Abstract
We have developed and validated a simple and sensitive stable isotope dilution liquid chromatography/tandem mass spectrometric (LC-MS/MS) method for the quantification of bumetanide in human serum. Samples were prepared with a simple acetonitrile based protein precipitation. The supernatant was then analyzed directly using LC-MS/MS. Chromatographic separation was achieved on a C18 reversed phase column using a methanol and water gradient. The detection was performed in selected reaction monitoring (SRM) mode via a positive electrospray ionization (ESI) interface. The method had a lower limit of quantification (LLOQ) of 1 ng/mL, linearity up to 1250 ng/mL, intra- and inter-day precision less than 10%, and accuracy within ±10%. This method was also demonstrated to be suitable for the analysis of bumetanide in rat serum and brain tissue. Bumetanide concentrations in rat serum and brain were determined for samples collected at several intervals following intraperitoneal (i.p.) injection of bumetanide, and were used to calculate bumetanide permeability through the blood brain barrier.
Keywords: bumetanide, liquid chromatography/tandem mass spectrometry (LC-MS/MS), stable isotope dilution
Introduction
Bumetanide (3-n-butylamino-4-phenoxy-5-sulfamylbenzoic acid) is a potent loop diuretic. It is often used in patients in whom high doses of furosemide are ineffective. It has demonstrated efficacy in the management of edema associated with congestive heart failure, hepatic cirrhosis, and renal insufficiency [1, 2, 3]. Bumetanide is also an inhibitor of the chloride (Cl−) co-transporter NKCC1 that functions in maintaining intracellular Cl− levels in the brain [4, 5]. Recent studies have shown bumetanide is effective in suppressing neonatal seizures in the rodent, and its clinical use as an anti-epileptic drug (AED) in neonates is currently being investigated [6, 7].
Various methods have been developed for the analysis of bumetanide in biological samples or in its pure form, such as high-performance liquid chromatography (HPLC) [8, 9], gas chromatography mass spectrometry [10, 11], capillary electrophoresis [12, 13], and liquid chromatography tandem mass spectrometry (LC-MS/MS) [14, 15]. These methods were developed either for the quantification of bumetanide in high concentrations in raw materials or pharmaceutical preparations, or for screening purposes for the detection of bumetanide in urine along with other diuretics. They typically require rather laborious sample preparation such as liquid-liquid or solid phase extraction, and solvent evaporation. Furthermore, these methods require a large sample size (> 50 µL) and have a relatively high lower limit of quantitation (LLOQ) (> 30 ng/mL).
The limitations of the aforementioned methods make the effective study of bumetanide difficult or impossible. For example, in a recent study of bumetanide as an AED, Brandt, et al analyzed bumetanide in rat brains using an HPLC method to evaluate the efficacy of bumetanide in treating epilepsy [7]. Due to the low brain permeability of bumetanide and the low sensitivity of the HPLC method (LLOQ of 100 ng/g brain), brain bumetanide levels could not be determined for most rats, even when a very high dose of bumetanide was administered (10 mg/kg). As both the pharmacokinetics and bioavailability of bumetanide are dose-dependent, the brain permeability study should utilize a bumetanide dose close to the therapeutic dosage. Although the therapeutic dosage of bumetanide as an AED has yet to be determined, animal studies have demonstrated doses between 0.15 and 0.30 mg/kg to be effective in suppressing seizures [5]. At this dose, the brain bumetanide concentration is expected to be in the range of 0.1 to 2 ng/g brain tissue, a level that none of the existing methods have sufficient sensitivity to measure.
