Abstract
Background
The introduction of technique of radiofrequency (RF) catheter ablation in 1990, has revolutionized management of different types of paroxysmal supraventricular tachycardia (PSVT). In spite of higher success rate, there were reported recurrences among different types of SVT. The aim of this study was to report the efficacy of RF ablation, its complications, recurrence rate and its predictors.
Methods
The material of this study (our 3rd registry) included patients who underwent electrophysiological study (EPS) and radiofrequency ablation of their supraventricular tachycardia in the past 5 years, starting from January 2002 to January 2007 at The Critical Care Medicine Department, Cairo University.
Results
Out of 400 pts studied, 381 (95%) had been subjected to radiofrequency ablation (RF) ablation while the remaining 19 pts (4.7%) refused ablation for fear of possible complications. Out of the 381 pts, 366 (96%) had their target tachycardia successfully terminated, from them 26 pts (7%) experienced recurrence after having successful RF ablation. Nine pts (34.6%) of total recurrence was reported in pts with AVNRT, 7 pts (26.9%) of total recurrence was reported in pts with AVRT utilizing septal accessory pathway (Rt AS and /or Rt PS AP), 4 pts (15.4%) was reported in pts with double AP, 2 pts (7.7%) of total recurrence was reported in pts with AFl, one pt (3.8%) of total recurrence was reported in cases of AT. Redo ablation have been carried out successfully in 25 pts (96.2%), and one pt (3.8%) refused ablation for fear of possible complications.
Conclusions
Although electrophysiological study and RF ablation eliminated different types of SVT. However, there may be increased incidence of recurrence among pts with AVNRT and AVRT utilizing concealed septal AP and multiple APs secondary to the complexity of AVN physiology, the critical location of septal AP, the clinical expertise, and poor electrophysiological criteria for good procedural success.
Keywords: Supraventricular tachycardia, Radiofrequency ablation, Recurrence
1. Introduction
Recurrences of supraventricular tachycardia (SVT) after ablation have been reported to vary widely (Blomstrom-Lundqvist et al., 2003; Clague et al., 2001; Calkins et al., 1999; Jackman et al., 1991; Estner et al., 2005; Josephson, 2002; Charme et al., 2003; Calkins et al., 2002; Braunwald and Zipes, 2007) and determinants of SVT recurrence after radiofrequency are largely unknown. Several studies have analyzed whether various clinical, electrophysiological, or procedural parameters could be used to predict the SVT recurrence but the results have been contradictory (Clague et al., 2001; Estner et al., 2005; Schwacke et al., 2002; Weismuller et al., 2002).
The performance of catheter ablation procedures requires skills that are developed over time. Several studies have shown that success rates improve and fluoroscopy times decrease with experience (Calkins et al., 1993; Katritsis et al., 1994). Although there are many determinants of arrhythmia recurrences, recurrence rates drop with operator experience (Rosenheck et al., 1997).
Cryoablation is emerging as an attractive treatment option for several arrhythmia substrates in children; acute success rates are comparable to radiofrequency ablation. But concerns subsist over potentially higher recurrence rates (Kriebel et al., 2005; Bar-Cohen et al., 2006; Papez et al., 2006). However, more investigation is needed in order to decrease the recurrence rate with cryoablation, since single cryoablation lesion does not seem to lead to comparable long-term outcome to radiofrequency ablation (RFA). The choice of larger tip cryoablation catheters may also need to be assessed while looking for possible solution for the long-term effectiveness (Volkan Tuzcu, 2007).
Patients with structural heart disease (SHD) and SVT who undergo RF ablation have diverse anatomic and arrhythmia substrates. Compared to patients with anatomically normal hearts, they have lower acute success rate and higher recurrence rate. Two major subgroups predominate: Ebstein’s anomaly and scar-related intra-atrial reentrant tachycardias (IART) after atrial surgery. In each of these groups, the lower acute success rates and higher recurrence rates as compared to patients without CHD are likely due to the complex arrhythmia substrate and multiple accessory pathways (Reich et al., 1998; Triedman et al., 2002).
