Abstract
Background
Serotonin transporter (SERT) plays a critical role in regulating serotonin (5-hydroxytryptamine, 5-HT) availability in the gut. Elevated 5-HT levels are associated with diarrheal conditions such as irritable bowel syndrome and enteric infections. Whether alteration in SERT activity contributes to the pathophysiology of diarrhea induced by the food-borne pathogen enteropathogenic E coli (EPEC) is not known. The present studies examined the effects of EPEC infection on SERT activity and expression in intestinal epithelial cells and elucidated the underlying mechanisms.
Methods
Caco-2 cells as a model of human intestinal epithelia and EPEC infected C57BL/6J mouse model of infection were utilized. SERT activity was measured as Na+ and Cl− dependent 3[H] 5-HT uptake. SERT expression was measured by real time QRT-PCR, Western blotting and immunofluorescence studies.
Results
Infection of Caco-2 cells with EPEC for 30-120 min decreased apical SERT activity (P<0.001) in a type 3 secretion system dependent manner and via involvement of protein tyrosine phosphatases. EPEC infection decreased Vmax of the transporter; while cell surface biotinylation studies revealed no alteration in the cellular or plasma membrane content of SERT in Caco-2 cells. EPEC infection of mice (24h) reduced SERT immunostaining with a corresponding decrease in SERT mRNA levels, 5-HT uptake and mucosal 5-HT content in the small intestine.
Conclusion
Our results demonstrate inhibition of SERT by EPEC and define the mechanisms underlying these effects. These data may aid in the development of a novel pharmacotherapy to modulate the serotonergic system in treatment of infectious diarrheal diseases.
Introduction
The gastrointestinal tract contains about 90% of the whole body content of 5-HT, the majority of which is produced and stored in enterochromaffin cells1. 5-HT is a key hormone of the GI tract that affects several physiological processes including absorption or secretion of fluids and electrolytes via its interactions with serotonin receptor subtypes 2. The physiological actions of 5-HT are terminated by the rapid uptake of serotonin through a highly selective sodium and chloride coupled serotonin transporter, SERT 3-5. This process facilitates 5-HT degradation by the intracellular enzymes. SERT, therefore, has been suggested to play a critical role in regulating 5-HT content and availability in the gut 6. Our recent studies demonstrated that SERT is apically localized and differentially expressed along the length of the human intestine with highest expression in the ileum 7.
Impairment of SERT function in pathogenesis of various diarrheal disorders has been increasingly acknowledged. For example, molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter have been described in ulcerative colitis and irritable bowel syndrome 8, 9. Also, animal models of post-infectious IBS revealed distinct changes in enterochromaffin cell density and decreased SERT expression in the small bowel of parasite infected mice10. These findings suggest that SERT may be an important target for enteric pathogens as well as for the development of therapeutics to modulate diarrhea.
In this regard, enteropathogenic Escherichia coli (EPEC) is an important food-borne pathogen and a major cause of infantile diarrhea. EPEC are non-toxigenic, but through a type 3 secretory system (T3SS), the bacterium injects virulence proteins into host cells 11, 12. Until recently, not much was known regarding the pathophysiology of early diarrhea associated with EPEC infection. Recent findings from our laboratory and others demonstrated that diarrhea induced by EPEC is multi-factorial and occurs in part from a decrease in intestinal absorption of Na+, Cl−, glucose and butyrate as well as disruption in barrier function 13-17. However, the involvement of SERT in pathophysiology of EPEC-induced diarrhea is unknown. The aim of the current study was, therefore, to investigate the direct effects of EPEC infection on the expression and activity of SERT in model intestinal epithelial Caco-2 monolayers and in a mouse model.
Materials and Methods
Models
i) Cell culture
Fully differentiated Caco-2 confluent monolayers grown on collagen coated Transwell inserts or 24-well plastic supports were used for experiments at day 10–12 post plating.
ii) Mouse model of EPEC infection
The previously published C57BL/6 mouse model of EPEC infection was used 18. All experiments involving mice were approved by Animal Care Committee of the University of Illinois at Chicago.
Bacterial culture and cell infection
The EPEC strains used were14, 16: (i) wild-type EPEC strain E2348/69, (ii) CVD452 (E2348/69 escN:Km), (iii) UMD864 (E2348/69 Δ48-759 espB1), (iv) UMD870 (E2348/69 espD1:aph-3(Km)), (v) espG (SE1114) (vi) espZ (vii) espF (UMD874), (viii) espH (SE651orf18) and (ix) map (orf19). Cell monolayers were infected at an MOI of 100 as described previously14, 16.
[3H] serotonin uptake in Caco-2 monolayers
Serotonin uptake was performed in Caco-2 cells as described previously19. Unless otherwise stated, uptake was initiated by the addition of 0.3 ml of medium containing 25-50 nM [3H] serotonin (Perkin Elmer, Waltham, MA) for 5 min. The 5-HT uptake was NaCl dependent with negligible activity seen with the omission of NaCl from the medium.
Na+-K+-ATPase activity assay
Na+-K+-ATPase activity was measured in a crude membrane preparation from Caco-2 cells as the rate of inorganic phosphate released in the presence or absence of ouabain as described 20, 21.
Cell-surface biotinylation studies
Biotinylation studies in Caco-2 cells were performed using sulfo-NH-SS-biotin (0.5 mg/ml; Pierce Biotechnology) as previously described 22. Anti-GFP (1:100, Cell signaling, MA) or anti-SERT (1:500. overnight at 4°C, Immunostar, WI) antibodies were utilized for Western blotting.
[3H]serotonin uptake in apical membrane vesicles (AMVs)
AMVs from the mouse small intestine were prepared from thawed mucosa as described previously 23, 24. The integrity of the membranes was assessed by measuring initial uptake of D-glucose 23, 24. [3H] serotonin uptake into intestinal AMVs was measured using a rapid filtration technique 7.
Immunofluorescence staining in mouse intestine
4-10-μm frozen sections of small intestinal tissue were fixed with 1% paraformaldehyde and stained as described previously 7. Tissues were incubated with rabbit anti-SERT antibody (1:100) and anti-villin antibody (1:100) in PBS with 1% NGS for 120 minutes at room temperature. After washing, sections were incubated with Alexa Fluor 594–conjugated goat anti-rabbit IgG, Alexa Fluor 488–conjugated goat anti-mouse IgG and Hoechst 33342 (Invitrogen) for 60 minutes. Sections were imaged using a_Zeiss_LSM_510_laser_scanning_confocal_microscope.
Small intestine mucosal serotonin content
5-HT content from mucosa was measured from both EPEC infected and control mice by immediately homogenizing 1 μg/ 10μl of mucosa in 0.2 N perchloric acid using the ELISA-based commercially available kit (Beckman Coulter, CA).
Statistics
Results were expressed as % of control (mean ± SEM). One-way ANOVA was used for statistical analysis. P < 0.05 was considered statistically significant.
Results
EPEC infection inhibits SERT activity
To directly examine the direct effect of early EPEC infection on SERT, fully differentiated Caco-2 monolayers were infected with wild type EPEC strain E2348/69. EPEC infection decreased apical 5-HT uptake as early as 15 min, reaching maximal inhibition at 30-60 min (Figure 1A). However, infection of Caco-2 cells with the nonpathogenic E. coli for 60 min had no effect (Figure 1B). Previous studies have demonstrated a functional serotonin uptake at both apical and basolateral membrane domains of Caco-2 monolayers 19. To examine whether EPEC infection differentially affects apical and basolateral uptake of 5-HT, monolayers grown on Transwells were utilized. EPEC infection for 60 min decreased apical SERT activity by 53% (Figure 1C), while basolateral SERT activity was inhibited to a significantly lesser degree (Figure 1D). Since, our recent studies showed that SERT was predominantly localized to the apical membrane in the human intestine 7, subsequent experiments examined mechanisms underlying the modulation of SERT activity (represented by apical 5-HT uptake) by EPEC.
Figure 1. EPEC infection inhibits serotonin reuptake in Caco-2 cells.
