Abstract
Objectives
The health hazards related to smoking are well known. Smoking is a recognized risk factor for coronary artery disease (CAD). Despite rejection of smoking by the Saudi community, we are still seeing smokers in our population. This study is designed to determine the prevalence of smoking in the Kingdom of Saudi Arabia (KSA), and to find out its relation to CAD. This study is part of the Coronary Artery Disease In Saudis (CADIS) study.
Methods
This health survey was conducted by collecting data regarding smoking status among adult Saudis aged between 30 and 70 years of both sexes in KSA over a five year period from 1995 up to 2000. The study sample was of normal distribution and representative of all regions of KSA. The data were analyzed to provide the prevalence of smoking and its relation with CAD.
Results
The total number of subjects was 17,350, and current smokers were 2217; accordingly the overall prevalence of smoking among Saudis was 12.8%. Males (1555) were significantly smoking more than females (662) with a prevalence of 18.7% and 7.3%, respectively (P < 0.0001). Smoking is more prevalent among Saudis living in urban, northern, western, and eastern regions compared to other regions of KSA. Smokers are more likely to develop CAD compared to non-smokers (P < 0.0001).
Conclusions
Smoking is a prevalent health problem among Saudis that requires intervention for eradication. We found clear association between cigarettes smoking and CAD particularly among males. Persistent education of the health hazards related to smoking is recommended particularly at early age in-order to prevent initiation of smoking.
Keywords: Smoking, Prevalence, Saudi Arabia, Coronary artery disease
1. Introduction
Tobacco is manufactured and produced in several different forms to make it suitable for smoking such as; cigarettes, cigars, pipe, or water pipe (sheesha). While the awareness of health hazards related to smoking is certainly well known to the public, one still sees smoking affecting large number of different age groups in both sexes. Several studies have shown the risk of smoking in the development of CAD, moreover, the disease risks associated with cigarette smoking are proportional to the intensity and duration of smoking (Tresch and Aronow, 1996; Bittner, 2002; Brown and Mensah, 2007; Burns, 2003; Jacobs et al., 1999; Jousilahti et al., 1999; Scheidt, 1997; Abrams et al., 1995; Rigotti and Pasternak, 1996; Woodward et al., 1999).
Cigarette smoking has been shown to be the leading preventable cause of death in the United States of America (USA), with increasing prevalence among young adults as nearly 80% of tobacco users initiate its use before the of age 18 years and cigarette smoking was the most prevalent form of tobacco use (Marshall et al., 2006).
Previous reports on smoking in KSA did not clearly address the problem of smoking epidemiologically as a risk factor for the development of CAD. Therefore, we designed this study to provide an estimated prevalence of smoking among different age groups of both sexes in urban and rural communities in KSA, with main emphasis on its statistical correlation with other risk factors of CAD. Moreover, we intended to identify the social as well as the educational backgrounds of smokers to target high risk individuals who are vulnerable for acquiring the habit of smoking in-order to develop preventive strategy. This study is part of the Coronary Artery Disease In Saudis (CADIS) study.
2. Methods
A 5-year national epidemiological health survey regarding CAD and its well known risk factors was conducted between 1995 and 2000 in KSA. Male and female Saudi adults aged (30–70-years), in rural and urban areas of KSA formed the target population for this study. For the purpose of the study, a Saudi is identified as a person holding a Saudi Nationality Identification Card (SNIC) or a dependent of a holder. A sample size of 20,000 participants was the target of the study to ensure a high reliability of our estimates of the prevalence of smoking in KSA.
The subjects were selected using two stages, stratified cluster sampling procedure, urban and rural areas being the stratifying factors. For practical and logistic reasons, the study population was drawn from the local primary health care centers’ catchments areas. The catchments population of each primary care center was taken as a cluster. KSA is subdivided into 14 administrative regions and samples were selected from each region. The first stage sampling units were 1623 primary health care centers (PHCC) uniformly distributed in KSA. Since the establishment of the PHCC was dictated by the population in each region, the allocation of the required number of PHCC were made proportional to be the number of PHCC in each region. Then, each region was stratified into urban and rural communities and a simple random sample of PHCC was selected. A total of 66 PHCC were selected from urban and 58 from rural areas. Then block (blocks) was randomly selected from the catchments areas of each selected primary health care center and used as a cluster. One hundred households from urban PHCC and 50 households from rural PHCC were selected from these blocks. All subjects (males and females) of age group 30–70 years of selected households were interviewed and examined.