It was found that persistence of residual slow pathway conduction and single AV nodal echo beats after ablation or other electrophysiological or procedural parameters did not predict the risk for developing AVNRT recurrences in a large series of patients during a long follow-up interval (Estner et al., 2005). Younger patients being at increased risk for developing AVNRT recurrences, (Estner et al., 2005) exact factors that predispose for AVNRT recurrence in younger patients as compared for older patients are not known, histological and functional studies of AV node, on the other hand, have shown age-related changes in the morphology and function of AV node increasing the predisposition for AV node reentry in young adults compared with children (Blaufox et al., 2000; Umetani et al., 1998). Another possible explanation of increased susceptibility for AVNRT recurrence in younger patients may be related to age-related changes in the underlying autonomic tone (Bonnemeier et al., 2003; Miller and Olgin, 2002).
The probability of AVRT recurrence after a successful ablation procedure was strongly influenced by the ablation target, with recurrence being more likely after ablation of right free wall, posteroseptal, septal APs, and with multiple APs. These differences in the likelihood of arrhythmia recurrence can be explained, in large part, by the target-dependent differences in the effectiveness of tissue heating (Calkins et al., 1999; Chen et al., 1999).
The most important feature which avoids the recurrence of flutter is the creation of a stable and complete block of isthmus conduction (Villacastin et al., 2000; Raviele, 2005). In fact when the validation of block was not made in the first ‘90s the recurrence rate of flutter ablation was very high (30%) Shih et al., 1998.
The patients with multiple atrial tachycardias also were more likely to have recurrence. Because patient’s age and presence of structural heart diseases were the significant predictors of multiple atrial tachycardias, they also were the significant predictors of recurrence (Hussein et al., 2000).
2. Patients and methods
This retrospective-cohort study included 543 studies involving 400 patients (214 males, 186 females; their mean age was 35.7 ± 14 years) who underwent electrophysiological study(ies) and/or radiofrequency ablation of SVT in 5-years duration (from January 2002 to January 2007) at the Critical Care Medicine Department, Cairo University.
All data are obtained from patients files including age, sex, proper history analysis and structural heart disease, pre and post catheter ECG, ECG with tachycardia if available, echocardiography, electrophysiological diagnosis, results of RF ablation, complications, and recurrence regarding predictors, redo ablation and results.
3. Electrophysiological study and radiofrequency ablation
All the studied patients underwent EPS for definitive diagnosis of their SVT. All patients were studied in post absorptive sedated state. All anti-arrhythmic drugs were discontinued for at least five half-lives. Three quadripolar catheters were inserted in high right atrium (HRA), atrioventricular junction (His bundle) and right ventricular apex (RV) together with a multipolar one in the coronary sinus (CS) for recording the left sided activation. For atrial flutter, a duodecopolar “Halo” catheter (Cordis-webster), 20 poles, 2 mm paired spacing was inserted in place of HRA catheter for detailed mapping of anterolateral RA wall and lateral part of the inferior vena cava–tricuspid isthmus (IVC–TA). The recording was made on a computerized multi-channel recorder (BARD medical system).
Electrical stimulation was delivered through a programmable stimulator (“Nihon Kohden” Japan).
The electrophysiological study consisted of: Analysis of intracardiac electrograms.
-
•
Antegrade curve i.e. (atrial pacing with atrial extrastimulus) to determine atrial, AV nodal refractory periods and evidence of dual AV nodal pathways.
-
•
Retrograde curve i.e. (ventricular pacing with ventricular extrastilmulus) to determine His bundle retrograde refractory period and to confirm or exclude retrograde conduction via an accessory pathway.
-
•
Induction of SVT by atrial and/or ventricular extrastimulation.
-
•
Incremental pacing (atrial and ventricular) to detect antegrade and retrograde Wenckbach respectively.
-
•
If all failed to induce tachycardia, isoprenaline (at graded doses, 1–4 mcg/min) or atropine (0.04 mg/kg) was given intravenously.
