Abstract
The sirtuins are a highly conserved family of NAD+-dependent enzymes that regulate lifespan in lower organisms. Recently, the mammalian sirtuins have been connected to an ever widening circle of activities that encompass cellular stress resistance, genomic stability, tumorigenesis and energy metabolism. Here we review the recent progress in sirtuin biology, the role these proteins have in various age-related diseases and the tantalizing notion that the activity of this family of enzymes somehow regulates how long we live.
“Chekhov’s gun” refers to the seemingly irrelevant prop or observation that is introduced early on in a drama (such as the gun in Act I of Uncle Vanya) but whose true significance is only revealed at the play’s conclusion. Often, science has its own version of the Russian playwright’s famous maxim. In our case, the drama begins in the autumn of 1914, with the seemingly irrelevant observations of a young professor describing the beneficial effects of rats placed on a calorie-restricted diet. Some suspect that these remarkable observations were largely ignored and forgotten because of concerns about the scientist himself. Indeed the young professor, F. Peyton Rous, had only a few years earlier shocked the scientific community by postulating that in some situations, cancer could be transmitted by viruses—a notion that was roundly ridiculed and derided by his older colleagues. For whatever reason, Rous’s curious observations remained dormant for nearly two decades. Over time, however, other scientists gradually extended these ideas and demonstrated the benefits of caloric restriction in species ranging from humans to yeast. Indeed, it was in the latter species that dissection of the molecular basis of caloric restriction first implicated a family of NAD+-dependent enzymes, now collectively termed the sirtuins. In the following sections, we review the recent progress in our understanding of sirtuin biology with particular emphasis on their role in mammalian species. Although much remains unanswered and controversies remain unresolved, as with Chekhov’s gun it is only with the passage of time that the true significance of Rous’s observations can finally be understood.
A new family of deacetylases
The founding member of the sirtuin family, yeast Sir2 (silent information regulator 2), was originally isolated in a screen for silencing factors1. Ultimately, four proteins (named Sir1–Sir4) were established as important regulators of silencing at the mating type locus as well as telomeric DNA. The Sir family was rescued from relative obscurity when Guarente and colleagues, using Saccharomyces cerevisiae as a model system, independently identified these proteins as key regulators of lifespan2,3. Subsequent analysis revealed that Sir2 functioned biochemically as a histone deacetylase, in a unique reaction requiring the energetic intermediate NAD+ as a co-factor4. Remarkably, analysis in other model organisms (including Caenorhabditis elegans and Drosophila) also implicated Sir2 homologues as a determinant of lifespan5. In both yeast and flies, Sir2 levels increased following caloric-restriction treatment. Perhaps more importantly, in sirtuin-deficient yeast and mice, extension of lifespan by caloric restriction is abolished5,6. Subsequent studies have qualified some of these observations and have demonstrated that in yeast, the effect of Sir2 in caloric restriction and lifespan extension might be strain-, species- and context-dependent7,8.
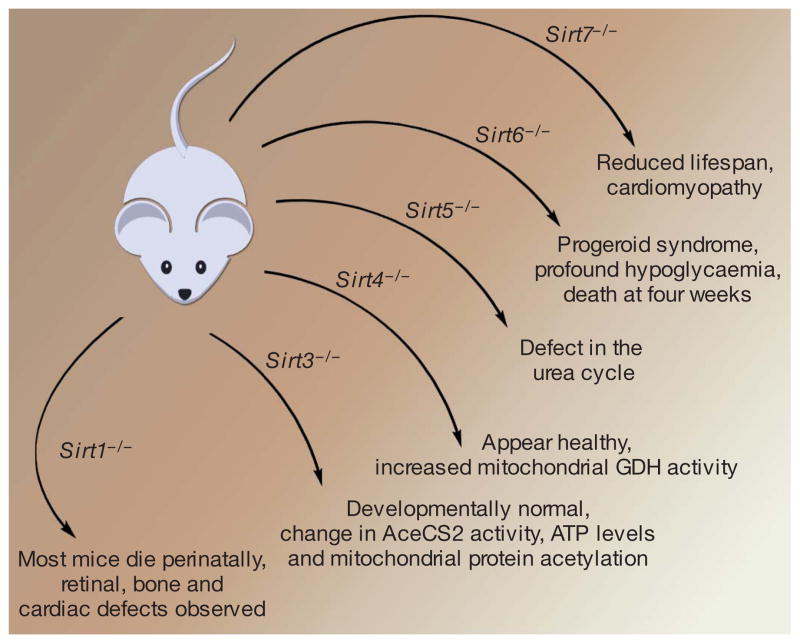
To date, seven mammalian homologues have been identified, with mammalian SIRT1 closest evolutionarily to yeast Sir2. Specific mouse models in which one or more sirtuin genes has been knocked out have been recently created (Fig. 1). Cell biological studies have further demonstrated different subcellular compartments for each family member, with SIRT6 and SIRT7 being nuclear proteins, SIRT3, SIRT4 and SIRT5 mitochondrial proteins, and SIRT1 and SIRT2 being found both in the nucleus and the cytoplasm, in a cell-and tissue-dependent context9. The observation that these proteins require NAD+ as part of their enzymatic action immediately suggested a mechanistic link between sirtuin activity and intracellular energetics. However, it is important to stress that currently there is no definitive evidence that these proteins play any direct role in mammalian lifespan regulation, as neither pharmacological sirtuin activators nor overexpression of SIRT1 has been demonstrated to extend lifespan in mice. Nonetheless, the explosion of interest in the sirtuins centres on the nagging notion that in some fashion, these proteins provide the connection between what we eat and how long we live.
