Ubiquitin-specific protease 18 inhibits ubiquitination of TAK1–TAB complexes to restrict IL-2 production and promote Th17 differentiation and autoimmune responses.
Abstract
Reversible ubiquitin modification of cell signaling molecules has emerged as a critical mechanism by which cells respond to extracellular stimuli. Although ubiquitination of TGF-β–activated kinase 1 (TAK1) is critical for NF-κB activation in T cells, the regulation of its deubiquitination is unclear. We show that USP18, which was previously reported to be important in regulating type I interferon signaling in innate immunity, regulates T cell activation and T helper 17 (Th17) cell differentiation by deubiquitinating the TAK1–TAB1 complex. USP18-deficient T cells are defective in Th17 differentiation and Usp18−/− mice are resistant to experimental autoimmune encephalomyelitis (EAE). In response to T cell receptor engagement, USP18-deficient T cells exhibit hyperactivation of NF-κB and NFAT and produce increased levels of IL-2 compared with the wild-type controls. Importantly, USP18 is associated with and deubiquitinates the TAK1–TAB1 complex, thereby restricting expression of IL-2. Our findings thus demonstrate a previously uncharacterized negative regulation of TAK1 activity during Th17 differentiation, suggesting that USP18 may be targeted to treat autoimmune diseases.
Reversible ubiquitin modification of cell signaling molecules has emerged as a critical mechanism by which cells respond to extracellular stimuli. For example, ubiquitination of TRAF6 allows the activation of downstream kinase TGF-β–activated kinase 1 (TAK1), which is required for activation of NF-κB in response to various stimuli including IL-1, TNF, and Toll-like receptor agonists (Wang et al., 2001). In addition, TAK1 plays a central role in adaptive immunity by mediating signaling from T and B cell receptors (Skaug et al., 2009; Dai et al., 2012). Under steady state, TAK1 is constitutively associated with TAB1 and TAB2 which contain ubiquitin-binding motifs. Upon activation, TAB recognizes and is recruited to free polyubiquitin chains or ubiquitin chains targeted to a specific protein, which makes TAK1 spatially closer and thereby results in mutual phosphorylation and activation of TAK1 (Kanayama et al., 2004; Xia et al., 2009). In this context, the E3 ubiquitin ligase RBCK1, inducing ubiquitination and degradation of TABs, inhibits TNF- or IL-1–triggered activation of NF-κB (Tian et al., 2007). In contrast, accumulating evidence also suggests that TAK1 undergoes K63-linked ubiquitination and the lysine residue at 158 is critical for TNF- and IL-1–induced activation of NF-κB (Fan et al., 2010; Ahmed et al., 2011; Li et al., 2011). Although it has been reported that USP4 deubiquitinates TAK1 and negatively regulates TNF- and IL-1–induced activation of NF-κB (Fan et al., 2011), how TAK1 ubiquitination is regulated in adaptive immune cells such as T cells and whether such a regulation regulates T cell–mediated immune response remain unknown.
Ubiquitin-specific proteases (USPs) are a large family of proteins whose functions have just begun to be studied and appreciated. Among them, USP18 was first identified in AML1-ETO leukemia (Liu et al., 1999). USP18 gene expression has been found at low levels in multiple tissues and cell lines (Schwer et al., 2000; Kang et al., 2001) and could be rapidly up-regulated by type I IFNs (Malakhov et al., 2002). USP18 shares significant homology with well characterized deubiquitinating enzymes and was initially suspected to have deubiquitinating enzymatic activity. However, later it was found in another study that USP18 deconjugates ISG15, a ubiquitin-like protein modifier (Malakhov et al., 2002). Although Usp18−/− cells have elevated ISGylation levels, the function of USP18 in innate immunity appears to be ISG15 independent. USP18-deficient mice were protective from lethal LCMV and VSV infection (Ritchie et al., 2004), which was not dependent on ISG15 deconjugation (Knobeloch et al., 2005; Kim et al., 2006). USP18 directly binds to IFN-α/β receptor 2 (IFNAR2) in human KT-1 cells and, by competing for JAK1 binding, inhibits type I IFN signaling (Kim et al., 2006; Malakhova et al., 2006). The function of USP18 in adaptive immune cells is unclear.
After activation, CD4+ helper T (Th) cells differentiate into distinct cytokine-producing effector subsets. In addition to Th1 and Th2 cells, Th17 cells have been identified as a unique lineage of T cells important for autoimmunity and clearance of mucosal infection by producing proinflammatory cytokines IL-17, IL-17F, IL-21, and IL-22 (Dong, 2008). Th17 cell differentiation is initiated by TGF-β and IL-6 signaling (Bettelli et al., 2006; Veldhoen et al., 2006). The transcription factor STAT3, downstream of IL-6 and IL-21, is essential for Th17 cell differentiation via induction of two orphan nuclear receptors RORγt and RORα (Ivanov et al., 2006; Nurieva et al., 2007). In contrast, TGF-β and IL-2, through STAT5, promotes Foxp3 expression and inhibits Th17 generation (Laurence et al., 2007). Despite this knowledge, it is still not clear how signaling pathways are regulated in T cells to control Th17 cell differentiation.
In this study, we have characterized the function of USP18 in T cell–mediated adaptive immune response and autoimmune disease. We found that USP18 is necessary for Th17 differentiation and autoimmune response. USP18 binds to and inhibits ubiquitination of the TAK1–TAB complex, thereby restricting IL-2 production and promoting IL-17 production. Our work thus identifies a novel negative regulator of TAK1 crucial for Th17 cell differentiation.
RESULTS
USP18 expression in T cells
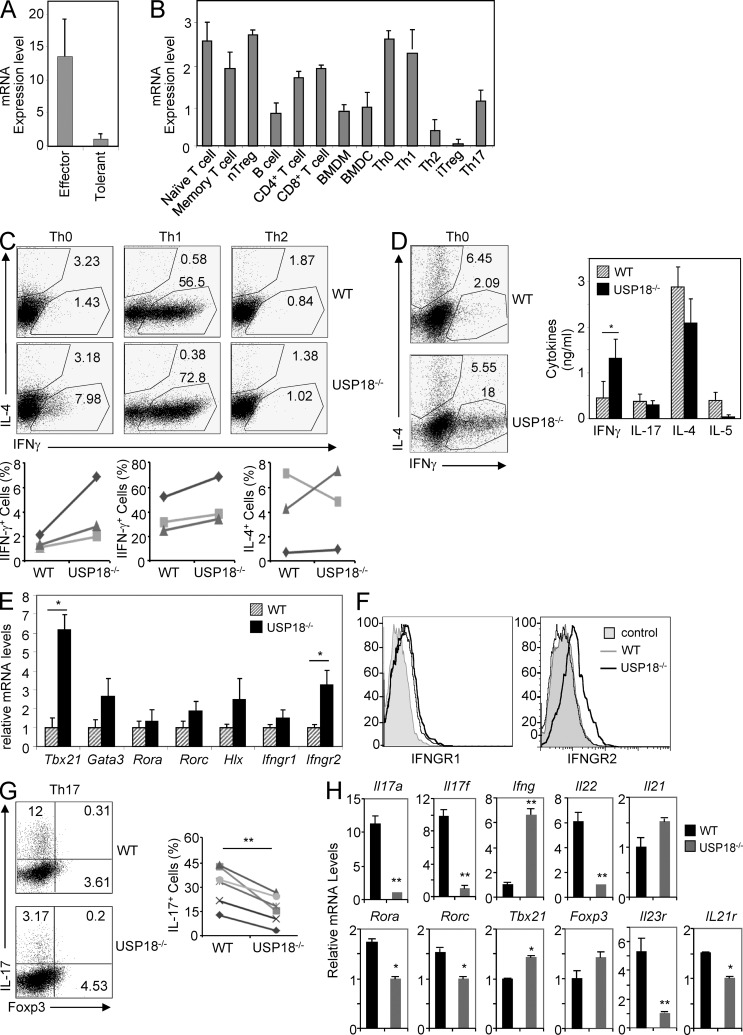
In our attempt to identify genes differentially expressed in different T cell subsets by microarray analysis (Nurieva et al., 2008), we found that USP18 mRNA was expressed at higher levels in Th1 and Th17 cells than in Th2 cells (unpublished data). Interestingly, when we compared Usp18 expression between effector and tolerant CD4+ T cells, which were generated by activating naive T cells in the presence or absence of CD28 and ICOS co-stimulation (Nurieva et al., 2006), respectively, tolerant cells expressed 10-fold less of USP18 mRNA in comparison with effector T cells (Fig. 1 A). This pattern of expression was opposite to that of E3 ubiquitin ligases Cbl-b and Grail (Nurieva et al., 2010), suggesting that USP18 may be important for effector T cell function. We thus further examined the expression levels of Usp18 in different lymphatic and hematopoietic populations, including splenic T and B cells, BM-derived DCs (BMDCs), and macrophages (BMDMs). Usp18 was expressed in all lymphocytes examined, including B cells, CD4+, and CD8+ T cells (Fig. 1 B). In T cells, Usp18 was highly expressed in naive (CD4+CD25−CD62L+CD44−), effector/memory (CD4+CD25−CD62L−CD44+), and natural regulatory T cells (CD4+CD25+CD62L−CD44−; nT reg cells; Fig. 1 B). Consistent with our microarray data, we found that the high-level expression of Usp18 was maintained in Th0, Th1, and Th17 cells, but diminished in Th2 cells and inducible regulatory T cells (iT reg cells; Fig. 1 B). Because Usp18 is expressed in various subsets of CD4+ T cells and the expression levels of Usp18 are differently regulated during T cell activation, tolerance, and effector differentiation, we speculated that USP18 might regulate T cell–mediated adaptive immune response.
Figure 1.