Conventional RF ablation was performed with a generator (“Stockert, ep shuttle” Biosense, Webster) connected to mapping ablation catheter 4 mm tip (Cordis-Webster). Other mapping/ablation catheters were used namely; Stinger, Bard, Blazer and EPT.
Three dimensional mapping system (CARTO XP – Biosense) connected to mapping/ablation 8 mm tip catheter (Navistar) was used for redo ablation of few recurrent difficult cases.
4. Statistical methods
Descriptive analysis for quantitative and qualitative data respectively in the form of (mean ± SD and percentage).
5. Results
5.1. Patients, characteristics
Twenty six patient (7.1%) with successful RF ablation of their SVT of total 366 pts experienced recurrence of their tachycardia.
Structural heart diseases were reported in 2 pts with AVNRT and systemic diseases were reported in 3 pts (2 with AVNRT and 1 with atrial flutter1 (AFl)) – Table 1.
Table 1.
Structural heart diseases and systemic diseases in recurrent cases.
| Type of SVT | No. | SHD | Systemic disease |
|---|---|---|---|
| AVRT | 4 | VSD in 1 LVH in 1 |
T2DM + HTN in 1 Hypothyroidism in 1 |
| AFl | 1 | – | HTN + chronic liver disease |
T2DM: type 2 diabetes mellitus; HTH: hypertension.
VSD: ventricular septal defect; LVH: LV hypertrophy.
Nine pts (34.6%) of 26 who had recurrence were reported in pts with AVNRT, 5 (19.2%) was reported in pts with anteroseptal AP, and 2 (7.7%) was reported in pts with right posteroseptal AP, left lateral AP, and AFl Table 2.
Table 2.
Recurrence rate in successfully ablated cases (26 pts).
| Type of SVT | Total number of successfully ablated cases | Recurrence rate among selective SVT | Percentage from total recurrence (26 pts) |
|---|---|---|---|
| AFl | 31 | 2 (6.5%) | 7.7 |
| AT | 13 | 1 (7.7%) | 3.8 |
| AVNRT | 144 | 9 (6.3%) | 34.6 |
| LL AP | 61 | 2 (3.3%) | 7.7 |
| RPS AP | 26 | 2 (7.7%) | 7.7 |
| AS AP | 26 | 5 (19.2%) | 19.2 |
| AVRT + AVNRT | 5 | 1 (20%) | 3.8 |
| LL AP + LPL AP | 1 | 1 (100%) | 3.8 |
| AS AP + MS AP | 1 | 1 (100%) | 3.8 |
| LL AP + AS AP | 1 | 1 (100%) | 3.8 |
| RPS AP + LPS AP | 1 | 1 (100%) | 3.8 |
5.1.1. Time of recurrence
Out of 26 pts, 15 (57.7%) had recurrence before 6 months, 8 pts (30.8%) had recurrence between 6 months and 2 years, and 3 pts (11.5%) had it more than 2 years respectively Table 3.
Table 3.
Time of recurrence in successfully ablated cases.
| Type of SVT | Total no. of recurrent cases | <6 months | 6 months–2 years | >2 years |
|---|---|---|---|---|
| AVNRT | 9 | 4 (44.4%) | 4 (44.4%) | 1 (11.1%) |
| AVRT | 13 | 9 (69.2%) | 2 (15.4%) | 2 (15.4%) |
| AT | 1 | 1 (100%) | 0 (0.0%) | 0 (0.0%) |
| AFl | 2 | 1 (50%) | 1 (50%) | 0 (0.0%) |
| AVRT + AVNRT | 1 | 0 (0.0%) | 1 (100%) | 0 (0.0%) |
5.1.2. Age distribution in recurrent cases
The age has been classified into three age groups:
| • Group I: | <20 years |
| • Group II: | between 20 and 40 years |
| • Group III: | >40 years |
-
•
Out 26 pts, 10 (38.4%) were in age group II (20–40), 8 (30.8%) were in age group I (0–20) as were as age group III (>40 years), Table 4.
-
•
All cases in age group I (0–20) (30.8%) were diagnosed as having AVRT.