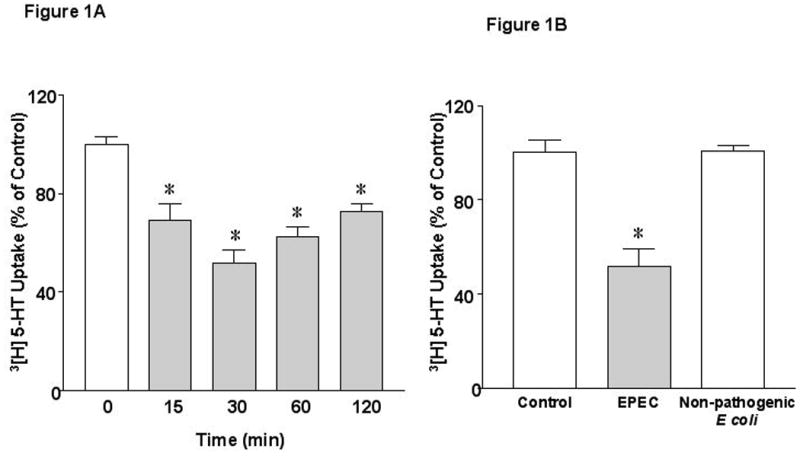
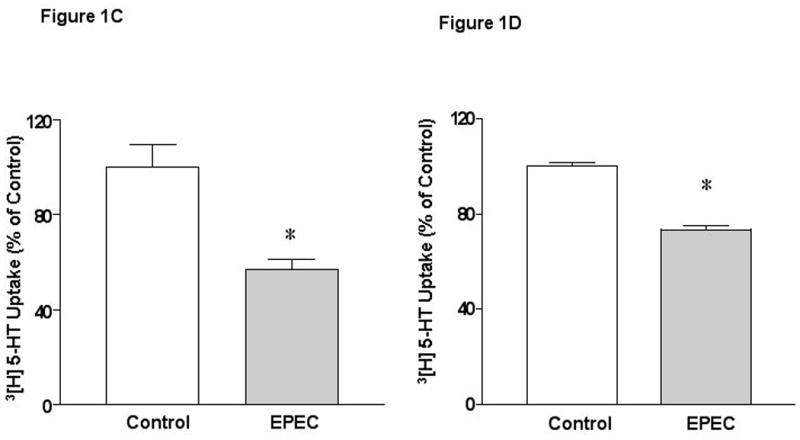
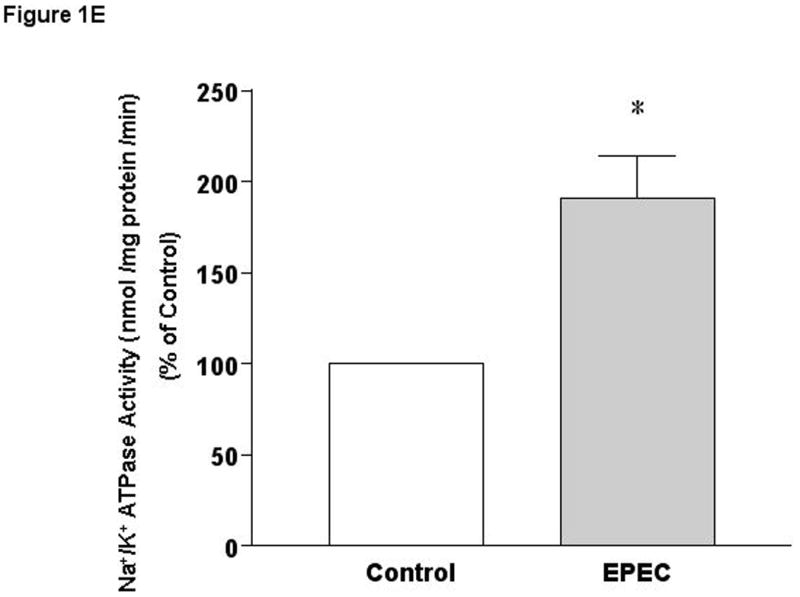
A) Time course of EPEC infection on 3[H]-5-HT uptake. B) Effects of non-pathogenic E coli on 3[H]-5-HT uptake. C and D) Effects of EPEC infection on apical and basolateral 5-HT uptake. Results are depicted as % of control. N=6-9. *P<0.001 vs control. [Control Values in pmol/mg protein/ 5 min: A) 0.27 ± 0.014; B) 0.33 ± 0.010; C) 0.22 ± 0.019 and D) 0.43 ± 0.005]. E). EPEC infection increases Na+/K+ATPase activity in Caco-2 cells. N=3. * P < 0.001 vs control.
SERT is a secondary active transporter that maintains its electrochemical gradient by the action of Na+/K+ATPase. It is possible that a decrease in the ionic gradient following EPEC infection might lead to reduced serotonin uptake. Intriguingly, Na+/K+ATPase activity was stimulated by ∼ 2 fold in Caco-2 cells infected with EPEC for 60 min (Figure 1E), indicating that the observed inhibition in SERT activity by EPEC could not be secondary to alterations in Na+/K+ATPase activity.
Effects of EPEC are functional TTSS dependent
To elucidate the mechanisms of inhibition of SERT activity by EPEC, Caco-2 cells were exposed to sterile culture supernatant (CS) of bacteria grown in DMEM 25. EPEC CS failed to inhibit SERT activity unlike infection with whole bacteria (not shown) indicating that the released soluble components do not mediate SERT inhibition. We next investigated if attachment of EPEC to host cells via plasmid-encoded bundle-forming pilus (BFP) 26-28 is necessary for SERT inhibition. Previous studies have shown that attachment of bfp mutant of EPEC to epithelial cells is significantly lower compared to wild type EPEC 18. Infection of Caco-2 cells with bfp mutant abrogated the inhibition of SERT activity (Figure 2A). EPEC also encodes a type 3 secretion system (T3SS) that injects E. coli secreted proteins (Esps) into the host cytosol. EscN is the putative ATPase that drives T3SS 29. Infection of Caco-2 cells with the escN mutant had no effect on SERT activity indicating that this response is T3SS dependent (Figure 2A). We further investigated the role of the structural components of T3SS namely EspA, (forms a filamentous extension of the T3SS)30, 31 and EspB and EspD, (provide a translocation pore)26, 32. Infection of cells with deletion mutants of espA, espB, or espD failed to inhibit SERT activity (Figure 2B). These results indicate that the structural components of the T3SS or the secreted effector molecules mediate the inhibitory effect of EPEC on SERT activity.
Figure 2. EPEC effects are T3SS Dependent.
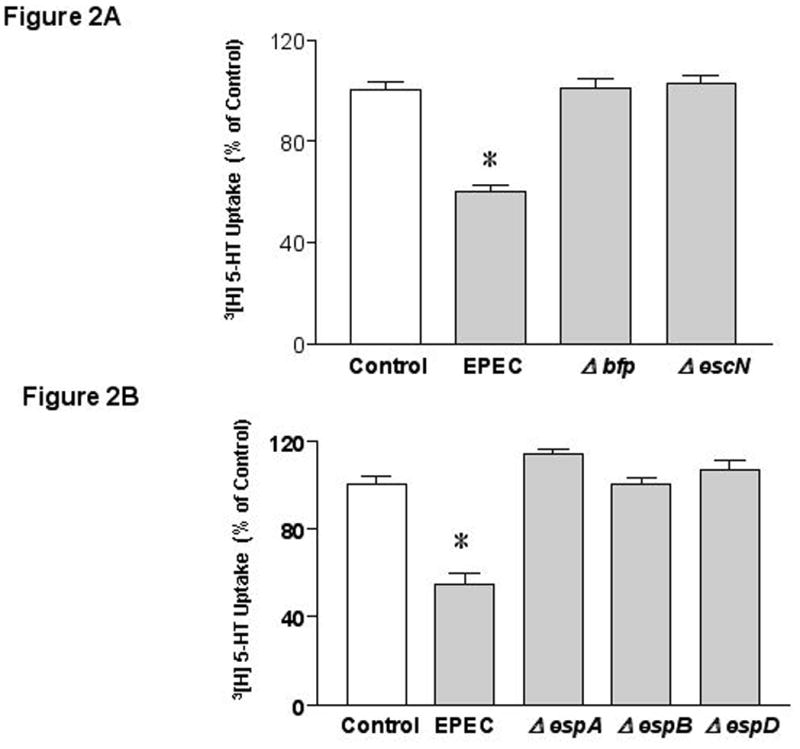
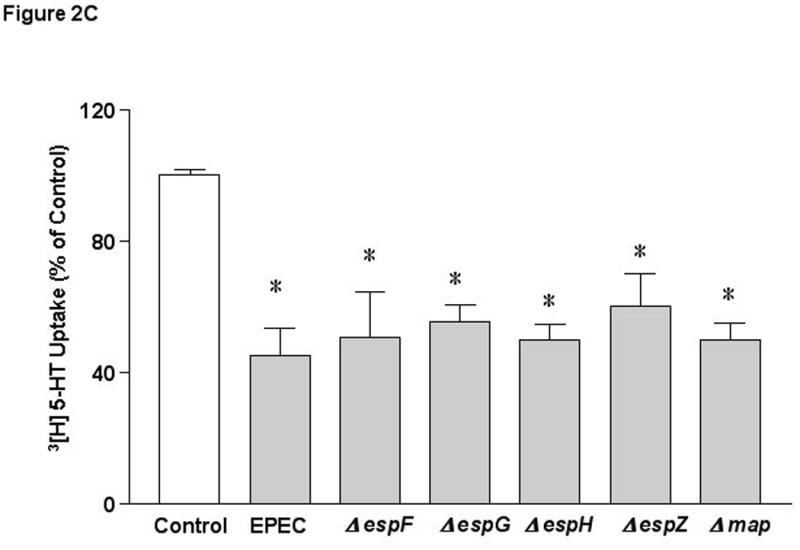
A) bfp and T3SS are essential for EPEC mediated effects. B) Structural components of T3SS are necessary for SERT inhibition. C) Role of effector molecules. N=6 * P < 0.01 vs control. [Control Values in pmol/mg protein/ 5 min: A) 0.39 ± 0.015; B) 0.35 ± 0.017; C) 0.320 ± 0.006].
