Abstract
Objective
To propose a unifying set of definitions for prescription adherence research utilizing electronic health record prescribing databases, prescription dispensing databases, and pharmacy claims databases and to provide a conceptual framework to operationalize these definitions consistently across studies.
Methods
We reviewed recent literature to identify definitions in electronic database studies of prescription-filling patterns for chronic oral medications. We then develop a conceptual model and propose standardized terminology and definitions to describe prescription-filling behavior from electronic databases.
Results
The conceptual model we propose defines two separate constructs: medication adherence and persistence. We define primary and secondary adherence as distinct sub-types of adherence. Metrics for estimating secondary adherence are discussed and critiqued, including a newer metric (New Prescription Medication Gap measure) that enables estimation of both primary and secondary adherence.
Discussion
Terminology currently used in prescription adherence research employing electronic databases lacks consistency. We propose a clear, consistent, broadly applicable conceptual model and terminology for such studies. The model and definitions facilitate research utilizing electronic medication prescribing, dispensing, and/or claims databases and encompasses the entire continuum of prescription-filling behavior.
Conclusion
Employing conceptually clear and consistent terminology to define medication adherence and persistence will facilitate future comparative effectiveness research and meta-analytic studies that utilize electronic prescription and dispensing records.
Keywords: medication adherence, medication persistence, medication discontinuation, refill compliance, refill persistence, administrative, database, electronic health record, computerized medical record systems
BACKGROUND
The construct of medication adherence comprises a set of inter-related health behaviors.1 One of these behaviors, the act of filling a medication prescription, can be estimated objectively using electronic databases such as electronic insurance claims or pharmacy dispensing databases. Numerous studies have assessed patterns of prescription refills among individuals who obtain at least one fill of a medication. 2-11 Over the last several years, advances in electronic prescribing and medication order entry within the electronic health record (EHR) have expanded our ability to assess whether or not patients obtain their initial prescriptions, another of the health behaviors within the adherence cluster.12-17 Although EHR prescribing databases vary in whether or not they contain information on dispensed prescriptions, their use can enhance widely available pharmacy dispensing and pharmacy insurance claims database to better describe the sequence of behaviors that are necessary to achieve desirable treatment outcomes.
The addition of this new source of information about prescription-filling emphasizes the long-recognized lack of uniform terminology and precise definitions to describe prescription-filling behavior.18,19 Organizations such as the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) and the World Health Organization (WHO) have put forward definitions of adherence and persistence; however these definitions did not encompass the entire range of data sources for adherence. Further, existing definitions do not define terms consistently, do not address the issues of medications that are prescribed but not dispensed, and do not address behaviors such as medication discontinuation.20,21 Finally, adherence-related publications sometimes neither define their terms carefully nor explain their choice of metrics, leaving readers to make assumptions about why metrics were chosen and how they were calculated.
Applying a uniform conceptual framework to electronic database studies of medication adherence and employing standardized terminology and definitions within that framework will enhance both rigor and generalizability of medication adherence research, as well as the ability of researchers to formally compare the findings of studies in systematic reviews. In this paper we propose a unifying set of definitions for use when studying prescription-filling for chronic oral medications in EHR, in pharmacy insurance claims databases, and in pharmacy dispensing databases and provide a framework to operationalize these definitions consistently across studies.
METHODS
Literature Review of Chronic Oral Medication Adherence Definitions in Electronic Database Studies
The purpose of this literature review was to inform development of the conceptual model by identifying definitions and metrics for terms such as medication adherence, persistence, and discontinuation used in published studies based on electronic databases, and the rationale provided by the authors of these studies for selecting definitions and measurement tools. To accomplish this, two authors of the current paper (JLK and MAR) searched published literature to identify EHR, pharmacy dispensing, and pharmacy insurance claims-based studies where medication adherence, persistence, and/or discontinuation was stated as a primary outcome. Although the purpose of this review was narrowly focused and a comprehensive literature review was neither intended nor undertaken, the authors utilized search techniques from the literature on systematic reviews. Retrospective observational studies, randomized controlled trials, and non-randomized comparative studies were included. The National Library of Medicine’s Medical Subject Headings (MeSH) keyword nomenclature developed for MEDLINE® and adapted for use in other databases was employed. The search was limited to studies published in English from January 1, 2000 through December 15, 2011, and to articles indexed in PubMed, the Cumulative Index to Nursing and Allied Health Literature (CINAHL®), Google Scholar, or the Web of Science. MeSH terms applied during the preliminary search were revised and refined based on expert input from a medical librarian and review of the MeSH terms identified from relevant publication titles retrieved in the preliminary search.