-
•
All atrial flutter cases (2 pts) were in age group III (>40 years).
Table 4.
Age distribution in recurrent cases.
| Type of SVT | Total no. of recurrent cases | Group I (0–20) | Group II (20–40) | Group III above 40 years |
|---|---|---|---|---|
| AVNRT | 9 | 0 (0.0%) | 3 (33.3%) | 6 (66.7%) |
| AVRT | 13 | 8 (61.5%) | 5 (38.5%) | 0 (0.0%) |
| AT | 1 | 0 (0.0%) | 1 (100%) | 0 (0.0%) |
| AFl | 2 | 0 (0.0%) | 0 (0.0%) | 2 (100%) |
| AVRT + AVNRT | 1 | 0 (0.0%) | 1 (100%) | 0 (0.0%) |
5.1.3. Gender distribution in recurrent cases
Out of 26 pts, 16 (61.6%) were males, 10 (38.4%) were females Table 5.
-
•
Out of 26 pts with recurrent SVT, 6 (23.1%) had different EP diagnosis in the second study.
-
•
Out of 26 pts, 24 (92.4%) had been successfully ablated; 21 pts (80.8%) were ablated in the 2nd study, 3 (11.5%) were ablated in the 3rd study respectively. The other 2 pts (7.7%), one refused ablation for fear of complications, the other one had failed ablation in the 2nd study and continued the anti-arrhythmic drugs (He was diagnosed as having AVRT with AS AP).
-
•
Two pts (7.7%) out of 26 who had experienced recurrence, were not completely ablated in the 1st study for fear of epicardial location of their accessory pathways but was completely ablated in the 2nd study.
-
•
Two pts who had AVRT (1 with left PL location and the other one with left PS location) had their accessory pathways reablated successfully using irrigated tip (8 mm) catheter (Navistar-thermo cool).
-
•
One pt with AFl who experienced recurrence after successful conventional linear ablation of IVC–tricuspid isthmus had second successful attempt using 3-D mapping system (CARTO XP) connected to 8 mm tip thermo dilution catheter – Fig. 1.
Table 5.
Sex distribution in recurrent cases of SVT.
| Type of SVT | Total no. of recurrent cases | Male | Female |
|---|---|---|---|
| AVNRT | 9 | 5 (55.6%) | 4 (44.4%) |
| AVRT | 13 | 9 (69.2%) | 4 (30.8%) |
| AT | 1 | 0 (0.0%) | 1 (100%) |
| AFl | 2 | 2 (100%) | 0 (0.0%) |
| AVRT + AVNRT | 1 | 0 (100%) | 1 (100%) |
Fig. 1.


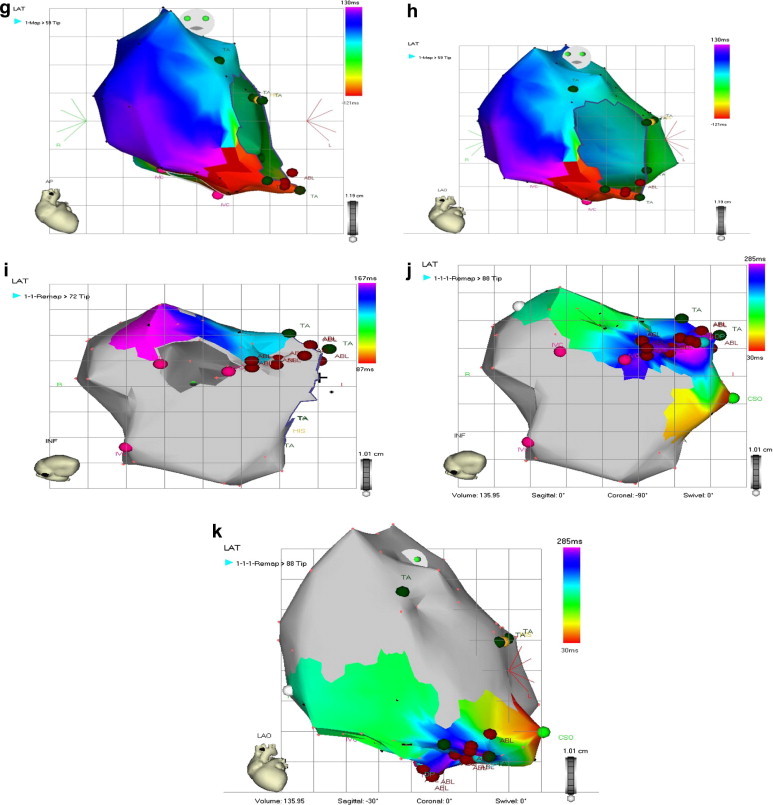
(a) 12-Lead ECG with counterclockwise flutter. (b) Intracardiac recording confirming early activation of the proximal Halo 9,10 during AFl with counterclockwise rotation. (c) Successful RF ablation of cavo–tricuspid ismuth with termination of AFl. (d) Validation of isthmus block: the distal Halo1,2 is activated later during PCS pacing. (e) Failed post ablation induction by IAP. (f) Post-ablation ECG in SR. (g) Standard AP view. Activation mapping during AFl in the same pt with early recurrence by 3-D electro-anatomic mapping system (CARTO). (h) LAO view activation mapping during AFl with counterclockwise rotation (early activation in red and late activation in violet). (i) Linear RF ablation of the cavo–tricuspid isthmus (tagged with red) with flutter termination. (j) Block validation during PCS pacing in inferior view. (k) Block validation was confirmed also in LAO view.
