu M, Omura K, Congdon E, Haas BW, Amin Z, Herrmann MJ, Constable RT, Lesch KP. Neural correlates of epigenesis. Proceedings of the National Academy of Sciences USA. 2006;103((43)):16033–16038. doi: 10.1073/pnas.0601674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli-Sforza L, Feldman M. Cultural transmission and evolution: A Quantitative Approach. Princeton: Princeton University Press; 1981. [PubMed] [Google Scholar]
- Chae DH, Lincoln KD, Adler NE, Syme SL. Do experiences of racial discrimination predict cardiovascular disease among African American men? The moderating role of internalized negative racial group attitudes. Social Science and Medicine. 2010;71((6)):1182–1188. doi: 10.1016/j.socscimed.2010.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon BK, Im D, Harada T, Kim J, Mathur VA, Scimeca JM, Parrish TB, Park H, Chiao JY. Cultural influences on neural basis of intergroup empathy. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.04.031. [DOI] [PubMed] [Google Scholar]
- Cheon BK, Livingston RW, Hong Y-Y, Chiao JY. Gene x environment interaction on intergroup bias: The role of 5-HTTLPR and perceived outgroup threat. doi: 10.1093/scan/nst111. (under review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao JY. At the frontier of cultural neuroscience: Introduction to the special issue. Social Cognitive and Affective Neuroscience. 2010;5((2–3)):109–110. doi: 10.1093/scan/nsq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao JY. Neural basis of social status hierarchy across species. Current Opinion in Neurobiology. 2010;20((6)):803–809. doi: 10.1016/j.conb.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Chiao JY. Cultural neuroscience: Visualizing culture-gene influences on brain function. In: Decety J, Cacioppo J, editors. Handbook of Social Neuroscience. UK: Oxford University Press; 2011. [Google Scholar]
- Chiao JY, Ambady N. Cultural neuroscience: Parsing universality and diversity across levels of analysis. In: Kitayama S, Cohen D, editors. Handbook of Cultural Psychology. NY: Guilford Press; 2007. pp. 237–254. [Google Scholar]
- Chiao JY, Blizinsky KD. Culture-gene coevolution of individualism-collectivism and the serotonin transporter gene (5-HTTLPR) Proceedings of the Royal Society B: Biological Sciences. 2010;277((1681)):529–537. doi: 10.1098/rspb.2009.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao JY, Blizinsky KD. Population disparities in mental health: Insights from cultural neuroscience. American Journal of Public Health. doi: 10.2105/AJPH.2013.301440. (under review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao JY, Cheon BK. The weirdest brains in the world. Behavioral and Brain Sciences. 2010;33:28–30. doi: 10.1017/S0140525X10000282. [DOI] [PubMed] [Google Scholar]
- Chiao JY, Harada T, Komeda H, Li Z, Mano Y, Saito DN, Parrish TB, Sadato N, Iidaka T. Neural basis of individualistic and collectivistic views of self. Human Brain Mapping. 2009;30((9)):2813–2820. doi: 10.1002/hbm.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao JY, Harada T, Komeda H, Li Z, Mano Y, Saito DN, Parrish TB, Sadato N, Iidaka T. Dynamic cultural influences on neural representations of the self. Journal of Cognitive Neuroscience. 2010;22((1)):1–11. doi: 10.1162/jocn.2009.21192. [DOI] [PubMed] [Google Scholar]