In the medical literature, the word adherence is applied to a broader variety of behaviors and regulatory topics than the focus of this work. Thus, studies that focused on adherence to or compliance with lifestyle, guidelines, exercise, diet, preventative screenings or follow-up, dental screenings/procedures, radiation/imaging, hospitalization/surgery, quality of care recommendations, medication reconciliation, drug administration/efficacy/adverse effects, device use, medical visits recommendations, isolation or hand washing precautions, cognitive/behavioral therapies, vaccination/immunization, and Health Insurance Portability and Accountability Act (HIPAA) regulations were excluded. Moreover, we excluded studies that used the degree of risk factor control as the indicator of adherence, rather than directly measuring medication use or non-use.
The title of each citation retrieved was reviewed and the abstract retrieved if the title indicated adherence or persistence to, or discontinuation of, chronic oral medications was the focus. Abstracts of articles dealing with validation of adherence measures used in electronic database studies were also included. Potentially relevant citations were imported into an electronic database. Abstracts of these citations were reviewed and the full texts of relevant articles were retrieved and read by the two individuals that conducted the literature search (MAR and JLK). As articles were read, the definitions and terminology used for adherence, persistence, and/or discontinuation in the articles were extracted and cataloged.
This literature scan retrieved 2484 articles, 315 (13%) of which utilized electronic data sources that included as the primary outcome chronic oral medication adherence or that evaluated adherence metrics. Studying the definitions and terminology we extracted from these publications confirmed our subjective impression of variation, inconsistency, and confusion in the terminology used. It also demonstrated that terms were used imprecisely and interchangeably to refer to different constructs in different papers. This background work reinforced the fact that a conceptual model of and uniform definitions for the adherence continuum were lacking. It also informed the standardized terminology and definitions we developed (Tables 1 and 2).
TABLE 1.
Recommendations for Standardized Terminology and Definitions of Medication Adherence and Persistence for Electronic Data
| Terminology for Medication Adherence or Persistence Construct |
Recommended Foundational Definition |
Components of Definition Requiring Study-Specific Decisions to Operationalize |
Examples of Other Terms Previously Applied to the Same Construct * |
|---|---|---|---|
|
Adherence
| |||
| Primary Adherence6,15 | A new prescription was dispensed (“filled” or sold) within a defined number of days after the medication was ordered |
Definition of “new prescription:” How far to look back to determine if the medication has been previously dispensed; 12 or 24 months commonly used Definition of “defined number of days:” The prescription must be dispensed within “x” days after the order was written, with “x” commonly60 or 30 days 6,13,17 |
First-fill adherence13 Adoption32 Initiation33 |
| Primary Non-adherence15- 17 |
Failure to have a new prescription dispensed (did not pick up the first prescription) within a defined number of days after the medication was ordered |
Definition of new prescription(see Primary Adherence) Definition of “defined number of days:” The prescription must not be dispensed within “x” days after order was written, with “x” commonly 60 or 30 days6,13,17 |
Dispensation delay34 |
| Secondary Adherence6 | Adherence measured among patients with Primary Adherence) and who have the prescription refilled within a defined number of days following the end of the days’ supply of the first dispensing. Secondary adherence is usually measured over 6 or 12 months or longer† |
Definition of “prescription refill…defined number of days:” The prescription must be refilled within “x” days after exhaustion of the days’ supply of the first dispensing. Sometimes referred to as the grace period |
Ongoing dispensing/use17 Implementation31 |
| Adequate Secondary Adherence |
Secondary adherence with either an overall a) gap in days of medication possession not exceeding 20% of the days between the date of initial dispensing and the date of the end of the measurement period (gap measures) or b) number of days of medication possession of no less than 80%of the days between the date of initial dispensing and date of the end of the measurement period (possession measures) |
By convention – only rarely with supporting evidence – the following cut-points have commonly been used: Gap: CMG < 20% NPMG < 20% Possession: MPR >= 80% PDC >= 80% |
Adequate adherence35 Adherence33 Ongoing adherence6 Compliance |
| Inadequate Secondary Adherence |
Secondary adherence with either an overall a) gap in days of medication possession exceeding 20% of the days between the date of initial dispensing and the date of the end of the measurement period or b) number of days of medication possession of less than 80% of the days between the date of initial dispensing and the date of the end of the measurement period |
By convention – only rarely with supporting evidence – the following cut-points have been used: CMG > 20% to < 60% or upper cut- point can be as high as 100% NPMG > 20% to < 60% or upper cut-point can be as high as < 100% MPR > 40% to < 80% or lower cut- point can be as low as 0%) PDC > 40% to < 80% or lower cut- point can be as low as 0%) Sometimes further subcategorized into MPR or PDC ≥ 60 – 80%, ≥ 40 – 60%, and < 40% |
