Abstract
Over the years, many studies have attempted to establish a link between tobacco smoking and an increased risk of nasopharyngeal carcinoma (NPC), but their results have been inconsistent. To clarify this link, we first conducted a comprehensive meta-analysis to integrate the findings of epidemiologic studies from the last half-century. The methodology used for this study followed the checklist proposed by the Meta-analysis of Observational Studies in Epidemiology (MOOSE) Group. Pooled risk estimates were generated using a random-effects model. Twenty-eight case-control studies and 4 cohort studies involving a total of 10,274 NPC cases and 415,266 comparison subjects were included. A substantial effect of smoking on the risk of NPC was identified in this study. The results showed that ever smokers had a 60% greater risk of developing the disease than never smokers (95% confidence interval: 1.38, 1.87); this was a robust dose-dependent association. More importantly, stronger associations were observed in low-risk populations and among persons with the predominant histological type of differentiated NPC than in high-risk populations and persons with an undifferentiated type; the odds ratios were 1.76 and 2.20, respectively, versus 1.29 and 1.27. In this comprehensive meta-analysis, well-established statistical evidence was provided about the role of tobacco smoking in the etiology of NPC.
Keywords: case-control studies, cohort studies, meta-analysis, nasopharyngeal carcinoma, odds ratio, tobacco smoking
While nasopharyngeal carcinoma (NPC) is rare across the globe, its incidence is high in southern China and Southeast Asia, particularly among the Cantonese population, making this type of cancer a leading cause of death in high-risk areas. Over the past half-century, epidemiologic studies have identified several risk factors for NPC, such as infection with Epstein-Barr virus and consumption of salted fish and other salt-preserved foods.
Long recognized as an important tool for evaluating risk factors' contributions to the development of disease, meta-analysis of epidemiologic data has been widely used in the search for potential causes of NPC. For instance, based on a meta-analysis of 16 case-control studies, Gallicchio et al. (1) suggested that persons in the highest category of preserved vegetable consumption exhibited an approximately 2-fold greater risk of NPC than those in the lowest category, providing convincing evidence that preserved food intake is one of the important risk factors for NPC. Meta-analysis has also been used to confirm that elevated NPC risk was associated with high alcohol consumption (2) and high exposure to formaldehyde (3–6).
Tobacco smoking accounts for 5% of cancer cases overall (7). Multiple lines of evidence have linked tobacco smoking to NPC risk. To date, there have been at least 29 studies on tobacco smoking and NPC risk, including 25 case-control studies conducted in China, the United States, Southeast Asia, and Europe and 4 cohort studies from Singapore, the United States, China (Guangdong), and Taiwan (8). Although most of the studies showed a link between tobacco smoking and an increased risk of NPC, the findings are conflicting. The differences may be due to variations in sample size, methodology, and validity across these studies. Therefore, unveiling the underlying patterns of the association between tobacco smoking and NPC using analytical epidemiologic studies is important for public health.
Although early studies (9–21) made several attempts to summarize the epidemiologic evidence on tobacco smoking and NPC risk, to our knowledge, no meta-analyses have ever been conducted in a standardized manner. To quantitatively assess this relationship, we conducted a comprehensive meta-analysis and performed a dose-response analysis on a large set of case-control studies and cohort studies conducted between 1979 and 2011.
MATERIALS AND METHODS
Search strategy and selection criteria
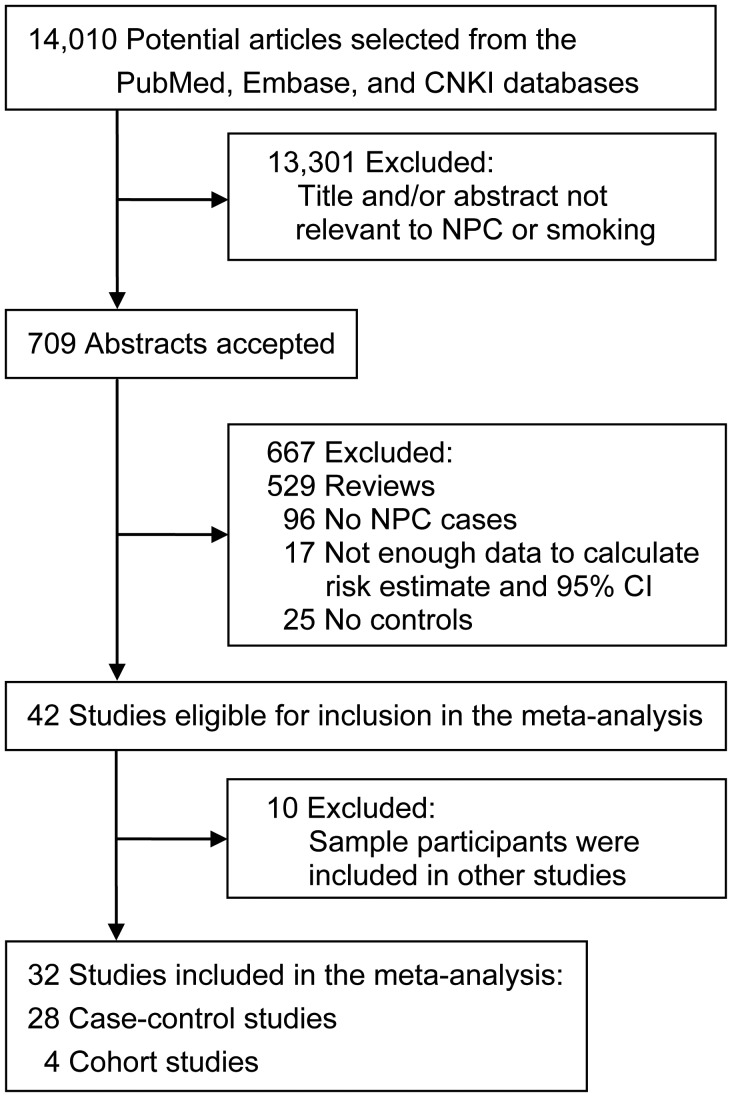
Following the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines, we performed a comprehensive review of the literature to identify all available risk estimates for tobacco smoking as a risk factor for NPC (22). A flow chart illustrating the study selection process is shown in Figure 1. Briefly, relevant publications were identified by searching PubMed (http://www.ncbi.nlm.nih.gov/pubmed/), Embase (http://www.embase.com/), and the China National Knowledge Infrastructure (http://dlib4.edu.cnki.net/) from the date of their earliest publication through December 2011, with no language restrictions. We combined terms related to nasopharyngeal carcinoma (“nasopharyngeal carcinoma,” “nasopharynx neoplasm,” “cancer,” “carcinoma,” and “neoplasm”) and smoking (“smoking,” “tobacco,” “nicotine,” “cigarette,” “nondietary,” “environmental,” and “behavioral”) to identify relevant articles. We also conducted a manual search to find references not available in PubMed.
Figure 1.
Selection of studies included in a meta-analysis of the association between smoking and risk of nasopharyngeal carcinoma (NPC), 1979–2011. CI, confidence interval; CNKI, China National Knowledge Infrastructure.
Observational studies referring to cohort studies and case-control studies were included only if the authors reported quantitative estimates and variability or if they provided the numbers of cases and controls so that we could calculate risk estimates for smoking as a risk factor for NPC. When multiple articles reported on the same study, we included the most recent article or the most informative publication. Studies that were unrelated to tobacco smoking and NPC and studies that provided insufficient information on the procedure used to determine the odds ratios were excluded. Because most studies used nonsmokers as the reference group, we excluded 1 study that compared heavy smokers with light smokers (17). One study that reported risk estimates for passive smoking only (23) was excluded from the overall analysis, but it was used for other analyses.
Data abstraction and quality assessment
Two reviewers independently extracted data from the included studies. The basic data included the following study characteristics: year of publication, author(s), study population, study design, sample size, methods used to identify NPC cases, methods used to assess smoking, type of control subjects (either population-based or hospital-based), variables used for matching, and variables used to control for confounding factors. For the cohort studies, we recorded source of the cohort, years of follow-up, variables used for adjustment, and proportion of subjects who failed to complete follow-up. Risk estimates (expressed as odds ratios or relative risks with 95% confidence intervals) adjusted for potential confounders, different categories of smoking, and specific pathological types were collected for the subgroup analyses. Assessment of the quality of the included studies was conducted using the Newcastle-Ottawa Scale (NOS) (24). Points (a maximum of 12) were assigned to each study based on the 3 parts of the NOS: selection, comparability, and exposure and outcome condition. The case-control studies were assessed for case definition, selection of controls, comparability between cases and controls, ascertainment of exposure, and response rate. The cohort studies were assessed for representativeness of the exposure cohort, ascertainment of exposure, comparability of cohorts, assessment of the outcome, number of years of follow-up, and adequacy of the follow-up cohort. Any discrepancies were resolved by reevaluating the original articles and discussing the disagreement with a third investigator.