The study protocol consisted of interviews, clinical examinations, laboratory tests, electrocardiograms (ECGs), and laboratory measurements. The questionnaire used included basic demographic and socio-economic data; a meticulous history of CAD and its risk factors. A person was defined as having anginal chest pain of ischemia secondary to CAD if the chest pain was typical in character, occurred on effort (exertional), and relieved by rest or sublingual nitroglycerine (Al-Nozha et al., 2004). Additional detailed history of smoking such as current, passive or ex-smoking was obtained, as well as the type of tobacco smoked, quantity, and the duration of smoking.
Well trained primary care physicians were responsible for filling up the questionnaire form. The questionnaire was developed, pre-tested, and validated in a pilot study. A complete physical examination was conducted at the primary health care center, included height, weight, blood pressure (BP), waist and hip circumferences. Weight was measured using ordinary scales with indoor clothing on without shoes on to the nearest 0.1 kg. Height, waist and hip measurements were carried out to the nearest mm by using a measuring tape.
Laboratory data included a fasting blood sample for glucose (FBG), total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL) and low-density lipoprotein (LDL). A 12 leads electrocardiographic tracing was carried out for every participant.
The data were analyzed using the Statistical Package for Social Sciences (Version 10.0) on PC. Both univariate and multivariate analysis were carried out. The frequency distribution tables of the variables measured in various age groups, gender, rural, and urban areas are presented. The estimate of smoking prevalence rate was calculated for the total sample, and sub-groups of gender, area of residence and age groups. A risk assessment model was developed using logistic regression.
3. Results
The total number of subjects included for final analysis was 17350 Saudi adults. Eight thousands three hundreds and seven subjects (47.9%) were males, while 11,812 were living in urban areas. Current male and female smokers were 2217; accordingly the overall prevalence of smoking among Saudis obtained from this study was 12.8%. Among current smokers, males (1555) were smoking significantly more than females (662) with a prevalence of 18.7% and 7.3%, respectively (P < 0.0001). Approximately three quarters (75.3%) of the respondents never smoked either actively or passively (Table 1). Urban males and females reported higher rates of smoking compared to their rural counterparts (Table 1). The highest rate of current smoking (23.7%) was reported by males in the age group between 40 and 49 years (Table 1).
Table 1.
Prevalence of smoking categorized by age, gender, residence in KSA.
| Factor | Never smoked | Current smoker | Ex-smoker | Passive smoker | P-value | Total |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 5226 (62.9) | 1555 (18.7) | 1375 (16.6) | 151 (1.8) | <0.0001 | 8307 (47.9) |
