Abstract
Changes in the lipid composition of cardiac myocytes have been reported during cardiac hypertrophy, cardiomyopathy, and infarction. Because a recent study indicates a relation between low phosphatidylinositol-bisphosphate (PIP2) levels and reduced intercellular coupling, we tested the hypothesis that agonist-induced changes in PIP2 can result in a reduction of the functional coupling of cardiomyocytes and, consequently, in changes in conduction velocity. Intercellular coupling was measured by Lucifer Yellow dye transfer in cultured neonatal rat cardiomyocytes. Conduction velocity was measured in cardiomyocytes grown on microelectrode arrays. Intercellular coupling was reduced by angiotensin II (43.7±9.3%, N=11) and noradrenaline (58.0±10.7%, N=11). To test if reduced intercellular coupling after agonist stimulation was caused by PIP2-depletion, myocytes were stimulated by angiotensin II (57.3±5.7%, N=14) and then allowed to recover in medium with or without wortmannin (an inhibitor of PIP2 synthesis). Intercellular coupling fully recovered in control medium (102.1±8.9%, N=10), whereas no recovery occurred in the presence of wortmannin (69.3±7.8%, N=12). Inhibition of PKC, calmodulin, or arachidonic acid production did not affect the response to either angiotensin II or noradrenaline. Furthermore, decreasing or increasing PIP2 also decreased and increased intercellular coupling, respectively. This supports the role of PIP2 in the regulation of intercellular coupling. In beating myocytes, conduction velocity was reduced by angiotensin II stimulation, and recovery after wash out was prevented by inhibition of PIP2 production. Reductions in PIP2 inhibit intercellular coupling in cardiomyocytes, and stimulation by physiologically relevant agonists reduces intercellular coupling by this mechanism. The reduction in intercellular coupling lowered conduction velocity.
Keywords: Connexin, Gap junctions, PIP2, Cardiomyocytes
Introduction
Arrhythmias after acute myocardial infarction can be divided into distinct phases based on the time of their occurrence. The acute arrhythmias are divided into phase 1a (early) and phase 1b (late) arrhythmias. Phase 1b arrhythmias occur after approximately 12–30 min of ischemia [23] and correlate with compromised intercellular coupling [25, 46]. It has been suggested that changes in the intracellular milieu (for example calcium and pH [6, 34, 39]), lipid metabolites [19, 33, 55], and in phosphorylation [1, 4] status cause this reduction in coupling.
Another important event during phase 1b is the release of catecholamines by reversal of the reuptake mechanism. The role of this release is unclear, but its prevention or the inhibition of α-adrenergic receptors reduces the occurrence of arrhythmias [40, 44]. The proarrhythmic effect of α-adrenergic stimulation has been suggested to partly rely on inhibition of intercellular coupling [8]. The mechanisms by which Gαq-coupled receptors, such as α-adrenergic receptors, reduce coupling are unclear. These receptors are expressed in the heart; their agonists include noradrenaline, endothelin, thrombin, and angiotensin II. The receptors activate phospholipase C (PLC), which breaks down PIP2, resulting in generation of diacylglycerol (DAG) and inositol trisphosphate (IP3). These messengers activate protein kinase C (PKC) and releases calcium from intracellular stores, respectively.
Besides acting as precursor for the production of IP3 and DAG, PIP2 also acts as a signaling molecule itself. Hilgeman and coworkers showed that PIP2 directly regulates the activity of cardiac Na/Ca exchangers [21]. Since then, several ion channels and transporters have been found to be regulated by PIP2 (for review see [47]). PIP2 is reported to regulate a number of channels that are expressed in cardiac tissues. These include inward rectifier potassium channels [22], hERG [5], and KCNQ channels [30].
Several studies indicate that PIP2 levels are significantly reduced in the heart during stimulation by agonists such as adenosine [38], endothelin, bradykinin, and phenylephrine [10]. Furthermore, pathophysiological conditions such as ischemia are associated with reductions in PIP2 [36], and similar decreases are observed during cardiac hypertrophy [13], cardiomyopathy [58], and heart failure subsequent to chronic infarction [49]. The pathophysiological role and significance of these findings for the electrophysiological properties of the heart has not yet been determined.
Moolenaar and coworkers [51] recently showed that connexin 43 (Cx43), the main protein subunit of cardiac gap junctions, is regulated by PIP2, and reductions in the PIP2 level resulted in reduced coupling in Rat-1 fibroblasts. Whether PIP2 regulates intercellular coupling in cardiac myocytes is unknown, but if it does, it would contribute to the increased risk of arrhythmia in states of reduced PIP2.
Therefore, the aim of this study was to investigate whether PIP2 levels affect intercellular coupling in cardiac tissue. Furthermore, we tested whether stimulation of Gαq-coupled receptors relevant to cardiac tissue affect intercellular coupling and thereby conduction velocity via this mechanism.
Methods and materials
Isolation and culture of neonatal rat cardiomyocytes
Ventricular myocytes were isolated by a method modified from Simpson and Savion [45] by multiple rounds of trypsin digestions of ventricles from neonatal Wistar rats. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Detailed description of isolation procedure and culture conditions can be found in [7]. Experiments were performed on myocytes between days 4 and 8 in culture.
Cardiomyocytes were seeded onto collagen-coated slides (140,000 cells/cm2). For collagen coating, collagen type VI (0.142 mg/mL in 0.1% acetic acid and 30% ethanol) was added to coverslips and incubated for 3 h at 37°C. Excess solution was removed and coverslips left to dry for 2–3 h.
Measurements of intercellular coupling by localized electroporation
Principle of EpiZap dye diffusion
Intercellular coupling in neonatal rat cardiomyocytes was measured using the EpiZap system (ASK Science, Kingston, Ontario, Canada). Coupling is measured as spread of the fluorescent dye Lucifer Yellow (LY) after localized electroporation of a subset of cells [43]. The principle is similar to that used in dye injections, but electroporation ensures delivery of dye to many cells. Therefore, the measurements reflect the coupling of a large population of cells instead of just one cell. The myocytes were beating spontaneously after electroporation, indicating that the treatment was well tolerated.
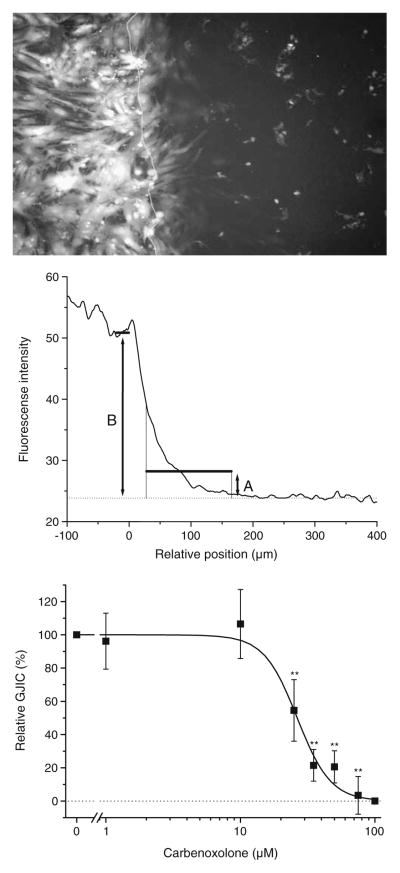
In the example shown in Fig. 1, the slide surface left of the white line is conductive, and cells growing on this surface are electroporated. The bright fluorescence of the cells in this area results from uptake of LY during electroporation. Dye was allowed to spread into neighboring myocytes for 2 min before images were captured at four different locations along the border line. The position of the border line was set to zero in each horizontal line of the image. Fluorescence was summed for each position and plotted. The resulting profile (Fig. 1, middle panel) shows high intensity in the electroporated cells (negative position values) which decays with increasing distance from the border. Intercellular coupling was quantified as mean intensity in region A divided by mean intensity in area B for comparison between experiments with different levels of electroporation. In 20 determinations, the ratio was 0.163±0.012 in control cells and 0.050±0.006 in cells treated with the gap junction uncoupler carbenoxolone (100 μmol/L, 30 min, P<0.0001 versus control in paired t test).
Fig. 1.