Data integration and statistical analysis
For the cohort studies, we used the relative risk as an estimate of the odds ratio because of the low incidence rate of NPC. All of the previous studies except 1 (17) estimated smoking risk by comparing NPC risk among ever smokers with NPC risk among never smokers. Some studies provided original data that could be used to calculate the risk estimates for ever smokers compared with never smokers. These calculated estimates were also pooled to summarize the odds ratios for tobacco smoking as a risk factor for NPC.
A total of 18 publications (11, 13, 15, 16, 18, 20, 21, 25–35) reported risk estimates for separate categories of smoking. Among them, 12 studies (15, 18, 21, 25–28, 30, 31, 33, 34, 36) divided smokers by the level of smoking intensity (cigarettes smoked per day), 11 studies (13, 18, 20, 27–30, 32–34) divided smokers by the cumulative amount of smoking (number of pack-years), 9 studies (15, 16, 18, 21, 25–28, 31) by years of smoking, and 5 (15, 18, 21, 26, 31) by the age at which the subjects began smoking. In the subgroup analyses, we combined data from the literature on smoking quantity into new categories, using the following methods: subjects with cumulative exposure of <30 pack-years were assigned to the low level, while subjects whose cumulative exposure was ≥30 pack-years were assigned to the high level. Similarly, subjects who smoked <15, <20, or <23 cigarettes per day (depending on the threshold the authors used) were designated the “low-exposure” group and subjects who smoked ≥15, ≥20, or ≥23 cigarettes per day were designated the “high-exposure” group. People with a smoking history of ≥20, ≥21, ≥25, ≥30, or ≥40 years (depending on the threshold the authors used) were categorized as long-term smokers. For age of smoking initiation, we designated subjects who began smoking at older ages (≥18 or ≥20 years) and at younger ages (<18 or <20 years) (depending on the threshold the authors used) as the medium group and the early group, respectively. Five studies (21, 23, 33, 34, 37) that provided odds ratios for passive smoking in childhood were also combined.
The odds ratios and 95% confidence intervals were pooled using the general variance-based method with a random-effects model (38). The heterogeneity of odds ratios across the studies was measured by means of the χ2 statistic and quantified with the I2 statistic (39). Subset analyses were conducted to assess the source of heterogeneity by study design (case-control or cohort), population of origin (from the NPC incidence rate), adjustment status (adjusted or unadjusted), NPC histological type (squamous-cell carcinoma or undifferentiated), quality of study methods (NOS score ≥8 or <8), smoking intensity, and passive smoking. A sensitivity analysis was performed by excluding each of the studies from the overall analysis one at a time to measure whether the relevant estimates were strongly influenced by any single study.
To explore the dose-response relationship between cumulative number of pack-years of smoking and risk of NPC, we conducted the generalized least-squares trend estimation analysis developed by Greenland and Longnecker (40, 41). Publication bias in the pooled analysis was investigated by creating funnel plots and evaluated using Egger's linear regression. The trim-and-fill method was used to adjust for publication bias if P < 0.1 in Egger's linear regression (42). All analyses were conducted using STATA software, version 10.0 (StataCorp LP, College Station, Texas).
RESULTS
Included studies
A total of 14,010 publications were retrieved from the initial database search. From these, 32 original articles that included data on the association between tobacco smoking and NPC were ultimately included in our meta-analysis, including 28 case-control studies (9–14, 16, 18–21, 25, 28–36, 38, 43–47) and 4 cohort studies (15, 26, 27, 48) (Figure 1). Three studies (27, 30, 49) focused exclusively on men, and 1 study (13) separated the findings by sex. One study that only reported the NPC risk associated with passive smoking during childhood (23) was excluded from the overall analysis but was used in the subgroup analysis for passive smoking.
The characteristics of the 32 studies are summarized in Tables 1 and 2. A total of 10,274 NPC cases and 415,266 comparison subjects were included in the overall analysis. All of the studies were published between 1979 and 2011. Fifteen studies (9, 12, 19, 21, 27–29, 32–36, 46–48) originated in China or Taiwan, 6 (11, 13, 15, 20, 30, 49) in the United States, 2 (14, 45) in Thailand, and 1 in each of the following states or regions: Malaysia (10), the Philippines (16), India (44), Turkey (38), Singapore (26), Serbia (23), Italy (31), Algeria (43), and North Africa (25). Four studies (13, 15, 35, 48) identified their cases using reports of NPC deaths, while the other cases were histologically confirmed. Smoking status was ascertained in face-to-face interviews using a structured questionnaire in 15 studies (10, 14, 16, 20, 21, 25, 26, 31–35, 38, 44, 46), using telephone interviews in 3 studies (20, 27, 49), and using a mailed questionnaire in 1 study (15). The methodological quality assessments yielded an average score of 7.5 for the case-control studies and 8.3 for the cohort studies. Thirteen studies (39%) were of very high quality (score ≥10) according to the NOS standards. Of the case-control studies, 54% used hospital-based controls or did not report the source of the controls; only 57% reported response rates. Of the cohort studies, half of the studies used death from NPC as the outcome.
Table 1.
Characteristics of Published Case-Control Studies Exploring the Association Between Smoking and Nasopharyngeal Carcinoma, 1979–2011
| First Author, Year (Reference No.) |
Region | No. of Cases | No. of Controls | Source of Controls | Quality Scorea | Matching and Adjustment Variables |
|---|---|---|---|---|---|---|
| Lin, 1979 (9) | Taiwan | 343 | 1,017 | Population-based | 8 | Age, sex, residence |
| Armstrong, 1983 (10) | Malaysia | 100 | 100 | Population-based | 7 | Age, sex, residence |
| Mabuchi, 1985 (11) | United States | 39 | 39 | Hospital-based | 5 | Age, sex, race, marital status |
| Ng, 1986 (12) | Hong Kong, China | 224 | 226 | Hospital-based | 5 | Age, sex, residence, race, admission time |
| Yu, 1990 (33) | Guangzhou, China | 306 | 306 | Population-based | 11 | Age, sex, residence, associated dietary factorsb |
| Nam, 1992c (13) | United States | 204 | 408 | Hospital-based | 4 | Age, sex, alcohol consumption |
| Sriamporn, 1992 (14) | Thailand | 120 | 120 | Hospital-based | 7 | Age, sex, alcohol consumption, consumption of salted fish, occupation |
| West, 1993 (16) | Philippines | 104 | 205 | Population- and hospital-based | 11 | Age, sex, residence, hospital ward type, education, associated dietary factors, exposure to dust, exposure to formaldehyde |
| Vaughan, 1996 (20) | United States | 231 | 246 | Population-based | 12 | Age, sex, residence, alcohol consumption |
| Zhu, 1997d (18, 49) | United States | 110 | 1,890 | Population-based | 10 | Age, residence, race, education, medical history, alcohol consumption, exposure to woodwork or asbestos, age at starting smoking/pack-years of smoking |
| Cai, 1996 (19) | Fujian, China | 115 | 115 | Hospital-based | 6 | Age, sex, month of diagnosis, exposure to products of combustion, associated dietary factors, chronic nasal disease |
| Cheng, 1999 (21) | Taiwan | 375 | 327 | Population-based | 12 | Age, sex, residence, race, education, alcohol consumption, family history of NPC |
| Chelleng, 2000 (44) | India | 47 | 94 | Population-based | 7 | Age, sex, race |
| Mirabelli, 2000d (30) | United States | 92 | 1,909 | Population-based | 10 | Age, residence |
| Yuan, 2000 (34) | Shanghai, China | 935 | 1,032 | Population-based | 11 | Age, sex, education, associated dietary factors, exposure to smoke from cooking, occupational exposure, history of chronic ear and nose conditions, family history of NPC |
| Zou, 2000 (35) | Yangjiang, China | 97 | 192 | Population-based | 7 | Age, sex |
| Huang, 2002 (46) | Guangxi, China | 175 | 350 | Hospital-based | 5 | Age, sex |
| Liao, 2005 (47) | Guangxi, China | 80 | 72 | NR | 3 | Age, sex |
| Feng, 2009 (25) | North Africa | 636 | 615 | Hospital-based | 10 | Age, sex, residence, associated dietary factors |
| Guo, 2009 (36) | Guangdong, China | 1,049 | 785 | Population-based | 11 | Age, sex, residence, positivity for Epstein-Barr virus, exposure to wood dust, exposure to solvents, associated dietary factors |
| Ekburanawat, 2010 (45) | Thailand | 327 | 327 | Hospital-based | 7 | Age, sex, residence, education |
| Nesic, 2010e (23) | Belgrade, Serbia | 45 | 90 | Hospital-based | 8 | Age, sex, residence, consumption of industrially manufactured food additives for enhancing flavor, consumption of white bread, olive oil, margarine, and cornbread |
| Ren, 2010 (32) | Guangdong, China | 1,845 | 2,275 | Population-based | 10 | Age, sex, residence, education, alcohol consumption, consumption of salted fish |
| Bendjemana, 2011 (43) | Algeria | 160 | 205 | NR | 3 | Age, sex |
| Ji, 2011 (28) | Wuhan, China | 1,044 | 1,095 | NR | 5 | Age, sex, alcohol consumption, family history of cancer |
| Ma, 2011 (29) | Guangxi, China | 855 | 1,036 | Population-based | 8 | Age, sex |
| Polesel, 2011 (31) | Italy | 150 | 450 | Hospital-based | 10 | Age, sex, residence, year of interview, education, alcohol consumption |
| Turkoz, 2011 (38) | Turkey | 183 | 183 | Hospital-based | 8 | Age, sex |
Abbreviations: NPC, nasopharyngeal carcinoma; NR, not reported.
