Abstract
Alterations of ovarian follicle morphology and function have been well documented in women with PCOS. These include increased numbers of growing preantral follicles, failure of follicle growth beyond the mid-antral stage, evidence of granulosa call degeneration, and theca cell hyperplasia. Functional abnormalities include paradoxical granulosa cell hyperresponsiveness to FSH which is clinically linked to ovarian hyperstimulation during ovulation induction. In addition, there is likely a primary theca cell defect that accounts for the majority of excess androgen production in this disorder.
The precise mechanisms responsible for altered follicle function are not completely clear. However, several factors appear to influence normal advancement of follicle development as well as impair ovarian steroidogenesis. These include intra- as well as extraovarian influences that distort normal ovarian growth and disrupt steroid production by follicle cells.
Keywords: PCOS, Granulosa cell, Theca cell, Androgen, Follicle, Insulin
1. Introduction
The classical description of women with polycystic ovary syndrome (PCOS) includes evidence of excess androgen production, irregular or absent menstrual bleeding as a result of chronic anovulation, and polycystic ovaries. The lack of regular ovulatory function in these women designates PCOS as the leading cause of anovulatory infertility (Franks et al., 2008). The mechanism(s) responsible for failure of ovulation is not known, although abnormalities of the follicle and its respective cellular investments – specifically, the oocyte, granulosa cell (GC), and theca cell (TC) – have been well documented. In particular, among early and mid-antral stage follicles, the degenerative appearance of GCs on histology belies their sustained functional ability to respond to FSH and LH stimulation. Nevertheless, in women with PCOS, alterations of GC function likely contribute to the poor response to gonadotropin administration during controlled ovarian hyperstimulation. Aberrant follicle growth and development does not, however, preclude successful ovarian responsiveness to careful gonadotropin stimulation during ovulation induction. Given these observations, it is likely that extra- or intraovarian factors and not inherent defects of GCs are responsible for disruption of progressive follicle development, leading to mid-antral arrest. In contrast, a primary defect of TC steroidogenesis appears to account for androgen excess in this disorder. Whether and to what degree local androgen overproduction may impact GC activity is not clearly known. However, evidence suggests that androgen excess facilitates abnormal follicle formation and, perhaps, follicle viability.
2. Morphology of the polycystic ovary
The ovaries of women with PCOS are slightly enlarged and contain numerous small (2–9 mm) antral follicles, which are commonly arranged on the periphery with increased central stroma. This distinctive appearance has been codified to establish diagnostic criteria for the polycystic ovary as more than 12 follicles per ovary or an ovarian volume over 10 ml (Rotterdam, 2004a,b). Histologically, growing preantral follicles appear similar to those of normal ovaries. In addition, early antral follicle formation may also appear normal. However, normal development beyond the mid-antral stage is not observed as the follicle begins to exhibit evidence of arrested growth and degenerative change. There is progressive accumulation of follicular fluid and expansion of the antrum. As the follicle enlarges, the GC layer undergoes apoptosis and becomes increasingly atretic. Eventually, the follicle wall may become devoid of GCs, leading to the appearance of a thin-walled cyst.
3. Increased follicle number
A distinctive feature of the polycystic ovary is a pronounced increase in follicle number as the population of growing preantral and antral follicles exceed by 2–3-fold that of normal ovaries (Hughesdon, 1982; Webber et al., 2003; Maciel et al., 2004). The process responsible for excessive follicle formation in PCOS ovaries has not been established; however, several possibilities have emerged, including increased primordial follicle activation, slowed preantral follicle development, increased follicle survival and/or decreased atresia, or a combination of these processes.
3.1. Preantral follicle population
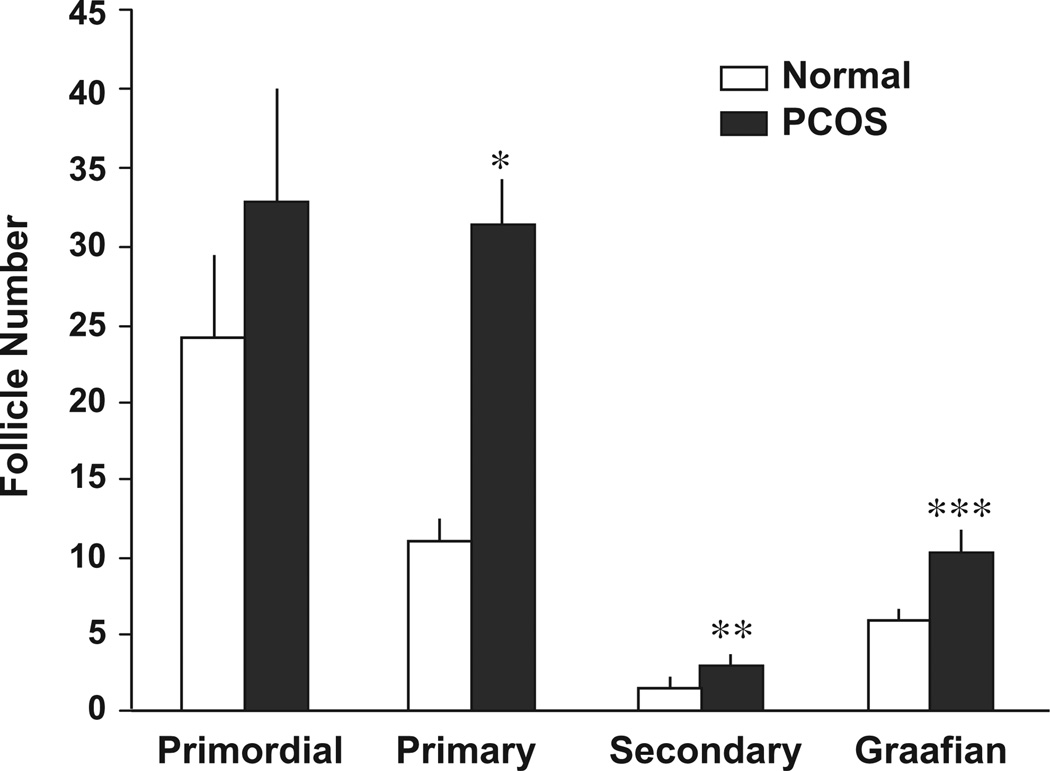
In one of the earliest descriptions of PCOS ovaries, Hughesdon found a 2-fold increase in the number of growing follicles, but the same number of primordial follicles in PCOS ovaries and normal ovaries (Hughesdon, 1982). These findings suggested that the increased number of preantral and early antral follicles in polycystic ovaries was not likely due to either an increased initial primordial pool or an accelerated rate of primordial follicle activation. Subsequent studies have shown increases in the number of total follicles; however efforts to determine the size of the primordial pool have been inconsistent. Webber et al. calculated that the number of healthy primordial follicles as a percentage of total follicles present was lower in both anovulatory and ovulatory polycystic ovaries compared to normal ovaries (Webber et al., 2003). This was interpreted to suggest that increased primordial follicle activation might contribute to the greater number of follicles in PCOS. An alternative explanation is that the growth of pre-antral follicles occurs more slowly in PCOS, resulting in an accumulation of growing follicles. This possibility is consistent with a study by Maciel et al., in which there were similar numbers of primordial follicles in PCOS and normal ovaries but a higher number of growing preantral follicles in PCOS (Fig. 1) (Maciel et al., 2004). As a result, the proportion of resting primordial follicles to the total number of growing follicles would remain decreased. Furthermore, there is no evidence that women with PCOS exhibit early menopause (Webber et al., 2003) as might be expected with accelerated primordial follicle recruitment. Taken together, these data clearly indicate that there are higher numbers of growing follicles in PCOS compared to normal ovaries with no consistent effect on primordial follicle number. However, the mechanism for this pattern remains to be determined.
Fig. 1.
The size (mean ± SE) of the follicle population (primordial, primary, secondary, and Graafian) in sections of ovaries from normal and PCOS patients. *P = 0.001; **P = 0.02; ***P < 0.001.
3.2. Prolonged follicle survival
The observation of an increased preantral follicle population in PCOS suggests either increased entry into or decreased exit from the growing follicle pool. To investigate the latter possibility, small growing follicles were obtained from normal and PCOS women and maintained in culture for approximately 2 weeks (Webber et al., 2007). Both PCOS and normal follicles exhibited increases in diameter and growth during culture. However, examination of atresia revealed a greater percentage of degenerating follicles from normal ovaries compared to that observed in PCOS follicles. Thus, cultured PCOS follicles sustained less atresia than similar staged follicles from normal ovaries. These data suggest that slowed follicle development though the primary stage, together with a prolonged survival pattern, may lead to an accumulation of preantral follicles and further explain the greater number of growing follicles in ovaries of women with PCOS.
3.3. Role of growth and differentiation factor-9
Intriguingly, independent studies have demonstrated that the greatest increase in growing follicles in PCOS is seen in the number of follicles at the primary stage, suggesting the transition from the primary to secondary stage might be slower in PCOS (Webber et al., 2003; Maciel et al., 2004). A possible explanation for slowed growth at the primary follicle stage was provided by Teixeira Filho et al. (2002). In PCOS and normal ovaries they examined the expression of growth differentiation factor-9 (GDF-9), an oocyte-specific member of the TGF-β family known to play a critical role in folliculogenesis. GDF-9 expression was decreased in PCOS ovaries compared to normals in oocytes at all developmental stages from transitional to the Graafian follicles. Studies in mice have suggested that adequate amounts of GDF-9 are required for growth beyond the primary follicle as female GDF-9 knock-out mice are infertile with a complete block in folliculogenesis at the primary stage (Dong et al., 1996). Filho et al. showed that only 8–12% of the oocytes of primary follicles in PCOS contained signal for GDF-9 compared to 96% of normal controls, and the signal, when present in more advanced follicles, was weaker than corresponding normal follicles (Teixeira Filho et al., 2002). Thus, defects in growth in PCOS may begin at the earliest stages of folliculogenesis, well before arrest of the antral follicle.