Partial adherence Poor adherence36 Inadequate adherence Non-compliance |
|
| |||
|
Persistence
| |||
| Early-stage Persistence6 | A new prescription was dispensed (Primary Adherence) and at least one refill of that prescription was dispensed over a time period consistent with (implying) current use of the drug |
Definition of time period allowed or considered between the new prescription dispensing and the one refill. |
Point-of-Time Persistence Early Persistence Persistence37 |
| Early-stage Non- persistence10,38 |
Failure to have the new prescription refilled over a time period consistent with current use of the drug |
Definition of time period allowed or considered between the new prescription dispensing and the one refill. |
Early Non-persistence |
| Later-stage Persistence | Two or more refills (i.e., the new prescription was dispensed and at least 2 refills of that prescription were dispensed) over a time period consistent with current use of the drug. The time period can span several refills that occur over 6 months, 12 months, or longer |
Definition of time periods allowed or considered between refills; can include definition of time period allowed after last refill in the measurement period |
Second stage persistence6 Refill compliance Persistent/Persistence31,38- 43 |
| Later-stage Non-persistence |
Failure to have two or more refills over a time period consistent with current use of the drug. Can imply either that the patient has discontinued the medication or that usage is inconsistent over time |
Definition of time periods allowed or considered between refills; can include definition of time period allowed after last refill in the measurement period |
Second stage non- persistence Suboptimal persistence Not persistent Non-persistence34,44 |
|
| |||
|
Discontinuation
| |||
| Discontinuation32,45-48 | Failure to have a medication dispensing within a defined number of days after exhaustion of the days’ supply of the previous dispensing (often includes exhaustion of any stockpiled medication accumulated from previous dispensings) |
Definition of “defined number of days after exhaustion of the days’ supply of the previous dispensing;” 180 days often used |
Termination40 End of therapy |
The terms listed in this column are provided as examples of terminology used in the published literature. The terms and their use within the cited publication(s) can be quite different from the Recommended Foundational Definition presented here. In some cases these example terms can have been used imprecisely or were incorrectly applied in the cited publication(s).
Secondary adherence can only be measured among patients who have at least early-stage persistence.
TABLE 2.
Metrics for Evaluating Medication Adherence and Persistence using Electronic Health Data
| Type and Name of Measure | Description and Calculation | Strengths | Weaknesses |
|---|---|---|---|
|
Based on Medication Possession
* | |||
| Medication Possession Ratio (MPR) and Medication Possession Ratio Modified (MPRm)18,23,26,28 |
Estimate of proportion (or percentage) of days’ supply obtained during a specified time period or over a period of refill intervals. Ratio of total days’ supply to number of days in observation period Variations:
Mathematically similar to MRA and CMA |
|
|
| Medication Refill Adherence (MRA)23,28 |
Total days’ supply divided by number of days in observation period and multiplied by 100 to obtain percent Mathematically similar formulas include MPR and CMA |
|
|
| MEDSUM49 | Number of daily doses dispensed in a period divided by number of days in period; if applied continuously, measures continuous med acquisition. |
|
|
| Proportion of Days Covered (PDC)18,23,26-28 |
Total number of days’ supply dispensed during specified observation period divided by number of days in patient’s observation period (i.e., this denominator is number of days between first dispensing during observation period and end of the observation period) multiplied by 100 to obtain percent; capped at 1 Rather than summing days’ supply across multiple drugs, should create time arrays to reflect dates encompassed by each dispensing of each drug within the patient’s observation period |
|
|
| Continuous multiple-refill- interval measure of Medication Availability OR Continuous measure of Medication Acquisition (CMA)23,28 |
Total days’ supply of medication obtained throughout study period divided by number of days from first dispensing until study completion date (number of days in observation period); mean of each patient’s CMA value provides overall study adherence value Mathematically similar formulas include MRA and MPR |
|
|
| Continuous, Single interval measure of medication Acquisition (CSA)28 |
Single-interval measure of medication availability; provides an adherence value for each patient between dispensings (not overall study period); mean of all dispensing adherence values provides overall study adherence value Number of days’ supply dispensed divided by number of days in interval from dispensing date up to (not including) next dispensing date |
|
|
| Compliance Rate or Compliance Ratio (CR)23 |
Sum of days’ supplies for each patient, minus days’ supply obtained at last dispensing divided by number of days from first up to (not including) last dispensing date Denominator can also be listed as: “last claim date minus index date” |
|
|
|
| |||
|
Based on Medication Gaps
* | |||
| New Prescription Medication Gap (NPMG)6 |