Figure 1. Mouse knockout models as tools for exploring sirtuin function.
Gene targeting of SIRT1, SIRT3, SIRT4, SIRT5, SIRT6 and SIRT7 has been reported. The phenotypes include a reduction in median lifespan, ranging from a usual survival of days (SIRT1) to weeks (SIRT6) or months (SIRT7). In contrast, although biochemical phenotypes have been reported, Sirt3−/− and Sirt4−/− mice appear outwardly normal. Initial reports suggest that Sirt5−/− mice also exhibit no obvious phenotype49.
Sirtuins in DNA repair
A role for sirtuins in maintaining genomic integrity was suggested by early work in model organisms. For instance, yeast Sir2 was shown to inhibit recombination of ribosomal DNA as well as to relocalize to sites of DNA breaks5,10. Furthermore, yeast sirtuin family members are capable of deacetylating histone proteins and the absence of this activity results in silencing defects, increased genomic instability and sensitivity to DNA damage5,10. These properties appear to also extend to the mammalian sirtuins. The first example came with analysis of SIRT6-deficient cells that demonstrated increased sensitivity to genotoxic damage and accumulation of chromosomal abnormalities11. Recent studies have shown that SIRT6 also acts as a histone deacetylase that can influence the telomeres of human cells12.
Mammalian SIRT1 also appears to influence genomic stability. First, SIRT1 can deacetylate various factors linked to the repair of DNA damage, including the Werner helicase and NBS1 (ref. 13). A role for SIRT1 in genomic integrity was further substantiated by the recent demonstration of increased chromosomal aberrations and impaired DNA repair in Sirt1−/− embryos14. In addition, following oxidative damage, SIRT1 has been shown to be recruited to sites of DNA breaks. Although such recruitment appears to be important in protecting against genomic instability, this response is accompanied by the de-repression of previously silenced genes15. These observations suggest that like yeast Sir2, SIRT1 appears to regulate epigenetic silencing and chromatin modification, and recent evidence suggests this is achieved, at least in part, through direct regulation of modifying enzymes, such as the histone methyltransferase SUV39H1 (refs 16, 17).
Sirtuins and cell fate
Mammalian sirtuins also appear to have an important role in regulating cellular stress resistance and modulating the threshold for cell death. In part, this increased stress resistance comes from the interaction with the Forkhead box class O (FOXO) family of transcription factors. These mammalian transcription factors regulate both energy status and stress resistance, two properties intimately connected to lifespan extension. SIRT1 can bind and deacetylate FOXO3a, leading to a selective augmentation of FOXO-regulated stress resistance genes18,19. Subsequent experiments have extended these observations to demonstrate that various different sirtuin and FOXO family members can interact. In addition, a recent report suggested that SIRT1 can also protect cells against stress by regulating the heat shock response20. The interaction of SIRT1 and p53 can also modulate the threshold for cell death in the setting of exogenous stress21,22. Indeed, FOXO proteins and p53 proteins can directly interact under stress conditions18,23, and p53 can regulate SIRT1 expression through various means that include p53-dependent microRNAs23–25. Besides p53, SIRT1 can regulate other targets linked to cell death, including Ku70, E2F1 and TGF-β signalling26. In addition, the coordinated action of SIRT3 and SIRT4 appear to inhibit cell death by maintaining mitochondrial NAD+ levels following stress27. Although in most examples sirtuin activity appears to antagonize stress-induced cell death pathways, SIRT1 can also deacetylate components of the NF-κB complex, leading to increased apoptosis28.