Several T3SS effector molecules have been previously described 12, 33-35. Deletion of espF (involved in barrier disruption)12, espG or espG2, (implicated in microtubulular network disruption)33, espH (involved in pedestal formation and filopodia formation) 35, espZ37 (unknown function) or map (alters mitochondrial membrane potential)34 had no effect on EPEC-mediated inhibition in SERT activity (Figure 2C). These studies rule out the involvement of these specific effector molecules in EPEC-induced inhibition of SERT.
EPEC infection decreases the maximal velocity of 5-HT uptake
The mechanism underlying EPEC-mediated inhibition of SERT activity was further examined by performing kinetic studies. EPEC infection significantly decreased SERT activity at all concentrations (25nM-400nM) (Figure 3A). Analysis of the kinetic parameters by GraphPad Prism revealed that maximal velocity of 5-HT uptake was significantly reduced (Vmax: in pmol.mg protein− 1.5min−1: 1.09±0.19 for control vs. 0.35* ± 0.06 in response to EPEC infection, *P< 0.001 vs control). In contrast, the apparent Michaelis constant (Km in nM) was unaltered (control 179 ± 20.8 vs. EPEC infected 135 ± 30.4). These results indicated that the number of active SERT available to the substrate is reduced by EPEC with no change in the affinity of the 5-HT for SERT.
Figure 3. Mechanisms of EPEC induced Inhibition of SERT.
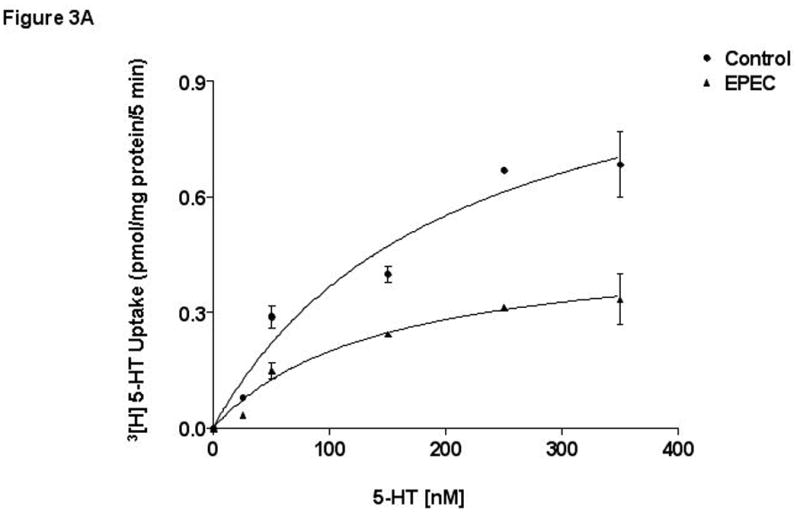
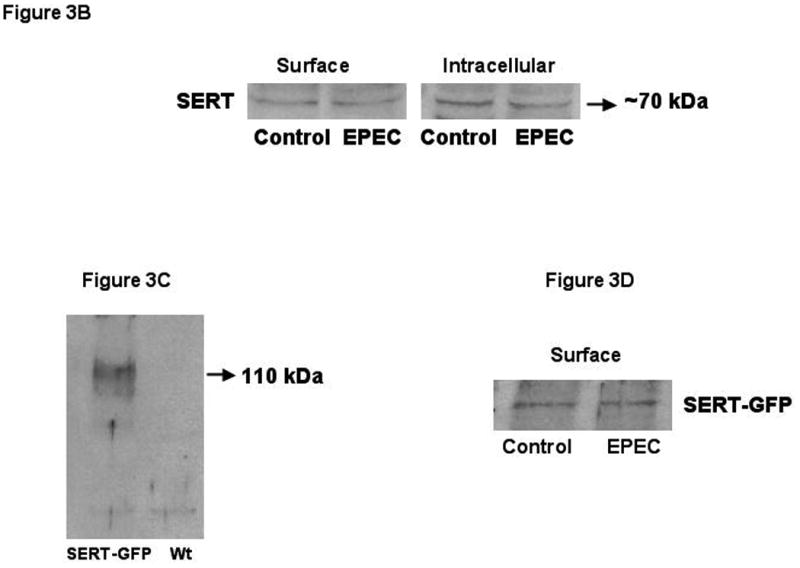
A)Kinetic studies of EPEC induced inhibition. A Michaelis–Menten plot from a representative experiment is shown. N=5. * P < 0.01 compared to control. B) Cell-surface expression of SERT. Caco-2 monolayers were infected with wild-type EPEC for 60 minutes and subjected to biotinylation. C) SERT expression in transiently transfected Caco-2 cells. SERT expression in wild type untransfected and cells transiently transfected with SERT-GFP construct utilizing ant-GFP antibodies. D) EPEC does not alter cell-surface expression of SERT-GFP: Representative blots from 3 different experiments are shown.
To investigate the possibility that EPEC modulates SERT expression, we first examined the SERT mRNA levels by real time QRT-PCR. EPEC infection of Caco-2 monolyers with EPEC for 1-4h did not alter the SERT mRNA expression [Relative SERT mRNA / Histone mRNA Expression: Control: 1.0; EPEC 1h: 1.25 ± 0.15; EPEC 4h: 1.34 ± 0.27].
Since SERT mRNA levels remained unaltered, we examined whether EPEC infected monolayers exhibit reduced SERT levels on the plasma membrane utilizing cell surface biotinylation studies. The anti-SERT antibody used to detect endogenous SERT expression showed multiple bands in Caco-2 cell extracts. However, the expected size band (∼70 kDa) of SERT was expressed at similar levels on the plasma membrane of control and EPEC-infected cells (Figure 3B). Biotinylation studies were also performed in Caco-2 cells transiently transfected with a SERT-GFP construct (supplemental methods) to enhance the specificity of detection. As shown in Figure 3C, a single band ∼110 kDa was observed in cells transiently transfected with SERT-GFP fusion construct as compared to untransfected cells. However, EPEC infection of Caco-2 cells did not alter SERT-GFP expression (∼110 kDa) on the plasma membrane (Figure 3D). Total cellular levels of SERT were also similar in control and EPEC-infected cells.
Role of signaling intermediates
SERT has been shown to be regulated by various signaling pathways such as PKC and MAP kinases 38, 39. EPEC is also known to induce numerous signaling molecules in host cells 16, 40-42. The inhibition of 5-HT uptake in response to EPEC infection was unaltered in the presence of inhibitors of PKC (BIM, 10 μM,) PKA (RpcAMP, 20 μM), microtubule (colchicine 100 μM), PI3 Kinase (LY294002, 20 μM), intracellular calcium, (BAPTA-AM, 10 μM), tyrosine kinase (herbimycin, 10 μM), pinocytosis (amiloride, 1mM) or endocytosis inhibitor (nystatin 5-50 μM) (data not shown).