Measurements of intercellular coupling by electroporation of Lucifer Yellow. Top panel: Fluorescence image showing cardiomyocytes after electroporation. The white line indicates the border between the conductive (left) and nonconductive surface of the slide. Cells growing on the conductive layer are electroporated and show bright fluorescence. The fluorescence right of the line is caused by diffusion of dye through gap junctions. Middle panel: Quantification of images. The border position was set to zero in each horizontal line in the image. Fluorescence was summed for each position and plotted in the graph. The ratio between average intensity in region A and average intensity in region B was used to calculate relative coupling. Bottom panel: Carbenoxolone dose-inhibition curve. Cardiomyocytes were exposed to varying carbenoxolone concentrations and coupling determined (N=4–9). IC50 was 26 μmol/L (Hill coefficient 3.5). Statistical analysis was performed by one-way ANOVA with Dunnet’s test post hoc (versus control). ** signifies P<0.01
On each experimental day, double determination was performed in untreated cells and in carbenoxolone-treated (100 μmol/L, 30 min) cells. Relative coupling was determined as:
where RatioX is the ratio of interest, Ratiocarb is the ratio in carbenoxolone-treated cells, and Ratiocontrol is the ratio in untreated cells. Thereby, 100% corresponds to the level of untreated myocyte cultures, and 0% corresponds to complete uncoupling.
Using this method, we determined the dose-inhibition curve to carbenoxolone (Fig. 1, bottom panel). This yielded an IC50 of 26 μmol/L which is in the expected range [11].
Experimental procedure
For each experimental condition, cardiomyocytes were incubated (37°C, 5% CO2) in serum-free medium with test reagents. A 23-μl drop of LY solution (12 mg/mL) was applied on the cells and the electrode placed on top of the slide. Cells were electroporated three times by discharging a capacitor (1 μF, 15 V). After electroporation, slides were rinsed and images acquired 2 min later, using a Micromax camera (Princeton Instruments, Trenton, NJ, USA) mounted on a DMRE microscope with an I3 filter cube (Leica Microsystems, Heidelberg, Germany). Electroporation was performed in calcium-free buffer to avoid calcium overload. Buffer contained (in mmol/L): NaCl (135), KCl (4), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (10), MgCl2 (0.8), NaH2PO4 (0.5), Glucose (5), ethylene glycol tetraacetic acid (1) and 10% fetal calf serum, pH adjusted to 7.4.
Measurements of conduction velocity
Multielectrode arrays (MEAs; Multi Channel Systems, Reutlingen, Germany) were used for field potential recordings from spontaneously active monolayers of neonatal rat cardiomyocytes [3, 20]. The MEAs used consisted of 60 electrodes (diameter 30 μm) which had an inter-electrode distance of 200 μm. MEAs were fibronectin coated (2.5 μg/ml in H2O). Experiments were conducted at 37°C and data stored online and analyzed offline with a customized toolbox programmed for MATLAB (The Mathworks, Natick, MA, USA) [16, 20]. Activation time contour plots revealed direction of excitation spread in cultures, and conduction velocity was obtained perpendicularly to the excitation wave front.
Chemicals
All chemicals were from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated. Candesartan was a gift from AstraZeneca (Gothenburg, Sweden).
Statistical analysis
Data are displayed as mean±SEM, and N denotes number of experiments on separately grown cell cultures. Statistical significance was tested by one-way analysis of variance (ANOVA) followed by Tukey’s or Dunnet’s Multiple Comparison Test (using GraphPad Prism 4.00) as indicated in figure legends.
Results
Stimulation of Gαq-coupled receptors reduces intercellular coupling
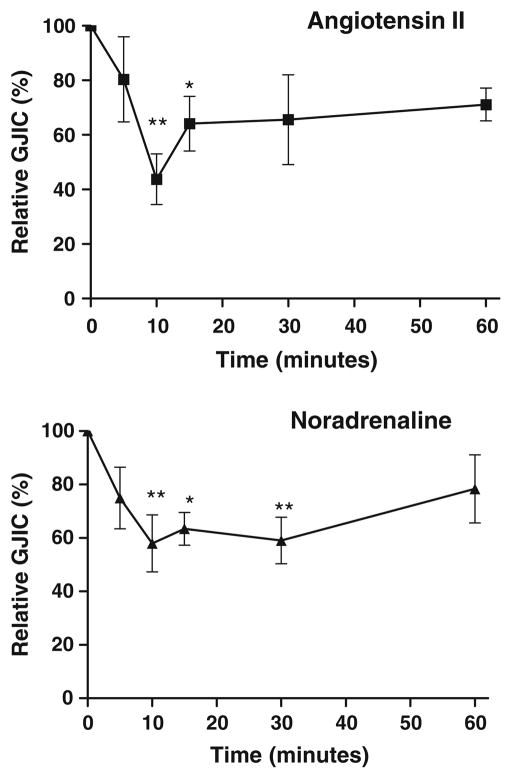
To stimulate Gαq-coupled receptors, cardiomyocytes were exposed to either angiotensin II (1 μmol/L) or noradrenaline (0.5 μmol/L) for periods ranging from 5 to 60 min. In all experiments with noradrenaline, propranolol (0.5 μmol/L) was added to prevent activation of β-adrenergic receptors. As shown in Fig. 2, both angiotensin II (N=10–11) and noradrenaline (N=9–11) decreased intercellular coupling in a time-dependent manner with maximal uncoupling after 10 min. Maximal uncoupling was 43.7±9.3% of control for angiotensin II and 58.0±10.7% for noradrenaline.
Fig. 2.
Angiotensin II and noradrenaline time-dependently reduce intercellular coupling. Top panel: Intercellular coupling as function of time during angiotensin II stimulation (1 μmol/L, N=10–11). Bottom panel: Intercellular coupling as function of time during noradrenaline stimulation (0.5 μmol/L, N=9–11). Propranolol (0.5 μmol/L) was included in noradrenaline experiments to prevent activation of β-adrenergic receptors. Statistical analysis was performed by one-way ANOVA with Dunnet’s test post hoc (versus control). * signifies P<0.05 and ** P<0.01
To test the specificity of the response, cardiomyocytes were pre-incubated with receptor antagonists, and the response to 10 min of agonist stimulation was evaluated. Pre-incubation with the AT1-receptor antagonist candesartan (20 nmol/L) abolished the response to angiotensin II (91.2±13.9%, N=9), showing that Gαq-coupled AT1-receptors mediate the observed response.
To evaluate the noradrenaline-induced signaling pathway, cells were pre-incubated with the α1-adrenergic receptor antagonist prazosin (0.5 μmol/L). This abolished the effect of noradrenaline stimulation (116.3±15%, N=17 with prazosin) supporting a Gαq-coupled signaling pathway. Neither candesartan (106.1±6.5%, N=8) nor prazosin (104.8±14.0%, N=7) alone affected intercellular coupling.
Intercellular coupling is regulated by PIP2
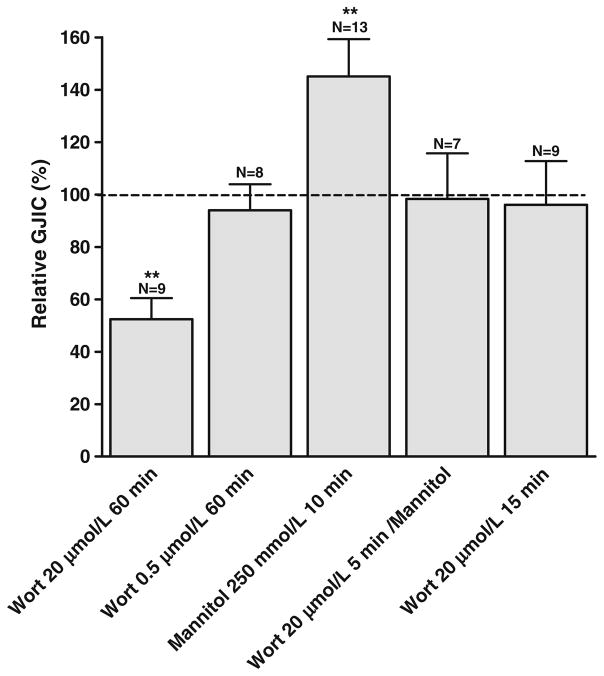
To test whether changes in PIP2 regulate intercellular coupling per se, cardiomyocytes were exposed to a high concentration of wortmannin (20 μmol/L) for 1 h before electroporation. At this concentration, wortmannin inhibits the PI4 kinase [37] and thereby synthesis of PIP2, which will result in a reduction of the plasma membrane pool of PIP2 over time. To accelerate this process, serum (5%) was added to the medium. After wortmannin treatment, intercellular coupling was reduced to 52.4±8% (N=9, P<0.01, see Fig. 3), indicating that a reduction in PIP2 inhibits intercellular coupling. This result shows that depletion of PIP2 by wortmannin resulted in an inhibition comparable to that induced by agonist stimulation and could therefore, in principle, underlie the agonist responses.