| Female | 7832 (86.6) | 662 (7.3) | 181 (2.0) | 368 (4.1) | 9043 (52.1) | |
| Residence | ||||||
| Urban | ||||||
| Male | 3498 (61.2) | 1155 (20.2) | 940 (16.5) | 119 (2.1) | <0.0001 | 5712 (48.4) |
| Female | 5197 (85.2) | 476 (7.8) | 126 (2.1) | 301 (4.9) | 6100 (51.6) | |
| Rural | ||||||
| Male | 1728 (66.6) | 400 (15.4) | 435 (16.8) | 32 (1.2) | <0.0001 | 2595 (46.9) |
| Female | 2635 (89.5) | 186 (6.3) | 55 (1.9) | 67 (2.3) | 2943 (53.1) | |
| Age groups | ||||||
| 30–39 | ||||||
| Male | 1409 (64.4) | 396 (18.1) | 309 (14.1) | 75 (3.4) | <0.0001 | 2189 (36.4) |
| Female | 3374 (88.0) | 171 (4.5) | 77 (2.0) | 211 (5.5) | 3833 (63.6) | |
| 40–49 | ||||||
| Male | 1251 (57.3) | 518 (23.7) | 374 (17.1) | 41 (1.9) | <0.0001 | 2184 (44.7) |
| Female | 2321 (85.9) | 255 (9.4) | 36 (1.3) | 89 (3.3) | 2701 (55.3) | |
| 50–59 | ||||||
| Male | 1253 (63.4) | 335 (17.0) | 372 (18.8) | 15 (0.8) | <0.0001 | 1975 (56.5) |
| Female | 1293 (85.2) | 144 (9.5) | 37 (2.4) | 44(2.9) | 1518(43.5) | |
| 60–70 | ||||||
| Male | 1313 (67.0) | 306 (15.6) | 320 (16.3) | 20 (1.0) | <0.0001 | 1959 (66.4) |
| Female | 844 (85.2) | 92 (9.3) | 31 (1.3) | 24 (2.4) | 991 (33.6) | |
| Total | 13,058 (75.3) | 2217 (12.8) | 1556 (9.0) | 519 (3.0) | 17,350 | |
About 20% of male subjects of monthly income less than SR 10,000 were current smokers. Both male and female subjects of monthly income group of more than SR 15,000 were found to have the lowest prevalence of smoking (Table 2a). Semi-skilled, unskilled workers and businessmen had the highest prevalence of smoking compared to other professions (Table 2b). Divorced subjects were more likely to be current smokers compared to other groups of marital status (Table 2c).
Table 2a.
Prevalence of smoking categorized by income.
| Factor | Never smoked | Current smoker | Ex-smoker | Passive smoker | P-value | Total |
|---|---|---|---|---|---|---|
| Income | ||||||
| Less than SR 2500 | ||||||
| Male | 1250 (62.5) | 375 (18.8) | 338 (16.9) | 37 (1.9) | <0.0001 | 2000 (42.1) |
| Female | 2366 (86.0) | 240 (8.7) | 65 (2.4) | 80 (2.9) | 2751 (57.9) | |
| SR 2500–4999 | ||||||
| Male | 1795 (62.0) | 564 (19.5) | 486 (16.8) | 48 (1.7) | <0.0001 | 2893 (50.6) |
| Female | 2438 (86.4) | 185 (6.6) | 65 (2.3) | 133 (4.7) | 2821 (49.4) | |
| SR 5000–7499 | ||||||
| Male | 1174 (63.7) | 338 (18.3) | 301 (16.3) | 29 (1.6) | <0.0001 | 1842 (51.6) |
| Female | 1491 (86.3) | 121 (7.0) | 28 (1.6) | 88 (5.1) | 1728 (48.4) | |
| SR 7500–9999 | ||||||
| Male | 468 (60.5) | 147 (19.0) | 134 (17.3) | 24 (3.1) | <0.0001 | 773 (51.4) |
| Female | 640 (87.4) | 56 (7.7) | 8 (1.1) | 28 (3.8) | 732 (48.6) | |
| SR 10,000–14,999 | ||||||
| Male | 398 (67.1) | 100 (16.9) | 88 (14.8) | 7 (1.2) | <0.0001 | 593 (49.4) |
| Female | 517 (85.2) | 47 (7.7) | 11 (1.8) | 32 (5.3) | 607 (50.6) | |
| SR 15,000 and above | ||||||
| Male | 125 (66.8) | 30 (16.0) | 26 (13.9) | 6 (3.2) | <0.0001 | 187 (46.9) |
| Female | 190 (89.6) | 11 (5.2) | 4 (1.9) | 7 (3.3) | 212 (53.1) | |
Table 2b.