3.4. Anti-mullerian hormone
Abnormal expression of anti-mullerian hormone (AMH) in PCOS may also play a role in increased follicle density. This TGF-beta peptide is produced exclusively by GCs of growing pre-antral and small antral follicles and serves to negatively regulate advancement of follicle maturation (Weenen et al., 2004). In primordial and primary follicles from PCOS ovaries, AMH expression was decreased compared with that of normal ovaries (Stubbs et al., 2005). The relative diminution of AMH expression in early stage follicles may provide a possible mechanism for increased numbers of preantral follicles in PCOS. Interestingly, in women with PCOS serum AMH levels are elevated 2–3-fold compared to values observed in normal women. While this has been attributed to increase follicle number in PCOS, recent evidence indicates that in vitro AMH production per GC is increased in this disorder (Pellatt et al., 2007). In other words, each small antral follicle from PCOS ovaries makes more AMH than a corresponding normal follicle. Collectively, these findings suggest that in women with PCOS AMH expression is reduced in early growing follicles, which might allow for advanced development. However, with advanced follicle growth to the antral stage, increased AMH production occurs as a result of increased follicle number as well as greater output on a per cell basis.
3.5. Androgen
Among local factors that might accelerate follicle growth, androgen excess is suggested from polycystic ovary morphology associated with hyperandrogenic women with congenital adrenal hyperplasia or androgen-producing ovarian tumors (Dunaif et al., 1984; Erickson et al., 1989; Barnes et al., 1994). Moreover, female-to-male transsexuals receiving long-term androgen treatment commonly exhibit polycystic ovary morphology (Futterweit and Deligdisch, 1986; Spinder et al., 1989; Chadha et al., 1994). Similar findings have been observed in nonhuman primates administered high doses of testosterone, where increased ovarian size was accompanied by greater numbers of preantral and antral follicles (Vendola et al., 1998). In addition, it was shown that androgen induced increased FSH receptor expression in GCs, which suggests a mechanism for this androgen effect.
4. Arrest of follicle development
It has been recognized that, in PCOS, follicle development fails to proceed beyond the mid-antral stage. Based on observational data, arrest of follicle growth typically occurs when follicular diameter reaches 5–8 mm. However, the mechanism is responsible for arrest or precisely when cessation of follicle development is initiated is not known. Possible mechanisms that have been suggested include premature terminal GC differentiation and insufficient FSH secretion.
4.1. Premature luteinization
In a series of elegant studies, steroid production from GCs of individual PCOS and normal follicles of varying size were assessed following LH stimulation (Willis et al., 1998). LH-stimulated progesterone (P4) production from small PCOS follicles (4 mm) was greater or, at least, equivalent to the amount of P4 generated by larger follicles from normal ovaries (9.5–10 mm). These findings suggested that GCs in follicles from PCOS ovaries had acquired LH receptors at an earlier stage of development and, subsequently, undergone luteinization prematurely. That LH secretion in women with PCOS is increased only underscores the likelihood of this mechanism as a prelude to terminal differentiation and arrested follicle development. This concept was reinforced by studies that revealed overexpression of LH receptor mRNA in PCOS GCs compared to GCs from normal follicles (Jakimiuk et al., 2001).
Precisely how GCs of small antral follicles might acquire increased LH receptors is unknown. It is well established that FSH induces LH receptor content in cultured GCs (Richards, 1994; Lindeberg et al., 2007). However, in women with PCOS, FSH secretion is reduced. Insulin has been shown to stimulate FSH-induced LH receptor in rat and porcine GCs although inhibition of LH receptors by insulin has also been reported (May et al., 1980; Sanders and Midgley, 1982; Davoren et al., 1986). The relevance of these findings in PCOS is unclear as androgen has been shown to inhibit FSH-induced LH receptor expression in cultured rat GCs (Jia et al., 1985). Nevertheless, in GCs from PCOS follicles, insulin and LH were synergistic in stimulating steroid production (Willis et al., 1996; Franks et al., 2008). These results raise the possibility that increased insulin secretion in women with PCOS promotes LH action on GCs, and by extension, contributes to follicle atresia and eventual arrest.
4.2. Inadequate FSH secretion
Failure of follicle maturation in PCOS has also been attributed to inadequate FSH stimulation as serum levels of FSH are decreased in women with PCOS. Yet, GCs of PCOS express increased FSH binding and are readily responsive to FSH in vitro and in vivo (Almahbobi et al., 1996; Willis et al., 1996; Coffler et al., 2003a). Additionally, it is well established that anovulation in PCOS women may be overcome with exogenous FSH administration. The relative suppression of FSH secretion, as well as increased LH secretion, may be a consequence of increased activity of the hypothalamic GnRH pulse generator observed in women with PCOS. LH and FSH sub-unit genes are differentially regulated as increased GnRH pulse activity inhibits FSH-β gene expression and increases LH-β gene expression, thereby accounting for inappropriate gonadotropin secretion PCOS (Kaiser et al., 1995). Further reduction of circulating FSH may also result from the chronic estrogen secretion characteristic of this condition. In PCOS women treated with estrogen over a two week interval, serum FSH levels declined progressively and the disparate release of the gonadotropins FSH and LH was enhanced (Chang et al., 1982).
4.3. Role of anti-mullerian hormone
Because AMH inhibits aromatase, it has been proposed that excessive local production of this protein, combined with reduced FSH secretion, could set the stage for follicle arrest and decreased estradiol (E2) production by the ovary (Grossman et al., 2008). This notion is consistent with in vitro studies showing that GCs incubated for 48 h in the presence of FSH exhibited reduced AMH production (Pellatt et al., 2007). In addition, during controlled ovarian hyperstimulation, serum AMH levels have been noted to decline with length of treatment (Fanchin et al., 2003; La Marca et al., 2004). However, these observations may also be explained by the decrease in AMH production seen in the final stages of follicle development in normal ovaries. The possible role of AMH in follicle arrest in PCOS is uncertain as endogenous factors that regulate this protein are currently unknown.
5. Granulosa cell steroidogenesis
Despite the observed degenerative changes, the functional integrity of GCs of PCOS follicles is retained and, to a significant extent, is even greater than that of normal GCs. Early in vitro studies demonstrated that E2 production by PCOS GCs following FSH stimulation was 6- to 10-fold greater compared to responses observed in normal cells (Erickson et al., 1990; Mason et al., 1994). This enhanced responsiveness was found predominantly in cells from anovulatory PCOS women and not hyperandrogenic women with ovulatory cycles.
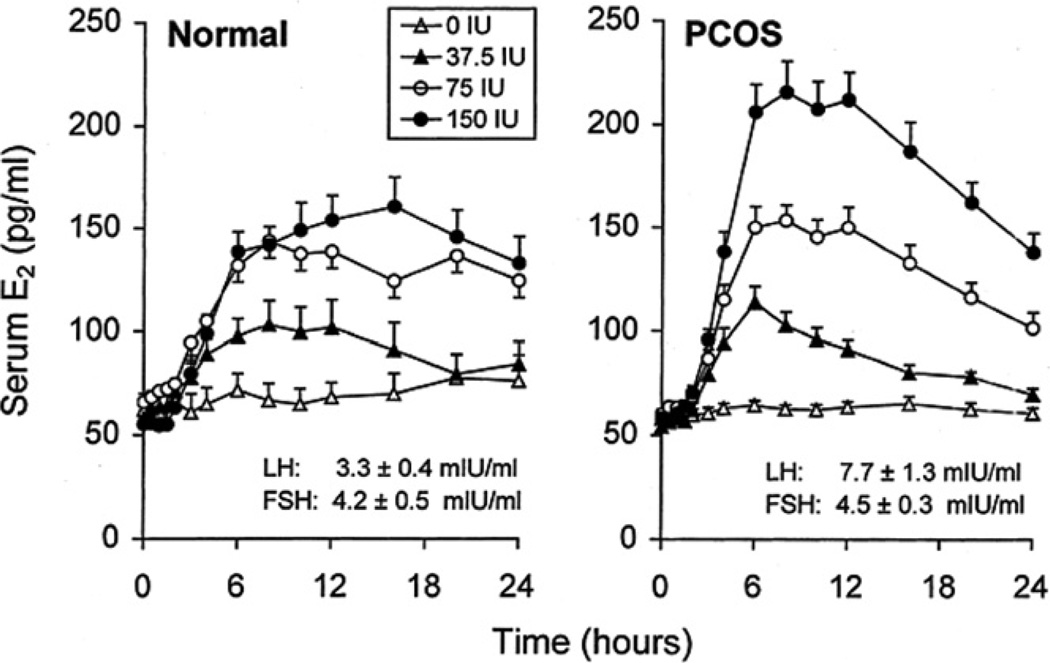
Subsequent clinical studies have been consistent with the results of these in vitro studies. When women with PCOS were acutely stimulated with increasing doses of intravenous FSH, the highest dose of FSH produced significantly greater E2 production than that observed in normal women (Fig. 2) (Coffler et al., 2003a). Interestingly, the time-course response revealed that, in PCOS women, increases of E2 were not sustained as peak levels were followed by an estimated 40% decrement while E2 levels in normal women persisted for 24 h after reaching maximal values. Thus, while hyperresponsive to FSH, PCOS GCs were unable to sustain E2 production, perhaps reflecting the underlying degenerative process of GC attrition in these cells.
Fig. 2.
Mean (±SE) serum E2 levels after intravenous administration of FSH in PCOS and normal controls. FSH administered after t = 0 h. The 0 IU dose of FSH is saline control. Mean (± SE) baseline levels of serum LH and FSH are also shown. (Conversion of E2 to SI units by factor of 3.67).
The mechanism by which GC sensitivity to FSH is increased in PCOS has not been clearly defined. Previous studies in rodents and non-human primates have shown that E2 production in GCs may be enhanced by several factors including insulin, estrogen, and androgen. These factors may contribute to increased GC sensitivity to FSH as women with PCOS exhibit chronic unopposed estrogen secretion, excessive androgen production, and insulin resistance with compensatory hyperinsulinemia.
5.1. Role of insulin
To address the role of insulin on GC function, a series of meticulous studies were performed on GCs obtained from ovaries of PCOS and normal women. Incubation with insulin alone was associated with increased E2 and P4 production from GCs of normal ovaries as well as those from ovulatory and anovulatory PCOS ovaries (Willis et al., 1996). When combined with FSH, there was an additive effect of insulin on normal and ovulatory PCOS GCs whereas a synergistic response to these combined hormones was exhibited by GCs from anovulatory women with PCOS. These findings are consistent with the observation that insulin resistance is more pronounced in anovulatory compared to ovulatory PCOS individuals and imply a role for insulin in GC steroidogenesis in this group of women.