Fig. 3.
Effect of wortmannin and hypertonic shock on intercellular coupling. Wortmannin treatment for 60 min significantly reduced intercellular coupling at 20 μmol/L, but had no effect at 0.5 μmol/L. Hypertonic shock for 10 min increased intercellular coupling, and this effect was prevented by wortmannin (20 μmol/L, 5 min pre-incubation and during hypertonic shock). Incubation with wortmannin (20 μmol/L) alone for 15 min had no effect on intercellular coupling. N indicates number of experiments in each group. Statistical analysis was performed by ANOVA with Dunnet’s test post hoc (versus control). ** signifies P<0.01
In contrast, exposure of cardiomyocytes to a low concentration of wortmannin (0.5 μmol/L), which completely inhibits the PI3 kinase but not the PI4 kinase [37], did not affect intercellular coupling (94.0±9.92%, N=8, Fig. 3). This result indicates that inhibition was likely caused by a reduction in PIP2 synthesis and not by PI3 kinase and its downstream signaling.
Hypertonic shock increases PIP2 by activation of PI kinase in cell lines as well as in cardiac cells [38, 56]. To test whether an increase in PIP2 increases intercellular coupling, cardiomyocytes were exposed to mannitol (250 mmol/L; 10 min), which increased intercellular coupling (145.0±14.3%, N=13, P<0.01, Fig. 3). Pre-incubation with wortmannin (20 μmol/L; 5 min) before the hypertonic shock, prevented the increase in intercellular coupling (98.3±17.4%, N=7). This result indicates that stimulation of PIP2 synthesis was involved in the upregulation of intercellular coupling. Wortmannin treatment alone (15 min) did not reduce intercellular coupling. These experiments indicate that intercellular coupling is sensitive to both increasing and decreasing levels of PIP2.
The uncoupling by Gαq-coupled receptor stimulation is mediated by PIP2
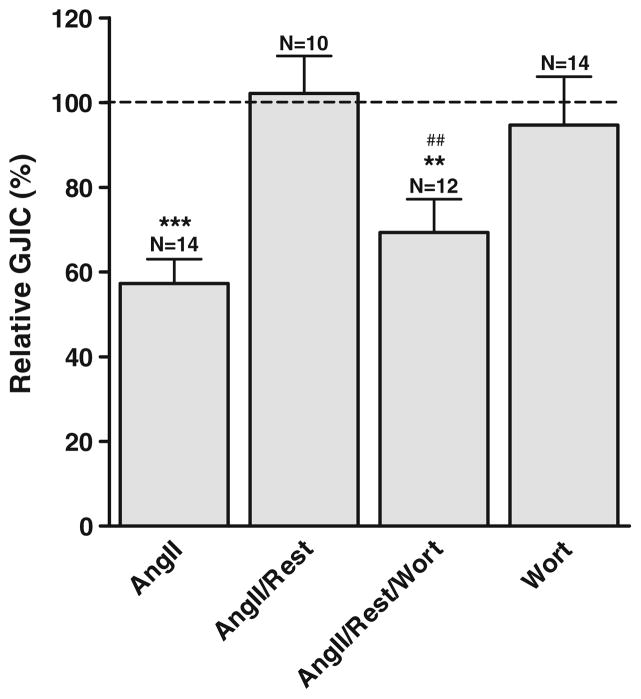
To evaluate whether PIP2 was involved in the uncoupling after receptor stimulation, experiments were conducted where cardiomyocytes recovered for 10 min after angiotensin II stimulation (1 μmol/L; 10 min) in angiotensin II-free medium. As shown in Fig. 4, stimulation by angiotensin II resulted in a reduction of intercellular coupling to 57.3±5.7% (N=14). This effect was reversible and intercellular coupling recovered completely within 10 min after removal of angiotensin II (102.1±8.9%, N= 10). However, when wortmannin (20 μmol/L) was added to the recovery medium, intercellular coupling remained reduced (69.3±7.8%, N=12). In contrast to prolonged exposures to wortmannin and serum (Fig. 2), wortmannin treatment for 10 min did not significantly affect intercellular coupling (94.7±11.4%, N=14). This result indicates that the recovery from inhibition by angiotensin II is dependent on the resynthesis of PIP2, underlining that the response to this agonist is at least partly mediated by a reduction of PIP2.
Fig. 4.
Recovery from angiotensin II stimulation is dependent on resynthesis of PIP2. Stimulation by angiotensin II (1 μmol/L) reduced intercellular coupling, but 10 min of rest in angiotensin II-free medium resulted in full recovery. When wortmannin (20 μmol/L) was added to the medium during rest, recovery was prevented. Wortmannin alone for 10 min did not affect intercellular coupling. N indicates number of experiments in each group. Statistical analysis was performed by ANOVA with Tukey’s multiple comparison test post hoc. ** signifies P<0.01 and *** P<0.001 versus control and ## P<0.01 versus angiotensin II/rest
The role of PKC in uncoupling by Gαq-coupled agonists
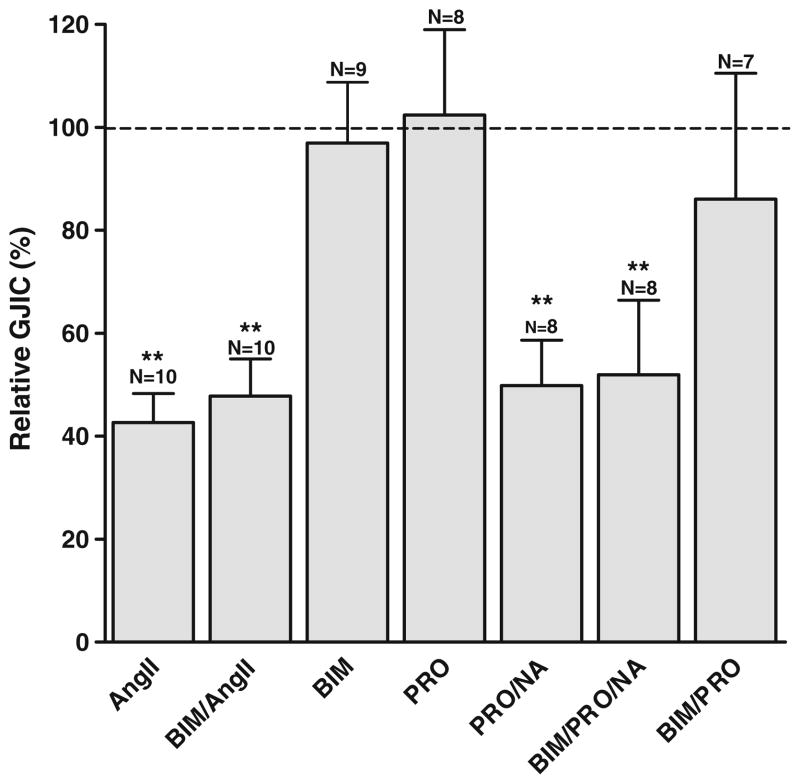
Stimulation with angiotensin II and noradrenaline also activates PKC. Specific inhibition of PKC can be obtained by bisindolylmaleimide (BIM). To evaluate the role of PKC, cardiomyocytes were pretreated with BIM (100 nmol/L) for 5 min before stimulation with angiotensin II (1 μmol/L; 10 min) in the continued presence of BIM (Fig. 5). No significant change in the angiotensin II response was detected, with a coupling of 47.8±7.2% in the presence of angiotensin II + BIM (N=10) versus 42.7±5.6% with angiotensin II alone (N=10). Similar experiments showed that the reduction in intercellular coupling induced by noradrenaline was also unaffected by inhibition of PKC (51.9±14.5%, N=8 with BIM, versus 49.8±14.5% with noradrenaline alone, N=8, Fig. 5). Figure 5 also shows that propranolol alone does not affect intercellular coupling (102.5±16.6%, N=8). BIM itself did not affect intercellular coupling (96.7±11.8%, N=9 in medium and 86.0±24.5%, N=7 in medium with propranolol). These data indicate that the decrease in intercellular coupling after Gαq-coupled receptor stimulation is not mediated by PKC.