USP18KO cells defects in Th17 generation in vitro. (A) Naive CD4+ T cells were sorted by flow cytometry (gated on CD4+CD25−CD44lowCD62Lhigh) and stimulated with anti-CD3 and APC from WT or mice lacking B7.1, B7.2, and B7h to generate effector or tolerant T cells. After 5 d of culture, cells were washed and stimulated with anti-CD3 for 5 h, followed by real-time PCR analysis. (B) CD4+ and CD8+ T cells, memory (gated on CD4+CD25−CD44lowCD62Lhigh), nT reg cells (CD4+CD25+CD44−CD62L−), and B220+ B cells were sorted by flow cytometry from splenocytes. BMDCs and BMDMs were differentiated from BM progenitor cells with GM-CSF or M-CSF. Th0, Th1, Th2, iT reg, and Th17 cells were prepared by culturing naive cells in these polarizing conditions for 5 d, followed by stimulation with anti-CD3 for 24 h, followed by real-time analysis or by PMA and ionomycin for 5 h, followed by intracellular cytokine staining (not depicted) to examine the differentiation efficiency. (C) WT and USP18KO (KO) naive CD4+ T cells were cultured under different polarizing conditions for 4 d. Cells were washed and stimulated with PMA plus ionomycin in the presence of Golgi stop for 5 h, followed by intracellular staining of the indicated antibodies. (D–F) Naive CD4+ T cells from WT or USP18KO (KO) mice were purified and stimulated with anti-CD3/CD28 for 4 d. Cells were washed and stimulated with PMA plus ionomycin for 5 h, followed by intracellular cytokine staining (left plots), with anti-CD3 for 24 h for ELISA (right graph; D), or with anti-CD3 for 4 h for real-time PCR analysis (E), or stained with anti-IFNGR1 and -IFNGR2 (F). (G) Naive CD4+ T cells from WT or USP18KO (KO) mice were purified and cultured under Th17 polarizing conditions (anti-CD3/CD28, TGF-β, and IL-6) for 4 d. Cells were stimulated with PMA and ionomycin for 5 h followed by flow cytometry analysis. (H) Naive CD4+ T cells from WT or USP18KO (KO) mice were differentiated under Th17 condition for 48 h. Cells were harvested and real-time PCR analysis was performed to determine the mRNA levels of the indicated cytokines. The level of the lower sample for each gene was set at 1 for comparison. Data are representative from two (A and B) or at least three independent experiments (C–H). Bar graphs show mean ± SD, n = 3. *, P < 0.05; **, P < 0.01 (unpaired Student’s t test).
USP18-deficient T cells are impaired in Th17 differentiation in vitro
Because the expression levels of Usp18 varied in different Th lineages, we assessed whether USP18 deficiency influenced the differentiation of naive T cells in vitro by using T cells from WT and Usp18−/− mice (KO). Usp18−/− T cells had increased percentages of IFN-γ–producing cells under neutral (Th0) and Th1 conditions, whereas the expression of IL-4 under Th0 or Th2 conditions was normal compared with WT cells (Fig. 1 C). Higher frequency of IFN-γ–producing cells under Th0 conditions in Usp18−/− cells (Fig. 1 D) was correlated with elevated levels of IFN-γ cytokines in the supernatants and increased Tbx21 mRNA in the cells (Fig. 1, D and E). USP18 has been reported to down-regulate type I IFN signaling through binding to IFNAR2 and competing for JAK binding without altering the expression levels of Ifnar2 (Kim et al., 2006). However, we found that the expression of IFNGR2 was potentiated at mRNA and protein levels in Usp18−/− T cells (Fig. 1, E and F), which might contribute to the elevated IFN-γ signaling as a feedback effect.
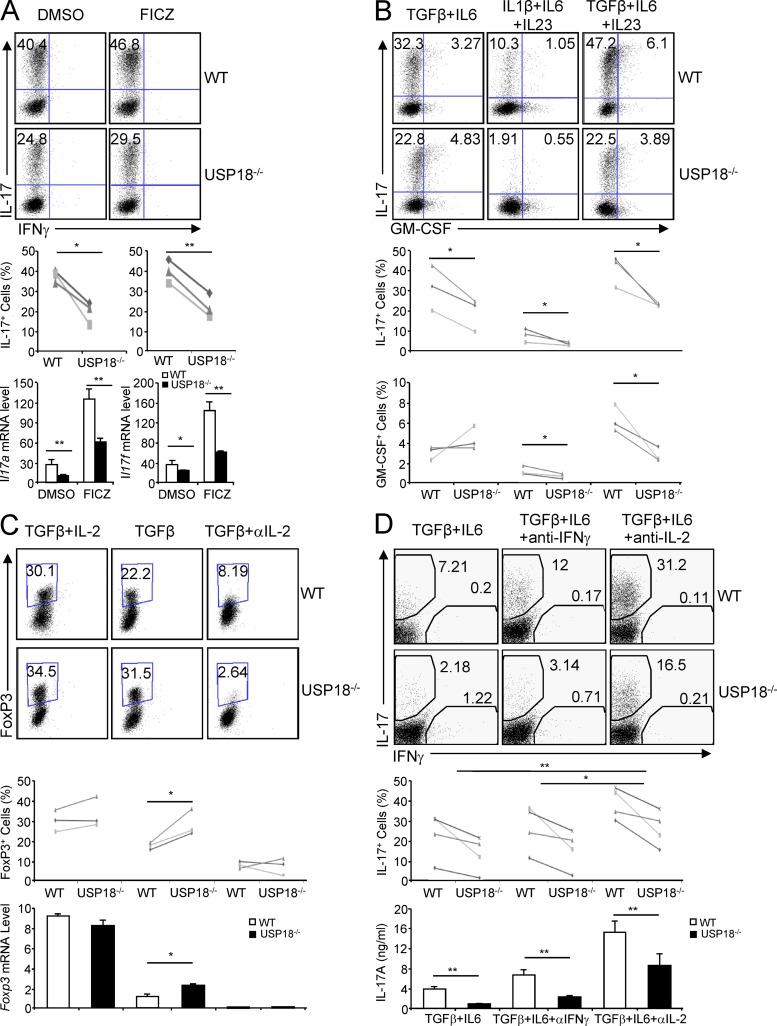
Interestingly, during Th17 differentiation, the IL-17–producing cells were reduced more than threefold in Usp18−/− cells in comparison with the WT controls (Fig. 1 G). To understand the basis for this defect, we then examined mRNA levels of different genes at day 2 after T cell polarization and found that Th17 signature cytokines, including IL-17, IL-17F, and IL-22, were all already decreased in cells deficient in USP18. Accordingly, the expression of Th17-specific transcription factors RORα and RORγt was also found reduced in Usp18−/− cells (Fig. 1 H). In contrast, there was little increase in Foxp3 expression in Usp18−/− cells under our Th17 polarized conditions (Fig. 1 H), indicating that USP18 regulates Th17 differentiation not through Foxp3. FICZ is an AHR ligand that promotes Th17 differentiation (Veldhoen et al., 2008, 2009). However, addition of the AHR agonist FICZ could not restore the defect of USP18-deficient naive T cell differentiation into Th17 cells (Fig. 2 A), suggesting that USP18 regulates Th17 differentiation through AHR-independent pathways. It has been demonstrated that IL-1 signaling promotes early Th17 cells differentiation, whereas IL-23 is important for Th17 maintenance and GM-CSF induction (Chung et al., 2009; Codarri et al., 2011; El-Behi et al., 2011). However, IL-1β or IL-23 could not overcome the IL-17– or GM-CSF–producing defect in USP18-deficient T cells compared with WT controls (Fig. 2 B and not depicted).
Figure 2.
Blockage of IL-2 partially restores Th17 generation defects of USP18KO cells. Naive CD4+ T cells from WT or USP18KO (KO) mice were sorted by flow cytometry and differentiated into Th17 or iT reg cells under various conditions. (A) Cells were cultured with TGF-β and IL-6 in the presence or absence of the AHR agonist FICZ for 4 d, followed either by PMA/ion stimulation and intracellular staining analysis or by anti-CD3 stimulation and real-time PCR analysis. (B) Cells were cultured with the indicated cytokines for 4 d, followed by PMA/ion stimulation and intracellular staining analysis. (C) Cells were cultured with TGF-β alone or TGF-β with IL-2 or anti–IL-2 for 4 d, followed either by PMA/ion stimulation and intracellular staining analysis or by anti-CD3 stimulation and real-time PCR analysis. (D) Cells were cultured with TGF-β plus IL-6 in the presence of anti–IFN-γ or anti–IL-2 for 4 d, followed either by PMA/ion stimulation and intracellular staining analysis or by anti-CD3 stimulation and ELISA analysis. Staining data shown are representatives of three or four independent experiments (paired Student’s t test). Bar graphs show mean ± SD, n = 3. *, P < 0.05; **, P < 0.01 (unpaired Student’s t test).
When we examined WT and USP18KO naive T cells differentiation into iT reg cells, we found that the expression level of Foxp3 was significantly higher in Usp18−/− cells compared with WT cells treated with TGF-β alone (Fig. 2 C). However, the expression levels of Foxp3 were comparable between WT and Usp18−/− cells after addition of exogenous IL-2, whereas addition of IL-2 neutralizing antibody strongly inhibited the expression of Foxp3 and diminished Foxp3 expression difference between WT and Usp18−/− cells at both mRNA and protein levels, indicating that IL-2 is responsible for promoting USP18-deficient cells differentiation into iT reg cells in vitro.
Because it has been well documented that IL-2 restricts Th17 differentiation in vitro and in vivo (Laurence et al., 2007; Liao et al., 2011a,b; Yang et al., 2011), we examined whether IL-2 was responsible for the defect of USP18KO T cells differentiation into Th17 cells. As shown in Fig. 2 D, blockage of IL-2 signaling by IL-2 antibody partially restored the Th17 differentiation defect by USP18 deficiency and increased expression of IL-17 at mRNA and protein levels. In contrast, adding IFN-γ blocking antibody during Th17 differentiation increased the percentages of IL-17–producing cells in WT but not significantly in USP18-deficient cells (Fig. 2 D), although there were increases of Tbx21 and Ifng mRNA in Usp18−/− cells at early Th17 differentiation stage (Fig. 1 H). Thus, USP18 regulates Th17 differentiation, at least partially, through influencing IL-2 cytokine production.