Time between date provider first prescribes medication until first of the following: end of follow- up, censoring due to patient being switched to alternate therapy or medication discontinued by prescriber; total days without sufficient supply summing across each refill interval within follow- up period, divided by total number of days from point of a new electronic prescription to end of follow-up |
|
|
| Continuous measure of Medication Gaps (CMG)28,51 |
Subtract total days’ supply obtained throughout study period from total number of days of observation period (gives number of days of treatment gaps); total days of treatment gaps is then divided by number of days of observation period The mean of each patient’s CMG value provides an overall study non-adherence value based on lack of available medication; 0% reflects complete adherence and 100% reflects complete non- adherence |
|
|
| Continuous Multiple interval measure of OverSupply (CMOS)23,28 |
Total number of days’ supply (if gap) or surplus divided by days in observation period or total days to next fill Mean of each patient’s CMOS value provides an overall study non-adherence value |
|
|
Other less commonly used medication possession measures include: Adherence Ratio, Refill Adherence, Adherence Index, Compliance Ratio, Compliance Index, Refill Compliance Rate (RCR), Refill compliance (ReComp), Medication-Total (MED_TOT), and Medication Interval (MED_INT). Other less commonly used gap measures include: Cumulative Gap Ratio, Medication Out (MED_OUT or MEDOUT), and Days Between fill adherence Rate (DBR)
Developing the Conceptual Model of Standardized Terminology and Definitions of Medication Adherence for Electronic Database Methods
The overall intent of developing a conceptual model was to set out a series of general definitions that could be broadly applied across the fields of electronic data methods and medical informatics and that were applicable to estimating adherence to oral medications for chronic diseases. These definitions were intended to encompass the many opportunities for patients to accept or decline medication within the prescribing and dispensing components of the adherence, persistence, and discontinuation continuum of behaviors. In developing the conceptual model, we applied the following principles: 1) Develop a clear, concise, and broadly applicable model that described a sequence of discrete behaviors over time, 2) Articulate key constructs and sub-constructs of the continuum, 3) Express and organize categories in a logical and systematic manner, 4) Provide sufficient detail to clarify the approach and enable its use, and 5) Avoid restricting appropriate study-specific decisions (e.g., observation windows).
Adherence and persistence definitions require study-specific decisions to yield operational definitions of, for example, the period of observation (“observation window”), the patient sample under study, and the period over which the prescription must be filled after the initial order is written. The duration of the observation window is often conditional on the context, type of medication or disease state, unique details (e.g., usual days’ supply of drug dispensed) and selection criteria (e.g., patients with at least two dispensings). Other study-specific decisions often include specifying an observation timeframe after the medication was dispensed as well as measurement metrics and tools such as formulas to calculate medication possession or gaps in medication availability. The definitions outlined here are focused on the structure of the definitions rather than on the specifics of the operational definitions. However, some operational definitions are provided to remind researchers that these decisions must also be clearly documented. Although the primary focus of developing this conceptual model was to provide broadly applicable definitions, we also identify and classify existing metrics that enable use of these definitions. The Kaiser Permanente Colorado Institutional Review Board (IRB) determined that the activities involved in writing this paper met the federal and institutional criteria for exemption from IRB review.
RESULTS
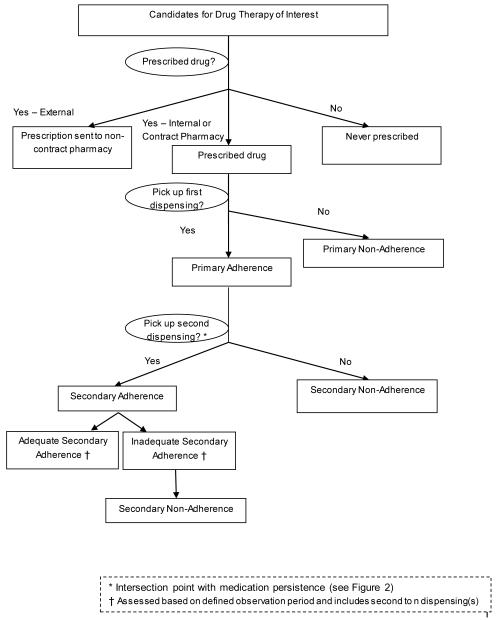
Our proposed conceptual model, terminology and definitions of medication adherence for electronic-data-based methods (Figure 1 and Table 1) is predicated on two key constructs: medication adherence and medication persistence. Adherence connotes the degree or extent to which the patient conforms to the medication use recommendations specified by the prescriber (e.g., frequency/interval of administration, time of day ingested, strength of dosage).20 In contrast, persistence encompasses the time over which a patient continues treatment or continues to re/fill the prescription, from starting to stopping therapy.20,22
Figure 1.
Medication Adherence Conceptual Model and Terminology for Electronic Data Methods
In this conceptual model (Figure 1), adherence is subdivided into two main categories: Primary adherence and secondary adherence. Primary adherence is a discrete event that assesses whether or not the patient received the first prescription. In contrast, secondary adherence is an ongoing process that measures whether or not the patient received dispensings or refills as prescribed during a defined observation period. Only after these adherence sub-constructs have been acknowledged and assessed can the next level of secondary adherence—whether it is adequate or inadequate—be assessed. In these proposed definitions, the converse of each adherence term is simply “non” as in “primary non-adherence” and “secondary non-adherence.”
