Abstract
Tissue engineering is an interdisciplinary field that combines the principles of engineering, material and biological sciences toward the development of therapeutic strategies and biological substitutes that restore, maintain, replace or improve biological functions. The association of biomaterials, stem cells, growth and differentiation factors have yielded the development of new treatment opportunities in most of the biomedical areas, including Dentistry. The objective of this paper is to present the principles underlying tissue engineering and the current scenario, the challenges and the perspectives of this area in Dentistry.
Significance
The growth of tissue engineering as a research field have provided a novel set of therapeutic strategies for biomedical applications. The emerging knowledge arisen from studies in the dental area may translate into new methods for caring or improving the alternatives used to treat patients in the daily clinic.
Keywords: Tissue engineering, stem cells, scaffolds, molecular biology, restorative dentistry
1. Introduction
The use of synthetic restorative materials as substitutes for dental structures is a practice nearly as old as Dentistry itself [1]. To date, most of the procedures performed in Dentistry are limited to the replacement of damaged tissues for biocompatible synthetic materials that may not present chemical, biological, or physical characteristics and behaviors similar to the host tissues. These discrepancies, together with the hostile environment of the oral cavity, result in relatively short-lived successful outcomes and frequent need for re-treatment. Tissue Engineering is a multi-disciplinary field focused on the development of materials and strategies to replace damaged or lost tissues for biological materials by merging principles, methods and knowledge of chemistry, physics, engineering and biology [2]. The achievements obtained by tissue engineering in the past few years has resulted in new therapies such as the production of skin to treat burns [3], bone grafts to replace large bone defects [4], small-caliber arteries to treat atherosclerotic vascular disease [5] and cartilage for plastic and reconstructive surgeries [6]. Important advances have been reported in Dentistry aiming the regeneration of temporo-mandibular joint [7], periodontal ligament [8, 9], dentin [10], enamel [11, 12], pulp [10, 13] and integrated tooth tissues [14, 15].
The concept underlying tissue engineering was first proposed in the United States in the mid-1980s in order to reduce the donor scarcity to organ transplantation [16]. The classical cell-based tissue engineering approach involves the seeding of biodegradable scaffolds with cells and/or growth factors, then, implanting it in order to induce and conduct the tissue growth [17]. Obtaining good responses from this model demands the fine orchestration of the three tissue engineering fundamental elements: cells, scaffold and cell signaling. The objective of this review is to present the fundaments of the tissue engineering components and their application in Dentistry.
2. Tissue Engineering
2.1 Cells
Stem cells are clonogenic cells capable of self-renewal and capable of generating differentiated progenies. These cells are responsible for normal tissue renewal as well as for healing and regeneration after injuries [18]. Some stem cells are said to be pluripotent, i.e. have the ability to differentiate into many different cell types while the range of others are more restricted. The most pluripotent cells are found in the inner cell mass of blastocyst during the early stages of embryo development [19]. They can differentiate into each of the more than 200 cell types of the adult body [20] when exposed to appropriate stimuli. Along with the potential applications of totipotent cells lies a strong ethical discussion regarding the use of human embryos. This issue has strengthened the rational for the use of adult stem cells, which have been identified in every tissue formed after embryonic development and can be used to the same purpose of embryonic stem cells.
Studies have showed that it is possible to isolate clonogenic and highly proliferative cells from dental pulp using similar research protocol to isolate and characterize bone marrow stem cells [21]. Dental pulp stem cells (DPSC) can differentiate into multiple cell lineages, such as adipocytes, chrondocytes, neurons and odontoblasts [22-24]. Stem cells from human exfoliated deciduous teeth (SHED) were also identified and isolated [24]. SHED has the advantage of being retrievable from naturally exfoliated teeth, which are one of the only disposable post-natal human tissues. As primary teeth are clearly a feasible source of post-natal stem cells, the interest toward the differentiation power of SHED cells has increased. Indeed, today we know that SHED can undergo adipogenic, chondrogenic, osteogenic, endothelial and odontoblastic differentiation [10, 25, 26]. The ability that these cells have to cross lineage boundaries expands the potential use of SHED for therapies involving a large number of tissues (Fig. 1).
Fig. 1. The principles of Tissue Engineering using dental stem cells may allow the regeneration of osseous, neural and tooth-related tissues.
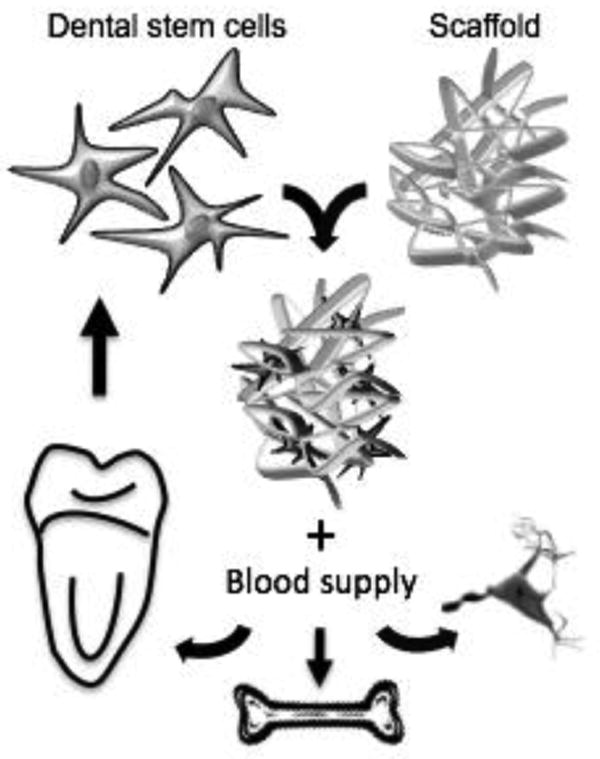
Although both DPSC and SHED cells are originated from the dental pulp, they present differences regarding the odontogenic differentiation and osteogenic induction. For example, the levels of alkaline phosphatase activity and osteocalcin production during osteogenic differentiation are higher for SHED than for DPSC [23]. However, the ability to regenerate a dentin-pulp-like complex found in DPSC [21, 24] is also observed in SHED cells [25]. Furthermore, SHED may present an osteoinductive potential once they were able to induce differentiation from recipient murine cells into bone-forming cells [24].
