Abstract
Without data from randomized trials, the long-term effects of fish consumption on coronary heart disease (CHD) need to be inferred from observational studies. We estimated CHD risk under different hypothetical interventions on fish consumption during mid- and later life in 2 prospective US cohorts of 25,797 men in the Health Professionals Follow-Up Study and 53,772 women in the Nurses’ Health Study. Participants provided information on risk factors and disease every 2 years and on diet every 4 years. We adjusted for baseline and time-varying risk factors for CHD by using the parametric g-formula (where g stands for “generalized”). We observed 1,865 incident CHD cases among men (in 1990–2008) and 1,891 CHD cases among women (in 1986–2008). The risk ratios for CHD when comparing the risk if everyone had consumed at least 2 servings of fish per week with the risk if no one consumed fish during the follow-up periods were 1.03 (95% confidence interval: 0.90, 1.15) for men and 0.87 (95% confidence interval: 0.76, 0.98) for women. Our results suggest that increasing fish consumption to at least 2 servings per week in mid- or later life may lower CHD risk in women but not in men. Our analytical approach allowed us to explicitly specify hypothetical interventions and to assess the effectiveness of dietary changes in midlife.
Keywords: causal inference, causal model, cohort, coronary artery disease, diet, intervention, lifestyle
In observational studies, fish consumption is associated with lower risk of death from coronary heart disease (CHD). The long-chain ω-3 polyunsaturated fatty acids (PUFAs) in fish may reduce cardiovascular risk through effects on vascular resistance, blood pressure, inflammation, and serum lipids (1). In a meta-analysis (2) of 13 randomized trials involving high-risk individuals, fish oil supplements were associated with 9% lower CHD mortality (95% confidence interval (CI): 2, 15).
In the absence of randomized trials to estimate the long-term effects of fish intake on the primary prevention of CHD in the general population, these effects need to be inferred from observational studies. Fish consumption has been associated with a lower risk of CHD and CHD mortality in observational studies (3, 4). However, associations in these studies may reflect the effects of lifelong dietary habits. A different question is the potential effect of altering fish consumption later in life (during middle age or later). To estimate the incidence of CHD, nonfatal CHD, and CHD mortality under several hypothetical interventions on fish consumption that start in midlife, we applied the parametric g-formula (5, 6), a generalization of standardization, to 2 prospective studies of US men and women. Unlike conventional statistical methods, the g-formula can naturally estimate the effect of longitudinal interventions while appropriately adjusting for measured time-varying confounders under the assumptions of no unmeasured confounding, no measurement error, and no model misspecification.
MATERIALS AND METHODS
Study populations
The Health Professionals Follow-Up Study is a prospective study of 51,529 US male dentists, pharmacists, veterinarians, optometrists, osteopathic physicians, and podiatrists who were aged 40–75 years at the time of enrollment in 1986. We excluded men with CHD, stroke, diabetes, cancer, missing dietary information, or implausible energy intakes (<500 kcal or >3,500 kcal daily) reported in 1986–1990 and those with missing values for risk factors in 1986–1990. To adjust for baseline diet, we followed participants from the time of return of the second dietary questionnaire in 1990 until CHD diagnosis, death, censoring by first skipped questionnaire, or June 2008, whichever happened first.
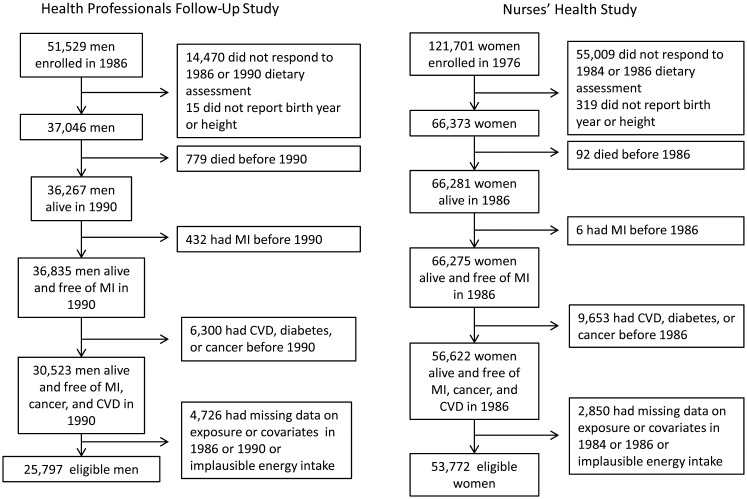
The Nurses’ Health Study is a prospective cohort that enrolled 121,701 US female registered nurses aged 30–55 years in 1976. We excluded women with CHD, diabetes, cancer, missing dietary information, or implausible energy intakes (<500 kcal or >3,500 kcal daily) reported in 1984–1986 and those with missing values for risk factors in 1982–1986. In the Nurses’ Health Study, diet was first assessed in 1980, and the dietary questionnaire was expanded in 1984. We followed participants from the time of the return of the 1986 dietary questionnaire until CHD diagnosis, death, censoring by first skipped questionnaire, or December 2008, whichever happened first. Figure 1 shows the flowchart for selection of study participants. Institutional review boards of the Harvard School of Public Health and Brigham and Women's Hospital (both in Boston, Massachusetts) approved both studies.
Figure 1.