Given the potential link between sirtuins and ageing, it is appealing to envisage a role for these proteins in mediating processes such as cellular senescence and stem cell function. Consistent with such a role, SIRT1 expression can antagonize the premature senescence seen in mouse embryonic fibroblasts following forced oncogene expression29. Similarly, through its regulation of NF-κB activity, knockdown of SIRT6 appears to augment senescence in primary keratinocytes30. However satisfying it may be to conclude that increased sirtuin activity can forestall senescence, it is important to note that an analysis of studies of Sirt1−/− mouse embryonic fibroblasts has come to the opposite conclusion31. With regards to differentiation, sirtuins have been shown to inhibit adipogenesis by modulating PPAR-γ (peroxisome proliferative activated receptor γ) and to effect muscle and neuronal differentiation32,33. Recent studies in mouse embryonic stem cells also suggest a role for SIRT1 in regulating stem cell reactive oxygen species homeostasis and differentiation34. The observation that SIRT1 can also interact with members of the polycomb family of repressors, a family of proteins intricately connected to stem cell self-renewal, suggests that additional links between sirtuins, differentiation and stem cell function are likely to emerge35.
Sirtuins and metabolic regulation
In mammals, blood glucose concentration is maintained within a narrow range under a variety of physiological conditions. During starvation, maintenance of serum glucose is achieved in part by implementing a program of hepatic gluconeogenesis. Increasing evidence suggests an important role for sirtuins in this physiological adaptation. The peroxisome proliferator-activated receptor gamma-coactivator-1α (PGC-1α) is a known target of SIRT1-dependent deacetylation36,37, and this coactivator also plays a fundamental part in regulating gluconeogenesis and fatty acid oxidation pathways within the liver. The ability of PGC-1α to modulate these latter two pathways appears to require SIRT1 (refs 37, 38). Recently, distinct roles for protein acetylation and SIRT1-dependent deacetylation have been shown to regulate the hepatic response to both short term (<6 h) and long term (>18 h) fasting39. In this case, the opposing actions of SIRT1 and the p300/CBP acetyltransferase choreograph hepatic glucose production in the setting of nutrient stress. Finally, the observation that SIRT6-deficient mice demonstrate severe hypoglycaemia suggests a potential role for other sirtuins in glucose production and homeostasis11.
In addition to regulating hepatic gluconeogenesis, sirtuins also modulate serum glucose levels by regulating pancreatic insulin secretion. A transgenic mouse with β cell-specific SIRT1-overexpression was noted to have increased glucose-stimulated insulin secretion and improved glucose tolerance compared to control mice40, while Sirt1−/− mice have impaired glucose-stimulated insulin secretion41. Finally, SIRT4 through its ADP-ribosylation of glutamate dehydrogenase (GDH)42 as well as its interactions with insulin degrading enzyme43 also appears to be involved in regulating pancreatic insulin secretion.
The sirtuin family has a much broader role in metabolism than the regulation of glucose homeostasis (Fig. 2). As was previously discussed, SIRT1 through its regulation of PPAR-γ and PGC-1α activity has a significant regulatory role in fat mobilization and fatty acid oxidation32,44. The regulation of PGC-1α activity also suggests a role for sirtuins in the generation of new mitochondria, as PGC-1α is a key regulator of mitochondrial biogenesis. These observations, coupled with recent links between SIRT1 and autophagy45, suggest that sirtuins might regulate the flux of mitochondria within cells by balancing PGC-1α mediated generation with autophagy-dependent clearance. In addition to the connection between SIRT1 and the mitochondria, SIRT3-dependent deacetylation regulates the activity of the mitochondrial enzyme acetyl coenzyme A synthetase 2 (AceCS2)46,47 as well as Complex I of the electron transport chain48. Mitochondrial lysates from Sirt3−/− mice reveal >30 proteins whose acetylation is markedly increased, suggesting that other important targets undoubtedly exist48,49. Finally, a very recent study has implicated SIRT5, another mitochondrial sirtuin, as an important regulator of the urea cycle50.
