Abstract
Background
Recent studies demonstrated a key role of ubiquitous isoform of Na+,K+,2Cl− co-transport (NKCC 1) in regulation of myogenic tone and peripheral resistance. We examined the impact of race, gender, and plasma lipid on NKCC 1 activity in French Canadians and African Americans with hypertension and dyslipidemia.
Methods
NKCC and passive erythrocyte membrane permeability to K+, measured as ouabain-resistant, bumetanide-sensitive, and (ouabain+bumetanide)-resistant 86Rb influx, respectively, were compared in 111 French-Canadian men, 107 French-Canadian women, 26 African-American men, and 45 African-American women with essential hypertension and dyslipidemia.
Results
The African-American men and women were 7 years younger and presented twofold decreased plasma triglycerides compared to their French-Canadian counterparts (P < 0.01) whereas body mass index (BMI), total cholesterol, low-density lipoprotein, and high-density lipoprotein (HDL) were not different. NKCC was respectively 50 and 38% lower in the African-American men and women than in the French Canadians (P < 0.005) without any differences in passive erythrocyte membrane permeability for K+. We did not observe any impact of age on NKCC in all groups under investigation, whereas plasma triglycerides correlated positively with the activity of this carrier in the French-Canadian men only.
Conclusions
NKCC 1 activity is lower in erythrocytes of African Americans with essential hypertension and dyslipidemia than in Caucasian counterparts. We suggest that decreased NKCC 1 may contribute to the feature of the pathogenesis of salt-sensitive hypertension seen in A frican Americans.
Keywords: African Americans; blood pressure; hypertension; Na+,K+,Cl− co-transport; plasma lipids; renal complications; salt retention
Since the initial observations of increased monovalent ion exchange across erythrocytes from spontaneously hypertensive rats,1,2 such cells lacking a nucleus and intracellular organelles have been widely studied for the functional characterization of ion transport pathways in human hypertension. These investigations revealed that in erythrocytes from Caucasians with primary (essential) hypertension, ion transport is altered by augmented Na+/Na+ exchange, measured as the rate 23Na+o/22Na+i exchange or Na+o -dependent Li+ efflux (Na+/Li+ countertransport), and the abnormal kinetic properties of Na+,K+,2Cl co-transport (NKCC) indicated by increased maximal rate of the carrier activity and decreased its affinity for intracellular Na+ (for review, see refs. 3–5).
Two NKCC isoforms have been cloned from vertebrate complementary DNA libraries. An ubiquitous NKCC1 has been detected in all types of cells studied so far, whereas renal-specific NKCC2 expression is limited to the apical membrane of epithelial cells from the thick ascending limb of Henle’s loop and the macula densa. Apart from NKCC, the superfamily of Cl−-coupled carriers, providing bidirectional electroneutral symport of monovalent ions, consists of Na+,Cl− (NCC) and K+, Cl− (KCC) co-transporters. Both NKCC1 and NKCC2 are inhibited by high-ceiling diuretics (HCD), such as bumetanide and furosemide, whereas NCC is resistant to HCD and inhibited by hydrochlorothiazide and other thiazide-derived diuretics (for review, see ref. 6). In contrast to NKCC, Na+/Li+ countertransporter has not yet been cloned, and its function remains poorly understood.4
It has been reported that hypertensive blacks are more frequently sodium-sensitive than hypertensive whites, and diuretics targeting NCC and NKCC2 remain the most reliable therapy either in combination or alone for hypertensive African Americans.7,8 These clinical data suggest that abnormal solute and fluid movement in absorptive and/or secretory epithelia contribute to enhanced salt sensitivity and sodium retention in African Americans with essential hypertension. This hypothesis is also consistent with the augmented activity of epithelial Na+ channels, i.e., major regulators of salt reabsorption in distal tubules, seen in hypertensive African Americans9 and decreased NKCC1-mediated sweat gland function in blacks compared to whites.10,11
It has been reported that NKCC is reduced in erythrocytes from blacks compared to whites (for review, see refs. 5,12). However, the limitations listed below complicate the comparative analysis of these data. First, hypertension was not controlled in several comparative black vs. white studies.13,14 Second, NKCC was shown to be higher in erythrocytes from Caucasian men compared to women.15 Mixed populations of men and women were investigated to analyze impact of race on erythrocyte ion transport in certain studies.13,14,16,17 Third, plasma lipids have been well documented to affect erythrocyte ion transport in Caucasian populations.18 The possible impact of distinct plasma lipid profiles has not been considered in white vs. black comparisons.
Keeping these observations in mind, we compared ion transport in erythrocytes from Caucasian and African-American men and women with essential hypertension and dyslipidemia. In contrast to nystatin- and 2,5-chloromercuribenzenesulfonic acid–treated erythrocytes employed in overwhelming number of previous investigations,13,14,16,17,19 we applied methods that allowed us to measure NKCC in intact cells with physiologically low intracellular Na+/K+ ratios. The possible influence of age, body mass index (BMI), and plasma lipids on the activity of ion transporters in erythrocytes from whites and blacks was examined by correlation analysis.