Flowchart for selection of study participants from the Health Professionals Follow-Up Study, 1990–2008, and the Nurses’ Health Study, 1986–2008. CVD, cardiovascular disease; MI, myocardial infarction.
Dietary assessment
Dietary information was collected with a 127–food item semiquantitative food frequency questionnaire that was first sent to participants in the Health Professionals Follow-Up Study in 1986, to participants in the Nurses’ Health Study in 1984 and 1986, and to all participants every 4 years afterward. This previously validated questionnaire (7, 8) evaluates average consumption of specified portions from a list of foods during the previous year by using 9 response categories ranging from “never or less than once a month” to “6 or more per day.”
Fish intake was defined as the sum of canned tuna; dark-meat fish, such as mackerel, salmon, sardines, bluefish, and swordfish; and other fish such as cod, haddock, and halibut. Starting in 1994, an additional question on the consumption of store-bought breaded fish, fish cakes, fish pieces, and fish sticks was added, and the consumption of this type of fish was added to the total fish intake. Red meat intake was estimated as the sum of processed meat, bacon, hot dog, hamburger, beef, pork, and salami consumption. Individuals with intake values of fish or meat higher than the respective 99th percentile were assigned this value.
Daily nutrient intakes were calculated by multiplying the frequency of consumption of each food by the nutrient content for the specified portion of each food by using values provided by the US Department of Agriculture (Washington, DC), manufacturers, published reports, and direct, year-specific fatty acid analyses of processed vegetable fats. Nutrient intakes derived from different foods were summed to obtain total nutrient and energy intakes. Total alcohol intake was calculated by using separate questions on the consumption of beer, wine, and spirits.
Assessment of nondietary risk factors
Participants from both studies reported lifestyle factors, medication use, and newly diagnosed diseases on mailed questionnaires every 2 years. Height and parental history of myocardial infarction before the age of 60 years were ascertained in 1986 in men and in 1976 in women. Weight, physical activity, cigarette smoking (including past smoking and number of cigarettes per day), and physician-diagnosed hypertension and high cholesterol were ascertained biennially. Self-reports of the last 2 diagnoses were found to be accurate compared with medical records (9). Weight and height were used to calculate body mass index (weight (kg)/height (m)2). A validated physical activity questionnaire was first used in 1986 and included in all questionnaires thereafter. Physical activity responses were transformed to weekly metabolic equivalents (10, 11). Regular aspirin use (in men and women), as well as the use of postmenopausal hormone therapy, multivitamins, and vitamin E supplements (in women), was assessed every 2 years.
Information on angina, percutaneous coronary angioplasty, and coronary artery bypass grafting surgery was based on unconfirmed self-reports because these have been shown to have high validity in the Nurses’ Health Study (9). Information on stroke was confirmed through medical record review if the abstracted information met the criteria of the National Survey of Stroke (12). Self-reports of diabetes were confirmed on the basis of information from a supplementary questionnaire by using National Diabetes Data Group diagnostic criteria (13, 14).
Outcome assessment
CHD deaths and nonfatal myocardial infarctions occurring between the return of the baseline questionnaire and 2008 were classified as CHD. Medical record review for confirmation of nonfatal myocardial infarction cases was conducted by physicians who were unaware of the self-reported risk-factor status. Diagnoses were confirmed by using World Health Organization criteria, supplemented after 1998 by guidelines accounting for troponin measurements.
Deaths were ascertained from relatives, postal authorities, or the National Death Index, and the cause of death was classified by using medical records, death certificates, and autopsies (15). CHD deaths were confirmed according to autopsy findings or medical records following criteria for definite fatal myocardial infarction, or when CHD was listed as a cause of death in persons with confirmed CHD and there was no other plausible cause of death. In addition to confirmed CHD deaths, we included deaths in which CHD was listed as the underlying cause but no records were available, as well as sudden deaths with no plausible cause other than CHD.
Hypothetical interventions on fish consumption
We estimated the 18-year risks (in men) and the 22-year risks (in women) of total, nonfatal, and fatal CHD had everyone's fish intake throughout the follow-up been changed to 1 of the following interventions: 1) 0 servings/week, 2) at least 1 serving/week, 3) at least 2 servings/week, 4) at least 3 servings/week, or 5) at least 5 servings/week. We also considered “isocaloric” interventions in which red meat intake was replaced by fish intake to attain 1 of the following serving amounts, which are numbered continuously from the previous list of interventions: 6) at least 1 serving/week, 7) at least 2 servings/week, 8) at least 3 servings/week, and 9) at least 5 servings/week.
These 9 hypothetical interventions are “threshold interventions” (16). At each 4-year interval, fish intake is increased to the threshold (e.g., 2 servings/week under intervention 3) in those individuals who eat fewer fish servings than the threshold; fish intake is not changed in those who meet or exceed the threshold (e.g., those who eat at least 2 servings/week). For example, under intervention 7, fish intake of an individual consuming 1.5 servings/week is increased to 2 servings/week, and red meat consumption is reduced by 0.5 servings/week. For individuals with fish intake below the threshold, fish intake is increased to the threshold unless they reported neither fish nor red meat intake. By using these interventions, we effectively assume that different types of fish and red meat, as well as cooking methods, will produce similar effects (17, 18)
Statistical methods
The parametric g-formula, a generalization of standardization for time-varying exposures and confounders (5, 6), can be used to estimate the standardized risk of CHD under hypothetical interventions. Briefly, the standardized risk is a weighted average of the risks conditional on the specified exposure history and the observed confounder history with the probability density functions of the time-varying confounders as weights. For computational reasons, the weighted average is approximated through a Monte Carlo simulation. The parametric g-formula has been previously applied to estimate the effects of various interventions on the risk of CHD (5, 19), diabetes (20), and clinical outcomes in human immunodeficiency virus–infected patients (21). As in previous applications, our estimator adjusts for informative censoring and takes into account the presence of competing risks by non-CHD deaths (22).