- Chiao JY, Harada T, Komeda H, Li Z, Mano Y, Saito DN, Parrish TB, Sadato N, Iidaka T. Neural basis of individualistic and collectivistic views of self. Human Brain Mapping. 2009a;30:2813–2820. doi: 10.1002/hbm.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao JY, Hariri AR, Harada T, Mano Y, Sadato N, Parrish TB, Iidaka T. Theory and methods in cultural neuroscience. Social Cognitive and Affective Neuroscience. 2010;5((2–3)):356–361. doi: 10.1093/scan/nsq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao JY, Hariri AR, Harada T, Mano Y, Hechtman LA, Sadato N, Parrish TB, Iidaka T. Cultural values predict amygdala response to emotion. (in revision) [Google Scholar]
- Chiao JY, Hechtman LA, Hariri AR, Harada T, Mano Y, Sadato N, Parrish TB, Iidaka T. Dynamic cultural values predict amygdala response to emotion. (in preparation) [Google Scholar]
- Chiao JY, Iidaka T, Gordon HL, Nogawa J, Bar M, Aminoff E, Sadato N, Ambady N. Cultural specificity in amygdala response to fear faces. Journal of Cognitive Neuroscience. 2008;20((12)):2167–2174. doi: 10.1162/jocn.2008.20151. [DOI] [PubMed] [Google Scholar]
- Chiao JY, Immordino-Yang MH. Modularity and the cultural mind: Contributions of cultural neuroscience to cognitive theory. Perspectives on Psychological Science. doi: 10.1177/1745691612469032. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins PY, Patel V, Joesti SS, March D, Insel TR, Daar AS on behalf of the Scientific Advisory Board and the Executive Committee of the Grand Challenges in Global Mental Health. Grand challenges in mental health. Nature. 2010;475(7354):27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B, Habel U, Robinson S, Windischberger C, Kryspin-Exner I, Gur RC, Moser E. Amygdala activation during recognition of emotions in a foreign ethnic group is associated with duration of stay. Social Neuroscience. 2012;4((4)):294–307. doi: 10.1080/17470910802571633. [DOI] [PubMed] [Google Scholar]
- Dovidio JF, Fiske ST. Under the radar: how unexamined biases in decision-making processes in interactions can contribute to health care disparities. American Journal of Public Health. 2012;102((5)):945–952. doi: 10.2105/AJPH.2011.300601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabant EM, Ramel W, Edge MD, Hyde LW, Kuo JR, Goldin PR, Hariri AR, Gross JJ. Neural mechanisms underlying 5-hTTLPR-related sensitivity to acute stress. American Journal of Psychiatry. 2012;169((4)):397–405. doi: 10.1176/appi.ajp.2011.10111699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DH, Kravitz EA. Serotonin, social status and aggression. Current Opinion in Neurobiology. 1997;7((6)):812–819. doi: 10.1016/s0959-4388(97)80140-7. [DOI] [PubMed] [Google Scholar]
- Exner DV, Dries DK, Domanski MJ, Cohen JN. Lesser response of angiotensin-converting-enzyme inhibitor therapy in black as compared with white patients with left ventricular dysfunction. New England Journal of Medicine. 2001;344:1351–1377. doi: 10.1056/NEJM200105033441802. [DOI] [PubMed] [Google Scholar]
- Fincher CL, Thornhill R, Murray DR, Schaller M. Pathogen prevalence predicts human cross-cultural variability in individualism/collectivism. Proceedings of the Royal Society B. 2008;275:1279–1285. doi: 10.1098/rspb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor JA. Modularity of Mind: An Essay on Faculty Psychology. Cambridge, Mass.: MIT Press; 1983. [Google Scholar]
- Fox E, Ridgewell A, Ashwin C. Looking on the bright side: biased attention and the human serotonin gene. Proceedings of the Royal Society B: Biological Sciences. 2009 doi: 10.1098/rspb.2008.1788. doi:10.1098/rspb.2008.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaniga MS, Ivry R, Mangun GR. Cognitive neuroscience: The biology of the mind. New York: Norton; 2002. [Google Scholar]