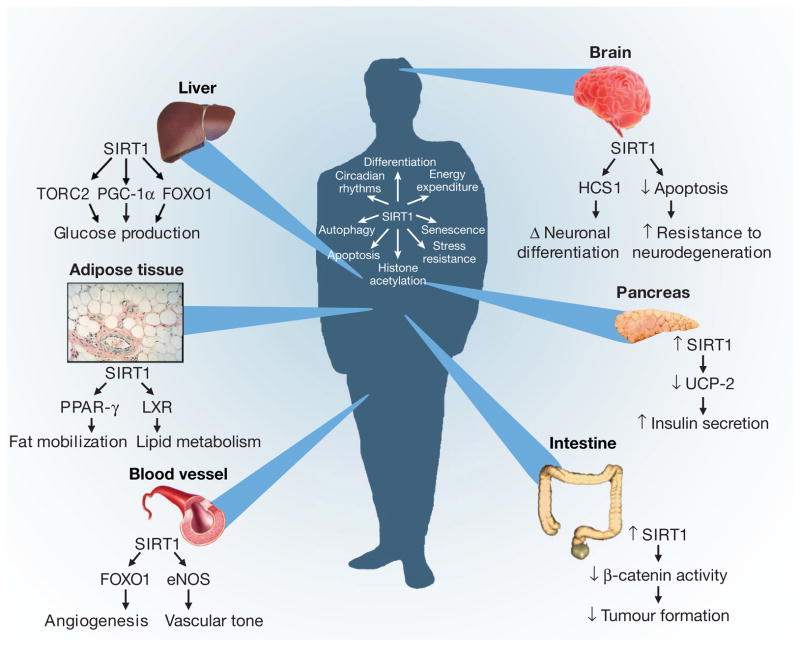
Figure 2. The diverse physiological roles of the sirtuins.
Shown are examples of the organ-specific physiology of SIRT1, along with some of the direct or indirect targets of sirtuin regulation (see text for details). In addition, examples of some of the SIRT1-regulated intracellular parameters are presented, ranging from modulation of progenitor differentiation to altering the threshold for apoptosis. At the beginning of a compound name or process (for example, ‘Tumour formation’ at bottom right), down-arrow indicates ‘decreasing’, up-arrow indicates ‘increasing’ and ‘Δ’ indicates ‘change in’.
Although the role of sirtuins in regulating metabolism has centred on key metabolic organs, such as liver and pancreas, early studies in human subjects undergoing voluntary caloric restriction suggest that levels of SIRT1 rise in tissues as diverse as skeletal muscle and circulating mononuclear cells51,52. The role of sirtuins in the metabolic adaptation of these cell types is largely unexplored. The intriguing connection between SIRT1 and circadian rhythms provides a glimpse of the wide spectrum of metabolic effects sirtuins may eventually be shown to regulate53,54. The observation that SIRT1 can directly deacetylate core components of the circadian clock machinery is particularly fascinating, as the ultimate goal of such rhythms is to coordinate the sleep-wake cycle of an organism with environmental cues, including coordinating and matching intracellular metabolism to external food availability.
Sirtuins and age-related diseases
Given the wealth of data connecting sirtuins to glucose homeostasis and insulin secretion, it seemed reasonable to suspect that these proteins might also regulate the susceptibility to developing insulin resistance and diabetes. Two recent studies using moderate transgenic overexpression of SIRT1 demonstrate that such engineered animals do indeed exhibit improved glucose tolerance when challenged with high-fat diets55,56. Interestingly, these two studies came to two different conclusions regarding the effects of moderately increased SIRT1 expression on basal energy homeostasis. In addition to these gain of function studies, inhibition of SIRT1 by genetic or pharmacological means can induce insulin resistance57. These data support the notion that manipulating sirtuin activity might result in protection from a host of metabolic derangements. Such notions have also been aided by the association of SIRT1 genetic variants with human energy expenditure and obesity58,59. In animal models, treatment with the sirtuin activator resveratrol appears to protect against diet-induced obesity and glucose intolerance60,61. Some concerns have been raised regarding whether resveratrol in mammalian systems works exclusively or even predominantly through regulation of sirtuin activity. For instance, resveratrol also activates the AMPK pathway, although recent evidence suggests that AMPK activity is intimately connected to sirtuin function62. Some of the concerns raised regarding the specificity of resveratrol have also been addressed by the use of newer and seemingly more specific sirtuin activators, which again appear to provide some protection in animal models of diet-induced obesity and insulin resistance63,64.