The periodontal ligament was found to be a source of que a novel population of dental stem cell (PDLSC - periodontal ligament stem cell). This cell express high levels of telomerase [27], a key molecule in mediating cell proliferation [28] and have the capacity to develop adipocytes and cementoblast/osteoblastic-like cells in vitro. In addition they also form collagen fibers, similar to Sharpey's fibers, and cementum/periodontal ligament-like tissue when transplanted into immunocompromised mice using hydroxyapatite/tricalcium phosphate (HA/TCP) scaffold [29, 30]
The stem cells from the apical papilla (SCAP) were recently isolated from the apical papilla of immature human permanent teeth [29]. The population seems to be the source of odontoblasts responsible for the formation of root dentin. [31]. These may be the reason why SCAP present similarities to DPSC regarding osteo/dentinogenic and growth factor receptor gene profiles. The in vivo implantation of SCAP with HA/TCP scaffold allowed the differentiation into odontoblast-like cells capable o regenerate a mineralized structure having a layer of dentin tissue formed over the surface of the HA/TCP besides connective tissue [32].
Additionally, the ability of SCAP to regenerate the periodontal ligament and alveolar bone in vivo [30]. Analogous to DPSC and SHED, SCAP express a wide variety of neurogenic markers attesting its neurogenic potential [32].
2.2 Scaffolds
Scaffolds are temporary frameworks used to provide a three-dimensional microenvironment where cells can proliferate, differentiate and generate the desired tissue [33]. The design of the ideal scaffold for each tissue to be formed is a challenging task. Ideally, a scaffold must allow cell attachment and migration, permit the localized and sustained delivery of growth factors, and enable the influx of oxygen to maintain the high metabolic demands of cells engaged in tissue regeneration.
Scaffolds are usually made from ceramics [34], natural or synthetic polymers [14], or composites from these materials [35]. The choice of scaffold material depends on the desired outcome thus physical (e.g. rheological behavior, mechanical properties, surface roughness and porosity) as well as chemical characteristics (e.g. mode, velocity and products of degradation) must be considered.
The scaffold's physical properties have to attend the needs of the target environment. It must present proper mechanical resistance to support in vivo stresses, and it should be mechanically compatible with the surrounding tissues [33, 36-38]. The scaffold's mechanical properties have a direct impact in tissue formation by affecting cell differentiation into the desired phenotype through mechanotransduction [33]. Therefore, linear elastic scaffolds are preferred when one attempts to generate bone, and nonlinear elastic or viscoelastic models are more suitable for soft tissues [37, 39]. Scaffold porosity is also critical to tissue generation. The quantity and extension of pores change the specific scaffold surface modifying its permeability and mechanical properties, having strong impact in cell seeding, nutrient diffusion and tissue ingrowth [36, 38, 40]. Notably, higher number and extension of pores allows for enhanced cellularity but reduces scaffold strength [41, 42]. A study suggested pore size ranging from 50 to 400 μm for the optimum bone growth into porous-surfaced metallic implants [43]. However, it has been described up to 80% of bone in-growth after 2 months from implanting scaffolds in mice, regardless the pore sizes, which ranged from 300 to 1,200 μm [44, 45]. It has been proposed that pore interconnectivity is even more important to sustain bone growth than size of the pore size itself [46].
The scaffold degradation is fundamental to achieve success in tissue engineering therapies [38]. The scaffold should ideally reabsorb once it has served its purpose of providing a template for tissue regeneration. Importantly, the degradation must occur at a rate compatible with the new tissue formation [47]. For example, the implantation of fast degrading scaffolds decreases the in vitro viability of primary smooth muscle cells resulting in less cell population and lower angiogenesis levels [48]. Furthermore, the degradation products should not be toxic and must be easily cleared or resorbed to minimize the risk of inflammatory response [39, 49]. It must be emphasized that during the scaffold degradation, the local pH should not be significantly lower than the physiological pH [39], otherwise cell death and protein degradation may occur.
2.3 Cell signaling
Cell signaling is part of a complex system of communication that governs cell activities and organizes their interactions (Fig. 2).
Fig. 2.