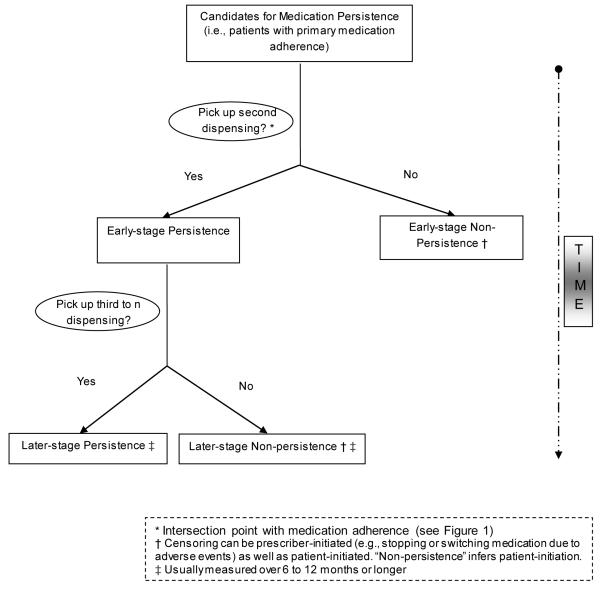
Medication persistence implies that the patient must have exhibited at least primary adherence because persistence over time cannot be measured unless the patient has received at least the first dispensing (Figure 2). We propose that early-stage persistence be defined to include individuals with at least two dispensings and later-stage persistence as including individuals with three or more dispensings of the medication and with evidence of medication availability. For consistent terminology, we propose the converse of each persistence category to also be “non” as in “early-stage non-persistence” and “later-stage non-persistence.”
Figure 2.
Medication Persistence Conceptual Model and Terminology for Electronic Data Methods
Medication discontinuation implies that a patient has terminated therapy as evidenced by not refilling a prescription, but no subjective inference regarding appropriateness is made, since discontinuation may be initiated either by the clinician or the patient. Further, in claims database studies, it is usually not possible to determine whether discontinuation was prescriber-initiated or patient-initiated. Medication discontinuation in electronic database studies can only be assessed within the context of a pre-specified operational definition for the required number of days without medication available. Thus, very low measured levels of adherence (MPR or PDC < 40%; CMG or NPMG > 60%) can in some circumstances represent, or be confused with, discontinuation.
Metrics that have been used to calculate medication adherence and/or persistence using electronic databases are summarized in Table 2. In general, these metrics enable calculation of either medication possession (i.e., possession measures) or gaps in medications availability (i.e., gap measures)18,23-25 and most estimate adherence only among individuals with secondary adherence. Most metrics are continuous measures, but they are often categorized (e.g., low or inadequate versus moderate versus high or adequate adherence). These measures require data including the date of medication dispensing, days’ supply dispensed with each dispensing, and previous (stockpiled) medications (or an indication that it will be set to zero) to estimate medication availability and consumption, usually estimated between the first and terminal dispensings within an observation window. A minority of metrics estimate availability within a single dispensing interval (e.g., the Continuous, Single interval measure of medication Acquisition, CSA). The metrics also vary in whether or not the days’ supply dispensed with the terminal dispensing is included in the calculation. The time between any one dispensing and the subsequent dispensing is known as the refill interval. Person-time is censored at the last dispensing date, at the time of exhaustion of the last days’ supply, or at a fixed number of days after exhaustion of the last days’ supply. Most gap measures of secondary adherence censor after the last dispensing once stockpiled medications have been exhausted.
The two most commonly used secondary adherence medication possession measures are the Medication Possession Ratio (MPR) and the Proportion of Days Covered (PDC),18,22,23,26-28 Both report medication availability by estimating the proportion of prescribed days’ supply obtained during a specified observation period over refill intervals and both are becoming widely applied in health care settings29 in large part because they are easily calculated (a SAS macro has been written for MPR and PDC). For example, as operationalized by the Pharmacy Quality Alliance,29 the PDC has been endorsed by the National Quality Forum as a tool to measure health care quality.30 The main difference between the PDC and the MPR is that with the PDC any oversupply is truncated, whereas adherence values of greater than 100 percent are allowed with the MPR. There is controversy about whether “over adherence,” often considered as MPR between 100 and 120 percent, has clinical meaning.26 A shortcoming of these (and other) secondary adherence measures is that, when integrating across several observation periods of multiple refills each, delayed dispensing(s) in one observation period can be numerically counterbalanced by early dispensing in a later observation period, thus potentially under-ascertaining adherence in one observation period and overestimating it in another. The converse can also occur. This drawback is of particular importance in longitudinal assessments where changes in adherence behavior are assessed across multiple observation periods by calculating the adherence metric separately within each period. Other strengths and weaknesses of the medication possession and gap measures are summarized in Table 2.