Methods
Patients
African Americans were examined at the Medical College of Wisconsin in Milwaukee, and French Canadians were investigated at Chicoutimi Hospital in the Saguenay–Lac-St-Jean region of Quebec, Canada, with identical protocols employed in both clinics. Hypertensive families were selected on the basis of having at least two siblings in the range from 18 to 55 years with hypertension and dyslipidemia, defined, respectively, as diastolic blood pressure >90 mm Hg, total cholesterol concentration >5.2 mmol/l, and high-density lipoprotein (HDL)-cholesterol <0.9 mmol/l. The exclusion criteria were secondary hypertension, diabetes mellitus, diastolic blood pressure >110 mm Hg on drug therapy, serum creatinine concentration >180 µmol/l, BMI >35 kg/m2, pregnancy, malignancy, substance abuse (including alcohol), and myocardial infarction or stroke in the last 6 months. Once at least two siblings in a family satisfied all the above-listed criteria, other siblings, not necessarily hypertensive, were also enrolled. Before the study, lipid-lowering medication was withdrawn for 1 month, and antihypertensive drugs for at least 1 week. For more details, see refs. (20,21). The protocol was approved by the Medical College of Wisconsin/Froedtert Memorial Lutheran Hospital institutional review board and the Human Investigation Committee of Chicoutimi Hospital.
Blood samples and ion transport measurement
Fasting blood samples were collected on an empty stomach in the morning into tubes containing 20–30 U/ml heparin as an anticoagulant, placed on ice and shipped for further analysis from both clinics to the Research Centre of the Centre hospitalier de l’Université de Montréal (CRCHUM). Previously, it was shown that blood storage with heparin at 2–4 °C for up to 4 days does not affect the erythrocyte ion transporters analyzed in the present study.15 Considering these data, ion transport was measured 24–48 h after blood withdrawal. NKCC and passive permeability for K+, mainly comprising KCC and lipid bilayer permeability, were quantified as ouabain-resistant, bumetanide-sensitive, and (ouabain+bumetanide)-resistant rates of 86Rb influx, respectively, as described in detail elsewhere.15
Western blot was performed in accordance with a previously described protocol22 using anti-NKCC1 and anti-NKCC2 antibodies from Santa Cruz Biotechnology, Santa Cruz, CA. Human erythrocyte ghosts and protein extracts from rat kidney, aorta, and brain were obtained as described elsewhere.23,24
Data analysis
Differences between African-American and French-Canadian subjects were examined by t-test (age, anthropometric parameters, lipids, ion transport, and blood pressure) and χ2 analysis (gender). To analyze the predictors of ion transport activity, we carried out stepwise, block, multiple regression. This analysis tests a predictive linear model between a dependent variable and its predictors (independent variables) in multiple steps. At each step, a block of independent variables is included into the model on the basis of its partial correlation with the dependent variable. In the present study, four blocks were used: (i) age, sex (forced into the equation for control purposes); (ii) BMI, waist-to-hip ratio; (iii) total cholesterol, triglycerides, HDL, and low-density lipoprotein; (iv) awake and asleep systolic and diastolic blood pressures. Prior to this analysis, the dependent variables were tested for normality, outliers were excluded, and, if necessary, the data were transformed.
Results
Clinical characteristics of hypertensive families
Fifty-five French-Canadian families comprising 218 hypertensive siblings (111 men and 107 women) and 23 African-American families comprising 71 hypertensive siblings (26 men and 45 women) were included in this study (Table 1). Neither BMI nor total plasma cholesterol, low-density lipoprotein, and HDL was different between African Americans and French Canadians. Plasma triglycerides were decreased by 40 and 50% in African-American men and women (P < 0.01 and 0.001, respectively). The African Americans were 7 years younger than the French Canadians.
Table 1.
Age, body mass index (BMI), and plasma lipids in French Canadians and African Americans with essential hypertension
| French Canadians | African Americans | |||
|---|---|---|---|---|
| Men (n = 111) | Women (n = 107) | Men (n = 26) | Women (n = 45) | |
| Age | 48.6 ± 4.6 (22–86) | 52.9 ± 5.2 (23–88) | 41.8 ± 8.4 (31–53) | 45.6 ± 6.9 (28–56) |
| BMI, kg/m2 | 28.64 ± 2.76 (21.90–38.76) | 28.65 ± 2.80 (17.03–43.74) | 29.55 ± 5.91 (18.92–43.67) | 29.55 ± 5.91 (20.60–40.81) |
| Triglycerides, mmol/l | 2.41 ± 0.23 (0.22–6.58) | 2.23 ± 0.23 (0.24–7.03) | 1.43 ± 0.29* (0.41–2.16) | 1.10 ± 0.17** (0.52–2.34) |
| Cholesterol, mmol/l | 5.21 ± 0.51 (0.62–7.81) | 5.82 ± 0.59 (0.68–11.36) | 4.99 ± 1.02 (2.34–8.22) | 4.89 ± 0.75 (2.73–7.12) |
| LDL, mmol/l | 3.22 ± 0.33 (0.38–5.50) | 3.41 ± 0.36 (0.32–9.38) | 3.30 ± 0.67 (1.24–5.77) | 3.36 ± 0.51 (1.36–5.38) |
| HDL, mmol/l | 1.05 ± 0.10 (0.18–2.17) | 1.39 ± 0.14 (0.21–4.37) | 1.02 ± 0.21 (0.51–2.22) | 1.04 ± 0.16 (0.73–1.77) |
Means ± s.e. values are given; extreme values are shown in parentheses.
HDL, high-density lipoprotein; LDL, low-density lipoprotein.
P < 0.01 and
P < 0.001 compared to French-Canadian men and women, respectively.