In this study we developed and validated a simple, sensitive, and quantitative method for the analysis of bumetanide in human serum based on stable isotope dilution LC-MS/MS technology. The method was also demonstrated to be suitable for the analysis of bumetanide in rat serum and brain tissue
2. Materials and methods
2.1. Chemicals and materials
Human serum samples were obtained from the Department of Laboratory Medicine with the approval of the Institutional Review Board (IRB) of Children’s Hospital Boston (CHB). All animal use protocols were approved and in accordance with the guidelines of the Animal Care and Use Committee at CHB and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Long Evans rats were obtained from Charles River Laboratories International, Inc (Wilmington, MA, USA). The d5-bumetanide (the five hydrogens on the phenol group were each replaced by a deuterium atom, > 98% chemical purity with 99% isotopic purity) was custom synthesized by Isosciences LLC (King of Prussia, PA, USA). Water was purified by Milli-Q system (Millipore, Bedford, MA, USA). Bumetanide (≥99%) was purchased from MP Biomedicals (Santa Ana, CA, USA). All other reagents were purchased from Sigma–Aldrich (St. Louis, MO, USA).
2.2. LC-MS/MS equipment and settings
A Waters Quattro Premier mass spectrometer equipped with an electrospray ionization source and a Waters Acquity UPLC system (Waters, Milford, MA, USA) was used in this study. The LC column (Waters, Atlantis T3, 100×2.1 mm, 3 µm) and the autosampler were operated at ambient temperature. The LC gradient settings are shown in Table 1.
Table 1.
Gradient settings for the liquid chromatography
| Time (min) | % of solvent A | Flow rate (mL/min) |
|---|---|---|
| 0 | 50 | 0.25 |
| 4 | 0 | 0.25 |
| 4.1 | 0 | 0.4 |
| 6.5 | 0 | 0.4 |
| 7 | 50 | 0.25 |
| 9 | 50 | 0.25 |
Solvent A: 0.1% formic acid in water; solvent B: 0.1% formic acid in methanol. Changes were linear between two consecutive time points. LC eluent was diverted to waste during time intervals 0 – 3.5 and 4.9 – 9.0 min to minimize the solvent gaining entry into the mass spectrometer.
The mass spectrometer was operated in positive ion mode with selected reaction monitoring (SRM) scanning. The SRM transitions were optimized using 1800 ng/mL of bumetanide and d5-bumentanide (internal standard or IS) dissolved in methanol/water (50/50, v/v) with 0.1% formic acid. Both bumetanide and the IS were monitored by two SRM transitions: 365.3 > 240.2 and 365.3 > 184.2 for bumetanide; 370.3 > 244.2 and 370.3 > 188.2 for the IS. Mass spectrometric settings were optimized to obtain the maximum signal for each of the SRM transition. The optimal mass spectrometric settings were as follows: capillary voltage 2.5 kv, source temperature 130°C, dissolvation gas temperature at 400°C with flow rate at 1000 L/hr. The optimal cone voltage was 27 volts for all four transitions with collision energy 22 ev for transitions 365.3 > 240.2 and 370.3 > 244.2, and 17 ev for 365.3 > 184.2 and 370.3 > 188.2. Signals of the primary transitions 365.3 > 240.2 and 370.3 > 244.2 were used in the calculation. The two remaining transitions were monitored mainly for confirmatory purposes when the primary transitions were in any doubt, particularly when the signal was very weak.
2.3. Calibration standards and internal standard working solution
The stock solutions of bumetanide and IS were both prepared in DMSO at concentrations of 54.66 µg/mL and 36.94 g/mL, respectively. To prepare calibration standards, a 5000 ng/mL level was first prepared by spiking 0.128 mL of 54.66 g/mL bumetanide in DMSO into 1.272 mL of human serum. Then other levels at 1250, 321.5, 78.1, 19.5, 6.5, 2.2, and 1.0 ng/mL were prepared by serial dilution using human serum as the diluent. Aliquots (80 µL) of these standard solutions were stored at −20°C until analysis. Each aliquot was discarded after four uses. The IS working solution was 74 ng/mL d5-bumetanide in acetonitrile: water (80/20, v/v).
2.4. Sample preparation
Calibrator standards, quality controls, and serum samples were thawed at ambient temperature. Samples were vortexed and 15 µL of each was added to a microcentrifuge tube, to which 135 µL of IS working solution was then added. The tubes were vortexed vigorously for 1 min and centrifuged at 16,000 g for 10 min. Supernatant was then transferred to an autosampler vial for LC-MS/MS analysis. The injection volume was 10 µL.