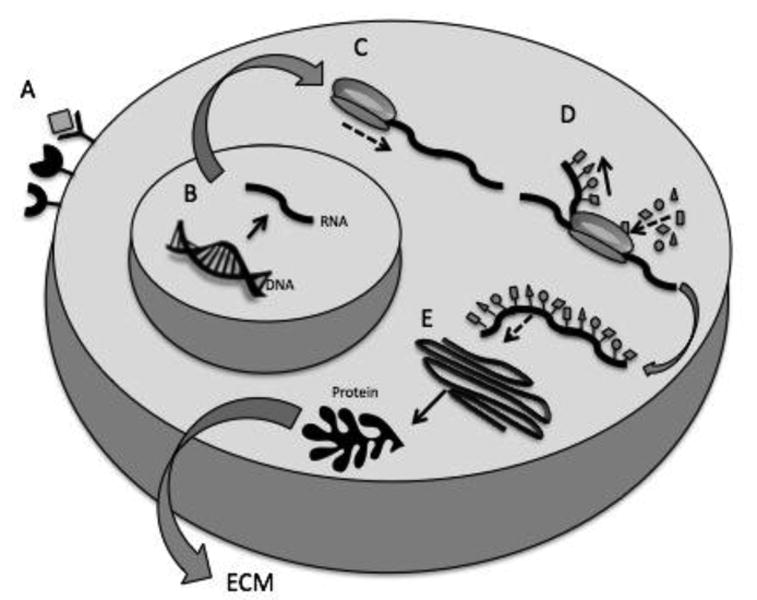
Flow of events leading to protein synthesis. A) Binding of ligand to its specific cell membrane receptor triggers intracellular signaling and activation of gene expression. B) Synthesis of new RNA template from transcription of the original strand of DNA. C) Assembling of the ribosome complex at the initiation codon of the mRNA molecule. D) Decoding of mRNA in sets of nucleotides forming a polypeptide chain. E) Folding in the Golgi apparatus to complete protein synthesis. This can be followed by protein secretion to the extracellular environment or “sequestration” within the cell itself.
Many extracellular molecules have been described in the literature. It has been shown that a pool of these extracellular molecules has a major role than a single protein in the differentiation of cells into a functional tissue. This could be observed when proteins present in dentin disks [50], dentin extract in EDTA [51] or a tooth-germ conditioned extract [52] were found to supplement the scaffolds as a mechanism of cellular induction. Yet, there still is missing information on how each factor acts in isolation. Among the different factors, the TGFβ-1 (Transforming Growth Factor 1) and BMP (Bone Morphogenetic Protein), seem to have an important role in the odontoblastic differentiation [53]. Moreover, there is evidence that the TGFβ-1 is released from the dentin after any injury [54] and that BMPs, more specifically the BMP-2, have dentin induction ability [55].
The understanding of intracellular events triggered by extracellular proteins is critical for tissue engineering. In general, it is described that BMPs act in the canonical TGF-β pathway modulating smads, leading to odontoblastic differentiation and inducing dentin formation [55, 56]. However, it is important to keep in mind that TGF-β proteins, including BMPs, have also well established roles in cancer progression [57]. Among these pathways, the Wnt pathway appears to be important for stem cell self-renewal [58] and cell differentiation [59]. The interactions and crosstalk between the canonical smad pathway and the Wnt pathway is probably one of the most studied, but there is little information on how these interactions affect DPSC. Additionally, it has been described that Wnt proteins are not able to induce DPSC differentiation [60]. On the other hand, there is a connection between BMPs with β-catenin, an intracellular protein that is part of the Wnt pathway, which has an important role in differentiation processes for other cell types [61].
3. Current trends and future applications of Tissue Engineering in Dentistry
Recent advances brought by tissue engineering suggest that significant changes in “traditional” clinical dentistry are beginning to occur. For example, the purpose of periodontal tissue engineering is to establish new therapies to manage periodontal diseases beyond the traditional approaches that are based solely upon infection control [62]. Chronic periodontitis is one of the most common oral disease worldwide, after dental decay, with a prevalence of 35% within the United States adult population with at least 6 teeth [63]. A periodontal stem cell-based therapy will congregate the inflammation and infection control to stem cells capable to regenerate new periodontal tissues. This approach can rapidly providing cells for to the damaged diseased site reducing the temporal gap between the start of treatment and the arrival of progenitor cells, which is frequently seen as one of the shortcomings of the traditional procedures [30, 64, 65]. One problem to be overcome is that residual PDLSC are limited in patiets with periodontitis due to the long-term inflammation [66]. However the achievements obtained using these cells for periodontal needs are exciting. It has been shown that the association of PDLSC and SCAP mixed in a HA/TCP scaffold allows for reconstruction of reconstruct a functional tooth with a root periodontal complex [30]. Similar protocol was responsible for regenerating for the regeneration of alveolar bone in dogs after a 8-week evaluation period [65]. The potential of this cells to treat periodontitis was shown by implanting PDLSC in HA/TCP scaffolds in periodontal bone defects for 12 weeks, it was possible to observe an osseous regeneration near to 50% of total original defect height of 7 mm [66].
To date, the regeneration of small- to moderate-sized periodontal defects using engineered cell-scaffold constructs is technically feasible, and some of the current concepts may represent alternatives for selected clinical scenarios. However, the predictable reconstruction of the normal structure and functionality of a tooth-supporting apparatus remains challenging [8, 62]. The future possibilities depend on improved understanding of cellular and molecular mechanisms involved in the regeneration of periodontal tissues, the differentiation potential of stem cells, and the interaction between stem cells and scaffold with host tissues. Major bone reconstructions because of trauma, cancer, or augmentation for dental implants are just few examples of how tissue engineering can be also be used for craniofacial applications [34].
In clinical scenarios involving normal bone, the long-term implant success and survival rates are above 90% [67]. However, conditions as smoking, diabetes, radiotherapy, and postmenopausal estrogen therapy may compromise bone quantity and quality leading to scenarios in which bone healing may become challenging [68]. Through developing biomaterials and strategies that may increase bone cell adhesion, modulate cell signaling, deliver growth factors and promote osteoblast differentiation followed by matrix deposition and mineralization [69, 70], it might be possible to place implants even under less than ideal anatomical or biological circumstances [71].