Although the link between sirtuins and metabolic disease has been intensely studied, there is a growing body of evidence that similar relationships may hold for a wide swath of age-related maladies. For instance, the ability of SIRT1 to regulate p53 activity potentially implicates the sirtuins in tumorigenesis. Indeed, depending on the context, arguments have been made that SIRT1 might either increase or decrease cancer risks65. Recently, the notion that SIRT1 might act as a non-traditional tumour suppressor was highlighted by observations that in the context of a p53+/− mouse, haploinsufficiency of SIRT1 resulted in increased tumour formation14 while overexpressed SIRT1 reduced tumours15. In addition, a direct connection between the tumour suppressor Brca1 and SIRT1 expression has recently been established66. Finally, overexpression of SIRT1 can inhibit tumori-genesis in a mouse model of colon cancer triggered by constitutive Wnt signalling67. With observations that SIRT6 regulates DNA repair, and that SIRT2 activity is necessary for proper cytokinesis, a strong possibility exists that other sirtuin family members will also affect cancer predisposition.
The sirtuins also appear to have a prominent role in vascular biology, and may regulate aspects of age-dependent atherosclerosis. Part of these effects may come through regulation of lipid and cholesterol metabolism, including the ability of SIRT1 to modulate the activity of the nuclear receptor LXR, a critical factor in reverse cholesterol transport68. In addition, a conditional deletion of SIRT1 in endothelial cells has been demonstrated to impair the angiogenic response following an ischaemic insult69. SIRT1 can also deacetylate and regulate endothelial nitric oxide synthase (eNOS) activity, a key regulator of vascular tone70. These results are particularly interesting, because in mouse models, caloric restriction has been shown to induce mitochondrial biogenesis through an eNOS-dependent pathway71. Other sirtuins can also affect cardiovascular physiology, as SIRT7-deficient mice exhibit cardiac abnormalities72 and the important vasoconstrictor angiotensin II regulates SIRT3 expression73.
A unified picture of the role of sirtuins in neurological diseases has yet to emerge. Initial reports had implicated SIRT1 in a specific model of axonal degeneration74, although subsequent studies have raised the possibility that the neuronal pathology observed in this model may occur through a sirtuin-independent pathway75. Similarly, although (as discussed above) SIRT1 is in general thought to be protective against oxidative stress, there is evidence both in vitro and in vivo that this may not hold true in the brain76. Despite these observations, in a mouse model of Alzheimer’s disease, brain specific overexpression of SIRT1 appears to reduce neurodegeneration77,78. Surprisingly, pharmacological or genetic inhibition of SIRT2 was also protective in both a cell-based and a Drosophila model of Parkinson’s disease79. These results suggest that increased SIRT1 or decreased SIRT2 both reduce neurodegeneration, potentially raising cautionary flags for the development of broadly acting sirtuin-based pharmaceuticals for these types of conditions.
Concluding remarks
From their humble origins as silencing factors in yeast, members of the sirtuin family have emerged as broad regulators of cellular fate and mammalian physiology. As impressive as the recent progress has been, much of our understanding has come from studies involving only SIRT1, whereas the other six mammalian sirtuins—presumably equally important—have received significantly less attention. Even for SIRT1, many questions remained unanswered, including a full understanding of how the activity of the enzyme is regulated under both normal and stress conditions, as well as during ageing (Fig. 3). The recent discovery of interacting proteins that can both positively and negatively regulate SIRT1 deacetylase activity80–82 suggests that similar interacting proteins may exist for the other sirtuin family members. Pharmacological manipulation of sirtuin activity that began with the use of resveratrol has now extended to a variety of newer agents that appear to have greater specificity. Some of these agents have already begun human clinical trials83. Whereas the primary benefit of these drugs may lie in preventing diet-induced metabolic disorders, direct effects on vascular health, tumorigenesis and even overall lifespan are at least within the realm of possibility, given the wide spectrum of sirtuin biology.