2.5. Method validation
The guidelines outlined in the US Food and Drug Administration’s Guidance for Industry: Bioanalytical Method Validation were followed in our assay validation to assess the specificity, linearity, LLOQ, precision, and accuracy of the assay, as well as the sample stability [16].
2.5.1. Selectivity
Five different human serum samples from individuals who had not taken bumetanide were processed with and without bumetanide and IS to ensure the absence of interference peaks. Each blank sample was processed, analyzed, and compared with those obtained by spiking bumetanide and IS into the corresponding serum samples.
2.5.2. Linearity and LLOQ
The eight levels of standard solutions described above were analzyed using the standard assay procedure. The calibration curve was constructed by plotting the bumetanide/IS peak area ratio versus the molar ratio of bumetanide/IS. The correlation coefficient and linear regression equations were calculated using a weighted least-squared linear regression method. Calibration curves established on five different days were analyzed. The LLOQ was defined as the lowest level that had a signal/noise ratio greater than 5:1 and a relative standard deviation (RSD) less than 20%[16].
2.5.3. Precision and accuracy
The precision and accuracy of the assay were evaluated by analyzing quality control samples at concentrations of 2, 50 and 500 ng/mL that were prepared by spiking bumetanide into human serum. These three levels were analyzed in duplicates (separately prepared samples) for a total of 10 days to assess the intra-run, inter-run precisions, and accuracy.
2.5.4. Stability
Freeze and thaw stability, short and long-term stability, and post-preparation stability were each assessed. Experimental details and results are summarized in the Supplemental Data.
2.6. Method suitable for rat serum
To demonstrate that the above assay was suitable for the analysis of bumetanide in rat serum, selectivity, precision and accuracy studies were performed with the same experiment design described in sections 2.5.1 and 2.5.3 using rat serum in lieu of human serum.
2.7. Method modified for rat brain tissue
To measure the bumetanide concentration in brains of postnatal day (P)70 Long Evans rats, a quarter of a rat brain (~0.5 g ) was weighed and added to a microcentrifuge tube (Eppendorf Safe-Lock Tubes). This was followed by the addition of 270 µl of 73.9 ng/mL IS in 100% acetonitrile and additional acetonitrile, so that the total volume of liquid added was twice the mass of the brain (2x v/m). After adding one 5 mm stainless steel bead (Qiagen Inc.) to each tube, samples were homogenized for 4 min at 20 Hz in a Qiagen TissueLyser. After homogenization the beads were removed using a magnet, and the samples were centrifuged at 16,200 rcf (Eppendorf, model #: 5417R, 24394xG) at 20°C for 10 min. The supernatant was transferred to an autosampler vial for LC-MS/MS analysis.
The specificity of the assay was evaluated similarly to the assay for human serum using brain tissues from five different rats. In addition, the assay was evaluated for accuracy by analyzing five different brains with spiked bumetanide concentrations at 0.25 and 1 ng/g of brain tissue (2 samples per level).
2.8. Application to study of bumetanide brain permeability
P70 Long Evans rats were treated with a single dose of bumetanide (0.30 mg/kg), and sacrificed at 0.5, 1, 3, and 4 h post-injection (2 rats per time point). Blood was collected via cardiac puncture and clotted to separate out serum. Rats were then perfused with 0.9% NaCl solution and brains were collected, halved (along the sagittal midline) and frozen at −80°C in microcentrifuge tubes. Bumetanide levels in rat serum and brain were measured using their respective assay procedures as described above.
3. Results and discussion
3.1. Method development
3.1.1. Serum sample preparation optimization
A simple protein precipitation by acetonitrile was developed for the preparation of human serum samples. The acetonitrile-to-water ratio was optimized to achieve quantitative recovery of bumetanide and optimal liquid chromatographic separation. Incomplete recoveries were observed when the acetonitrile percentage was too low (< 60%). Presumably, when the acetonitrile percentage is too low bumetanide binds to proteins too tightly and does not come off into solution due to its strong hydrophobicity. When the acetonitrile percentage was too high (>80%), bumetanide and IS did not adequately bind to the LC column, causing partial elution at the void volume. In the optimized extraction solution, acetonitrile was ~70%.