Prevalence of smoking categorized by occupation.
| Factor | Smoked | Current smoker | Ex-smoker | Passive smoker | P-value | Total |
|---|---|---|---|---|---|---|
| Occupation | ||||||
| Skilled | ||||||
| Male | 151 (62.7) | 39 (16.2) | 44 (18.3) | 7 (2.9) | <0.0001 | 241 (73.7) |
| Female | 72 (83.7) | 4 (4.7) | 3 (3.5) | 7 (8.1) | 86 (26.3) | |
| Semi-skilled | ||||||
| Male | 1853 (60.7) | 616 (20.2) | 17 (16.9) | 67 (2.2) | <0.0001 | 3053 (95.4) |
| Female | 105 (71.4) | 22 (15.0) | 8 (5.4) | 12 (8.2) | 147 (4.6) | |
| Unskilled | ||||||
| Male | 840 (60.6) | 270 (19.5) | 251 (18.1) | 24 (1.7) | <0.0001 | 1385 (87.2) |
| Female | 182 (89.2) | 11 (5.4) | 8 (3.9) | 3 (1.5) | 204 (12.8) | |
| Unemployed | ||||||
| Male | 670 (67.3) | 156 (15.7) | 158 (15.9) | 12 (1.2) | <0.0001 | 996 (81.8) |
| Female | 192 (86.5) | 14 (6.3) | 12 (5.4) | 4 (1.8) | 222 (18.2) | |
| Business | ||||||
| Male | 684 (59.3) | 236 (20.5) | 216 (18.7) | 17 (1.5) | <0.0001 | 1153 (97.6) |
| Female | 18 (64.3) | 8 (28.6) | 2 (7.1) | 28 (2.4) | ||
Table 2c.
Prevalence of smoking categorized by marital status.
| Factor | Smoked | Smoker | Ex-smoker | Smoker | P-value | Total |
|---|---|---|---|---|---|---|
| Marital status | ||||||
| Single | ||||||
| Male | 114 (68.3) | 32 (19.2) | 18 (10.8) | 3 (1.8) | <0.0001 | 167 (44.3) |
| Female | 189 (90.0) | 7 (3.3) | 2 (1.0) | 12 (5.7) | 210 (55.7) | |
| Married | ||||||
| Male | 5021 (62.8) | 1490 (18.6) | 1344 (16.8) | 145 (1.8) | <0.0001 | 8000 (51.0) |
| Female | 6695 (86.9) | 535 (6.9) | 147 (1.9) | 323 (4.2) | 7700 (49.0) | |
| Divorced | ||||||
| Male | 19 (52.8) | 11 (30.6) | 4 (11.1) | 2 (5.6) | <0.0001 | 36 (17.6) |
| Female | 134 (79.3) | 17 (10.1) | 9 (5.3) | 9 (5.3) | 169 (82.4) | |
| Widowed | ||||||
| Male | 26 (72.2) | 8 (22.2) | 2 (5.6) | 0 (0.0) | <0.0001 | 36 (4.4) |
| Female | 667 (85.1) | 89 (11.4) | 15 (1.9) | 13 (1.7) | 784 (95.6) | |
Saudi males living in northern region had the highest prevalence of smoking followed by eastern and western regions (Table 2d). In contrary, only 6.6% of Southern region’s males were current smokers (Table 2d).
Table 2d.
Prevalence of smoking categorized by region.
| Factor | Never smoked | Smoker | Ex-smoker | Smoker | P-value | Total |
|---|---|---|---|---|---|---|
| Region | ||||||
| Central | ||||||
| Male | 1339 (65.5) | 346 (16.9) | 327 (16.0) | 32 (1.6) | <0.0001 | 2044 (51.0) |
| Female | 1840 (93.7) | 72 (3.7) | 20 (1.0) | 31 (1.6) | 1963 (49.0) | |
| Northern | ||||||
| Male | 352 (49.8) | 196 (27.7) | 145 (20.5) | 14 (2.0) | <0.0001 | 707 (45.6) |
| Female | 654 (77.4) | 118 (14.0) | 31 (3.7) | 42 (5.0) | 845 (54.4) | |
| Southern | ||||||
| Male | 1262 (73.9) | 112 (6.6) | 312 (18.3) | 22 (1.3) | <0.0001 | 1708 (47.5) |
| Female | 1801 (95.3) | 30 (1.6) | 20 (1.1) | 39 (2.1) | 1890 (52.5) | |
| Western | ||||||
| Male | 1585 (59.2) | 608 (22.7) | 441 (16.5) | 45 (1.7) | <0.0001 | 2679 (48.2) |
| Female | 2416 (84.1) | 286 (10.0) | 74 (2.6) | 98 (3.4) | 2874 (51.8) | |
| Eastern | ||||||
| Male | 688 (58.9) | 293 (25.1) | 150 (12.8) | 38 (3.3) | <0.0001 | 1169 (44.3) |
| Female | 1121 (76.2) | 156 (10.6) | 36 (2.4) | 158 (10.7) | 1471 (55.7) | |
Cigarettes were the most common type of tobacco smoked by our subjects compared to other types of tobacco. The percentages of different types of tobacco smoking for cigarette, sheesha, cigar and pipe were 84.6%, 19.9%, 1.9% and 3.6%, respectively. Virtually 10% of current smokers used more than one type of smoking. Our data showed that 87.3% of males smoked cigarettes compared to 77.3% of females. Current smokers reported an average amount of 23.3 cigarettes per day (nearly one pack) for an average of 30 years duration. About 7% of males were smoking sheesha nearly 11 times per day for approximately 25 years.