The probability density functions of outcome, exposure, and confounders were estimated via regression models. Pooled logistic and linear regressions (with normal distribution for the errors) were used to estimate the distribution of dichotomous and continuous variables, respectively. All regression models included the time-varying data on smoking history; physical activity; body mass index; alcohol intake and aspirin use; newly diagnosed hypertension, hypercholesterolemia, diabetes, angina, stroke and coronary artery bypass grafting; and meat, fish, energy, trans-fat, and cereal fiber intakes. Analyses in women were additionally adjusted for menopausal status, hormone replacement therapy, and vitamin E and multivitamin use. History was assumed to constitute the most recent measurement (in men) or the 2 most recent measurements (in women) of these time-varying risk factors.
All models also included baseline age, period of follow-up, parental history of myocardial infarction before age 60 years, smoking, and baseline values of all time-varying covariates. When a time-varying covariate was not assessed in all 2-year periods (e.g., dietary variables), only the baseline and most recent measurements were included in the models together with a product term between the most recent measurement and the time since that measurement. The functional form used for each covariate and parameter estimates from all models are listed in the Web Appendix available at http://aje.oxfordjournals.org/.
We conducted a Monte Carlo simulation of 10,000 individuals (Web Figure 1). Baseline covariate values were assigned by using the empirical distribution. Time-varying values for each 2-year interval were drawn from the distribution estimated via the regression models after setting fish intake to the value specified by the intervention. Dichotomous risk factors were assigned a value of 1 if the predicted probability was greater than a random number drawn from a uniform distribution; continuous risk factors were the predicted value plus the standard error multiplied by a random number drawn from a normal (0, 1) distribution. Simulated continuous values were truncated so they did not fall outside the observed range.
For each intervention, we estimated the CHD risk, the population risk ratio with the risk under no intervention as the reference, and the risk ratio with the risk under no fish intake as the reference. If the assumptions of no unmeasured confounding, no model misspecification, and no measurement error nearly hold, our estimates can be interpreted as arising from a randomized experiment in which participants were randomly assigned and adhered to the above interventions.
We used a nonparametric bootstrap procedure based on 500 samples to obtain percentile-based 95% confidence intervals. To assess the possibility of model extrapolation, we estimated the proportion of individuals who would need to change their fish intakes at some point during the follow-up to reach the intervention threshold and the average proportion of individuals who would need to change their fish intakes in a single 2-year period after having been above the threshold in previous periods. To assess model misspecification, we compared the observed means of all time-varying variables with the model-based predicted means under no intervention on fish or meat (Web Figure 2). For comparison purposes, we also fit conventional pooled logistic models to estimate the association between fish intake and CHD risk in 2-year intervals (Web Tables 1 and 2).
RESULTS
Men
At baseline, the average age of male participants was 56.5 (standard deviation, 9.3) years; 7% were smokers; mean physical activity was 7.3 hours/week; and average alcohol intake and fish consumption were 10.1 g/day and 2.4 servings/week, respectively (Table 1). Of 25,797 eligible men, 1,865 had incident total CHD, 1,011 had nonfatal CHD, 928 had fatal CHD, 3,536 were lost to follow-up, and 3,456 died from causes other than CHD. The observed 18-year risks of total CHD, nonfatal CHD, and fatal CHD were 7.7%, 4.1%, and 3.9%, respectively. The corresponding estimated risks under no intervention on actual fish intake were 7.9%, 4.3%, and 4.1%. We estimated CHD population risk ratios with the risk under no fish intervention (no changes in fish consumption from the observed) as the reference and risk ratios with the risk under an intervention in which no one consumes fish as the reference (Table 2).
Table 1.
Baseline Characteristics of Eligible Participants in the Health Professionals Follow-Up Study, 1990, and the Nurses’ Health Study, 1986, United States
| Characteristic | HPFS (n = 25,797 men) |
NHS (n = 53,772 women) |
||
|---|---|---|---|---|
| % | Mean (SD) | % | Mean (SD) | |
| Age, years | 56.5 (9.3) | 52.1 (7.1) | ||
| Family history of MI (at age ≤60 years) | 12 | 19 | ||
| History of oral contraceptive use | 50 | |||
| Menopausal status | 55 | |||
| Postmenopausal hormone use | 17 | |||
| Current smoker | 7 | 21 | ||
| Daily aspirin use | 12 | 9 | ||
| Vitamin E supplement use | 18 | 18 | ||
| Multivitamin supplement use | 39 | 43 | ||
| Body mass indexa | 25.5 (3.2) | 25.1 (4.6) | ||
| Physical activity, hours/week | 7.3 (8.2) | 1.9 (3.0) | ||
| Dietary intakes | ||||
| Energy, kcal/day | 1,892 (536) | 1,769 (512) | ||
| Trans-fatty acids, mg/day | 1.5 (0.6) | 1.7 (0.5) | ||
| Alcohol, g/day | 10.1 (14.0) | 6.4 (10.8) | ||
| Cereal fiber, g/day | 6.9 (4.4) | 4.5 (3.1) | ||
| Red meat, servings/week | 6.2 (4.4) | 6.2 (3.6) | ||
| Fish, servings/week | 2.4 (1.9) | 2.1 (1.6) | ||
Abbreviations: HPFS, Health Professionals Follow-Up Study; MI, myocardial infarction; NHS, Nurses’ Health Study; SD, standard deviation.
a Body mass index is weight (kg)/height (m)2.