Fig. 5.
Inhibition of PKC does not affect the response to angiotensin II and noradrenaline. Angiotensin II (1 μmol/L) and noradrenaline [0.5 μmol/L, with propranolol (0.5 μmol/L)] reduced intercellular coupling, and this reduction was unaffected by inhibition of PKC by BIM (100 nmol/L). BIM alone did not affect intercellular coupling with or without propranolol (0.5 μmol/L). N indicates number of experiments in each group. Statistical analysis was performed by ANOVA with Dunnet’s test post hoc (versus control). ** signifies P<0.01
The role of calcium in uncoupling by Gαq-coupled agonists
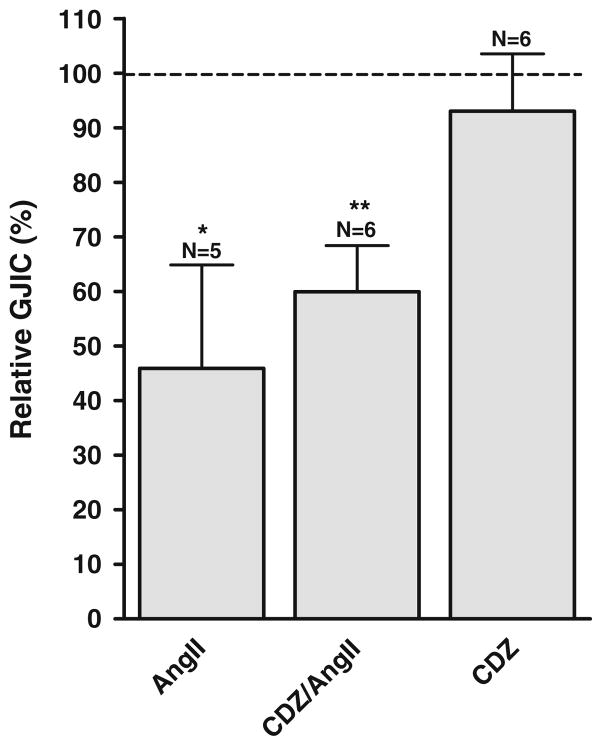
Activation of phosholipase C also leads to the generation of IP3, which induces the release of calcium from intracellular stores. Because gap junctions are sensitive to calcium, this could inhibit coupling. Lurtz and Louis [31] showed that inhibition by calcium is mediated by calmodulin, and to evaluate the role of this pathway, we used the calmodulin-inhibitor calmidazolium (CDZ, 5 μmol/L). Pretreatment with CDZ had no effect on the response to angiotensin II (1 μmol/L, 10 min; see Fig. 6). Angiotensin II reduced coupling to 45.9±19.0% (N=5), and the response in CDZ-treated cultures was not significantly different (59.9±8.5%, N=6). CDZ alone did not affect coupling (93.0±10.5%, N=6). Thus, we conclude that the decrease in intercellular coupling after agonist stimulation is not due to increased intracellular calcium.
Fig. 6.
The decreased coupling after angiotensin II stimulation is not mediated by calmodulin. Angiotensin II (1 μmol/L) reduced intercellular coupling, and this reduction was unaffected by inhibition of calmodulin by calmidazolium (CDZ, 5 μmol/L). N indicates number of experiments in each group. Statistical analysis was performed by ANOVA with Dunnet’s test post hoc (versus control). * signifies P< 0.05 and ** P<0.01
Role of arachidonic acid in the response to Gαq-coupled agonists
An alternative explanation to the observed responses could be a build up of arachidonic acid (AA), which is known to directly inhibit intercellular coupling [19, 33]. AA could increase after stimulation of PLA2, which releases AA directly from phospholipids, or by release from DAG by the action of DAG lipase and monoacylglycerol lipase [9].
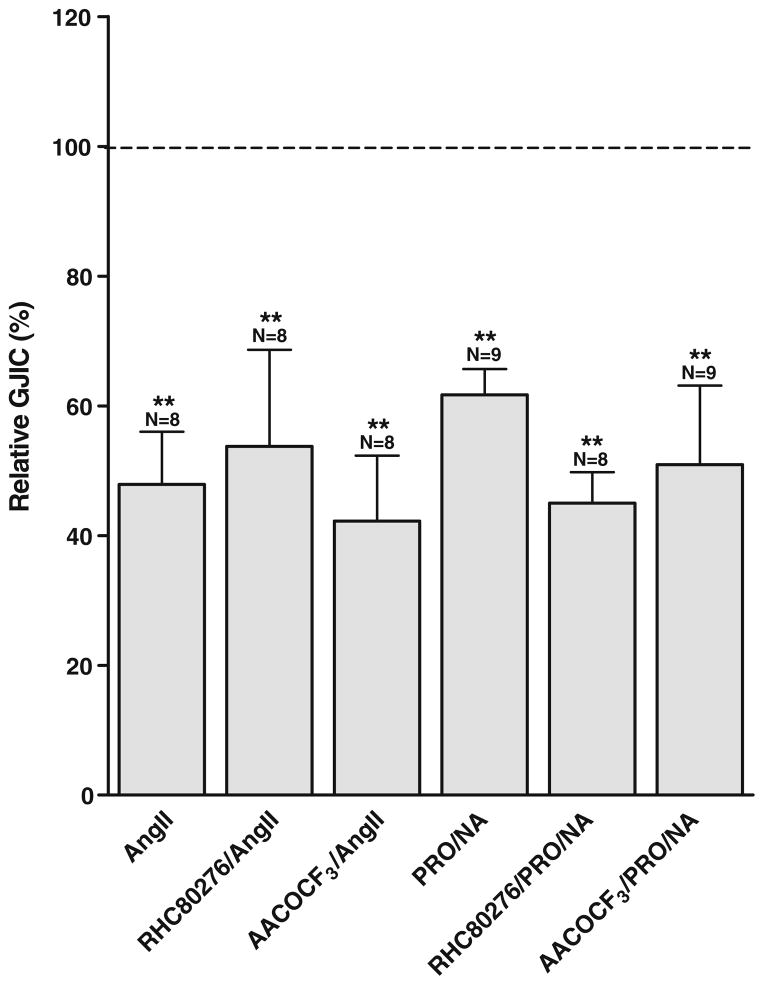
To test the involvement of PLA2 and DAG lipase, we employed the respective inhibitors arachidonyl trifluoromethyl ketone (AACOCF3) and RHC80267. As shown in Fig. 7, 5 min pre-incubation with AACOCF3 (50 μmol/L) did not affect the response to angiotensin II which was 47.9± 8.1% for angiotensin II alone (N=8) and 42.3±10.1% in the presence of AACOCF3 (N=8). Similar results were observed with RHC80267 (25 μmol/L), where the response to angiotensin II was 53.7±14.9% in the presence of RHC80267 (N=8).
Fig. 7.
Arachidonic acid is not involved in the response to angiotensin II and noradrenaline. Angiotensin II (1 μmol/L) and noradrenaline (0.5 μmol/L, with propranolol (0.5 μmol/L)) reduced intercellular coupling, and this reduction was unaffected by inhibition of PLA2 by AACOCF3 (50 μmol/L) or DAG lipase by RHC80267 (25 μmol/L). N indicates number of experiments in each group. Statistical analysis was performed by ANOVA with Dunnet’s test post hoc (versus control). ** signifies P<0.01
Also, the effect of noradrenaline was insensitive to inhibition of PLA2 and DAG lipase. Noradrenaline reduced intercellular coupling to 61.7±3.9% alone (N=9), 50.9± 12.2% with AACOCF3 (N=9), and 44.9±4.8% in the presence of RHC80267 (N=8).