One interesting strategy to increase osteointegration of titanium implants is to coat them with extracellular matrix components, such as collagen, BMP, TGF-B or chondroitin sulphate [72, 73]. BMPs are known to improve healing, to induce peri-implant bone formation and to enhance osseointegration of endosseous implants [72]. TGF-B is produced in response to factors that stimulate osteoclastic bone resorption and plays a key role in osteoblastic bone formation, inhibiting osteoclast formation and activity [74]. Chondroitin sulphate has an anti-inflammatory effect, accelerates bone repair and increases bone regeneration [75]. The addition of these growth factors on implant surface is certainly an exciting perspective, but many questions still remain unanswered. It is known that BMP and TGF-B act synergistically but this action depends on the temporal sequence and timing of growth factor delivery [76]. Thus, to gain further advantage on implant osseointegration using growth factors, it is necessary to understand beyond the application mechanisms of these proteins and to orchestrate their release pattern, increasing the time that they are bioactive and maximizing their potential [72]. It is important to consider the release kinetics and degradation rate to optimize the amount of extracellular matrix components over the implant surface [73, 77]. As the cost of proteins is high, to achieve maximum bone formation at minimal concentration is crucial to reduce the toxicity and side effects for this approach to become affordable and clinically safe.
Autogenous bone transplantation has been used for repairing the bone defects for a long time. Although this technique may provide to the patient its own scaffold, growth factors and cells [78] it also present some shortcomings such as: the sacrifice of healthy tissue, limited availability of donor tissue, post-surgical bone resorption, and donor-site morbidity [79]. Tissue engineering may circumvent many of the limitations of the available techniques and its power has been explored to minimize the need of surgery to obtain the bone graft [69, 70]. In vivo studies observed stem cell differentiation towards osteoblast-like cell producing a 30-mm long repair in a mandibular segmental defect of a dog 32 weeks after implantation of scaffolds with bone marrow stem cells [80, 81]. Other reports showed that mesenchymal stem-cells derived from bone marrow or peripheral adipose tissue were able to regenerate tissue in critical-sized craniofacial defects of mice without the addition of exogenous growth or morphogenetic factors [82, 83]. These findings allow foreseeing that tissue engineering can become a breakthrough approach to reconstruct bone deformities in a more effective and less traumatic way.
Restorative dentistry is looking for techniques and materials to regenerate the dentin-pulp complex in a biological manner. Tissue engineering-based approaches have the potential to do it (Fig. 3). It has been reported that DPSC and SHED can be induced to differentiate into odontoblasts [10, 26]. SHED seeded in tooth slices containing scaffolds and implanted in immunodeficient mice are able to produce a dental pulp tissue [10, 13]. In addition, it has been also shown that SHED cells can differentiate into odontoblasts that generate tubular dentin in vivo [10, 84]. It was shown that DPSC are able to produce pulp/dentin-like tissues in an emptied human root canal with the deposition of a layer of mineralized tissue on the canal walls [85]. One important step towards regenerative endodontics was achieved when SHED mixed with nanofiber peptide scaffold and injected into full-length root canals were able to generate a dental pulp. The original data presented here in Figure 3 shows the presence of a pulp tissue fulfilling the hollow passageway of the root canal, with proliferative activity and blood network maturity comparable to the ones observed in a young human dental pulp. The dental pulp engineered was able to deposit organized dentin along walls (data not shown). These findings are exciting once SHED may be readily retrievable from decidous tooth, the only “disposable” tissue and injectable scaffolds will allow dentists to easily deliver stem cells inside root canals disregarding its internal anatomy.
Fig. 3.
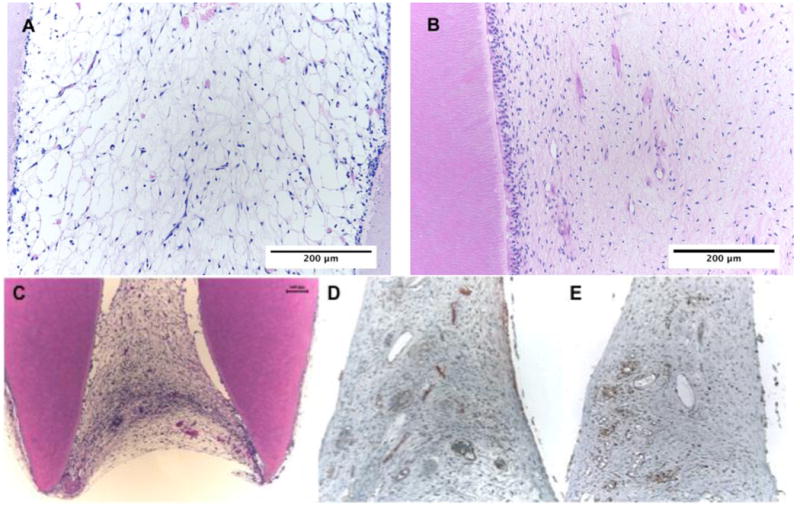
Dental pulp tissue engineered for 35 days inside root canal using SHED cells (A) and natural dental pulp from a young premolar (B). It is possible to observe the formation of a healthy tissue without inflammatory signs and a densification of odontoblast-like cell along dentin walls in the SHED originated tissue similar to the control. The engineered tissue occupies the whole apical portion (C) and immunohistochemistry with PCNA (proliferating cell nuclear activity) and Factor VIII show a proliferative tissue with well estabilished and mature blood network (D and E).
These findings support the concept that the regeneration of the dentin pulp complex with stem cells might be clinically achievable. However, this may not be applicable to all clinical scenarios. For example, older teeth with small pulp chamber and root canals and with closed apex may present a formidable challenge to tissue engineering. However, engineering a dental pulp to deposit dentin and continue the natural root growth of non-infected, accidently injured young teeth is apparently more feasible.