6. Discussion
The results of this retrospective-cohort study (our 2nd published registry) from the Critical Care Center at Cairo University Hospitals 34 and the prior studies indicate that RF catheter ablation can be done with a high success rate and low incidence of complications. In this study, the reported acute success rate was high (96.1%), the recurrence rate was low (7.1%), and the incidence of complications was relatively low (4.2%).
The study also identified some types of SVT that are predictive of ablation success as AVNRT (99.3%), left lateral AP (100%), right posteroseptal (100%) and anteroseptal AP (96.3%). It also identified some types of SVT that are predictive of ablation failure as, atrial tachycardia (18.75%). It also identified the clinical variables that predict development of arrhythmia recurrences and complications as structural heart disease, multiple ablation targets, septal AP, and AVNRT.
The recurrence rate in our series was 7.1%, the highest rate of recurrence in multiple APs (50%) followed by anteroseptal AP (19.2%), atrial tachycardia and right posteroseptal AP (7.7%), then AFl 6.5%. The lowest rate of recurrence was reported in AVNRT (6.3%).
Calkins et al. (1999) found that total recurrence in SVT was 6% (47 pts); where 31 pts (7.8%) who had RF ablation for an AP, and 16 pts (4.6%) who had RF ablation of AVNRT. The study did not show the recurrence rate in different individual types of APs.
The recurrence rates in atrial flutter and atrial tachycardia were previously shown in Tables 6 and 7 respectively. The higher recurrence rates (in the previous study in comparison to our study) in atrial flutter and atrial tachycardia cases may reflect the difference in experience, in technique used during mapping and ablation, and the small sample size (limited number of patients). Introducing the 3-D mapping/ablation system (CARTO) in our center has revolutionized management of do novo cases of AT and atypical atrial flutter with higher success rate and lower incidence of recurrence. At present, Conventional mapping/ablation technique has never used for cases of AT.
Table 6.
Comparison of results of atrial flutter ablation in different series.
| Author | Year | No. of pts | Success rate (%) | Recurrence |
|
|---|---|---|---|---|---|
| No. | % | ||||
| Chen et al. | 1994 | 18 | 94 | 5/18 | 94 |
| Steinberg et al. | 1995 | 16 | 100 | 4/16 | 100 |
| Poty et al. | 1995 | 12 | 100 | 1/12 | 100 |
| Poty et al. | 1996 | 44 | 98 | 4/43 | 98 |
| Willems et al. | 1999 | 24 | 100 | 2/24 | 100 |
| Hussein et al., Magdi et al. (2004) | 2000 | 44 | 86.4 | 2/38 | 86.4 |
| Magdy et al., Hussein et al. (2004) | 2002 | 10 | 80 | 3/8 | 80 |
| Present study | 2007 | 34 | 91.2 | 2/31 | 91.2 |
Table 7.