Because most measures of adherence require at least two dispensings, the least adherent patients (primary non-adherent) are excluded. Within the last few years measures have been developed that include patients with either primary or secondary (non-)adherence.6 One such metric, the New Prescription Medication Gap (NPMG) measure, is defined as the proportion of days within an interval bounded by the prescriber’s initial EHR prescription medication order date and the end of the observation period (or end of follow-up if censored or the therapy is switched or discontinued).6 As with older gap measures, NPMG is a continuous measure, ranging from one hundred percent for patients who obtain no medication to zero percent for those who consistently refill their medication in a timely fashion. Unlike secondary adherence measures, NPMG was designed to evaluate medication supply starting at prescribing and ending at a fixed censoring point, thus comprehensively capturing (non)-adherence for those who never start the prescribed medication or who discontinue it early as well as for those who have at least two dispensings. An additional strength of NPMG is that because it enables evaluation from the point of prescribing in the EHR, person-time can be censored if the prescriber switches or discontinues therapy and documents those orders in the EHR.
DISCUSSION
In this paper we offer a standardized set of definitions and terminology for assessing medication adherence and persistence in electronic database studies, whether the databases employed are medications ordered within an EHR, medications dispensed and documented in a pharmacy database, or pharmacy claims processed through an insurance database. These conceptual models and terminology are more comprehensive than current, commonly used definitions. The models we propose include clear and systematic definitions of adherence and persistence developed to facilitate EHR-based research, are specific to medication adherence, and extend to adherence and persistence subcategories and medication discontinuation. We also point out the importance and utility of developing precise operational definitions for adherence research as these definitions enhance the precision of ascertaining whether a patient was likely exposed to a specific medication on a particular date for a specific research purpose (e.g., on the date of some clinical measure, event, or outcome).
Research focusing on adherence is voluminous. As obtaining the initial prescription medication and taking the medication are prerequisite health behaviors for medication effectiveness, these are key explanatory variables when observed effectiveness is lower than the efficacy demonstrated in controlled trials. As a consequence, comparative effectiveness studies may be designed to evaluate the effectiveness of various interventions to improve these adherence behaviors in their own right. Meta-analytic studies are also useful in assessing adherence as an outcome, and the level of adherence necessary to achieve treatment goals. To facilitate these types of studies, it is critical that the adherence measure be accurately estimated and consistent across studies.
There are limitations to this work. Our conceptual model is specific to prescription-filling and has not been compared with frameworks for other prescription behaviors such as medication-taking.20,31 Definitions are only part of the decision-making process in adherence research. Many important methodological considerations that should be addressed were beyond the scope of this paper such as identifying clinically meaningful categorizations for adherence (e.g., < 20% CMG; >= 80% MPR) based on observed relationships between adherence and clinical outcomes for specific disease states, the role of informative censoring (e.g., medication stop orders and switches), comprehensive assessment of discrete events (e.g., appropriate time frames to consider for medication discontinuation), and bias associated with assuming dispensing data are complete (e.g., prescriptions transferred outside an integrated healthcare system, paying cash for prescriptions resulting in no prescription insurance claims being filed).
CONCLUSION
We offer a set of standardized medication adherence terminology and definitions for use with electronic database research. The medication adherence and persistence conceptual models and definitions we present will enable future meta-analytic and comparative effectiveness research, as standardized terminology facilitates rigorous comparisons. As such, this paper is foundational for adherence methods.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Steiner JF. Rethinking adherence. Ann Intern Med. 2012;157:580–585. doi: 10.7326/0003-4819-157-8-201210160-00013. [DOI] [PubMed] [Google Scholar]
- 2.McGinnis B, Olson KL, Magid D, et al. Factors related to adherence to statin therapy. Ann Pharmacother. 2007;41:1805–1811. doi: 10.1345/aph.1K209. [DOI] [PubMed] [Google Scholar]
- 3.Batal HA, Krantz MJ, Dale RA, et al. Impact of prescription size on statin adherence and cholesterol levels. BMG Health Services Research. 2007;7:175. doi: 10.1186/1472-6963-7-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briesacher BA, Andrade SE, Fouayzi H, et al. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacother. 2008;28:437–443. doi: 10.1592/phco.28.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan DC, Shrank WH, Cutler D, et al. Patient, physician, and payment predictors of statin adherence. Med Care. 2010;48:196–202. doi: 10.1097/MLR.0b013e3181c132ad. [DOI] [PubMed] [Google Scholar]