3.1.2. HPLC column
The main objective of liquid chromatography in the assay was to separate the bumetanide and IS from any other components remaining in the samples after protein precipitation (such as salts, lipids, amino acids, etc), so that ion suppression was minimized. An Atlantis T3 column was chosen for this study, as it provided sufficient affinity for bumetanide and IS with a high percentage of organic solvent present in the sample (~70% acetonitrile).
3.2. Method validation
3.2.1. Selectivity
Under the mentioned chromatographic conditions, bumetanide and IS were eluted at ~ 4.15 min (Fig. 1), with IS being eluted slightly earlier than bumetanide. No interference peaks were observed on any of the SRM transitions.
Fig. 1. Representative LC-MS/MS chromatograms.

SRM transitions 365.3 > 240.2 (bumetanide) and 370.3 > 244.2 (IS) are represented by the solid and dotted lines, respectively. (a) and (b) were the human serum and rat brain samples prepared with the respective assay procedures where IS was omitted from the IS working solutions; (c), bumetanide spiked human serum sample at 312 ng/mL; (d) and (e) were rat serum samples with measured bumetanide concentration of 2 and 210 ng/mL, respectively; and (f) was rat brain sample with measured bumetanide concentration of 0.24 ng/mL. The inserts in (d) and (f) are the zoomed in chromatograms of bumetanide SRM transition.
3.2.2. Linearity and LLOQ
Calibration curves were linear within the concentration range of 1.0 – 1250 ng/mL (corresponding to a bumetanide/IS molar ratio of 0.0015 to 1.875) using linear least-square regression. The mean (±S.D.) regression equation for calibration curves in serum was: y = (0.4886 ± 0.0132) x, r2 = 0.9998±0.0001 (N = 5), where y is the measured bumetanide/IS peak area ratio and x is the bumetanide/IS molar ratio. The LLOQ was 1.0 ng/mL, at which level the accuracy was 95% and the inter-day RSD was 14.5% (N =5).
3.2.3. Precision and accuracy
Data for inter- and intra-day precision and accuracy of the method are presented in Table 2. At the two higher levels (50 and 500 ng/mL), the measured concentrations were within 2% of the actual values with inter- and intra-day RSD < 3.5%. At the level of 2 ng/mL, the measured concentration deviated by less than 10% from the actual concentration and the inter- and intra-day RSD were within 10%. The absolute recovery of bumetanide from human serum was evaluated by comparing extracted serum sample spiked with bumetanide versus unextracted standards prepared in 70% acetonitrile water solution (2, 50 and 500 ng/mL, N = 3 at each level). The mean absolute recovery of bumetanide was determined to be > 97%.
Table 2.
Accuracy and precision of the analysis of bumetanide in human serum quality control samples (10 days, two replicates per day)
| Spiked (ng/mL) |
Measured (ng/mL) |
Inter-day RSD (%) |
Intra-day RSD (%) |
Accuracy percent error (%) |
|---|---|---|---|---|
| 2 | 2.18 | 9.4 | 4.3 | 8.9 |
| 50 | 50.6 | 3.3 | 1.9 | 1.1 |
| 500 | 494 | 3.4 | 1.4 | −1.2 |
3.3. Method suitable for rat serum
The assay was demonstrated to be suitable for the analysis of bumetanide in rat serum. As in human serum samples, analysis of five rat serum samples prepared without IS showed no detectable peaks in any of the SRM transitions. In addition, quantitative recoveries were obtained when rat serum samples spiked with bumetanide were analyzed using the same assay procedure. Accuracy (and intra-day %CV) of the three bumetanide-spiked rat serum levels at 2, 50, and 500 ng/mL were 111.5% (8.5%), 97.2% (2.2%), and 97.4% (1.6%), respectively. The accuracy and precision values were consistent with the results obtained with human serum.