- Gelfand MJ, Raver JL, Nishii L, Leslie LM, Lun J, Lim BC, et al. Differences between tight and loose cultures: A 33-nation study. Science. 2011;332((6033)):1100–1104. doi: 10.1126/science.1197754. [DOI] [PubMed] [Google Scholar]
- Gelfand MJ, Shteynberg G, Lee T, Lun J, Lyons S, Bell C, Chiao JY, Bruss CB, Al Dabbagh M, Aycan Z, Abdel-Latif AH, Dagher M, Khashan H, Soomro N. The cultural contagion of conflict. Philosophical Transactions of the Royal Society B: Biological Sciences. 2012;367(1589):692–703. doi: 10.1098/rstb.2011.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golby AJ, Gabrieli JDE, Chiao JY, Eberhardt JL. Differential fusiform responses to same- and other-race faces. Nature Neuroscience. 2001;4((8)):845–850. doi: 10.1038/90565. [DOI] [PubMed] [Google Scholar]
- Handy TC, editor. Event-Related Potentials: A Methods Handbook. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- Harada T, Li Z, Chiao JY, editors. Differential dorsal and ventral medial prefrontal representations of the implicit self modulated by individualism and collectivism: an fMRI study. Social Neuroscience. 2010;22:1–15. doi: 10.1080/17470910903374895. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana BS, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hechtman LA, Pornpattanangkul N, Chiao JY. Psychological constructionism and cultural neuroscience. Behavioral and Brain Sciences. 2012;35((3)):152–153. doi: 10.1017/S0140525X11001713. [DOI] [PubMed] [Google Scholar]
- Heeger DJ, Ress D. What does fMRI tell us about neuronal activity? Nature Reviews Neuroscience. 2002;3:142–151. doi: 10.1038/nrn730. [DOI] [PubMed] [Google Scholar]
- Henrich J, Heine S, Norenzayan A. The weirdest people in the world? Behavioral and Brain Sciences. 2010;33((2–3)):61–83. doi: 10.1017/S0140525X0999152X. [DOI] [PubMed] [Google Scholar]
- Hofstede G. Culture’s consequences: Comparing values, behaviors, institutions and organizations across nations. Thousand Oaks, CA: Sage Publications; 2001. [Google Scholar]
- Hong L, Page SE. Groups of diverse problem solvers can outperform groups of high-ability problem solvers. Proceedings of National Academy of Sciences. 2004;101((46)):16385–16389. doi: 10.1073/pnas.0403723101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahoda G. Culture, biology and development across history. In: Keller H, Poortinga YH, Schoemerich A, editors. Between culture and biology: Perspectives on ontogenetic development. Cambridge, UK: Cambridge University Press; 2002. pp. 13–29. [Google Scholar]
- Jarrell H, Hoffman JB, Kaplan JR, Berga S, Kinkead B, Wilson ME. Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in female rhesus monkeys. Physiology & Behavior. 2008;93((4–5)):807–819. doi: 10.1016/j.physbeh.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N. Inaugural Article: Functional specificity in the human brain: A window into the functional architecture of the mind. Proceedings of the National Academy of Sciences USA. 2010;107((25)):11163–11170. doi: 10.1073/pnas.1005062107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Ustun TB. The WHO World Mental Health Surveys: global perspectives on the epidemiology of mental disorders. New York: Cambridge University Press; 2008. [Google Scholar]
- Kim HS, Sherman DK, Mojaverian T, Sasaki JY, Park J, Suh EM, Taylor S. Gene-cultur interaction: Oxytocin receptor polymorphism (OXTR) and emotion regualtion. Social Psychological and Personality Science. 2011;2:665–672. [Google Scholar]