Impact of race
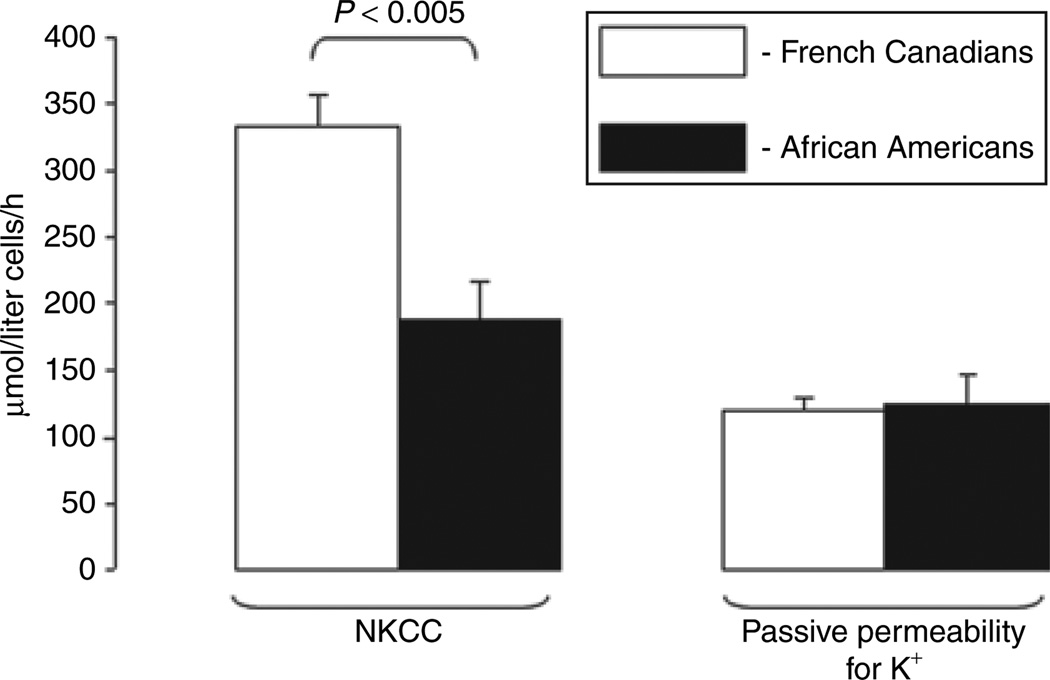
NKCC activity was 45% lower in the mixed population of African-American men and women with essential hypertension than in their French-Canadian counterparts (P < 0.005, Figure 1). Importantly, the values of NKCC activity in French Canadians were consistent with activity of this carrier measured in other Caucasian populations.16,19 We did not observe any difference between the African Americans and French Canadians in passive erythrocyte permeability for K+ (Figure 1).
Figure 1.
NKCC and passive permeability of erythrocyte membranes for K+ in French Canadians and African Americans with essential hypertension. Means ± s.e. are shown. NKCC, Na+,K+,2Cl− co-transport.
Impact of gender
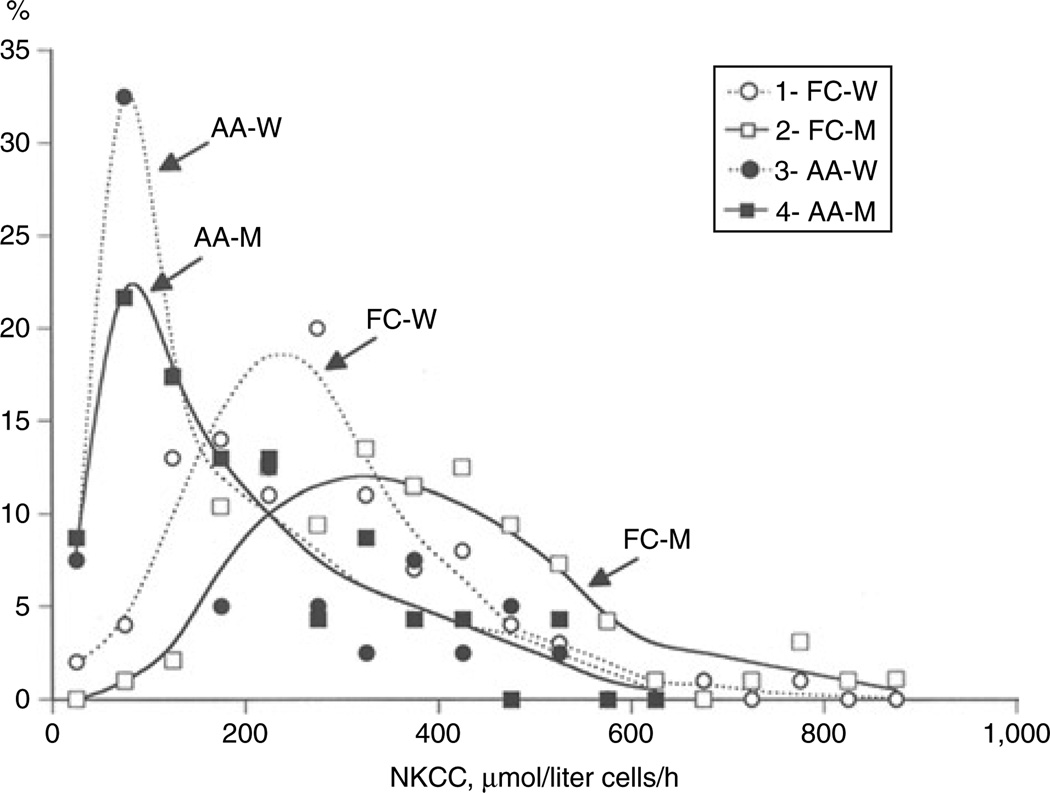
Previously, we reported that NKCC is increased in Caucasian men compared to women and sibling resemblance of NKCC in French-Canadian families with hypertension and dyslipidemia is higher in men than in women (r = 0.39 and 0.03, respectively).15 Considering this, we analyzed the data for Caucasian and African-American men and women separately. Table 2shows that NKCC was elevated by ~34% in the French-Canadian men compared to the women (P > 0.05). In contrast to whites, we did not find any difference in the activity of this carrier between the African-American men and women. Figure 2 illustrates the distribution pattern of NKCC activity among the French-Canadian and African-American men and women. In 40% of the African-American women and in 31% of the African-American men, the carrier’s activity was <100 µmol/liter of cells/h. In contrast, only 2% of the French-Canadian men and 6% of the French-Canadian women showed such low NKCC. In both races, passive permeability of erythrocytes for K+ was not affected by gender (Table 2).