USP18-deficient mice are defective in Th17 generation in vivo and are resistant to experimental autoimmune encephalomyelitis (EAE)
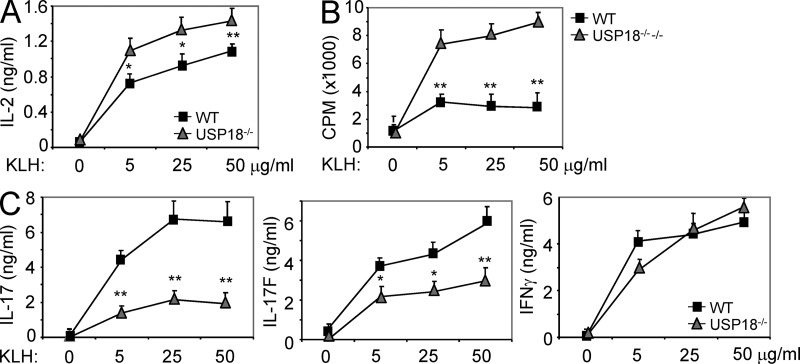
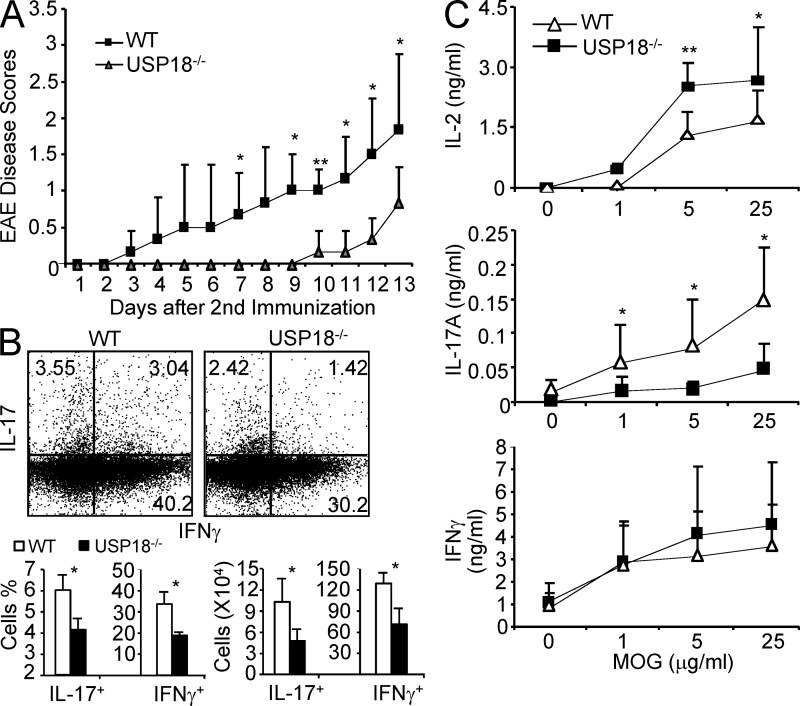
To examine T cell differentiation in vivo, WT and USP18 KO mice (Kim et al., 2005) were immunized with KLH emulsified in CFA subcutaneously. Splenocytes were harvested and restimulated with KLH 7 d after immunization. We found that USP18KO T cells produced increased levels of IL-2 and proliferated more robustly compared with WT controls (Fig. 3, A and B). In contrast, IL-17 and IL-17F productions were greatly reduced in splenocytes from USP18 KO mice, whereas IFN-γ levels were similar between the two groups (Fig. 3 C).
Figure 3.
USP18KO T cells are hyperactive and defect in Th17 generation after KLH immunization. WT and USP18KO (KO) mice were immunized with KLH in CFA subcutaneously. 7 d later, the mice were killed and splenocytes were restimulated with KLH. Cytokines and proliferation were measured at 72 h with [3H]thymidine added at the last 7 h of culture. The data are representative of two individual experiments (n = 5). Graphs show mean ± SD, n = 5. *, P < 0.05; **, P < 0.01 (unpaired Student’s t test).
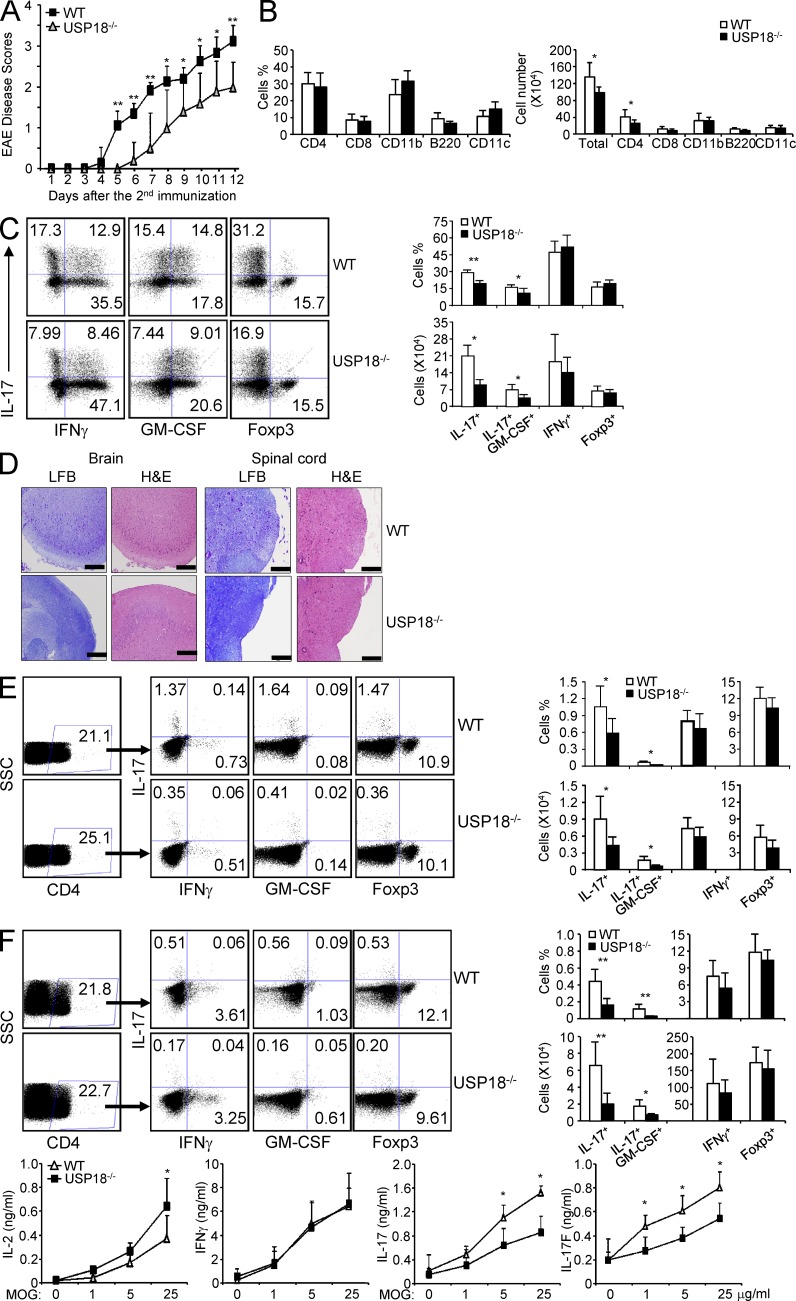
Given the greatly reduced IL-17 generation in USP18 KO mice, we examined whether the deficiency contributed to the development of autoimmune disease EAE, which is mediated by Th17 cells. We found that USP18 KO mice were delayed in disease onset and progression compared with littermate controls (Fig. 4 A). We then analyzed the infiltrating cells in central nervous system (CNS) and found that the percentages of various immune cells (CD4+, CD8+, CD11b+, B220+, and CD11c+) infiltrated into CNS were comparable between USP18KO mice and littermate controls (Fig. 4 B). However, the numbers of infiltrating cells and CD4+ cells in the CNS of USP18KO mice were significantly less than those of littermate controls (Fig. 4 B). The percentages and absolute numbers of IL-17+ and IL-17+GM-CSF+ cells were significantly reduced in the CNS from USP18KO mice (Fig. 4 C). Our histology analysis showed that the brain and spinal cord from USP18KO mice were protected from demyelination and inflammation (Fig. 4 D). When the cells from draining lymph nodes and spleen were restimulated with MOG, we found that the percentages and numbers of IL-17+ or IL-17+GM-CSF+ CD4+ cells were significantly reduced by USP18 deficiency (Fig. 4, E and F). Splenocytes from USP18KO mice produced substantially decreased amounts of IL-17 and IL-17F but increased amounts of IL-2 compared with those from WT mice, whereas IFN-γ levels were similar between the two groups (Fig. 4 F). In contrast, the numbers and percentages of Foxp3-expressing CD4+ T cells in CNS, spleen, or dLN were comparable between USP18KO mice and littermate controls (Fig. 4, C–F), supporting a specific role of USP18 in Th17 cell response in vivo.
Figure 4.
USP18KO mice are resistant to EAE induction. WT and USP18KO (KO) mice were induced EAE through immunization with MOG emulsified in CFA. (A) The mice were monitored daily for EAE syndrome. (B) CNS infiltrates from EAE mice were isolated on day 12 after the second immunization and stained for various surface markers (CD4, CD8, CD11b, B220, or CD11c) and the absolute numbers of different types of cells were calculated. (C) CNS infiltrates were stimulated with PMA/ion plus golgi stop for 6 h, followed by surface staining for CD4 and intracellular staining for IL-17, IFN-γ, GM-CSF, or Foxp3, and the numbers of different cell populations were calculated. (D) Brain or spinal cord from EAE mice were sectioned and stained with luxol fast blue (LFB) or hematoxylin and eosin (H&E). Bars, 200 µM. (E) Cells from draining lymph nodes of the EAE mice were stimulated with 50 µg/ml MOG for 20 h and treated with golgi stop for 6 h, followed by surface (CD4) and intracellular (IL-17, IFN-γ, GM-CSF, and Foxp3) staining. The absolute numbers of different cell populations were calculated. (F) Splenocytes from EAE mice were stimulated with 50 µg/ml MOG for 20 h and treated with golgi stop for 6 h, followed by surface (CD4) and intracellular (IL-17, IFN-γ, GM-CSF, and Foxp3) staining. Splenocytes from the EAE mice were stimulated with the indicated concentrations of MOG peptide, and cytokine expression levels in the supernatants were measured by ELISA at 72 h after culture. Data shown are representative of at least three independent experiments. Graphs show mean ± SD, n = 5. *, P < 0.05; **, P < 0.01 (unpaired Student’s t test).
A T cell–intrinsic role of USP18 in regulation of EAE development
USP18 has an important function in the innate immune system. To confirm that the resistance to EAE by USP18 KO mice is caused by a T cell–intrinsic defect, we adoptively transferred CD4+ T cells from WT or USP18 KO mice into Rag1−/− mice and subsequently induced EAE in the recipient mice. We found that the recipient mice transferred with USP18 KO CD4+ T cells had significantly less severe disease and delayed disease onset compared with the control group (Fig. 5 A), indicating that deficiency of USP18 in T cells reduces EAE. The frequencies and numbers of IL-17– or IFN-γ– producing cells in the CNS was significantly reduced in the recipients with Usp18−/− CD4+ T cells (Fig. 5 B). Consistently, splenocytes from mice with Usp18−/− CD4+ T cells produced decreased levels of IL-17 but increased levels of IL-2 after MOG restimulation (Fig. 5 C). In contrast, the production of IFN-γ in MOG-stimulated splenocytes was comparable between the two groups (Fig. 5 C). These data together suggest that USP18 regulates EAE development and Th17 cell differentiation in a T cell–intrinsic manner.
Figure 5.