- Kim HS, Sherman DK, Sasaki JY, Xu J, Chu TQ, Ryu C, Suh EM, Graham K, Taylor SE. Culture, Distress, and Oxytocin Receptor Polymorphism (OXTR) Interact to Influence Emotional Support Seeking. Proceedings of the National Academy of Sciences. 2010;107:15717–15721. doi: 10.1073/pnas.1010830107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Sherman DK, Taylor SE. Culture and social support. American Psychologist. 2008;63:518–526. doi: 10.1037/0003-066X. [DOI] [PubMed] [Google Scholar]
- Kim HS, Sherman DK, Ko D, Taylor SE. Pursuit of Happiness and Pursuit of Harmony: Culture, relationships, and social support seeking. Personality and Social Psychology Bulletin. 2006;32:1595–1607. doi: 10.1177/0146167206291991. [DOI] [PubMed] [Google Scholar]
- Kiser D, Steemers B, Branchi I, Homberg JR. The reciprocal interaction between serotonin and social behaviour. Neuroscience & Biobehavioral Reviews. 2012;36((2)):786–798. doi: 10.1016/j.neubiorev.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Williams KA, Lacerda F, Stevens KN, Lindblom B. Linguistic experience alters phonetic perception in infants by 6 months. Science. 1992;255:606–608. doi: 10.1126/science.1736364. [DOI] [PubMed] [Google Scholar]
- Laland KN, Odling-Smee J, Feldman MW. Niche construction, biological evolution and cultural change. Behavioural and Brain Sciences. 2000;23((1)):131–146. doi: 10.1017/s0140525x00002417. [DOI] [PubMed] [Google Scholar]
- Larson ET, Summers CH. Serotonin reverses dominant social status. Behavioural Brain Research. 2001;121((1–2)):95–102. doi: 10.1016/s0166-4328(00)00393-4. [DOI] [PubMed] [Google Scholar]
- Li SC. Biocultural orchestration of developmental plasticity across levels: The interplay of biology and culture in shaping the mind and behavior across the life span. Psychological Bulletin. 2003;129((2)):171–194. doi: 10.1037/0033-2909.129.2.171. [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: A meta-analytic review. Behavioral and Brain Sciences. 2012;35:121–143. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. What are emotions and how are they created in the brain. Behavioral and Brain Sciences. 2012;35:172–185. doi: 10.1017/s0140525x1100183x. [DOI] [PubMed] [Google Scholar]
- Livingston R, Drwecki B. Why are some individuals not raciallly biased? Susceptibility to affective conditioning prediccts non prejudice toward Blacks. Psychological Science. 2007;18((9)):816–823. doi: 10.1111/j.1467-9280.2007.01985.x. [DOI] [PubMed] [Google Scholar]
- Lumsden CJ, Wilson EO. Genes, Mind and Culture: The Coevolutionary Process. Cambridge: Harvard University Press; 1981. [Google Scholar]
- Ly M, Haynes MR, Barter JW, Weinberger DR, Zink CF. Subjective socioeconomic status predicts human ventral striatal responses to social status information. Current Biology. 2011;21((9)):704–707. doi: 10.1016/j.cub.2011.03.050. [DOI] [PubMed] [Google Scholar]
- Ma Y, Wang C, Han S. Neural responses to perceived pain in others predict real-life monetary donations in different socioeconomic contexts. Neuroimage. 2011;57:1273–1280. doi: 10.1016/j.neuroimage.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Byrd D, Touradji P, Stern Y. Acculturation, reading level, and neuropsychological test performance among African American elders. Applied Neuropsychology. 2004;11:37–46. doi: 10.1207/s15324826an1101_5. [DOI] [PubMed] [Google Scholar]
- Markus HR, Kitayama S. Culture and the self: implications for cognition, emotion and motivation. Psychological Review. 1991;98:224–253. [Google Scholar]