Table 2.
Risk Estimatesa for Coronary Heart Disease Among 25,797 Participants in the Health Professionals Follow-Up Study, 1990–2008, United States
| Intervention | 18-Year Risk, % | Population Risk Ratio | Risk Ratio | 95% CI |
|---|---|---|---|---|
| Total coronary heart diseaseb | ||||
| No intervention | 8.0 | 1 | ||
| 0 Fish servings/week | 7.9 | 0.99 | 1 | |
| ≥1 Fish serving/week | 8.0 | 1.01 | 1.02 | 0.92, 1.12 |
| ≥2 Fish servings/week | 8.1 | 1.01 | 1.03 | 0.90, 1.15 |
| ≥3 Fish servings/week | 8.4 | 1.05 | 1.06 | 0.92, 1.21 |
| ≥5 Fish servings/week | 8.5 | 1.07 | 1.08 | 0.86, 1.29 |
| Meat replaced with fish to attain ≥1 serving/week | 7.9 | 1.00 | 1.01 | 0.92, 1.12 |
| Meat replaced with fish to attain ≥2 servings/week | 8.0 | 1.01 | 1.02 | 0.90, 1.14 |
| Meat replaced with fish to attain ≥3 servings/week | 8.3 | 1.04 | 1.05 | 0.91, 1.20 |
| Meat replaced with fish to attain ≥5 servings/week | 8.3 | 1.05 | 1.06 | 0.84, 1.27 |
| Nonfatal coronary heart diseasec | ||||
| No intervention | 4.3 | 1 | ||
| 0 Fish servings/week | 4.1 | 0.95 | 1 | |
| ≥1 Fish serving/week | 4.3 | 1.01 | 1.06 | 0.91, 1.22 |
| ≥2 Fish servings/week | 4.4 | 1.04 | 1.09 | 0.90, 1.29 |
| ≥3 Fish servings/week | 4.5 | 1.04 | 1.09 | 0.88, 1.33 |
| ≥5 Fish servings/week | 4.7 | 1.11 | 1.16 | 0.83, 1.52 |
| Meat replaced with fish to attain ≥1 serving/week | 4.3 | 1.01 | 1.06 | 0.91, 1.22 |
| Meat replaced with fish to attain ≥2 servings/week | 4.4 | 1.04 | 1.09 | 0.90, 1.29 |
| Meat replaced with fish to attain ≥3 servings/week | 4.4 | 1.04 | 1.09 | 0.88, 1.33 |
| Meat replaced with fish to attain ≥5 servings/week | 4.7 | 1.10 | 1.15 | 0.83, 1.50 |
| Fatal coronary heart diseased | ||||
| No intervention | 4.1 | 1 | ||
| 0 Fish servings/week | 4.1 | 1.00 | 1 | |
| ≥1 Fish serving/week | 4.1 | 1.00 | 1.00 | 0.86, 1.15 |
| ≥2 Fish servings/week | 4.1 | 0.99 | 0.99 | 0.83, 1.17 |
| ≥3 Fish servings/week | 4.3 | 1.06 | 1.05 | 0.86, 1.27 |
| ≥5 Fish servings/week | 4.4 | 1.07 | 1.07 | 0.78, 1.40 |
| Meat replaced with fish to attain ≥1 serving/week | 4.1 | 1.00 | 0.99 | 0.86, 1.14 |
| Meat replaced with fish to attain ≥2 servings/week | 4.0 | 0.99 | 0.98 | 0.83, 1.16 |
| Meat replaced with fish to attain ≥3 servings/week | 4.2 | 1.03 | 1.03 | 0.84, 1.25 |
| Meat replaced with fish to attain ≥5 servings/week | 4.2 | 1.02 | 1.02 | 0.75, 1.37 |
Abbreviation: CI, confidence interval.
a Estimates based on the parametric g-formula adjusted for age, parental history of myocardial infarction, physical activity, smoking, body mass index (weight (kg)/height (m)2), high blood pressure, high cholesterol, diabetes, angina or coronary artery bypass grafting, stroke, aspirin use, and intakes of calories, trans-fats, alcohol, cereal fiber, red meat, and fish.
b Observed risk of 7.7% among 1,865 cases accounting for 203,225 years of person-time.
c Observed risk of 4.1% among 1,011 cases accounting for 203,228 years of person-time.
d Observed risk of 3.9% among 928 cases accounting for 207,145 years of person-time.
Overall, CHD risk did not decrease with increasing fish consumption. The risk ratio for total CHD comparing the risk had everyone consumed at least 2 servings/week (the current dietary recommendation) compared with the risk had no one consumed fish during the follow-up was 1.03 (95% CI: 0.90, 1.15). For interventions that increased fish intake to more than 2 servings/week, the risk ratio was above 1, in particular for nonfatal CHD. The risk ratio estimates did not materially change when we estimated the effects of increasing fish consumption in place of red meat (Table 2) and after stratification by baseline fish intake (data not shown).