3.4. Method modified for rat brain tissue
Knowing that bumetanide could be quantitatively extracted from serum samples with ~70% acetonitrile aqueous solution, we added two volumes of acetonitrile to one volume of brain (assuming 1 mL/g brain) for the homogenization step. This condition proved to be efficient in recovering bumetanide from spiked brain samples. At spiked levels of 0.25 and 1 ng/g of brain tissue (N = 2), the measured values were 0.25 (RSD: 12%) and 1.05 ng/g (RSD: 3.4%), corresponding to 100 and 105% accuracy, respectively. No ion suppression or interference peaks were detected in any of the SRM transitions when the brain samples were prepared using this assay procedure (N = 5, data not shown). The LLOQ was determined to be 0.10 ng/g brain (calculated based on 10 times the response from blank samples). Because the recoveries in both the human serum and rat brain assays were essentially quantitative, and no ion suppression was observed in either assay, the ULOQ could be estimated based on the ULOQ of the assay for human serum. An ULOQ of 375 ng/g was estimated by taking into account both the size of samples and final volumes for both assay procedures.
3.5. Application to study of bumetanide brain permeability
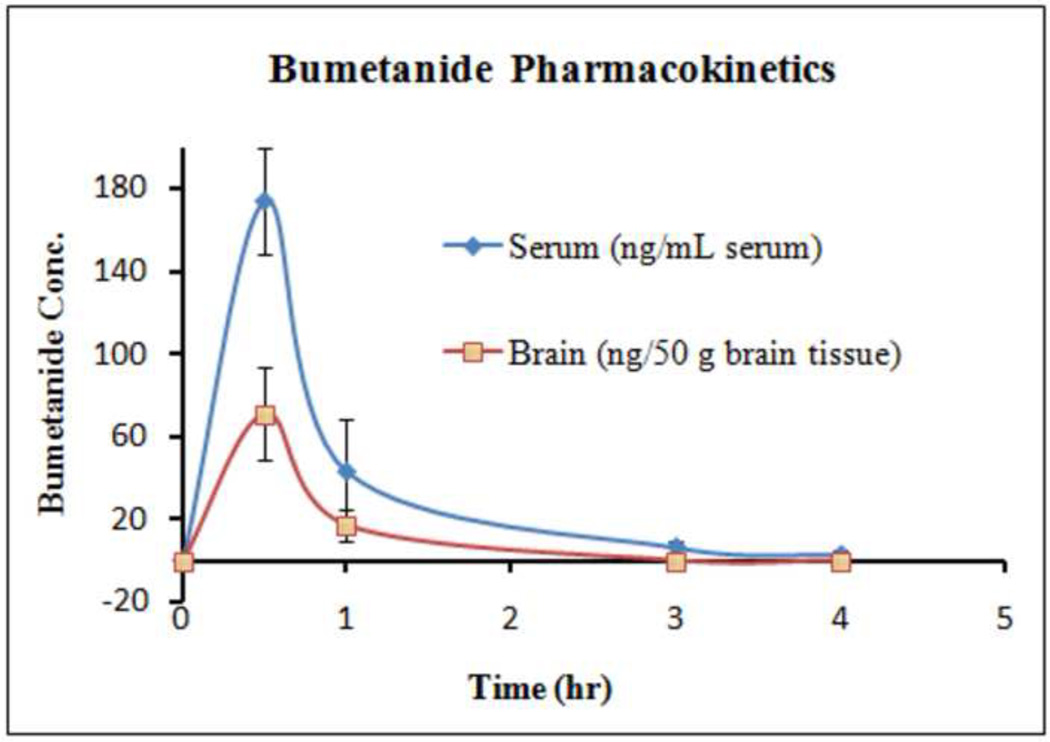
Bumetanide concentrations in rat serum and brain after bumetanide administration are presented in Fig. 2. Bumetanide was shown to reach peak concentration within 0.5 h and was then rapidly eliminated in both serum and brain. More than a 75% decrease in bumetanide was observed between 0.5 and 1 h in both serum and brain. At the dosage of 0.3 mg/kg, the brain:serum ratio was 0.0068 ± 0.0028 (mean SD, N = 4) for the samples that were collected at 0.5 and 1 h, indicating that bumetanide does cross the blood brain barrier, but has a fairly low permeability.