Table 2.
Ion transport in erythrocytes of French Canadians and African Americans with essential hypertension
| Subjects | Ion transport, µmol/liter of cells/h | |
|---|---|---|
| NKCC | Passive permeability for K+ |
|
| French-Canadian men | 381 ± 37 (86–891) | 102 ± 10 (58–157) |
| French-Canadian women | 285 ±28* (28–823) | 128 ± 12 (63–417) |
| African-American men | 191 ± 39 (21–507) | 125 ± 26 (50–248) |
| African-American women | 176 ± 27 (2–559) | 125 ± 19 (73–281) |
| P1,3 | <0.001 | NS |
| P2,4 | <0.01 | NS |
Means ± s.e. values are given; extreme values are shown in parentheses.
NKCC, Na+,K+,2Cl− co-transport; NS, not significant.
P < 0.05 compared to French-Canadian men.
Figure 2.
Distribution of African-American and French-Canadian men and women according to NKCC activity. The 50 µmol/liter cells/h increment of NKCC was used to calculate the percentage of patients with the carrier’s activity, indicated by white and black circles (French-Canadian and African-American women, respectively) and white and black squares (French-C anadian and African-American men, respectively). AA-M, African-American men; AA-W, African-American women; FC-M, French-Canadian men; FC-W, French-Canadian women; NKCC, Na+,K+,2Cl− co-transport.
Impact of age, BMI, and plasma lipids
The African-American men and women were 7 years younger and exhibited approximately twofold decreased plasma triglycerides compared to their French-Canadian counterparts (Table 1). To analyze the implication of these differences in altered NKCC, we undertook intraindividual correlation analyses separately for men and women. The possible impact of BMI, total cholesterol, low-density lipoprotein, and HDL was also subjected to correlation analysis, which led to several conclusions.
First, the correlation between passive erythrocyte permeability for K+ was found to be race and gender dependent. Thus, this parameter correlated negatively with age in the French-Canadian men but positively in the African-American women (Table 3). We observed a highly significant correlation of plasma triglycerides with passive permeability to K+ (P = 0.00099, Table 4) in the African-American women but not in the other groups subjected to this analysis.
Table 3.
Age, BMI, and erythrocyte ion transport in French-Canadian and African-American men and women with essential hypertension: correlation analysis
| NKCC | Passive permeaility for K+ |
NKCC | Passive permeability for K+ |
|
|---|---|---|---|---|
| French-Canadian men | African-American men | |||
| Age | r = 0.066 | r = −0.222 | r = 0.139 | r = −0.215 |
| P = 0.49 | P = 0.02 | P = 0.51 | P = 0.30 | |
| BMI | r = 0.186 | r = −0.073 | r = −0.315 | r = 0.14 |
| P = 0.05 | P = 0.45 | P = 0.12 | P = 0.51 | |
| French-Canadian women | African-American women | |||
| Age | r = −0.057 | r = −0.026 | r = 0.067 | r = 0.145 |
| P = 0.56 | P = 0.78 | P = 0.67 | P = 0.35 | |
| BMI | r = 0.165 | r = −0.154 | r = −0.059 | r = 0.438 |
| P = 0.09 | P = 0.12 | P = 0.71 | P = 0.03 | |
Correlations with P < 0.05 appear in boldface.
BMI, body mass index; NKCC, Na+,K+,2Cl− co-transport.
Table 4.
Ion transport and plasma lipids in French-Canadian and African-American men and women with essential hypertension: correlation analysis
| NKCC | Passive permeability for K+ |
NKCC | Passive permeability for K+ |
|
|---|---|---|---|---|
| French-Canadian men | African-American men | |||
| Triglycerides | r = 0.245 | r = 0.150 | r = −0.235 | r = 0.267 |
| P = 0.01 | P = 0.12 | P = 0.27 | P = 0.21 | |
| Cholesterol | r = 0.205 | r = 0.099 | r = −0.120 | r = 0.14 |
| P = 0.03 | P = 0.31 | P = 0.22 | P = 0.51 | |
| LDL | r = 0.06 | r = 0.051 | r = −0.103 | r = 0.201 |
| P = 0.56 | P = 0.61 | P = 0.63 | P = 0.35 | |
| HDL | r = 0.197 | r = −0.062 | r = 0.051 | r = −0.273 |
| P = 0.04 | P = 0.52 | P = 0.81 | P = 0.20 | |
| French-Canadian women | African-American women | |||
| Triglycerides | r = 0.139 | r = 0.066 | r = 0.093 | r = 0.490 |
| P = 0.17 | P = 0.51 | P = 0.56 | P = 0.00099 | |
| Cholesterol | r = 0.101 | r = 0.08 | r = −0.194 | r = 0.175 |
| P = 0.31 | P = 0.43 | P = 0.22 | P = 0.27 | |
| LDL | r = 0.06 | r = 0.049 | r = −0.152 | r = 0.100 |
| P = 0.56 | P = 0.64 | P = 0.34 | P = 0.53 | |
| HDL | r = 0.026 | r = 0.039 | r = 0.326 | r = − 0.068 |
| P = 0.79 | P = 0.70 | P = 0.03 | P = 0.67 | |
Correlations with P < 0.05 appear in boldface.