4. Future direction in Dentistry
The future of tissue engineering in Dentistry is exciting. It is certainly possible that, once dentist-scientists bring together the new discoveries in material's sciences, genetics, molecular and cell biology, new alternatives for regeneration of bone and soft tissues [34, 83], management of periodontal disease [8, 62, 65] and restorative procedures to regenerate enamel, dentin and pulp will become available for clinical application [2, 10]. However, one of the important considerations will certainly be the cost of these procedures. It does not only applies to the cost of treatment itself, but the aggregate costs required to introduce such technology to clinicians and students and to build facilities for dental stem cell obtaining and banking and to produce scaffolds at affordable prices. History has shown that most of the revolutionary technologies became more affordable as they have become more popular, and, perhaps, this will be also true for tissue engineering in Dentistry. It is difficult to predict at this time the full impact of tissue engineering to the future of Dentistry.
References
- 1.Majumdar SK. History of dentistry: an overview. Bull Indian Inst Hist Med Hyderabad. 2002;32:31–42. [PubMed] [Google Scholar]
- 2.Nör JE. Tooth regeneration in operative dentistry. Oper Dent. 2006;31:633–642. doi: 10.2341/06-000. [DOI] [PubMed] [Google Scholar]
- 3.Hohlfeld J, de Buys Roessingh A, Hirt-Burri N, Chaubert P, Gerber S, Scaletta C, Hohlfeld P, Applegate LA. Tissue engineered fetal skin constructs for paediatric burns. Lancet. 2005;366:840–842. doi: 10.1016/S0140-6736(05)67107-3. [DOI] [PubMed] [Google Scholar]
- 4.Warnke PH, Springer IN, Wiltfang J, Acil Y, Eufinger H, Wehmoller M, Russo PA, Bolte H, Sherry E, Behrens E, Terheyden H. Growth and transplantation of a custom vascularised bone graft in a man. Lancet. 2004;364:766–770. doi: 10.1016/S0140-6736(04)16935-3. [DOI] [PubMed] [Google Scholar]
- 5.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 6.Cao Y, Vacanti JP, Paige KT, Upton J, Vacanti CA. Transplantation of chondrocytes utilizing a polymer-cell construct to produce tissue-engineered cartilage in the shape of a human ear. Plast Reconstr Surg. 1997;100:297–302. doi: 10.1097/00006534-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Abukawa H, Terai H, Hannouche D, Vacanti JP, Kaban LB, Troulis MJ. Formation of a mandibular condyle in vitro by tissue engineering. J Oral Maxillofac Surg. 2003;61:94–100. doi: 10.1053/joms.2003.50015. [DOI] [PubMed] [Google Scholar]
- 8.Park CH, Rios HF, Jin Q, Bland ME, Flanagan CL, Hollister SJ, Giannobile WV. Biomimetic hybrid scaffolds for engineering human tooth-ligament interfaces. Biomaterials. 2010;31:5945–5952. doi: 10.1016/j.biomaterials.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu B, Nadiri A, Kuchler-Bopp S, Perrin-Schmitt F, Peters H, Lesot H. Tissue engineering of tooth crown, root, and periodontium. Tissue Eng. 2006;12:2069–2075. doi: 10.1089/ten.2006.12.2069. [DOI] [PubMed] [Google Scholar]
- 10.Sakai VT, Zhang Z, Dong Z, Neiva KG, Machado M, Shi S, Santos CF, Nör JE. SHED differentiate into functional odontoblast and endothelium. J Dent Res. 2010;89:791–796. doi: 10.1177/0022034510368647. [DOI] [PubMed] [Google Scholar]
- 11.Vaahtokari A, Aberg T, Jernvall J, Keranen S, Thesleff I. The enamel knot as a signaling center in the developing mouse tooth. Mech Dev. 1996;54:39–43. doi: 10.1016/0925-4773(95)00459-9. [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Tang Z, Liu J, Sun K, Chang SR, Peters MC, Mansfield JF, Czajka-Jakubowska A, Clarkson BH. Acellular synthesis of a humamn enamel-like microstructure. Adv Mater. 2006;18:1846–1851. [Google Scholar]
- 13.Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith AJ, Nor JE. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod. 2008;34:962–969. doi: 10.1016/j.joen.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Duailibi MT, Duailibi SE, Young CS, Bartlett JD, Vacanti JP, Yelick PC. Bioengineered teeth from cultured rat tooth bud cells. J Dent Res. 2004;83:523–528. doi: 10.1177/154405910408300703. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda E, Morita R, Nakao K, Ishida K, Nakamura T, Takano-Yamamoto T, Ogawa M, Mizuno M, Kasugai S, Tsuji T. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc Natl Acad Sci U S A. 2009;106:13475–13480. doi: 10.1073/pnas.0902944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 17.Langer R, Vacanti JP. Artificial organs. Sci Am. 1995;273:130–133. [PubMed] [Google Scholar]
- 18.van der Kooy D, Weiss S. Why stem cells? Science. 2000;287:1439–1441. doi: 10.1126/science.287.5457.1439. [DOI] [PubMed] [Google Scholar]
- 19.Pera MF, Reubinoff B, Trounson A. Human embryonic stem cells. J Cell Sci. 2000;113(Pt 1):5–10. doi: 10.1242/jcs.113.1.5. [DOI] [PubMed] [Google Scholar]