Women
The average age of female participants at baseline was 52.1 (standard deviation, 7.1) years; 21% were smokers; mean physical activity was 1.9 hours/week; and alcohol intake and fish consumption were 6.4 g/day and 2.1 servings/week, respectively (Table 1). Fifty-five percent of women were postmenopausal and 17% used hormone replacement therapy. Among 53,772 women, there were 1,891 incident total CHD cases, 1,145 nonfatal CHD cases, 823 fatal CHD cases, 5,101 participants lost to follow-up, and 6,110 deaths from causes other than CHD. The observed 22-year risks of total, nonfatal, and fatal CHD were 3.7%, 2.2%, and 1.6%, respectively. The corresponding estimated risks under no fish intervention (no changes in fish consumption from the observed) were 3.9%, 2.3%, and 1.7%. We estimated population risk ratios and risk ratios for CHD (Table 3).
Table 3.
Risk Estimatesa for Coronary Heart Disease Among 53,772 Participants in the Nurses’ Health Study, 1986–2008, United States
| Intervention by Outcome | 22-Year Risk, % | Population Risk Ratio | Risk Ratio | 95% CI |
|---|---|---|---|---|
| Total coronary heart diseaseb | ||||
| No intervention | 3.9 | 1 | ||
| 0 Fish servings/week | 4.1 | 1.08 | 1 | |
| ≥1 Fish serving/week | 3.8 | 0.98 | 0.91 | 0.83, 1.00 |
| ≥2 Fish servings/week | 3.6 | 0.94 | 0.87 | 0.76, 0.98 |
| ≥3 Fish servings/week | 3.4 | 0.88 | 0.82 | 0.67, 0.95 |
| ≥5 Fish servings/week | 3.4 | 0.88 | 0.82 | 0.61, 1.07 |
| Meat replaced with fish to attain ≥1 serving/week | 3.8 | 0.98 | 0.91 | 0.83, 1.01 |
| Meat replaced with fish to attain ≥2 servings/week | 3.6 | 0.92 | 0.86 | 0.75, 0.97 |
| Meat replaced with fish to attain ≥3 servings/week | 3.4 | 0.88 | 0.81 | 0.68, 0.95 |
| Meat replaced with fish to attain ≥5 servings/week | 3.4 | 0.88 | 0.81 | 0.62, 1.05 |
| Nonfatal coronary heart diseasec | ||||
| No intervention | 2.3 | 1 | ||
| 0 Fish servings/week | 2.5 | 1.05 | 1 | |
| ≥1 Fish serving/week | 2.3 | 0.99 | 0.94 | 0.82, 1.07 |
| ≥2 Fish servings/week | 2.2 | 0.93 | 0.88 | 0.74, 1.07 |
| ≥3 Fish servings/week | 2.1 | 0.90 | 0.86 | 0.69, 1.07 |
| ≥5 Fish servings/week | 2.3 | 0.98 | 0.93 | 0.64, 1.30 |
| Meat replaced with fish to attain ≥1 serving/week | 2.3 | 0.98 | 0.94 | 0.82, 1.06 |
| Meat replaced with fish to attain ≥2 servings/week | 2.2 | 0.94 | 0.90 | 0.74, 1.06 |
| Meat replaced with fish to attain ≥3 servings/week | 2.1 | 0.90 | 0.86 | 0.69, 1.06 |
| Meat replaced with fish to attain ≥5 servings/week | 2.2 | 0.97 | 0.92 | 0.61, 1.26 |
| Fatal coronary heart diseased | ||||
| No intervention | 1.7 | 1 | ||
| 0 Fish servings/week | 1.9 | 1.10 | 1 | |
| ≥1 Fish serving/week | 1.6 | 0.97 | 0.88 | 0.74, 1.00 |
| ≥2 Fish servings/week | 1.5 | 0.87 | 0.80 | 0.65, 0.97 |
| ≥3 Fish servings/week | 1.4 | 0.80 | 0.73 | 0.57, 0.94 |
| ≥5 Fish servings/week | 1.3 | 0.72 | 0.66 | 0.36, 0.98 |
| Meat replaced with fish to attain ≥1 serving/week | 1.7 | 0.97 | 0.89 | 0.75, 1.00 |
| Meat replaced with fish to attain ≥2 servings/week | 1.6 | 0.91 | 0.83 | 0.66, 0.97 |
| Meat replaced with fish to attain ≥3 servings/week | 1.4 | 0.83 | 0.76 | 0.58, 0.95 |
| Meat replaced with fish to attain ≥5 servings/week | 1.3 | 0.75 | 0.68 | 0.37, 0.99 |
Abbreviation: CI, confidence interval.
a Estimates based on the parametric g-formula adjusted for age, parental history of myocardial infarction, oral contraceptive use, body mass index (weight (kg)/height (m)2), smoking, menopausal status, hormone replacement therapy, physical activity, aspirin use, vitamin E supplement use, multivitamin supplement use, high blood pressure, high cholesterol, diabetes, angina or coronary artery bypass grafting, stroke, and intakes of calories, trans-fats, alcohol, cereal fiber, red meat, and fish.
b Observed risk of 3.7% among 1,865 cases accounting for 528,425 years of person-time.
c Observed risk of 2.2% among 1,145 cases accounting for 541,751 years of person-time.
d Observed risk of 1.6% among 830 cases accounting for 546,230 years of person-time.