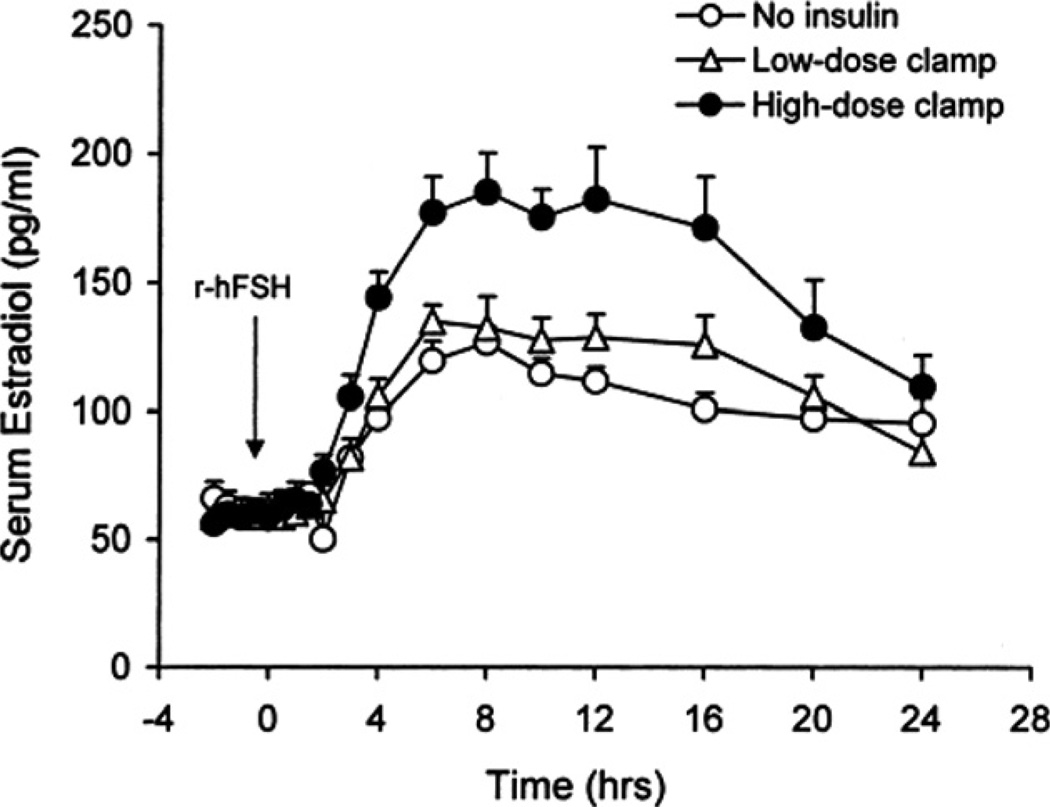
To determine the clinical effect of insulin on GC responses to FSH, anovulatory PCOS and normal women were administered a 10-h hyperinsulinemic-euglycemic clamp combined with FSH stimulation (Coffler et al., 2003b). After induced hyperinsulinemia, E2 responses to FSH were unaltered compared to responses observed without insulin infusion (Fig. 3). Subsequently, the PCOS women were treated with an insulin lowering drug, pioglitazone, for 12 weeks, after which insulin infusion and FSH stimulation were repeated. Serum E2 responses to FSH were significantly greater than that observed before treatment (Fig 3). In addition, it was also noted that with piogltiazone, the duration of maximal E2 response was more sustained and similar to that observed in untreated normal women. Notably, initial E2 responsiveness to FSH was more rapid and of greater magnitude compared to that occurring without insulin infusion. These results suggest that hyperinsulinemia may not contribute to enhanced E2 production in PCOS women, but that initial GC responsiveness to FSH may be improved by reducing insulin resistance, as might occur during ovulation induction.
Fig. 3.
Time course of mean (±SE) 24 h serum E2 responses after injection of intravenous FSH, 75 IU, to PCOS women treated with pioglitazone without insulin infusion and 2 h after initiation of low-dose and high-dose hyperinsulinemic-euglycemic clamps administered for 10 h. The integrated E2 response as determined by area under the curve was significantly greater in subjects receiving high-dose insulin infusion compared with those observed for women without insulin or with low-dose insulin infusion. (Conversion of E2 to SI units by factor of 3.67).
As previously mentioned, cultured PCOS GCs appeared to be prematurely luteinized based on P4 responsiveness to LH and FSH stimulation (Willis et al., 1998). In women with PCOS we have examined P4 responses to FSH before and after hyperinsulinemia. Under basal conditions, women with PCOS exhibited significantly increased FSH-stimulated P4 production whereas incremental changes in normal women were not apparent. Insulin infusion failed to impact P4 responses to FSH. In addition, treatment with pioglitazone which improved insulin sensitivity was not associated with greater P4 responses either prior to or during a hyperinsulinemic clamp compared to pretreatment responses. The observation of greater P4 responses to FSH in PCOS women compared to that of normal women is consistent with prematurely luteinized GCs in this disorder. However, absence of an effect of hyperinsulinemia suggests that insulin has a limited, if any, role in FSH-mediated P4 release in PCOS women.
5.2. Role of androgen
Co-incubation of androgen with GCs from rodents and non-human primates has been shown to enhance estrogen responses to FSH stimulation (Erickson and Hsueh, 1978; Daniel and Armstrong, 1980; Hillier and De Zwart, 1981; Harlow et al., 1986). This ability of androgen to enhance GC function is not limited to aromatizable compounds as the non-aromatizable androgen, dihydrotestosterone, produced the same effects. Subsequent studies have revealed that, in GCs of non-human primates with implanted silastic capsules containing testosterone, FSH receptor expression was increased and co-localized with that of the androgen receptor (Vendola et al., 1998; Weil et al., 1999). Thus, excess androgen production may contribute to greater ovarian responsiveness to FSH in hyperandrogenic women with PCOS.
In an indirect attempt to assess the influence of hyperandrogenemia on GC steroid production, E2 responses to FSH stimulation were assessed in women with PCOS prior to and following flutamide treatment for 6 weeks (Mehta et al., 2006). Baseline and maximally stimulated E2 responses were not affected by flutamide treatment. Among androgen responses, the only significant change was decreased serum DHEA-S levels, which denoted bioeffectiveness of flutamide administration. These findings indicate that, contrary to findings in vitro, GC hyperresponsiveness to FSH observed in women with PCOS may not be attribus to increased circulating androgens.
5.3. Role of estrogen
It has been previously shown that estrogen can enhance ovarian responses to FSH in vitro (Richards et al., 1979; Adashi and Hsueh, 1982; Jonassen et al., 1982; Daniel and Armstrong, 1983). In cultured GCs, synergy between E2 and FSH has been demonstrated with regards to increased FSH receptor binding, induction of LH receptors, increased aromatase activity, and progestin synthesis (Richards et al., 1976; Adashi and Hsueh, 1982). The mechanism for this synergy has not been completely elucidated although an effect on adenylate cyclase or estrogen-induced GC proliferation was suggested since FSH receptor expression did not appear to be altered (Richards, 1980; Daniel and Armstrong, 1983). Alternatively, other investigators have demonstrated increased FSH-binding capacity per GC following stimulation by FSH using estrogen-treated immature rats (Knecht et al., 1984). Both processes may serve to amplify FSH receptor number in GCs of developing follicles. In humans, increased FSH binding to GCs derived from antral follicles of anovulatory women with PCOS compared to those of sized-matched follicles from normal ovaries has been reported (Almahbobi et al., 1996). The precise mechanism for greater FSH binding is not obvious.
In an attempt to show whether estrogen may influence GC responsiveness to FSH in women with PCOS, we examined E2 responsiveness to FSH during ovarian recovery from GnRH agonist administration before and after estrogen supplementation (unpublished data). Maximal suppression at 4 weeks was followed by a gradual rise of basal and stimulated E2 levels returning to pretreatment values by 8 weeks (Fig. 4). Subsequently, the protocol was repeated with application of transdermal estrogen 2 weeks after GnRH agonist injection to provide serum E2 levels comparable to basal values observed in PCOS women. In E2-treated women, FSH-stimulated E2 production during recovery was unchanged from maximally suppressed values at 4 weeks post-GnRH agonist. Thus, early restoration of estrogen exposure following ovarian suppression did not accelerate GC responses to FSH during recovery. However, the concentrations achieved with transdermal E2 likely inhibited recovery of gonadotropin secretion and reflected extreme sensitivity of GnRH-FSH interaction to negative E2 feedback, much like that which occurs during early puberty.
Fig. 4.
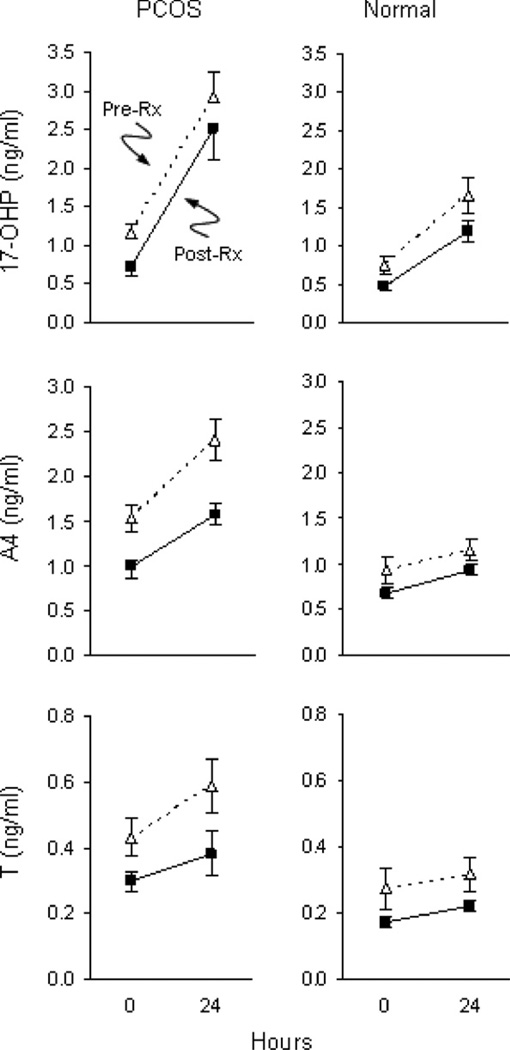
Mean (±SE) baseline and 24-h serum 17-OHP, A4, T, DHEA, E2, and P4 responses to intravenous administration of 1, 10, 25, 100, and 250 µg of hCG in PCOS and normal women. Using linear mixed-effect models analyses, significant increases of 17-OHP, E2, and P4 were observed in both groups. By comparison, incremental A responses were significant only in PCOS women. Between groups, 17-OHP and A responses were significantly greater in PCOS women. Serum T responses were also higher compared to normal women, but the difference did not achieve statistical significance. Significant differences between groups in response to a fixed dose of hCG are indicated by a (P < 0.05), b (P < 0.01), and c (P < 0.001). (Conversion of 17-OHP, A4, T, DHEA, E2, and P4 to SI units by factors of 3.03, 3.49, 3.47, 3.47, 3.67, and 3.18, respectively).