EPEC infection is also known to induce tyrosine dephosphorylation of various host proteins 42. To examine the involvement of protein tyrosine phosphatases (PTPs) in EPEC mediated effects, phenylarsine oxide (PAO) that cross-links vicinal thiol groups and thereby inactivates phosphatases possessing XCysXXCysX motifs was utilized. EPEC-induced inhibition of 5-HT uptake was abrogated in the presence of 5-10 μM concentrations of PAO suggesting the involvement of PTPs in EPEC mediated effects (Figure 4A). The specificity of action of PAO was examined by using other inhibitors of PTPases including ortho-vanadate (Figure 4B) and 3,4 dephostatin (DP) [% of Control (EPEC: 60* ± 5; DP: 105 ± 10; EPEC + DP*: 83 ± 9], which significantly attenuated the inhibition of SERT.
Figure 4. Protein phosphotyrosine phosphatases (PTP) mediate the effects of EPEC.
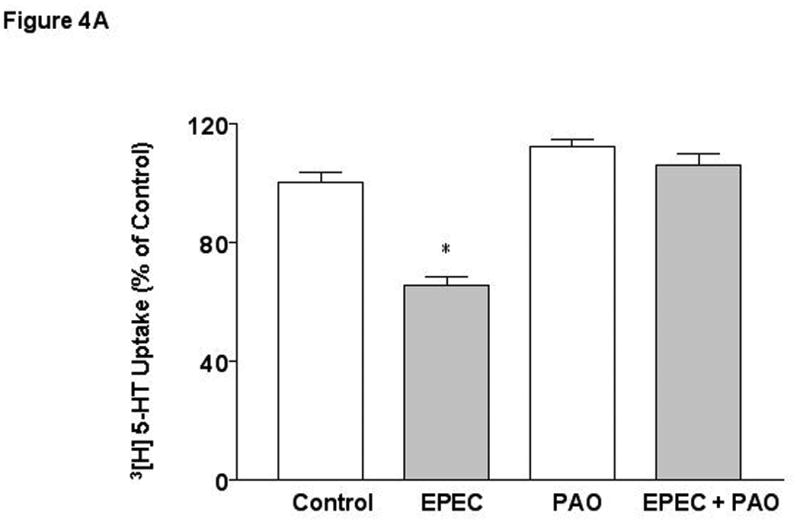
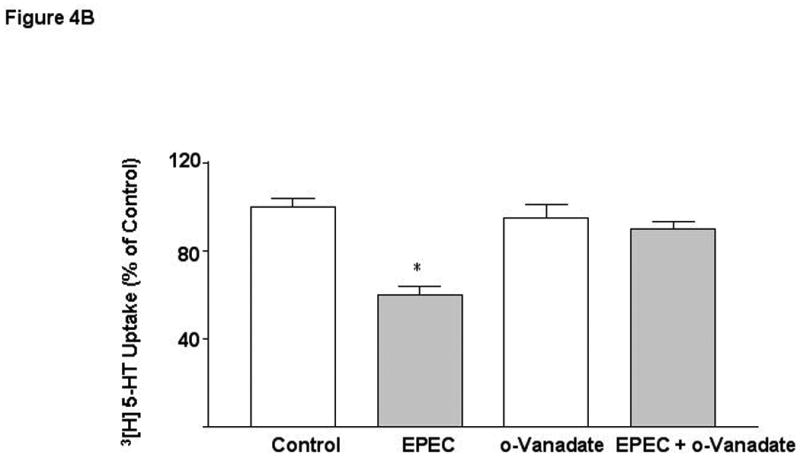
Caco-2 monolayers were pre-treated with specific PTP inhibitors A) Phenyl-arsineoxide (PAO) and B) o-vanadate for 30 min and then infected with EPEC in the presence or absence for inhibitors for another 60 min. N=3. * P < 0.01 vs control. [Control Values in pmol/mg protein/ 5 min: A) 0.27 ± 0.006; B 0.38 ± 0.018].
Effects of EPEC infection in in vivo mouse model
SERT mRNA expression: Our previous studies demonstrated that SERT is expressed in the human duodenum and ileum with negligible expression in the colon 7. However, a comprehensive study regarding SERT expression in different regions of the mouse intestine is lacking. SERT mRNA expression was found to be markedly higher in the proximal and distal small intestine compared to colon (Figure 5A). EPEC infection of mice significantly decreased small intestinal SERT mRNA levels at 14h and 18h post-infection that returned to baseline at 24h (Figure 5B).
Figure 5. SERT mRNA expression in murine model of EPEC infection.
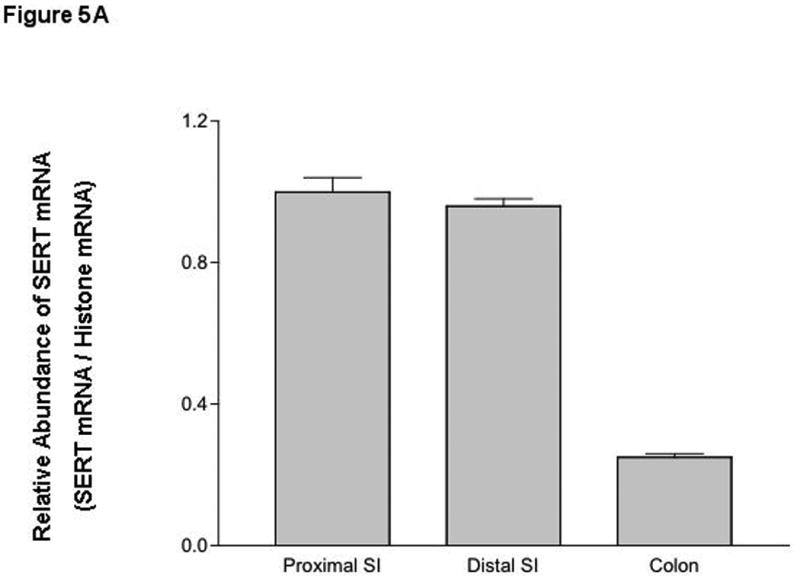
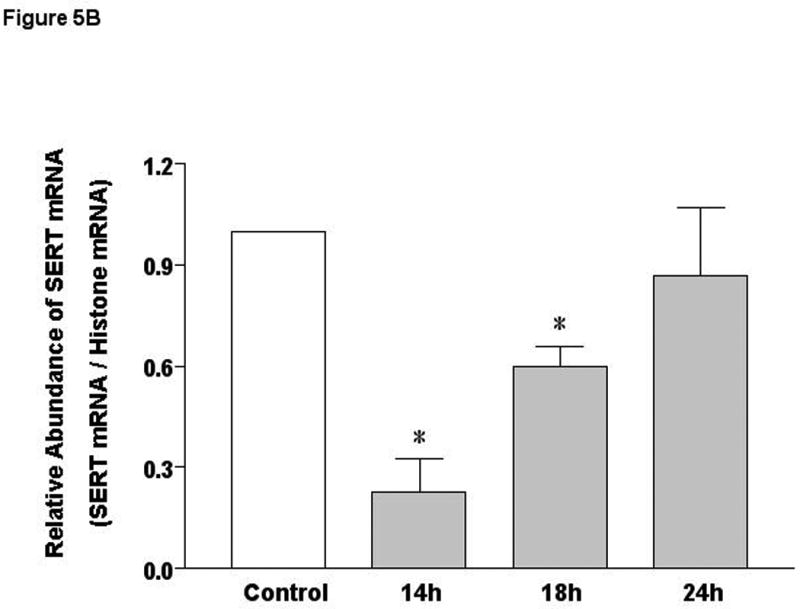
A) SERT mRNA expression in native mouse intestine. B) Time course of EPEC infection on SERT mRNA expression. N=3-7. *P< 0.001 vs control.
SERT protein expression: Immunofluorescence studies showed that SERT was detected primarily on the apical membranes (red) co-localized with the apical membrane marker villin (green) in mouse small intestine. EPEC infection of mice for 24h, however, led to a significant loss of SERT protein (red) on the apical membranes of small intestinal epithelial cells. This decrease in SERT expression was not associated with reduced villin content (structural marker of brush border membrane)56 (Figure 6). This is important since EPEC is known to efface intestinal microvilli.