USP18 regulates Th17 differentiation and EAE development in a T cell–intrinsic manner. (A) CD4+ T cells from WT or USP18 KO (KO) mice were transferred into Rag−/− mice followed by EAE induction and the mice were monitored daily for EAE syndrome. (B) CNS infiltrates were stimulated with PMA/ion plus golgi stop for 6 h, followed by surface staining for CD4 and intracellular staining for IL-17 and IFN-γ, and the numbers of different cell populations were calculated. (C) Splenocytes from the EAE mice were stimulated with MOG peptide and cytokine expression levels in the supernatants were measured by ELISA at day 3 of culture. Data shown are a representative of three individual experiments. Graphs show mean ± SD, n = 5. *, P < 0.05; **, P < 0.01 (unpaired Student’s t test).
USP18 regulates TCR signaling pathways and IL-2 production
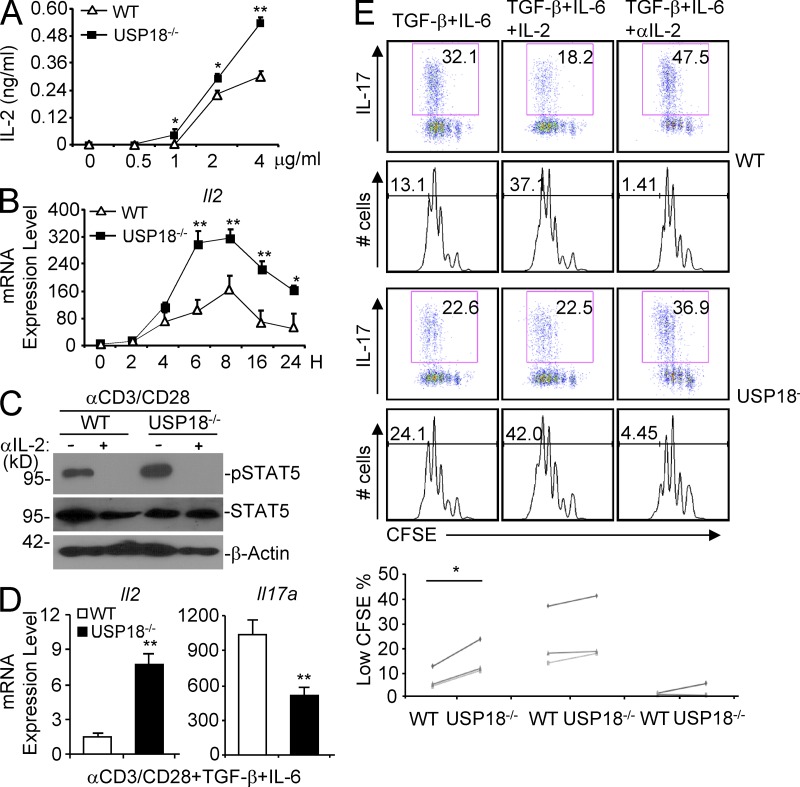
Because we found that IL-2 blockage could restore Th17 generation in USP18KO mice in vitro (Fig. 2), and there was more IL-2 production by splenocytes of USP18 KO mice immunized with KLH or MOG as well as by splenocytes from Rag1−/− mice with transferred Usp18−/− CD4+ T cells (Figs. 3–5), we examined IL-2 production directly in purified WT and USP18-deficient naive T cells in vitro. Interestingly, USP18KO cells secreted increased amounts of IL-2 after anti-CD3 and anti-CD28 stimulation (αCD3/CD28; Fig. 6 A). To rule out the possibility that increased IL-2 expression in the supernatants was a result of decreased consumption of IL-2 by USP18KO cells, we measured the IL-2 mRNA expression and observed that there was significantly higher expression of Il2 in USP18KO cells during T cell activation compared with WT controls (Fig. 6 B). Consistent with these observations, the phosphorylation of STAT5 caused by IL-2 in the supernatant was enhanced in activated Usp18−/− cells compared with controls (Fig. 6 C).
Figure 6.
USP18 regulates IL-2 expression in naive and Th17-polarizing cells. Naive CD4+ T cells were isolated from WT and USP18KO mice. (A) Cells were stimulated with plate-bound anti-CD3/CD28 for 24 h, and IL-2 protein levels in the culture supernatants were measured by ELISA. (B) Cells were stimulated with 2 µg/ml of plate-bound anti-CS3/CD28 and harvested at the indicated time points. Il2 mRNA was measured by real-time PCR analysis. (C) Cells were stimulated by plate-bound 4 µg/ml anti-CD3 and anti-CD28 with or without anti–IL-2 for 24 h before cells were harvested and lysed. The lysates were analyzed by immunoblot with the indicated antibodies. (D) Cells were polarized under Th17 conditions (TGF-β plus IL-6). On day 3, cells were washed and stimulated with plate-bound anti-CD3 for 4 h before real-time PCR was performed. (E) Cells were stained with 2.5 µM CFSE and cultured under the indicated Th17 polarizing conditions for 3 d. Cells were harvested and stimulated with PMA/ion for 5 h, followed by intracellular staining analysis. The percentages of low CFSE cells were statistically analyzed (paired Student’s t test). Data shown are representatives of at least three individual experiments. Graphs show mean ± SD, n = 3. *, P < 0.05; **, P < 0.01 (unpaired Student’s t test).
We next examined whether USP18 also regulates Il2 expression in cells under Th17 polarizing conditions. As shown in Fig. 6 D, the expression of Il2 was significantly higher in Usp18−/− cells compared with WT controls. Consistently, Usp18−/− cells showed hyperproliferation under Th17 polarizing conditions, and addition of IL-2 or anti–IL-2 abrogated the difference (Fig. 6 E). These data demonstrate that USP18 inhibits IL-2 expression and T cell proliferation in T cells after TCR stimulation as well as under Th17 polarizing conditions.
USP18 deficiency results in hyperactivation of NF-κB and NFAT
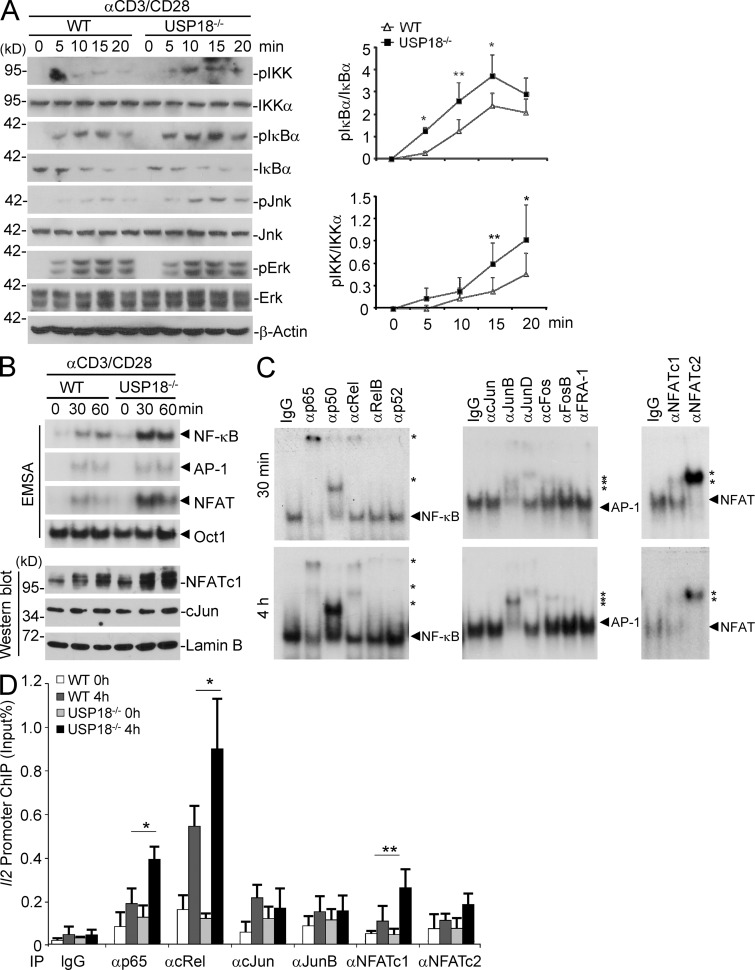
To understand the molecular mechanisms by which USP18 reduces IL-2 production in T cells, we first examined the activation of TCR-proximal signaling events. In naive T cells, USP18 deficiency had no appreciable effect on TCR down-regulation or phosphorylation of ZAP70 and PLC-γ (unpublished data), two key components involved in TCR proximal signaling. We then examined the activation of MAP kinases and NF-κB after cross-linking of TCR/CD28 in WT and USP18KO cells. Interestingly, in the USP18KO T cells, NF-κB became more activated, as measured by the phosphorylation of IKK, and phosphorylation and degradation of IκBα (Fig. 7 A). In addition, phosphorylation of MAP kinase JNK but not ERK was potentiated in USP18KO cells compared with WT cells (Fig. 7 A). To substantiate the regulation of NF-κB by USP18, NF-κB translocation and activation in the nucleus were measured by EMSA from WT or USP18KO CD4+ T cells stimulated with anti-CD3/CD28. Clearly, there was more nuclear NF-κB binding to the κB probes in the nuclear extract from USP18-deficient cells (Fig. 7 B). Interestingly, we also observed increased nuclear NFAT binding to NFAT probes from USP18KO cells (Northrop et al., 1994), whereas nuclear AP-1 binding to the probes was comparable between WT and USP18KO T cells with stimulation of αCD3/CD28 for 0.5–1 h (Fig. 7 B). Results from antibody supershift experiments suggested that the canonical NF-κB proteins p65, p50, and cRel, AP-1 proteins JunB, JunD, cFos, and FosB, and NFATc1 and NFATc2 were activated in USP18KO T cells with stimulation of αCD3/CD28 for 0.5 or 4 h (Fig. 7 C). Consistent with these observations, there was significantly increased binding of p65, cRel, and NFATc1 but not cJun or JunB to the Il2 gene promoter (Fig. 7 D). These data together demonstrate that USP18 negatively regulates TCR-induced activation of NF-κB and NFAT, thereby restricting IL-2 production.
Figure 7.
USP18 deficiency results in hyperactivation of NF-κB, JNK, and NFAT after TCR stimulation. Naive CD4+ T cells were isolated from WT or USP18KO (KO) mice and rested in complete medium for overnight at 4°C. Cells were warmed at 37°C for 2 h, followed by various experiments. (A) Cells were stimulated with cross-linked anti-CD3/CD28 for the indicated time points followed by immunoblot assays with antibodies against the indicated proteins. The expression intensities of pIKK, IKKα, pIκBα, and IκBα were quantified and the ratios of pIKK/IKKα and pIκBα/IκBα were calculated. (B) Cells were stimulated with cross-linked anti-CD3/CD28 for the indicated time points. Nuclear extracts were subjected to wither EMSA or Western blot to determine the activity of NF-κB, AP-1, and NFAT. Oct-1 and Lamin B were included as loading controls, respectively. (C) USP18KO CD4+ T cells were stimulated with cross-linked anti-CD3/CD28 for 30 min or 4 h. Nuclear extracts were incubated with antibodies against NF-κB, AP-1, or NFAT followed by supershift assays. Stars indicate shifted probes and arrowheads indicate unshifted probes. (D) Cells were left untreated or stimulated with cross-linked anti-CD3/CD28 for 4 h, followed by ChIP assay with the indicated antibodies and real-time PCR analysis. Data shown are representatives of three (A) and two (B–D) independent experiments. Graphs show mean ± SD, n = 5. *, P < 0.05; **, P < 0.01 (unpaired Student’s t test).