In contrast to men, women had decreased risk estimates with greater fish consumption. Compared with no fish intake, the risk ratio for at least 2 servings/week was 0.87 (95% CI: 0.76, 0.98) for total CHD, 0.88 (95% CI: 0.74, 1.07) for nonfatal CHD, and 0.80 (95% CI: 0.65, 0.97) for fatal CHD. For fatal CHD, the risk ratio was further lowered to 0.73 (95% CI: 0.57, 0.94) for increases in consumption to at least 3 servings/week and 0.66 (95% CI: 0.37, 0.09) for increases in consumption to at least 5 servings/week.
In analyses stratified by baseline fish intake, the risk ratio for at least 2 servings/week compared with no fish intake was 0.81 (95% CI: 0.67, 0.96) among women who consumed less than 2 servings/week in 1984 and 1.03 (95% CI: 0.81, 1.29) among women who reported at least 2 servings/week in 1984 (P for heterogeneity = 0.12). The risk ratio estimates did not materially change for joint interventions on fish consumption and aspirin use (data not shown) or for interventions that replaced red meat with fish (Table 3).
Table 4 presents the proportions of men and women who would have been required to change their observed fish and meat intakes to adhere to our hypothetical interventions. For example, increasing to at least 2 servings of fish required an intervention in 90% of men and 94% of women at some point during the follow-up. Among those adhering to the intervention in the past, 46% of men and women would have had to change their current diets to keep consuming at least 2 servings of fish per week. Web Figures 2 and 3 show that the predicted means under no intervention were similar to the observed means for all time-varying variables.
Table 4.
Percentage of Persons Subject to Each Intervention on Fish Consumption in the Health Professionals Follow-Up Study, 1990–2008, and the Nurses’ Health Study, 1986–2008, United States
| Intervention | HPFS (n = 25,797 men) |
NHS (n = 53,772 women) |
||
|---|---|---|---|---|
| Interventions, %a | Interventions per Period, mean %b | Interventions, %a | Interventions per Period, mean %b | |
| No intervention | 0 | 0 | 0 | 0 |
| 0 Fish servings/week | 100 | 84 | 100 | 80 |
| ≥1 Fish serving/week | 57 | 20 | 75 | 26 |
| ≥2 Fish servings/week | 90 | 46 | 94 | 46 |
| ≥3 Fish servings/week | 97 | 64 | 99 | 65 |
| ≥5 Fish servings/week | 100 | 77 | 100 | 86 |
| Meat replaced with fish to attain ≥1 serving/week | 57 | 19 | 75 | 26 |
| Meat replaced with fish to attain ≥2 servings/week | 90 | 46 | 94 | 46 |
| Meat replaced with fish to attain ≥3 servings/week | 98 | 64 | 99 | 65 |
| Meat replaced with fish to attain ≥5 servings/week | 100 | 77 | 100 | 86 |
Abbreviations: HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study.
a Percentage of persons who received the intervention.
b Average number of individuals per period in which an intervention was necessary.
Sensitivity analyses
The above effect estimates did not materially change in any of the following situations: 1) when we modeled dietary variables by using restricted cubic splines with 3–4 knots at different locations; 2) when we modeled fish intake by using a 2-stage procedure that first estimated the probability of non-0 fish intake and then fit a linear regression only among fish consumers (in women) or as a continuous variable (in men); 3) when we further adjusted for a baseline healthy behavior score (regular multivitamin use, routine physical examinations, and rectal examination or mammography and sigmoidoscopy/colonoscopy for screening); and 4) when we changed the order of the modeling strategy for concurrently measured variables. Data on fish intake were missing in 8% of the eligible periods in the Health Professionals Follow-Up Study and 4.8% of the eligible periods in the Nurses’ Health Study. In those cases, we carried forward the value from the previous dietary questionnaire. Censoring at first missing fish intake resulted in similar estimates (data not shown). The estimates from conventional pooled logistic models were qualitatively similar to ours, though conventional estimates for men were further from the null (Web Tables 1 and 2).
DISCUSSION
No randomized trial has directly evaluated the effect of fish intake on the primary prevention of CHD risk in a relatively healthy population over a long period. We emulated such a trial by using observational data and estimated a 15% reduction in CHD risk among women, but not men, for a change in midlife to sustained consumption of 2 or more servings of fish per week compared with no fish consumption over a 22-year period.