Figure 6. SERT immunostaining in EPEC infected mouse small intestine.
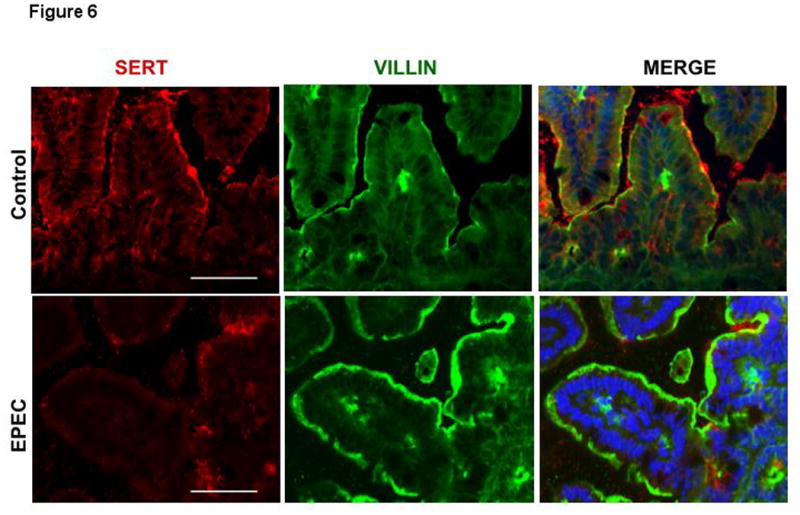
Immunofluorescent staining for SERT (red) and villin (green) with blue counterstained nuclei was performed on OCT sections of control and EPEC infected mouse distal small intestine. Scale bar = 50 μm. A representative of 4 different experiments is shown.
SERT function and 5-HT content: Corresponding with decreased SERT expression, EPEC infection for 24h significantly decreased initial uptake of 3[H]-5-HT in the purified small intestinal AMVs (Figure 7A). A decrease in SERT function and expression would result in a decrease in mucosal 5-HT content leading to an increased luminal 5-HT availability. In parallel, a significant decrease was observed in mucosal serotonin content in the small intestinal mucosa of EPEC-infected mice compared to uninfected controls (Figure 7B).
Figure 7. Effects of EPEC infection on SERT function and 5-HT mucosal content.
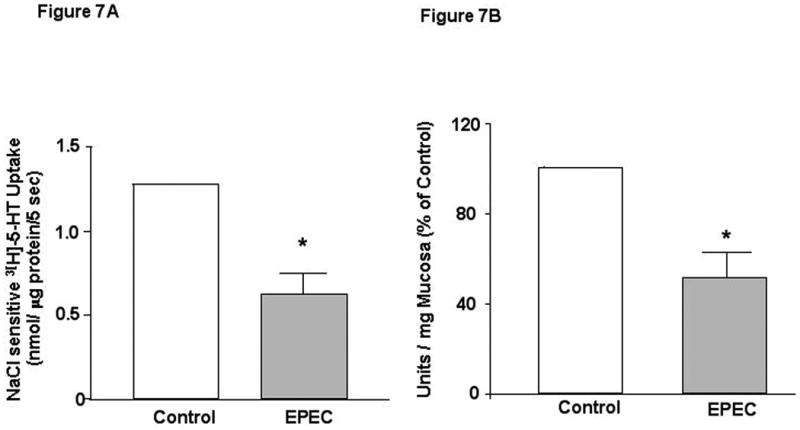
A) EPEC inhibits [3H]5-HT uptake. N=3. B) 5-HT mucosal content in response to EPEC infection. N=3. * P < 0.05 vs control.
Mechanistic link between in vitro vs in vivo mouse model of EPEC infection
The differences in data obtained from our in vitro and in vivo models were carefully considered. Most striking was the decrease in SERT mRNA following EPEC infection of mice but not cultured intestinal epithelial cells. The effect of decreased SERT expression is an increase in luminal 5-HT. However, Caco-2 cells do not secrete 5-HT. We hypothesized that the absence of 5-HT in the in vitro cell culture model may account for the differences in SERT mRNA levels in in vitro and in vivo following EPEC infection. To test this hypothesis, Caco-2 cells were treated with different concentrations of 5-HT. SERT mRNA expression was transiently down-regulated by 5-HT in Caco-2 cells at 18h which returned to baseline at 24h (Figure 8A). Correlative functional studies were performed whereby, SERT activity was measured in Caco-2 cells treated with 5-HT (10 μM) for 24h. As shown in Figure 8B, SERT activity was significantly decreased following 5-HT exposure. Previous studies have also shown a decrease in SERT function in response to long-term treatment of Caco-2 cells with serotonin 48. These data suggest that increased 5-HT levels in the lumen may contribute to the inhibition of SERT function and expression induced by EPEC infection.
Figure 8. 5-HT inhibits SERT function and expression in Caco-2 cells.
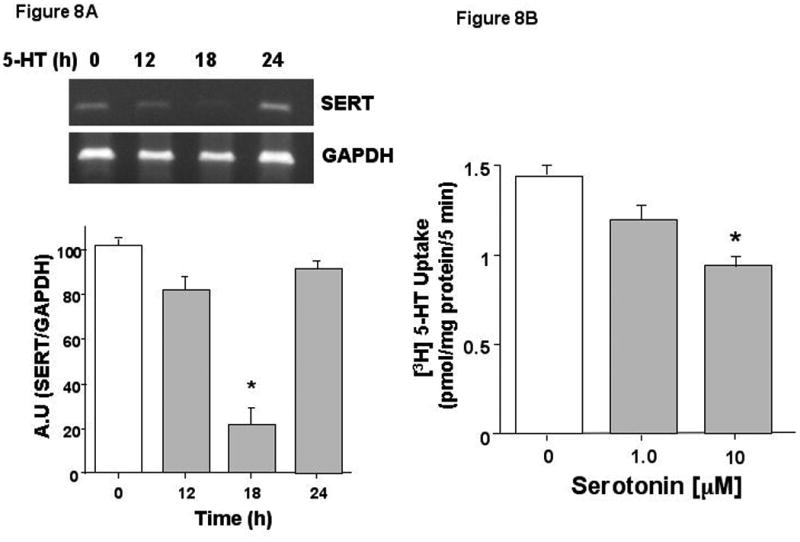
A) Semi-Quantitative RT-PCR was performed with total RNA extracted from control and 5-HT treated Caco-2 cells (n=3). B) Caco-2 cells were treated with 5-HT (0.1-10 μM) for 24 h and uptake of apical 5-HT was assessed with 200 nM of [3H] 5-HT for 5min (n=4-6). *P<0.05.
Discussion
Rapid onset of diarrhea is the predominant symptom of EPEC infection. EPEC is non-toxigenic 11 but produces a characteristic attaching and effacing lesion26 that was originally presumed to cause diarrhea due to loss of overall absorptive surface. However, studies in human volunteers showed that the incubation period between EPEC ingestion and onset of diarrhea is less than 4h 43, suggestive of alterations in ion transport mechanisms rather than a generalized effect of microvilli effacement. In this regard, we have previously shown that EPEC inhibits the absorption of Na+, Cl− and butyrate 14-16, 44. Dean et al. 17 reported that EPEC rapidly inactivates the sodium-D-glucose cotransporter (SGLT-1) in human intestinal epithelial cells. Further, infection of susceptible mice with C. rodentium (mouse homolog of EPEC) was associated with impaired intestinal ion transport and fatal fluid loss 45. In fact, microarray analysis revealed that amongst the differentially expressed genes in the C. rodentium infected susceptible and resistant mice, alterations in intestinal transport genes were over-represented compared to even the immune response related genes 45.
Current studies demonstrating that EPEC infection modulates SERT function represent another novel illustration of epithelial-microbial interactions that underlie the pathophysiology of associated diarrhea. Our in vitro studies utilizing Caco-2 cells, delineated the immediate events reducing SERT function following EPEC infection (30 min-2h). Since, SERT is predominantly expressed in the human small intestine as compared to colon 7,46, Caco-2 monolayers provided an excellent model system, representing the enterocyte phenotype upon differentiation, to assess SERT function and regulation 19, 47-49. Caco-2 cells have also been extensively used previously to study the function and regulation of various small intestinal ion and nutrient transporters 4, 14, 50-54. Assessment of the net impact of EPEC infection on SERT was performed utilizing in vivo mouse model of EPEC infection14, 55. It is impossible to replicate the in vitro models of infection in in vivo because of the complex native tissue and requirement of the passage of bacterium through the stomach plus additional barriers to adherence. We selected 14–24 post infection time period based on our previous studies showing that EPEC colonizes mouse ileum and colon as early as 24h 14, 55.