Neither AACOCF3 nor RHC80267 affected coupling when applied alone (data not shown). Data are summarized in Fig. 7, showing that AA is unlikely to mediate the observed reduction in coupling after stimulation of Gαq-coupled receptors.
Effect of Gαq-coupled agonists on conduction velocity
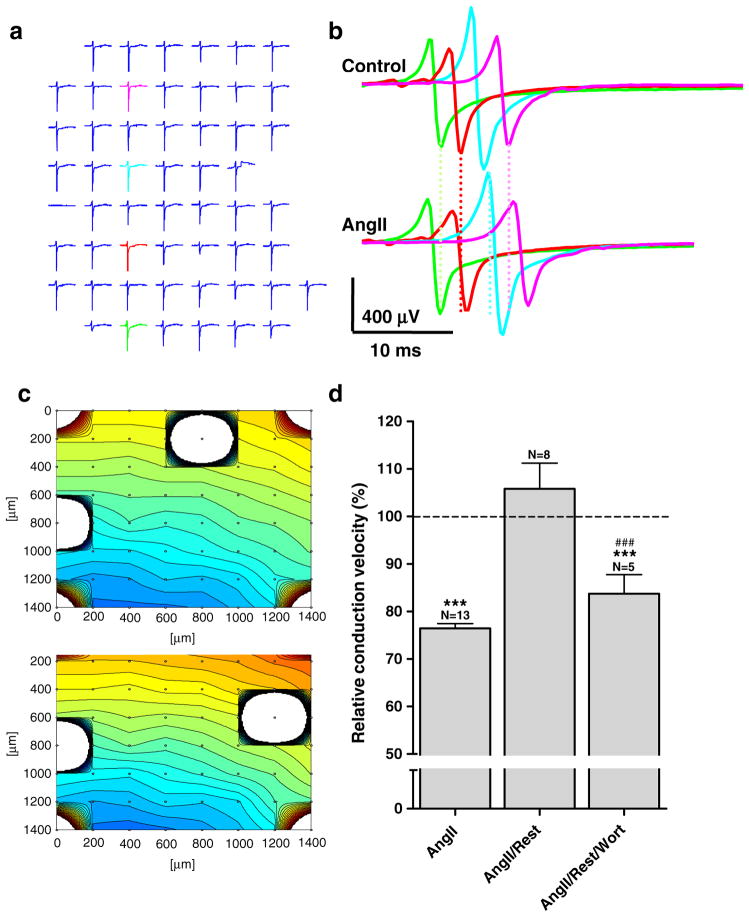
Neonatal cardiomyocytes grown on MEAs form spontaneously active monolayers. We determined the excitation spread and conduction velocity under control conditions and after 10 min incubation with angiotensin II (1 μmol/L, Fig. 8). Under control conditions, conduction velocity was 16.1±2.6 cm/s (N=8), but stimulation with angiotensin II reduced conduction velocity to 13.0±2.9 cm/s (N=8). This decrease was reversible after wash out of angiotensin II with a recovery of conduction velocity to 17.8±3.2 cm/s (N=8). Because changes in conduction velocity can also result from changes in the excitability of the cells, we further analyzed the effect of angiotensin II on the spontaneous activity, but we observed no significant change in the beating frequency during the application of angiotensin II (control=2.25±0.29 Hz; angiotensin II=2.16±0.30 Hz). These data indicate that the reduction in intercellular coupling after agonist stimulation had functional consequences by reducing action potential propagation. To test if, also in this case, the effect was mediated by metabolism of PIP2, experiments were conducted in which wortmannin was included in the wash out medium. In these experiments, conduction velocity was 22.8±3.2 cm/s (N=5) in untreated cells, and stimulation with angiotensin II reduced conduction velocity to 17.4±2.2 cm/s (N=5). Wash out of angiotensin II in the presence of wortmannin only resulted in a partial recovery to 19.0±3.0 cm/s (N=5), indicating that resynthesis of PIP2 was needed to reestablish normal conduction velocity. Thus, the decrease in intercellular coupling after reducing PIP2 was sufficiently robust to affect the propagation of the impulse wave front.
Fig. 8.
Angiotensin II reduces conduction velocity, and recovery is dependent on resynthesis of PIP2. a 8×8 plot showing original voltage recordings from all 60 MEA electrodes. b The electrodes marked in color are superimposed for control (top) and angiotensin II conditions (bottom); lines illustrate the increasing delay of excitation spread between the two conditions. c Contour plots for the two conditions also show the decreased conduction velocity (0.18 m/s vs. 0.13 m/s) in the presence of angiotensin II. d Stimulation by angiotensin II (1 μmol/L) reduced conduction velocity, but 10 min of rest in angiotensin II-free medium resulted in full recovery. When wortmannin (20 μmol/L) was added to the medium during rest, recovery was prevented. Statistical analysis was performed by ANOVA with Tukey’s multiple comparison test post hoc. *** signifies P<0.001 versus control and ### P<0.001 versus angiotensin II/rest
Discussion
The aim of this study was to investigate whether PIP2 levels affect intercellular coupling in cardiac tissue. We provide evidence that agonists acting on Gαq-coupled receptors reduce intercellular communication by lowering the levels of PIP2 in the cell membrane. This is a novel mechanism that may partly explain the uncoupling observed during acute ischemia and other conditions where PIP2-levels are reduced. The resulting reduction in conduction velocity could increase the susceptibility to cardiac arrhythmia.
All tested conditions expected to change the level of PIP2 were associated with corresponding changes in intercellular coupling. This was the case not only for interventions expected to reduce PIP2 such as prolonged wortmannin treatment or stimulation of Gαq-coupled receptors, but also for hypertonic shock which has been shown to increase PIP2 [38] via stimulation of PIP5 kinase [56]. These independent approaches indicate that PIP2 regulates intercellular coupling.
Stimulation of Gαq-coupled AT1 and α1-adrenergic receptors reduced intercellular coupling in a time-dependent manner. Maximal reduction was seen after 10 min of incubation and was of the same magnitude as observed when PIP2 was reduced by wortmannin. The partial recovery beyond the 10-min time point likely reflects internalization of receptors or activation of compensatory resynthesis of PIP2. In the case of angiotensin II, its low stability may also have reduced its effective concentration over time.
The inhibition of intercellular coupling after stimulation of Gαq-coupled receptors was likely caused by reduced PIP2 levels in the plasma membrane because recovery from inhibition of intercellular coupling and conduction velocity was sensitive to the PI4-kinase inhibitor wortmannin. Wortmannin was originally found to be an inhibitor of the PI3-kinase when used in concentrations in the nanomolar range [42]. However, it was later discovered that higher concentrations led to inhibition of the PI4-kinase [37]. This kinase is essential in the synthesis of PIP2 and mediates phosphorylation of PI in the 4′ position, generating PIP, which is further phosphorylated to PIP2. At lower concentrations, wortmannin inhibits only the PI3-kinase, an essential component in the activation of the akt/PKB signaling cascade. However, the inhibitory effect of wortmannin was not mediated by PI3-kinase, as low concentrations of the compound did not affect intercellular coupling. Wortmannin inhibits other proteins besides the PI3 and -4 kinases. Myosin light chain kinase and Polo-like kinase can be inhibited by wortmannin; but as for PI3 kinase, they are inhibited by low concentrations [2] and therefore unlikely to be involved.
Wortmannin has been used extensively to document the involvement of PIP2 in the regulation of many membrane proteins. In some of these studies, other approaches, such as application of PIP2 to the inside of macropatches [21, 57] or expression of components of the PIP2 metabolic pathways [48, 54], confirmed the involvement of PIP2. Moolenaar and coworkers recently showed that activation of Gαq-coupled receptors in Rat-1 fibroblasts reduced Cx43-mediated intercellular coupling [51]. This reduction was dependent on a ZO-1 mediated interaction between Cx43 and PLC-β3. Furthermore, they showed that specific activation of PIP5-phosphatase reduced coupling and that overexpression of PIP5-kinase reduced the ability of Gαq-coupled receptors to inhibit intercellular coupling. The study thus supports the concept that PIP2 regulates intercellular coupling, which is further substantiated by the present study on intercellular coupling in cardiac myocytes.
