Abstract
Mitral valve disease is the second most common valvular heart disease after the aortic valve worldwide. Mitral valve has historically been a structure of interest by pioneers in echocardiography. One of the earliest applications of echocardiography was in the diagnosis of valvular heart disease, particularly mitral stenosis. In this review we wish to take the reader through the structural and hemodynamic evaluation of the normal mitral valve.
Keywords: Echocardiography, Normal mitral valve, Mitral valve disease
1. Introduction
The name of mitral valve (MV) is derived from the word mitre (miter, mitra), a Greek name for a type of ceremonial headdress of bishops in the Catholic and Orthodox churches, which is a tall folding cap consisting of two similar parts (the front and back) rising to a peak and sewn together at the side. This name was probably first used by Andreas Vesalius for left ventricular inflow valve.
Mitral valve disease is the second most common valvular heart disease after the aortic valve in the Western countries. Mitral valve has historically been a structure of interest by pioneers in echocardiography. Due to its location, which is perpendicular to echo beam from the parasternal view, it was the first cardiac structure identified by initial investigation of Edler and Hertz in 1953. Echocardiography with different advanced modalities is still the backbone for structural and hemodynamic evaluation of mitral valve and decision making for medical and surgical interventions. The purposes of this article in this issue and the upcoming issues are to deal with echocardiographic assessment of the following:
-
(1)
Normal mitral valve
-
(2)
Mitral stenosis
-
(3)
Mitral regurgitation
-
(4)
Role of 3-D echocardiography
2. Normal mitral valve
2.1. Normal functional anatomy
The mitral valve (MV) is located between the left atrium (LA) and the left ventricle (LV). The MV opens during ventricular diastole when blood flows from LA into LV. During ventricular systole, the MV closes as blood is ejected through the AV.
Normal mitral valve has three components:
-
(a)
Mitral annulus is saddle ellipsoid shape with antero-posterior dimension of three-quarter of medial–lateral dimension. Furthermore the dimensions of mitral annulus change during cardiac cycle. The posterior mitral annulus is in close proximity with circumflex coronary artery at posterolateral side and with coronary sinus at posteromedial side. Mitral annulus is in continuity with aortic and tricuspid valves by aortomitral fibrosa and right and left fibrous trigon. The maximum normal diameter of mitral annulus at medial–lateral axis is about 3.0 cm.
-
(b)
Mitral leaflets consist of an anterior leaflet (AMVL) which is longer and a posterior leaflet (PMVL) of a shorter length. AMVL is attached to about 45% of circumference of mitral annulus while PMVL occupies the other 55%. Normal mitral leaflet thickness is about 3–5 mm. Different classifications and nomenclatures for anatomic assessment of mitral valve has been introduced but Carpentier’s classification is widely accepted by most echocardiologists and cardiac surgeons (Carpentier, 2001). Based on this classification, mitral leaflets are divided into eight segments (Fig. 1). PMVL has three scallops: medial scallop (P3), middle scallop, the largest of the three (P2), and lateral scallop (P1). AMVL is in turn divided arbitrarily into three segments: anteromedial (A3), anteromiddle (A2), and anterolateral (A1). There is no real scallop in AMVL. Mitral leaflets have two commissural segments called posteromedial commissure (PMC) and anterolateral commissure (ALC). Note the landmark of ALC which is left atrial appendage in surgical view.
-
(c)
Subvalvular apparatus consists of chorda tendina and papillary muscles (PM). Chorda are thin filaments, which are primary and secondary, connecting mitral leaflets to the papillary muscles. There are twenty-five primary chorda with fan-like distribution at the end attached to the margins of the leaflets. Nine chorda go to AMVL, fourteen to PMVL, and one to each commissural segments. Elongation or rupture of these chorda creates lack of leaflets coaptation and flail segments resulting in mitral regurgitation (MR). All chorda of mitral valve are attached to two papillary muscles. Distribution of chorda is in such a way that the medial half of both leaflets is attached to the posteromedial PM and lateral half of them is connected to the anterolateral one. Each PM may have one or two heads. Displacement of papillary muscles as well as partial or complete rupture of them creates MR.
Figure 1.