Table 3 shows the correlation between smoking and CAD. Among Saudi males who had CAD; 63.9% were cigarettes smokers compared to 59.5% of non-smokers (P < 0.0001). On the contrary, among Saudi females who had CAD; 37.9% were cigarettes smokers compared to 80.7% of non-smokers (Table 3). Overall; 55.7% of cigarettes smokers had CAD compared to 68.5% of non-smokers, while 48.3% of cigarettes smokers had no evidence of CAD compared to 75.7% of non-smokers (Table 3). There was no statistically significant correlation between CAD and sheesha, cigar, or pipe smoking among our subjects in this study (Table 3).
Table 3.
Correlation of CAD with type of smoking.
| Factor | Cigarettes | P-value | Sheesha | P-value | Cigar | P-value | Pipe | P-value | Non-smoker | P-value |
|---|---|---|---|---|---|---|---|---|---|---|
| CAD | ||||||||||
| Male | 193 (63.9) | <0.0001 | 40 (19.1) | 0.491 | 3 (1.5) | 0.074 | 6 (3.1) | <0.0001 | 322 (59.5) | <0.0001 |
| Female | 53 (37.9) | 18 (22.8) | 3 (3.8) | 6 (7.5) | 322 (80.7) | |||||
| No CAD | ||||||||||
| Male | 2284 (60.5) | <0.0001 | 527 (21.8) | 0.055 | 52 (2.2) | 0.244 | 30 (1.3) | 0.101 | 4817 (63.2) | <0.0001 |
| Female | 763 (30.2) | 193 (18.9) | 13 (1.3) | 98 (9.6) | 7394 (86.9) | |||||
| Total | ||||||||||
| CAD | 246 (55.7) | 0.003 | 58 (20.1) | 0.756 | 6 (2.2) | 0.803 | 12 (4.3) | 0.680 | 644 (68.5) | <0.0001 |
| No CAD | 3047 (48.3) | 720 (20.9) | 65 (2.0) | 128 (3.8) | 12211 (75.7) | |||||
The correlation between smoking and CAD is further simplified in Figs. 1 and 2 that clearly show the effect of smoking in the development of CAD. Among patients with CAD of our population, 68.5% were non-smokers compared to 16.3% who were smokers, 12.1% were ex-smokers, and 3.1% were passive smokers (Fig. 1). Alternatively, among all subjects in our study, only 5% of non-smokers had CAD compared to 7% of smokers, 7.5% of ex-smokers, and 5.7% of passive smokers (Fig. 2).
Figure 1.
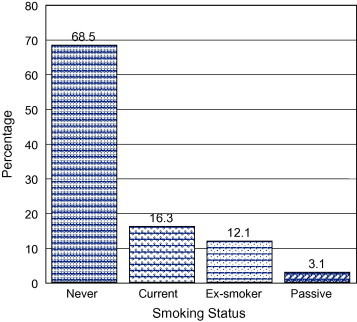
Percentage of smokers & non-smokers among patients with CAD.