6. Theca cell androgen production
Evidence of normal TC growth is initially seen during secondary follicle development as thin, elongated stromal cells begin to emerge next to the basement membrane and surround the follicle. The mechanism by which follicles acquire TCs is not known. With continued growth of the follicle, the TCs differentiate into the theca interna, which has steroidogenic capacity and the ability to generate androgens. Unlike the appearance of normal TCs, antral follicles of polycystic ovaries are surrounded by a theca layer of considerably greater cellularity and thickness, usually exceeding the width of the corresponding GC layer (Erickson and Yen, 1984). Little is known about the genesis of theca hyperplasia. Evidence indicates that abnormally increased pituitary LH secretion certainly plays a role in excess ovarian androgen production (Rebar et al., 1976; de Ziegler et al., 1989). However, there is now substantial evidence to also suggest a primary defect in TC steroidogenesis in PCOS, leading to excessive responses to gonadotropin stimulation. Intriguingly, gonadotropins appear to act both directly and indirectly to influence androgen production by TCs.
6.1. Role of insulin
In PCOS, the most notable extraovarian factor implicated in androgen overproduction is insulin. The association between hyperinsulinemia and hyperandrogenemia in women with PCOS might be explained by the finding that the activity of a single serine kinase may result in both hyperandrogenemia and insulin resistance as it appears to phosphorylate CYP17, activating androgen production, as well as the insulin receptor-β, blocking insulin signaling (Dunaif et al., 1995; Bremer and Miller, 2008). However, in vitro studies suggest a more direct relationship exists. Studies involving culture of normal human theca tissue have demonstrated that insulin is capable of enhancing androgen production in response to LH as well as independently stimulating androgen production (Barbieri et al., 1984, 1986; Bergh et al., 1993). In TCs from women with PCOS, insulin was shown to markedly increase androgen production. The relationship in vivo, however, is less clear. The reduction of hyperinsulinemia in women with PCOS treated with insulin-lowering drugs is associated with significant decreases of serum androgen levels without corresponding changes in LH levels. This observation indirectly suggests a role for insulin in LH-stimulated androgen synthesis in women with this disorder. However, induction of marked hyperinsulinemia in women with PCOS has not been associated with increases in serum androgen levels (Nestler et al., 1987; Dunaif and Graf, 1989). More studies are needed to resolve these discrepant results.
6.2. Primary theca cell defect
There is good evidence to suggest that excess ovarian androgen results from a primary defect of TC steroidogenesis in women with PCOS. Initial studies established that cultured TCs from PCOS ovaries produced significantly more androgen both at baseline and following LH stimulation per cell than that generated by TCs from normal ovaries (Barbieri et al., 1984; Gilling-Smith et al., 1994). To determine the nature of excess ovarian androgen production in vivo, GnRH agonist was administered to women with PCOS, normal women and normal men followed by frequent blood sampling over 24 h (Barnes et al., 1989). PCOS women exhibited significant increases in serum 17-hydroxyprogesterone (17-OHP) and androstenedione (A4) concentrations compared to those of normal women. Moreover, these responses were similar to those observed in normal men. Interestingly, differences in testosterone (T) responses between women with PCOS and normal women were not found. Examination of the patterns of individual steroid hormone responses pointed to alterations in both 17-hydroxylase and C-17, 20-lyase activity. As CYP17 regulates the activity of enzymes, it was proposed that androgen excess may arise de novo from the ovary as a result of dysregulation of CYP17 activity in PCOS. Consistent with this hypothesis, a subsequent study of PCOS women showed that 17-OHP production in response to human chorionic gonadotropin (hCG), a surrogate for LH stimulation, was elevated compared to that of normal women and remained amplified following suppression of endogenous gonadotropins by GnRH agonist (Gilling-Smith et al., 1997). In contrast, the post-GnRH agonist response in normal women was significantly lowered. Taken together, these clinical results support the concept of an inherent dysregulation of CYP17 in TCs leading to increased androgen production in response to gonadotropin stimulation in women with PCOS.
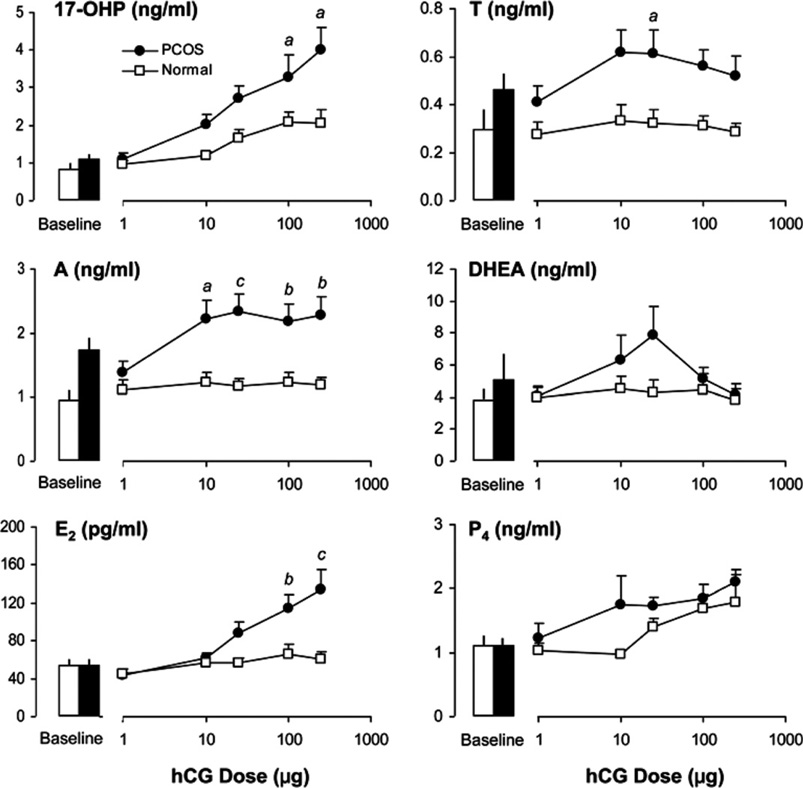
While PCOS TCs are more responsive on a per cell basis in vitro, it was still unclear whether TCs were more sensitive to LH secretion in women with PCOS compared to normal women. To further investigate this possibility, we conducted a dose–response study in PCOS and normal women to determine androgen production following intravenous administration of hCG at doses of 1, 10, 25, 100, and 250 µg (Rosencrantz et al., 2011). Our results revealed that 17-OHP responsiveness to hCG increased in a dose dependent manner in PCOS women and was greater than that observed in normal women (Fig. 5). A shift in the response curve was not observed, which indicated greater TC responsiveness and not increased sensitivity to hCG. Additionally, it was noted that peak A4 and T responses in PCOS women occurred at a low dose of hCG whereas maximal 17-OHP responses were observed at higher doses of hCG. Interestingly, hCG-stimulated responses of A4 and T in normal women were not observed. These results suggested exquisite TC responsiveness to hCG in PCOS women compared to minimal stimulated androgen production in normal women. Previous clinical studies have suggested a lack of 17, 20-lyase activity based on GnRH-stimulated rises of A4 that were relatively less than that of 17-OHP (Rosenfield et al., 1994). Using propagated PCOS TCs, it was demonstrated that radiolabeled pregnenolone was more rapidly metabolized to 17-OH pregnenolone and DHEA and, eventually, to A4 compared to normal cells (Nelson et al., 1999). This increased steroid output was accompanied by greater enzymatic activities of CYP11A, 3β-HSD, and 17β-HSD. The inefficiency of del-ta-4 17, 20-lyase activity may explain, at least in part, the discordant dose–responses of 17-OHP compared to those exhibited by A4 and T in PCOS women. The accumulation of A4 and T with minimal 17-OHP production at a low dose of hCG is compatible with hyperactivity of the delta-5 steroid pathway combined with increased 3β-HSD enzyme activity, a stable property of the PCOS theca cell (Nelson et al., 1999). At higher doses of hCG, incremental changes in 17-OHP responsiveness likely arose from combined over-expression of both 17-hydroxylase and 3β-HSD enzymes.
Fig. 5.
Mean (±SE) serum 17-OHP, A4, and T levels following administration of hCG, 25 µg intravenously, prior to and after treatment with GnRH antagonist in women with PCOS and normal controls. The rise from baseline values is significant (P < 0.05) for all pre- and post-treatment responses in both groups except for the T response in normal women before GnRH antagonist. (Conversion of 17-OHP, A, T, to SI units by factors of 3.03, 3.49, and 3.47, respectively).
6.3. Role of LH secretion
To further determine the contribution of endogenous LH to androgen production, we examined TC response to IV hCG stimulation in PCOS and normal women prior to and 2 days following administration of a GnRH antagonist (unpublished data). Antagonist treatment significantly lowered LH and moderately decreased FSH. As expected, basal estrogen and androgen levels were also significantly reduced (Fig. 6). Prior to antagonist injection, hCG stimulated clear increases of serum 17-OHP, A4, and T in PCOS women while changes in normal women were not significant. After LH suppression by antagonist administration, both groups demonstrated reduced basal levels of androgens. Accordingly, peak androgen responses to hCG were lower in each group. However, the incremental fold-change for both PCOS and normal women was unaltered. Our results are not surprising as amplified 17-OHP responses to GnRH agonist stimulation in PCOS women were reported to be unaltered despite ovarian suppression for one month (Gilling-Smith et al., 1997). In addition, after 3 months of ovarian suppression by long acting GnRH agonist, women with PCOS exhibited 17-OHP responses to hCG that remained greater than those of normal women albeit at substantially lower circulating values (Hirsh-feld-Cytron et al., 2009). Collectively, these results suggest that endogenous gonadotropin secretion contributes to basal androgen secretion and, even in the presence of lowered gonadotropin levels, maintains ovarian androgen synthesis and responsiveness to gonadotropin stimulation. This is consistent with primary TC dysfunction leading to excessive ovarian androgen production in women with PCOS.