USP18 interacts with the TAK1–TAB complex
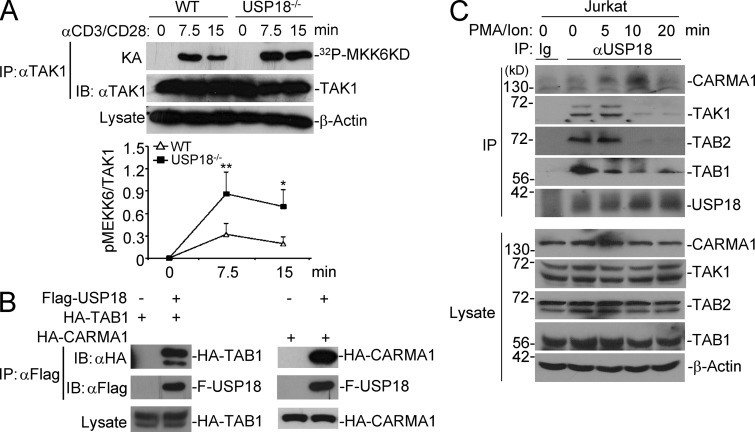
It has been reported that TAK1 is required for IKK and JNK activation by TCR stimulation, and the TAK1–TAB1 complex is sufficient for NFAT activation in Jurkat cells (Liu et al., 2006; Wan et al., 2006; Liu et al., 2009). Because USP18 deficiency potentiated activation of molecules such as IKK, JNK, and NFAT distal from TCR, but not TCR-proximal signaling complex ZAP70 or PLC-γ, we reasoned that USP18 might target some key molecules in between the proximal and distal signaling complexes, of which TAK1 is a promising candidate. We thus tested whether TAK1 was hyperactivated in USP18KO T cells. Results from the in vitro kinase assay indicated that the kinase activity of TAK1 was significantly increased in USP18KO cells compared with WT cells stimulated with anti-CD3/CD28 (Fig. 8 A).
Figure 8.
USP18 interacts with the TAK1–TAB complex. (A) TAK1 was isolated by immunoprecipitation from cells stimulated with anti-CD3/CD28 for the indicated time points, followed by kinase assay using recombinant MKK6 as a substrate. The intensities of the indicated proteins were quantified and the ratio of pMEKK6/TAK1 was calculated. (B) 293T cells were transfected with the indicated plasmids. 24 h later, cells were lysed and immunoprecipitation was performed with anti-Flag. The immunoprecipitants were analyzed by immunoblot with the indicated antibodies. The expression levels of TAK1 in the lysates were analyzed by immunoblot with anti-HA. (C) Jurkat T cells were stimulated with PMA and ionomycin for the indicated time points, followed by immunoprecipitation by control IgG (Ig) or anti-USP18 (αUSP18). The immunoprecipitants were analyzed by immunoblot with antibodies against the indicated proteins. The expression levels of CARMA1, TAK1, TAB1, TAB2, and Actin were analyzed by immunoblot with antibodies against the indicated proteins. Data shown are representatives of three (A and B) and two (C) independent experiments. Graphs show mean ± SD, n = 3. *, P < 0.05; **, P < 0.01 (unpaired Student’s t test).
We next examined whether USP18 interacted with TAK1. In overexpression and co-immunoprecipitation assays, USP18 interacted with TAB1 and CARMA1 but not with NEMO constitutively (Fig. 8 B and not depicted). TAB1 is a chaperone protein for TAK1, which constitutively associates with and induces autophosphorylation and activation of TAK1. To confirm the association of USP18 with endogenous TAK1–TAB1 complex and CARMA1, we stimulated Jurkat cells with PMA and ionomycin and performed immunoprecipitation assays with control IgG or anti-USP18 followed by immunoblot analysis with the immunoprecipitants. The results indicated that USP18 interacted with TAK1, TAB1, and TAB2 without stimulation and PMA/ion stimulation reduced but did not totally abolish their association, whereas USP18 interacted with CARMA1 weakly in unstimulated cells and PMA/ion stimulation first enhanced their interaction and then resulted in their disassociation (Fig. 8 C). It is likely that USP18 keeps TAK1–TAB complexes in check to prevent TAK1 activation in steady conditions and is recruited to CARMA1 together with TAK1–TABs after TCR activation. Collectively, these results suggest that USP18 interacts with the TAK1–TAB complex and regulates the kinase activity of TAK1.
USP18 catalyzes deubiquitination of the TAK1–TAB1 complex
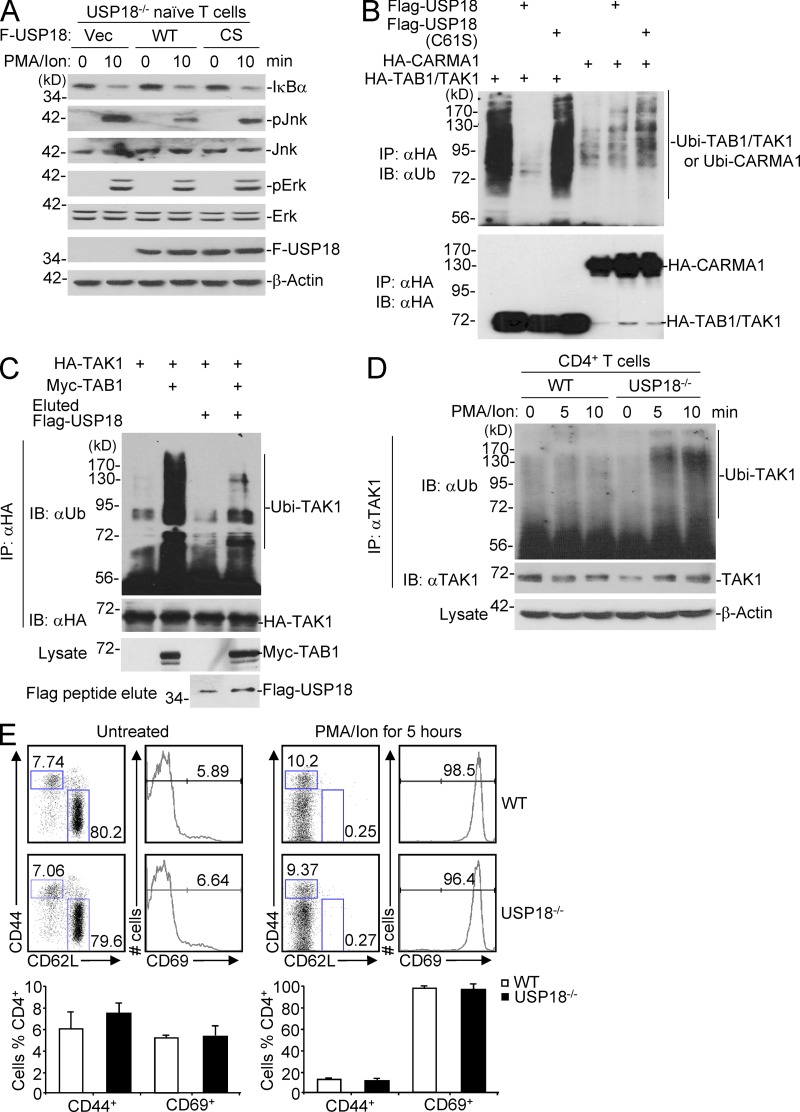
USP18 was originally identified as a deubiquitination enzyme (DUB) and later proved to deconjugate ISG15 modification from targeted proteins, and the Cys61 of USP18 is critical for it enzymatic activity (Schwer et al., 2000; Malakhov et al., 2002; Zhang and Zhang, 2011). It has been suggested that TAK1 is a ubiquitin-dependent kinase, and polyubiquitination of TAK1 results in its autoactivation (Wang et al., 2001; Thiefes et al., 2006). We suspected that USP18 might regulate TAK1–TAB1 ubiquitination through its DUB enzymatic activity. To test this possibility, we examined whether the catalytic activity of USP18 is required for regulating TCR signaling. USP18-deficient naive CD4+ T cells were reconstituted with empty vector (Vec) or Flag-tagged USP18 (WT) or USP18(C61S)(CS) by retrovirus-mediated gene transfer followed by PMA and ionomycin stimulation. Results from immunoblot experiments indicated that PMA/ion-induced phosphorylation of Jnk but not Erk was substantially inhibited by reconstitution of USP18(WT) but not USP18(C61S) (Fig. 9 A), indicating that the catalytic activity of USP18 is required for its function. Interestingly, however, PMA/ion-induced degradation of IκBα was similar between cells reconstituted with USP18(WT) and USP18(C61S) (Fig. 9 A). In our experiments, the T cells were activated with anti-CD3/CD28 before transfection (see Materials and methods). In this context, a previous study demonstrates that TAK1 is required for phosphorylation of JNK but dispensable for NF-κB activation in effector or activated T cells (Wan et al., 2006).
Figure 9.
USP18 catalyzes deubiquitination of TAK1. (A) Naive CD4+ T cells from USP18KO were sorted and transfected with empty vector (Vec), Flag-tagged USP18 (WT), or USP18(C61S) (CS). The transfected cells were sorted and stimulated with PMA and ionomycin (PMA/ion) for 10 min, followed by immunoblot analysis with antibodies against the indicated proteins. (B) 293T cells were transfected with USP18 together with HA-tagged TAK1 and TAB1, or CARMA1. TAK1 complex and CARMA1 were isolated by immunoprecipitation with anti-HA, followed by immunoblot analysis with anti-ubiquitin (αUb). The expression levels of TAK1, TAB1, and CARMA1 in lysates were analyzed by immunoblot with anti-HA. (C) 293T cells were transfected with HA-TAK1 with or without Myc-TAB1. The HA–TAK1 complex was immunoprecipitated with anti-HA. Flag-USP18 was purified by immunoprecipitation with anti-Flag agarose followed by elution with Flag peptide from 293T cells transfected with Flag-USP18. The immunoprecipitants were incubated with or with out Flag-USP18 protein followed by immunoblot with anti-HA or anti-ubiquitin (αUb). (D) WT and USP18KO (KO) CD4+ T cells were stimulated with PMA/ion for the indicated time points. The cells were lysed and cell lysates were immunoprecipitated with anti-TAK1. The immunoprecipitants were analyzed with anti-ubiquitin or anti-TAK1. The expression levels of β-actin were analyzed by immunoblot. (E) Splenocytes from WT and USP18KO (KO) mice left untreated or stimulated with PMA and ionomycin for 5 h were stained with fluorescence-labeled anti-CD4, CD44, CD69, and CD62L, followed by flow cytometry analysis. The flow blots show one representative of WT and KO mice, respectively. Graphs show mean ± SD, n = 4. Data shown are representatives of three (A–C) and two (D and E) independent experiments.