Comparison of results of atrial tachycardia ablation in different series.
| Author | Year | No. of pts | Success rate (%) | Recurrence |
|---|---|---|---|---|
| Poty et al. | 1996 | 36 | 86 | – |
| Shaban et al. | 1997 | 11 | 81 | – |
| Kalman et al. | 1998 | 27 | 96 | – |
| Hussein et al. | 2000 | 9 | 66 | 2/6 (33%) |
| Magdy et al. | 2002 | 30 | 70 | 2/21 (13%) |
| Present study | 2007 | 16 | 81.3 | 1/13 (7.7%) |
Recurrence occurred in 57.7% before 6 months and in 89.5% before 2 years. It was up to 90% before 6 months as reported in Calkins series, (Calkins et al., 1999) and in Van Hare series (Van Hare et al., 2004).
Thirty eight percent of recurrent cases were reported in middle age group, of which, 30.8% were in young and in old age group. In AVNRT there were no recurrent cases in young age group in contradictory to Estner series where recurrence rate was higher in young age group. The difference may be due to small sample size.
7. The limitations of the study
-
1.
It was a retrospective study lacking follow-up and detection of echocardiographic changes following the catheter ablations as valvular damage, pericardial effusion and recently developed RWMAs which might occur without detection.
-
2.
Some intra-cath procedures were not properly assessed as the procedure time, number of RF pulses, and the exposure time.
8. Conclusion
There is increased incidence of recurrence among pts with AVNRT and AVRT utilizing concealed septal AP and multiple APs secondary to the complexity of AV nodal physiology, the critical location of septal AP and poorly defined electrophysiological criteria for procedural success.
Identification of groups of patients with higher rates of recurrence after catheter ablation will stimulate further investigations of the potential predictors of recurrence and how to decrease it.
References
- Bar-Cohen Y., Cecchin F., Alexander M.E. Cryoablation for accessory pathways located near normal conduction tissues or within the coronary venous system in children and young adults. Heart Rhythm. 2006;3:253–258. doi: 10.1016/j.hrthm.2005.11.029. [DOI] [PubMed] [Google Scholar]
- Blaufox A.D., Rhodes J.F., Fishberger S.B. Age related changes in dual AV nodal physiology. PACE. 2000;23(4 Pt 1):477–480. doi: 10.1111/j.1540-8159.2000.tb00830.x. [DOI] [PubMed] [Google Scholar]
- Blomstrom-Lundqvist C., Scheinman M.M., Aliot E.M. The ACC/AHA/ESC Guidelines for the management of patients with supraventricular arrhythmias – executive summary. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology, Committee for practice guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Supraventricular Arrhythmias) J. Am. Coll. Cardiol. 2003;42:1493–1531. doi: 10.1016/j.jacc.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Bonnemeier H., Richardt G., Potratz J. Circadian profile of cardiac autonomic nervous modulation in healthy subjects: different effects of aging and gender on heart rate variability. J. Cardiovasc. Electrophysiol. 2003;14:791–799. doi: 10.1046/j.1540-8167.2003.03078.x. [DOI] [PubMed] [Google Scholar]
- Braunwald E., Zipes D.P., editors. Therapy for Cardiac Arrhythmias: Heart Disease: A Textbook for Cardiovascular Medicine. eighth ed. WB Saunders; Philadelphia, PA: 2007. pp. 803–818. (Chapter 33) [Google Scholar]
- Calkins H., El-Atassi R., Kalbfleisch S.J. Effect of operator experience on outcome of radiofrequency catheter ablation of accessory pathways. Am. J. Cardiol. 1993;71:1104–1105. doi: 10.1016/0002-9149(93)90581-v. [DOI] [PubMed] [Google Scholar]