Fig. 6.
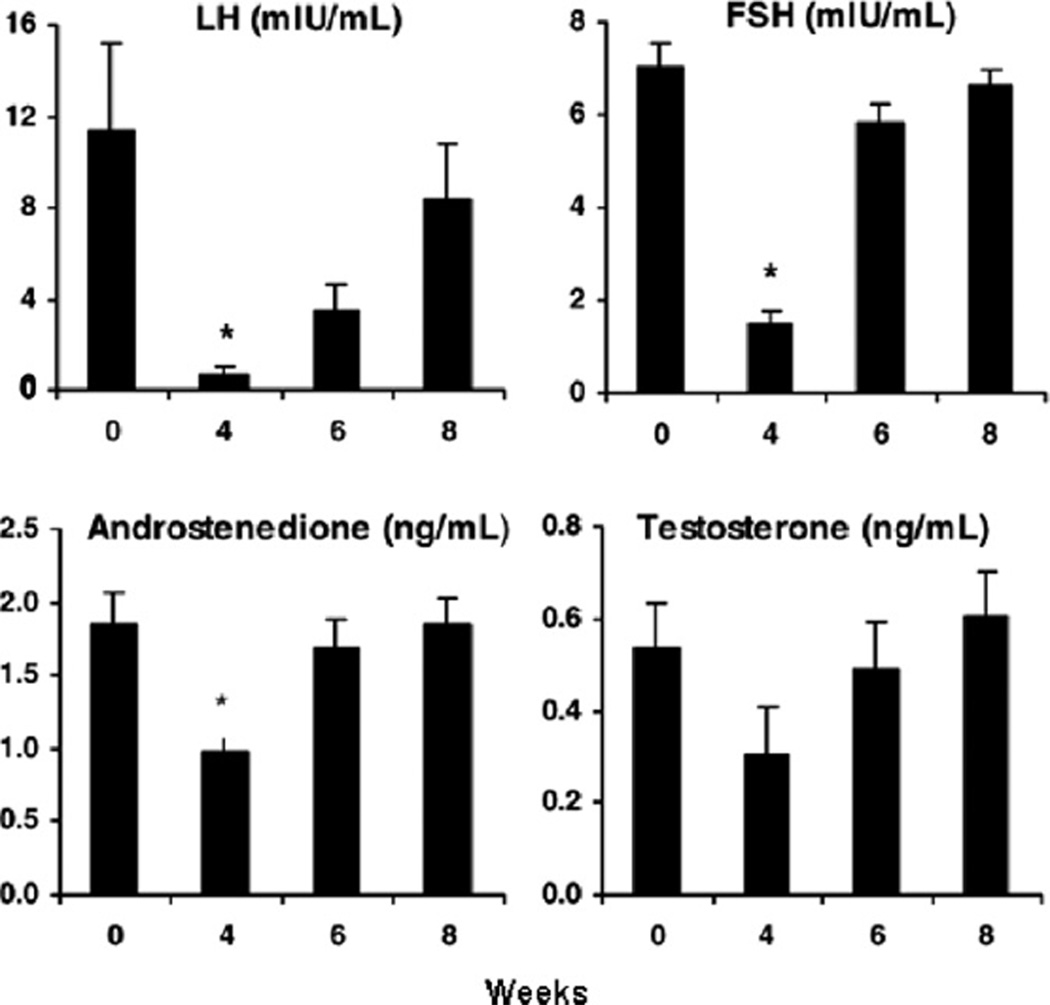
Mean (±SE) basal serum levels of LH, FSH, A and T before and 4, 6 and 8 weeks after treatment with a single injection of a long-acting GnRH agonist (depot Lupron, 3.75 mg, i.m.) in six PCOS women. *P < 0.05 versus 0 weeks. (Conversion of A4 and T to SI units by factors of 3.49 and 3.47, respectively).
7. Intrafollicular paracrine interaction
We have previously examined the pattern of hormone recovery following ovarian suppression by GnRH agonist in women with PCOS. As expected, we noted maximal suppression at 4 weeks after agonist administration followed by recovery of steroidogenesis, with serum E2 levels returning to pretreatment values 8 weeks (Fig. 4). Basal serum A4 and T levels, as well as basal FSH levels, were restored to pretreatment values by 6 weeks. In contrast, serum LH remained significantly suppressed at 6 weeks and only resumed pretreatment levels at 8 weeks. These results demonstrated that recovery of ovarian steroidogenesis was marked by an accelerated return of androgen production that was more rapid than that of estrogen and coincided with the rapid return of FSH.
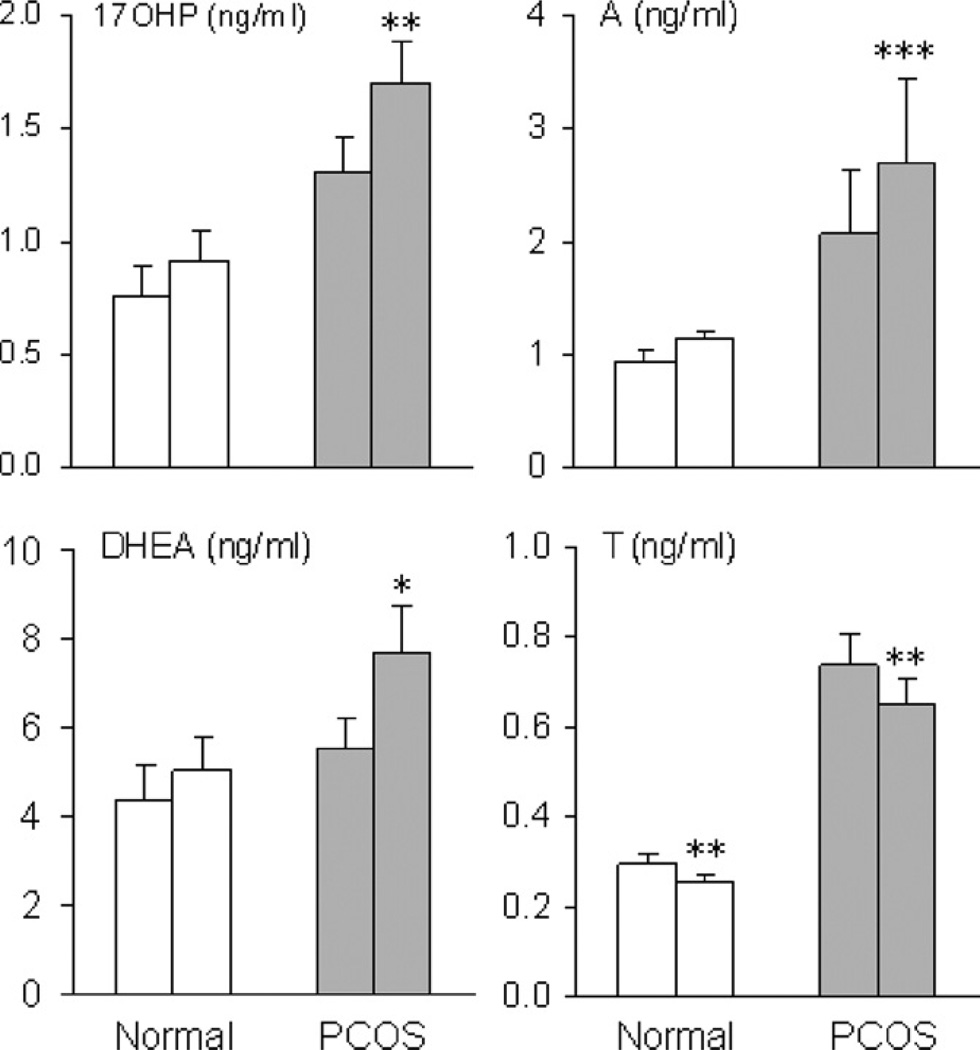
The coupling of androgen and FSH recovery patterns, particularly in the absence of restored LH secretion, suggested that FSH may induce TC androgen production. To address this possibility, androgen responses to FSH were tested in women with PCOS and normal women. In the PCOS group, significant, incremental increases in 17-OHP, A4, and DHEA following FSH stimulation were observed whereas androgen responses to FSH were unchanged in normal women (Fig. 7) (Wachs et al., 2008). By comparison, FSH provoked a significant reduction in T production in both groups, which might reflect conversion to E2 by FSH-activated aromatase. Surprisingly, these findings provide evidence that, in PCOS women, TC androgen production is enhanced by FSH administration and suggest a paracrine mechanism by which GC factors produced in response to FSH stimulate TC androgen production.
Fig. 7.
Mean (±SE) serum 17-OHP, A4, DHEA, and T levels before and 24 h after administration of hFSH, 150 IU, in PCOS and normal women. A significant change from baseline is denoted by an asterisk. *P < 0.05; **P < 0.02; ***P < 0.001. (Conversion of 17-OHP, A4, T, and DHEA to SI units by factors of 3.03, 3.49, 3.47, and 3.47, respectively).