Figure 2.
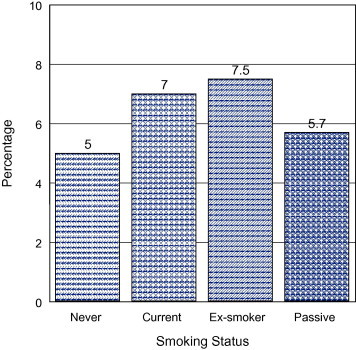
Prevalence of CAD among smokers & non-smokers.
4. Discussion
Our study clearly demonstrates that smoking is an active problem among adult population in KSA. Despite lower rates of current and active smoking among females compared to males, our data show that Saudi females are significantly more likely to be exposed to passive smoking from actively smoking male relatives. Passive smoking has been reported to carry similar health hazards as active smoking (He et al., 1999; He and Whelton, 1999; Coggins, 1998; Wells, 1994).
The predominance of cigarette smoking compared to other types of smoking is likely to be explained by ease of access (availability and price) to cigarettes.
Clearly, the factors that contribute to the increase of smoking prevalence can be identified from our study by being first; more commonly affecting males compared to females probably due to more social acceptance for males to smoke compared to females. Second; more prevalent in northern, eastern, and western regions compared to other regions of KSA is likely to be due to influence of cultural exposures to expatriates who are smokers and come in direct contact with Saudis living in these areas. Third; the presence of social stress either by occupation or by marital problems, as our study showed higher prevalence among divorcee & widowed, may be a major contributing factor to smoking initiation or continuation. Fourth; Saudi males and females are more likely to initiate smoking at their forth decade, probably due to lack of health hazards education at the time of initiation of smoking; while Saudis above the age of 50 are less likely to smoke because it is considered an antisocial behavior back then. Finally; smoking is not influenced by income or occupations in KSA.
Smoking is a worldwide prevalent problem that has been recognized and addressed by many countries and health authorities with variable control and success. For instance, in USA, findings from the National Youth Tobacco Survey indicate that the prevalence of smoking is up to 34.5% among high school students, and cigarette smoking is the most prevalent form of tobacco use (National Youth Tobacco Survey Investigators, 2000). Another study in USA surveyed a sample of 2816 adolescents reported the prevalence of cigarette smoking among Vietnamese males (27.9%) which was similar to that for Caucasian males (28.3%) but was higher than that for Hispanic males (19.7%) or African-American males (18.9%) (Wiecha, 1996).
Recent survey from Pakistan (random sample of 576 male college students) reported rather very high prevalence of smoking reaching 65% among adolescents more than or equal to 21 years of age (Rozi et al., 2007). One more recently published study on school students from Cyprus reported a prevalence of current cigarettes smoking of 36% among males and 23% among females in high schools (Christophi et al., 2008). Moreover, the overall reported prevalence of smoking in Nairobi (Kenya) among a sample of 5311 students attending high schools was 38.6% of males and 17.9% of females (Kwamanga et al., 2003).
Previously published data from KSA were different from our current study in either methodology or selected samples that were not reflecting community based survey. One study conducted in the northern region of KSA (sample of 1505 secondary school students’ in Tabuk) reported smoking prevalence of 34% for males, and 11.1% for females (Abdalla et al., 2007). Moreover, another study from KSA reported an overall 25.3% prevalence of smoking in a sample of 1534 adults aged 15 years and above residing in Riyadh (Saeed et al., 1996). The prevalence of smoking in Bahrain was reported as follows; cigarettes (21.0%), water-pipes (13.0%) and cigars (1.6%) among male secondary school students in Bahrain (Al-Haddad and Hamadeh, 2003). Similar rates of estimated smoking prevalence were reported from Yemen; 15.5% among females and 21.9% among males in a sample of 1000 secondary-school students (Bawazeer et al., 1999).
The data obtained from our study may reflect a more realistic prevalence of smoking habit in KSA due to the normal distribution of the sample selected as well as covering all the regions of KSA, despite being less than most would expect, or previously reported rates either from KSA or other countries.