a Summary score for the 6 aspects of methodological quality in the Newcastle-Ottawa Scale guidelines (24), including case definition, control selection, comparability of cases and controls, adjustment, ascertainment of exposure, and response rate. A score of 12 represented the highest-quality studies.
b Consumption of salted fish, other preserved foods, or fresh vegetables that may be related to NPC.
c Odds ratios were reported for men and women separately.
d Results were limited to men.
e This study was not included in the overall analysis but was included in the subgroup analysis for passive smoking.
Table 2.
Characteristics of Published Cohort Studies Exploring the Association Between Smoking and Nasopharyngeal Carcinoma, 1979–2011
| First Author, Year (Reference No.) | Region | Source of Cohort | No. of Cases | Cohort Size | Quality Scorea | Adjustment Variables |
|---|---|---|---|---|---|---|
| Chow, 1993 (15) | United States | Veterans | 48 | 248,046 | 5 | Age, calendar year |
| Zhang, 2004 (48) | Guangzhou, China | Workers | 75 | 80,987 | 5 | Age, sex, education, marital status, alcohol consumption, occupation, exposure to dust |
| Friborg, 2007 (26) | Singapore | Population-based | 173 | 61,320 | 11 | Age, sex, dialect group, year of interview, consumption of preserved protein foods, consumption of fresh vegetables, family history of nasopharyngeal carcinoma |
| Hsu, 2009b (27) | Taiwan | Population-based | 32 | 9,622 | 12 | Age, combination of 2 anti–Epstein-Barr virus seromarkers |
a Summary score for the 6 aspects of methodological quality in the Newcastle-Ottawa Scale guidelines (24), including case definition, representativeness of the exposed cohort, adjustment, measurement of the exposure, follow-up period, and the proportion of subjects lost to follow-up. A score of 12 represented the highest-quality studies.
b Results were limited to men.
Association between tobacco smoking and elevated NPC risk
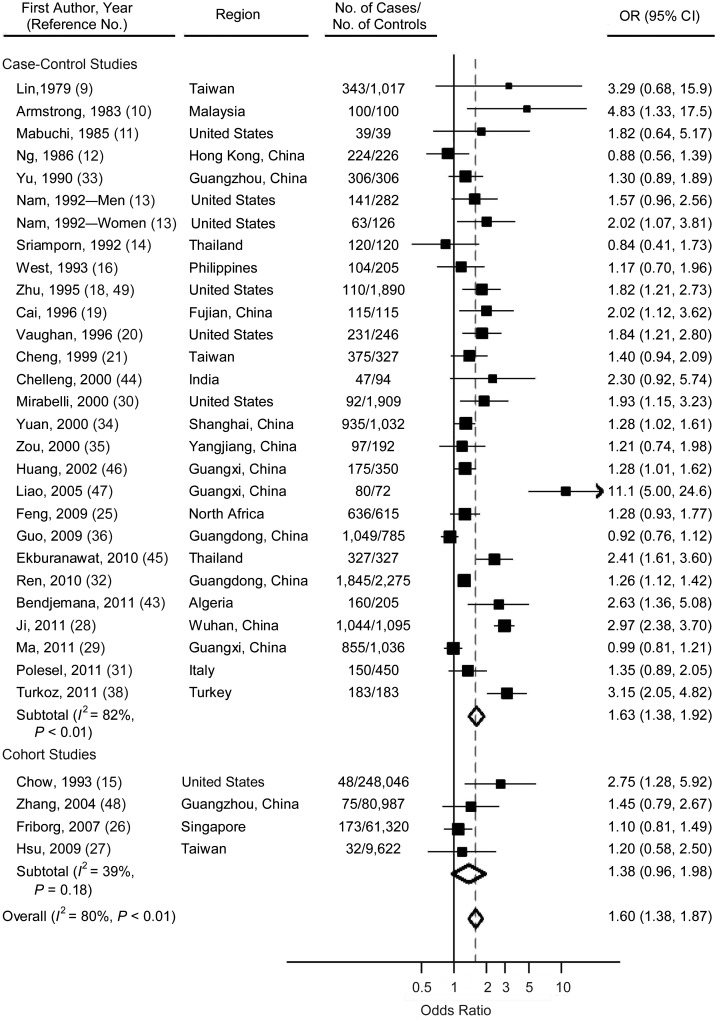
The overall analysis of all 32 studies, including the case-control and cohort studies, found an increased risk of NPC for ever smokers compared with never smokers (odds ratio (OR) = 1.60, 95% confidence interval (CI): 1.38, 1.87). Heterogeneity across all of the studies was significant (P < 0.01, I2 = 80%). Specifically, the pooled estimate for case-control studies was 1.63 (95% CI: 1.38, 1.92; heterogeneity: P < 0.01, I2 = 82%), and for cohort studies, the estimate was 1.38 (95% CI: 0.96, 1.98; heterogeneity: P = 0.18, I2 = 39%) (Figure 2). No significant difference was detected between the results from the case-control studies and those from the cohort studies (P = 0.41, I2 = 0%) (Table 3).
Figure 2.
Odds ratios (ORs) for nasopharyngeal carcinoma among ever smokers versus nonsmokers in individual studies and a meta-analysis, 1979–2011. Subtotals represent the pooled OR within each subcategory, based on a random-effects model. Heterogeneity for the subgroup analysis comparing the case-control studies with the cohort studies: P = 0.41, I2 = 0%. Bars, 95% confidence interval (CI).
Table 3.