- 6.Karter AJ, Parker MM, Moffet HH, et al. New prescription medication gaps: A comprehensive measure of adherence to new prescriptions. Health Serv Res. 2009;44:1640–1661. doi: 10.1111/j.1475-6773.2009.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kane S, Shaya F. Medication non-adherence is associated with increased medical health care costs. Dig Dis Sci. 2008;53:1020–1024. doi: 10.1007/s10620-007-9968-0. [DOI] [PubMed] [Google Scholar]
- 8.Ho PM, Magid DJ, Masoudi FA, et al. Adherence to cardioprotective medications and mortality among patients with diabetes and ischemic heart disease. BMC Cardiovascular Disorders. 2006;6 doi: 10.1186/1471-2261-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kramer JM, Hammill B, Anstrom KJ, et al. National evaluation of adherence to beta-blocker therapy for 1 year after acute myocardial infarction in patients with commercial health insurance. Am Heart J. 2006;152:454.e1–8. doi: 10.1016/j.ahj.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 10.Raebel MA, Carroll NM, Ellis JL, et al. Importance of early non-adherence in estimations of medication adherence. Ann Pharmacother. 2011;45:1053–1060. doi: 10.1345/aph.1Q146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegel D, Lopez J, Meier J. Antihypertensive medication adherence in the Department of Veterans Affairs. Am J Med. 2007;120:26–32. doi: 10.1016/j.amjmed.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 12.Shah NR, Hirsch AG, Zacker C, et al. Predictors of first-fill adherence for patients with hypertension. Am J Hypertens. 2009;22:392–396. doi: 10.1038/ajh.2008.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah NR, Hirsch AG, Zacker C, et al. Factors associated with first-fill adherence rates for diabetic medications: a cohort study. J Gen Intern Med. 2009;24:233–237. doi: 10.1007/s11606-008-0870-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carroll NM, Ellis JL, Luckett C, et al. Feasibility of determining medication adherence from electronic health record medications orders. J Am Med Inform Assoc. 2011;18:717–720. doi: 10.1136/amiajnl-2011-000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer MA, Stedman MR, Lii J, et al. Primary Medication Non-Adherence: Analysis of 195,930 Electronic Prescriptions. J Gen Intern Med. 2010;25:284–290. doi: 10.1007/s11606-010-1253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karter AJ, Parker MM, Adams AS, et al. Primary non-adherence to prescribed medications. J Gen Intern Med. 2010;25:763. doi: 10.1007/s11606-010-1381-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raebel MA, Ellis JL, Carroll NM, et al. Characteristics of patients with primary nonadherence to medications for hypertension, diabetes, and lipid disorders. J Gen Intern Med. 2012;27:57–64. doi: 10.1007/s11606-011-1829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrade SE, Kahler KH, Frech F, et al. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15:565–574. doi: 10.1002/pds.1230. [DOI] [PubMed] [Google Scholar]
- 19.Caetano PA, Lain JMC, Morgan SG. Toward a standard definition and measurement of persistence with drug therapy: examples from research on statin and antihypertensive utilization. Clin Ther. 2006;28:1411–1424. doi: 10.1016/j.clinthera.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization [Accessed November 14, 2012];Adherence to long-term therapies: Evidence for action. 2003 http://www.who.int/chp/knowledge/publications/adherence_report/en/
- 22.Peterson AM, Nau DP, Cramer JA, et al. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10:3–12. doi: 10.1111/j.1524-4733.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 23.Hess LM, Raebel MA, Conner DA, et al. Measurement of adherence in pharmacy administrative databases: A proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40:1280–1288. doi: 10.1345/aph.1H018. [DOI] [PubMed] [Google Scholar]
- 24.Karve S, Cleves MA, Helm M, et al. Prospective validation of eight different adherence measures for use with administrative claims data among patients with schizophrenia. Value Health. 2009;12:989–995. doi: 10.1111/j.1524-4733.2009.00543.x. [DOI] [PubMed] [Google Scholar]
- 25.Hansen RA, Kim MM, Song L, et al. Comparison of methods to assess medication adherence and classify nonadherence. Ann Pharmacother. 2009;43:413–422. doi: 10.1345/aph.1L496. [DOI] [PubMed] [Google Scholar]
- 26.Nau DP. [Accessed February 11, 2013];Proportion of days covered (PDC) as a preferred method of measuring medication adherence. 2011 Available at: http://www.pqaalliance. org/images/uploads/files/PQA%20PDC%20vs%20%20MPR.pdf.
- 27.Benner JS, Glynn RJ, Mogun H, et al. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288:455–461. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- 28.Karve S, Cleves MA, Helm M, et al. An empirical basis for standardizing adherence measures derived from administrative claims data among diabetic patients. Med Care. 2008;46:1125–1133. doi: 10.1097/MLR.0b013e31817924d2. [DOI] [PubMed] [Google Scholar]
- 29.Pharmacy Quality Alliance [Accessed February 11, 2013];PQA measures used by CMS in the STAR ratings. 2012 http://pqaalliance.org/measures/cms.asp.
- 30.National Quality Forum [Accessed February 11, 2013]; Available at: http://www.qualityforum.org/home.aspx.