- Matsumoto D, Yoo SH, Fontaine J, Anguas-Wong AM, Arriola M, Ataca B, Bond MH, Boratav HB, Breugelmans SM, Cabecinhas R, Chae J, Chin WH, Comunian AL, DeGere DN, Djunaidi A, Fok HK, Friedlmeier W, Ghosh A, Glamcevski M, Granskaya JV, Groenvynck H, Harb C, Haron F, Joshi R, Kakai H, Kashima E, Khan W, Kurman J, Kwantes CT, Mahmud SH, Mandaric M, Nizharadze G, Odusanya JOT, Ostrosky-Solis F, Palaniappan AK, Papastylianou D, Safdar S, Setiono K, Shigemasu E, Singelis TM, Polackova Solcova Iva, Spieβ E, Sterkowicz S, Sunar D, Szarota P, Vishnivetz B, Vohra N, Ward C, Wong S, Wu R, Zebian S, Zengeya A. Mapping expressive differences around the world: The relationship between emotional display rules and Individualism v. Collectivism. Journal of Cross-Cultural Psychology. 2008;39:55–74. [Google Scholar]
- Mathur VA, Harada T, Lipke T, Chiao JY. Neural basis of extraordinary empathy and altruistic motivation. Neuroimage. 2010;51((4)):1468–1475. doi: 10.1016/j.neuroimage.2010.03.025. [DOI] [PubMed] [Google Scholar]
- Mathur VA, Harada T, Chiao JY. Racial identification modulates default network activity for same- and other-race faces. Human Brain Mapping. 2012;33((8)):1883–1893. doi: 10.1002/hbm.21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz DS, Pinard G, Zuroff DC, Annable L, Young SN. The effect of tryptophan on social interaction in everyday life: a placebo-controlled study. Neuropsychopharmacology. 2001;25((2)):277–289. doi: 10.1016/S0893-133X(01)00219-6. [DOI] [PubMed] [Google Scholar]
- Mrazek AJ, Chiao JY, Blizinsky KD, Lun J, Gelfand MJ. Culture-gene coevolution of tightness-looseness and the serotonin transporter gene. doi: 10.1007/s40167-013-0009-x. (in revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Brown SM, Hariri AR. Serotonin transporter (5HTTLPR) genotype and amygdala activation: a meta-analysis. Biological Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Clark T, Flint J. Does measurement instrument moderate the association between the serotonin transporter gene and anxiety-related personality traits? A meta-analysis. Molecular Psychiatry. 2005;10:415–419. doi: 10.1038/sj.mp.4001627. [DOI] [PubMed] [Google Scholar]
- Muscatell KA, Morelli SA, Falk EB, Way BM, Pfeifer JH, Galinsky AD, Lieberman MD, Dapretto M, Eisenberger NI. Social status modulates neural activity in the mentalizing network. Neuroimage. 2012;60((3)):1771–1777. doi: 10.1016/j.neuroimage.2012.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaidis GA, Gray JR. ADHD and the DRD4 exon III 7-repeat polymorphism: An International meta-analysis. Social Cognitive & Affective Neuroscience. 2009;5((2–3)):188–193. doi: 10.1093/scan/nsp049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbett RE, Peng K, Choi I, Norenzayan A. Culture and systems of thought: Holistic versus analytic cognition. Psychological Review. 2001;108((2)):291–310. doi: 10.1037/0033-295x.108.2.291. [DOI] [PubMed] [Google Scholar]
- Odling-Smee FJ, Laland KN, Feldman MW. Niche construction: The neglected process in evolution. Princeton University Press; 2003. [Google Scholar]
- Olsson A, Ebert JP, Banaji MR, Phelps EA. The Role of Social Groups in the Persistence of Learned Fear. Science. 2005;309:785–787. doi: 10.1126/science.1113551. [DOI] [PubMed] [Google Scholar]
- Phinney JS. The Multigroup Ethnic Identity Measure: A New Scale for Use with Diverse Groups. Journal of Adolescent Research. 1992;7((2)):156–176. [Google Scholar]
- Pratto F, Sidanius J, Stallworth LM, Malle BF. Social dominance orientation: A personality variable predicting social and political attitudes. Journal of Personality and Social Psychology. 1994;67:741–763. [Google Scholar]