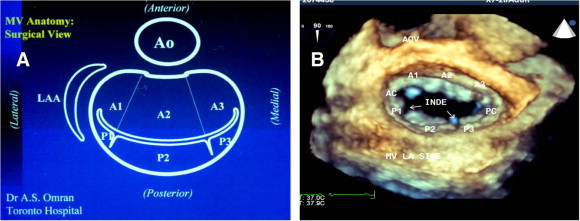
(A) Diagrammatic illustration of mitral valve in surgical view after opening left atrium. Note: left atrial appendage (LAA) is located at the left side of surgeon and aorta is located anterior. (B) Corresponding view acquired by 3D-TEE (zoom mode). Note: normal indentations between posterior scallops during diastole which are closed in systole without causing mitral regurgitation. AOV = aortic valve, A1 = anterolateral segment, A2 = anteromiddle segment, A3 = anteromedial segment, AC = anterolateral commissure, PC = posteromedial commissure, P1 = posterolateral scallop, P2 = posteromiddle scallop, P3 = posteromedial scallop.
Integrity of the entire mitral valve consisting of annulus, chorda, and papillary muscles with harmonic synchronized function is essential for normal performance of mitral valve. Any abnormality in each of these components creates pathologic consequences.
2.2. Echocardiographic assessment of normal mitral valve
2.2.1. Transthoracic echocardiography
Transthoracic echocardiography is the main tool to assess mitral valve pathology in day to day practice of cardiology. The following views are essential for assessment of mitral valve:
-
(a)
Parasternal long axis view is the first view to assess MV. In this view thickness of leaflets and their motion during the cardiac cycle can be evaluated. Dimension of the left atrium which will be affected by MV pathology is evaluated in this view as well (Fig. 2). Applying the color Doppler adds more information about mitral inflow in diastole. Although M-mode echo is used less frequently nowadays, it is still important in terms of accurate timing of mitral events during the cardiac cycle (Fig. 3).
-
(b)
Parasternal short axis view reveals mitral leaflets motion and their attachment to the chorda. Short axis view at the level of mitral leaflets shows their restrictions and splitting of commissures. Mitral valve area can be measured in this view by planimetry at the level of leaflets tip. At the midventricular level, presence of two papillary muscles, their orientation, and the number of their heads can be illustrated (Fig. 4).
-
(c)
Apical four chamber view visualizes both mitral leaflets and their coaptation, which should be at the level of the plain of the mitral annulus (Fig. 5). MV area by pressure-half time and LV diastolic parameters including mitral inflow Doppler can be assessed in this view (Fig. 6).
-
(d)
Apical long axis view (three chamber view) and apical two chamber view are necessary to evaluate different segments of mitral leaflets and the degree of possible mitral regurgitation (Fig. 7).
Figure 2.
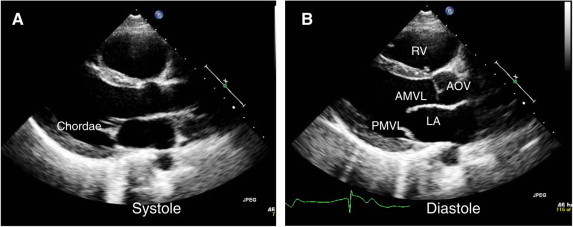
Parasternal view of normal mitral valve. (A) In systole, leaflets are closed and chordae are barely visible. (B) In diastole, leaflets are open. RV = right ventricle; AOV = aortic valve; AMVL = anterior mitral leaflet; PMVL = posterior mitral leaflet.
Figure 3.
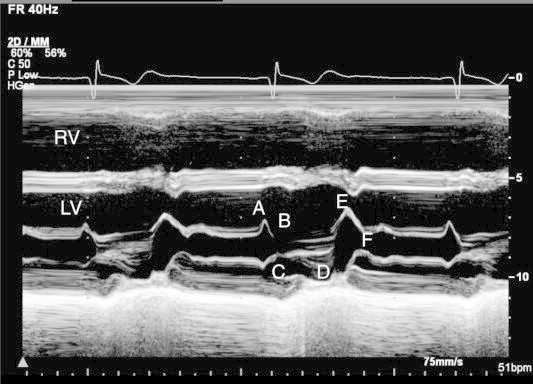
M-mode display of mitral valve. Anterior mitral valve leaflet (AMVL) during diastole moves anteriorly like letter of “M” and posterior mitral valve leaflet moves backward like letter of “W”. Mitral leaflets motion during one cardiac cycle is labeled as A–E, and F. Note: leaflet closure line (C–D line) in systole is straight and towards the interventricular septum.
