Abstract
Monocyte-derived macrophages represent an important cell type of the innate immune system. Mouse models studying macrophage biology suffer from the phenotypic and functional differences between murine and human monocyte-derived macrophages. Therefore, we here describe an in vitro model to generate and study primary human macrophages. Briefly, after density gradient centrifugation of peripheral blood drawn from a forearm vein, monocytes are isolated from peripheral blood mononuclear cells using negative magnetic bead isolation. These monocytes are then cultured for six days under specific conditions to induce different types of macrophage differentiation or polarization. The model is easy to use and circumvents the problems caused by species-specific differences between mouse and man. Furthermore, it is closer to the in vivo conditions than the use of immortalized cell lines. In conclusion, the model described here is suitable to study macrophage biology, identify disease mechanisms and novel therapeutic targets. Even though not fully replacing experiments with animals or human tissues obtained post mortem, the model described here allows identification and validation of disease mechanisms and therapeutic targets that may be highly relevant to various human diseases.
Keywords: Immunology, Issue 76, Infection, Medicine, Cellular Biology, Molecular Biology, Inflammation, Monocyte-Macrophage Precursor Cells, Myeloid Cells, Immune System, Macrophages, Mononuclear Phagocyte System, Cells, in vitro model, human, cell culture, differentiation, polarization
Introduction
Monocyte-derived macrophages represent an important cellular component of the innate immune system and contribute to many acute or chronic inflammatory processes 1. Macrophages play an important role in many inflammatory diseases like atherosclerosis or cancer 2. Macrophages show a high degree of plasticity and are able to assume different phenotypes depending on the local micromilieu 3. Thus, studying macrophage differentiation and heterogeneity is essential for increasing our knowledge of the pathophysiology of many diseases and to allow identification of novel therapeutic targets and development of novel therapies.
In many cases, murine models are used to investigate the pathophysiology of specific diseases. However, studying macrophage biology using mouse models is accompanied by several shortcomings: (1) The proportion of leukocyte subset numbers (i.e. monocytes and granulocytes) in peripheral blood of mice or humans differs significantly suggesting different roles of monocytes in murine and human pathophysiology. (2) There are substantial differences in gene expression between murine and human peripheral blood monocytes suggesting substantial differences in their function during health and disease 4. (3) A number of markers that are used to identify murine monocytes and macrophages (F4/80, LyC, etc.) does not exist in human myeloid cells, making the transfer of findings in mouse models to the human situation rather difficult.
Thus, in order to increase our understanding of macrophage differentiation and heterogeneity in human disease, we need to make use of models working with human macrophages. Therefore, we here describe a model of human primary macrophage generation that is easy to use and allows study of human monocyte-derived macrophages in vitro under various conditions resulting in different macrophage polarization types. In several studies, we have used the in vitro model of monocyte-derived primary human macrophages to analyze macrophage biology and its potential relevance to human atherosclerosis 5-7.
Even though not fully replacing experiments with animals or human tissues obtained post mortem, the model described here allows identification and validation of disease mechanisms and therapeutic targets that may be highly relevant to various human diseases.
Protocol
1. Protocol
- Prepare Buffers as follows:
- Prepare buffer for PBMC isolation: "Wash buffer" = 0.02% EDTA in PBS (use 0.5 M EDTA).
- Prepare buffer for monocyte isolation: "MACS rinsing buffer" = 0.5% BSA (250 mg) + 2 mM EDTA (200 μl) + PBS (50 ml). Degas the buffer.
- Prepare buffer for FACS staining and storage of cells: "FACS buffer" = 10% FCS in PBS and "fixation buffer" = 1% PFA in PBS.
Draw 30 ml whole blood from a forearm vein. Use EDTA as anticoagulant.
- Isolate PBMC (histopaque) as follows:
- Prepare two sterile 50 ml tubes (no poly-styrene).
- Dilute blood from step 2 with PBS 1:1.
- Add 25 ml histopaque + 25 ml whole blood/PBS per tube. Make sure that histopaque and blood do not mix.
- Centrifuge at 400 x g for 30 min at RT, no brake.
- Aspirate plasma and discard.
- Aspirate opaque interface and pipette into clean 50 ml tube.
- Add 20 ml of 0.02% EDTA in PBS.
- Centrifuge at 250 x g for 5 min at 4 °C.
- Discard supernatant.
- Add 10 ml of 0.02% EDTA in PBS.
- Count cells as follows:
- Mix well by vortexing for 10 sec.
- Take 200 μl cell suspension + 300 μl 0.02% EDTA in PBS + 500 μl of Trypan blue (200-500 cells/10 squares, otherwise change dilution factor).