- 20.Mobasheri A, Bondy AC, Moley K, Mendes AF, Rosa SC, Richardson SM, Hoyland JA, Barrett-Jolley R, Shakibaei M. Facilitative Glucose Transporters in Articular Chondrocytes. Vol. 200. New York: Springer Berlin Heidelberg; 2008. [PubMed] [Google Scholar]
- 21.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiraly M, Porcsalmy B, Pataki A, Kadar K, Jelitai M, Molnar B, Hermann P, Gera I, Grimm WD, Ganss B, Zsembery A, Varga G. Simultaneous PKC and cAMP activation induces differentiation of human dental pulp stem cells into functionally active neurons. Neurochem Int. 2009;55:323–332. doi: 10.1016/j.neuint.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 23.Koyama N, Okubo Y, Nakao K, Bessho K. Evaluation of pluripotency in human dental pulp cells. J Oral Maxillofac Surg. 2009;67:501–506. doi: 10.1016/j.joms.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakai VT, Zhang Z, Dong Z, Neiva KG, Machado M, Shi S, Santos CF, Nör JE. SHED differentiate intro functional odontoblast and endothelium. J Dent Res. 2009 doi: 10.1177/0022034510368647. [DOI] [PubMed] [Google Scholar]
- 26.Casagrande L, Demarco FF, Zhang Z, Araujo FB, Shi S, Nör JE. Dentin-derived BMP-2 and odontoblastic differentiation of SHED. J Dent Res. 2010;89:603–608. doi: 10.1177/0022034510364487. [DOI] [PubMed] [Google Scholar]
- 27.Shi S, Gronthos S, Chen S, Reddi A, Counter CM, Robey PG, Wang CY. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat Biotechnol. 2002;20:587–591. doi: 10.1038/nbt0602-587. [DOI] [PubMed] [Google Scholar]
- 28.Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Wang S, Shi S. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1:e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 30.Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Shi S, Wang S. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1:e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang GT, Sonoyama W, Liu Y, Liu H, Wang S, Shi S. The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod. 2008;34:645–651. doi: 10.1016/j.joen.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GT. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166–171. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kemppainen JM, Hollister SJ. Tailoring the mechanical properties of 3D-designed poly(glycerol sebacate) scaffolds for cartilage applications. J Biomed Mater Res A. 2010;94:9–18. doi: 10.1002/jbm.a.32653. [DOI] [PubMed] [Google Scholar]
- 34.Xu HH, Weir MD, Simon CG. Injectable and strong nano-apatite scaffolds for cell/growth factor delivery and bone regeneration. Dent Mater. 2008;24:1212–1222. doi: 10.1016/j.dental.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leong NL, Jiang J, Lu HH. Polymer-ceramic composite scaffold induces osteogenic differentiation of human mesenchymal stem cells. Conf Proc IEEE Eng Med Biol Soc. 2006;1:2651–2654. doi: 10.1109/IEMBS.2006.259459. [DOI] [PubMed] [Google Scholar]
- 36.Saito E, Kang H, Taboas JM, Diggs A, Flanagan CL, Hollister SJ. Experimental and computational characterization of designed and fabricated 50:50 PLGA porous scaffolds for human trabecular bone applications. J Mater Sci Mater Med. 2010;21:2371–2383. doi: 10.1007/s10856-010-4091-8. [DOI] [PubMed] [Google Scholar]
- 37.Miranda P, Pajares A, Saiz E, Tomsia AP, Guiberteau F. Mechanical properties of calcium phosphate scaffolds fabricated by robocasting. J Biomed Mater Res A. 2008;85:218–227. doi: 10.1002/jbm.a.31587. [DOI] [PubMed] [Google Scholar]
- 38.Russias J, Saiz E, Deville S, Gryn K, Liu G, Nalla RK, Tomsia AP. Fabrication and in vitro characterization of three-dimensional organic/inorganic scaffolds by robocasting. J Biomed Mater Res A. 2007;83:434–445. doi: 10.1002/jbm.a.31237. [DOI] [PubMed] [Google Scholar]
- 39.Wiesmann HP, Lammers L. Scaffold Structure and Fabrication. New York: Springer; 2009. [Google Scholar]
- 40.Xu HH, Quinn JB, Takagi S, Chow LC, Eichmiller FC. Strong and macroporous calcium phosphate cement: Effects of porosity and fiber reinforcement on mechanical properties. J Biomed Mater Res. 2001;57:457–466. doi: 10.1002/1097-4636(20011205)57:3<457::aid-jbm1189>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 41.Salerno A, Iannace S, Netti PA. Open-pore biodegradable foams prepared via gas foaming and microparticulate templating. Macromol Biosci. 2008;8:655–664. doi: 10.1002/mabi.200700278. [DOI] [PubMed] [Google Scholar]
- 42.Ripamonti U, Ma S, Reddi AH. The critical role of geometry of porous hydroxyapatite delivery system in induction of bone by osteogenin, a bone morphogenetic protein. Matrix. 1992;12:202–212. doi: 10.1016/s0934-8832(11)80063-6. [DOI] [PubMed] [Google Scholar]
- 43.Bobyn JD, Pilliar RM, Cameron HU, Weatherly GC. The optimum pore size for the fixation of porous-surfaced metal implants by the ingrowth of bone. Clin Orthop Relat Res. 1980:263–270. [PubMed] [Google Scholar]