Our findings suggest that infection with EPEC alters SERT function in vitro without altering SERT gene expression, whereas, in vivo SERT gene expression is also decreased. We have identified a plausible explanation i.e differences in luminal 5-HT concentrations in the intestinal lumen for the discrepancy between the data from our in vitro and in vivo models. EPEC infection of mice decreased 5-HT mucosal content (which increases 5-HT availability in the gut lumen) a scenario lacking in the in vitro model of infected Caco-2 cells, as these cells do not secrete 5-HT. Interestingly, 5-HT treatment of Caco-2 monolayers directly inhibited SERT function via down-regulating SERT mRNA expression in Caco-2 cells at 18h that returned to baseline at 24h; the same pattern of SERT mRNA expression that occurred in the EPEC-infected mouse model. The transient decrease in SERT mRNA following 5-HT treatment of Caco-2 cells or EPEC infection could be explained by 5-HT receptor desensitization in response to high levels of 5-HT. Another possible contributing factor is an increase in pro-inflammatory cytokines such as TNF-α, which have been reported previously to down-regulate SERT mRNA in Caco-2 cells 47. In addition, EPEC has been shown to increase TNF-α expression in the murine model 18. It is also likely that infection may alter enterochromaffin cell density or function that may contribute to the diarrhea associated with EPEC infection in vivo. Importantly, our data from both in vitro and in vivo models demonstrate EPEC induced inhibition of SERT function (albeit by different mechanisms) that may underlie the diarrheal phenotype of the pathogen.
EPEC mediated inhibition of SERT could be secondary to modulation of other ion transporters. In this regard, 5-HT is known to modulate both Na+ and Cl− transport processes in the intestinal epithelial cells 57, 58. However, EPEC-mediated effects on epithelial ion transport system appear to be specific. Our previous studies demonstrated that EPEC exhibited differential effects on NHE isoform activity, with an inhibition in NHE3 activity via EspF 36 and stimulation in NHE2 activity via PKC 16. However, the effects of EPEC on Cl−/OH− exchange activity were induced by EspG/EspG2 14. In contrast, the known EPEC effector molecule mediating SERT inhibition has not been determined. Also, EPEC mediated effects on SERT appear to be not attributed to alterations in the electrochemical gradient as Na+-K+-ATPase activity in Caco-2 cells was increased. In parallel, EPEC infection of mice increased Na+-K+-ATPase mRNA expression in the small intestine (supplemental Figure 1).
Notably, our findings for the first time demonstrate the involvement of protein tyrosine phosphatases (PTP) in inhibition of SERT by EPEC. These data are in accordance with previous studies that identified tyrosine phosphorylation and dephosphorylation events following EPEC infection in HeLa and Caco-2 cells 42. S. typhimurium, is also known to secrete a modular effector protein SptP, homologous to the catalytic domains of PTP 59. Additionally, Yersinia spp. has been shown to induce the tyrosine dephosphorylation of host proteins as virulent mechanism 60. Interestingly, SERT has been shown to undergo basal phosphorylation that is acutely increased by treatments with PKC activators and protein phosphatase inhibitors 61. Previous studies utilizing HEK-293 cells have demonstrated that SERT phosphorylation by PKC activation leads to SERT internalization, while SERT dephosphorylation by p38 MAPK inhibition attenuates SERT insertion to the plasma membrane 38, 39. We previously showed that EPEC infection decreases Cl− and butyrate absorption in human intestinal epithelial cells via internalization of SLC26A314 and monocarboxylate transporter44 respectively. However, our current data provide evidence that EPEC-mediated inhibition of SERT activity is independent of membrane trafficking events. Nonetheless, a decrease in Vmax of the transporter following EPEC infection indicates that EPEC-induced dephosphorylation events may have a direct effect on SERT, resulting in a lowering of the transporter membrane turnover rate or changes in the distribution of the transporter at the plasma membrane level such as via lipid ratfs.
In conclusion, our findings suggest a two-phase model of SERT inhibition following EPEC infection. In the first phase, SERT function is rapidly reduced via protein tyrosine phosphatases followed by a decrease in SERT expression as evidenced in the in vivo mouse model. Our studies are significant in providing mechanistic insights that may offer novel strategies for treatment of diarrheal diseases. Further, EPEC may provide a prototype for understanding the regulation of SERT, an effective pharmacological target of gastrointestinal disorders.
Supplementary Material
Acknowledgments
These studies were supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-074459 (R. K. Gill), P01 DK-067887 (G. A. Hecht, J. R. Turner), DK-09930 (W. A. Alrefai), DK 061931 and DK068271 (J. R. Turner).
Footnotes
No Conflict of Interest exists for: Ali Esmaili, Saad Nazir, Alip Borthakur, Dan Yu, Jerrold Turner, Seema Saksena, Amika Singla, Gail Hecht, Waddah Alrefai and Ravinder Gill.
Contribution of Authors: Ali Esmaili (Acquisition of data, analysis, drafting of manuscript), Saad Nazir (Acquisition of data), Alip Borthakur (Technical support), Dan Yu (Acquisition of data), Jerrold Turner (Acquisition of data and manuscript preparation), Seema Saksena (Technical support and preparation of manuscript), Amika Singla (Technical support), Gail Hecht (Data analysis and interpretation, preparation of manuscript), Waddah Alrefai (Critical revision of manuscript and data analysis), and Ravinder Gill (Concept, design, acquisition of data, analysis and interpretation, statistical analysis, critical revision)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tobe T, Fujiwara M, Tanaka C. Distribution of serotonin (5-hydroxytryptamine) in the human gastroinetestinal tract. Am J Gastroenterol. 1966;46:34–7. [PubMed] [Google Scholar]
- 2.Gill R, Hadjiagapiou C, Alrefai WA, Saksena S, Carrol R, Goldstein J, Ramaswamy K, Dudeja PK. Expression and membrane localization of MCT isoforms along the length of the human intestine. Am J Physiol, (cell) 2005 May 18; doi: 10.1152/ajpcell.00112.2005. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 3.Chen JX, Pan H, Rothman TP, Wade PR, Gershon MD. Guinea pig 5-HT transporter: cloning, expression, distribution, and function in intestinal sensory reception. Am J Physiol. 1998;275:G433–48. doi: 10.1152/ajpgi.1998.275.3.G433. [DOI] [PubMed] [Google Scholar]
- 4.Gill RK, Saksena S, Tyagi S, Alrefai WA, Malakooti J, Sarwar Z, Turner JR, Ramaswamy K, Dudeja PK. Serotonin inhibits Na+/H+ exchange activity via 5-HT4 receptors and activation of PKC alpha in human intestinal epithelial cells. Gastroenterology. 2005;128:962–74. doi: 10.1053/j.gastro.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Takayanagi S, Hanai H, Kumagai J, Kaneko E. Serotonin uptake and its modulation in rat jejunal enterocyte preparation. J Pharmacol Exp Ther. 1995;272:1151–9. [PubMed] [Google Scholar]
- 6.Miwa J, Echizen H, Matsueda K, Umeda N. Patients with constipation-predominant irritable bowel syndrome (IBS) may have elevated serotonin concentrations in colonic mucosa as compared with diarrhea-predominant patients and subjects with normal bowel habits. Digestion. 2001;63:188–94. doi: 10.1159/000051888. [DOI] [PubMed] [Google Scholar]