Considering the phenotypes observed with Tak−/− and Usp18−/− T cells or Usp18−/− T cells transfected with USP18(WT) or USP18(C61S), we further hypothesized that USP18 targets TAK1 complex and catalyzes deubiquitination of TAK1. As shown in previous studies (Wang et al., 2001; Thiefes et al., 2006), ectopic expression of TAB1 together with TAK1 induced constitutive polyubiquitination of TAB1–TAK1 (Fig. 9 B). Co-expression of USP18 but not USP18(C61S) potently abolished the polyubiquitination modification of TAK1–TAB1 (Fig. 9 B). In contrast, USP18 did not influence the much weaker ubiquitin modification of CARMA1 and overexpression of USP18 did not affect CARMA1-induced activation of NF-κB in reporter assays (Fig. 9 B and not depicted). In this context, we have observed that CARMA1 induces activation of NF-κB independently of TAK1 (Shambharkar et al., 2007). These data together exclude CARMA1 as a target of USP18.
To further examine whether USP18 deubiquitinates the TAK1–TAB1 complex, we purified Flag-tagged USP18 from 293T cells transiently transfected with Flag-USP18 plasmid by immunoprecipitation with anti-Flag agarose and elution with Flag peptide. The eluted protein was incubated with the immunoprecipitants of HA-TAK1 alone or the HA-TAK1–Myc-TAB1 complex from 293T cells transiently transfected with these constructs. As shown in Fig. 9 C, USP18 efficiently removed the polyubiquitin modifications from the TAK1–TAB1 complex. Conversely, PMA/ionomycin (PMA/ion)-induced ubiquitination of TAK1 was potentiated in USP18-deficient T cells compared with WT T cells, whereas the activation states were comparable between WT and USP18-deficient T cells before or after PMA and ionomycin stimulation (Fig. 9, D and E). Collectively, these data suggest that USP18 targets the TAB1–TAK1 complex and inhibits TAK1 polyubiquitination modification and kinase activity, thereby restricting TCR-mediated NF-κB and NFAT activation and subsequent expression of IL-2.
DISCUSSION
In this study, we demonstrated a critical role for USP18 in adaptive immunity by controlling Th17 cell differentiation and autoimmune disease. USP18KO T cells were hypersensitive to TCR stimulation, which resulted in defective Th17 generation in vitro and in vivo. USP18-deficient mice and Rag1−/− mice adoptively transferred with Usp18−/− T cells were thus resistant to EAE induction. Mechanistically, we found that USP18 interacted with and inhibited ubiquitination and activation of TAK1–TAB complexes.
In innate cells, it has been reported that type I IFNs or viral infection induces expression of Usp18. Considering Usp18 expression was maintained in cells under Th17 condition (TGF-β plus IL-6) but not in cells under iT reg cell condition (TGF-β plus IL-2), we speculate that IL-6 may regulate expression of Usp18 in T cells. However, the expression of Usp18 in naive CD4+ T cells could not be maintained with treatment of IL-6 alone or IL-6 plus plate-bound anti-CD3/CD28 stimulation (unpublished data). It is possible that other Th17 signature cytokines, such as IL-21 or IL-22, may be involved in expression of Usp18. In addition, the expression of Usp18 was also maintained in T cells under Th0 and Th1 conditions, indicating that the regulation of Usp18 is complicated and requires further investigations.
It has been demonstrated that IL-2 inhibits Th17 differentiation through at least three different mechanisms. First, IL-2 induces activation of STAT5, which competes for STAT3 (activated by IL-6) binding sites in the Il17a gene locus and restricts transcription of Il17a (Laurence et al., 2007; Yang et al., 2011). Second, IL-2 induces Tbx21 (encoding T-bet), which inhibits RUNX1-mediated RORγt-dependent transcription (Lazarevic et al., 2011; Liao et al., 2011a,b). Third, IL-2 inhibits expression of IL-6Ra and gp130, which form a functional receptor for IL-6 (Liao et al., 2011b). In our experiments, we found that USP18-deficient naive CD4+ T cells produced increased amounts of IL-2 compared with the WT counterparts stimulated with anti-CD3/CD28 or under Th17 polarizing conditions. The expression of Tbx21 was increased in USP18KO T cells compared with WT cells under Th17 polarizing conditions. In addition, STAT5 was more profoundly phosphorylated in USP18-deficient naive CD4+ T cells compared with WT counterparts after anti-CD3/CD28 stimulation and treatment of anti–IL-2 diminished the differences. Consistent with these observations, USP18KO naive CD4+ T cells showed a decreased percentage of IL-17+ populations under various Th17 polarizing conditions and addition of anti–IL-2 could partially restore the defect. In our KLH immunization or EAE induction model, splenocytes from USP18KO mice produced increased amounts of IL-2 but reduced amounts of IL-17 and IL-17F after KLH or MOG stimulation. These data together support our conclusion that USP18 regulates Th17 differentiation, at least partially, through IL-2. It is worthy to note that there is an IL-2–independent mechanism underlying the defective IL-17 production in USP18KO T cells, as blockage of IL-2 only partially restored Th17 generation in these cells. In this context, the activation of NF-κB and NFAT was potentiated in USP18KO cells, which may activate expression of other genes that are important for inhibition of Th17 generation.
TAK1 has been reported as an indispensable kinase for T cell development and activation, through mediating the activation of IKK complex and JNK in T cells (Liu et al., 2006; Wan et al., 2006). Indeed, CYLD KO T cells displayed constitutive TAK1 activity, which was associated with constitutive activation of both IKK and JNK pathways (Reiley et al., 2007). In this study, however, we found that USP18KO T cells displayed elevated TAK1 activation after stimulation, which significantly increased activation of NF-κB and phosphorylation of JNK (Liu et al., 2006; Wan et al., 2006). In addition, deficiency of USP18 also led to enhanced activation of NFAT and nuclear translocation of NFATc1 which is activated by Ca2+-calcineurin upon TCR stimulation (Smith-Garvin et al., 2009). It has been reported that the TAK1–TAB complex interacts with RCAN1-calcineurin and activates calcineurin-NFAT signaling in Jurkat cells (Liu et al., 2009). It might be possible that TAK1 is involved in NFAT activation in T cells stimulated with TCR signals, in which USP18 plays as an essential negative regulator.
Polyubiquitination of TAK1 is required for its activity (Wang et al., 2001; Fan et al., 2010). Our data demonstrated that USP18 interacted with TAK1 signaling complex and removed polyubiquitin chain from TAK1 in vivo or in vitro, indicating that USP18 regulates ubiquitination of TAK1. We observed that USP18 interacted with the TAK1–TAB complex without stimulation and their association was attenuated after PMA and ionomycin stimulation. It is likely that USP18 keeps TAK1–TAB complexes in check to prevent TAK1 ubiquitination and activation in steady conditions. Upon TCR activation, USP18, together with the TAK1–TAB complex, is recruited to the CARMA1 signaling complex. At the same time, most USP18 is disassociated from the TAK1–TAB complex, leading to full activation of TAK1 and expression of downstream genes for T cell activation, whereas the residual USP18 keeps interacting with the TAK1–TAB1 complex to prevent excessive ubiquitination and activation of TAK1. Whether USP18 regulates TCR signaling-activated TAK1-dependent IKK-, JNK-, and NFAT-independent signaling is currently unknown. Nonetheless, our data have clearly demonstrated that USP18 regulates TAK1 ubiquitination to restrict activation of NF-κB, NFAT, and JNK as well as expression of Il2 in T cells after TCR activation. To our knowledge, USP18 is by far the only deubiquitylating enzyme that regulates TAK1 activity through directly binding to TAB1 in T cells. Considering the divergent functions of USP18 in innate response, T cell activation, and Th17-mediated autoimmunity, USP18 might control the threshold and extent of the inflammation.
As the only protease identified thus far that removes ISG15 conjugation from proteins, however, USP18 functions through an ISGylation-independent manner, as indicated by USP18/ube1L double KO mice still showing similar enhanced innate immunity to that of USP18KO mice (Malakhova et al., 2006). It has been shown that USP18 does not remove single ubiquitin from fusion proteins in vitro. We also found that USP18 immunoprecipitated from 293T cells could not digest either K48- or K63-linked penta- or octa-ubiquitin chain (unpublished data). However, USP18 binds to TAB1 and inhibits the ubiquitination of the TAB1–TAK1 complex. In contrast, USP18(C61S), carrying a single point mutation in the protease domain, could not prevent the polyubiquitination of the TAB1–TAK1 complex. We speculate that USP18 might deubiquitinate TAB1–TAK1 directly in a substrate-specific manner, which could not be observed in synthesized polyubiquitin chains, or other associated cofactors facilitate this regulation in vivo.
In summary, we report here a crucial function of USP18 in T cell activation and Th17 generation, and evidence for USP18 regulation of TAB1–TAK1 activity. These results may benefit understanding and treatment of inflammatory diseases.
MATERIALS AND METHODS
Mice.
USP18KO mice were of a mixed background of C57BL/6 and 129Sv and survived >12 wk without obvious neurological abnormalities, as previously described (Kim et al., 2005). Rag1−/− mice were purchased from The Jackson Laboratory. All mice were housed in the specific pathogen-free animal facility at MD Anderson Cancer Center, and the animal experiments were performed with protocols approved by Institutional Animal Care and Use Committee. 8–12-wk-old mice were used in the experiments.
T cell differentiation.