7.1. Inhibin
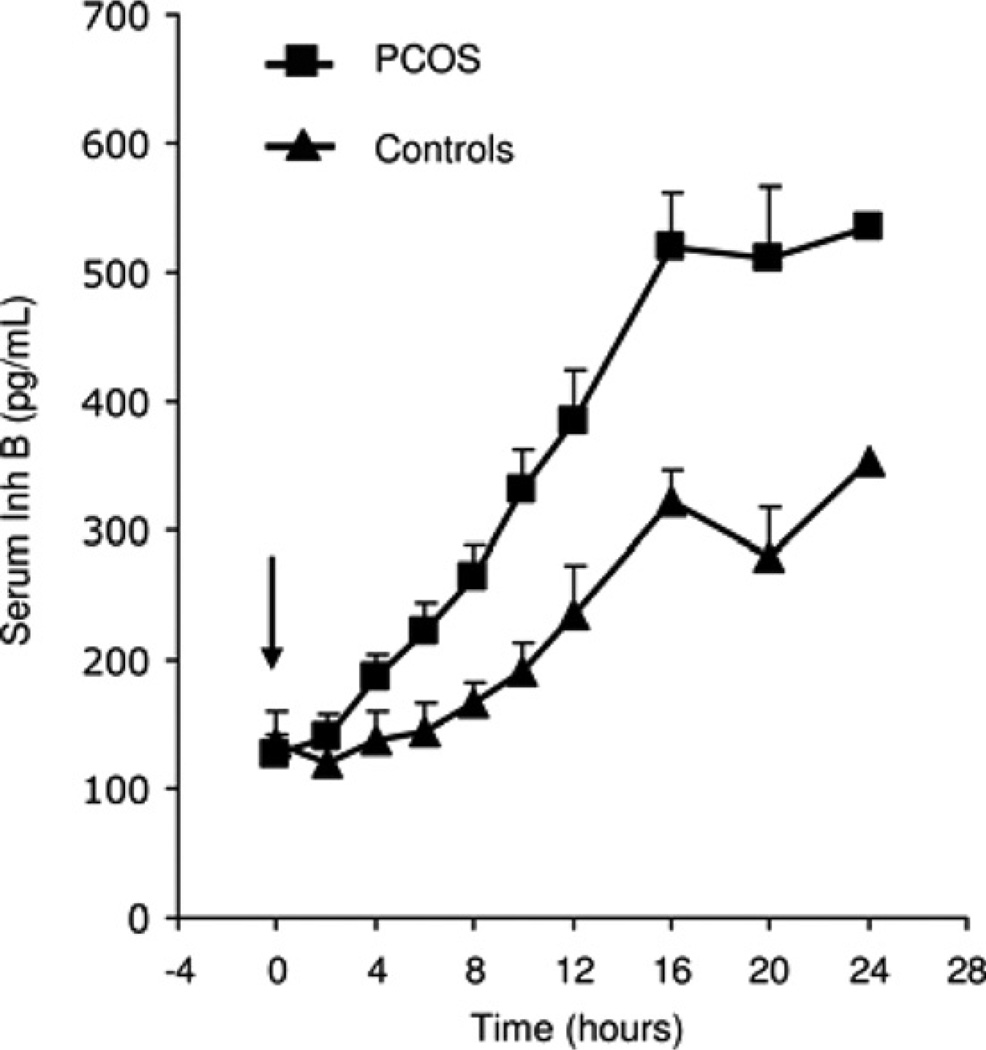
In vitro and in vivo studies in animals have shown that, among factors produced by GCs, inhibin may enhance LH-stimulated TC androgen production (Hillier and De Zwart, 1981). However, reports of serum inhibin B levels in women with PCOS have been inconsistent in that some have shown increased concentrations and others no change (Magoffin and Jakimiuk, 1998; Laven et al., 2001; Welt et al., 2005). These conflicting findings were clarified by examining inhibin B and E2 responses to FSH stimulation in PCOS and normal women. In women with PCOS, inhibin B levels rose 5-fold after FSH (Fig. 8), which was significantly greater than the 3-fold increase observed in normal women (Wachs et al., 2006).
Fig. 8.
Mean (±SE) serum Inh B levels after administration of r-hFSH, 150 IU, in PCOS and normal women. Arrow indicates injection of FSH. (Conversion of Inh B to SI units by factor of 1).
A direct effect of inhibin B on TC androgen production is unlikely as a receptor for this protein has not been identified. Rather, inhibin B has been shown to bind a membrane bound protein, beta-glycan, to form a complex that has high affinity for activin (Act) type II receptors (Lewis et al., 2000). Sequestration of Act type II receptors prevents formation of the Act type II/type I receptor complex, activin signaling, and inhibition of CYP17 in the TC. This provides a possible mechanism for FSH-stimulated androgen production in PCOS women. However, documentation of this interaction in human ovarian tissue and whether inhibin/activin signaling is altered in TCs of PCOS women has not been studied.
7.2. Bone morphogenetic proteins
Other factors that utilize Act type II receptors may also be inactivated through the competitive action of inhibin B. In particular, some bone morphogenetic proteins (BMPs) bind to not only BMP type II receptors, but also Act type II receptors to initiate their divergent actions (Shimasaki et al., 2004). It has been reported BMPs may inhibit CYP17 mRNA expression and androgen production in ovarian TC culture (Dooley et al., 2000; Glister et al., 2005). Whether the inhibitory effect of BMPs on androgen production involves BMP or Act type II receptors is unknown, however, the possibility that inhibin B may act to antagonize BMP signaling through binding to beta-glycan is an intriguing hypothesis under investigation (Wiater and Vale, 2003).
7.3. Insulin-like growth factors
In addition to inhibin B, insulin-like growth factors (IGF) I and II also may act to influence TC androgen production (Cara and Rosenfield, 1988; Bergh et al., 1993; Nahum et al., 1995). In vitro, IGF-I has been shown to synergize with LH to enhance androgen production from cultured rodent TCs (Hillier et al., 1991). However, because IGF-I mRNA and protein do not appear to be expressed in human GCs, it is more likely that IGF-II is the active paracrine factor. In human TC tissue, IGF-II was shown to stimulate basal androgen release as well as enhance LH-induced androgen production much like that of IGF-I (Nahum et al., 1995). In the same study, the stimulatory effects of IGF-I and IGF-II on TC androgen production were greatly enhanced by inhibin B, which supports a predominant role for this protein among potential GC mediators of TC androgen production in PCOS.
8. Summary
Disordered follicle development is a cardinal feature of women with PCOS. The distinctive polycystic ovary morphology is accompanied by alterations in steroid hormone production that have been characterized in vitro as well as in vivo. However, the precise mechanisms responsible for increased preantral follicle number, arrested follicle growth, and abnormal steroidogenesis remain incompletely understood. Clearly, there are inherent defects within the ovary of women with PCOS although evidence for disrupted GC/TC paracrine interaction and roles for extraovarian factors are compelling.
Acknowledgment
This research was supported by the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement (U54 HD12303–28) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research and in part by NIH Grant MO1 RR00827.
References
- Adashi EY, Hsueh AJW. Estrogens augment the stimulation of ovarian aromatase activity by follicle-stimulating hormone in cultured rat granulose cells. J. Biol. Chem. 1982;257:6077–6083. [PubMed] [Google Scholar]
- Almahbobi G, Anderiesz C, Hutchinson P, McFarlane JR, Wood C, Trounson AO. Functional integrity of granulosa cells from polycystic ovaries. Clin. Endocrinol. 1996;44:571–580. doi: 10.1046/j.1365-2265.1996.724545.x. [DOI] [PubMed] [Google Scholar]
- Barbieri RL, Makris A, Ryan KJ. Insulin stimulates androgen accumulation in incubations of human ovarian stroma and theca. Obstet. Gynecol. 1984;64:73S–80S. doi: 10.1097/00006250-198409001-00019. [DOI] [PubMed] [Google Scholar]
- Barbieri RL, Makris A, Randall RW, Daniels G, Kistner RW, Ryan KJ. Insulin stimulates androgen accumulation in incubations of ovarian stroma obtained from women with hyperandrogenism. J. Clin. Endocrinol. Metab. 1986;62:904–910. doi: 10.1210/jcem-62-5-904. [DOI] [PubMed] [Google Scholar]
- Barnes RB, Rosenfield RL, Burstein S, Ehrmann DA. Pituitary-ovarian responses to nafarelin testing in the polycystic ovary syndrome. N. Engl. J. Med. 1989;320:559–565. doi: 10.1056/NEJM198903023200904. [DOI] [PubMed] [Google Scholar]
- Barnes RB, Rosenfield RL, Ehrmann DA, Cara JF, Cuttler L, Levitsky LL, Rosenthal IM. Ovarian hyperandrogynism as a result of congenital adrenal virilizing disorders: evidence for perinatal masculinization of neuroendocrine function in women. J. Clin. Endocrinol. Metab. 1994;79:1328–1333. doi: 10.1210/jcem.79.5.7962325. [DOI] [PubMed] [Google Scholar]
- Bergh C, Carlsson B, Olsson JH, Selleskog U, Hillensjo T. Regulation of androgen production in cultured human thecal cells by insulin-like growth factor I and insulin. Fertil. Steril. 1993;59:323–331. doi: 10.1016/s0015-0282(16)55675-1. [DOI] [PubMed] [Google Scholar]
- Bremer AA, Miller WL. The serine phosphorylation hypothesis of polycystic ovary syndrome: a unifying mechanism for hyperandrogenemia and insulin resistance. Fertil. Steril. 2008;89:1039–1048. doi: 10.1016/j.fertnstert.2008.02.091. [DOI] [PubMed] [Google Scholar]
- Cara JF, Rosenfield RL. Insulin-like growth factor I and insulin potentiate luteinizing hormone-induced androgen synthesis by rat ovarian thecal-interstitial cells. Endocrinology. 1988;123:733–739. doi: 10.1210/endo-123-2-733. [DOI] [PubMed] [Google Scholar]
- Chadha S, Pache TD, Huikeshoven FJM, Brinkmann AO, van der Kwast TH. Androgen receptor expression in human ovarian and uterine tissue of long term androgen-treated transsexual women. Human Path. 1994;25:1198–1204. doi: 10.1016/0046-8177(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Chang RJ, Mandel FP, Lu JK, Judd HL. Enhanced disparity of gonadotropin secretion by estrone in women with polycystic ovarian disease. J. Clin. Endocrinol. Metab. 1982;54:490–494. doi: 10.1210/jcem-54-3-490. [DOI] [PubMed] [Google Scholar]
- Coffler MS, Patel KS, Dahan MH, Malcom PJ, Kawashima T, Deutsch R, Chang RJ. Evidence for abnormal granulosa cell responsiveness to follicle stimulating hormone in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2003a;88:1742–1747. doi: 10.1210/jc.2002-021280. [DOI] [PubMed] [Google Scholar]
- Coffler MS, Patel KS, Dahan MH, Yoo RY, Malcom PJ, Chang RJ. Enhanced granulosa cell responsiveness to follicle stimulating hormone during insulin infusion in women with polycystic ovary syndrome treated with pioglitazone. J. Clin. Endocrinol. Metab. 2003b;88:5624–5631. doi: 10.1210/jc.2003-030745. [DOI] [PubMed] [Google Scholar]
- Daniel S, Armstrong D. Enhancement of follicle-stimulating hormone-induced aromatase activity by androgens in cultured rat granulosa cells. Endocrinology. 1980;107:1027–1033. doi: 10.1210/endo-107-4-1027. [DOI] [PubMed] [Google Scholar]
- Daniel SA, Armstrong DT. Involvement of estrogens in the regulation of granulosa cell aromatase activity. Can. J. Physiol. Pharmacol. 1983;61:507–511. doi: 10.1139/y83-077. [DOI] [PubMed] [Google Scholar]
- Davoren JB, Kasson BG, Li CH, Hsueh AJ. Specific insulin-like growth factor (IGF) I- and II-binding sites on rat granulosa cells: relation to IGF action. Endocrinology. 1986;119:2155–2162. doi: 10.1210/endo-119-5-2155. [DOI] [PubMed] [Google Scholar]