- Perform negative isolation of monocytes as follows:
- Centrifuge cells obtained in step 3.10 for 10 min, 120 x g. Discard supernatant.
- Wash cell pellet 2x by adding 10 ml of PBS + 0.02% EDTA and centrifuge for 10 min, 120 x g.
- Add 9 ml sterile water for 3 sec, add 1 ml 10X PBS.
- Wash once more with PBS + 0.02% EDTA and centrifuge 10 min, 120 x g.
- Count during centrifugation.
- Dilute in EasySep buffer at 5 x 107/ml.
- Add 50 μl/ml monocyte enrichment cocktail.
- Incubate for 10 min at 4 °C.
- Vortex beads for 30 sec.
- Add 50 μl/ml beads.
- Incubate for 5 min at 4 °C.
- Fill up with EasySep buffer to complete volume of 2.5 ml. The solution now appears brownish.
- Put into magnet.
- Wait 2.5 min at RT. During this time non-monocytic cells bound to the magnetic bead will move to the tube wall and stick there.
- Pour buffer with non-binding monocytes into fresh sterile tube. This solution looks whitish.
- Wash once with PBS + 0.02% EDTA and centrifuge for 10 min, 120 x g.
Plate cells in a plastic dish or multiwall plate at a density of 0.5 x 106/cm2. Add 1 ml of culture media/1 x 106 cells.
Culture cells under conditions of interest for 6-14 days.
Change media after 3 days by replacing 50% of the media with fresh media (see Table 1 for details).
After six days harvest cells for mRNA isolation, flow cytometry, Western blotting, etc.
Representative Results
Using the protocol described above, we routinely obtain 25.1 x 106 ± 2.2 x 106 monocytes/100 ml blood (average ± standard error from 26 independent experiments, Figure 1A). Monocyte purity as determined by flow cytometric staining for CD14 is routinely greater than 95% (97.1 ± 0.4%, average ± standard error from 3 independent experiments, Figure 1B). Cell viability of freshly isolated monocytes as determined by trypan blue staining is routinely greater than 95% (data not shown). While initially cells are round-shaped and float, they usually start adhering within a few hours. Adherence is associated with a shape change toward a "fried egg" or spindle-like shape (Figure 2). After six days in culture with M-CSF (100 ng/ml) with or without addition of oxidized LDL (100 μg/ml for the last 24 hr), roughly 80% of the cells not exposed to oxLDL are viable, whereas treatment with oxLDL resulted in about 70% viable cells (Figure 3). Flow cytometry after six days of culture with M-CSF demonstrates that while the cells keep expressing the leukocyte marker CD45RO, they downregulate the monocyte marker CD14 (Figure 4). Specific conditions may induce macrophage polarization - thus M1- or M2-polarized macrophages express typical markers like IL-6, TNF-alpha (M1) or CD36 and CD206 (M2) (Figure 5). Macrophages can further be studied in functional experiments. For example, uptake of oxidized LDL can be studied using fluorescently labels LDL (Figure 6).
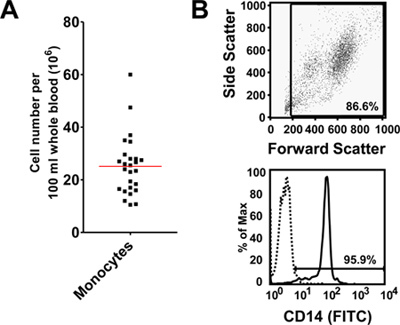 Figure 1. (A) Numbers of peripheral monocytes obtained after negative bead isolation. Cell numbers are normalized for 100 ml of peripheral blood. Data of 26 independent experiments. (B) Purity of monocytes obtained using negative bead isolation as determined by flow cytometry. Representative forward side scatter plot and histogram of CD14 staining, negative control shown as dotted line.
Figure 1. (A) Numbers of peripheral monocytes obtained after negative bead isolation. Cell numbers are normalized for 100 ml of peripheral blood. Data of 26 independent experiments. (B) Purity of monocytes obtained using negative bead isolation as determined by flow cytometry. Representative forward side scatter plot and histogram of CD14 staining, negative control shown as dotted line.
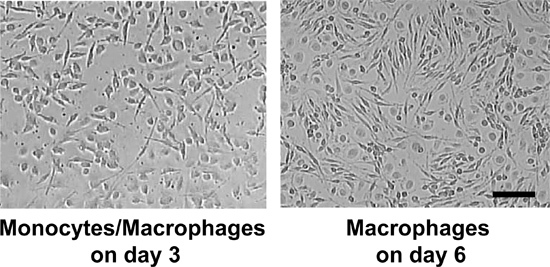 Figure 2. Cells after 3 and 6 days culture with M-CSF. Scale bar = 50 μm.