Pooled Odds Ratios for the Association Between Smoking and Nasopharyngeal Carcinoma, by Study Subgroup, 1979–2011
| Subgroup | No. of Studies | OR | 95% CI | Test for Heterogeneity |
Egger's Test P Value | Adjusted for Publication Bias |
||
|---|---|---|---|---|---|---|---|---|
| P Value | I2, % | OR | 95% CI | |||||
| Overall | 32 | 1.60 | 1.38, 1.87 | <0.01 | 80 | 0.05 | 1.23 | 1.04, 1.46 |
| Study design | ||||||||
| Case-control | 28 | 1.63 | 1.38, 1.92 | <0.01 | 82 | 0.05 | 1.23 | 1.03, 1.48 |
| Cohort | 4 | 1.38 | 0.96, 1.98 | 0.18 | 39 | 0.25 | ||
| Pheterogeneitya | 0.41 | 0 | ||||||
| Regional NPC prevalence (cases/100,000 person-years) | ||||||||
| <1 | 8 | 1.76 | 1.47, 2.10 | 0.84 | 0 | 0.20 | ||
| 1–<5 | 8 | 1.94 | 1.37, 2.75 | <0.01 | 85 | 0.99 | ||
| 5–10 | 5 | 1.44 | 1.12, 1.86 | 0.52 | 0 | 0.38 | ||
| >10 | 11 | 1.29 | 1.05, 1.58 | <0.01 | 79 | 0.18 | ||
| Pheterogeneity | 0.08 | 56 | ||||||
| Sex | ||||||||
| Men | 3 | 1.77 | 1.35, 2.31 | 0.84 | 0 | 0.93 | ||
| Women | 1 | 2.02 | 1.07, 3.81 | |||||
| Pheterogeneity | 0.71 | 0 | ||||||
| Histological type | ||||||||
| Squamous-cell carcinoma | 4 | 2.20 | 1.63, 2.98 | 0.84 | 0 | 0.98 | ||
| Undifferentiated | 2 | 1.27 | 0.98, 1.66 | 0.14 | 40 | |||
| Pheterogeneity | <0.01 | 86 | ||||||
| Quality assessment scoreb | ||||||||
| <8 (low quality) | 18 | 1.93 | 1.47, 2.55 | <0.01 | 85 | 0.23 | ||
| ≥8 (high quality) | 14 | 1.29 | 1.15, 1.45 | 0.06 | 40 | 0.09 | 1.19 | 1.05, 1.34 |
| Pheterogeneity | <0.01 | 88 | ||||||
| Adjustment for possible confounding factors | ||||||||
| Adjusted | 15 | 1.51 | 1.20, 1.90 | <0.01 | 83 | 0.73 | ||
| Unadjusted | 15 | 1.58 | 1.31, 1.90 | <0.01 | 79 | 0.81 | ||
| Pheterogeneity | 0.77 | 0 | ||||||
Abbreviations: CI, confidence interval; NPC, nasopharyngeal carcinoma; OR, odds ratio.
a Test for heterogeneity between subgroups.
b The methodological quality of the included studies was assessed according to the Newcastle-Ottawa Scale guidelines (24).
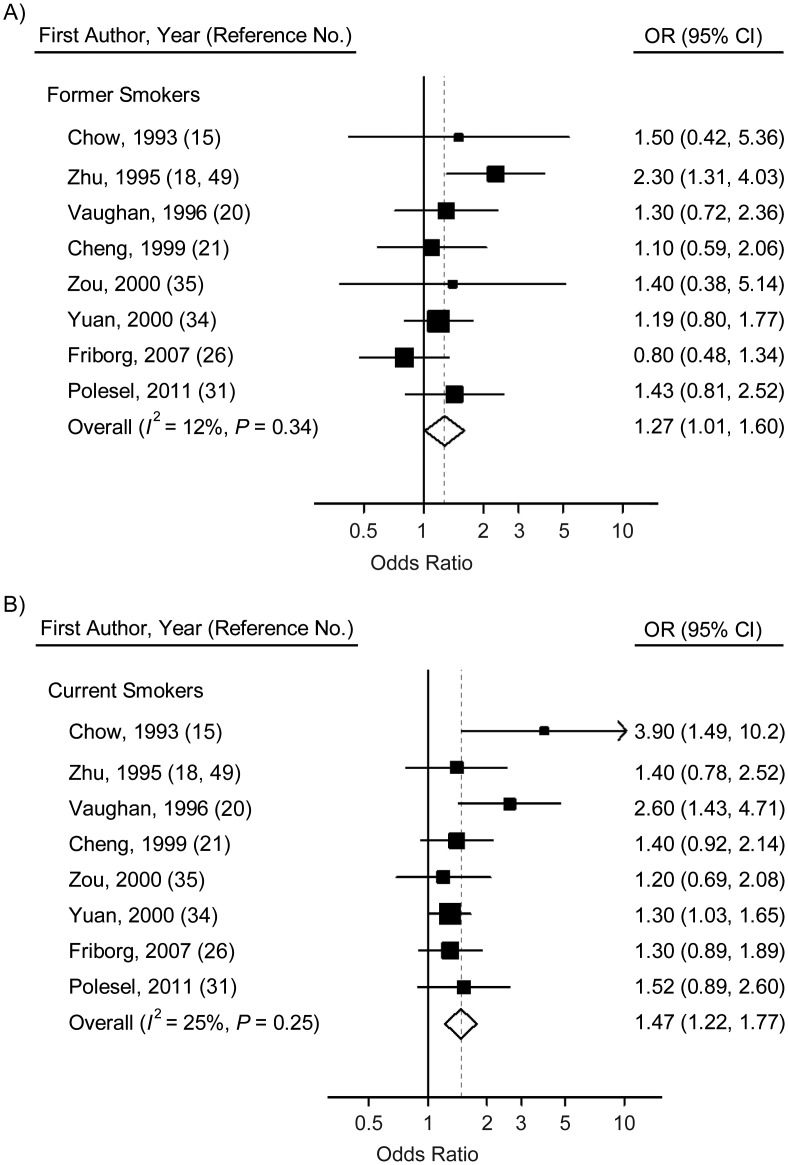
For former smokers, the odds ratio for NPC was 1.27 (95% CI: 1.01, 1.60), while for current smokers, the odds ratio was slightly higher at 1.47 (95% CI: 1.22, 1.77) (Figure 3, Table 4). The forest plots of the NPC odds ratios for ever smokers (including former and current smokers) versus nonsmokers further confirmed the positive association between smoking and risk of NPC (Figure 3, Table 4). However, no significant heterogeneity was detected in former smokers (P = 0.34, I2 = 12%) or current smokers (P = 0.25, I2 = 25%). Egger's test reflected significant publication bias only for current smokers (P = 0.06) (Table 4).
Figure 3.
Odds ratios (ORs) for nasopharyngeal carcinoma among former smokers (A) and current smokers (B) versus nonsmokers in individual studies and a meta-analysis, 1979–2011. Bars, 95% confidence interval (CI).
Table 4.
Pooled Odds Ratios for the Association Between Smoking and Nasopharyngeal Carcinoma, by Smoking Status and Related Characteristics, 1979–2011
| Smoking-related Characteristic | No. of Studies |
OR | 95% CI | Test for Heterogeneity |
Egger's Test P Value | Adjusted for Publication Bias |
||
|---|---|---|---|---|---|---|---|---|
| P Value | I2, % | OR | 95% CI | |||||
| Smoking status | ||||||||
| Former smoker | 8 | 1.27 | 1.01, 1.60 | 0.34 | 12 | 0.66 | ||
| Current smoker | 8 | 1.47 | 1.22, 1.77 | 0.25 | 25 | 0.06 | 1.47 | 1.22, 1.77 |
| Pheterogeneitya | 0.34 | 0 | ||||||
| Cumulative amount of smoking, pack-years | ||||||||
| <30 | 8 | 1.29 | 1.04, 1.62 | 0.70 | 0 | 0.62 | ||
| ≥30 | 10 | 2.93 | 1.87, 4.61 | <0.01 | 89 | 0.11 | ||
| Pheterogeneity | 0.01 | 90 | ||||||
| Intensity of smoking, cigarettes/day | ||||||||
| Low exposureb | 12 | 1.31 | 1.07, 1.60 | <0.01 | 64 | 0.38 | ||
| High exposureb | 12 | 1.98 | 1.31, 2.98 | <0.01 | 89 | 0.62 | ||
| Pheterogeneity | 0.07 | 69 | ||||||
| Age at initiation of smoking | ||||||||
| Medium groupb | 3 | 1.58 | 1.10, 2.26 | 0.63 | 0 | 0.72 | ||
| Early groupb | 5 | 1.17 | 0.78, 1.75 | 0.03 | 62 | 0.99 | ||
| Pheterogeneity | 0.28 | 16 | ||||||
| Long duration of smokingb | 9 | 2.26 | 1.32, 3.84 | <0.01 | 88 | 0.27 | ||
| Passive smoking during childhood | 5 | 1.29 | 0.80, 2.09 | <0.01 | 75 | 0.25 | ||
Abbreviations: CI, confidence interval; OR, odds ratio.
a Test for heterogeneity between subgroups.
b Exact cutpoint depended on the threshold used in each study; see Materials and Methods section.
To evaluate the stability of the estimated effect sizes, we conducted a sensitivity analysis by removing each study sequentially and then evaluating the associations in the remaining data. The overall estimates ranged from 1.64 (95% CI: 1.40, 1.92) to 1.53 (95% CI: 1.34, 1.75) (data not shown). The results did not change significantly when we omitted any single study from the overall analysis. Likewise, excluding the studies that used death from NPC to define cases also did not affect the pooled estimate significantly (OR = 1.60, 95% CI: 1.35, 1.89) (data not shown).