- de Ziegler D, Steingold K, Cedars M, Lu JK, Meldrum DR, Judd HL, Chang RJ. Recovery of hormone secretion after chronic gonadotropin-releasing hormone agonist administration in women with polycystic ovarian disease. J. Clin. Endocrinol. Metab. 1989;68:1111–1117. doi: 10.1210/jcem-68-6-1111. [DOI] [PubMed] [Google Scholar]
- Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- Dooley CA, Attia GR, Rainey WE, Moore DR, Carr BR. Bone morphogenetic protein inhibits ovarian androgen production. J. Clin. Endocrinol. Metab. 2000;85:3331–3337. doi: 10.1210/jcem.85.9.6835. [DOI] [PubMed] [Google Scholar]
- Dunaif A, Graf M. Insulin administration alters gonadal steroid metabolism independent of changes in gonadotropin secretion in insulin-resistant women with the polycystic ovary syndrome. J. Clin. Invest. 1989;83:23–29. doi: 10.1172/JCI113864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaif A, Scully RE, Andersen RN, Chapin DS, Crowley WF., Jr. The effects of continuous androgen secretion on the hypothalamic-pituitary axis in women: evidence from a luteinized the coma of the ovary. J. Clin. Endocrinol. Metab. 1984;59:389–393. doi: 10.1210/jcem-59-3-389. [DOI] [PubMed] [Google Scholar]
- Dunaif A, Xia J, Book CB, Schenker E, Tang Z. Excessive insulin receptor serine phosphorylation in cultured fibroblasts and in skeletal muscle. A potential mechanism for insulin resistance in the polycystic ovary syndrome. J. Clin. Invest. 1995;96:801–810. doi: 10.1172/JCI118126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson GF, Hsueh AJ. Stimulation of aromatase activity by follicle stimulating hormone in rat granulosa cells in vivo and in vitro. Endocrinology. 1978;102:1275–1282. doi: 10.1210/endo-102-4-1275. [DOI] [PubMed] [Google Scholar]
- Erickson GF, Yen SSC. New data on follicle cells in polycystic ovaries: a proposed mechanism for the genesis of cystic follicles. Semin. Reprod. Endocrinol. 1984;2:231–243. [Google Scholar]
- Erickson GF, Magoffin DA, Jones KL. Theca function in polycystic ovaries of a patient with virilizing congenital adrenal hyperplasia. Fertil. Steril. 1989;51:173–176. doi: 10.1016/s0015-0282(16)60450-8. [DOI] [PubMed] [Google Scholar]
- Erickson GF, Magoffin DA, Cragun JR, Chang RJ. The effects of insulin and insulin-like growth factors-I and -II on estradiol production by granulose cells of polycystic ovaries. J. Clin. Endocrinol. Metab. 1990;70:894–902. doi: 10.1210/jcem-70-4-894. [DOI] [PubMed] [Google Scholar]
- Fanchin R, Schonauer LM, Righini C, Frydman N, Frydman R, Taieb J. Serum anti-Mullerian hormone dynamics during controlled ovarian hyperstimulation. Hum. Reprod. 2003;18:328–332. doi: 10.1093/humrep/deg043. [DOI] [PubMed] [Google Scholar]
- Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum. Reprod. Update. 2008;14:367–378. doi: 10.1093/humupd/dmn015. [DOI] [PubMed] [Google Scholar]
- Futterweit W, Deligdisch L. Histopathological effects of exogenously administered testosterone in 19 female to male transsexuals. J. Clin Endocrinol. Metab. 1986;62:16–21. doi: 10.1210/jcem-62-1-16. [DOI] [PubMed] [Google Scholar]
- Gilling-Smith C, Willis DS, Beard RW, Franks S. Hypersecretion of androstenedione by isolated thecal cells from polycystic ovaries. J. Clin. Endocrinol. Metab. 1994;79:1158–1165. doi: 10.1210/jcem.79.4.7962289. [DOI] [PubMed] [Google Scholar]
- Gilling-Smith C, Story H, Rogers V, Franks S. Evidence for a primary abnormality of thecal cell steroidogenesis in the polycystic ovary syndrome. Clin. Endocrinol. 1997;47:93–99. doi: 10.1046/j.1365-2265.1997.2321049.x. [DOI] [PubMed] [Google Scholar]
- Glister C, Richards SL, Knight PG. Bone morphogenetic proteins (BMP) −4, −6, and −7 potently suppress basal and luteinizing hormone-induced androgen production by bovine theca interna cells in primary culture: could ovarian hyperandrogenic dysfunction be caused by a defect in thecal BMP signaling? Endocrinology. 2005;146:1883–1892. doi: 10.1210/en.2004-1303. [DOI] [PubMed] [Google Scholar]
- Grossman MP, Nakajima ST, Fallat ME, Siow Y. Mullerian-inhibiting substance inhibits cytochrome P450 aromatase activity in human granulosa lutein cell culture. Fertil. Steril. 2008;89:1364–1370. doi: 10.1016/j.fertnstert.2007.03.066. [DOI] [PubMed] [Google Scholar]
- Harlow C, Hillier S, Hodges J. Androgen modulation of follicle-stimulating hormone-induced granulosa cell steroidogenesis in the primate ovary. Endocrinology. 1986;119:1403–1405. doi: 10.1210/endo-119-3-1403. [DOI] [PubMed] [Google Scholar]
- Hillier S, De Zwart F. Evidence that granulosa cell aromatase induction/activation by follicle- stimulating hormone is an androgen receptor-regulated process in-vitro. Endocrinology. 1981;109:1303–1305. doi: 10.1210/endo-109-4-1303. [DOI] [PubMed] [Google Scholar]
- Hillier SG, Yong EL, Illingworth PJ, Baird DT, Schwall RH, Mason AJ. Effect of recombinant inhibin on androgen synthesis in cultured human thecal cells. Mol. Cell. Endocrinol. 1991;75:R1–R6. doi: 10.1016/0303-7207(91)90234-j. [DOI] [PubMed] [Google Scholar]
- Hirshfeld-Cytron J, Barnes RB, Ehrmann DA, Caruso A, Mortensen MM, Rosenfield RL. Characterization of functionally typical and atypical types of polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2009;94:1587–1594. doi: 10.1210/jc.2008-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughesdon PE. Morphology and morphogenesis of the Stein-Leventhal ovary and of so- called ‘‘hyperthecosis’’. Obstet. Gynecol. Surv. 1982;37:59–77. doi: 10.1097/00006254-198202000-00001. [DOI] [PubMed] [Google Scholar]
- Jakimiuk AJ, Weitsman SR, Navab A, Magoffin DA. Luteinizing hormone receptor, steroidogenesis acute regulatory protein, and steroidogenic enzyme messenger ribonucleic acids are overexpressed in thecal and granulosa cells from polycystic ovaries. J. Clin. Endocrinol. Metab. 2001;86:1318–1323. doi: 10.1210/jcem.86.3.7318. [DOI] [PubMed] [Google Scholar]
- Jia XC, Kessel B, Welsh TH, Jr., Hsueh AJ. Androgen inhibition of follicle-stimulating hormone-stimulated luteinizing hormone receptor formation in cultured rat granulosa cells. Endocrinology. 1985;117:13–22. doi: 10.1210/endo-117-1-13. [DOI] [PubMed] [Google Scholar]
- Jonassen JA, Bose K, Richards JS. Enhancement and desensitization of hormone-responsive adenylate cyclase in granulosa cells of preantral and antral ovarian follicles: effects of estradiol and follicle-stimulating hormone. Endocrinology. 1982;111:74–79. doi: 10.1210/endo-111-1-74. [DOI] [PubMed] [Google Scholar]
- Kaiser U, Sabbagh E, Katzenellenbogen R, Conn P, Chin W. A mechanism for the differential regulation of gonadotropin subunit gene expression by gonadotropin-releasing hormone. Proc. Natl. Acad. Sci. USA. 1995;92:12280–12284. doi: 10.1073/pnas.92.26.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht M, Darbon J-M, Ranta T, Baukal AJ, Catt KJ. Estrogens enhance the adenosine 3′, 5′-monophosphate-mediated induction of follicle-stimulating hormone and luteinizing hormone receptors in rat granulosa cells. Endocrinology. 1984;115:41–49. doi: 10.1210/endo-115-1-41. [DOI] [PubMed] [Google Scholar]
- La Marca A, Malmusi S, Giulini S, Tamaro LF, Orvieto R, Levratti P, Volpe A. Anti-Müllerian hormone plasma levels in spontaneous menstrual cycle and during treatment with FSH to induce ovulation. Hum. Reprod. 2004;19:2738–2741. doi: 10.1093/humrep/deh508. [DOI] [PubMed] [Google Scholar]
- Laven JS, Imani B, Eijkemans MJ, de Jong FH, Fauser BC. Absent biologically relevant associations between serum inhibin B concentrations and characteristics of polycystic ovary syndrome in normogonadotrophic anovulatory infertility. Hum. Reprod. 2001;16:1359–1364. doi: 10.1093/humrep/16.7.1359. [DOI] [PubMed] [Google Scholar]
- Lewis KA, Gray PC, Blount AL, MacConell LA, Wiater E, Bilezikjian LM, Vale W. Betaglycan binds inhibin and can mediate functional antagonism of activin signalling. Nature. 2000;404:411–414. doi: 10.1038/35006129. [DOI] [PubMed] [Google Scholar]
- Lindeberg M, Carlstrom K, Ritvos O, Hovatta O. Gonadotrophin stimulation of non-luteinized granulosa cells increases steroid production and the expression of enzymes involved in estrogen and progesterone synthesis. Hum. Reprod. 2007;22:401–406. doi: 10.1093/humrep/del408. [DOI] [PubMed] [Google Scholar]
- Maciel GA, Baracat EC, Benda JA, Markham SM, Hensinger K, Chang RJ, Erickson GF. Stockpiling of transitional and classic primary follicles in ovaries of women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2004;89:5321–5327. doi: 10.1210/jc.2004-0643. [DOI] [PubMed] [Google Scholar]