Figure 2. Cells after 3 and 6 days culture with M-CSF. Scale bar = 50 μm.
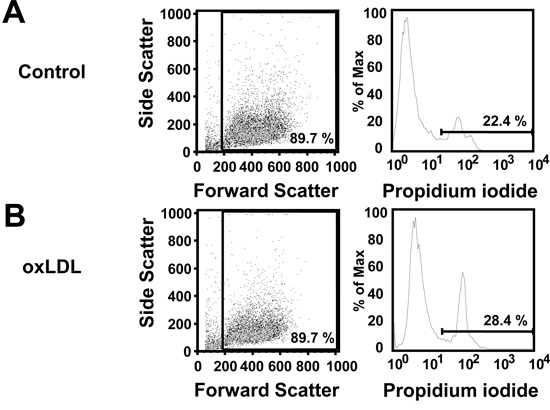 Figure 3. Viability of macrophages after 6 days in culture with M-CSF or after 6 days in culture with M-CSF and oxLDL (100 μg/ml) added for the last 24 hr as determined by propidium iodide (PI) staining. Representative forward side scatter plots and histograms for PI staining.
Figure 3. Viability of macrophages after 6 days in culture with M-CSF or after 6 days in culture with M-CSF and oxLDL (100 μg/ml) added for the last 24 hr as determined by propidium iodide (PI) staining. Representative forward side scatter plots and histograms for PI staining.
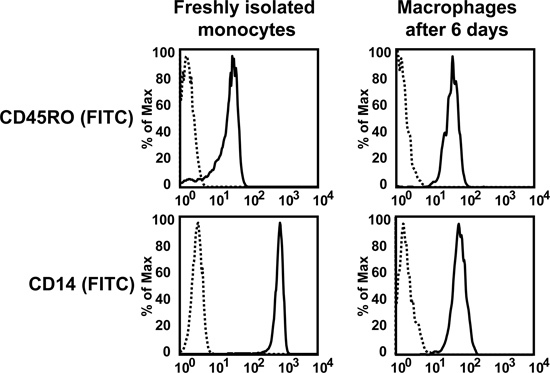 Figure 4. Flow cytometry for the monocyte marker CD14 on freshly isolated monocytes or monocyte-derived macrophages after six days in culture with M-CSF (100 ng/ml).
Figure 4. Flow cytometry for the monocyte marker CD14 on freshly isolated monocytes or monocyte-derived macrophages after six days in culture with M-CSF (100 ng/ml).
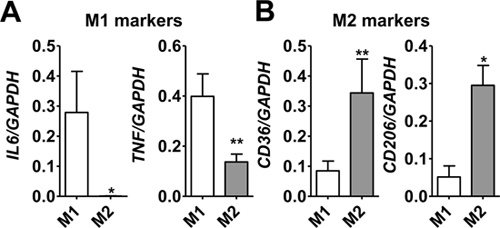 Figure 5. Gene expression of IL-6, TNF-alpha, CD36 and CD206 (mannose receptor) in primary human macrophages differentiated with M-CSF (100 ng/ml, 6 days) and the polarized toward an M1 phenotype (LPS, 100 ng/ml, and IFN-gamma, 20 ng/ml, for the last 18 hr) or M2 (IL-4, 20 ng/ml for the last 18 hr). Gene expression was measured by quantitative PCR. *P<0.05, **P<0.01.
Figure 5. Gene expression of IL-6, TNF-alpha, CD36 and CD206 (mannose receptor) in primary human macrophages differentiated with M-CSF (100 ng/ml, 6 days) and the polarized toward an M1 phenotype (LPS, 100 ng/ml, and IFN-gamma, 20 ng/ml, for the last 18 hr) or M2 (IL-4, 20 ng/ml for the last 18 hr). Gene expression was measured by quantitative PCR. *P<0.05, **P<0.01.
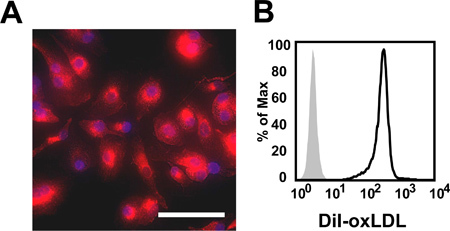 Figure 6. (A) Immunofluorescence image of M-CSF-induced macrophages human macrophages. Cells were exposed to 10 μg/ml DiI-labeled oxLDL for 4 hr. Nuclei were stained with DAPI. Scale bar = 50 μm. (B) Representative histogram of flow cytometric measurement of DiI-oxLDL uptake by M-CSF-induced human macrophages. Grey = untreated control, black line = oxLDL-treated macrophages.