Saudi Adults must be aware of the extent of smoking-related risks and the benefits of smoking cessation as an important intervention. Furthermore, despite the fact that most smokers expressed a strong desire to stop smoking, only few succeeded to achieve this goal. Therefore, it is important to emphasize that preventing initiation of smoking is more important task than achieving cessation.
The correlation between cigarettes smoking and the development of CAD is evident from the results of our study among current and passive smokers; nonetheless, it is less obvious particularly among females, probably due to their low smoking prevalence. Moreover, we were expecting to see reversal of the relationship upon cessation of smoking expressed as reduction of CAD among ex-smokers. However, our data actually showed a higher percentage of CAD among ex-smokers compared to current smokers.
Possibly, this finding may be explained by failure to maintain smoking cessation among ex-smokers leading to more percentage of CAD in this group along with longer duration of cigarettes smoked, enforcing the fact that not to start smoking is unquestionably much better than trying to stop smoking.
In summary, smoking is current and active problem in Saudi Arabia, more prevalent in males than females. Despite lower prevalence of smoking in KSA compared to other countries, the higher rates of other risk factors particularly diabetes mellitus and hypertension, makes any additional risk factor for CAD, such as smoking, of major adversity. Moreover, we found indisputable relationship between cigarettes smoking and CAD among Saudi males participated in this study, and this relationship extends to ex-smokers as well as passive smokers.
We recommend aggressive campaign addressing the health hazards of smoking beginning at younger than starter age that is estimated to be less than 12 years of age to prevent initiation of a deadly habit. Moreover, active measures should be established to provide professional help to quit smoking addiction rather than just advising against smoking.

Acknowledgements
We are grateful to all who assisted in conducting this national project including administrators at health directorates, primary care physicians, technicians, and nurses. We would like to thank King Abdulaziz City for Science and Technology (KACST) for the generous grant extended to this project.
References
- Abdalla A.M., Al-Kaabba A.F., Saeed A.A., Abdulrahman B.M., Raat H. Gender differences in smoking behavior among adolescents in Saudi Arabia. Saudi Med. J. 2007;28(7):1102–1108. [PubMed] [Google Scholar]
- Abrams J., Vela B.S., Coultas D.B., Samaan S.A., Malhotra D., Roche R.J. Coronary risk factors and their modification: lipids, smoking, hypertension, estrogen, and the elderly. Curr. Probl. Cardiol. 1995;20(8):533–610. [PubMed] [Google Scholar]
- Al-Haddad N., Hamadeh R.R. Smoking among secondary-school boys in Bahrain: prevalence and risk factors. East Mediterr. Health J. 2003;9(1–2):78–86. [PubMed] [Google Scholar]
- Al-Nozha M.M., Arafah M.R., Al-Mazrou Y.Y., Al-Maatouq M.A., Khan N.B., Khalil M.Z. Coronary artery disease in Saudi Arabia. Saudi Med. J. 2004;25(9):1165–1171. [PubMed] [Google Scholar]
- Bawazeer A.A., Hattab A.S., Morales E. First cigarette smoking experience among secondary-school students in Aden, Republic of Yemen. East Mediterr. Health J. 1999;5(3):440–449. [PubMed] [Google Scholar]
- Bittner V. Women and coronary heart disease risk factors. J. Cardiovasc. Risk. 2002;9(6):315–322. doi: 10.1097/01.hjr.0000044514.34172.4d. [DOI] [PubMed] [Google Scholar]