ω-3 PUFAs from fish may have antiarrhythmic (23, 24) and antiinflammatory (25) actions, as well as beneficial effects on circulating lipids, inflammation, vascular resistance, and blood pressure (1). Effects of ω-3 PUFA supplements (but not fish consumption) on secondary prevention of CHD have been evaluated in several randomized trials of relatively short duration (median, 2.4 years). A meta-analysis (2) of these trials that included 3,480 cases estimated that ω-3 PUFAS reduce the risk of cardiovascular mortality by 9%. In contrast to our study, trials have been conducted in high-risk populations, especially in patients with preexisting cardiovascular disease. The recent Alpha Omega Trial (671 cases) (26) conducted in the Netherlands among individuals who had prior myocardial infarctions found that the consumption of margarines supplemented with <400 mg of ω-3 PUFAs had no effect on cardiovascular mortality compared with margarines containing no ω-3 PUFAs. The hazard ratios were 0.98 (95% CI: 0.72, 1.33) overall, 1.06 (95% CI: 0.89, 1.25) for men, and 0.82 (95% CI: 0.58, 1.16) for women. The average Dutch diet is high in ω-3 PUFAs from both plant and animal sources (27). The only randomized trial that has estimated the effect of ω-3 PUFAs on CHD risk found a hazard ratio for CHD of 0.81 (95% CI: 0.69, 0.95) for ω-3 PUFAs (1,800 mg/day, which is roughly equivalent to 1 serving of fatty fish daily) plus statins versus statins alone in a hypercholesterolemic population (69% women) (28). The hazard ratios were 0.87 (95% CI: 0.68, 1.13) for women and 0.76 (95% CI: 0.62, 0.94) for men.
Observational studies have compared the CHD rates among individuals reporting different levels of fish consumption. In previous analyses, the CHD hazard ratios for individuals who consumed >5 servings/week versus those who consumed <1 serving/month were 0.69 (95% CI: 0.52, 0.93) in the Nurses’ Health Study (29) and 1.14 (95% CI: 0.86, 1.51) in the Health Professionals Follow-Up Study (30). In contrast, a meta-analysis (4) of 13 prospective cohorts based on 222,364 individuals estimated that the CHD mortality hazard ratio was 0.62 (95% CI: 0.46, 0.82) for those who consumed 5 servings/week versus <1 serving/month and found no differences by sex, with mortality hazard ratios of 0.65 (95% CI: 0.46, 0.92) for men and 0.55 (95% CI: 0.33, 0.91) for women.
None of these observational analyses attempted to estimate the effects of fish interventions starting in middle age or later, which are arguably the most relevant effects to assess the comparative effectiveness of preventive measures in adults who may or may not have previously followed public health recommendations. Our g-formula analysis differs from previous observational analyses in that it estimates the effects of dietary changes in adults in mid- and later life, while appropriately adjusting for measured time-dependent confounders (31). However, as in all observational studies, the validity of our estimates relies on the assumption of no unmeasured confounding, requires that the exposures and important confounders are correctly measured, and requires that all models are correctly specified.
Measurement bias may be of concern in these analyses because the true between-person variation of dietary change may be less than the within-person variation because of measurement error. Dietary change can be particularly challenging to quantify because it is affected by measurement error at both points in time. However, we have previously shown that our dietary questionnaire is sensitive enough to detect changes in fish and red meat intakes after cardiometabolic diagnoses (32). Also, our sensitivity analyses showed that our estimates were robust to various modeling decisions, and our models closely reproduce the observed data, which suggests the absence of gross model misspecification under no intervention.
The difference in our effect estimates in men and women could be consistent with a lack of beneficial effect of fish intake in men. Alternatively, the men in the Health Professionals Follow-Up Study cohort may have been more focused on preventing cardiovascular disease than were women and, thus, more likely to make dietary changes in the presence of slightly elevated blood pressure and serum glucose and lipid levels. Such “heart healthy” behaviors may be imperfectly captured in our data, which would result in upward biased estimates due to residual confounding.
In summary, we estimated that an increase in fish intake starting in mid- or later life reduces the risk of CHD in women but not in men. The parametric g-formula allowed us to explicitly specify hypothetical interventions to evaluate the effectiveness of dietary changes over long periods.
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Martin Lajous, Walter C. Willett, James Robins, Jessica G. Young, Eric Rimm, Dariush Mozaffarian, Miguel A. Hernán); Center for Research on Population Health, National Institute of Public Health, Cuernavaca, Mexico (Martin Lajous); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, Gustave-Roussy Cancer Institute, Villejuif, France (Martin Lajous); Channing Laboratory, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (Walter C. Willett, Eric Rimm, Dariush Mozaffarian); Department of Nutrition, Harvard School of Public Health, Boston, Massachusetts (Walter C. Willett, Eric Rimm, Dariush Mozaffarian); Department of Biostatistics, Harvard School of Public Health, Boston, Massachusetts (James Robins, Miguel A. Hernán); Division of Cardiovascular Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (Dariush Mozaffarian); and Harvard-Massachusetts Institute of Technology Health Sciences and Technology, Boston, Massachusetts (Miguel A. Hernán).
This study was supported by grants R01-ES-014433, 3R01-ES014433-03S, CA055075, HL35464, and HL080644 from the National Institutes of Health (Bethesda, Maryland). M. L. was supported by the National Council for Science and Technology (Mexico City, Mexico), the National Institute of Public Health (Cuernavaca, Mexico), the Ministry of Health (Mexico City, Mexico), the Department of Epidemiology at the Harvard School of Public Health (Boston, Massachusetts), and the Bernard Lown Fund for Cardiovascular Health in the Developing World (Boston, Massachusetts). D. M. receives funding from the Searle Scholars Program (Chicago, Illinois).
The authors are indebted to Roger Logan, Sarah Taubman, and Goodarz Danaei for their technical programming support and advice.
Conflict of interest: none declared.