Figure 6. (A) Immunofluorescence image of M-CSF-induced macrophages human macrophages. Cells were exposed to 10 μg/ml DiI-labeled oxLDL for 4 hr. Nuclei were stained with DAPI. Scale bar = 50 μm. (B) Representative histogram of flow cytometric measurement of DiI-oxLDL uptake by M-CSF-induced human macrophages. Grey = untreated control, black line = oxLDL-treated macrophages.
| Type | Conditions | Refs |
| M0 |
|
7-8 |
| M1 |
|
8 |
|
9-10 | |
|
9 | |
|
10 | |
|
10 | |
| M2a |
|
9-10 |
|
8 | |
|
10 | |
|
8 | |
| M2b |
|
8 |
| M2c |
|
10 |
| M4 |
|
7 |
| M-Hb |
|
11 |
| M-ox |
|
12 |
| Other macrophage types |
|
13 |
Table 1. Example for conditions and typical markers of established M1 or M2 macrophage polarization types (please see references for specific isolation procedures or cell culture conditions).
Discussion
Monocyte-derived macrophages represent the key cell type of the innate immune system. They play an important role in many inflammatory diseases including atherosclerosis or cancer 2. Thus, studying macrophage biology is essential for increasing our knowledge on the pathophysiology of many diseases and to allow development of novel therapies.
Many studies apply of mouse models overexpressing or lacking certain genes of interest. In the case of monocyte-derived macrophages, this seems not the best way to study processes relevant to human disease as there are substantial differences between murine and human monocytes and monocyte-derived cells 4. Thus, valid models making use of primary human cells seem a good alternative to study disease-relevant mechanisms of macrophage differentiation.
A clear advantage of the in vitro model presented here is that it does not rely on immortalized cell lines like THP-1 or others 14-15. While cell lines can be easily obtained and circumvent the need for regular blood draws, cell lines are phenotypically and functionally not identical to primary cells. Thus, Cho et al. have compared the gene signatures of primary human macrophages and compared them with previously published data obtained from THP-1 12,14. They found a significant, but moderate correlation between both cell types suggesting that THP-1 cells may not be the ideal model to study macrophage differentiation and heterogeneity in many cases. Also, there are various models how to differentiate non-adherent monocyte-like THP-1 cells towards macrophages. Even when using the probably most applicable protocol in which THP-1 cells are treated with PMA followed by five days resting in culture without PMA (PMAr), cells do not entirely behave like primary cells 16. Taken together, these findings suggest that for study of human disease mechanisms on a cellular level, primary macrophages seem more suitable than cell lines.
One has to be aware of other methods that allow isolation of primary human monocytes: adherence to plastic, positive bead isolation, or counter flow centrifugation elutriation 17. The monocyte enrichment cocktail used here contains antibodies against CD2, CD3, CD16, CD19, CD20, CD56, CD66b, CD123, glycophorin A and dextran-coated magnetic particles. These antibodies bind to epitopes on leukocytes other than monocytes that can subsequently bind the magnetic particles and are held in the tube by the magnetic field while all unbound monocyte can be transferred into a second tube. Furthermore, the enrichment cocktail contains antibody to human Fc receptors (which are highly expressed by monocytes) that prevent non-specific binding to monocytes. Accordingly, the method presented here yields "untouched" non-activated monocytes with a high degree of purity.
While adherence to plastic is easy and cost efficient, it results in cell activations. Thus, adherence to plastic induces gene and protein expression through binding and activation of integrins. Positive bead isolation is similar to the method described in this paper. However, as the antibodies used bind directly to the cell type of interest it may also result in cell activation. This may affect behavior of the cells; furthermore, bound antibodies may interfere with subsequent experiments. Finally, cells can be isolated by counter flow centrifugation elutriation. While this method does not require antibodies or chemicals resulting in high recovery of untouched cells, it does not allow specific isolation of monocyte subsets which have similar sedimentation properties.
There are some points that need to be kept in mind when applying this model. Ficoll/Histopaque centrifugation should be performed at room temperature. EDTA in the wash buffer is necessary to inhibit integrin-dependent binding of platelets to monocytes. It is important to seed the monocytes at the right density in order to achieve optimal differentiation. Both seeding monocytes too loosely or too densely results in suboptimal growth and differentiation. Options for changes include seeding the cells on specific substrates or using different growth factors (e.g. GM-CSF 18) or addition of specific cell activators (e.g. cytokines or LPS 8, Table 1).