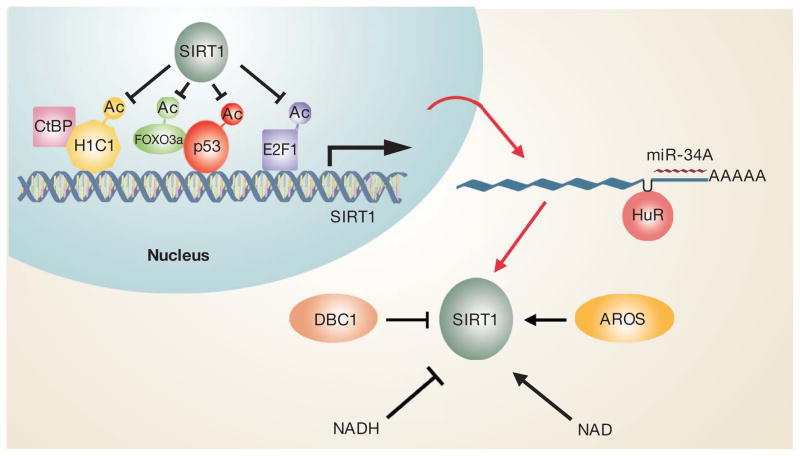
Figure 3. Complex regulation of SIRT1 activity.
The promoter of SIRT1 is positively and negatively regulated by the binding of various transcription factors and repressors, including H1C1, CtBP, p53, FOXO3A and E2F1. The acetylation and hence activity of many of these factors are in turn controlled by SIRT1. The SIRT1 message is also regulated by the RNA binding protein HuR and the p53-regulated microRNA miR-34A. Finally, SIRT protein activity is regulated positively and negatively by interacting proteins such as AROS and DBC1, as well as by the overall metabolic state, as reflected in the NAD/NADH ratio.
From an evolutionary standpoint, it remains unclear why the sirtuin family has emerged as a key regulator of so many seemingly varied processes. Perhaps some of this prominence can be attributed to the enzyme’s dependence on NAD+, forever linking sirtuin activity to the underlying metabolic state of the cell. In the 1940s and 50s, it became clear that ingested sugar, fat and protein was eventually reduced to a single, simple and versatile intermediate. Fifty years later, it was realized that this same intermediate, acetyl-CoA, could also be used to modify histones and hence regulate gene expression. As such, acetylation and NAD+-dependent deacetylation have emerged as perhaps the most immediate and versatile connection between intracellular energetics and intracellular fate. Indeed, the evolving and intricate connection between acetylation, energetics and gene expression is now just beginning to be revealed84. In this regard, although numerous individual studies have linked sirtuins to changes in gene expression, regulation of genomic stability or alterations in intracellular metabolism, the real excitement lies in the understanding that these disparate functions are perhaps all interconnected—that sirtuins serve as the bridge between what we eat and what we are. More twists and turns await us in this drama, as we are indeed far from the final curtain. But for now at least, many of the curious observations of the past are beginning to make sense. After waiting for nearly a century, the significance of Chekhov’s gun may have finally been realized.
Acknowledgments
We apologize to our colleagues for being unable to cite all appropriate references owing to space limitations. Highlighted references are a subjective appraisal of some of the most interesting manuscripts published in the last year. We are grateful to I. Rovira for help with figures. This work was supported by NIH Intramural funds (T.F, C.-X.D.), The Ellison Medical Foundation (T.F.), The Sidney Kimmel Cancer Research Foundation (R.M.) and the V Foundation (R.M.).
Footnotes
Reprints and permissions information is available at www.nature.com/reprints.
Author Contributions All authors contributed to the writing of this Review.