The suggestion that the sole effect of wortmannin is prevention of the resynthesis of PIP2 has important implications. The lack of recovery after angiotensin II stimulation in the presence of wortmannin indicates that the inhibition of intercellular coupling is mainly due to a reduction of PIP2. This is a surprising conclusion because of the large volume of evidence that activation of PKC leads to hyperphosphorylation of Cx43 and inhibition of coupling measured by dye diffusion (for review see [27]).
Originally, uncoupling due to PKC activation was shown using phorbol esters as activators [18], and many subsequent studies have used this approach. Phorbol esters such as TPA activate a number of PKC subtypes [32] by mimicking DAG binding. The reduction in dye transfer after stimulation with TPA correlates with phosphorylation of serine 368 in the Cx43 C-terminus, and mutation of this serine to alanine abolishes the effect of TPA [28].
We found that stimulation via specific Gαq-coupled receptors reduces intercellular coupling independently of PKC, as the response to angiotensin II and noradrenaline was unaffected by PKC inhibition. The reason for this lack of PKC involvement could be that stimulation via specific receptors leads to a subthreshold level of activation of PKC or that these receptors activate specific subtypes of PKC that either do not affect intercellular coupling or have opposing effects. Studies have shown that different PKC subtypes may exert different effects on intercellular coupling. Along these lines, PKC-α reduces intercellular coupling in cardiac cells after stimulation by growth factors [15], whereas PKC-α increases intercellular coupling after stimulation with anti-arrhythmic peptide analogs [14, 53]. Lack of PKC involvement after stimulation of Gαq-coupled receptors was also reported by Postma et al. [41].
Electroporation was performed in calcium-free solution to avoid calcium overload. This means that calcium was likely to be low during the actual measurement of coupling. This does, however, not exclude that IP3 could increase calcium by activation of IP3 receptors in the sarcoplasmic reticulum. Other studies show that calcium increases in cultured neonatal rat cardiac myocytes after stimulation with, for example, angiotensin II [24, 50]. Lurtz and Louis [31] showed that the effect of calcium is mediated by calmodulin. Therefore, we tested the possible involvement of calcium by inhibiting calmodulin with CDZ. This did not affect the response to angiotensin II, and therefore, we conclude that calcium is not involved in the observed inhibition of intercellular coupling.
Activation of Gαq-coupled receptors can activate release of AA either directly by activating PLA2 or indirectly by producing DAG which can be further metabolized to AA in a two-step reaction by diacylglycerol lipase and mono-acylglycerol lipase [9]. AA directly inhibits intercellular coupling [19, 33] and could represent an alternative to PIP2 as a mediator of the response to Gαq-coupled receptors. However, inhibition of PLA2 and DAG lipase did not affect the reductions in intercellular coupling seen after stimulation with angiotensin II or noradrenaline, making it unlikely that AA plays a role in the response.
Previous studies have shown that the ability to pass dyes and current do not necessarily correlate [17, 26]. In the present study, however, changes in intercellular coupling after angiotensin II stimulation, measured as dye diffusion, were accompanied by a conduction velocity reduction, indicating that electrical coupling was also reduced. This is supported by a study by De Mello showing that acute angiotensin II stimulation reduces electrical coupling in freshly isolated hamster cardiomyocytes [12].
Decreased conduction velocity can also be induced by inhibition of voltage-dependent sodium or calcium channels. The constant beating frequency of the monolayers even in the presence of angiotensin II implies that voltage-dependent ion channels are not affected by agonist stimulation. Although we suspect that noradrenaline would decrease conduction velocity, as angiotensin II does, this was not tested in the current study.
An acute and massive stimulation of Gαq receptors occurs during ischemia, where reversal of uptake mechanism for noradrenaline leads to liberation of large amounts of this agonist. This condition could be worsened by release of other substances such as endothelin, ATP, and thrombin. On top of these agonists that can potentially reduce PIP2, other factors such as lysophosphatidylcholine [29] and oxidative stress [35] reduces PIP2 levels. The risk of reentry arrhythmia is increased whenever the wavelength (conduction velocity×refractory period) of the excitation is reduced. We did not investigate the effect of agonist stimulation on refractory period, but the slowing of conduction velocity would decrease the wavelength. If this occurs, it could increase the risk that the heart can harbor a reentrant circuit. Also, in the case of adrenergic stimulation during phase 1b, Verkerk and coworkers suggested that the reduced coupling enhances development of afterdepolarizations [52]. This way, we speculate that changes in intercellular coupling downstream of PIP2 changes may also contribute to occurrence of phase 1b arrhythmias by ectopic mechanisms.
We conclude that stimulation of Gαq-coupled receptors reduces intercellular coupling in cultured neonatal rat cardiac myocytes by reducing levels of PIP2 in the plasma membrane. Whether this mechanism plays a physiological role in cardiac cells in situ remains to be investigated. But if so, it could play an important role in several pathological conditions where the risk of arrhythmias is increased by impaired coupling.
Acknowledgments
We thank Professor Donald Marsh for valuable suggestions and comments to the manuscript. Ninna Buch Petersen and Trine Eidsvoll are thanked for excellent technical assistance.
Grants This work was supported by: The Danish National Research Foundation; The John and Birthe Meyer Foundation; The Danish Natural and Health Sciences Research Councils; The Velux Foundation; The Danish Heart Association; The Novo Nordisk Foundation; The A.P. Møller Foundation.
Footnotes
Disclosures There are no conflicts of interest.
Contributor Information
Johannes P. Hofgaard, The Danish National Research Foundation Centre for Cardiac Arrhythmia and Department of Biomedical Sciences, Faculty of Health Sciences, University of Copenhagen, Copenhagen, Denmark
Kathrin Banach, Department of Cellular and Molecular Physiology, Stricht School of Medicine, Loyola University Chicago, Chicago, IL, USA.
Sarah Mollerup, The Danish National Research Foundation Centre for Cardiac Arrhythmia and Department of Biomedical Sciences, Faculty of Health Sciences, University of Copenhagen, Copenhagen, Denmark.
Helene Korvenius Jørgensen, The Danish National Research Foundation Centre for Cardiac Arrhythmia and Department of Biomedical Sciences, Faculty of Health Sciences, University of Copenhagen, Copenhagen, Denmark.
Søren Peter Olesen, The Danish National Research Foundation Centre for Cardiac Arrhythmia and Department of Biomedical Sciences, Faculty of Health Sciences, University of Copenhagen, Copenhagen, Denmark.
Niels-Henrik Holstein-Rathlou, The Danish National Research Foundation Centre for Cardiac Arrhythmia and Department of Biomedical Sciences, Faculty of Health Sciences, University of Copenhagen, Copenhagen, Denmark.
Morten Schak Nielsen, Email: schak@mfi.ku.dk, The Danish National Research Foundation Centre for Cardiac Arrhythmia and Department of Biomedical Sciences, Faculty of Health Sciences, University of Copenhagen, Copenhagen, Denmark. The Danish National Research Foundation Centre for Cardiac Arrhythmia and Department of Biomedical Sciences, Blegdamsvej 3C, DK-2200 Copenhagen, Denmark.