Naive CD4+CD25−CD62LhiCD44lo T cells were sorted by flow cytometry from spleen and peripheral lymph nodes. Naive T cells were activated with 1 µg/ml of plate-bound anti-CD3 (2C11; BioXCell), 1 µg/ml of anti-CD28 (37.51; BioXCell), and 30 IU/ml of recombinant human IL-2 (except in Th17 condition) in the presence of polarizing cytokines or/and blocking antibodies. For Th1 polarization, 10 µg/ml anti–IL-4 (11B11; BioXCell) and 5 ng/ml IL-12 (PeproTech) were used. For Th2 polarization, 10 µg/ml anti–IFN-γ (XMG1.2; BioXCell) and 5 ng/ml IL-4 were used. For Th-17 polarization, 10 ng/ml IL-1b (PeproTech), 5 ng/ml IL-23, 20 ng/ml IL-6 (PeproTech), and 2.5 ng/ml TGF-β (PeproTech) with or without anti–IFN-γ and anti–IL-2 were used. For iT reg cell differentiation, 5 ng/ml TGF-β and 5 U/ml hIL-2 (PeproTech), or 20 ng/ml anti–IL-2 (BD) were used. After 4 d of culture, cells were washed and restimulated with plate-bound 2 µg/ml anti-CD3 for 4 h, and then collected for RNA extraction. For cytokine measurement by ELISA, culture supernatants were collected at 24 h. For intracellular cytokine analysis, cells were restimulated with 50 ng/ml PMA and 500 ng/ml ionomycin in the presence of Golgiplug (BD) for 5 h. Cells were then permeabilized and analyzed for the expression of IL-4, IL-17, GM-CSF, or IFN-γ with the Cytofix/Cytoperm kit (BD), and for the expression of Foxp3 with Foxp3 Staining Buffer Set (eBioscience).
KLH immunization.
Mice (five per group) were immunized subcutaneously at the tail base with 2 mg/ml KLH emulsified in 0.5 mg/ml CFA (50 µl/site, two sites each/mouse). 7 d later, splenocytes from the immunized mice were cultured for an additional 3 d in vitro in the presence of 0–50 µg/ml of KLH and cytokine expression was analyzed by ELISA. IL-2 was measured at 24 h, and proliferation was measured at 72 h with [3H]thymidine added at the last 7 h of culture.
EAE induction.
6–8-wk-old USP18KO mice and littermate controls were immunized subcutaneously at the dorsal flanks with 150 µg of MOG peptide in CFA at days 0 and 7. Pertussis toxin (500 ng per mouse) was given i.p. at days 1 and 8. Signs of EAE were assigned scores on a scale of 1–5 as follows: 0, none; 1, limp tail or waddling gait with tail tonicity; 2, wobbly gait; 3, hind limb paralysis; 4, hind limb and forelimb paralysis; 5, death. To analyze CNS infiltrates, both the brain and spinal cord were collected from perfused mice, and mononuclear cells were prepared by Percoll gradient. In the adoptive transfer studies, CD4+ T cells were isolated from WT or USP18KO mice and intravenously transferred into Rag1−/− mice (5 × 106 cells per mouse). 1 d later, the recipient mice were induced EAE.
Quantitative real-time PCR.
Gene expression was examined with an iCycler Optical system with an iQ SYBR Green Real-Time PCR kit (Bio-Rad Laboratories). Data were normalized to expression of Actin or GAPD (reference genes). Primers were Usp18 forward, 5′-CCTGGAAGGATGTCCAGTGT-3′, and reverse, 5′-TTGAAATGCAGCAGACAAGG-3′; Hlx forward, 5′-CTCGTGGTCCCGTGCTGTCTTTTC-3′, and reverse, 5′-GTTCCCTCAGTCCGTTCCGTGTCG-3′; Ifngr1 forward, 5′-TGTTACCTAAGTCCTTGCTC-3′, and reverse, 5′-TCTTCCTGTTCTGCTGCTTC-3′; Ifngr2 forward, 5′-CCAGACCAATTCATCTTAGA-3′, and reverse, 5′-AGCACATCATCTCGCTCCTTT-3′; Isg15 forward, 5′-GACTCCTTAATTCCAGGGGACCT-3′, and reverse, 5′-GGGCAATCTGCTTCTTCAGTTCT-3′; and GAPD forward, 5′-GAGAACTTTGGCATTGTGG-3′, and reverse 5′-ATGCAGGGATGATGTTCTG-3′. Actin, Il-2, Ifng, Il4, Il-17a, T-bet, Gata-3, Rora, Rorc, Il-17f, Il-22, Il-21, Foxp3, Il-23r, and Il-21r were amplified as described previously (Nurieva et al., 2007, 2008).
Co-immunoprecipitation and immunoblot experiments.
The cells were lysed in a buffer containing 50 mM Hepes, pH 7.4, 250 mM NaCl, 1% Nonidet P-40, 1 mM EDTA, 1 mM Na3VO4, 0.5 mM PMSF, 0.5 mM dithiothreitol, 2 µg/ml Aprotenin, and 10 µg/ml Leupeptin. The cell lysates were subjected to SDS-PAGE and immunoblot analysis was performed with the following Abs: anti-USP18, ubiquitin, IκBα, TAB1, TAB2, Actin, STAT5, NFATc1, IKKα, Lamin B, ZAP70, and PLC-γ (Santa Cruz Biotechnology, Inc.); and anti-pSTAT5, pIKK, pIκB, pJNK, pP38, pERK, pZAP70, pPLC-γ, pIKK, cJun, and CARMA1 (Cell Signaling Technology). The lysates were immunoprecipitated with control IgG or various antibodies against Flag (Sigma-Aldrich) and TAK1 (Cell Signaling Technology), and the precipitants were washed with lysis buffer four times followed by immunoblot analysis.
In vitro kinase assay.
TAK1 was immunoprecipitated from variously stimulated cells as indicated at 4°C for 3 h. The immunoprecipitates were washed three times with lysis buffer and once with kinase buffer containing 10 mM Hepes, pH 7.4, 1 mM MnCl2, 5 mM MgCl2, 12.5 mM glycerol-2-phosphate, 0.1 mM Na3VO4, 4 mM NaF, and 1 mM dithiothreitol. Bacterially expressed and purified His-MKK6KD (provided by B. Darnay, University of Texas M.D. Anderson Cancer Center, Houston, TX) was used as the substrate. The immunoprecipitated TAK1s were incubated with the substrates in the kinase buffer with 20 µM of cold or 32P-γ-ATP at 30°C for 30 min. The reactions were stopped by adding 2× SDS loading buffer and the samples were boiled for 5 min. The samples were fractionated on SDS-PAGE and transferred to nitrocellulose membranes, followed by immunoblotting or autoradiograph.
EMSA, supershift, and ChIP analysis.
20 × 106 CD4+ T cells were stimulated with 2 µg/ml of cross-linked anti-CD3 and CD28 for various time points. The cells were lysed in lysis buffer (10 mM Hepes, pH 7.9, 10 mM KCl, 0.1 mM EDTA, and 0.4% NP-40) for 10 min on ice followed by centrifuge at 15,000 g for 1 min. The supernatants were saved as cytosol fraction. The pellets were washed once with lysis buffer, resuspended in 20–50 µl of extract buffer (20 mM Hepes, pH 7.9, 0.4 M NaCl, and 1 mM EDTA), and shaken vigorously every 30 s for 15 min, followed by centrifuge at 15,000 g for 10 min. The supernatant was subjected to EMSA with 32P-radiolabeled oligonucleotide probes κB (Promega), AP-1 (Promega), Oct-1 (Promega), and NFAT (5′-CGCCCAAAGAGGAAAATTTGTTTCATA-3′; Northrop et al., 1994) or immunoblot analysis. For supershift analysis, the nuclear extracts were incubated with 1 µg of the indicated antibodies and 32P-radiolabeled κB, AP-1, or NFAT probes followed by autoradiophotography analysis. The ChIP assay was performed with a ChIP kit (Millipore) as previously described (Wang et al., 2012). The antibodies used for supershift and ChIP are as follows: anti-p65, p50, cRel, RelB, p52, cJun, JunD, JunB, NFATc1, and NFATc2 . The ChIP PCR primers for mouse Il2 promoter are: forward, 5′-ATGGGAGGCAATTTATACTG-3′; and reverse, 5′-CCATTCAGTCAGTGTATGGG-3′.
Statistical analysis.
The statistical significance of differences between WT and KO groups was calculated with the two-tailed Student’s t test. P-values of 0.05 or less were considered significant.