Dose dependency
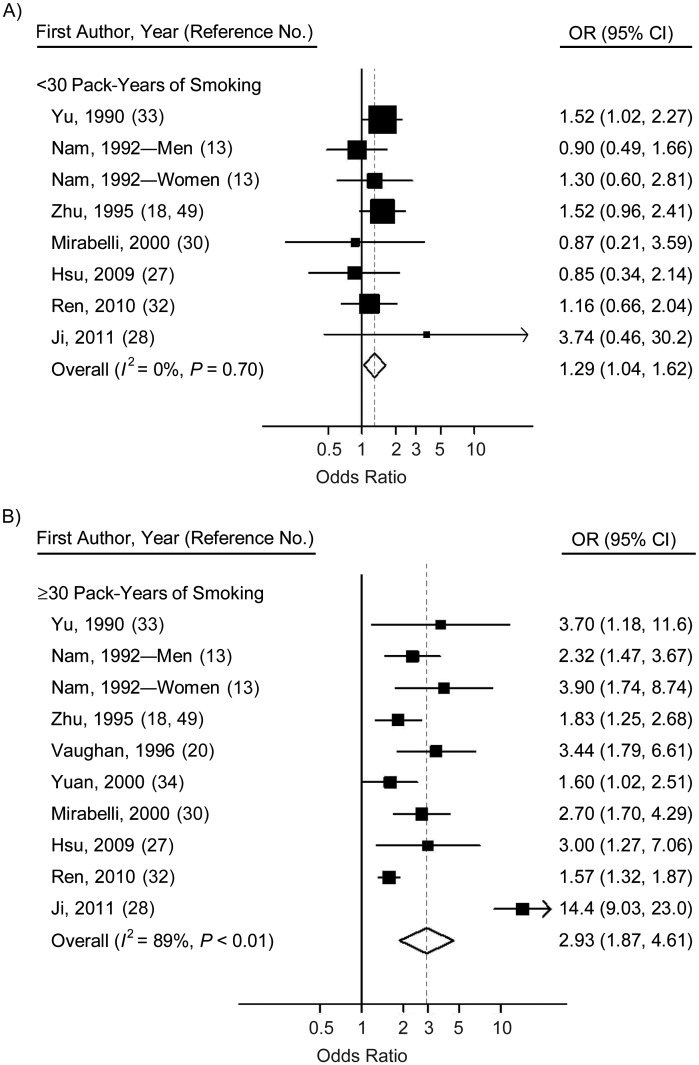
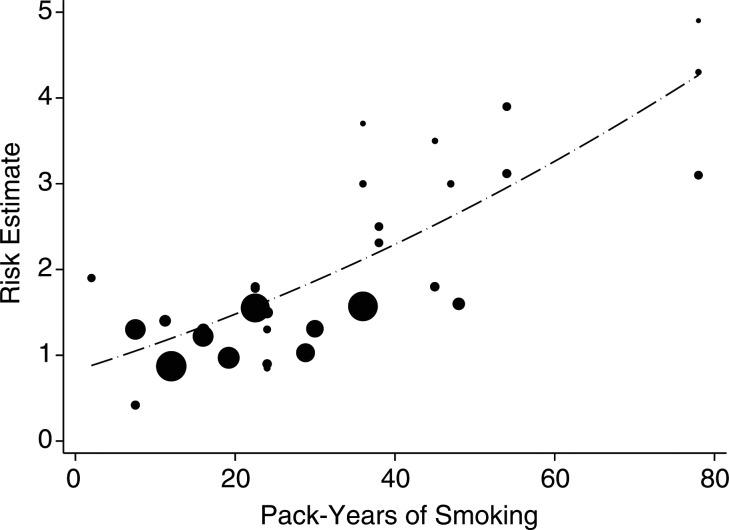
When stratified by cumulative number of pack-years and smoking intensity, the pooled analyses suggested a positive, dose-dependent association between smoking and NPC risk. We found that smokers with cumulative exposure of ≥30 pack-years had approximately twice the risk (OR = 2.93, 95% CI: 1.87, 4.61) of smokers with exposure of <30 pack-years (OR = 1.29, 95% CI: 1.04, 1.62) (Figure 4). While the low-cumulative-smoking subgroup appeared to have no heterogeneity (P = 0.70, I2 = 0%), substantial heterogeneity appeared in the high-cumulative-smoking subgroup (P < 0.01, I2 = 89%) (Table 4, Figure 4). No evidence of publication bias was observed in either subgroup analysis. Comparing the NPC risk for smokers by smoking intensity (cigarettes per day) with that for never smokers, we found that the risk estimates for medium smokers and heavier smokers were 1.31 (95% CI: 1.07, 1.60) and 1.98 (95% CI: 1.31, 2.98), respectively, with no significant difference between the subgroups (P = 0.07). Our results also suggested that long-term smoking increased the risk of NPC by 126% (Table 4). The dose-response analysis revealed a significant positive relationship between the cumulative number of pack-years smoked and risk of NPC (P < 0.001), with no evidence of heterogeneity (P = 0.384) (data not shown). The pooled risk estimate (dashed line) for NPC associated with each 1-pack-year increment of smoking was 1.019 (95% CI: 1.016, 1.021) (Figure 5).
Figure 4.
Odds ratios (ORs) for nasopharyngeal carcinoma among smokers who consumed <30 pack-years (A) and smokers who consumed ≥30 pack-years (B) in individual studies and a meta-analysis, 1979–2011. Bars, 95% confidence interval (CI).
Figure 5.
Dose-response relationship between pack-years of smoking and risk of nasopharyngeal carcinoma in individual studies and a meta-analysis, 1979–2011. The dots represent the odds ratios (ORs) corresponding to the pack-years in each study. The sizes of the dots are inversely proportional to the logarithm of the variance of the ORs. The dashed line depicts the pooled risk estimate or nasopharyngeal carcinoma associated with each 1-pack-year increment of smoking (OR = 1.019, 95% confidence interval: 1.016, 1.021).
Associations for different risk populations
The subgroup analyses demonstrated a diverse pattern of association between smoking and NPC risk in different populations (Table 3). In Italy, the United States, and populations in which NPC incidence was less than 1 per 100,000 person-years, the combined odds ratio from 7 studies (11, 13, 15, 18, 20, 30, 31) was 1.76 (95% CI: 1.47, 2.10), with mild heterogeneity (P = 0.84, I2 = 0%). By contrast, the findings from 11 studies (10, 12, 26, 29, 32, 33, 35, 36, 46–48) conducted in southern Chinese (Guangdong, Guangxi, and Hong Kong), Malaysian Chinese, and Singapore Chinese, in whom the incidence of NPC was more than 10 per 100,000 person-years, yielded the lowest risk (OR = 1.29, 95% CI: 1.05, 1.58; heterogeneity: P < 0.01, I2 = 79%). Pooled odds ratios in regions where NPC incidence rates were 1–<5 per 100,000 person-years (14, 25, 28, 34, 38, 43–45) and 5–10 per 100,000 person-years (9, 16, 19, 21, 27) were 1.94 (95% CI: 1.37, 2.75; heterogeneity: P < 0.01, I2 = 85%) and 1.44 (95% CI: 1.12, 1.86; heterogeneity: P = 0.52, I2 = 0%), respectively, with no significant publication bias.
Association between tobacco smoking and undifferentiated NPC
A significant association with smoking was observed only for squamous-cell NPC (OR = 2.20, 95% CI: 1.63, 2.98), with no observed heterogeneity or publication bias (heterogeneity: P = 0.84, I2 = 0%; Egger's test: P = 0.98). The odds ratio for undifferentiated carcinoma in smokers was significantly lower (OR = 1.27, 95% CI: 0.98, 1.66; test for subgroup difference: P < 0.01), with mild heterogeneity (P = 0.14, I2 = 40%) (Table 3).
Study quality and publication bias
To evaluate the impact of publication quality, we ranked the publications by their NOS scores, with a threshold of 8. The high-quality studies (NOS score ≥8) had an odds ratio of 1.29 (95% CI: 1.15, 1.45; heterogeneity: P = 0.06, I2 = 40%), while the low-quality studies had a higher odds ratio of 1.93 (95% CI: 1.47, 2.55; heterogeneity: P < 0.01, I2 = 85%).