References
- 1.Axelsen LN, Stahlhut M, Mohammed S, Larsen BD, Nielsen MS, Holstein-Rathlou NH, Andersen S, Jensen ON, Hennan JK, Kjolbye AL. Identification of ischemia-regulated phosphorylation sites in connexin43: A possible target for the antiarrhythmic peptide analogue rotigaptide (ZP123) J Mol Cell Cardiol. 2006;40:790–798. doi: 10.1016/j.yjmcc.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banach K, Halbach MD, Hu P, Hescheler J, Egert U. Development of electrical activity in cardiac myocyte aggregates derived from mouse embryonic stem cells. Am J Physiol Heart Circ Physiol. 2003;284:H2114–H2123. doi: 10.1152/ajpheart.01106.2001. [DOI] [PubMed] [Google Scholar]
- 4.Beardslee MA, Lerner DL, Tadros PN, Laing JG, Beyer EC, Yamada KA, Kleber AG, Schuessler RB, Saffitz JE. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res. 2000;87:656–662. doi: 10.1161/01.res.87.8.656. [DOI] [PubMed] [Google Scholar]
- 5.Bian J, Cui J, McDonald TV. HERG K(+) channel activity is regulated by changes in phosphatidyl inositol 4,5-bisphosphate. Circ Res. 2001;89:1168–1176. doi: 10.1161/hh2401.101375. [DOI] [PubMed] [Google Scholar]
- 6.Burt JM. Block of intercellular communication: interaction of intracellular H + and Ca2+ Am J Physiol. 1987;253:C607–C612. doi: 10.1152/ajpcell.1987.253.4.C607. [DOI] [PubMed] [Google Scholar]
- 7.Calloe K, Nielsen MS, Grunnet M, Schmitt N, Jorgensen NK. KCNQ channels are involved in the regulatory volume decrease response in primary neonatal rat cardiomyocytes. Biochim Biophys Acta. 2007;1773:764–773. doi: 10.1016/j.bbamcr.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Carmeliet E. Cardiac ionic currents and acute ischemia: from channels to arrhythmias. Physiol Rev. 1999;79:917–1017. doi: 10.1152/physrev.1999.79.3.917. [DOI] [PubMed] [Google Scholar]
- 9.Chau LY, Tai HH. Release of arachidonate from diglyceride in human platelets requires the sequential action of a diglyceride lipase and a monoglyceride lipase. Biochem Biophys Res Commun. 1981;100:1688–1695. doi: 10.1016/0006-291x(81)90713-0. [DOI] [PubMed] [Google Scholar]
- 10.Clerk A, Sugden PH. Regulation of phospholipases C and D in rat ventricular myocytes: stimulation by endothelin-1, bradykinin and phenylephrine. J Mol Cell Cardiol. 1997;29:1593–1604. doi: 10.1006/jmcc.1997.0395. [DOI] [PubMed] [Google Scholar]
- 11.Davidson JS, Baumgarten IM. Glycyrrhetinic acid derivatives: a novel class of inhibitors of gap-junctional intercellular communication. Structure–activity relationships. J Pharmacol Exp Ther. 1988;246:1104–1107. [PubMed] [Google Scholar]
- 12.De Mello WC. Renin–angiotensin system and cell communication in the failing heart. Hypertension. 1996;27:1267–1272. doi: 10.1161/01.hyp.27.6.1267. [DOI] [PubMed] [Google Scholar]
- 13.Dent MR, Dhalla NS, Tappia PS. Phospholipase C gene expression, protein content, and activities in cardiac hypertrophy and heart failure due to volume overload. Am J Physiol Heart Circ Physiol. 2004;287:H719–H727. doi: 10.1152/ajpheart.01107.2003. [DOI] [PubMed] [Google Scholar]
- 14.Dhein S, Larsen BD, Petersen JS, Mohr FW. Effects of the new antiarrhythmic peptide ZP123 on epicardial activation and repolarization pattern. Cell Commun Adhes. 2003;10:371–378. doi: 10.1080/cac.10.4-6.371.378. [DOI] [PubMed] [Google Scholar]
- 15.Doble BW, Ping P, Kardami E. The ε subtype of protein kinase C is required for cardiomyocyte connexin-43 phosphorylation. Circ Res. 2000;86:293–301. doi: 10.1161/01.res.86.3.293. [DOI] [PubMed] [Google Scholar]
- 16.Egert U, Knott T, Schwarz C, Nawrot M, Brandt A, Rotter S, Diesmann M. MEA-Tools: an open source toolbox for the analysis of multi-electrode data with MATLAB. J Neurosci Methods. 2002;117:33–42. doi: 10.1016/s0165-0270(02)00045-6. [DOI] [PubMed] [Google Scholar]
- 17.Ek-Vitorin JF, King TJ, Heyman NS, Lampe PD, Burt JM. Selectivity of connexin 43 channels is regulated through protein kinase C-dependent phosphorylation. Circ Res. 2006;98:1498–1505. doi: 10.1161/01.RES.0000227572.45891.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enomoto T, Yamasaki H. Phorbol ester-mediated inhibition of intercellular communication in BALB/c 3T3 cells: relationship to enhancement of cell transformation. Cancer Res. 1985;45:2681–2688. [PubMed] [Google Scholar]
- 19.Fluri GS, Rudisuli A, Willi M, Rohr S, Weingart R. Effects of arachidonic acid on the gap junctions of neonatal rat heart cells. Pflugers Arch. 1990;417:149–156. doi: 10.1007/BF00370692. [DOI] [PubMed] [Google Scholar]
- 20.Halbach M, Egert U, Hescheler J, Banach K. Estimation of action potential changes from field potential recordings in multicellular mouse cardiac myocyte cultures. Cell Physiol Biochem. 2003;13:271–284. doi: 10.1159/000074542. [DOI] [PubMed] [Google Scholar]
- 21.Hilgemann DW, Ball R. Regulation of cardiac Na+, Ca2+ exchange and KATP potassium channels by PIP2. Science. 1996;273:956–959. doi: 10.1126/science.273.5277.956. [DOI] [PubMed] [Google Scholar]
- 22.Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbetagamma. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 23.Kaplinsky E, Ogawa S, Balke CW, Dreifus LS. Two periods of early ventricular arrhythmia in the canine acute myocardial infarction model. Circulation. 1979;60:397–403. doi: 10.1161/01.cir.60.2.397. [DOI] [PubMed] [Google Scholar]
- 24.Kem DC, Johnson EI, Capponi AM, Chardonnens D, Lang U, Blondel B, Koshida H, Vallotton MB. Effect of angiotensin II on cytosolic free calcium in neonatal rat cardiomyocytes. Am J Physiol. 1991;261:C77–C85. doi: 10.1152/ajpcell.1991.261.1.C77. [DOI] [PubMed] [Google Scholar]
- 25.Kleber AG, Riegger CB, Janse MJ. Electrical uncoupling and increase of extracellular resistance after induction of ischemia in isolated, arterially perfused rabbit papillary muscle. Circ Res. 1987;61:271–279. doi: 10.1161/01.res.61.2.271. [DOI] [PubMed] [Google Scholar]
- 26.Kwak BR, van Veen TA, Analbers LJS, Jongsma HJ. TPA increases conductance but decreases permeability in neonatal rat cardiomyocyte gap junction channels. Exp Cell Res. 1995;220:456–463. doi: 10.1006/excr.1995.1337. [DOI] [PubMed] [Google Scholar]
- 27.Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol. 2004;36:1171–1186. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lampe PD, TenBroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J Cell Biol. 2000;149:1503–1512. doi: 10.1083/jcb.149.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu SY, Yu CH, Hays JA, Panagia V, Dhalla NS. Modification of heart sarcolemmal phosphoinositide pathway by lysophosphatidylcholine. Biochim Biophys Acta. 1997;1349:264–274. doi: 10.1016/s0005-2760(97)00142-2. [DOI] [PubMed] [Google Scholar]
- 30.Loussouarn G, Park KH, Bellocq C, Baro I, Charpentier F, Escande D. Phosphatidylinositol-4,5-bisphosphate, PIP2, controls KCNQ1/KCNE1 voltage-gated potassium channels: a functional homology between voltage-gated and inward rectifier K+ channels. EMBO J. 2003;22:5412–5421. doi: 10.1093/emboj/cdg526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lurtz MM, Louis CF. Intracellular calcium regulation of connexin43. Am J Physiol Cell Physiol. 2007;293:C1806–C1813. doi: 10.1152/ajpcell.00630.2006. [DOI] [PubMed] [Google Scholar]