- Perrachione TK, Chiao JY, Wong PC. Asymmetric cultural effects on perceptual expertise underlie an own-race bias for voices. Cognition. 2010;114((1)):42–55. doi: 10.1016/j.cognition.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh MJ, McGuire MT, Brammer GL, Pollack DB, Yuwiler A. Serotonergic mechanisms promote dominance acquisition in adult male vervet monkeys. Brain Research. 1991;559((2)):181–190. doi: 10.1016/0006-8993(91)90001-c. [DOI] [PubMed] [Google Scholar]
- Ray RD, Shelton AL, Hollon NG, Matsumoto D, Frankel CB, Gross JJ, Gabrieli JD. Interdependent self-construal and neural representations of self and mother. Social Cognitive and Affective Neuroscience. 2010;5((2–3)):318–323. doi: 10.1093/scan/nsp039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losin EA, Iacoboni M, Martin A, Dapretto M. Own-gender imitation activates the brain’s reward circuitry. Social Cognitive and Affective Neuroscience. 2012;7((7)):804–810. doi: 10.1093/scan/nsr055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losin EA, Iacoboni M, Martin A, Dapretto M. Race modulates neural activity during imitation. NeuroImage. 2012;59((4)):3594–3603. doi: 10.1016/j.neuroimage.2011.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki JY, Kim H, Xu J. Religion and well-being: An analysis of an oxytocin receptor polymorphism (OXTR) and culture. Journal of Cross-Cultural Psychology. 2011;42:1394–1405. [Google Scholar]
- Sasaki JY, Kim HS, Mojaverian T, Kelley LDS, Park IY, Janušonis S. Religion priming differentially increases prosocial behavior among variants of the dopamine D4 receptor (DRD4) gene. Social Cognitive and Affective Neuroscience. doi: 10.1093/scan/nsr089. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savani K, Markus HR, Conner AL. Let your preference be your guide? Preferences and choices are more tightly linked for North Americans than for Indians. Journal of Personality and Social Psychology. 2008;95:861–876. doi: 10.1037/a0011618. [DOI] [PubMed] [Google Scholar]
- Saxe R, Schulz LE, Jiang YV. Reading minds versus following rules: Dissociating theory of mind and executive control in the brain. Social Neuroscience. 2006;1((3–4)):284–298. doi: 10.1080/17470910601000446. [DOI] [PubMed] [Google Scholar]
- Sellers RM, Shelton JN. The role of racial identity in perceived racial discrimination. Journal of Personality and Social Psychology. 2003;84((5)):1079–1092. doi: 10.1037/0022-3514.84.5.1079. [DOI] [PubMed] [Google Scholar]
- Sellers RM, Smith MA, Shelton JN, Rowley SAJ, Chavous TM. Multidimensional model of racial identity: A reconceptualization of African American racial identity. Personality and Social Psychology Review. 1998;2((1)):18. doi: 10.1207/s15327957pspr0201_2. [DOI] [PubMed] [Google Scholar]
- Shields AE, Fortun M, Hammonds E, King PA, Lerman C, Rapp R, Sullivan PF. The use of race variables in genetic studies of complex traits and the goal of reducing health disparities: A transdisciplinary perspective. American Psychologist. 2005;6((1)):77–103. doi: 10.1037/0003-066X.60.1.77. [DOI] [PubMed] [Google Scholar]
- Smith CO, Levine DW, Smith EP, Dumas J, Prinz RJ. A developmental perspective of the relationship of racial-ethnic identity to the self-construct, achievement, and behavior in African-American children. Cultural Diversity and Ethnic Minority Psychology. 2009;15((2)):145–157. doi: 10.1037/a0015538. [DOI] [PubMed] [Google Scholar]
- Snibbe A, Markus HR. You can't always get what you want: Educational attainment, agency, and choice. Journal of Personality and Social Psychology. 2005;88:703–720. doi: 10.1037/0022-3514.88.4.703. [DOI] [PubMed] [Google Scholar]