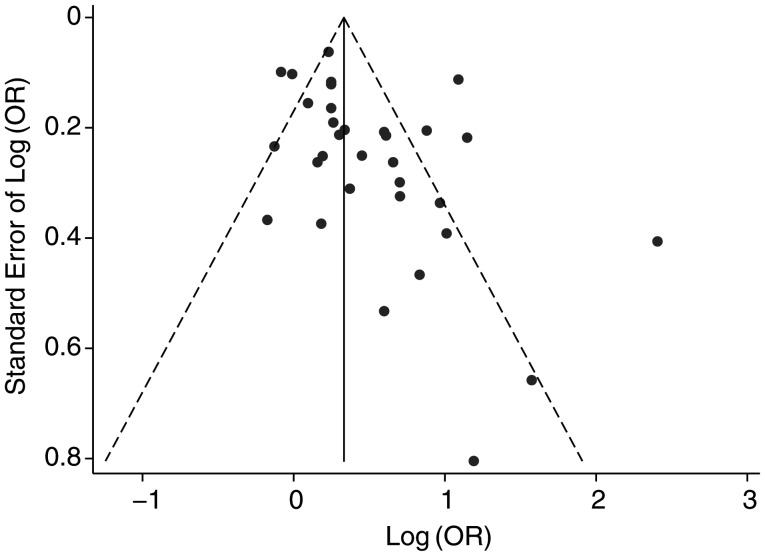
Funnel plots (Figure 6) showed that there was significant publication bias in our overall analysis (P = 0.05). The trim-and-fill analysis identified 5 imputed studies and generated an adjusted estimate of 1.23 (95% CI: 1.04, 1.46). Egger's weighting regression also found a moderate level of bias among the case-control studies (P = 0.05), which had an adjusted odds ratio of 1.23 (95% CI: 1.03, 1.48). No publication bias was detected in the cohort studies (P = 0.25).
Figure 6.
Funnel plot of the risk of nasopharyngeal carcinoma (log odds ratio (OR)) associated with ever having smoked in a meta-analysis, 1979–2011.
DISCUSSION
In the current meta-analysis, we reviewed 32 epidemiologic studies on tobacco smoking and NPC risk conducted in the last 50 years, involving a total of 10,274 NPC cases and 415,266 comparison subjects. We added to the evidence base by comprehensively evaluating the effect of tobacco smoking on NPC risk, with reasonable quality control. Our results confirmed that ever smokers had a significantly higher risk of NPC than never smokers, and this association was relatively stable across almost all of the subgroup analyses, with a robust dose-dependent pattern. To our knowledge, this was the first meta-analysis to evaluate the association between cigarette smoking and NPC risk. Although previous studies have investigated this association, because of the limitations of small sample sizes and other confounding factors, several studies did not report a significant increase in the risk of NPC (9, 11, 12, 14, 16, 21, 25–27, 31, 35, 36, 44, 48), or they demonstrated a significantly higher risk only for smokers with very high levels of smoking (more than 30 pack-years or 20 cigarettes per day) (13, 18, 27, 28, 30–32, 34). By combining evidence, meta-analysis is a valuable tool for increasing the reliability and accuracy of reported associations. Our study found that both former smokers and low-dose smokers had an increased risk of NPC; moreover, the risk rose by 1%–2% with each pack-year of smoking (P < 0.001), which seems to indicate that this meta-analysis made a more accurate assessment of the association than any single study.
The subgroup analyses showed that the observed heterogeneity in the overall analysis and among the case-control studies could be partially explained by the differences in study region and research quality. The risk estimates for ever smokers versus nonsmokers seemed to be adversely related to the local NPC incidence rate, with the odds ratios ranging from 1.29 to 1.94, suggesting that tobacco smoking may have a greater impact on NPC risk among populations in low-risk areas than among populations in high-risk areas.
This phenomenon could be mainly due to the different histological types in high- and low-risk areas. Undifferentiated carcinoma (type III) comprises over 95% of the NPC cases in high-incidence regions, while differentiated carcinoma (type I) is predominant in low-incidence regions (50). Four (20, 25, 28, 31) of the 32 studies provided estimates of the histology-specific risk of NPC for smoking; all of these studies showed a slightly higher risk of differentiated NPC than of undifferentiated NPC. However, because of the small sample sizes, none of the studies found a significant difference between the associations for different histological types. Our subgroup analyses showed an odds ratio for differentiated NPC that was nearly 2-fold higher than the odds ratio for undifferentiated NPC, which strongly supports the observation made in previous studies. Note that similar results have been widely observed for lung cancer, with the odds ratio for the heaviest smokers ranging from 18.3 for squamous-cell carcinoma to 4.10 for adenocarcinoma (51, 52). Previous pooled analyses have also found that smoking-related odds ratios for squamous-cell carcinoma were greater for esophageal cancer (53) and cervical cancer (54) than for other histological types.
We speculate that the differences in the effects of smoking on NPC risk might be due to the different etiologies of the 2 histological types. Epstein-Barr virus infection is strongly related to undifferentiated carcinoma in high-risk areas. It has been documented that viral oncogenes, such as latent membrane protein 1 (LMP1) and Epstein-Barr nuclear antigen 1 (EBNA1), play a crucial role in carcinogenesis (55). While a different pathway that is less involved with Epstein-Barr virus may contribute to the development of squamous-cell carcinoma in low-risk areas, a classic pathway similar to the pathways involved in other smoking-related cancers may be part of the carcinogenic process for smoking. Further studies have revealed that various genetic variants play crucial roles in smoking-related carcinogenesis for different types of lung cancer. Mutations in the epidermal growth factor receptor gene (EGFR) are more frequently observed in never-smoker patients (mainly adenocarcinoma), while mutations in the V-Ki-ras2 Kirsten rat sarcoma (KRAS) and protein 53 (P53) genes are more common in patients with smoking-related lung cancer (mainly squamous-cell lung cancer and small-cell lung cancer) (56). However, to our knowledge, there have been no studies exploring the histology-specific genetic variants for NPC. Well-designed, large-scale epidemiologic studies and experimental research are needed to confirm this notion.
We conducted a meta-regression analysis to further evaluate the sources of heterogeneity and their impacts on the level of risk. The results suggested that heterogeneity still existed among the study regions (P = 0.051) and the quality of research (P = 0.049), consistent with previous subgroup analyses. In addition, the risk estimate decreased as NPC incidence increased among different regions, and it also decreased as the quality of the publications increased.
A quality assessment using the NOS quality criteria was conducted in our meta-analysis. The results showed that 14 of the 32 studies included were high-quality, with 13 studies scoring more than 10 points (the highest score was 12). When we included only the high-quality studies (NOS score ≥8), the risk estimate for NPC associated with tobacco smoking was 1.29 (95% CI: 1.15, 1.45), as compared with 1.93 (95% CI: 1.47, 2.55) using data from the studies with low NOS scores. This suggests that study quality may change the effect sizes for smoking but not the significance of the positive association. For case definition, the NPC cases in 28 studies (88%) were pathologically confirmed. The cases and controls were matched by age and sex in all of the case-control studies, and all 4 cohort studies adjusted for potentially confounding factors; thus, the studies were fairly comparable. To measure smoking exposure, 69% of the studies collected the relevant information in an interview, and 50% of the studies used structured questionnaires.
Confounding is one of the most important issues affecting the risk estimates. When we pooled the adjusted and unadjusted odds ratios, similar results were obtained (OR = 1.51 vs. OR = 1.58), suggesting that the confounding factors might not have significantly affected the results. Moreover, using a meta-analysis, we cannot assess any confounding factors that were not measured in the original publications. Epstein-Barr virus infection is the factor most closely related to NPC development, and in most parts of the world, more than 95% of NPC cases are seropositive for Epstein-Barr virus (57). Investigators in case-control studies are not likely to adjust for Epstein-Barr virus positivity as a factor; prospective cohort studies offer a better study design. In fact, in a 22-year follow-up study carried out in Taiwan, Hsu et al. (27) reported that smoking was an independent risk factor for NPC, indicating that Epstein-Barr virus was unlikely to modify the risk estimate for smoking. To our knowledge, there have not been any previous epidemiologic studies on the interactions between smoking and other risk factors.
Our study had several strengths. It used the largest number of subjects to date to evaluate the effect size of the NPC risk associated with tobacco smoking. Moreover, meaningful subgroup analyses were conducted to explore the potential factors that might modify the effect of smoking on NPC risk. Finally, the subgroup analyses of various smoking habits (smoking status, cumulative amount of smoking, smoking intensity, etc.) produced a more precise quantification of the relationship between smoking and NPC risk than was provided in any single study.