- 32.Mackay K, Mochly-Rosen D. Localization, anchoring, and functions of protein kinase C isozymes in the heart. J Mol Cell Cardiol. 2001;33:1301–1307. doi: 10.1006/jmcc.2001.1400. [DOI] [PubMed] [Google Scholar]
- 33.Massey KD, Minnich BN, Burt JM. Arachidonic acid and lipoxygenase metabolites uncouple neonatal rat cardiac myocyte pairs. Am J Physiol. 1992;263:C494–C501. doi: 10.1152/ajpcell.1992.263.2.C494. [DOI] [PubMed] [Google Scholar]
- 34.Maurer P, Weingart R. Cell pairs isolated from adult guinea pig and rat hearts: effects of [Ca2+]i on nexal membrane resistance. Pflugers Arch. 1987;409:394–402. doi: 10.1007/BF00583793. [DOI] [PubMed] [Google Scholar]
- 35.Mesaeli N, Tappia PS, Suzuki S, Dhalla NS, Panagia V. Oxidants depress the synthesis of phosphatidylinositol 4, 5-bisphosphate in heart sarcolemma. Arch Biochem Biophys. 2000;382:48–56. doi: 10.1006/abbi.2000.2012. [DOI] [PubMed] [Google Scholar]
- 36.Mouton R, Huisamen B, Lochner A. The effect of ischaemia and reperfusion on sarcolemmal inositol phospholipid and cytosolic inositol phosphate metabolism in the isolated perfused rat heart. Mol Cell Biochem. 1991;105:127–135. doi: 10.1007/BF00227752. [DOI] [PubMed] [Google Scholar]
- 37.Nakanishi S, Catt KJ, Balla T. A wortmannin-sensitive phosphatidylinositol 4-kinase that regulates hormone-sensitive pools of inositolphospholipids. Proc Natl Acad Sci U S A. 1995;92:5317–5321. doi: 10.1073/pnas.92.12.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nasuhoglu C, Feng S, Mao Y, Shammat I, Yamamato M, Earnest S, Lemmon M, Hilgemann DW. Modulation of cardiac PIP2 by cardioactive hormones and other physiologically relevant interventions. Am J Physiol Cell Physiol. 2002;283:C223–C234. doi: 10.1152/ajpcell.00486.2001. [DOI] [PubMed] [Google Scholar]
- 39.Noma A, Tsuboi N. Dependence of junctional conductance on proton, calcium and magnesium ions in cardiac paired cells of guinea-pig. J Physiol (Lond) 1987;382:193–211. doi: 10.1113/jphysiol.1987.sp016363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penny WJ. The deleterious effects of myocardial catecholamines on cellular electrophysiology and arrhythmias during ischaemia and reperfusion. Eur Heart J. 1984;5:960–973. doi: 10.1093/oxfordjournals.eurheartj.a061616. [DOI] [PubMed] [Google Scholar]
- 41.Postma FR, Hengeveld T, Alblas J, Giepmans BN, Zondag GC, Jalink K, Moolenaar WH. Acute loss of cell-cell communication caused by G protein-coupled receptors: a critical role for c-Src. J Cell Biol. 1998;140:1199–1209. doi: 10.1083/jcb.140.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Powis G, Bonjouklian R, Berggren MM, Gallegos A, Abraham R, Ashendel C, Zalkow L, Matter WF, Dodge J, Grindey G. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 1994;54:2419–2423. [PubMed] [Google Scholar]
- 43.Raptis LH, Brownell HL, Firth KL, MacKenzie LW. A novel technique for the study of intercellular, junctional communication: electroporation of adherent cells on a partly conductive slide. DNA Cell Biol. 1994;13:963–975. doi: 10.1089/dna.1994.13.963. [DOI] [PubMed] [Google Scholar]
- 44.Sheridan DJ, Penkoske PA, Sobel BE, Corr PB. Alpha adrenergic contributions to dysrhythmia during myocardial ischemia and reperfusion in cats. J Clin Invest. 1980;65:161–171. doi: 10.1172/JCI109647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simpson P, Savion S. Differentiation of rat myocytes in single cell cultures with and without proliferating nonmyocardial cell. Cross-striations, ultrastructure, and chronotropic response to isoproterenol. Circ Res. 1982;50:101–116. doi: 10.1161/01.res.50.1.101. [DOI] [PubMed] [Google Scholar]
- 46.Smith WT, Fleet WF, Johnson TA, Engle CL, Cascio WE. The Ib phase of ventricular arrhythmias in ischemic in situ porcine heart is related to changes in cell-to-cell electrical coupling. Experimental Cardiology Group, University of North Carolina. Circulation. 1995;92:3051–3060. doi: 10.1161/01.cir.92.10.3051. [DOI] [PubMed] [Google Scholar]
- 47.Suh BC, Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr Opin Neurobiol. 2005;15:370–378. doi: 10.1016/j.conb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Suh BC, Inoue T, Meyer T, Hille B. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science. 2006;314:1454–1457. doi: 10.1126/science.1131163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tappia PS, Liu SY, Shatadal S, Takeda N, Dhalla NS, Panagia V. Changes in sarcolemmal PLC isoenzymes in postinfarct congestive heart failure: partial correction by imidapril. Am J Physiol. 1999;277:H40–H49. doi: 10.1152/ajpheart.1999.277.1.H40. [DOI] [PubMed] [Google Scholar]
- 50.Touyz RM, Fareh J, Thibault G, Tolloczko B, Lariviere R, Schiffrin EL. Modulation of Ca2+ transients in neonatal and adult rat cardiomyocytes by angiotensin II and endothelin-1. Am J Physiol. 1996;270:H857–H868. doi: 10.1152/ajpheart.1996.270.3.H857. [DOI] [PubMed] [Google Scholar]
- 51.van Zeijl L, Ponsioen B, Giepmans BN, Ariaens A, Postma FR, Varnai P, Balla T, Divecha N, Jalink K, Moolenaar WH. Regulation of connexin43 gap junctional communication by phosphatidylinositol 4,5-bisphosphate. J Cell Biol. 2007;177:881–891. doi: 10.1083/jcb.200610144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verkerk AO, Veldkamp MW, Coronel R, Wilders R, van Ginneken AC. Effects of cell-to-cell uncoupling and catecholamines on Purkinje and ventricular action potentials: implications for phase-1b arrhythmias. Cardiovasc Res. 2001;51:30–40. doi: 10.1016/s0008-6363(01)00246-2. [DOI] [PubMed] [Google Scholar]
- 53.Weng S, Lauven M, Schaefer T, Polontchouk L, Grover R, Dhein S. Pharmacological modification of gap junction coupling by an antiarrhythmic peptide via protein kinase C activation. FASEB J. 2002;16:1114–1116. doi: 10.1096/fj.01-0918fje. [DOI] [PubMed] [Google Scholar]
- 54.Winks JS, Hughes S, Filippov AK, Tatulian L, Abogadie FC, Brown DA, Marsh SJ. Relationship between membrane phosphatidylinositol-4,5-bisphosphate and receptor-mediated inhibition of native neuronal M channels. J Neurosci. 2005;25:3400–3413. doi: 10.1523/JNEUROSCI.3231-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu J, McHowat J, Saffitz JE, Yamada KA, Corr PB. Inhibition of gap junctional conductance by long-chain acylcarnitines and their preferential accumulation in junctional sarcolemma during hypoxia. Circ Res. 1993;72:879–889. doi: 10.1161/01.res.72.4.879. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto M, Chen MZ, Wang YJ, Sun HQ, Wei Y, Martinez M, Yin HL. Hypertonic stress increases phosphatidylinositol 4,5-bisphosphate levels by activating PIP5KIbeta. J Biol Chem. 2006;281:32630–32638. doi: 10.1074/jbc.M605928200. [DOI] [PubMed] [Google Scholar]
- 57.Zhang H, He C, Yan X, Mirshahi T, Logothetis DE. Activation of inwardly rectifying K+ channels by distinct PtdIns (4,5)P2 interactions. Nat Cell Biol. 1999;1:183–188. doi: 10.1038/11103. [DOI] [PubMed] [Google Scholar]
- 58.Ziegelhoffer A, Tappia PS, Mesaeli N, Sahi N, Dhalla NS, Panagia V. Low level of sarcolemmal phosphatidylinositol 4,5-bisphosphate in cardiomyopathic hamster (UM-X7.1) heart. Cardiovasc Res. 2001;49:118–126. doi: 10.1016/s0008-6363(00)00209-1. [DOI] [PubMed] [Google Scholar]