HDL, high-density lipoprotein; LDL, low-density lipoprotein; NKCC, Na+,K+,2Cl− co-transport.
Second, NKCC correlated positively with plasma triglycerides in the French-Canadian men (P = 0.01, Table 4). However, neither the French-Canadian nor the African-American women showed any significant correlation between these two parameters. Moreover, in the African-American men, this correlation tended to be negative (r = −0.235, P = 0.27) rather than positive (Table 4).
Third, in the French-Canadian men, NKCC activity correlated positively with BMI (P = 0.05, Table 3) as well as with total cholesterol (P = 0.03) and HDL (P = 0.04, Table 4). A positive correlation between HDL and NKCC was also observed in African-American women (P = 0.03, Table 4). It should be underlined, however, that neither BMI nor total cholesterol and HDL were significantly different between the African Americans and French Canadians (Table 1).
Viewed collectively, these results argue against the impact of age, BMI, and plasma lipids on the decreased NKCC activity seen in African Americans with essential hypertension.
Western blot analysis
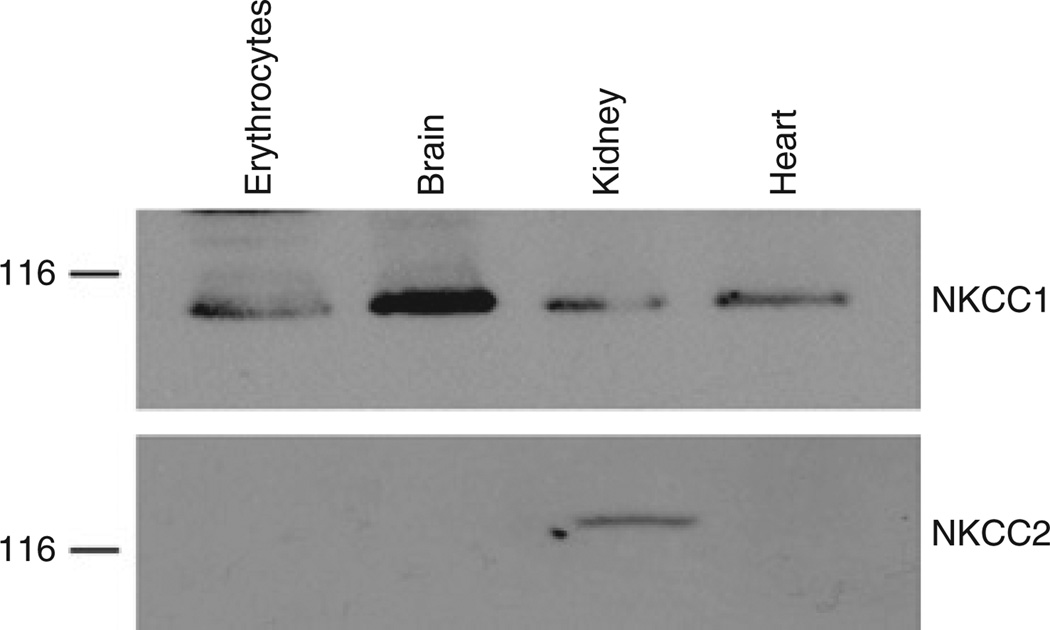
Human erythrocytes as well as rat brain, kidney, and aorta expressed ~ 116 kDa protein interacting with anti-NKCC1 antibodies (Figure 3). In contrast to NKCC1, the presence of NKCC2-immunoreactive protein was limited to the rat kidney.
Figure 3.
Representative western blots demonstrating the presence of NKCC1 and NKCC2 immunoreactive proteins in human erythrocytes and rat heart, kidney, and brain. NKCC, Na+,K+,2Cl− co-transport.
Discussion
Western blot analysis showed that NKCC1 is the only isoform of NKCC expressed in human erythrocytes (Figure 3). This conclusion is consistent with the lack of bumetanide-sensitive component of 86Rb uptake in erythrocytes isolated from NKCC1−/− mice.25,26 Our results show that NKCC1 activity is sharply reduced in African Americans with essential hypertension compared to French-Canadian Caucasians (Figure 1). In French Canadians, the activity of this carrier is affected by gender (Table 2, Figure 2). In contrast, we did not observe any significant difference in NKCC activity between the African-American men and women (Table 2). These results strongly suggest that racial differences in the activity of this carrier, seen in our study as well as in previous investigations of mixed groups of men and women,13,14,16,17,19 are gender independent.
Our French-Canadian and African-American groups were well matched for BMI but were slightly different for age (Table 1). However, the lack of NKCC correlation with age in all groups under investigation (Table 3) and with BMI in French-Canadian women and in African-American women and men (Table 3) goes against the impact of age and BMI in racial differences of this carrier’s activity.