- Calkins H., Yong P., Miller J.M., Atakr Multicenter Investigators Group Catheter ablation of accessory pathways, atrioventricular nodal reentrant tachycardia, and the atrioventricular junction: final results of a prospective, multicenter clinical trial. Circulation. 1999;99:262–270. doi: 10.1161/01.cir.99.2.262. [DOI] [PubMed] [Google Scholar]
- Calkins Hugh, Ajit Kumar V.K., Francis Johnson. Radiofrequency catheter ablation of supraventricular tachycardia. Ind Pacing Electrophysiol. J. 2002;2(2):45–49. ISSN:0972-6292. [PMC free article] [PubMed] [Google Scholar]
- Charme G., Seguel M., Gonzalez R. Clinical and electrophysiological characteristics of patients with atrioventricular nodal reentry tachycardia who underwent slow pathway ablation. Rev. Med. Chil. 2003;131:1237–1242. [PubMed] [Google Scholar]
- Chen J., De Chillou C., Basiouny T. Cavotricuspid isthmus mapping to assess bidirectional block during common atrial flutter radiofrequency ablation. Circulation. 1999;100:2507–2513. doi: 10.1161/01.cir.100.25.2507. [DOI] [PubMed] [Google Scholar]
- Clague J.R., Dagres N., Kottkamp H. Targeting the slow pathway for atrioventricular nodal reentrant tachycardia: initial results and long-term follow-up in 379 consecutive patients. Eur. Heart J. 2001;22:82–88. doi: 10.1053/euhj.2000.2124. [DOI] [PubMed] [Google Scholar]
- Estner H.L., Ndrepepa G., Dong J. Acute and long term results of slow pathway ablation in patients with atrioventricular nodal reentrant tachycardia: an analysis of the predictive factors for arrhythmia recurrence. Pacing Clin. Electrophysiol. 2005;28:102–110. doi: 10.1111/j.1540-8159.2005.09364.x. [DOI] [PubMed] [Google Scholar]
- Hussein, K., Hammouda, M., Nagi, H.K., et al., 2000. Atrial tachycardias electrophysiological characteristics, and the effect of radiofrequency catheter ablation 2000, MD thesis.
- Hussein Khaled, Mohamed Hammouda, Wahid Radwan. Supraventricular tachycardia with multiple pathways (prevalence and outcome of radiofrequency ablation). A 10-year-registry. Egypt. Heart J. 2004;56(3):417–422. [Google Scholar]
- Jackman W.M., Wang X.Z., Friday K.J. Catheter ablation of accessory atrioventricular pathways (Wolff-Parkinson-White syndrome) by radiofrequency current. N. Engl. J. Med. 1991;324:1605–1611. doi: 10.1056/NEJM199106063242301. [DOI] [PubMed] [Google Scholar]
- Josephson M.E. Clinical Cardiac Electrophysiology: Techniques and Interpretations. third ed. Lippincott Williams and Wilkins; Philadelphia, PA: 2002. Catheter and surgical ablation in the therapy of arrhythmias; p. 710. [Google Scholar]
- Katritsis D., Bashir Y., Heald S. Radiofrequency ablation of accessory pathways: implications of accumulated experience and time dedicated to procedures. Eur. Heart J. 1994;15:339–344. doi: 10.1093/oxfordjournals.eurheartj.a060500. [DOI] [PubMed] [Google Scholar]
- Kriebel T., Broistedt C., Kroll M. Efficacy and safety of cryoenergy in the ablation of atrioventricular reentrant tachycardia substrates in children and adolescents. J. Cardiovasc. Electrophysiol. 2005;16(9):960–966. doi: 10.1111/j.1540-8167.2005.50054.x. [DOI] [PubMed] [Google Scholar]
- Magdi, M., Hussein, K., Hammouda, M., et al., 2004. Electrophysiologic diagnosis and radiofrequency ablation of supraventricular tachycardia. An eight-year-follow-up 2004, M.Sc.