- 44.Hollister SJ, Lin CY, Saito E, Schek RD, Taboas JM, Williams JM, Partee B, Flanagan CL, Diggs A, Wilke EN, Van Lenthe GH, Muller R, Wirtz T, Das S, Feinberg SE, Krebsbach PH. Engineering craniofacial scaffolds. Orthod Craniofac Res. 2005;8:162–173. doi: 10.1111/j.1601-6343.2005.00329.x. [DOI] [PubMed] [Google Scholar]
- 45.Schek RM, Wilke EN, Hollister SJ, Krebsbach PH. Combined use of designed scaffolds and adenoviral gene therapy for skeletal tissue engineering. Biomaterials. 2006;27:1160–1166. doi: 10.1016/j.biomaterials.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 46.Li JP, Habibovic P, van den Doel M, Wilson CE, de Wijn JR, van Blitterswijk CA, de Groot K. Bone ingrowth in porous titanium implants produced by 3D fiber deposition. Biomaterials. 2007;28:2810–2820. doi: 10.1016/j.biomaterials.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 47.Freed LE, Vunjak-Novakovic G, Biron RJ, Eagles DB, Lesnoy DC, Barlow SK, Langer R. Biodegradable polymer scaffolds for tissue engineering. Biotechnology (N Y) 1994;12:689–693. doi: 10.1038/nbt0794-689. [DOI] [PubMed] [Google Scholar]
- 48.Sung HJ, Meredith C, Johnson C, Galis ZS. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials. 2004;25:5735–5742. doi: 10.1016/j.biomaterials.2004.01.066. [DOI] [PubMed] [Google Scholar]
- 49.Nof M, Shea LD. Drug-releasing scaffolds fabricated from drug-loaded microspheres. J Biomed Mater Res. 2002;59:349–356. doi: 10.1002/jbm.1251. [DOI] [PubMed] [Google Scholar]
- 50.Gonçalves SB, Dong Z, Bramante CM, Holland GR, Smith AJ, Nor JE. Tooth slice-based models for the study of human dental pulp angiogenesis. J Endod. 2007;33:811–814. doi: 10.1016/j.joen.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 51.Liu J, Jin T, Ritchie HH, Smith AJ, Clarkson BH. In vitro differentiation and mineralization of human dental pulp cells induced by dentin extract. In Vitro Cell Dev Biol Anim. 2005;41:232–238. doi: 10.1290/0502014.1. [DOI] [PubMed] [Google Scholar]
- 52.Yu J, Deng Z, Shi J, Zhai H, Nie X, Zhuang H, Li Y, Jin Y. Differentiation of dental pulp stem cells into regular-shaped dentin-pulp complex induced by tooth germ cell conditioned medium. Tissue Eng. 2006;12:3097–3105. doi: 10.1089/ten.2006.12.3097. [DOI] [PubMed] [Google Scholar]
- 53.Nakashima M, Reddi AH. The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol. 2003;21:1025–1032. doi: 10.1038/nbt864. [DOI] [PubMed] [Google Scholar]
- 54.Magloire H, Romeas A, Melin M, Couble ML, Bleicher F, Farges JC. Molecular regulation of odontoblast activity under dentin injury. Adv Dent Res. 2001;15:46–50. doi: 10.1177/08959374010150011201. [DOI] [PubMed] [Google Scholar]
- 55.Iohara K, Nakashima M, Ito M, Ishikawa M, Nakasima A, Akamine A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J Dent Res. 2004;83:590–595. doi: 10.1177/154405910408300802. [DOI] [PubMed] [Google Scholar]
- 56.Yang X, Yang F, Walboomers XF, Bian Z, Fan M, Jansen JA. The performance of dental pulp stem cells on nanofibrous PCL/gelatin/nHA scaffolds. J Biomed Mater Res A. 2009 doi: 10.1002/jbm.a.32535. [DOI] [PubMed] [Google Scholar]
- 57.Guo X, Wang XF. Signaling cross-talk between TGF-β/BMP and other pathways. Cell Res. 2008;19:71–78. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 59.Luo Q, Kang Q, Si W, Jiang W, Park JK, Peng Y, Li X, Luu HH, Luo J, Montag AG, Haydon RC, He TC. Connective tissue growth factor (CTGF) is regulated by Wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279:55958–55968. doi: 10.1074/jbc.M407810200. [DOI] [PubMed] [Google Scholar]
- 60.Scheller EL, Chang J, Wang CY. Wnt/beta-catenin inhibits dental pulp stem cell differentiation. J Dent Res. 2008;87:126–130. doi: 10.1177/154405910808700206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dupont S, Mamidi A, Cordenonsi M, Montagner M, Zacchigna L, Adorno M, Martello G, Stinchfield MJ, Soligo S, Morsut L, Inui M, Moro S, Modena N, Argenton F, Newfeld SJ, Piccolo S. FAM/USP9x, a deubiquitinating enzyme essential for TGFbeta signaling, controls Smad4 monoubiquitination. Cell. 2009;136:123–135. doi: 10.1016/j.cell.2008.10.051. [DOI] [PubMed] [Google Scholar]
- 62.Chen FM, Jin Y. Periodontal Tissue Engineering and Regeneration: Current Approaches and Expanding Opportunities. Tissue Eng Part B Rev. 2009 doi: 10.1089/ten.TEB.2009.0562. [DOI] [PubMed] [Google Scholar]
- 63.Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988-1994. J Periodontol. 1999;70:13–29. doi: 10.1902/jop.1999.70.1.13. [DOI] [PubMed] [Google Scholar]
- 64.Kawaguchi H, Hirachi A, Hasegawa N, Iwata T, Hamaguchi H, Shiba H, Takata T, Kato Y, Kurihara H. Enhancement of periodontal tissue regeneration by transplantation of bone marrow mesenchymal stem cells. J Periodontol. 2004;75:1281–1287. doi: 10.1902/jop.2004.75.9.1281. [DOI] [PubMed] [Google Scholar]