The striking gender-, age-, and BMI-independent differences in NKCC observed between the African Americans and French Canadians with essential hypertension could be due to altered plasma lipids affecting ion fluxes across erythrocyte membranes.18 Indeed, plasma triglycerides were almost twofold lower in the African-American men and women compared to their French-Canadian counterparts (Table 1). However, the absence of correlation between plasma triglycerides and NKCC in the French-Canadian women and the African-American women and men (Table 4) argues against this hypothesis. It should be acknowledged, however, that the absence of statistical significance in some of the African-American correlations may reflect the smaller sample size (71 African Americans vs. 218 French Canadians).
Several working hypotheses might be proposed after analysis of the possible implication of decreased NKCC1 activity in features of the pathogenesis of essential hypertension and its cardiovascular and renal complications documented in African Americans.
First, NKCC1 plays a key role in transport of salt and osmotically obliged water across the basolateral membrane of secretory epithelium in distal tubule27 and sweat glands.12 Thus, decreased NKCC1 activity may lead to salt retention and the prevalence of salt-sensitive hypertension documented in African Americans.7,8 It should be stressed, however, that salt retention in African Americans with essential hypertension is accompanied by decreased plasma renin activity compared to their Caucasian counterparts, including French Canadians from the Saguenay–Lac-St-Jean region of Quebec.28 In contrast, plasma renin activity is elevated by approximately threefold in NKCC1 null mice27,29 that might be caused by direct involvement of NKCC1 in renin secretion demonstrated in comparative analysis of cultured juxtaglomerular granular cells from NKCC1+/+ and NKCC1−/− mice.29 Increased plasma renin activity as well as decreased plasma concentration of atrial natriuretic peptide and augmented expression of major renal Na+ transporters such as NKCC2, NCC, Na+/H+ exchanger (NHE3), and α1 subunit of Na+, K+-ATPase in kidney lysates from NKCC1−/− mice27 should increase vascular volume and blood pressure, thus minimizing hypotension demonstrated in these genetically engineering animals by Flagella et al. and Wall et al.25,27 Importantly, augmented salt sensitivity7,8 and NKCC2-mediated reabsorption of salt and osmotically obliged water30 detected in blacks possessing decreased NKCC1 activity are consistent with increased expression of NKCC2 (ref. 27) and salt-induced hypertension31 seen in NKCC1-deficent mice.
Second, it is well documented that HCD decrease [Cl−]i and hyperpolarize vascular smooth muscle cells. They also attenuate Ca2+ influx via voltage-gated L-type channels and reduce smooth muscle contractions triggered by diverse stimuli, including modest depolarization, α-adrenoceptor agonists, histamine, angiotensin II, thromboxane A2, and oxytocin (for complete list of references, see refs. 32–35). More recently, we reported that these actions of HCD are absent in NKCC1 null mice.26 Thus, decreased NKCC1 seen in African Americans may contribute to attenuation rather than elevation of blood pressure via its manifestation in vascular smooth muscle cells involved in regulation of peripheral resistance.
Third, because of its key role in the maintenance of capillary blood flow and hydrostatic pressure,36 the involvement of abnormal myogenic tone responses in protection against hypertension-induced organ damage is widely disputed.37 Recently, it has been reported that HCD sharply lower myogenic tone in mouse mesenteric arteries26 and completely abolish the myogenic responses of renal afferent arterioles of in vitro perfused hydronephrotic rat kidneys.35 Importantly, this action of HCD was absent in mesenteric arteries isolated from NKCC1−/− mice.26 Viewed collectively, these data allowed us to propose that decreased NKCC1 contributes to attenuated myogenic tone that, in turn, leads to high blood pressure–induced kidney damage and approximately fourfold greater prevalence of end-stage renal disease documented in hypertensive African Americans compared to their Caucasian counterpart.21,38,39
In conclusion, our study results show that NKCC1 activity in erythrocytes of African Americans with essential hypertension is twofold lower than in their French-Canadian counterparts. They also demonstrate that these differences are not caused by altered plasma lipid profile. Further experiments should be performed to uncover the mechanism of this phenomenon and its relationship to features of the pathogenesis of hypertension and its renal complications seen in African Americans.