Acknowledgments
This work was supported by grants from the National Institutes of Health and MD Anderson Cancer Center (AR050772 and AI50761 to C. Dong; AI050848 and GM065899 to X. Lin). B. Zhong is an Odyssey Fellow supported by the CFP foundation at the MD Anderson Cancer Center. C. Dong is a Leukemia and Lymphoma Society Scholar and holds the Olga and Harry Distinguished University Chair in Cancer Research at the MD Anderson Cancer Center.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- CNS
- central nervous system
- EAE
- experimental autoimmune encephalomyelitis
- IFNAR2
- IFN-α/β receptor 2
- iT reg cell
- inducible regulatory T cell
- TAK1
- TGF-β–activated kinase 1
- USP
- ubiquitin-specific protease
References
- Ahmed N., Zeng M., Sinha I., Polin L., Wei W.Z., Rathinam C., Flavell R., Massoumi R., Venuprasad K. 2011. The E3 ligase Itch and deubiquitinase Cyld act together to regulate Tak1 and inflammation. Nat. Immunol. 12:1176–1183 10.1038/ni.2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441:235–238 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- Chung Y., Chang S.H., Martinez G.J., Yang X.O., Nurieva R., Kang H.S., Ma L., Watowich S.S., Jetten A.M., Tian Q., Dong C. 2009. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 30:576–587 10.1016/j.immuni.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codarri L., Gyülvészi G., Tosevski V., Hesske L., Fontana A., Magnenat L., Suter T., Becher B. 2011. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 12:560–567 10.1038/ni.2027 [DOI] [PubMed] [Google Scholar]
- Dai L., Aye Thu C., Liu X.Y., Xi J., Cheung P.C. 2012. TAK1, more than just innate immunity. IUBMB Life. 64:825–834 10.1002/iub.1078 [DOI] [PubMed] [Google Scholar]
- Dong C. 2008. Regulation and pro-inflammatory function of interleukin-17 family cytokines. Immunol. Rev. 226:80–86 10.1111/j.1600-065X.2008.00709.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Behi M., Ciric B., Dai H., Yan Y., Cullimore M., Safavi F., Zhang G.X., Dittel B.N., Rostami A. 2011. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 12:568–575 10.1038/ni.2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Yu Y., Shi Y., Sun W., Xie M., Ge N., Mao R., Chang A., Xu G., Schneider M.D., et al. 2010. Lysine 63-linked polyubiquitination of TAK1 at lysine 158 is required for tumor necrosis factor alpha- and interleukin-1beta-induced IKK/NF-kappaB and JNK/AP-1 activation. J. Biol. Chem. 285:5347–5360 10.1074/jbc.M109.076976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y.H., Yu Y., Mao R.F., Tan X.J., Xu G.F., Zhang H., Lu X.B., Fu S.B., Yang J. 2011. USP4 targets TAK1 to downregulate TNFα-induced NF-κB activation. Cell Death Differ. 18:1547–1560 10.1038/cdd.2011.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I.I., McKenzie B.S., Zhou L., Tadokoro C.E., Lepelley A., Lafaille J.J., Cua D.J., Littman D.R. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 126:1121–1133 10.1016/j.cell.2006.07.035 [DOI] [PubMed] [Google Scholar]
- Kanayama A., Seth R.B., Sun L., Ea C.K., Hong M., Shaito A., Chiu Y.H., Deng L., Chen Z.J. 2004. TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol. Cell. 15:535–548 10.1016/j.molcel.2004.08.008 [DOI] [PubMed] [Google Scholar]
- Kang D., Jiang H., Wu Q., Pestka S., Fisher P.B. 2001. Cloning and characterization of human ubiquitin-processing protease-43 from terminally differentiated human melanoma cells using a rapid subtraction hybridization protocol RaSH. Gene. 267:233–242 10.1016/S0378-1119(01)00384-5 [DOI] [PubMed] [Google Scholar]
- Kim K.I., Malakhova O.A., Hoebe K., Yan M., Beutler B., Zhang D.E. 2005. Enhanced antibacterial potential in UBP43-deficient mice against Salmonella typhimurium infection by up-regulating type I IFN signaling. J. Immunol. 175:847–854 [DOI] [PubMed] [Google Scholar]
- Kim K.I., Yan M., Malakhova O., Luo J.K., Shen M.F., Zou W., de la Torre J.C., Zhang D.E. 2006. Ube1L and protein ISGylation are not essential for alpha/beta interferon signaling. Mol. Cell. Biol. 26:472–479 10.1128/MCB.26.2.472-479.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobeloch K.P., Utermöhlen O., Kisser A., Prinz M., Horak I. 2005. Reexamination of the role of ubiquitin-like modifier ISG15 in the phenotype of UBP43-deficient mice. Mol. Cell. Biol. 25:11030–11034 10.1128/MCB.25.24.11030-11034.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence A., Tato C.M., Davidson T.S., Kanno Y., Chen Z., Yao Z., Blank R.B., Meylan F., Siegel R., Hennighausen L., et al. 2007. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 26:371–381 10.1016/j.immuni.2007.02.009 [DOI] [PubMed] [Google Scholar]
- Lazarevic V., Chen X., Shim J.H., Hwang E.S., Jang E., Bolm A.N., Oukka M., Kuchroo V.K., Glimcher L.H. 2011. T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORγt. Nat. Immunol. 12:96–104 10.1038/ni.1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Yan J., Mao A.P., Li C., Ran Y., Shu H.B., Wang Y.Y. 2011. Tripartite motif 8 (TRIM8) modulates TNFα- and IL-1β-triggered NF-κB activation by targeting TAK1 for K63-linked polyubiquitination. Proc. Natl. Acad. Sci. USA. 108:19341–19346 10.1073/pnas.1110946108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W., Lin J.X., Leonard W.J. 2011a. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr. Opin. Immunol. 23:598–604 10.1016/j.coi.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W., Lin J.X., Wang L., Li P., Leonard W.J. 2011b. Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nat. Immunol. 12:551–559 10.1038/ni.2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.H., Xie M., Schneider M.D., Chen Z.J. 2006. Essential role of TAK1 in thymocyte development and activation. Proc. Natl. Acad. Sci. USA. 103:11677–11682 10.1073/pnas.0603089103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.Q., Ilaria R., Jr, Kingsley P.D., Iwama A., van Etten R.A., Palis J., Zhang D.E. 1999. A novel ubiquitin-specific protease, UBP43, cloned from leukemia fusion protein AML1-ETO-expressing mice, functions in hematopoietic cell differentiation. Mol. Cell. Biol. 19:3029–3038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Busby J.C., Molkentin J.D. 2009. Interaction between TAK1-TAB1-TAB2 and RCAN1-calcineurin defines a signalling nodal control point. Nat. Cell Biol. 11:154–161 10.1038/ncb1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malakhov M.P., Malakhova O.A., Kim K.I., Ritchie K.J., Zhang D.E. 2002. UBP43 (USP18) specifically removes ISG15 from conjugated proteins. J. Biol. Chem. 277:9976–9981 10.1074/jbc.M109078200 [DOI] [PubMed] [Google Scholar]
- Malakhova O.A., Kim K.I., Luo J.K., Zou W., Kumar K.G., Fuchs S.Y., Shuai K., Zhang D.E. 2006. UBP43 is a novel regulator of interferon signaling independent of its ISG15 isopeptidase activity. EMBO J. 25:2358–2367 10.1038/sj.emboj.7601149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrop J.P., Ho S.N., Chen L., Thomas D.J., Timmerman L.A., Nolan G.P., Admon A., Crabtree G.R. 1994. NF-AT components define a family of transcription factors targeted in T-cell activation. Nature. 369:497–502 10.1038/369497a0 [DOI] [PubMed] [Google Scholar]
- Nurieva R., Thomas S., Nguyen T., Martin-Orozco N., Wang Y., Kaja M.K., Yu X.Z., Dong C. 2006. T-cell tolerance or function is determined by combinatorial costimulatory signals. EMBO J. 25:2623–2633 10.1038/sj.emboj.7601146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R., Yang X.O., Martinez G., Zhang Y., Panopoulos A.D., Ma L., Schluns K., Tian Q., Watowich S.S., Jetten A.M., Dong C. 2007. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 448:480–483 10.1038/nature05969 [DOI] [PubMed] [Google Scholar]
- Nurieva R.I., Chung Y., Hwang D., Yang X.O., Kang H.S., Ma L., Wang Y.H., Watowich S.S., Jetten A.M., Tian Q., Dong C. 2008. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 29:138–149 10.1016/j.immuni.2008.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R.I., Zheng S., Jin W., Chung Y., Zhang Y., Martinez G.J., Reynolds J.M., Wang S.L., Lin X., Sun S.C., et al. 2010. The E3 ubiquitin ligase GRAIL regulates T cell tolerance and regulatory T cell function by mediating T cell receptor-CD3 degradation. Immunity. 32:670–680 10.1016/j.immuni.2010.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiley W.W., Jin W., Lee A.J., Wright A., Wu X., Tewalt E.F., Leonard T.O., Norbury C.C., Fitzpatrick L., Zhang M., Sun S.C. 2007. Deubiquitinating enzyme CYLD negatively regulates the ubiquitin-dependent kinase Tak1 and prevents abnormal T cell responses. J. Exp. Med. 204:1475–1485 10.1084/jem.20062694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie K.J., Hahn C.S., Kim K.I., Yan M., Rosario D., Li L., de la Torre J.C., Zhang D.E. 2004. Role of ISG15 protease UBP43 (USP18) in innate immunity to viral infection. Nat. Med. 10:1374–1378 10.1038/nm1133 [DOI] [PubMed] [Google Scholar]
- Schwer H., Liu L.Q., Zhou L., Little M.T., Pan Z., Hetherington C.J., Zhang D.E. 2000. Cloning and characterization of a novel human ubiquitin-specific protease, a homologue of murine UBP43 (Usp18). Genomics. 65:44–52 10.1006/geno.2000.6148 [DOI] [PubMed] [Google Scholar]
- Shambharkar P.B., Blonska M., Pappu B.P., Li H., You Y., Sakurai H., Darnay B.G., Hara H., Penninger J., Lin X. 2007. Phosphorylation and ubiquitination of the IkappaB kinase complex by two distinct signaling pathways. EMBO J. 26:1794–1805 10.1038/sj.emboj.7601622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaug B., Jiang X., Chen Z.J. 2009. The role of ubiquitin in NF-kappaB regulatory pathways. Annu. Rev. Biochem. 78:769–796 10.1146/annurev.biochem.78.070907.102750 [DOI] [PubMed] [Google Scholar]
- Smith-Garvin J.E., Koretzky G.A., Jordan M.S. 2009. T cell activation. Annu. Rev. Immunol. 27:591–619 10.1146/annurev.immunol.021908.132706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiefes A., Wolf A., Doerrie A., Grassl G.A., Matsumoto K., Autenrieth I., Bohn E., Sakurai H., Niedenthal R., Resch K., Kracht M. 2006. The Yersinia enterocolitica effector YopP inhibits host cell signalling by inactivating the protein kinase TAK1 in the IL-1 signalling pathway. EMBO Rep. 7:838–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Zhang Y., Zhong B., Wang Y.Y., Diao F.C., Wang R.P., Zhang M., Chen D.Y., Zhai Z.H., Shu H.B. 2007. RBCK1 negatively regulates tumor necrosis factor- and interleukin-1-triggered NF-kappaB activation by targeting TAB2/3 for degradation. J. Biol. Chem. 282:16776–16782 10.1074/jbc.M701913200 [DOI] [PubMed] [Google Scholar]
- Veldhoen M., Hocking R.J., Atkins C.J., Locksley R.M., Stockinger B. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 24:179–189 10.1016/j.immuni.2006.01.001 [DOI] [PubMed] [Google Scholar]
- Veldhoen M., Hirota K., Westendorf A.M., Buer J., Dumoutier L., Renauld J.C., Stockinger B. 2008. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 453:106–109 10.1038/nature06881 [DOI] [PubMed] [Google Scholar]
- Veldhoen M., Hirota K., Christensen J., O’Garra A., Stockinger B. 2009. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J. Exp. Med. 206:43–49 10.1084/jem.20081438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y.Y., Chi H., Xie M., Schneider M.D., Flavell R.A. 2006. The kinase TAK1 integrates antigen and cytokine receptor signaling for T cell development, survival and function. Nat. Immunol. 7:851–858 10.1038/ni1355 [DOI] [PubMed] [Google Scholar]
- Wang C., Deng L., Hong M., Akkaraju G.R., Inoue J., Chen Z.J. 2001. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 412:346–351 10.1038/35085597 [DOI] [PubMed] [Google Scholar]
- Wang X., Zhang Y., Yang X.O., Nurieva R.I., Chang S.H., Ojeda S.S., Kang H.S., Schluns K.S., Gui J., Jetten A.M., Dong C. 2012. Transcription of Il17 and Il17f is controlled by conserved noncoding sequence 2. Immunity. 36:23–31 10.1016/j.immuni.2011.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z.P., Sun L., Chen X., Pineda G., Jiang X., Adhikari A., Zeng W., Chen Z.J. 2009. Direct activation of protein kinases by unanchored polyubiquitin chains. Nature. 461:114–119 10.1038/nature08247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.P., Ghoreschi K., Steward-Tharp S.M., Rodriguez-Canales J., Zhu J., Grainger J.R., Hirahara K., Sun H.W., Wei L., Vahedi G., et al. 2011. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat. Immunol. 12:247–254 10.1038/ni.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Zhang D.E. 2011. Interferon-stimulated gene 15 and the protein ISGylation system. J. Interferon Cytokine Res. 31:119–130 10.1089/jir.2010.0110 [DOI] [PMC free article] [PubMed] [Google Scholar]