- 7.Gill RK, Pant N, Saksena S, Singla A, Nazir TM, Vohwinkel L, Turner JR, Goldstein J, Alrefai WA, Dudeja PK. Function, expression, and characterization of the serotonin transporter in the native human intestine. Am J Physiol Gastrointest Liver Physiol. 2008;294:G254–62. doi: 10.1152/ajpgi.00354.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellini M, Rappelli L, Blandizzi C, Costa F, Stasi C, Colucci R, Giannaccini G, Marazziti D, Betti L, Baroni S, Mumolo MG, Marchi S, Del Tacca M. Platelet serotonin transporter in patients with diarrhea-predominant irritable bowel syndrome both before and after treatment with alosetron. Am J Gastroenterol. 2003;98:2705–11. doi: 10.1111/j.1572-0241.2003.08669.x. [DOI] [PubMed] [Google Scholar]
- 9.Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM, Moses PL. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–64. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Wheatcroft J, Wakelin D, Smith A, Mahoney CR, Mawe G, Spiller R. Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterol Motil. 2005;17:863–70. doi: 10.1111/j.1365-2982.2005.00719.x. [DOI] [PubMed] [Google Scholar]
- 11.Hecht G. Microbes and microbial toxins: paradigms for microbial-mucosal interactions. VII. Enteropathogenic Escherichia coli: physiological alterations from an extracellular position. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1–7. doi: 10.1152/ajpgi.2001.281.1.G1. [DOI] [PubMed] [Google Scholar]
- 12.McNamara BP, Koutsouris A, O'Connell CB, Nougayrede JP, Donnenberg MS, Hecht G. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J Clin Invest. 2001;107:621–9. doi: 10.1172/JCI11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borthakur A, Gill RK, Hodges K, Ramaswamy K, Hecht G, Dudeja PK. Enteropathogenic Escherichia coli inhibits butyrate uptake in Caco-2 cells by altering the apical membrane MCT1 level. Am J Physiol Gastrointest Liver Physiol. 2006;290:G30–5. doi: 10.1152/ajpgi.00302.2005. [DOI] [PubMed] [Google Scholar]
- 14.Gill RK, Borthakur A, Hodges K, Turner JR, Clayburgh DR, Saksena S, Zaheer A, Ramaswamy K, Hecht G, Dudeja PK. Mechanism underlying inhibition of intestinal apical Cl/OH exchange following infection with enteropathogenic E. coli. J Clin Invest. 2007;117:428–37. doi: 10.1172/JCI29625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hecht G, Hodges K, Gill RK, Kear F, Tyagi S, Malakooti J, Ramaswamy K, Dudeja PK. Differential regulation of Na+/H+ exchange isoform activities by enteropathogenic E. coli in human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G370–8. doi: 10.1152/ajpgi.00432.2003. [DOI] [PubMed] [Google Scholar]
- 16.Hodges K, Gill R, Ramaswamy K, Dudeja PK, Hecht G. Rapid activation of Na+/H+ exchange by EPEC is PKC mediated. Am J Physiol Gastrointest Liver Physiol. 2006;291:G959–68. doi: 10.1152/ajpgi.00274.2005. [DOI] [PubMed] [Google Scholar]
- 17.Dean P, Maresca M, Schuller S, Phillips AD, Kenny B. Potent diarrheagenic mechanism mediated by the cooperative action of three enteropathogenic Escherichia coli-injected effector proteins. Proc Natl Acad Sci U S A. 2006;103:1876–81. doi: 10.1073/pnas.0509451103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savkovic SD, Villanueva J, Turner JR, Matkowskyj KA, Hecht G. Mouse model of enteropathogenic Escherichia coli infection. Infect Immun. 2005;73:1161–70. doi: 10.1128/IAI.73.2.1161-1170.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martel F, Monteiro R, Lemos C. Uptake of serotonin at the apical and basolateral membranes of human intestinal epithelial (Caco-2) cells occurs through the neuronal serotonin transporter (SERT) J Pharmacol Exp Ther. 2003;306:355–62. doi: 10.1124/jpet.103.049668. [DOI] [PubMed] [Google Scholar]
- 20.Esmann M. ATPase and phosphatase activity of Na+,K+-ATPase: molar and specific activity, protein determination. Methods Enzymol. 1988;156:105–15. doi: 10.1016/0076-6879(88)56013-5. [DOI] [PubMed] [Google Scholar]
- 21.Newaz MA, Ranganna K, Oyekan AO. Relationship between PPARalpha activation and NO on proximal tubular Na+ transport in the rat. BMC Pharmacol. 2004;4:1. doi: 10.1186/1471-2210-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borthakur A, Gill RK, Tyagi S, Koutsouris A, Alrefai WA, Hecht GA, Ramaswamy K, Dudeja PK. The probiotic Lactobacillus acidophilus stimulates chloride/hydroxyl exchange activity in human intestinal epithelial cells. J Nutr. 2008;138:1355–9. doi: 10.1093/jn/138.7.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berteloot A, Bennetts RW, Ramaswamy K. Transport characteristics of papain-treated brush border membrane vesicles: Non-involvement of g-glutamyltransferase in leucine transport. Biochim Biophys Acta. 1980;601:592–604. doi: 10.1016/0005-2736(80)90561-1. [DOI] [PubMed] [Google Scholar]
- 24.Schmitz JC, Preiser H, Maestracci D, Ghosh BK, Cerdo JJ, Crane RK. Purification of human intestinal brush border membrane. Biochem Biophys Acta. 1973;322:98–112. doi: 10.1016/0005-2736(73)90434-3. [DOI] [PubMed] [Google Scholar]
- 25.Sharma R, Tesfay S, Tomson FL, Kanteti RP, Viswanathan VK, Hecht G. Balance of bacterial pro- and anti-inflammatory mediators dictates net effect of enteropathogenic Escherichia coli on intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2006;290:G685–94. doi: 10.1152/ajpgi.00404.2005. [DOI] [PubMed] [Google Scholar]
- 26.Frankel G, Phillips AD, Rosenshine I, Dougan G, Kaper JB, Knutton S. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol Microbiol. 1998;30:911–21. doi: 10.1046/j.1365-2958.1998.01144.x. [DOI] [PubMed] [Google Scholar]
- 27.Donnenberg MS, Kaper JB, Finlay BB. Interactions between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol. 1997;5:109–14. doi: 10.1016/S0966-842X(97)01000-7. [DOI] [PubMed] [Google Scholar]
- 28.Donnenberg MS, Zhang HZ, Stone KD. Biogenesis of the bundle-forming pilus of enteropathogenic Escherichia coli: reconstitution of fimbriae in recombinant E. coli and role of DsbA in pilin stability--a review. Gene. 1997;192:33–8. doi: 10.1016/s0378-1119(96)00826-8. [DOI] [PubMed] [Google Scholar]
- 29.Gauthier A, Puente JL, Finlay BB. Secretin of the enteropathogenic Escherichia coli type III secretion system requires components of the type III apparatus for assembly and localization. Infect Immun. 2003;71:3310–9. doi: 10.1128/IAI.71.6.3310-3319.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daniell SJ, Takahashi N, Wilson R, Friedberg D, Rosenshine I, Booy FP, Shaw RK, Knutton S, Frankel G, Aizawa S. The filamentous type III secretion translocon of enteropathogenic Escherichia coli. Cell Microbiol. 2001;3:865–71. doi: 10.1046/j.1462-5822.2001.00168.x. [DOI] [PubMed] [Google Scholar]
- 31.Sekiya K, Ohishi M, Ogino T, Tamano K, Sasakawa C, Abe A. Supermolecular structure of the enteropathogenic Escherichia coli type III secretion system and its direct interaction with the EspA-sheath-like structure. Proc Natl Acad Sci U S A. 2001;98:11638–43. doi: 10.1073/pnas.191378598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDaniel TK, Kaper JB. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol Microbiol. 1997;23:399–407. doi: 10.1046/j.1365-2958.1997.2311591.x. [DOI] [PubMed] [Google Scholar]