Acknowledgments
This study was supported by grants from the National Institutes of Health (SCOR Program), and the Canadian Institutes of Health Research (MOP-14654—P.H. and S.N.O. and MOP-81392—S.N.O., J.T., and P.H.) and the Russian Foundation for Fundamental Research (09-0073/04—S.N.O.). O.A.A. is a recipient of a fellowship from the Kidney Foundation of Canada. The skilled technical work of Nathalie Bourcier, Sonia Girouard, Gilles Corbeil, and Monique Poirier; and the editorial assistance of Ovid Da Silva are appreciated.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Postnov YU, Orlov S, Gulak P, Shevchenko A. Altered permeability of the erythrocyte membrane for sodium and potassium ions in spontaneously hypertensive rats. Pflugers Arch. 1976;365:257–263. doi: 10.1007/BF01067026. [DOI] [PubMed] [Google Scholar]
- 2.Friedman SM, Nakashima M, McIndoe RA, Friedman CL. Increased erythrocyte permeability to Li and Na in the spontaneously hypertensive rat. Experientia. 1976;32:476–478. doi: 10.1007/BF01920806. [DOI] [PubMed] [Google Scholar]
- 3.Orlov SN, Adragna NC, Adarichev VA, Hamet P. Genetic and biochemical determinants of abnormal monovalent ion transport in primary hypertension. Am J Physiol. 1999;276:C511–C536. doi: 10.1152/ajpcell.1999.276.3.C511. [DOI] [PubMed] [Google Scholar]
- 4.Orlov SN. Hypertension. In: Bernhardt I, Ellory JC, editors. Red Cell Membrane Transport in Health and Disease. Springer: Berlin; 2003. pp. 587–602. [Google Scholar]
- 5.Garay RP, Alda O. What can we learn from erythrocyte Na-K-Cl cotransporter NKCC1 in human hypertension. Pathophysiology. 2007;14:167–170. doi: 10.1016/j.pathophys.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Gamba G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev. 2005;85:423–493. doi: 10.1152/physrev.00011.2004. [DOI] [PubMed] [Google Scholar]
- 7.Watson KE. Cardiovascular risk reduction among African Americans: a call to action. J Natl Med Assoc. 2008;100:18–26. doi: 10.1016/s0027-9684(15)31170-6. [DOI] [PubMed] [Google Scholar]
- 8.Krakoff LR. Diuretics for hypertension. Circulation. 2005;112:e127–e129. doi: 10.1161/CIRCULATIONAHA.105.570192. [DOI] [PubMed] [Google Scholar]
- 9.Pratt JH. Central role for ENaC in development of hypertension. J Am Soc Nephrol. 2005;16:3154–3159. doi: 10.1681/ASN.2005050460. [DOI] [PubMed] [Google Scholar]
- 10.Dill DB, Yousef MK, Goldman A, Hillyard SD, Davis TP. Volume and composition of hand sweat of White and Black men and women in desert walks. Am J Phys Anthropol. 1983;61:67–73. doi: 10.1002/ajpa.1330610107. [DOI] [PubMed] [Google Scholar]
- 11.Singh J, Gross M, Sage B, Davis HT, Maibach HI. Effect of saline iontophoresis on skin barrier function and cutaneous irritation in four ethnic groups. Food Chem Toxicol. 2000;38:717–726. doi: 10.1016/s0278-6915(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 12.Orlov SN. Decreased Na+,K+,2Cl- cotransport and salt retention in Blacks: a provocative hypothesis. J Hypertens. 2005;23:1929–1930. doi: 10.1097/01.hjh.0000181324.51786.29. [DOI] [PubMed] [Google Scholar]
- 13.Canessa ML. The Na-K-Cl cotransport in essential hypertension: cellular functions and genetic environment interactions. Int J Cardiol. 1989;25(Suppl 1):S37–S45. doi: 10.1016/0167-5273(89)90091-0. [DOI] [PubMed] [Google Scholar]
- 14.Garay RP, Nazaret C, Dagher G, Bertrand E, Meyer P. A genetic approach to the geography of hypertension: examination of Na+-K+ cotransport in Ivory Coast Africans. Clin Exp Hypertens. 1981;3:861–870. doi: 10.3109/10641968109033708. [DOI] [PubMed] [Google Scholar]
- 15.Orlov SN, Pausova Z, Gossard F, Gaudet D, Tremblay J, Kotchen T, Cowley A, Larochelle P, Hamet P. Sibling resemblance of erythrocyte ion transporters in French-Canadian sibling-pairs affected with essential hypertension. J Hypertens. 1999;17:1859–1865. doi: 10.1097/00004872-199917121-00013. [DOI] [PubMed] [Google Scholar]
- 16.Tuck ML, Gross C, Maxwell MH, Brickman AS, Krasnoshtein G, Mayes D. Erythrocyte Na+,K+ cotransport and Na+,K+ pump in black and caucasian hypertensive patients. Hypertension. 1984;6:536–544. doi: 10.1161/01.hyp.6.4.536. [DOI] [PubMed] [Google Scholar]
- 17.Canessa M, Spalvins A, Adragna N, Falkner B. Red cell sodium countertransport and cotransport in normotensive and hypertensive blacks. Hypertension. 1984;6:344–351. doi: 10.1161/01.hyp.6.3.344. [DOI] [PubMed] [Google Scholar]
- 18.Zicha J, Kunes J, Devynck MA. Abnormalities of membrane function and lipid metabolism in hypertension: a review. Am J Hypertens. 1999;12:315–331. doi: 10.1016/s0895-7061(98)00178-2. [DOI] [PubMed] [Google Scholar]
- 19.Weder AB, Torretti BA, Julius S. Racial differences in erythrocyte cation transport. Hypertension. 1984;6:115–123. doi: 10.1161/01.hyp.6.1.115. [DOI] [PubMed] [Google Scholar]