Our findings may have several limitations. First, publication bias is one concern, because studies with positive results are more likely to be published than studies with negative results. To limit publication bias, we screened a large number of publications manually to collect all available studies related to tobacco smoking and NPC risk. Egger's test suggested a potential publication bias in the overall analysis (P = 0.05) and the case-control studies (P = 0.05). Publication bias would still be an issue of concern even if Egger's test revealed no evidence. The trim-and-fill method infers the existence of unpublished hidden studies and yields unbiased pooled estimates. The odds ratios changed slightly after adjustment for publication bias, but the significance of the results remained (for all studies, OR = 1.23 vs. OR = 1.60; for case-control studies, OR = 1.23 vs. OR = 1.63). Second, the types of bias in the individual studies may have also been included in the meta-analysis (i.e., recall bias in retrospective studies). Because 28 of the 32 included studies were retrospective, it is likely that recall bias affected this study and may have influenced the risk estimates to some extent, but it should not have influenced the positive association between smoking and NPC. Nevertheless, because of the potential limitations of the studies, conclusions must be drawn with caution, and well-designed studies with large sample sizes should be conducted for further validation.
To summarize, we systematically reviewed 28 case-control studies and 4 cohort studies of tobacco smoking and NPC conducted between 1979 and 2011 and performed a meta-analysis. This study provided robust evidence that tobacco smoking is one of the most important risk factors for NPC. The cumulative effect of exposure to tobacco smoking on the risk of NPC is substantial, and a dose-dependent pattern was observed. Moreover, a stronger association was observed in the low-risk population and for the predominant histological type of differentiated NPC.
ACKNOWLEDGMENTS
Author affiliations: State Key Laboratory of Oncology in South China, Department of Experimental Research, Sun Yat-Sen University Cancer Center, Guangzhou, China (Wen-Qiong Xue, Hong-Lian Ruan, Wei-Hua Jia); and Unit of Statistical Genomics, Division of Intramural Research Programs, National Institute of Mental Health, National Institutes of Health, Bethesda, Maryland (Hai-De Qin, Yin Yao Shugart).
Wen-Qiong Xue and Dr. Hai-De Qin contributed to this work equally.
This work was supported by the National Natural Science Foundation of China (grant 81220108022), the Chinese National High Technology Research and Development Program (grant 863, programs 2012AA02A206 and 2012AA02A501), and the National Basic Research Program of China (grant 973, program 2011CB504303).
The views expressed in this article do not necessarily represent the views of the National Institute of Mental Health, the National Institutes of Health, the US Department of Health and Human Services, or the US Government.
Conflict of interest: none declared.
REFERENCES
- 1.Gallicchio L, Matanoski G, Tao XG, et al. Adulthood consumption of preserved and nonpreserved vegetables and the risk of nasopharyngeal carcinoma: a systematic review. Int J Cancer. 2006;119(5):1125–1135. doi: 10.1002/ijc.21946. [DOI] [PubMed] [Google Scholar]
- 2.Chen L, Gallicchio L, Boyd-Lindsley K, et al. Alcohol consumption and the risk of nasopharyngeal carcinoma: a systematic review. Nutr Cancer. 2009;61(1):1–15.. doi: 10.1080/01635580802372633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blair A, Saracci R, Stewart PA, et al. Epidemiologic evidence on the relationship between formaldehyde exposure and cancer. Scand J Work Environ Health. 1990;16(6):381–393. doi: 10.5271/sjweh.1767. [DOI] [PubMed] [Google Scholar]
- 4.Partanen T. Formaldehyde exposure and respiratory cancer—a meta-analysis of the epidemiologic evidence. Scand J Work Environ Health. 1993;19(1):8–15. doi: 10.5271/sjweh.1500. [DOI] [PubMed] [Google Scholar]
- 5.Bosetti C, McLaughlin JK, Tarone RE, et al. Formaldehyde and cancer risk: a quantitative review of cohort studies through 2006. Ann Oncol. 2008;19(1):29–43. doi: 10.1093/annonc/mdm202. [DOI] [PubMed] [Google Scholar]
- 6.Collins JJ, Acquavella JF, Esmen NA. An updated meta-analysis of formaldehyde exposure and upper respiratory tract cancers. J Occup Environ Med. 1997;3(7):639–651. doi: 10.1097/00043764-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 7.American Cancer Society. Global Cancer Facts and Figures. 2nd ed. Atlanta, GA: American Cancer Society; 2011. [Google Scholar]
- 8.Jia WH, Qin HD. Non-viral environmental risk factors for nasopharyngeal carcinoma: a systematic review. Semin Cancer Biol. 2012;22(2):117–126. doi: 10.1016/j.semcancer.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Lin TM, Yang CS, Tu SM, et al. Interaction of factors associated with cancer of the nasopharynx. Cancer. 1979;44(4):1419–1423. doi: 10.1002/1097-0142(197910)44:4<1419::aid-cncr2820440437>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong RW, Armstrong MJ, Yu MC, et al. Salted fish and inhalants as risk factors for nasopharyngeal carcinoma in Malaysian Chinese. Cancer Res. 1983;43(6):2967–2970. [PubMed] [Google Scholar]
- 11.Mabuchi K, Bross DS, Kessler II. Cigarette smoking and nasopharyngeal carcinoma. Cancer. 1985;55(12):2874–2876. doi: 10.1002/1097-0142(19850615)55:12<2874::aid-cncr2820551228>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Ng TP. A case-referent study of cancer of the nasal cavity and sinuses in Hong Kong. Int J Epidemiol. 1986;15(2):171–175. doi: 10.1093/ije/15.2.171. [DOI] [PubMed] [Google Scholar]
- 13.Nam JM, McLaughlin JK, Blot WJ. Cigarette smoking, alcohol, and nasopharyngeal carcinoma: a case-control study among U.S. whites. J Natl Cancer Inst. 1992;84(8):619–622. doi: 10.1093/jnci/84.8.619. [DOI] [PubMed] [Google Scholar]
- 14.Sriamporn S, Vatanasapt V, Pisani P, et al. Environmental risk factors for nasopharyngeal carcinoma: a case-control study in northeastern Thailand. Cancer Epidemiol Biomarkers Prev. 1992;1(5):345–348. [PubMed] [Google Scholar]
- 15.Chow WH, McLaughlin JK, Hrubec Z, et al. Tobacco use and nasopharyngeal carcinoma in a cohort of US veterans. Int J Cancer. 1993;55(4):538–540. doi: 10.1002/ijc.2910550403. [DOI] [PubMed] [Google Scholar]
- 16.West S, Hildesheim A, Dosemeci M. Non-viral risk factors for nasopharyngeal carcinoma in the Philippines: results from a case-control study. Int J Cancer. 1993;55(5):722–727. doi: 10.1002/ijc.2910550504. [DOI] [PubMed] [Google Scholar]
- 17.Ye W, Yi Y, Zhou T, et al. A case-control study on risk condition of nasopharyngeal carcinoma in the southern area of Fujian Province. J Fujian Med College. 1995;29(2):179–182. [Google Scholar]
- 18.Zhu K, Levine RS, Brann EA, et al. A population-based case-control study of the relationship between cigarette smoking and nasopharyngeal cancer (United States) Cancer Causes Control. 1995;6(6):507–512. doi: 10.1007/BF00054158. [DOI] [PubMed] [Google Scholar]
- 19.Cai L, Yi Y. A matched study with various controls in nasopharyngeal carcinoma epidemiology in Fujian Province [in Chinese] J Fujian Med College. 1996;30(2):199–202. [Google Scholar]
- 20.Vaughan TL, Shapiro JA, Burt RD, et al. Nasopharyngeal cancer in a low-risk population: defining risk factors by histological type. Cancer Epidemiol Biomarkers Prev. 1996;5(8):587–593. [PubMed] [Google Scholar]
- 21.Cheng YJ, Hildesheim A, Hsu MM, et al. Cigarette smoking, alcohol consumption and risk of nasopharyngeal carcinoma in Taiwan. Cancer Causes Control. 1999;10(3):201–207. doi: 10.1023/a:1008893109257. [DOI] [PubMed] [Google Scholar]