- 65.Kim SH, Kim KH, Seo BM, Koo KT, Kim TI, Seol YJ, Ku Y, Rhyu IC, Chung CP, Lee YM. Alveolar bone regeneration by transplantation of periodontal ligament stem cells and bone marrow stem cells in a canine peri-implant defect model: a pilot study. J Periodontol. 2009;80:1815–1823. doi: 10.1902/jop.2009.090249. [DOI] [PubMed] [Google Scholar]
- 66.Liu Y, Zheng Y, Ding G, Fang D, Zhang C, Bartold PM, Gronthos S, Shi S, Wang S. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells. 2008;26:1065–1073. doi: 10.1634/stemcells.2007-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Melo MD, Shafie H, Obeid G. Implant survival rates for oral and maxillofacial surgery residents: a retrospective clinical review with analysis of resident level of training on implant survival. J Oral Maxillofac Surg. 2006;64:1185–1189. doi: 10.1016/j.joms.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 68.Moy PK, Medina D, Shetty V, Aghaloo TL. Dental implant failure rates and associated risk factors. Int J Oral Maxillofac Implants. 2005;20:569–577. [PubMed] [Google Scholar]
- 69.Weir MD, Xu HH. Culture human mesenchymal stem cells with calcium phosphate cement scaffolds for bone repair. J Biomed Mater Res B Appl Biomater. 2010;93:93–105. doi: 10.1002/jbm.b.31563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao L, Weir MD, Xu HH. An injectable calcium phosphate-alginate hydrogel-umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials. 2010;31:6502–6510. doi: 10.1016/j.biomaterials.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Joos U. Tissue Engineering Strategies in Dental Implantology. New York: Springer; 2009. [Google Scholar]
- 72.Stadlinger B, Pilling E, Mai R, Bierbaum S, Berhardt R, Scharnweber D, Eckelt U. Effect of biological implant surface coatings on bone formation, applying collagen, proteoglycans, glycosaminoglycans and growth factors. J Mater Sci Mater Med. 2008;19:1043–1049. doi: 10.1007/s10856-007-3077-7. [DOI] [PubMed] [Google Scholar]
- 73.Liu Y, Huse RO, de Groot K, Buser D, Hunziker EB. Delivery mode and efficacy of BMP-2 in association with implants. J Dent Res. 2007;86:84–89. doi: 10.1177/154405910708600114. [DOI] [PubMed] [Google Scholar]
- 74.Mundy GR. The effects of TGF-beta on bone. Ciba Found Symp. 1991;157:137–143. discussion 143-151. [PubMed] [Google Scholar]
- 75.Nagai M, Hayakawa T, Fukatsu A, Yamamoto M, Fukumoto M, Nagahama F, Mishima H, Yoshinari M, Nemoto K, Kato T. In vitro study of collagen coating of titanium implants for initial cell attachment. Dent Mater J. 2002;21:250–260. doi: 10.4012/dmj.21.250. [DOI] [PubMed] [Google Scholar]
- 76.Kim GY, Lee HH, Cho SW. Differential effects of transforming growth factor-beta 1 and bone morphogenetic proteins in cultured rat osteogenic sarcoma and mink lung epithelial cells. Biochem Mol Biol Int. 1994;33:253–261. [PubMed] [Google Scholar]
- 77.Stadlinger B, Pilling E, Huhle M, Mai R, Bierbaum S, Scharnweber D, Kuhlisch E, Loukota R, Eckelt U. Evaluation of osseointegration of dental implants coated with collagen, chondroitin sulphate and BMP-4: an animal study. Int J Oral Maxillofac Surg. 2008;37:54–59. doi: 10.1016/j.ijom.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 78.Vaccaro AR. The role of the osteoconductive scaffold in synthetic bone graft. Orthopedics. 2002;25:s571–578. doi: 10.3928/0147-7447-20020502-05. [DOI] [PubMed] [Google Scholar]
- 79.Conway JD. Autograft and nonunions: morbidity with intramedullary bone graft versus iliac crest bone graft. Orthop Clin North Am. 2010;41:75–84. doi: 10.1016/j.ocl.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 80.Yuan J, Cui L, Zhang WJ, Liu W, Cao Y. Repair of canine mandibular bone defects with bone marrow stromal cells and porous beta-tricalcium phosphate. Biomaterials. 2007;28:1005–1013. doi: 10.1016/j.biomaterials.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 81.Yuan J, Zhang W, Liu G, Wei M, Qi Z, Liu W, Cui L, Cao Y. Repair of Canine Mandibular Bone Defects with Bone Marrow Stromal Cells and Coral. Tissue Eng Part A. 2009 doi: 10.1089/ten.TEA.2009.0472. [DOI] [PubMed] [Google Scholar]
- 82.Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, Quarto N, Contag CH, Wu B, Longaker MT. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 83.Krebsbach PH, Mankani MH, Satomura K, Kuznetsov SA, GRobey P. Repair of craniotomy defects using bone marrow stromal cells. Transplantation. 1998;66:1272–1278. doi: 10.1097/00007890-199811270-00002. [DOI] [PubMed] [Google Scholar]
- 84.Rosa V, Botero TM, Nör JE. Regenerative endodontics in light of the stem cell paradigm. Int Dent J. 2011;61:23–28. doi: 10.1111/j.1875-595X.2011.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang G, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan R, Shi S. Stem/progenitor Cell-Mediated De Novo Regeneration of Dental Pulp with Newly Deposited Continuous Layer of Dentin in an In Vivo Model. Tissue Eng Part A. 2009 doi: 10.1089/ten.tea.2009.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]