- 20.Pausova Z, Jomphe M, Houde L, Vezina H, Orlov SN, Gossard F, Gaudet D, Tremblay J, Kotchen TA, Cowley AW, Bouchard G, Hamet P. A genealogical study of essential hypertension with and without obesity in French Canadians. Obes Res. 2002;10:463–470. doi: 10.1038/oby.2002.64. [DOI] [PubMed] [Google Scholar]
- 21.Kotchen TA, Piering AW, Cowley AW, Grim CE, Gaudet D, Hamet P, Kaldunski ML, Kotchen JM, Roman RJ. Glomerular hyperfiltration in hypertensive African Americans. Hypertension. 2000;35:822–826. doi: 10.1161/01.hyp.35.3.822. [DOI] [PubMed] [Google Scholar]
- 22.Akimova OA, Grygorczyk A, Bundey RA, Bourcier N, Gekle M, Insel PA, Orlov SN. Transient activation and delayed inhibition of Na+,K+,Cl- cotransport in ATP-treated C11-MDCK cells involve distinct P2Y receptor subtypes and signaling mechanisms. J Biol Chem. 2006;281:31317–31325. doi: 10.1074/jbc.M602117200. [DOI] [PubMed] [Google Scholar]
- 23.Orlov SN, Pokudin NI, Kotelevtsev YV, Gulak PV. Volume-dependent regulation of ion transport and membrane phosphorylation in human and rat erythrocytes. J Membr Biol. 1989;107:105–117. doi: 10.1007/BF01871716. [DOI] [PubMed] [Google Scholar]
- 24.Solban N, Jia HP, Richard S, Tremblay S, Devlin AM, Peng J, Gossard F, Guo DF, Morel G, Hamet P, Lewanczuk R, Tremblay J. HCaRG, a novel calcium-regulated gene coding for a nuclear protein, is potentially involved in the regulation of cell proliferation. J Biol Chem. 2000;275:32234–32243. doi: 10.1074/jbc.M001352200. [DOI] [PubMed] [Google Scholar]
- 25.Flagella M, Clarke LL, Miller ML, Erway LC, Giannella RA, Andringa A, Gawenis LR, Kramer J, Duffy JJ, Doetschman T, Lorenz JN, Yamoah EN, Cardell EL, Shull GE. Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J Biol Chem. 1999;274:26946–26955. doi: 10.1074/jbc.274.38.26946. [DOI] [PubMed] [Google Scholar]
- 26.Koltsova SV, Kotelevtsev SV, Tremblay J, Hamet P, Orlov SN. Excitation-contraction coupling in resistance mesenteric arteries: evidence for NKCC1-mediated pathway. Biochem Biophys Res Commun. 2009;379:1080–1083. doi: 10.1016/j.bbrc.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 27.Wall SM, Knepper MA, Hassell KA, Fischer MP, Shodeinde A, Shin W, Pham TD, Meyer JW, Lorenz JN, Beierwaltes WH, Dietz JR, Shull GE, Kim YH. Hypotension in NKCC1 null mice: role of the kidneys. Am J Physiol Renal Physiol. 2006;290:F409–F416. doi: 10.1152/ajprenal.00309.2005. [DOI] [PubMed] [Google Scholar]
- 28.Kotchen TA, Kotchen JM, Grim CE, Krishnaswami S, Kidambi S. Aldosterone and alterations of hypertension-related vascular function in African Americans. Am J Hypertens. 2009;22:319–324. doi: 10.1038/ajh.2008.327. [DOI] [PubMed] [Google Scholar]
- 29.Castrop H, Lorenz JN, Hansen PB, Friis U, Mizel D, Oppermann M, Jensen BL, Briggs J, Skøtt O, Schnermann J. Contribution of the basolateral isoform of the Na-K-2Cl- cotransporter (NKCC1/BSC2) to renin secretion. Am J Physiol Renal Physiol. 2005;289:F1185–F1192. doi: 10.1152/ajprenal.00455.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chun TY, Bankir L, Eckert GJ, Bichet DG, Saha C, Zaidi SA, Wagner MA, Pratt JH. Ethnic differences in renal responses to furosemide. Hypertension. 2008;52:241–248. doi: 10.1161/HYPERTENSIONAHA.108.109801. [DOI] [PubMed] [Google Scholar]
- 31.Kim SM, Eisner C, Faulhaber-Walter R, Mizel D, Wall SM, Briggs JP, Schnermann J. Salt sensitivity of blood pressure in NKCC1-deficient mice. Am J Physiol Renal Physiol. 2008;295:F1230–F1238. doi: 10.1152/ajprenal.90392.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chipperfield AR, Harper AA. Chloride in smooth muscle. Prog Biophys Mol Biol. 2000;74:175–221. doi: 10.1016/s0079-6107(00)00024-9. [DOI] [PubMed] [Google Scholar]
- 33.Akar F, Jiang G, Paul RJ, O’Neill WC. Contractile regulation of the Na(+)-K(+)-2Cl(−) cotransporter in vascular smooth muscle. Am J Physiol Cell Physiol. 2001;281:C579–C584. doi: 10.1152/ajpcell.2001.281.2.C579. [DOI] [PubMed] [Google Scholar]
- 34.Orlov SN. NKCC1 as a regulator of vascular tone and a novel target for antihypertensive therapeutics. Am J Physiol Heart Circ Physiol. 2007;292:H2035–H2036. doi: 10.1152/ajpheart.00157.2007. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Breaks J, Loutzenhiser K, Loutzenhiser R. Effects of inhibition of the Na+/K+/2Cl- cotransporter on myogenic and angiotensin II responses of the rat afferent arteriole. Am J Physiol Renal Physiol. 2007;292:F999–F1006. doi: 10.1152/ajprenal.00343.2006. [DOI] [PubMed] [Google Scholar]
- 36.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- 37.Loutzenhiser R, Griffin K, Williamson G, Bidani A. Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1153–R1167. doi: 10.1152/ajpregu.00402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boone CA. End-stage renal disease in African-Americans. Nephrol Nurs J. 2000;27:597–600. [PubMed] [Google Scholar]
- 39.Barri YM. Hypertension and kidney disease: a deadly connection. Curr Hypertens Rep. 2008;10:39–45. doi: 10.1007/s11906-008-0009-y. [DOI] [PubMed] [Google Scholar]