- 22.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) Group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 23.Nesic V, Sipetic S, Vlajinac H, et al. Risk factors for the occurrence of undifferentiated carcinoma of nasopharyngeal type: a case-control study. Srp Arh Celok Lek. 2010;138(1-2):6–10. doi: 10.2298/sarh1002006n. [DOI] [PubMed] [Google Scholar]
- 24.Wells G, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa, Ontario, Canada: Ottawa Health Research Institute; 2000. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. (Accessed November 1, 2011) [Google Scholar]
- 25.Feng BJ, Khyatti M, Ben-Ayoub W, et al. Cannabis, tobacco and domestic fumes intake are associated with nasopharyngeal carcinoma in North Africa. Br J Cancer. 2009;101(7):1207–1212. doi: 10.1038/sj.bjc.6605281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friborg JT, Yuan JM, Wang R, et al. A prospective study of tobacco and alcohol use as risk factors for pharyngeal carcinomas in Singapore Chinese. Cancer. 2007;109(6):1183–1191. doi: 10.1002/cncr.22501. [DOI] [PubMed] [Google Scholar]
- 27.Hsu WL, Chen JY, Chien YC, et al. Independent effect of EBV and cigarette smoking on nasopharyngeal carcinoma: a 20-year follow-up study on 9,622 males without family history in Taiwan. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1218–1226. doi: 10.1158/1055-9965.EPI-08-1175. [DOI] [PubMed] [Google Scholar]
- 28.Ji X, Zhang W, Xie C, et al. Nasopharyngeal carcinoma risk by histologic type in central China: impact of smoking, alcohol and family history. Int J Cancer. 2011;129(3):724–732. doi: 10.1002/ijc.25696. [DOI] [PubMed] [Google Scholar]
- 29.Ma F, Zhang H, Zhai Y, et al. Functional polymorphism −31C/G in the promoter of BIRC5 gene and risk of nasopharyngeal carcinoma among Chinese. PLoS One. 2011;6(2):e16748. doi: 10.1371/journal.pone.0016748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mirabelli MC, Hoppin JA, Tolbert PE, et al. Occupational exposure to chlorophenol and the risk of nasal and nasopharyngeal cancers among U.S. men aged 30 to 60. Am J Ind Med. 2000;37(5):532–541. doi: 10.1002/(sici)1097-0274(200005)37:5<532::aid-ajim9>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 31.Polesel J, Franceschi S, Talamini R, et al. Tobacco smoking, alcohol drinking, and the risk of different histological types of nasopharyngeal cancer in a low-risk population. Oral Oncol. 2011;47(6):541–545. doi: 10.1016/j.oraloncology.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 32.Ren ZF, Liu WS, Qin HD, et al. Effect of family history of cancers and environmental factors on risk of nasopharyngeal carcinoma in Guangdong, China. Cancer Epidemiol. 2010;34(4):419–424. doi: 10.1016/j.canep.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 33.Yu MC, Garabrant DH, Huang TB, et al. Occupational and other non-dietary risk factors for nasopharyngeal carcinoma in Guangzhou, China. Int J Cancer. 1990;45(6):1033–1039. doi: 10.1002/ijc.2910450609. [DOI] [PubMed] [Google Scholar]
- 34.Yuan JM, Wang XL, Xiang YB, et al. Non-dietary risk factors for nasopharyngeal carcinoma in Shanghai, China. Int J Cancer. 2000;85(3):364–369. [PubMed] [Google Scholar]
- 35.Zou J, Sun Q, Akiba S, et al. A case-control study of nasopharyngeal carcinoma in the high background radiation areas of Yangjiang, China. J Radiat Res (Tokyo) 2000;41(suppl):53–62. doi: 10.1269/jrr.41.s53. [DOI] [PubMed] [Google Scholar]
- 36.Guo X, Johnson RC, Deng H, et al. Evaluation of nonviral risk factors for nasopharyngeal carcinoma in a high-risk population of Southern China. Int J Cancer. 2009;124(12):2942–2947. doi: 10.1002/ijc.24293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armstrong RW, Imrey PB, Lye MS, et al. Nasopharyngeal carcinoma in Malaysian Chinese: occupational exposures to particles, formaldehyde and heat. Int J Epidemiol. 2000;29(6):991–998. doi: 10.1093/ije/29.6.991. [DOI] [PubMed] [Google Scholar]
- 38.Turkoz FP, Celenkoglu G, Dogu GG, et al. Risk factors of nasopharyngeal carcinoma in Turkey—an epidemiological survey of the Anatolian Society of Medical Oncology. Asian Pac J Cancer Prev. 2011;12(11):3017–3021. [PubMed] [Google Scholar]
- 39.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 40.Nicola O, Rino B, Sander G. Generalized least squares for trend estimation of summarized dose-response data. Stata J. 2006;6(1):40–57. [Google Scholar]
- 41.Berlin JA, Longnecker MP, Greenland S. Meta-analysis of epidemiologic dose-response data. Epidemiology. 1993;4(3):218–228. doi: 10.1097/00001648-199305000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bendjemana K, Satta D, Adjabi K, et al. Epidemiology of nasopharyngeal carcinoma and impact of food factors in North-East of Algeria [in French] J Afr Cancer. 2011;3(1):59–62. [Google Scholar]
- 44.Chelleng PK, Narain K, Das HK, et al. Risk factors for cancer nasopharynx: a case-control study from Nagaland, India. Natl Med J India. 2000;13(1):6–8. [PubMed] [Google Scholar]
- 45.Ekburanawat W, Ekpanyaskul C, Brennan P, et al. Evaluation of non-viral risk factors for nasopharyngeal carcinoma in Thailand: results from a case-control study. Asian Pac J Cancer Prev. 2010;11(4):929–932. [PubMed] [Google Scholar]
- 46.Huang Z, Jiang Y, Fang Y. An epidemiological study on risk factors of nasopharyngeal carcinoma in Guangxi [in Chinese] Ind Health Occup Dis. 2002;28(4):193–196. [Google Scholar]
- 47.Liao Z, Deng Z, Wei Y, et al. Variants of GSTT1, GSTM1 and risk of nasopharyngeal carcinoma [in Chinese] J Guangxi Med Coll. 2005;22(3):372–374. [Google Scholar]
- 48.Zhang WS, Jiang CQ, Hing LT, et al. A prospective cohort study on the comparison of risk of occupational dust exposure and smoking to death [in Chinese] Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25(9):748–752. [PubMed] [Google Scholar]
- 49.Zhu K, Levine RS, Brann EA, et al. Cigarette smoking and nasopharyngeal cancer: an analysis of the relationship according to age at starting smoking and age at diagnosis. J Epidemiol. 1997;7(2):107–111. doi: 10.2188/jea.7.107. [DOI] [PubMed] [Google Scholar]
- 50.Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765–1777. doi: 10.1158/1055-9965.EPI-06-0353. [DOI] [PubMed] [Google Scholar]
- 51.Khuder SA. Effect of cigarette smoking on major histological types of lung cancer: a meta-analysis. Lung Cancer. 2001;31(2-3):139–148. doi: 10.1016/s0169-5002(00)00181-1. [DOI] [PubMed] [Google Scholar]
- 52.Hecht SS. Cigarette smoking: cancer risks, carcinogens, and mechanisms. Langenbecks Arch Surg. 2006;391(6):603–613. doi: 10.1007/s00423-006-0111-z. [DOI] [PubMed] [Google Scholar]
- 53.Lubin JH, Cook MB, Pandeya N, et al. The importance of exposure rate on odds ratios by cigarette smoking and alcohol consumption for esophageal adenocarcinoma and squamous cell carcinoma in the Barrett's Esophagus and Esophageal Adenocarcinoma Consortium. Cancer Epidemiol. 2012;36(3):306–316. doi: 10.1016/j.canep.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Appleby P, Beral V, Berrington de Gonzalez A, et al. Carcinoma of the cervix and tobacco smoking: collaborative reanalysis of individual data on 13,541 women with carcinoma of the cervix and 23,017 women without carcinoma of the cervix from 23 epidemiological studies. Int J Cancer. 2006;118(6):1481–1495. doi: 10.1002/ijc.21493. [DOI] [PubMed] [Google Scholar]
- 55.Tsao SW, Tsang CM, Pang PS, et al. The biology of EBV infection in human epithelial cells. Semin Cancer Biol. 2012;22(2):137–143. doi: 10.1016/j.semcancer.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 56.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers—a different disease. Nat Rev Cancer. 2007;7(10):778–790. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 57.Raab-Traub N. Epstein-Barr virus in the pathogenesis of NPC. Semin Cancer Biol. 2002;12(6):431–441. doi: 10.1016/s1044579x0200086x. [DOI] [PubMed] [Google Scholar]