REFERENCES
- 1.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58(20):2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 2.Rizos EC, Ntzani EE, Bika E, et al. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012;308(10):1024–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296(15):1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 4.He K, Song Y, Daviglus ML, et al. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation. 2004;109(22):2705–2711. doi: 10.1161/01.CIR.0000132503.19410.6B. [DOI] [PubMed] [Google Scholar]
- 5.Taubman SL, Robins JM, Mittleman MA, et al. Intervening on risk factors for coronary heart disease: an application of the parametric g-formula. Int J Epidemiol. 2009;38(6):1599–1611. doi: 10.1093/ije/dyp192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robins JM, Hernán MA. Estimation of the causal effects of time-varying exposures. In: Fitzmaurice G, Davidian M, Verbeke G, et al., editors. Longitudinal Data Analysis. Boca Raton, FL: Chapman and Hall/CRC; 2009. pp. 553–599. [Google Scholar]
- 7.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 8.Rimm EB, Giovannucci EL, Stampfer MJ, et al. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. Discussion 1127–1136. [DOI] [PubMed] [Google Scholar]
- 9.Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123(5):894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 10.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 11.Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7(1):81–86. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Walker AE, Robins M, Weinfeld FD. The National Survey of Stroke. Clinical findings. Stroke. 1981;12(2 Pt 2 suppl 1):I13–I44. [PubMed] [Google Scholar]
- 13.Hu FB, Stampfer MJ, Solomon CG, et al. The impact of diabetes mellitus on mortality from all causes and coronary heart disease in women: 20 years of follow-up. Arch Intern Med. 2001;161(14):1717–1723. doi: 10.1001/archinte.161.14.1717. [DOI] [PubMed] [Google Scholar]
- 14.Manson JE, Rimm EB, Stampfer MJ, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991;338(8770):774–778. doi: 10.1016/0140-6736(91)90664-b. [DOI] [PubMed] [Google Scholar]
- 15.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax nationwide death search. Am J Epidemiol. 1994;140(11):1016–1019. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 16.Taubman SL, Robins JM, Mittleman MA, et al. Alternative approaches to estimating effects in hypothetical interventions [abstract] 2008 Presented at Joint Statistical Meetings, Denver, Colorado, August 3–7. [Google Scholar]
- 17.Hernán MA, VanderWeele TJ. Compound treatments and transportability of causal inference. Epidemiology. 2011;22(3):368–377. doi: 10.1097/EDE.0b013e3182109296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.VanderWeele TJ, Hernán MA. Causal inference under multiple versions of treatment. J Causal Inference. doi: 10.1515/jci-2012-0002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robins J, Hernán MA, Seibert U. Effects of multiple interventions. In: Murray CJ, Ezzati M, Lopez AD, et al., editors. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Geneva, Switzerland: World Health Organization; 2003. pp. 2191–2230. [Google Scholar]
- 20.Danaei G, Pan A, Hu FB, et al. Hypothetical lifestyle interventions in middle-aged or elderly women and risk of type 2 diabetes: a 24-year prospective study. Epidemiology. 2013;40(1):122–128. doi: 10.1097/EDE.0b013e318276c98a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westreich D, Cole SR, Young JG, et al. The parametric g-formula to estimate the effect of highly active antiretroviral therapy on incident AIDS or death. Stat Med. 2012;31(18):2000–2009. doi: 10.1002/sim.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.Pepe S, McLennan PL. Dietary fish oil confers direct antiarrhythmic properties on the myocardium of rats. J Nutr. 1996;126(1):34–42. doi: 10.1093/jn/126.1.34. [DOI] [PubMed] [Google Scholar]
- 24.Reiffel JA, McDonald A. Antiarrhythmic effects of omega-3 fatty acids. Am J Cardiol. 2006;98(4A):50i–60i. doi: 10.1016/j.amjcard.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 25.Mori TA, Woodman RJ, Burke V, et al. Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated-hypertensive type 2 diabetic subjects. Free Radic Biol Med. 2003;35(7):772–781. doi: 10.1016/s0891-5849(03)00407-6. [DOI] [PubMed] [Google Scholar]
- 26.Kromhout D, Giltay EJ, Geleijnse JM. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363(21):2015–2026. doi: 10.1056/NEJMoa1003603. [DOI] [PubMed] [Google Scholar]
- 27.Petrova S, Dimitrov P, Willett WC, et al. The global availability of n-3 fatty acids. Public Health Nutr. 2011;14(7):1157–1164. doi: 10.1017/S1368980010003678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369(9567):1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 29.Hu FB, Bronner L, Willett WC, et al. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287(14):1815–1821. doi: 10.1001/jama.287.14.1815. [DOI] [PubMed] [Google Scholar]
- 30.Ascherio A, Rimm EB, Stampfer MJ, et al. Dietary intake of marine n-3 fatty acids, fish intake, and the risk of coronary disease among men. N Engl J Med. 1995;332(15):977–982. doi: 10.1056/NEJM199504133321501. [DOI] [PubMed] [Google Scholar]
- 31.Robins JM, Hernán MA. Estimation of causal effects of time-varying exposures. In: Fitzmaurice G, Davidian M, Verbeke G, et al., editors. Advances in Longitudinal Data Analysis. New York, NY: Chapman and Hall/CRC Press; 2009. [Google Scholar]
- 32.Lajous M, Hernán MA, Robins JM, et al. Changes in fish and red meat intake over 20 years after cardiometabolic diseases in men and women: Do people change their diets? 2011 EPI/NPAM Scientific Sessions, Atlanta, Georgia, March 22–25. [Google Scholar]