Using cells obtained from different individuals may result in a greater degree of variation regarding specific cellular functions. Thus, Martín-Fuentes et al. have demonstrated that monocyte-derived macrophages from different individuals display different capacities of LDL uptake 19. Interestingly, these differences remain stable over time suggesting that variation between cells from different donors are not due to different experimental conditions, but rather reflect individual genetic or epigenetic differences. On the one hand, this larger variation requires larger numbers to obtain significant results in specific experiments like oxLDL uptake. By contrast, these differences also enable researchers to study differences between healthy and diseased individuals on a cellular level thereby revealing novel disease mechanisms.
Overall, the protocol described here is easy to use, reproducible, and useful to obtain extremely pure monocyte populations that may then be differentiated towards macrophages. Depending on the culture conditions, various types of monocyte-derived macrophages can be studied allowing identification of specific physiological or pathophysiological processes relevant to human disease.
Disclosures
The authors do not have to disclose any conflict of interest.
Acknowledgments
We thank Nadine Wambsganss for excellent technical assistance. This work was supported by a grant from the Deutsche Forschungsgemeinschaft (GL599/1-1) and in part by a grant from the Innovation Fund FRONTIER (University of Heidelberg) to C.A.G.
References
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Gordon S, Mantovani A. Diversity and plasticity of mononuclear phagocytes. European Journal of Immunology. 2011;41:2470–2472. doi: 10.1002/eji.201141988. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front. Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- Ingersoll MA, et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115:10–19. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleissner CA, Sanders JM, Nadler J, Ley K. Upregulation of aldose reductase during foam cell formation as possible link among diabetes, hyperlipidemia, and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2008;28:1137–1143. doi: 10.1161/ATVBAHA.107.158295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleissner CA, et al. CXCL4 downregulates the atheroprotective hemoglobin receptor CD163 in human macrophages. Circ. Res. 2009. [DOI] [PMC free article] [PubMed]
- Gleissner CA, Shaked I, Little KM, Ley K. CXC chemokine ligand 4 induces a unique transcriptome in monocyte-derived macrophages. J. Immunol. 2010;184:4810–4818. doi: 10.4049/jimmunol.0901368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. J. Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- Verreck FAW, et al. Human il-23-producing type 1 macrophages promote but il-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4560–4565. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey-Giraud F, Hafner M, Ries CH. In vitro generation of monocyte-derived macrophages under serum-free conditions improves their tumor promoting functions. PLoS One. 2012;7:e42656. doi: 10.1371/journal.pone.0042656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle JJ, et al. Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. American Journal of Pathology. 2009;174:1097–1108. doi: 10.2353/ajpath.2009.080431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, et al. Induction of dendritic cell-like phenotype in macrophages during foam cell formation. Physiol Genomics. 2007;29:149–160. doi: 10.1152/physiolgenomics.00051.2006. [DOI] [PubMed] [Google Scholar]
- Vogt G, Nathan C. In vitro differentiation of human macrophages with enhanced antimycobacterial activity. The Journal of Clinical Investigation. 2011;121:3889–3901. doi: 10.1172/JCI57235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikita T, Porter G, Lawn RM, Shiffman D. Oxidized low density lipoprotein exposure alters the transcriptional response of macrophages to inflammatory stimulus. Journal of Biological Chemistry. 2001;276:45729–45739. doi: 10.1074/jbc.M106114200. [DOI] [PubMed] [Google Scholar]
- Qin ZY. The use of THP-1 cells as a model for mimicking the function and regulation of monocytes and macrophages in the vasculature. Atherosclerosis. 2012;221:2–11. doi: 10.1016/j.atherosclerosis.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Daigneault M, Preston JA, Marriott HM, Whyte MKB, Dockrell DH. The identification of markers of macrophage differentiation in pma-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One. 2010;5:e8668. doi: 10.1371/journal.pone.0008668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl LM, Wahl SM, Smythies LE, Smith PD. Current Protocols in Immunology. John Wiley & Sons, Inc; 2001. Isolation of human monocyte populations. [DOI] [PubMed] [Google Scholar]
- Waldo SW, et al. Heterogeneity of human macrophages in culture and in atherosclerotic plaques. Am. J. Pathol. 2008;172:1112–1126. doi: 10.2353/ajpath.2008.070513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Fuentes P, et al. Individual variation of scavenger receptor expression in human macrophages with oxidized low-density lipoprotein is associated with a differential inflammatory response. The Journal of Immunology. 2007;179:3242–3248. doi: 10.4049/jimmunol.179.5.3242. [DOI] [PubMed] [Google Scholar]