- Stephens NM, Markus HR, Fryberg SA. Social class disparities in health and education: Reducing inequality by applying a sociocultural self model of behavior. Psychological Review. 2012;119:723–744. doi: 10.1037/a0029028. [DOI] [PubMed] [Google Scholar]
- Stone L, Lurquin PF, Cavalli-Sforza LL. Genes, culture and human evolution: A synthesis. Oxford, UK: Wiley-Blackwell; 2007. [Google Scholar]
- Taylor SE, Sherman DK, Kim HS, Jarcho J, Takagi K, Dunagan MS. Culture and social support: Who seeks it and why? Journal of Personality and Social Psychology. 2004;87:354–362. doi: 10.1037/0022-3514.87.3.354. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Welch WT, Kim HS, Sherman DK. Cultural differences in the impact of social support on psychological and biological stress responses. Psychological Science. 2007;18:831–837. doi: 10.1111/j.1467-9280.2007.01987.x. [DOI] [PubMed] [Google Scholar]
- Tishkoff SA, Kidd KK. Implications of biogeography of human populations for ‘race’ and medicine. Nature Genetics. 2004;36:S21–S27. doi: 10.1038/ng1438. [DOI] [PubMed] [Google Scholar]
- Triandis HC, Gelfand MJ. Converging measurement of horizontal and vertical individualism and collectivism. Journal of Personality and Social Psychology. 1998;74:118–128. [Google Scholar]
- Valderas JM. Why do team-authored papers get cited more? Science. 2007;317(5844):1496–1498. doi: 10.1126/science.317.5844.1496b. [DOI] [PubMed] [Google Scholar]
- Wang VA, Sue S. In the eye of the storm: Race and the genomics in research and practice. American Psychologist. 2005;60:37–45. doi: 10.1037/0003-066X.60.1.37. [DOI] [PubMed] [Google Scholar]
- Watson KK, Ghodasra JH, Platt ML. Serotonin transporter genotype modulates social reward and punishment in rhesus macaques. PLoS ONE. 2009;4((1)):e4156. doi: 10.1371/journal.pone.0004156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way BM, Taylor SE. A polymorphism in the serotonin transporter gene moderates cardiovascular reactivity to psychosocial stress. Psychosomatic Medicine. 2011;73((4)):310–317. doi: 10.1097/PSY.0b013e31821195ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way B, Lieberman MD. Is there a genetic contribution to cultural differences? Collectivism, individualism, and genetic markers of social sensitivity. Social Cognitive and Affective Neuroscience. 2010;5:203–211. doi: 10.1093/scan/nsq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, Joyce PR, Karam EG, Lee CK, Lellouch J, Lepine JP, Newman SC, Rubio-Stipec M, Wells JE, Wickramaratne PJ, Wittchen HU, Yeh EK. Cross-national epidemiology of major depression and bipolar disorder. Journal of American Medical Association. 1996;276:293–299. [PubMed] [Google Scholar]
- Williams DR, John DA, Oyserman D, Sonnega J, Mohammed SA, Jackson JS. Research on discrimination and health: an exploration study of unresolved conceptual and measurement issues. American Journal of Public Health. 2012;102((5)):975–978. doi: 10.2105/AJPH.2012.300702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winberg S, Nilsson G, Olsén K. Social rank and brain levels of monoamines and monoamine metabolites in Arctic charr, Salvelinus alpinus (L.) Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 1991;168((2)):241–246. doi: 10.1007/BF00190404. [DOI] [PubMed] [Google Scholar]
- Woolley AW, Chabris CF, Pentland A, Hashmi N, Malone TW. Evidence for a collective intelligence factor in the performance of human groups. Sciences. 2010;330(6004):686–688. doi: 10.1126/science.1193147. [DOI] [PubMed] [Google Scholar]
- Wutchy S, Jones BF, Uzzi B. The increasing dominance of teams in production of knowledge. Science. 2007;316(5827):1036–1039. doi: 10.1126/science.1136099. [DOI] [PubMed] [Google Scholar]