- 31.Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73:691–705. doi: 10.1111/j.1365-2125.2012.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillipson TJ, Mozaffari E, Maclean JR. Pharmacy cost sharing, antiplatelet therapy utilization, and health outcomes for patients with acute coronary syndrome. Am J Manag Care. 2010;16:290–297. [PubMed] [Google Scholar]
- 33.Schneeweiss S, Patrick AR, Maclure M, et al. Adherence to beta-blocker therapy under drug cost-sharing in patients with and without acute myocardial infarction. Am J Manag Care. 2007;13:445–452. [PMC free article] [PubMed] [Google Scholar]
- 34.Yu AP, Yu YF, Nichol MB, et al. Delay in filling the initial prescription for a statin: a potential early indicator of medication nonpersistence. Clin Ther. 2008;30:761–774. doi: 10.1016/j.clinthera.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 35.Briesacher BA, Andrade SE, Harrold LR, et al. Adherence and occurrence of fractures after switching to once-monthly oral bisphophonates. Pharmacoepidemiol Drug Saf. 2010;19:1233–1240. doi: 10.1002/pds.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huybrechts KF, Ishak KJ, Caro JJ. Assessment of compliance with osteoporosis treatment and its consequences in a managed care population. Bone. 2006;38:922–928. doi: 10.1016/j.bone.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 37.Deambrosis P, Saramin C, Terrazzani G, et al. Evaluation of the prescription and utilization patterns of statins in an Italian local health unit during the period 1994–2003. Eur J Clin Pharmacol. 2007;63:197–203. doi: 10.1007/s00228-006-0239-3. [DOI] [PubMed] [Google Scholar]
- 38.Hertz RP, Unger AN, Lustik MB. Adherence with pharmacotherapy for type 2 diabetes: a retrospective cohort study of adults with employer-sponsored health insurance. Clinical Therapeutics. 2005;27:1064–1073. doi: 10.1016/j.clinthera.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Carnahan RM, Lund BC, Chrischilles EA, et al. Consistency of antidepressant and chronic nonpsychiatric medication use in a high-risk clinical population. Res Soc Admin Pharm. 2008;4:367–374. doi: 10.1016/j.sapharm.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Christensen L, Sasane R, Hodgkins P, et al. Pharmacological treatment patterns among patients with attention-deficit/hyperactivity disorder: retrospective claims-based analysis of a managed care population. Curr Med Res Opin. 2010;26:977–989. doi: 10.1185/03007991003673617. [DOI] [PubMed] [Google Scholar]
- 41.Erkens JA, Panneman MMJ, Klungel OH, et al. Differences in antihypertensive drug persistence associated with drug class and gender: a PHARMO study. Pharmacoepidemiol Drug Saf. 2005;14:795–803. doi: 10.1002/pds.1156. [DOI] [PubMed] [Google Scholar]
- 42.Gold DT, Martin BC, Frytak JR, et al. A claims database analysis of persistence with alendronate therapy and fracture risk in post-menopausal women with osteoporosis. CMRO. 2007;23:585–594. doi: 10.1185/030079906X167615. [DOI] [PubMed] [Google Scholar]
- 43.Yeaw J, Benner JS, Walt JG, et al. Comparing Adherence and Persistence Across 6 Chronic Medication Classes. J Manag Care Pharm. 2009;15:728–740. doi: 10.18553/jmcp.2009.15.9.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Wijka BLG, Shrank WH, Klungela OH, et al. A cross-national study of the persistence of antihypertensive medication use in the elderly. J Hypertens. 2008;26:145–153. doi: 10.1097/HJH.0b013e32826308b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barron TI, Connolly RM, Bennett K, et al. Early discontinuation of tamoxifen: a lesson for oncologists. Cancer. 2007;109:839. doi: 10.1002/cncr.22485. [DOI] [PubMed] [Google Scholar]
- 46.Boccuzzi SJ, Wogen J, Fox J, et al. Utilization of oral hypoglycemic agents in a drug-insured US population. Diabetes Care. 2001;24:1411–1415. doi: 10.2337/diacare.24.8.1411. [DOI] [PubMed] [Google Scholar]
- 47.El-Serag H, Fitzgerald S, Richardson P. The extent and determinants of prescribing and adherence with acid-reducing medications: a national claims database study. Am J Gastroenterol. 2009;104:2167. doi: 10.1038/ajg.2009.312. [DOI] [PubMed] [Google Scholar]
- 48.Kalsekar ID, Madhavan SS, Amonkar MM, et al. Impact of depression on utilization patterns of oral hypoglycemic agents in patients newly diagnosed with type 2 diabetes Mmellitus: a retrospective cohort analysis. Clin Ther. 2006;28:306–318. doi: 10.1016/j.clinthera.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 49.Bryson CL, Au DH, Young B, et al. A refill adherence algorithm for multiple short intervals to estimate refill compliance (ReComp) Med Care. 2008;45:497–503. doi: 10.1097/MLR.0b013e3180329368. [DOI] [PubMed] [Google Scholar]
- 50.Leslie RS. [Accessed April 26, 2012];Using arrays to calculate medication utilization. SAS Paper 043-2007. Available at: http://www2.sas.com/proceedings/forum2007/043-2007.pdf.
- 51.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]