Figure 4.
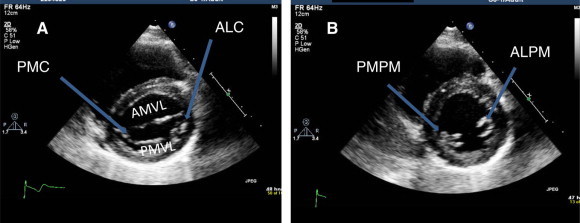
Parasternal short axis views. (A) At the level of mitral valve showing anterior (AMVL) and posterior (PMVL) leaflets. Anterolateral (ALC) and posteromedial (PMC) commissures (arrows) are widely open. (B) At the level of papillary muscle showing anterolateral (ALPM) and posteromedial (PMPM) papillary muscles.
Figure 5.
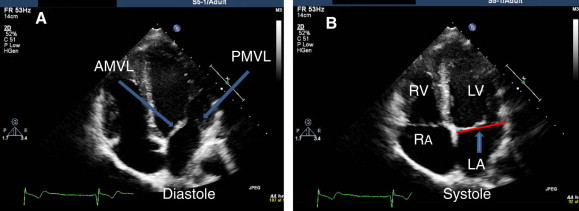
Apical four chamber view. (A) In diastole, mitral leaflets are open. Anterior mitral leaflet (AMVL) and posterior mitral leaflet are shown (arrows). (B) In systole, leaflets are closed. Note: coaptation line of leaflets are at LV side of mitral annular plane (arrow).
Figure 6.
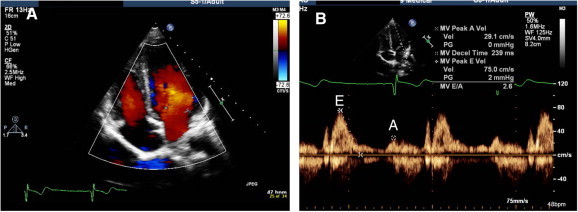
(A) Apical four chamber view in diastole, showing laminar flow from LA to LV. (B) Spectral Doppler of mitral inflow at the level of leaflets tip showing early rapid filling (E) and late filling after atrial contraction (A).
Figure 7.
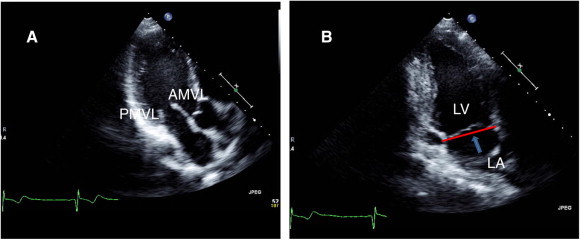
(A) Apical three chamber view showing mitral leaflets. (B) Apical two chamber view showing level of leaflets coaptation and mitral annulus (arrow).
2.2.2. Transesophageal echocardiography
Transesophageal echocardiography (TEE) has a great role in accurately predicting mitral valve anatomy and decision making for possible intervention. Multiplane TEE allows imaging in numerous scan planes, which can be optimally aligned to specific anatomy orientations to provide detailed information about pathology of mitral valve. Schematic diagram of mitral valve in Figs. 8–11 shows how scallops and segments of mitral valve can be investigated by different angle orientations (Omran et al., 2002).
Figure 8.
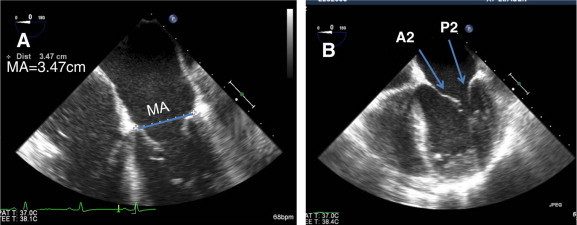
(A) Mid esophageal TEE view of mitral valve in four chamber view (0°) to measure mitral annulus (MA). (B) Mitral leaflets at 0° visualizing A2 and P2 segments.