- 33.Elliott SJ, Krejany EO, Mellies JL, Robins-Browne RM, Sasakawa C, Kaper JB. EspG, a novel type III system-secreted protein from enteropathogenic Escherichia coli with similarities to VirA of Shigella flexneri. Infect Immun. 2001;69:4027–33. doi: 10.1128/IAI.69.6.4027-4033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kenny B, Jepson M. Targeting of an enteropathogenic Escherichia coli (EPEC) effector protein to host mitochondria. Cell Microbiol. 2000;2:579–90. doi: 10.1046/j.1462-5822.2000.00082.x. [DOI] [PubMed] [Google Scholar]
- 35.Tu X, Nisan I, Yona C, Hanski E, Rosenshine I. EspH, a new cytoskeleton-modulating effector of enterohaemorrhagic and enteropathogenic Escherichia coli. Mol Microbiol. 2003;47:595–606. doi: 10.1046/j.1365-2958.2003.03329.x. [DOI] [PubMed] [Google Scholar]
- 36.Hodges K, Alto NM, Ramaswamy K, Dudeja PK, Hecht G. The enteropathogenic Escherichia coli effector protein EspF decreases sodium hydrogen exchanger 3 activity. Cell Microbiol. 2008;10:1735–45. doi: 10.1111/j.1462-5822.2008.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanack KJ, Crawford JA, Tatsuno I, Karmali MA, Kaper JB. SepZ/EspZ is secreted and translocated into HeLa cells by the enteropathogenic Escherichia coli type III secretion system. Infect Immun. 2005;73:4327–37. doi: 10.1128/IAI.73.7.4327-4337.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jayanthi LD, Samuvel DJ, Blakely RD, Ramamoorthy S. Evidence for biphasic effects of protein kinase C on serotonin transporter function, endocytosis, and phosphorylation. Mol Pharmacol. 2005;67:2077–87. doi: 10.1124/mol.104.009555. [DOI] [PubMed] [Google Scholar]
- 39.Samuvel DJ, Jayanthi LD, Bhat NR, Ramamoorthy S. A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: evidence for distinct cellular mechanisms involved in transporter surface expression. J Neurosci. 2005;25:29–41. doi: 10.1523/JNEUROSCI.3754-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Celli J, Olivier M, Finlay BB. Enteropathogenic Escherichia coli mediates antiphagocytosis through the inhibition of PI 3-kinase-dependent pathways. Embo J. 2001;20:1245–58. doi: 10.1093/emboj/20.6.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malladi V, Shankar B, Williams PH, Balakrishnan A. Enteropathogenic Escherichia coli outer membrane proteins induce changes in cadherin junctions of Caco-2 cells through activation of PKCalpha. Microbes Infect. 2004;6:38–50. doi: 10.1016/j.micinf.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 42.Kenny B, Finlay BB. Intimin-dependent binding of enteropathogenic Escherichia coli to host cells triggers novel signaling events, including tyrosine phosphorylation of phospholipase C-gamma1. Infect Immun. 1997;65:2528–36. doi: 10.1128/iai.65.7.2528-2536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goosney DL, Gruenheid S, Finlay BB. Gut feelings: enteropathogenic E. coli (EPEC) interactions with the host. Annu Rev Cell Dev Biol. 2000;16:173–89. doi: 10.1146/annurev.cellbio.16.1.173. [DOI] [PubMed] [Google Scholar]
- 44.Borthakur A, Hodges K, Gill R, Ramaswamy K, Hecht G, Dudeja PK. Enteropathogenic E coli (EPEC) infection inhibits butyrate uptake in Caco-2 cells. Gastroenterology. 2004;126:A-295. [Google Scholar]
- 45.Borenshtein D, Fry RC, Groff EB, Nambiar PR, Carey VJ, Fox JG, Schauer DB. Diarrhea as a cause of mortality in a mouse model of infectious colitis. Genome Biol. 2008;9:R122. doi: 10.1186/gb-2008-9-8-r122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meier Y, Eloranta JJ, Darimont J, Ismair MG, Hiller C, Fried M, Kullak-Ublick GA, Vavricka SR. Regional distribution of solute carrier mRNA expression along the human intestinal tract. Drug Metab Dispos. 2007;35:590–4. doi: 10.1124/dmd.106.013342. [DOI] [PubMed] [Google Scholar]
- 47.Foley KF, Pantano C, Ciolino A, Mawe GM. IFN-gamma and TNF-alpha decrease serotonin transporter function and expression in Caco2 cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G779–84. doi: 10.1152/ajpgi.00470.2006. [DOI] [PubMed] [Google Scholar]
- 48.Iceta R, Aramayona JJ, Mesonero JE, Alcalde AI. Regulation of the human serotonin transporter mediated by long-term action of serotonin in Caco-2 cells. Acta Physiol (Oxf) 2008;193:57–65. doi: 10.1111/j.1748-1716.2007.01793.x. [DOI] [PubMed] [Google Scholar]
- 49.Iceta R, Mesonero JE, Alcalde AI. Effect of long-term fluoxetine treatment on the human serotonin transporter in Caco-2 cells. Life Sci. 2007;80:1517–24. doi: 10.1016/j.lfs.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 50.Said HM, Ortiz A, Kumar CK, Chatterjee N, Dudeja PK, Rubin S. Transport of thiamine in human intestine: mechanism and regulation in intestinal epithelial cell model Caco-2. Am J Physiol. 1999;277:C645–51. doi: 10.1152/ajpcell.1999.277.4.C645. [DOI] [PubMed] [Google Scholar]
- 51.Said HM, Ortiz A, Ma TY. A carrier-mediated mechanism for pyridoxine uptake by human intestinal epithelial Caco-2 cells: regulation by a PKA-mediated pathway. Am J Physiol Cell Physiol. 2003;285:C1219–25. doi: 10.1152/ajpcell.00204.2003. [DOI] [PubMed] [Google Scholar]
- 52.Saksena S, Gill R, Tyagi S, Alrefai WA, Sarwar Z, Ramaswamy K, Dudeja PK. Involvement of 5-HT3/4 receptor subtypes in the serotonin induced inhibition of Cl(-)/OH(-) exchange activity in Caco-2 cells. J Biol Chem. 2005;280:11859–68. doi: 10.1074/jbc.M411553200. [DOI] [PubMed] [Google Scholar]
- 53.Turner JR, Black ED. NHE3-dependent cytoplasmic alkalinization is triggered by Na(+)-glucose cotransport in intestinal epithelia. Am J Physiol Cell Physiol. 2001;281:C1533–41. doi: 10.1152/ajpcell.2001.281.5.C1533. [DOI] [PubMed] [Google Scholar]
- 54.Pinto M, Robine-leon S, Tappay M. Enterocyte-like Differentiation and Polarization of the Human Colon Cell Line Caco2 in Culture. Bio Cell. 1983;47:323–330. [Google Scholar]
- 55.Shifflett DE, Clayburgh DR, Koutsouris A, Turner JR, Hecht GA. Enteropathogenic E. coli disrupts tight junction barrier function and structure in vivo. Lab Invest. 2005;85:1308–24. doi: 10.1038/labinvest.3700330. [DOI] [PubMed] [Google Scholar]
- 56.Coudrier E, Kerjaschki D, Louvard D. Cytoskeleton organization and submembranous interactions in intestinal and renal brush borders. Kidney Int. 1988;34:309–20. doi: 10.1038/ki.1988.183. [DOI] [PubMed] [Google Scholar]
- 57.Gill RK, Saksena S, Tyagi S, Alrefai WA, Malakooti J, Sarwar Z, Turner JR, Ramaswamy K, Dudeja PK. Serotonin inhibits NHE activity via 5-HT4 receptors and activation of PKCalpha in human intetsinal epithelial cells. Gastroenterology. 2005;128:962–74. doi: 10.1053/j.gastro.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 58.Saksena S, Gill RK, Tyagi S, Alrefai WA, Sarwar Z, Ramaswamy K, Dudeja PK. Involvement of c-Src and protein kinase C delta in the inhibition of Cl(-)/OH- exchange activity in Caco-2 cells by serotonin. J Biol Chem. 2005;280:11859–68. doi: 10.1074/jbc.M411553200. [DOI] [PubMed] [Google Scholar]
- 59.Kaniga K, Uralil J, Bliska JB, Galan JE. A secreted protein tyrosine phosphatase with modular effector domains in the bacterial pathogen Salmonella typhimurium. Mol Microbiol. 1996;21:633–41. doi: 10.1111/j.1365-2958.1996.tb02571.x. [DOI] [PubMed] [Google Scholar]
- 60.Black DS, Marie-Cardine A, Schraven B, Bliska JB. The Yersinia tyrosine phosphatase YopH targets a novel adhesion-regulated signalling complex in macrophages. Cell Microbiol. 2000;2:401–14. doi: 10.1046/j.1462-5822.2000.00061.x. [DOI] [PubMed] [Google Scholar]
- 61.Vaughan RA. Phosphorylation and regulation of psychostimulant-sensitive neurotransmitter transporters. J Pharmacol Exp Ther. 2004;310:1–7. doi: 10.1124/jpet.103.052423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