- Brown D.W., Mensah G.A. Smoking among adults with coronary heart disease. Prev. Med. 2007;4(1):85–86. doi: 10.1016/j.ypmed.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Burns D.M. Tobacco-related diseases. Semin. Oncol. Nurs. 2003;19(4):244–249. doi: 10.1053/j.soncn.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Christophi C.A., Kolokotroni O., Alpert H.R., Warren C.W., Jones N.R., Demokritou P. Prevalence and social environment of cigarette smoking in Cyprus youth. BMC Public Health. 2008;8(2):190. doi: 10.1186/1471-2458-8-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggins C.R. A prospective study of passive smoking and coronary heart disease. Circulation. 1998;97(18):1870–1871. (May 12) author reply 1872-3. [PubMed] [Google Scholar]
- He J., Whelton P.K. Passive cigarette smoking increases risk of coronary heart disease. Eur. Heart J. 1999;20(24):1764–1765. doi: 10.1053/euhj.1999.1825. [DOI] [PubMed] [Google Scholar]
- He J., Vupputuri S., Allen K., Prerost M.R., Hughes J., Whelton P.K. Passive smoking and the risk of coronary heart disease – a meta-analysis of epidemiologic studies. N. Engl. J. Med. 1999;340(12):920–926. doi: 10.1056/NEJM199903253401204. [DOI] [PubMed] [Google Scholar]
- Jacobs E.J., Thun M.J., Apicella L.F. Cigar smoking and death from coronary heart disease in a prospective study of US men. Arch. Intern. Med. 1999;159(20):2413–2418. doi: 10.1001/archinte.159.20.2413. [DOI] [PubMed] [Google Scholar]
- Jousilahti P., Vartiainen E., Korhonen H.J., Puska P., Tuomilehto J. Is the effect of smoking on the risk for coronary heart disease even stronger than was previously thought? J. Cardiovasc. Risk. 1999;6(5):293–298. doi: 10.1177/204748739900600503. [DOI] [PubMed] [Google Scholar]
- Kwamanga D.H., Odhiambo J.A., Amukoye E.I. Prevalence and risk factors of smoking among secondary school students in Nairobi. East Afr. Med. J. 2003;80(4):207–212. doi: 10.4314/eamj.v80i4.8644. [DOI] [PubMed] [Google Scholar]
- Marshall L., Schooley M., Ryan H., Cox P., Easton A., Healton C. Disease control and prevention. Youth tobacco surveillance – United States, 2001–2002. MMWR Surveill. Summ. 2006;55(3):1–56. [PubMed] [Google Scholar]
- National Youth Tobacco Survey Investigators, 2001. Youth tobacco surveillance – United States, 2000. MMWR CDC Surveill. Summ. 50 (4), 1–84. [PubMed]
- Rigotti N.A., Pasternak R.C. Cigarette smoking and coronary heart disease: risks and management. Cardiol. Clin. 1996;14(1):51–68. doi: 10.1016/s0733-8651(05)70260-5. [DOI] [PubMed] [Google Scholar]
- Rozi S., Butt Z.A., Akhtar S. Correlates of cigarette smoking among male college students in Karachi, Pakistan. BMC Public Health. 2007;7(1):312. doi: 10.1186/1471-2458-7-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed A.A., Khoja T.A., Khan S.B. Smoking behaviour and attitudes among adult Saudi nationals in Riyadh City, Saudi Arabia. Tob. Control. 1996;5(3):215–219. doi: 10.1136/tc.5.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidt S. Changing mortality from coronary heart disease among smokers and nonsmokers over a 20-year interval. Prev. Med. 1997;26(4):441–446. doi: 10.1006/pmed.1997.0185. [DOI] [PubMed] [Google Scholar]
- Tresch D.D., Aronow W.S. Smoking and coronary artery disease. Clin. Geriatr. Med. 1996;12(1):23–32. [PubMed] [Google Scholar]
- Wells A.J. Passive smoking as a cause of heart disease. J. Am. Coll. Cardiol. 1994;24(2):546–554. doi: 10.1016/0735-1097(94)90315-8. [DOI] [PubMed] [Google Scholar]
- Wiecha J.M. Differences in patterns of tobacco use in Vietnamese, African-American, Hispanic, and Caucasian adolescents in Worcester, Massachusetts. Am. J. Prev. Med. 1996;12(1):29–37. [PubMed] [Google Scholar]
- Woodward M., Moohan M., Tunstall-Pedoe H. Self-reported smoking, cigarette yields and inhalation biochemistry related to the incidence of coronary heart disease: results from the Scottish Heart Health Study. J. Epidemiol. Biostat. 1999;4(4):285–295. [PubMed] [Google Scholar]