Figure 9.
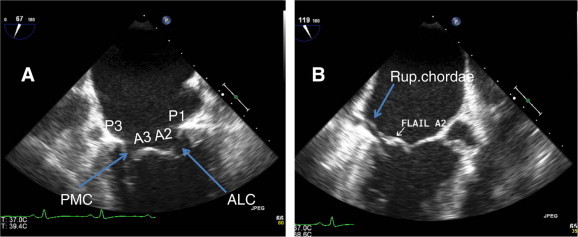
(A) Mid esophageal TEE view (bicommissural view, 75°) showing P1, P3, A2, A3 and both commissures (ALC and PMC). (B) Long axis view at 120° showing flail A2 and ruptured chorda of A2.
Figure 10.
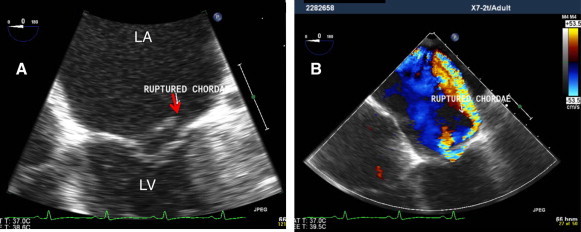
(A) TEE zoom view of mitral valve at 0°, showing flail A2 with ruptured chordae (red arrow). (B) Same view with color, showing severe very eccentric (hugging jet) of posteriorly directed MR.
Figure 11.
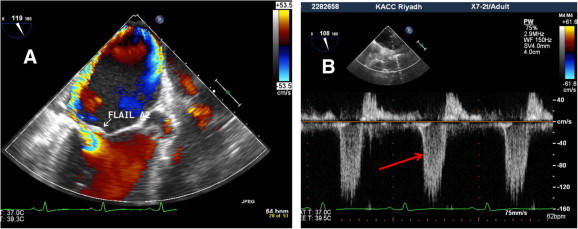
(A) Midesophageal long axis view of mitral valve showing flail A2 with severe MR. (B) Mitral inflow Doppler sampling from right upper pulmonary vein showing systolic reversal flow confirming severe MR (red arrow).
3-D TEE is an emerging modality which offers comprehensive evaluation of MV by providing surgical views and common language between cardiologists and cardiac surgeons in the operating room (Salcedo et al., 2009). The use of 3-D has facilitated the preoperative surgical planning of treating complex mitral pathology. Fig. 12 shows example of a patient with degenerative mitral valve, flail A2 with ruptured chordae and severe prolapse of A3 causing severe mitral regurgitation.
Figure 12.
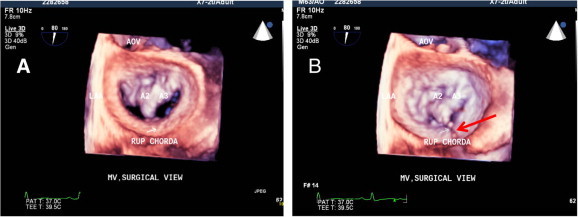
(A) 3-D zoom mode acquisition showing en-face surgical view of mitral valve with flail A2 and severe prolapse of A3. (B) Same mitral valve at the end of systole showing ruptured chorda attached to the A2 (red arrow).
References
- Carpentier A. Cardiac calve surgery – the French correction. J. Thoracic Cardiovasc. Surg. 2001;122:8–15. [PubMed] [Google Scholar]
- Omran A.S., Woo A., David T.E. Intraoperative transesophageal echocardiography accurately predicts mitral valve anatomy and suitability for repair. J. Am. Soc. Echocardiogr. 2002;15:950–957. doi: 10.1067/mje.2002.121534. [DOI] [PubMed] [Google Scholar]
- Salcedo E.E., Quaife R.A., Seres T. A framework for systematic characterization of the mitral valve by real-time three-dimensional transesophageal echocardiography. J. Am. Soc. Echocardiogr. 2009;22:1087–1099. doi: 10.1016/j.echo.2009.07.007. [DOI] [PubMed] [Google Scholar]