- Magoffin DA, Jakimiuk AJ. Inhibin A, inhibin B and activin A concentrations in follicular fluid from women with polycystic ovary syndrome. Hum. Reprod. 1998;13:2693–2698. doi: 10.1093/humrep/13.10.2693. [DOI] [PubMed] [Google Scholar]
- Mason HD, Willis DS, Beard RW, Winston RM, Margara R, Franks S. Estradiol production by granulosa cells of normal and polycystic ovaries: relationship to menstrual cycle history and concentrations of gonadotropins and sex steroids in follicular fluid. J. Clin. Endocrinol. Metab. 1994;79:1355–1360. doi: 10.1210/jcem.79.5.7962330. [DOI] [PubMed] [Google Scholar]
- May JV, McCarty K, Jr., Reichert LE, Jr., Schomberg DW. Follicle-stimulating hormone-mediated induction of functional luteinizing hormone/human chorionic gonadotropin receptors during monolayer culture of porcine granulosa cells. Endocrinology. 1980;107:1041–1049. doi: 10.1210/endo-107-4-1041. [DOI] [PubMed] [Google Scholar]
- Mehta RV, Malcom PJ, Chang RJ. The effect of androgen blockade on granulosa cell estradiol production after follicle-stimulating hormone stimulation in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2006;91:3503–3506. doi: 10.1210/jc.2006-0752. [DOI] [PubMed] [Google Scholar]
- Nahum R, Thong KJ, Hillier SG. Metabolic regulation of androgen production by human thecal cells in vitro. Hum. Reprod. 1995;10:75–81. doi: 10.1093/humrep/10.1.75. [DOI] [PubMed] [Google Scholar]
- Nelson VL, Legro RS, Strauss JF, 3rd, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol. Endocrinol. 1999;13:946–957. doi: 10.1210/mend.13.6.0311. [DOI] [PubMed] [Google Scholar]
- Nestler JE, Clore JN, Strauss JF, Blackard WG. The effects of hyperinsulinemia on serum testosterone, progesterone, dehydroepiandrosterone sulfate, and cortisol levels in normal women and in a woman with hyperandrogenism, insulin resistance, and acanthosis nigricans. J. Clin. Endocrinol. Metab. 1987;64:180–184. doi: 10.1210/jcem-64-1-180. [DOI] [PubMed] [Google Scholar]
- Pellatt L, Hanna L, Brincat M, Galea R, Brain H, Whitehead S, Mason H. Granulosa cell production of anti-Mullerian hormone is increased in polycystic ovaries. J. Clin. Endocrinol. Metab. 2007;92:240–245. doi: 10.1210/jc.2006-1582. [DOI] [PubMed] [Google Scholar]
- Rebar R, Judd HL, Yen SS, Rakoff J, Vandenberg G, Naftolin F. Characterization of the inappropriate gonadotropin secretion in polycystic ovary syndrome. J. Clin. Invest. 1976;57:1320–1329. doi: 10.1172/JCI108400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JS. Maturation of ovarian follicles: actions and interactions of pituitary and ovarian hormones on follicular cell differentiation. Physiol. Rev. 1980;60:51–89. doi: 10.1152/physrev.1980.60.1.51. [DOI] [PubMed] [Google Scholar]
- Richards JS. Hormonal control of gene expression in the ovary. Endocr. Rev. 1994;15:725–751. doi: 10.1210/edrv-15-6-725. [DOI] [PubMed] [Google Scholar]
- Richards JS, Ireland JJ, Rao MC, Bernath GA, Midgley ARJ, Reichert LEJ. Ovarian follicular development in the rat: hormone receptor regulation by estradiol, follicle stimulating hormone and luteinizing hormone. Endocrinology. 1976;99:1562–1570. doi: 10.1210/endo-99-6-1562. [DOI] [PubMed] [Google Scholar]
- Richards JS, Jonassen JA, Rolfes AI, Kersey K, Reichert LE., Jr. Adenosine 3′,5′-monophosphate, luteinizing hormone receptor, and progesterone during granulosa cell differentiation: effects of estradiol and follicle-stimulating hormone. Endocrinology. 1979;104:765–773. doi: 10.1210/endo-104-3-765. [DOI] [PubMed] [Google Scholar]
- Rosencrantz MA, Coffler MS, Haggan A, Duke KB, Donohue MC, Shayya RF, Su HI, Chang RJ. Clinical evidence for predominance of Delta-5 steroid production in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2011;96:1106–1113. doi: 10.1210/jc.2010-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfield RL, Barnes RB, Ehrmann DA. Studies of the nature of 17-hydroxyprogesterone hyperresonsiveness to gonadotropin-releasing hormone agonist challenge in functional ovarian hyperandrogenism. J. Clin. Endocrinol. Metab. 1994;79:1686–1692. doi: 10.1210/jcem.79.6.7989476. [DOI] [PubMed] [Google Scholar]
- Rotterdam. ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004a;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Rotterdam. ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Hum. Reprod. 2004b;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- Sanders MM, Midgley AR., Jr. Rat granulosa cell differentiation: an in vitro model. Endocrinology. 1982;111:614–624. doi: 10.1210/endo-111-2-614. [DOI] [PubMed] [Google Scholar]
- Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr. Rev. 2004;25:72–101. doi: 10.1210/er.2003-0007. [DOI] [PubMed] [Google Scholar]
- Spinder T, Spijkstra JJ, van den Tweel JG, Burger CW, van Kessel H, Hompes PG, Gooren LJ. The effects of long term testosterone administration on pulsatile luteinizing hormone secretion and on ovarian histology in eugonadal female to male transsexual subjects. J. Clin. Endocrinol. Metab. 1989;69:151–157. doi: 10.1210/jcem-69-1-151. [DOI] [PubMed] [Google Scholar]
- Stubbs SA, Hardy K, Da Silva-Buttkus P, Stark J, Webber LJ, Flanagan AM, Themmen AP, Visser JA, Groome NP, Franks S. Anti-mullerian hormone protein expression is reduced during the initial stages of follicle development in human polycystic ovaries. J. Clin. Endocrinol. Metab. 2005;90:5536–5543. doi: 10.1210/jc.2005-0907. [DOI] [PubMed] [Google Scholar]
- Teixeira Filho FL, Baracat EC, Lee TH, Suh CS, Matsui M, Chang RJ, Shimasaki S, Erickson GF. Aberrant expression of growth differentiation factor-9 in oocytes of women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2002;87:1337–1344. doi: 10.1210/jcem.87.3.8316. [DOI] [PubMed] [Google Scholar]
- Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J. Clin. Invest. 1998;101:2622–2629. doi: 10.1172/JCI2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachs DS, Coffler MS, Malcom PJ, Chang RJ. Comparison of follicle-stimulating-hormone-stimulated dimeric inhibin and estradiol responses as indicators of granulosa cell function in polycystic ovary syndrome and normal women. J. Clin. Endocrinol. Metab. 2006;91:2920–2925. doi: 10.1210/jc.2006-0442. [DOI] [PubMed] [Google Scholar]
- Wachs DS, Coffler MS, Malcom PJ, Shimasaki S, Chang RJ. Increased androgen response to follicle-stimulating hormone administration in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2008;93:1827–1833. doi: 10.1210/jc.2007-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber LJ, Stubbs S, Stark J, Trew GH, Margara R, Hardy K, Franks S. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362:1017–1021. doi: 10.1016/s0140-6736(03)14410-8. [DOI] [PubMed] [Google Scholar]
- Webber LJ, Stubbs SA, Stark J, Margara RA, Trew GH, Lavery SA, Hardy K, Franks S. Prolonged survival in culture of preantral follicles from polycystic ovaries. J. Clin. Endocrinol. Metab. 2007;92:1975–1978. doi: 10.1210/jc.2006-1422. [DOI] [PubMed] [Google Scholar]
- Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, Kramer P, Fauser BC, Themmen AP. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol. Hum. Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- Weil S, Vendola K, Zhou J, Bondy CA. Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J. Clin. Endocrinol. Metab. 1999;84:2951–2956. doi: 10.1210/jcem.84.8.5929. [DOI] [PubMed] [Google Scholar]
- Welt CK, Taylor AE, Fox J, Messerlian GM, Adams JM, Schneyer AL. Follicular arrest in polycystic ovary syndrome is associated with deficient inhibin A and B biosynthesis. J. Clin. Endocrinol. Metab. 2005;90:5582–5587. doi: 10.1210/jc.2005-0695. [DOI] [PubMed] [Google Scholar]
- Wiater E, Vale W. Inhibin is an antagonist of bone morphogenetic protein signaling. J. Biol. Chem. 2003;278:7934–7941. doi: 10.1074/jbc.M209710200. [DOI] [PubMed] [Google Scholar]
- Willis D, Mason H, Gilling-Smith C, Franks S. Modulation by insulin of follicle-stimulating hormone and luteinizing hormone actions in human granulosa cells of normal and polycystic ovaries. J. Clin. Endocrinol. Metab. 1996;81:302–309. doi: 10.1210/jcem.81.1.8550768. [DOI] [PubMed] [Google Scholar]
- Willis DS, Watson H, Mason HD, Galea R, Brincat M, Franks S. Premature response to luteinizing hormone of granulosa cells from anovulatory women with polycystic ovary syndrome: relevance to mechanism of anovulation. J. Clin. Endocrinol. Metab. 1998;83:3984–3991. doi: 10.1210/jcem.83.11.5232. [DOI] [PubMed] [Google Scholar]