- Miller J.M., Olgin J.E. Catheter ablation of free-wall accessory pathways and Mahaim fibres. In: Zipes D.P., Haissaguerre M., editors. Catheter Ablation of Arrhythmias. Futura Publishing Co, Inc.; New York: 2002. pp. 277–303. [Google Scholar]
- Papez A.L., Al-Ahdab M., Dick M., 2nd Transcatheter cryotherapy for the treatment of supraventricular tachyarrhythmias in children: a single center experience. J. Interv. Cardiac Electrophysiol. 2006;15:191–196. doi: 10.1007/s10840-006-9012-x. [DOI] [PubMed] [Google Scholar]
- Raviele, Antonio, 2006. Cardiac Arrhythmias 2005. In: Proceedings of the Nineth International Workshop on Cardiac Arrhythmias. Springer-Verlag, Italia, p. 22.
- Reich J.D., Auld D., Hulse E., Pediatric Electrophysiology Society The pediatric radiofrequency ablation Registry’s experience with Ebstein’s anomaly. J. Cardiovasc. Electrophysiol. 1998;9:1370–1377. doi: 10.1111/j.1540-8167.1998.tb00113.x. [DOI] [PubMed] [Google Scholar]
- Rosenheck S., Rose M., Sharon Z. The ongoing influence of staff training on the performance of radiofrequency catheter ablation. Pacing Clin. Electrophysiol. 1997;20:1312–1317. doi: 10.1111/j.1540-8159.1997.tb06785.x. [DOI] [PubMed] [Google Scholar]
- Schwacke H., Brandt A., Rameken M. Long-term outcome of AV node modulation in 387 consecutive patients with AV nodal reentrant tachycardia. Z. Kardiol. 2002;91:389–395. doi: 10.1007/s00392-002-0792-4. [DOI] [PubMed] [Google Scholar]
- Shih A.C., Ching T.T., Chern E.C. Focal atrial tachycardia: reanalysis of the clinical and electrophysiologic characteristics and prediction of successful radiofrequency ablation. J. Cardiovasc. Electrophysiol. 1998;9(4):1998. doi: 10.1111/j.1540-8167.1998.tb00924.x. [DOI] [PubMed] [Google Scholar]
- Triedman J.K., Alexander M.E., Love B.A. Influence of patient factors and ablative technologies on outcomes of radiofrequency ablation of intra-atrial re-entrant tachycardia in patients with congenital heart disease. J. Am. Coll. Cardiol. 2002;39:1827–1835. doi: 10.1016/s0735-1097(02)01858-2. [DOI] [PubMed] [Google Scholar]
- Umetani K., Singer D.H., McCraty R. Twenty-four hour time domain heart rate variability and heart rate: Relations to age and gender over nine decades. J. Am. Coll. Cardiol. 1998;31:593–601. doi: 10.1016/s0735-1097(97)00554-8. [DOI] [PubMed] [Google Scholar]
- Van Hare G.F., Javitz H., Carmelli D. Prospective assessment after pediatric cardiac ablation: demographic, medical profiles, and inititial outcome. J. Cardiovasc. Electrophysiol. 2004;15:759–770. doi: 10.1046/j.1540-8167.2004.03645.x. [DOI] [PubMed] [Google Scholar]
- Villacastin J., Almendral J., Arenal A. Usefulness of unipolar electrograms to detect isthmus block after radiofrequency ablation of typical atrial flutter. Circulation. 2000;102:3080–3085. doi: 10.1161/01.cir.102.25.3080. [DOI] [PubMed] [Google Scholar]
- Volkan Tuzcu M.D. Cryoablation of accessory pathways in children. PACE. 2007;30:1129–1135. doi: 10.1111/j.1540-8159.2007.00824.x. [DOI] [PubMed] [Google Scholar]
- Weismuller P., Kuly S., Brandts B. Is electrical stimulation during administration of catecholamines required for the evaluation of success after ablation of atrioventricular node re-entrant tachycardias? J. Am. Coll. Cardiol. 2002;39:689–694. doi: 10.1016/s0735-1097(01)01798-3. [DOI] [PubMed] [Google Scholar]