Fig. 2.
Bumetanide concentration in rat serum and brain after a single dose of bumetanide (0.3 mg/kg). Each point is the average of two rats, with standard deviation shown as the error bars.
4. Conclusions
This is the first study in which a stable isotope labeled internal standard was employed in an LC-MS/MS method for the quantitative analysis of bumetanide. This lent the method unparalleled quantitation features for measuring bumetanide in serum: The LLOQ was as low as 1 ng/mL, with linearity up to 1250 ng/mL, intra- and inter-day precision less than 10%, and accuracy within ±10%. Due to its excellent capability for quantitation and sensitivity, this method should prove to be of great use in clinical applications, such as therapeutic drug monitoring, and in future clinical and animal studies wherein bumetanide, particularly at low levels, needs to be accurately determined.
Supplementary Material
Acknowledgements
This project was funded by grants UL1 RR025758-01 and 5RC1NS068938-02 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Asbury MJ, Gatenby PB, O'Sullivan S, Bourke E. Br. Med. J. 1972;5794:211. doi: 10.1136/bmj.1.5794.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flamenbaum W, Friedman R. Pharmacotherapy. 1982;2:213. doi: 10.1002/j.1875-9114.1982.tb03188.x. [DOI] [PubMed] [Google Scholar]
- 3.Halstenson CE, Matzke GR. Drug Intell. Clin. Pharm. 1983;17:786. doi: 10.1177/106002808301701101. [DOI] [PubMed] [Google Scholar]
- 4.Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. Nat. Med. 2005;11:1205. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- 5.Dzhala VI, Brumback AC, Staley KJ. Ann. Neurol. 2008;63:222. doi: 10.1002/ana.21229. [DOI] [PubMed] [Google Scholar]
- 6.Soul J, Staley KJ, Jensen F. Clinicaltrials.gov. identifier# NCT00830531.
- 7.Brandt C, Nozadze M, Heuchert N, Rattka M, Löscher W. J. Neurosci. 2010;30:8602. doi: 10.1523/JNEUROSCI.0633-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith DE. J. Pharm. Sci. 1982;71:520. doi: 10.1002/jps.2600710510. [DOI] [PubMed] [Google Scholar]
- 9.Legorburu MJ, Alonso RM, Jiménez RM, Ortiz E. J. Chromatogr. Sci. 2001;39:425. doi: 10.1093/chromsci/39.10.425. [DOI] [PubMed] [Google Scholar]
- 10.Hagedorn HW, Schulz R. J. Anal. Toxicol. 1992;6:194. doi: 10.1093/jat/16.3.194. [DOI] [PubMed] [Google Scholar]
- 11.Lisi AM, Trout GJ, Kazlauskas R. J. Chromatogr. 1991;563:257. doi: 10.1016/0378-4347(91)80033-9. [DOI] [PubMed] [Google Scholar]
- 12.Sirén H, Luomanperä K, Työppönen T, Rovio S, Vastamäki P, Savolahti P. J. Biochem. Biophys. Methods. 2004;60:295. doi: 10.1016/j.jbbm.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Tolba K, Belder D. Electrophoresis. 2007;28:2934. doi: 10.1002/elps.200600520. [DOI] [PubMed] [Google Scholar]
- 14.Deventer K, Delbeke FT, Roels K, Van Eenoo P. J. Biomed. Chromatogr. 2002;16:529. doi: 10.1002/bmc.201. [DOI] [PubMed] [Google Scholar]
- 15.Sanz-Nebot V, Toro I, Bergés R, Ventura R, Segura J, Barbosa J. J. Mass Spectrom. 2001;36:652. doi: 10.1002/jms.166. [DOI] [PubMed] [Google Scholar]
- 16.Guidance for Industry: Bioanalytical Method Validation. Rockville, USA: US Department of Health and Human Services, Food and Drug Administration, CDER; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.