References
- 1.Rine J, Strathern JN, Hicks JB, Herskowitz I. A suppressor of mating-type locus mutations in Saccharomyces cerevisiae: evidence for and identification of cryptic mating-type loci. Genetics. 1979;93:877–901. doi: 10.1093/genetics/93.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennedy BK, Austriaco NR, Jr, Zhang J, Guarente L. Mutation in the silencing gene SIR4 can delay aging in S cerevisiae. Cell. 1995;80:485–496. doi: 10.1016/0092-8674(95)90499-9. [DOI] [PubMed] [Google Scholar]
- 3.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 5.Guarente L, Picard F. Calorie restriction—the SIR2 connection. Cell. 2005;120:473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 6.Boily G, et al. SirT1 regulates energy metabolism and response to caloric restriction in mice. PLoS One. 2008;3:e1759. doi: 10.1371/journal.pone.0001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Ageing Dev. 2007;128:546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Kaeberlein M, Kirkland KT, Fields S, Kennedy BK. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2004;2:E296. doi: 10.1371/journal.pbio.0020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haigis MC, Guarente LP. Mammalian sirtuins—emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 10.Denu JM. Linking chromatin function with metabolic networks: Sir2 family of NAD+-dependent deacetylases. Trends Biochem Sci. 2003;28:41–48. doi: 10.1016/s0968-0004(02)00005-1. [DOI] [PubMed] [Google Scholar]
- 11.Mostoslavsky R, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 12.Michishita E, et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature. 2008;452:492–496. doi: 10.1038/nature06736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan Z, Zhang X, Sengupta N, Lane WS, Seto E. SIRT1 regulates the function of the Nijmegen breakage syndrome protein. Mol Cell. 2007;27:149–162. doi: 10.1016/j.molcel.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang RH, et al. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell. 2008;14:312–323. doi: 10.1016/j.ccr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oberdoerffer P, et al. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell. 2008;135:907–918. doi: 10.1016/j.cell.2008.10.025. Refs 14 and 15 describe a role for SIRT1 in genomic stability and how disruption of this activity might contribute to cancer and ageing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murayama A, et al. Epigenetic control of rDNA loci in response to intracellular energy status. Cell. 2008;133:627–639. doi: 10.1016/j.cell.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 17.Vaquero A, et al. SIRT1 regulates the histone methyl-transferase SUV39H1 during heterochromatin formation. Nature. 2007;450:440–444. doi: 10.1038/nature06268. [DOI] [PubMed] [Google Scholar]
- 18.Brunet A, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 19.Motta MC, et al. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 20.Westerheide SD, Anckar J, Stevens SM, Jr, Sistonen L, Morimoto RI. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science. 2009;323:1063–1066. doi: 10.1126/science.1165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo J, et al. Negative control of p53 by Sir2α promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 22.Vaziri H, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 23.Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004;306:2105–2108. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- 24.Chen WY, et al. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell. 2005;123:437–448. doi: 10.1016/j.cell.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci USA. 2008;105:13421–13426. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang H, et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeung F, et al. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langley E, et al. Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. EMBO J. 2002;21:2383–2396. doi: 10.1093/emboj/21.10.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawahara TL, et al. SIRT6 links histone H3 lysine 9 deacetylation to NF-κB-dependent gene expression and organismal life span. Cell. 2009;136:62–74. doi: 10.1016/j.cell.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chua KF, et al. Mammalian SIRT1 limits replicative life span in response to chronic genotoxic stress. Cell Metab. 2005;2:67–76. doi: 10.1016/j.cmet.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Feige JN, Auwerx J. Transcriptional targets of sirtuins in the coordination of mammalian physiology. Curr Opin Cell Biol. 2008;20:303–309. doi: 10.1016/j.ceb.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prozorovski T, et al. Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nature Cell Biol. 2008;10:385–394. doi: 10.1038/ncb1700. [DOI] [PubMed] [Google Scholar]
- 34.Han MK, et al. SIRT1 regulates apoptosis and Nanog expression in mouse embryonic stem cells by controlling p53 subcellular localization. Cell Stem Cell. 2008;2:241–251. doi: 10.1016/j.stem.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuzmichev A, et al. Composition and histone substrates of polycomb repressive group complexes change during cellular differentiation. Proc Natl Acad Sci USA. 2005;102:1859–1864. doi: 10.1073/pnas.0409875102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J Biol Chem. 2005;280:16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- 37.Rodgers JT, et al. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 38.Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci USA. 2007;104:12861–12866. doi: 10.1073/pnas.0702509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, et al. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature. 2008;456:269–273. doi: 10.1038/nature07349. A detailed look at the role of protein acetylation and SIRT1-dependent deacetylation in the hepatic response to starvation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moynihan KA, et al. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–117. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Bordone L, et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006;4:e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haigis MC, et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126:941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 43.Ahuja N, et al. Regulation of insulin secretion by SIRT4, a mitochondrial ADP-ribosyl transferase. J Biol Chem. 2007;282:33583–33592. doi: 10.1074/jbc.M705488200. [DOI] [PubMed] [Google Scholar]
- 44.Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett. 2008;582:46–53. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee IH, et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci USA. 2008;105:3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwer B, Bunkenborg J, Verdin RO, Andersen JS, Verdin E. Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2. Proc Natl Acad Sci USA. 2006;103:10224–10229. doi: 10.1073/pnas.0603968103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hallows WC, Lee S, Denu JM. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc Natl Acad Sci USA. 2006;103:10230–10235. doi: 10.1073/pnas.0604392103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahn BH, et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci USA. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lombard DB, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27:8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakagawa T, Lomb DJ, Haigis MC, Guarente L. SIRT5 deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell. 2009;137:560–570. doi: 10.1016/j.cell.2009.02.026. A recent demonstration of the expanding connection between sirtuin family members and metabolic regulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crujeiras AB, Parra D, Goyenechea E, Martinez JA. Sirtuin gene expression in human mononuclear cells is modulated by caloric restriction. Eur J Clin Invest. 2008;38:672–678. doi: 10.1111/j.1365-2362.2008.01998.x. [DOI] [PubMed] [Google Scholar]
- 52.Civitarese AE, et al. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Asher G, et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 54.Nakahata Y, et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. Refs 53 and 54 provide the first link between sirtuins and circadian rhythms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Banks AS, et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun C, et al. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307–319. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 58.Weyrich P, et al. SIRT1 genetic variants associate with the metabolic response of Caucasians to a controlled lifestyle intervention — the TULIP Study. BMC Med Genet. 2008;9 doi: 10.1186/1471-2350-9-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peeters AV, et al. Association of SIRT1 gene variation with visceral obesity. Hum Genet. 2008;124:431–436. doi: 10.1007/s00439-008-0567-8. [DOI] [PubMed] [Google Scholar]
- 60.Lagouge M, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 61.Baur JA, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Canto C, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. Provides a link between sirtuins and other energy sensing pathways in the cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feige JN, et al. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 64.Milne JC, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deng CX. SIRT1, is it a tumor promoter or tumor suppressor? Int J Biol Sci. 2009;5:147–152. doi: 10.7150/ijbs.5.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang RH, et al. Interplay among BRCA1, SIRT1, and Survivin during BRCA1-associated tumorigenesis. Mol Cell. 2008;32:11–20. doi: 10.1016/j.molcel.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Firestein R, et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS One. 2008;3:e2020. doi: 10.1371/journal.pone.0002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li X, et al. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell. 2007;28:91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 69.Potente M, et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644–2658. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mattagajasingh I, et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nisoli E, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 72.Vakhrusheva O, et al. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res. 2008;102:703–710. doi: 10.1161/CIRCRESAHA.107.164558. [DOI] [PubMed] [Google Scholar]
- 73.Benigni A, et al. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119:524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- 75.Fainzilber M, Twiss JL. Tracking in the Wlds—the hunting of the SIRT and the luring of the Draper. Neuron. 2006;50:819–821. doi: 10.1016/j.neuron.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 76.Li Y, Xu W, McBurney MW, Longo VD. SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell Metab. 2008;8:38–48. doi: 10.1016/j.cmet.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Ham TJ, et al. C. elegans model identifies genetic modifiers of α-synuclein inclusion formation during aging. PLoS Genet. 2008;4:e1000027. doi: 10.1371/journal.pgen.1000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim D, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBOJ. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Outeiro TF, et al. Sirtuin 2 inhibitors rescue α-synuclein-mediated toxicity in models of Parkinson’s disease. Science. 2007;317:516–519. doi: 10.1126/science.1143780. [DOI] [PubMed] [Google Scholar]
- 80.Zhao W, et al. Negative regulation of the deacetylase SIRT1 by DBC1. Nature. 2008;451:587–590. doi: 10.1038/nature06515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim JE, Chen J, Lou Z. DBC1 is a negative regulator of SIRT1. Nature. 2008;451:583–586. doi: 10.1038/nature06500. Refs 80 and 81 demonstrate the role of protein–protein interactions in modulating SIRT1 function and suggest that this mode of regulation probably exists for other sirtuins. [DOI] [PubMed] [Google Scholar]
- 82.Kim EJ, Kho JH, Kang MR, Um SJ. Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Mol Cell. 2007;28:277–290. doi: 10.1016/j.molcel.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 83.Elliott PJ, Jirousek M. Sirtuins: novel targets for metabolic disease. Curr Opin Investig Drugs. 2008;9:371–378. [PubMed] [Google Scholar]
- 84.Wellen KE, et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]